Abstract
Activation of CD8+ cytolytic T lymphocytes (CTLs) by antigen is triggered by the interaction of clonotypic αβ T cell receptors (TCRs) with antigenic peptides bound to MHC class I molecules (pMHC complexes). Fluorescent multimeric pMHC complexes have been shown to specifically stain antigen-specific CTLs by directly binding the TCR. In tumor-infiltrating lymphocytes from a melanoma patient we found a high frequency of tyrosinase368–376 peptide-specific cells as detected by IFN-γ ELISPOT, without detectable staining with the corresponding A2/peptide multimers. Surprisingly, these T cells were able to lyse tyrosinase368–376 peptide-pulsed target cells as efficiently as other specific T cells that were stained by multimers. Analysis of the staining patterns under different conditions of incubation time and temperature revealed that these results were explained by major differences in TCR-multimeric ligand interaction kinetics among the clones. Whereas no direct quantitative correlation between antigenic peptide concentration required for CTL effector functions and equilibrium multimer binding was observed interclonally, the latter was profoundly affected by the kinetics of TCR-ligand interaction. More importantly, our data indicate that similar levels of T cell activation can be achieved by independent CD8+ T cell clonotypes displaying different TCR/pMHC complex dissociation rates.
CD8+ T lymphocytes specifically recognize antigen through the interaction of clonotypic αβ T cell receptors (TCRs) with antigen-derived peptides bound to MHC class I (pMHC) molecules. After binding of TCR to pMHC complex, recognition is translated into intracellular biochemical events by other polypeptides in the TCR complex, i.e., the CD3 chain that possess immunoreceptor tyrosine-based activation sequence motifs located in the cytoplasmic domain. The CD8 coreceptor is also part of the TCR complex and can contribute to T cell activation by slowing the dissociation rate of pMHC ligand from the TCR (1, 2) and/or by recruiting intracellular signaling molecules (3, 4). The complexity of the TCR signaling machinery is most likely required to discriminate among numerous ligands that can initiate a variety of quantitatively and qualitatively different T cell responses that span from partial or full activation to inhibition of activation (5).
Soluble pMHC complexes represent the ideal natural ligand for the analysis of the relationship between the primary event (binding of the TCR to pMHC complex) and T cell activation. Initial efforts to stain the TCR by using this reagent in its monomeric form, however, failed because of the low affinity of this interaction. More recently fluorescent multimeric arrays of pMHC class-I complexes have been shown to bind to cytolytic T lymphocytes (CTLs) sufficiently well to allow their direct labeling. Multimers have already been widely used to quantitate and characterize CTL responses (6–8). In contrast, few studies have attempted to elucidate the relationship between efficiency of multimer staining and CTL effector functions (9–11).
In this study we found a clear discrepancy between CTL effector functions measured both as cytokine secretion and specific lytic activity and staining with multimers in tumor-infiltrating lymphocytes (TILs) from a melanoma patient. Through a detailed analysis of the efficiency of staining on representative clonal populations under various conditions of incubation time and temperature we detected major differences in the kinetics of pMHC-multimer interaction with TCR among specific clones. An important finding was that clonotypes recognizing pMHC with similar efficiency can display very different kinetics of TCR-ligand interaction, resulting in differential staining with multimers.
Materials and Methods
Patients, Tissues, and Cells.
TIL Me 336 was obtained from a surgically resected melanoma metastatic lesion from patient LAU 156. TILs were prepared as described (12). Briefly, the sample was finely minced with needles in sterile RPMI 1640 medium supplemented with 10% FCS. The cell suspension was placed in a 24-well tissue culture plate (Costar) in 2 ml/well of Iscove's Dulbecco medium (Life Technologies, Basel, Switzerland) supplemented with 8% pooled human A+ serum (CTL medium), 100 units/ml human recombinant IL-2 (Glaxo; kindly provided by M. Nabholz, Institut Suisse de Recherches Experimentales sur le Cancer, Epalinges, Switzerland), and 10 ng/ml human recombinant IL-7 (R & D Systems). Cells were cultured 3 weeks before analysis. CTL clone LAU 156/34 was derived from TIL Me 336 by limiting dilution culture in the presence of irradiated allogeneic peripheral blood mononuclear cells, phytohemagglutinin, and human recombinant IL-2 as described (21). Clones LAU 132/1D5/1 and LAU 132/1G4/1 were similarly derived from A2/tyrosinase368–376/370D multimer+ cells isolated by cell sorting from tumor-infiltrated lymph node of melanoma patient LAU 132 (12).
51Cr Release Assay.
Labeled target cells (1,000 cells in 50 μl) were incubated in the presence of various concentrations of peptide (50 μl) for 15 min at room temperature before the addition of effector cells (10,000 cells in 50 μl). Chromium release was measured in supernatants (100 μl) harvested after 4-h incubation at 37°C. The percent specific lysis was calculated as: 100 × [(experimental − spontaneous release)/(total − spontaneous release).
Multimers, mAbs, and Flow Cytometry Immunofluorescence Analysis.
HLA-A2/peptide multimers were synthesized as described (6, 13). As the antigenic peptides, the Melan-A26–35 27L (ELAGIGILTV; ref. 14), Camel1–11 (MLMAQEALAFL; ref. 15), NY-ESO-1157–165 (SLLMWITQC; ref. 16), and tyrosinase368–376 (YMXGTMSQV, X = 370D or 370N; ref. 17) were used. Cells were stained with multimers in 20 μl of PBS, 2% BSA, and 0.2% azide (staining buffer) for the indicated time and at the indicated dose and temperature. In the case of polyclonal populations 20 μl of anti-CD8FITC (Becton Dickinson) was added at the end of the incubation with multimers, and the samples were incubated for an additional 30 min at 4°C. In all cases, at the end of the incubation period, cells were washed once in the same buffer and analyzed by flow cytometry. Data analysis was performed by using cell quest software. For dissociation experiments cells were stained with multimers at the indicated dose during 4 h at 4°C in 100 μl of staining buffer. Cells were washed two times (at 4°C) in 1 ml/sample of the same buffer to eliminate unbound multimers. Cells were then resuspended in 75 μl of buffer, and an aliquot (5 μl, corresponding to time to) was taken. Then 25 μl of buffer was added and incubation was pursued at room temperature for 3 h. During this period, aliquots were collected at different time points, and the cells were washed and resuspended in PBS, 10% formalin, 2% glucose and 0.3% sodium azide (fixing buffer). Intensity of multimer staining was measured by flow cytometry. The intensity of multimer fluorescence at each time point was expressed as the percentage of multimer fluorescence obtained at time t0. The rates of TCR-multimer dissociation observed were independent of the dose of multimer used as dissociation experiments performed by using serial multimer dilutions for the staining gave rise to curves with similar slopes for a given clone (not shown).
IFN-γ ELISPOT Assay.
IFN-γ ELISPOT assay (18) was performed in nitrocellulose-lined 96-well microplates (Millipore MAHA S45) by using a IFN-γ ELISPOT kit (Diaclone, Besancone, France) according to the manufacturer's instructions with minor modifications. Plates were coated overnight with antibody to human IFN-γ and washed six times. C1R.A2 cells (19) (5 × 104/well) were then added together with the indicated number of responder T cells (104/well) and peptide (1 μM where indicated) and incubated for 20 h at 37°C. Cells were then removed and plates were developed with a second antibody to human IFN-γ (biotinylated) and streptavidin-alkaline phosphatase. Spots were counted by using Bioreader 2000 (BioSys, Frankfurt, Germany).
Results and Discussion
Discrepancy Between IFN-γ ELISPOT and Multimer Staining Analysis of TIL Me 336.
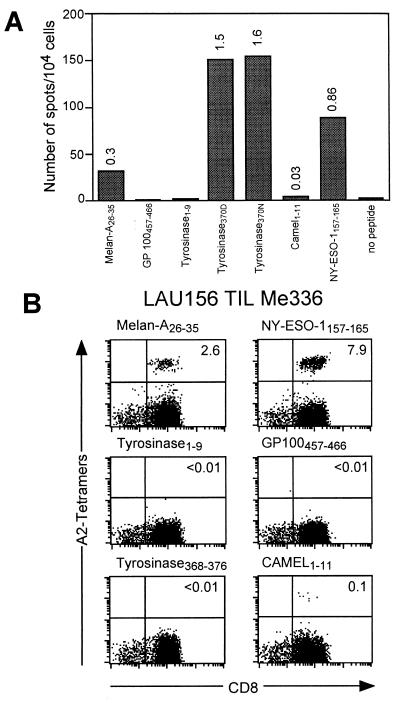
Melanoma patient LAU 156 (HLA*0201+) had an ocular melanoma that evolved slowly with recurrent metastases all localized at a single paravertebral site that were resected at different time points. TILs from one of these lesions (Me 336) were cultured in the presence of IL-2 and IL-7 for 3 weeks. At the end of this culture period TIL Me 336 contained >90% CD3+CD8+ T lymphocytes. This culture was simultaneously assayed for the presence of CD8+ T cells specific for several known HLA-A2-restricted epitopes from tumor antigens frequently expressed in melanoma, by IFN-γ ELISPOT and by staining with A2/peptide multimers incorporating each of the corresponding synthetic peptides. As illustrated in Fig. 1A, IFN-γ producing cells were clearly detected in the presence of peptides Melan-A26–35 (20), Camel1–11 (15), NY-ESO-1157–165 (16), and tyrosinase368–376/370D and tyrosinase368–376/370N (17). In agreement with these data, CD8+ Melan-A26–35, Camel1–11, and NY-ESO-1157–165 multimer+ T cell populations were clearly detected by staining with the corresponding multimers. In contrast, no specific staining was detected with A2/tyrosinase370D multimers. Because the ELISPOT analysis underestimates the frequency of antigen-specific cells by a factor of 4- to 7-fold, it could be estimated that a population of specific T cells representing 6–10% of the cultured TILs was undetectable by staining with A2/tyrosinase370D multimers under our conventional staining conditions (1 h incubation at room temperature).
Figure 1.
Discrepancy between IFN-γ ELISPOT and multimer staining analysis of TIL Me 336. (A) TIL Me 336 was obtained from a cell suspension prepared from a melanoma metastasis of patient LAU 156 as detailed in Materials and Methods. TILs (104 cells/well) were tested by IFN-γ ELISPOT assay in the presence of antigen-presenting cells alone (C1R.A2, 5 × 104 cells/well) or antigen-presenting cells plus the indicated peptide (1 μM) in duplicate cultures. Numbers on bars represent the percentages of antigen-specific cells calculated as the mean number of spot of duplicate cultures divided by the total number of cells in each well × 100. (B) For multimer staining analysis TILs were stained with the indicated multimers together with anti-CD8FITC as detailed in Materials and Methods. Numbers in the upper right quadrant indicate the percentage of multimer+ cells within CD8+ T lymphocytes.
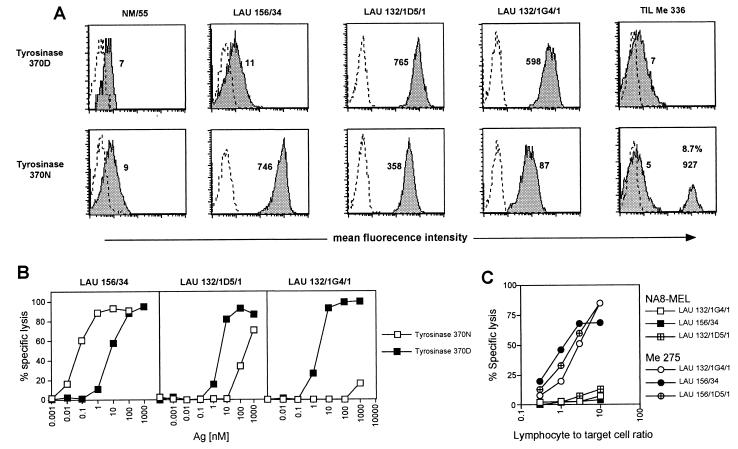
Monoclonal CD8+ T cell populations were isolated by limiting dilution cloning of the TIL in the presence of phytohemagglutinin and allogeneic feeder cells, as described (21). As autologous tumor cell lines from patient LAU 156 were not available, tumor-reactive clones were selected by testing single growing monoclonal populations for their capacity to specifically lyse an HLA*0201-matched tumor cell line (Me 275, which expresses Melan-A, tyrosinase, and NY-ESO-1, Fig. 2C). Antigen specificity of tumor reactive clones was subsequently assessed on T2 cells (HLA*0201+ and Melan-A, tyrosinase and NY-ESO-1 negative) pulsed or not with the corresponding synthetic peptide (not shown). Among tumor-reactive monoclonal populations, a monoclonal tyrosinase-specific CD8+ T cell population (clone LAU 156/34) was isolated. Consistent with the results obtained with the TIL, clone LAU 156/34 was not stained by A2/tyrosinase370D multimers, whereas two previously described clones (LAU 132/1D5/1 and LAU 132/1G4/1) (12), derived from A2/tyrosinase370D multimer guided cell sorting from tumor-infiltrated lymph node of melanoma patient LAU 132, were efficiently stained (Fig. 2A). Unexpectedly, this result could not be explained by differential avidity of antigen recognition because the dose of peptide tyrosinase370D required to obtain half-maximal lysis in a standard 51Cr release assay was similar for the three clonal populations (2–6 nM, Fig. 2B).
Figure 2.
Comparison between fine specificity of peptide recognition and tumor lysis by tyrosinase-specific T cell clones and staining with A2/peptide multimers incorporating tyrosinase370D or tyrosinase370N peptide variants. (A) T cell clones or TILs were stained with the indicated multimers as detailed in Materials and Methods. Dotted = unstained cells; shaded = stained cells. Numbers indicate mean fluorescence values for the different populations. In the case of TILs the percentage of A2/tyrosinase370N multimer+ cells is also indicated. The level of nonspecific multimer binding in this assay is illustrated by the signal obtained on CTL clone NM55, which is specific for an unrelated influenza matrix peptide. (B) The efficiency of antigen recognition by clones LAU 156/34, LAU 132/1D5/1, and LAU 132/1G4/1 was tested in a chromium release assay as detailed in Materials and Methods. Lysis of chromium-labeled target cells was assessed at a lymphocyte-to-target cell ratio of 10:1 in the presence of serial dilutions of peptide tyrosinase370N (□) or peptide tyrosinase370D (■). (C) Specific lysis of tyrosinase+ (circles) or tyrosinase− (squares) melanoma cell lines by tyrosinase370N- or tyrosinase370D-specific CTLs was assessed in a chromium release assay as detailed in Materials and Methods. Tyrosinase-specific CTLs were added to melanoma cells at the indicated lymphocyte-to-target cell ratio.
For peptide tyrosinase368–376, the sequence identified by MS in melanoma cell peptide eluates differs from the gene-encoded sequence as the result of posttranslational modification of amino acid residue 370 (asparagine [N] to aspartic acid [D]). The two peptide variants display comparable binding to HLA-A2 molecules but can be recognized by CTL with different efficacy (17). Interestingly, the clones differed in terms of fine specificity of recognition of the two peptide variants. Indeed, whereas clones LAU 132/1D5/1 and LAU 132/1G4/1 recognized peptide tyrosinase370D more efficiently than tyrosinase370N, clone LAU 156/34 recognized the latter 1,000-fold more efficiently than tyrosinase370D (Fig. 2B). The same hierarchy of peptide recognition was observed in CD3 down-regulation experiments (Fig. 5A, which is published as supplemental data on the PNAS web site, www.pnas.org). It is of note that, similarly to what was observed in standard 51Cr release assay, comparable levels of CD3 down-regulation were measured for the different clones upon stimulation with tyrosinase370D peptide-pulsed antigen-presenting cells. Similar results were obtained in cytokine (IFN-γ/tumor necrosis factor α) secretion experiments (Fig. 5B). The three clones were efficiently stained by tyrosinase370N multimers, including clone LAU 132/1G4/1 that recognized peptide tyrosinase370N very poorly (Fig. 2B). In this case, however, the intensity of multimer staining correlated, at least qualitatively, with the efficacy of recognition of peptide tyrosinase370N by the CTL clones. In addition, staining of TIL Me 336 with tyrosinase370N multimers resulted in the detection of a population of CD8+ multimer+ T cells that constituted 8.7% of the total CD8+ T cells (Fig. 2A).
Clone LAU 156/34 was tumor-reactive. Indeed, although its ability to specifically lyse the autologous tumor could not be assessed because of the unavailability of tumor cell lines from patient LAU 156, the clone was able to specifically lyse six of nine A2+tyrosinase+ melanoma lines tested (Fig. 2C and unpublished work). In addition, several other clones recognizing tyrosinase370N more efficiently than tyrosinase370D were isolated from peptide-stimulated peripheral blood mononuclear cells of several other melanoma patients, thus showing that these clones are a regular component of the tyrosinase-specific CD8+ T cell repertoire.
Dissociation Between CTL Effector Functions and TCR-Ligand Interaction Kinetics.
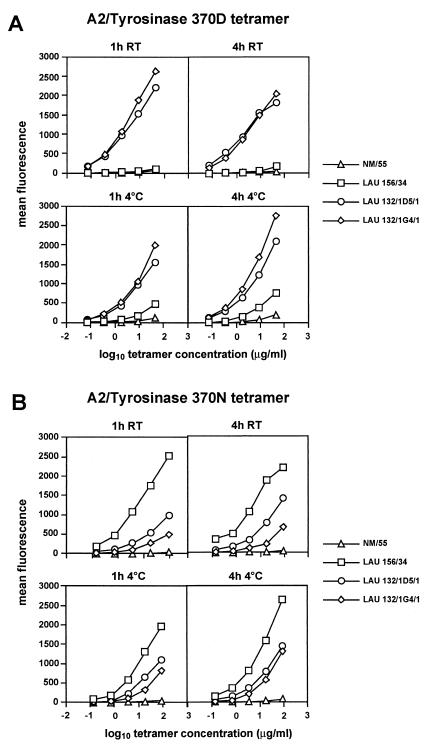
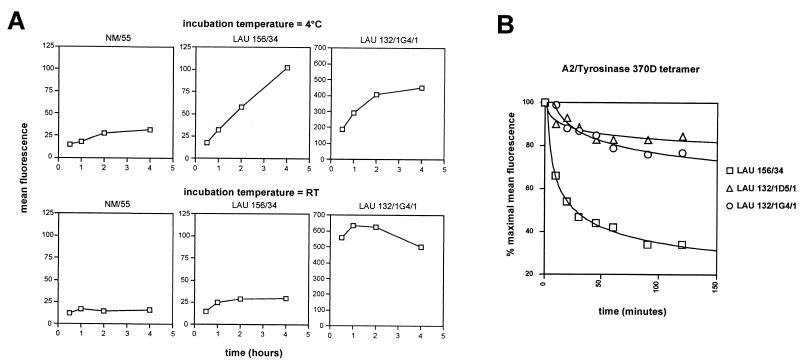
The molecular bases for the ability of specific clones displaying the same apparent avidity for peptide tyrosinase370D to be stained or not with pMHC fluorescent multimers were investigated by incubating them with serial dilutions of multimers under different conditions of time and temperature. Efficient staining was obtained with multimers incorporating peptide tyrosinase370D on both clones LAU 132/1G4/1 and LAU 132/1D5/1 upon staining during 1 h at room temperature and was slightly less intense after 4 h. On the contrary, clone LAU 156/34 showed a very modest level of specific staining (as compared with a influenza matrix-specific clone, NM55, used as an internal control) only after 4 h. Incubation for longer periods failed to further increase the fluorescent signal for all populations (data not shown). Staining of the same populations at 4°C resulted in a less intense staining of all clones after 1 h as compared with 4 h. However, in this case specific staining on clone LAU 156/34 was clearly detectable (Fig. 3A). The three clones were specifically stained by multimers incorporating peptide tyrosinase370N, but also in this case the intensity of multimer staining varied, depending on the incubation time and temperature. For example, a 10-fold higher dose of tyrosinase370N multimer was required to obtain a comparable level of staining of clone LAU 132/1G4/1 than on clone LAU 132/1D5/1 upon incubation during 1 h at room temperature, but the difference was only 2-fold upon staining at 4°C (Fig. 3B). In the experiments illustrated in Fig. 4 we followed more closely the interaction of multimers incorporating peptide tyrosinase370D with clone LAU 156/34 over time. Whereas incubation at room temperature resulted in only a modest increase of specific staining, as compared with internal controls, incubation at 4°C resulted in a significant and sustained increase in the mean fluorescence over time. These results clearly suggested that the inability of clone LAU 156/34 to be stained with multimers incorporating peptide tyrosinase370D could be caused by a relative instability of the complexes formed between its TCR and the fluorescent multimers as compared with other clones. To directly verify this hypothesis, we incubated the clones with multimers during 4 h at 4°C, washed off the unbound multimers and then followed the dissociation of multimers from the TCR at room temperature. Indeed, under these test conditions, multimers dissociated from clone LAU 156/34 significantly faster than from clones LAU 132/1G4/1 and LAU 132/1/D5/1 (Fig. 4B). Thus, because of the different off-rates of multimers incorporating a given peptide variant from individual clonally distributed TCR, T cell clones recognizing a given antigenic peptide (in this case tyrosinase370D) with similar avidity can display a very different ability to be stained with multimers incorporating that peptide. It is of note that at the clonal level, however, multimer off-rates directly correlated with the potency of individual ligands (Fig. 6, which is published as supplemental data on the PNAS web site).
Figure 3.
The efficiency of A2/tyrosinase peptide multimer binding to tyrosinase- specific clones varies with time and temperature conditions. T cell clones were stained with serial dilutions of A2/tyrosinase370D (A) or tyrosinase 370N (B) peptide multimers starting at a concentration of 50 μg/ml for tyrosinase370D and 100 μg/ml for tyrosinase370N under the indicated conditions as detailed in Materials and Methods. At the end of the incubation periods the cells were washed and maintained at 4°C until flow cytometry analysis. Data are shown as relative mean fluorescence.
Figure 4.
Interclonal heterogeneity of TCR-ligand binding kinetics. (A) T cell clones or TILs were stained with A2/tyrosinase370D multimers under the indicated conditions as detailed in Materials and Methods. At the end of each incubation period the cells were washed and fixed. After the last incubation cells were simultaneously analyzed by flow cytometry. Data are shown as relative mean fluorescence where each point represents the mean of duplicates. (B) T cell clones were stained by using a suboptimal dose of multimer to obtain a relative mean fluorescence of ≈200 (clone LAU 156/34: 5 μg/ml; clone LAU 132/1D5/1: 0.5 μg/ml; clone LAU 132/1G4/1: 5 μg/ml). TCR-multimer dissociation was performed as detailed in Materials and Methods. Data are shown as percentage of cell-associated fluorescence at different times of incubation. The results shown are from one of three independent experiments giving comparable results.
The results presented in this study are globally explained by differential kinetics of interaction of the TCR with pMHC multimeric ligands. TCRs that form relatively stable pMHC complexes are efficiently stained with multimers under conventional staining conditions, whereas TCRs that form more unstable complexes are relatively poorly stained or not stained at all depending on the staining conditions. These data clearly show that the kinetics of interaction of TCRs with pMHC complexes has to be taken into account when analyzing T cells with multimers.
The most surprising observation of this study, however, is that TCR-ligand interactions displaying different kinetics result in a comparable level of T cell activation that involves both early and late activation events (from TCR down-regulation to cytokine secretion), indicating that in all cases efficient signaling have occurred. Several models have been proposed to explain how the TCR is able to determine the potency of a given ligand. These models fall into two main groups: occupancy models and kinetic models. Occupancy models suggest that the potency of a TCR ligand basically follows the law of mass action and is hence the result of the product of its concentration and affinity (22–25). Thus, according to this model, the biochemical events within the cell would depend on the number of receptors occupied at one given time. The kinetic model suggests instead that the potency of a ligand is primarily determined by the off-rate of the TCR-ligand interaction (26–28). According to this model, a minimal time of interaction (corresponding to a certain off-rate) is required for a complete signal to be sent to the T cell. The ligand will in this case be considered a full agonist. Faster off-rates would result in weak agonism or antagonism (28). In addition, it has been recently proposed that off-rates slower than optimal could result in suboptimal signaling (23, 29, 30).
Whereas occupancy and kinetic models are not mutually exclusive and measurements of TCR-ligand kinetics do not always allow to distinguish between them, experimental data would mostly be in favor of off-rates being the main determinant of TCR-ligand potency. Indeed, Kersh et al. (31) have shown that ligands with similar affinities for the TCR can differ drastically with respect to their potency for T cell activation. In contrast, several studies have demonstrated that the biological effect of a TCR-ligand interaction often correlates with the half-life of the interaction (23, 28, 31, 32). It is of note that these studies have been conducted mostly by comparing the potency of different ligands with respect to a single TCR. The data reported here are only in apparent conflict with the kinetic model: in reality they do not inherently contradict but refine this concept. Indeed, in our study we observed a direct correlation between TCR-ligand potency and off-rates intraclonally. In contrast, multimers incorporating a ligand of similar potency for different clonal populations dissociated from the latter with significantly different off-rates. Therefore, our data indicate that the optimal time of TCR-ligand interaction required for full T cell activation would differ for different TCRs. The molecular and biochemical basis of this phenomenon, however, remain to be further elucidated.
Supplementary Material
Acknowledgments
We thank Dr. C. Servis for peptide synthesis, N. Montandon and K. Muehlethaler for excellent technical assistance, and M. Van Overloop for assistance in manuscript preparation. We also thank Dr. P. Batard for assistance in the FACS analysis. We are grateful to melanoma patients LAU 132 and LAU 156 for their generous participation in this research project.
Abbreviations
- CTL
cytolytic T lymphocyte
- pMHC
peptide/MHC
- TCR
T cell receptor
- TIL
tumor-infiltrating lymphocyte
References
- 1.Garcia K C, Scott C A, Brunmark A, Carbone F R, Peterson P A, Wilson I A, Teyton L. Nature (London) 1996;384:577–581. doi: 10.1038/384577a0. [DOI] [PubMed] [Google Scholar]
- 2.Luescher I F, Vivier E, Layer A, Mahiou J, Godeau F, Malissen B, Romero P. Nature (London) 1995;373:353–356. doi: 10.1038/373353a0. [DOI] [PubMed] [Google Scholar]
- 3.Chalupny N J, Ledbetter J A, Kavathas P. EMBO J. 1991;10:1201–1207. doi: 10.1002/j.1460-2075.1991.tb08061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoeveler A, Malissen B. Mol Immunol. 1993;30:755–764. doi: 10.1016/0161-5890(93)90147-4. [DOI] [PubMed] [Google Scholar]
- 5.Sloan-Lancaster J, Allen P M. Annu Rev Immunol. 1996;14:1–27. doi: 10.1146/annurev.immunol.14.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 7.Callan M F, Tan L, Annels N, Ogg G S, Wilson J D, O'Callaghan C A, Steven N, McMichael A J, Rickinson A B. J Exp Med. 1998;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallimore A, Glithero A, Godkin A, Tissot A C, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yee C, Savage P A, Lee P P, Davis M M, Greenberg P D. J Immunol. 1999;162:2227–2234. [PubMed] [Google Scholar]
- 10.Whelan J A, Dunbar P R, Price D A, Purbhoo M A, Lechner F, Ogg G S, Griffiths G, Phillips R E, Cerundolo V, Sewell A K. J Immunol. 1999;163:4342–4348. [PubMed] [Google Scholar]
- 11.Burrows S R, Kienzle N, Winterhalter A, Bharadwaj M, Altman J D, Brooks A. J Immunol. 2000;165:6229–6234. doi: 10.4049/jimmunol.165.11.6229. [DOI] [PubMed] [Google Scholar]
- 12.Valmori D, Pittet M J, Vonarbourg C, Rimoldi D, Lienard D, Speiser D, Dunbar R, Cerundolo V, Cerottini J C, Romero P. Cancer Res. 1999;59:4050–4055. [PubMed] [Google Scholar]
- 13.Romero P, Dunbar P R, Valmori D, Pittet M, Ogg G S, Rimoldi D, Chen J-L, Liénard D, Cerottini J C, Cerundolo V. J Exp Med. 1998;188:1641–1650. doi: 10.1084/jem.188.9.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valmori D, Fonteneau J F, Lizana C M, Gervois N, Lienard D, Rimoldi D, Jongeneel V, Jotereau F, Cerottini J C, Romero P. J Immunol. 1998;160:1750–1758. [PubMed] [Google Scholar]
- 15.Aarnoudse C A, van den Doel P B, Heemskerk B, Schrier P I. Int J Cancer. 1999;82:442–448. doi: 10.1002/(sici)1097-0215(19990730)82:3<442::aid-ijc19>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 16.Jager E, Chen Y T, Drijfhout J W, Karbach J, Ringhoffer M, Jager D, Arand M, Wada H, Noguchi Y, Stockert E, et al. J Exp Med. 1998;187:265–270. doi: 10.1084/jem.187.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skipper J C, Hendrickson R C, Gulden P H, Brichard V, Van Pel A, Chen Y, Shabanowitz J, Wolfel T, Slingluff C L, Jr, Boon T, et al. J Exp Med. 1996;183:527–534. doi: 10.1084/jem.183.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Czerkinsky C, Andersson G, Ekre H P, Nilsson L A, Klareskog L, Ouchterlony O. J Immunol Methods. 1988;110:29–36. doi: 10.1016/0022-1759(88)90079-8. [DOI] [PubMed] [Google Scholar]
- 19.Storkus W J, Howell D N, Salter R D, Dawson J R, Cresswell P. J Immunol. 1987;138:1657–1659. [PubMed] [Google Scholar]
- 20.Romero P, Gervois N, Schneider J, Escobar P, Valmori D, Pannetier C, Steinle A, Wolfel T, Lienard D, Brichard V, et al. J Immunol. 1997;159:2366–2374. [PubMed] [Google Scholar]
- 21.Valmori D, Dutoit V, Liénard D, Rimoldi D, Pittet M, Champagne P, Ellefsen U, Sahin U, Speiser D, Lejeune F, et al. Cancer Res. 2000;60:4499–4506. [PubMed] [Google Scholar]
- 22.Sykulev Y, Cohen R J, Eisen H N. Proc Natl Acad Sci USA. 1995;92:11990–11992. doi: 10.1073/pnas.92.26.11990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alam S M, Travers P J, Wung J L, Nasholds W, Redpath S, Jameson S C, Gasciogne N R J. Nature (London) 1996;381:616–620. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 24.Schodin B A, Tsomides T J, Kranz D M. Immunity. 1996;5:137–146. doi: 10.1016/s1074-7613(00)80490-2. [DOI] [PubMed] [Google Scholar]
- 25.Liu C P, Crawford F, Marrack P, Kappler J. Proc Natl Acad Sci USA. 1998;95:4522–4526. doi: 10.1073/pnas.95.8.4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKeithan T W. Proc Natl Acad Sci USA. 1995;92:5042–5046. doi: 10.1073/pnas.92.11.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabinowitz J D, Beeson C, Lyons D S, Davis M M, McConnell H M. Proc Natl Acad Sci USA. 1996;93:1401–1405. doi: 10.1073/pnas.93.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyons D S, Lieberman S A, Hampl J, Boniface J J, Chien Y, Berg L J, Davis M M. Immunity. 1996;5:53–61. doi: 10.1016/s1074-7613(00)80309-x. [DOI] [PubMed] [Google Scholar]
- 29.Sykulev Y, Vugmeyster Y, Brunmark A, Ploegh H L, Eisen H N. Immunity. 1998;9:475–483. doi: 10.1016/s1074-7613(00)80631-7. [DOI] [PubMed] [Google Scholar]
- 30.Kessler B M, Bassanini P, Cerottini J C, Luescher I F. J Exp Med. 1997;185:629–640. doi: 10.1084/jem.185.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kersh G J, Kersh E N, Fremont D H, Allen P M. Immunity. 1998;9:817–826. doi: 10.1016/s1074-7613(00)80647-0. [DOI] [PubMed] [Google Scholar]
- 32.Matsui K, Boniface J J, Steffner P, Reay P A, Davis M M. Proc Natl Acad Sci USA. 1994;91:12862–12866. doi: 10.1073/pnas.91.26.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.