Abstract
Cardiac muscle regeneration after injury is limited by “irreversible” cell cycle exit. Telomere shortening is one postulated basis for replicative senescence, via down-regulation of telomerase reverse transcriptase (TERT); telomere dysfunction also is associated with greater sensitivity to apoptosis. Forced expression of TERT in cardiac muscle in mice was sufficient to rescue telomerase activity and telomere length. Initially, the ventricle was hypercellular, with increased myocyte density and DNA synthesis. By 12 wk, cell cycling subsided; instead, cell enlargement (hypertrophy) was seen, without fibrosis or impaired function. Likewise, viral delivery of TERT was sufficient for hypertrophy in cultured cardiac myocytes. The TERT virus and transgene also conferred protection from apoptosis, in vitro and in vivo. Hyperplasia, hypertrophy, and survival all required active TERT and were not seen with a catalytically inactive mutation. Thus, TERT can delay cell cycle exit in cardiac muscle, induce hypertrophy in postmitotic cells, and promote cardiac myocyte survival.
Irreversible cell cycle exit limits the restoration of pump function after myocardial infarction (cardiac cell death from ischemia or reperfusion injury) or after chronic myocyte loss to apoptosis in heart failure (1). Thus, apart from fundamental interests, mechanisms underlying cardiac cell cycle exit hold importance given the therapeutic potential of regenerative cardiac muscle growth. Terminal differentiation involves the retinoblastoma family of tumor-suppressor pocket proteins and cyclin-dependent protein kinases (Cdks) that modulate their function (2). Although the role of pocket proteins and Cdks has been substantiated in cardiac cell cycle control (1, 3, 4), neither suffices to explain and impose the timing of the postmitotic phenotype in vivo. The likely need for multiple activators acting in concert and the coexistence of multiple inhibitors together create a formidable barrier to ventricular myocyte proliferation beyond the immediate perinatal period.
One mechanism that can cooperate with these, and mediates replicative senescence in other systems, is down-regulation of telomerase reverse transcriptase (TERT) and the resulting loss of telomerase activity (5–8). The telomere is a specialized DNA-protein complex, in species with linear genomes, that prevents linear chromosome ends from being sensed as a DNA strand break, triggering growth arrest and other responses (7–10). In vertebrates, the telomere comprises a conserved TTAGGG repeat, in a large duplex loop that buries the 3′ end in a lariat structure (9). As a putative mitotic clock (5–8), cell division erodes the telomeric repeat because of incomplete replication of the distal 3′ strand (the “end-replication” problem). During active replicative growth, TERT utilizes an intrinsic RNA component (TERC) as template, to restore the repeat and maintain telomere length. Telomere repeat-binding factors (TRF) 1 and 2 stabilize the lariat structure (7); TRF2, especially, is important for telomeric signaling to DNA damage checkpoint controls (10). At least in yeast, telomeric signals also involve transcriptional silencing of genes positioned near the telomere (7).
In adult humans, telomerase activity is found predominantly in germ cells, tumor cells, and stem cells with limitless proliferative capacity, but not somatic cells that ultimately senesce. In adult mice, telomerase activity is more widespread in tissue-specific patterns that correspond to the potential for sustained proliferation (11). Failure to express TERT is the principal mechanism for low levels of telomerase activity seen with replicative senescence. Telomerase activity can be reconstituted by ectopic expression of TERT (5, 12); thus, the catalytic subunit ordinarily is limiting. In mouse embryonic stem cells, even hemizygosity for TERT leads to telomere erosion (13). However, prolongation of cell lifespan can require other alterations in addition to TERT.
The need for whole-animal studies of telomerase function is underscored by discrepancies in long-term cell culture, used to model replicative senescence (8), plus differences among species in telomere biology (8, 14). The role of telomerase in mice has been questioned, in part because telomere length is greater in Mus musculus than in wild-derived species (11) or humans. Nevertheless, essential roles have been shown for mice, especially in highly proliferative organs, aging, and pathophysiological settings with high cell turnover (14). Of note, defects in mice lacking the RNA component of telomerase involve apoptosis, not just proliferation defects (14, 15), and telomerase may protect cells against at least some causes of programmed cell death (16).
Given that telomerase activity and TERT expression are lacking or are markedly decreased in the adult heart (17, 18), we postulated that preventing the down-regulation of TERT might delay or prevent the loss of telomerase activity and, if so, delay or prevent ventricular myocytes' exit from the cell cycle. To test this, exogenous TERT was forcibly expressed in mouse myocardium. TERT maintained telomerase activity in the adult heart and delayed ventricular myocytes' exit from the cell cycle in the first month after birth. Less expectedly, cardiac myocyte enlargement (hypertrophy) was provoked at later ages, without mechanical dysfunction or fibrosis as initiating factors. Hypertrophy also was elicited by TERT in cultured cardiac myocytes after viral gene transfer, suggesting additional functions of TERT beyond those reported to date. Consistent with other evidence for a cytoprotective effect of telomerase, TERT conferred protection from cardiac myocyte apoptosis. All three functions—delayed cell cycle exit, hypertrophy, and survival—required active TERT and were not evoked by a catalytically defective mutation.
Materials and Methods
Northern Blot Analysis.
Twenty μg aliquots of cardiac RNA were size-fractionated in agarose/formaldehyde gels and transferred to Hybond-N+ membranes (Amersham Pharmacia Biotech). TERT, atrial natriuretic factor (ANF), myosin heavy chain (MHC), c-myc, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probes were amplified by reverse transcription (RT)-PCR (19–21). Other probes were amplified by PCR, using the following primers: catalytic subunit of DNA-dependent protein kinase (DNA-PKcs), forward 5′-ATCAGAAGGTCTAAGGCTGGAAT-3′, reverse 5′-CGTACGGTGTTGGCTACTGC-3′; Ku70, forward 5′-GAGCATCCAGTGTATCCAGA-3′ and reverse 5′-CAGCATGATCCTCTTGTGAC-3′; Ku80, forward 5′-TCACAGTGTGCAGACACCTG-3′ and reverse 5′-AACTGCAGAGAGATGCCAGA-3′; TRF1, forward 5′-CATGGACTACACAGACTTAC-3′ and reverse 5′-ATCTGGCCTATCCTTAGACG-3′; and TRF2, forward 5′-TGTCTGTCGCGCATTGAAGA-3′ and reverse 5′-GCTGGAAGACCTCATAGGAA-3′.
Telomerase Activity.
Telomerase activity was measured by a modified telomeric repeat amplification protocol (TRAP; TRAPeze; Intergen, Purchase, NY; ref. 22), using 1 μg of cell or tissue extract, PCR amplification for 28 cycles, and nondenaturing PAGE.
Telomere Length.
Ten μg aliquots of cardiac DNA were digested with RsaI, which yields 25–30-kbp telomeric fragments in mice (23), fractionated by 0.5% agarose gel electrophoresis, and transferred to Hybond-N+ membranes. Hybridization was performed by using a 32P-labeled (TTAGGG)4 telomeric probe (24).
Generation of Active and Inactive TERT Transgenic Mice.
The cardiac-specific TERT transgene was constructed using the human TERT coding sequence from pGRN145 (25), cloned 3′ to the 5.5-kbp mouse αMHC promoter (26) and 5′ to the human growth hormone (GH) polyadenylation sequence. Catalytically inactive TERT cDNA was produced by site-directed mutagenesis (aspartic acid to alanine at codon 868; D868A). Expression cassettes were released with KpnI and NotI, and microinjected into the pronuclei of fertilized FVB/N oocytes. Tail DNA from 3-wk-old mice was subjected to Southern or dot blot analysis with full-length αMHC-TERT and αMHC-D868A.
Western Blot and Immune Complex Kinase Assays.
Abs against TERT, Cdks, p70 S6K, JNK1, and ERK1/2 were from Santa Cruz Biotechnology. Abs for Akt, p38, and phosphorylated proteins were from New England Biolabs. Fifty μg aliquots were size-fractionated by SDS/PAGE and transferred to Immobilon-P membranes (Millipore). Membranes were incubated with primary Abs (1:500–1,000) then horseradish peroxidase-conjugated second Abs. Protein expression was visualized with enhanced chemiluminescence reagents (Amersham Pharmacia Biotech). Immune complex assays for Cdk activity were performed as described (3).
Cardiac Function and Structure.
Transgenic animals and littermate controls underwent M-mode and Doppler echocardiography (27, 28). For histology, hearts were perfused with PBS followed by 10% (vol/vol) buffered formalin and embedded in paraffin. Myocardium was sectioned parallel to the plane of the atrioventricular valves at the level of the papillary muscles and was stained with hematoxylin and eosin or Sirius red.
Immunofluorescence Microscopy.
Laminin staining was performed by using rabbit primary Ab (1:100; Sigma) and FITC-conjugated Ab to rabbit IgG (Molecular Probes). Nuclei were stained with 2 μg/ml of 4′,6-diamidino-2-phenylindole (DAPI). Images were captured with a Zeiss Axioplan 2 epifluorescence microscope. Myocyte diameter was measured by using the transnuclear width of random myocytes in cross-section at the papillary muscle level (≥300 myocytes, using 2 hearts from each of 2 lines, by 2 blinded observers; ref. 29).
For phosphorylated histone H3, sections were digested with 0.05% trypsin and incubated with Abs to the Ser-10 phosphopeptide (1:1,000; Upstate Biotechnology, Lake Placid, NY; ref. 30) and sarcomeric MHC (1 μg/ml; MF20; University of Iowa Hybridoma Bank), then FITC- and Texas-red-conjugated Abs to rabbit and mouse IgG. BrdUrd (0.1 mg/g body weight) was administered i.p. 3 h before euthanasia and was detected with FITC-conjugated primary Ab (Roche Molecular Biochemicals).
Flow Cytometry.
Ventricular myocytes were isolated by perfusion with 0.17% type I collagenase (CL1; Worthington; ref. 4). For each assay, 1 × 106 cells were fixed, labeled with FITC-MF20 and propidium iodide (PI) in the presence of RNaseA, and analyzed by two-color flow cytometry (3), using MODFIT 2.0 (Verity, Topsham, ME). DNA histograms were derived from the authenticated cardiac myocytes.
Cardiac Myocyte Culture and Adenoviral Gene Transfer.
Ventricular myocytes from 2-day-old Sprague–Dawley rats were purified as described (3). Wild-type and catalytically inactive human TERT cDNAs were subcloned into pAdTrack-cytomegalovirus (CMV) and pShuttle-CMV (provided by Bert Vogelstein, Johns Hopkins Oncology Center, Baltimore), i.e., with and without coexpression of green fluorescent protein (GFP). Cardiac myocytes (106 cells per 60-mm dish) were infected for 6 h at 20 plaque-forming units per cell with adenovirus encoding wild-type TERT, D868A TERT, or the empty vector, and then were cultured for 24 h in serum-free medium, except where indicated. Infection efficiency was 100%, determined by coexpression of GFP or immunostaining for exogenous TERT.
Apoptosis.
For cell culture, nucleosomal DNA fragmentation was monitored by electrophoresis in 1.5% agarose (31), and cell viability was monitored by trypan blue exclusion.
Ligation of the left anterior descending coronary artery was performed as described (28). The prevalence of myocyte apoptosis in the area perfused by the affected vessel was determined after 6 h of ischemia, using the in situ ligase reaction (32), which may be more specific than nick end-labeling for apoptotic strand breaks, especially when necrosis coexists (28). Twenty-four hours after ligation, the areas of infarction and ischemic risk were determined by using 1.5% triphenyltetrazolium chloride (TTC) staining and 1% Evan blue perfusion (28).
Statistical Analyses.
Data are reported as the mean ± SE. Comparisons were analyzed by ANOVA and Scheffe's test, using a significance level of P < 0.05.
Results
Telomerase Activity, Telomere Length, and TERT Gene Expression Are Developmentally Regulated in Mouse Myocardium.
Measured by the TRAP assay (22), telomerase activity was readily detected in embryonic (E16.5) and neonatal (2-day) mouse myocardium, with little or none in adult (8-wk) cardiac tissue (Fig. 1A), as reported for the rat (17, 18). Pretreatment of extracts with RNaseA, to confirm that the amplified products are attributable to telomerase, prevented formation of the TRAP ladder. Telomerase activity was down-regulated >65% by day 2 after birth. Telomeric restriction fragments (24) were analyzed by Southern blotting of cardiac DNA (Fig. 1B). As expected from the TRAP assay, cardiac telomere fragments were largest in embryos, and shortened with age.
Figure 1.
Cardiac-specific overexpression of TERT restores telomerase activity and maintains telomere length. (A) Telomerase activity. Extracts from embryo (E16.5), neonate (day 2), and adult (8 wk) heart were assayed by a TRAP protocol, with (+) or without (−) RNaseA. IC, internal control template. (B) Telomere length. DNA extracted from myocardium was analyzed by Southern blotting for the telomeric repeat. (C and D) Northern blot analysis of TERT and telomerase-related factors during mouse cardiac development. Shown for comparison is 28S rRNA. (E) Western blot analysis of TERT transgene expression. TERT-infected cardiac myocytes were used as the positive control, and a nontransgenic littermate was used as the negative control. Total sarcomeric plus cytoplasmic actin is shown for comparison. (F) Northern blot analysis of TERT transgene expression. H, heart; B, brain; Lu, lung; Li, liver; S, spleen; K, kidney; T, testes; con, nontransgenic littermate. (G) Reconstitution of myocardial telomerase activity by TERT (Left) but not inactive TERT (D868A, Right). TRAP assays were performed as for A. (H) Restoration of telomere length in αMHC-TERT transgenic mice. In E, G, and H, numbers 1–4 denote 4 independent transgenic lines or the corresponding control littermates.
Consistent with the loss of telomerase activity and decreased telomere length, TERT mRNA was most prominent in embryonic myocardium, was down-regulated 58% by day 2 after birth, and was not detected in adult heart by Northern blotting (Fig. 1C). Other telomere-associated factors—the catalytic subunit of DNA-PK, its telomere-binding subunits (Ku70 and Ku80), and TRFs 1 and 2—were expressed even in postmitotic myocardium (Fig. 1D), as was the RNA component of telomerase (not shown), although the three components of DNA-PK were reduced in adult heart.
TERT Can Maintain Telomerase Activity and Telomere Length in the Adult Heart.
The cardiac-specific αMHC promoter was used to direct the expression of wild-type human TERT vs. catalytically inactive TERT (D868A), and four founders were identified for each genotype. Transgene expression and tissue specificity were confirmed by Western blotting (Fig. 1 E and F). Telomerase activity was rescued in all four lines with wild-type TERT, but not by the D868A mutation at comparable levels (Fig. 1G). Southern blot analysis confirmed that telomere shortening in adult myocardium was suppressed by TERT (Fig. 1H).
TERT Can Delay Cardiac Myocyte Cell Cycle Exit, Then Leads to Late-Onset Cardiac Hypertrophy.
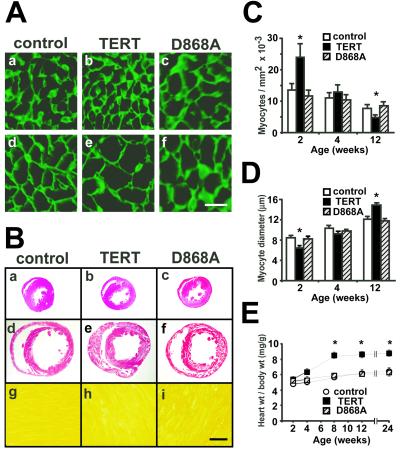
To delineate cardiac myocyte boundaries, immunolabeling of laminin was performed (Fig. 2 A, C, and D) (29). At 2 wk of age, myocyte density increased by two-thirds in TERT transgenic mice vs. controls, with myocyte diameter decreased, reciprocally. Similar conclusions were reached by using DAPI plus Ab to sarcomeric MHC (not shown). D868A transgenic mice were indistinguishable from transgene-negative animals. Given that cardiac dimensions and mass were unchanged at this age (Fig. 2 B and E), an increase in myocyte density suggests an increase in myocyte number (33).
Figure 2.
TERT induces hypercellular myocardium, followed by cardiac hypertrophy. (A, C, and D) Laminin staining at 2 (a–c) and 12 (d–f) wk of age. TERT increased myocyte density at the younger age, but myocyte diameter at the older age. [Bar = 10 μm.] (B) Heart size, at 2 (a–c) and 12 (d–f) wk of age. Concentric biventricular hypertrophy was seen in 12-wk-old αMHC-TERT mice (e). B g–i show the lack of interstitial fibrosis in each genotype (Sirius red). [Bar = 1 mm, a–f; 20 μm, g–i.] (E) The heart weight per body weight ratio increased as a late response to TERT. *, P < 0.05 vs. transgene-negative littermates.
Subsequently, the heart weight to body weight ratio increased, by one-third at 8 to 24 wk (Fig. 2E), with concentric hypertrophy in both ventricles (Fig. 2B). Myocyte diameter was likewise increased by TERT (12 wk; Fig. 2 A, C, and D), without tissue fibrosis (Fig. 2B g–i) or myocyte disarray (not shown). Echocardiography was performed to assess ventricular function (27), as hypertrophy could occur secondary to mechanical defects; no difference was seen in heart rate, ventricular performance, or chamber diameter (not shown). Although myocyte dropout from apoptosis also can be a cause of compensatory growth, no increase occurred in terminal deoxynucleotidyltransferase-mediated dUTP nick end-labeling (TUNEL)-positive cells (not shown). Thus, the early hyperplastic phenotype was followed by later hypertrophy, with neither cell loss nor discernible mechanical dysfunction as an explanation. Heart weight, wall thickness, and myocyte diameter were increased only by active TERT, not the inactive mutation.
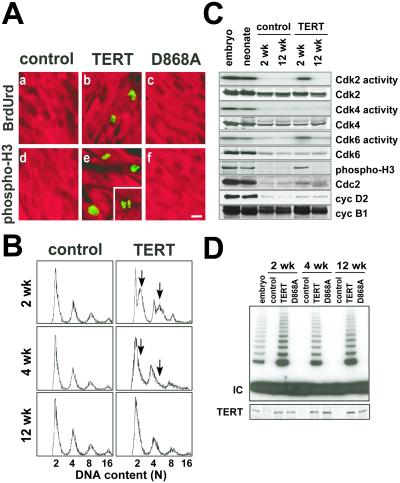
Immunocytochemistry was performed for BrdUrd incorporation and histone H3 phosphorylation on Ser-10, markers of S phase and mitosis, respectively (Fig. 3A; Table 1). Neither was detected in age-matched littermate controls. Both BrdUrd incorporation and histone H3 phosphorylation were seen in αMHC-TERT ventricular myocytes, with a prevalence of ≈4,000 and 3,000/106, respectively, at 2 wk of age (Table 1). Similar results were confirmed in 3 additional lines (1,800–2,500/106 at 3 wk of age). Less frequently (125/106), myocyte nuclei suggestive of late telophase were seen (Fig. 3A e Inset). The prevalence of BrdUrd incorporation and histone H3 phosphorylation declined >50% by 4 wk of age; neither was detected at 12 wk of age, in either genotype. Thus, TERT delays, but (by itself) cannot prevent, cardiac cell cycle exit. All effects were specific to catalytically active TERT.
Figure 3.
Cardiac myocyte DNA synthesis and mitoses in αMHC-TERT transgenic mice. (A) Immunofluorescence detection of BrdUrd incorporation (green; a–c) and mitotic phosphorylation of histone H3 (green; d–f) in cardiac myocytes at 2 wk of age, identified with MF20 Ab to sarcomeric MHC (red). Neither BrdUrd incorporation nor histone H3 phosphorylation was detected in nontransgenic controls or D868A mice. A cardiac myocyte in late telophase is seen in e (Inset). [Bar =10 μm.] (B) Dissociated cardiac myocytes were analyzed by flow cytometry. Arrows highlight the increase in DNA content by TERT. (C) TERT prolongs the activity of endogenous Cdk6, Cdk2, and Cdc2 in myocardium. Cdk2/4/6 activities were monitored by immune complex kinase assays, and Cdc2 activity was monitored by Ser-10 phosphorylation of histone H3. Western blots indicate an increase in cyc D2, cyc B1, and Cdc2 but not other Cdks. (D) The shift from hyperplasia to hypertrophy does not entail down-regulation of TERT expression (Western blot Bottom) or activity (TRAP assay Top).
Table 1.
TERT induces DNA synthesis and mitotic phosphorylation of histone H3 in postnatal ventricular myocytes
| Age, wk | BrdUrd (+) myocytes per 106
|
Phospho-H3 (+) myocytes per 106
|
||||
|---|---|---|---|---|---|---|
| Control | αMHC-TERT | αMHC-D868A | Control | αMHC-TERT | αMHC-D868A | |
| 2 | 0 | 3,711 | 0 | 0 | 2,752 | 0 |
| 4 | 0 | 1,543 | 0 | 0 | 1,000 | 0 |
| 12 | 0 | 0 | 0 | 0 | 0 | 0 |
Transgenic mice and their littermates were analyzed by epifluorescence microscopy, using Abs against BrdUrd or the histone H3 Ser-10 phosphopeptide, plus Ab to sarcomeric MHC, a marker of myocyte identity. No change from the controls was seen in liver or small intestine (not shown).
By using flow cytometry for DNA content (Fig. 3B), multiple peaks were seen with wild-type myocardium, as reported (4), corresponding to 2, 4, 8, or 16 haploid genomes per event. The prevalence of myocytes in S phase (2 < n < 4, plus 4 < n < 8) was increased 3-fold by TERT at 2 wk, with a smaller shift to the right at 4 wk. Similar results were seen in two independent lines. By 12 wk, the distribution was no different from littermate controls.
To test whether TERT affected endogenous cell cycle regulators, myocardium was studied by Western blot analysis and immune complex kinase assays (Fig. 3C). Mitotic phosphorylation of histone H3 was sustained by TERT at 2 but not 12 wk, as was true by immunocytochemistry; this marker of Cdc2 function was accompanied by increased Cdc2 expression at the younger age. Cdk6 and Cdk2 activities increased 2- and 4-fold, respectively, at 2 wk, with no increase at 12 wk. Unlike Cdc2, these Cdks are ordinarily expressed even in adult myocardium, and their expression was not increased by TERT. Cyclins B1 and D2 were expressed at higher levels in αMHC-TERT myocardium at 2 and 12 wk. TERT did not affect expression of Cdk4, cyc A, cyc D1, cyc E, retinoblastoma, p21, or p27.
TERT expression and activity were sustained in transgenic mice at 2–12 wk, with no decrease between the hyperplastic and hypertrophic stages (Fig. 3D).
TERT Triggers Hypertrophic Growth in Cultured Cardiac Myocytes.
Because cardiac hypertrophy required active TERT but could not be explained by a described function of the protein, we also tested whether TERT might acutely induce hypertrophy in a postmitotic background, using viral gene transfer to neonatal rat ventricular myocytes. Under the conditions used, these are already refractory to G1 exit in response to mitogenic serum (3). Telomerase activity was increased by wild-type TERT but not D868A TERT (Fig. 4A). TERT did not trigger DNA synthesis (Fig. 4B Top), but did cause myocyte enlargement (Fig. 4B Middle and Bottom), in agreement with the hypertrophic effect in vivo. Hypertrophy was produced by two independent TERT viruses, with and without green fluorescent protein to mark infected cells.
Figure 4.
Phosphorylation of p70 S6K and JNK during TERT-induced hypertrophy in vivo and in vitro. (A) Telomerase activity in rat ventricular myocytes after viral gene transfer, measured by the TRAP assay. IC, internal control template. (B) TERT elicits cardiac hypertrophy, not G1 exit, in cultured ventricular myocytes. (Top) DNA histograms after viral gene transfer, using FITC-MF20 plus propidium iodide (PI) for flow cytometry. Cells were visualized with MF20 Ab to sarcomeric MHC (red; Middle) or coexpressed green fluorescent protein (green; Bottom). [Bar = 20 μm.] (C) Northern blot analysis of myocardium, showing induction of ANF and βMHC by the TERT transgene. (D) Northern blot analysis of myocardium, showing no change in c-myc expression by TERT. (E) Northern blot analysis of cultured cardiac myocytes, showing induction of ANF by viral delivery of TERT. (F and G) Western blot analysis showing phosphorylation of p70 S6K and JNK in myocardium of TERT transgenic mice (F) and in myocytes 24 h after viral delivery of TERT (G). In G, cardiac myocytes were treated with serum for 30 min as a positive control. Blots were reprobed for TERT to confirm equal expression of the wild-type and mutant proteins.
TERT Activates Molecular Markers and Mediators of Cardiac Hypertrophy.
As with other triggers of hypertrophy (27, 34), the TERT transgene provoked ANF and βMHC expression in the heart (Fig. 4C). Although c-myc can regulate cell size and results from TERT in some cell types (35), no increase occurred in TERT transgenic mice, at either 2 or 12 wk (Fig. 4D), which concurs with the lack of Myc induction by TERT in a recent gain of function study (36). TERT also induced ANF acutely in culture (Fig. 4E).
Among potential mediators of hypertrophic growth, marked phosphorylation was seen for p70 S6K (37, 38), a pivotal regulator of translation, at T389, the rapamycin-sensitive residue (ref. 39; Fig. 4F). Akt activation was not increased, implicating an alternative p70 S6K kinase or decreased signaling by a p70 S6K phosphatase. Smaller but consistent increases were seen in activation of JNK and ERK, but not p38. Phosphorylation of p70 S6K and JNK also was increased by viral delivery of TERT (Fig. 4G). Inactive TERT had no effect.
TERT Alleviates Cardiac Apoptosis.
Although mechanisms coupling telomeric signals to cell survival are only beginning to be understood (10), we tested whether exogenous TERT might have salutary effects for cardiac muscle. In cell culture, nucleosomal DNA fragmentation at 24 h and cell death by 48 h were induced by serum-free insulin-free medium (Fig. 5 A and B). Protection was conferred by TERT, measured by both parameters, and required the active protein. Based on this cell culture result, we then tested the prediction that TERT might protect against cardiac myocyte apoptosis in vivo, after ischemic injury (28). The prevalence of apoptosis after coronary ligation was reduced 50% by TERT (Fig. 5C), and the subsequent area of infarction was reduced by 23% (Fig. 5D). Similar results were seen in all four TERT transgenic lines.
Figure 5.
TERT inhibits cardiac myocyte apoptosis in vitro and in vivo. (A) Nucleosomal DNA fragmentation. Cardiac myocytes were infected for 6 h then cultured for 18 h. DNA fragmentation induced by serum-free insulin-free medium was blocked by active TERT. (B) Cardiac myocyte survival. Cardiac myocytes were infected as shown, cultured in serum-free insulin-free medium for the indicated times, and analyzed by trypan blue exclusion. (C Left) Myocardium analyzed by the in situ ligase method 6 h after coronary artery occlusion. The arrows indicate Apoptotic nuclei (brown); sections were counterstained with nuclear fast red. (Right) Prevalence of myocyte apoptosis 6 h after coronary occlusion. *, P < 0.01; n ≥ 7. (D) Infarct size in wild-type and TERT transgenic mice 24 h after coronary occlusion, expressed as a percentage of the area at risk. *, P < 0.05; n ≥ 6.
Discussion
Mechanisms that impose a postmitotic phenotype in cardiac myocytes include decreased expression or function of essential cell cycle mediators and increased expression of cell cycle inhibitors (1). Progress toward cardiac myocyte regeneration has been slow, perhaps because of confounding redundancies among the cyclins, Cdks, Cdk inhibitors, pocket proteins, and E2F transcription factors. An alternative explanation may be a requirement for factors outside these alone, and telomerase activity thus has become an important focus of research on cell senescence and immortalization (5–10, 14). Our investigations establish that forcible expression of TERT, by itself, can prevent the loss of telomerase activity in postmitotic myocardium, maintain telomere length, and delay cardiac cell cycle exit. Prolongation of Cdk2 and Cdk6 activity, yet not Cdk4, conforms to its respective role in terminal differentiation by erythroid cells (40). By 3 months of age, however, Cdk activity and proliferation subsided despite equivalent telomerase activity. This result suggests the lack of an impetus to G1 exit in adult myocardium, or perhaps further inhibitory signals, and concurs with other evidence for proliferative signaling by TERT only under mitogenic conditions (36). Overcoming senescence can require not just TERT, but also inactivation of pocket proteins or loss of INK4 Cdk inhibitors (12, 41). It will be intriguing to test whether such synergy exists in postmitotic cardiac muscle and whether endogenous TERT is induced by genes that promote cardiac hyperplasia, like Myc (33), which activates TERT transcription directly (7).
Less expected was the later hypertrophy induced by TERT, also produced by viral delivery in cultured cardiac myocytes. A feature common to both settings was phosphorylation of p70 S6K at the key rapamycin-sensitive residue. p70 S6K controls the translation of mRNAs containing a 5′ oligopyrimidine tract, and the predominant effect of S6K in Drosophila is on cell size not cell number (39). In cardiac myocytes, p70 S6K is activated during diverse forms of hypertrophy (37, 38): inhibition by rapamycin underscores its essential function in hypertrophic growth but also that transcriptional “reprogramming” in hypertrophy occurs by an independent means (37). The concurrent activation of JNK by TERT, in vitro and in vivo, suggests one explanation for this observation (29). We have not detected reactivation of endogenous TERT in several mouse models of hypertrophy (not shown), but these do not emulate the increase in ploidy found in human heart failure.
In summary, preventing the down-regulation of TERT delays the timing of cardiac cell cycle exit and the postmitotic phenotype. Subsequent hypertrophy elicited by TERT was well tolerated, unlike many forms of induced cardiac growth (27, 34). Directly or indirectly, TERT also protected cardiac myocytes from apoptosis, consistent with evidence that telomerase or associated proteins promote cell survival (15, 16) at least in some settings (35). Relief of growth arrest could be independent of relief from apoptosis or might be linked, e.g., by components of a DNA damage-checkpoint pathway (10). For mice, where telomere length is much greater than for humans, alternatives to telomere length per se are attractive to explain effects of TERT on cell growth and survival, such as acting via telomere structure instead. A second, potentially related possibility would be the existence in mammals of telomeric silencing, as in yeast (7).
Acknowledgments
We thank Calvin Harley (Geron, Menlo Park, CA) and Jeffrey Robbins (Univ. of Cincinnati) for reagents, and Steve Elledge, Wade Harper, and Vicki Lundblad for suggestions. This work was supported in part by National Institutes of Health grants (to M.D.S.) and by the M. D. Anderson Foundation Professorship.
Abbreviations
- ANF
atrial natriuretic factor
- Cdk
cyclin-dependent kinase
- MHC
myosin heavy chain
- TERT
telomerase reverse transcriptase
- TRAP
telomeric repeat amplification protocol
- TRF
telomere repeat-binding factor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.MacLellan W R, Schneider M D. Annu Rev Physiol. 2000;62:289–320. doi: 10.1146/annurev.physiol.62.1.289. [DOI] [PubMed] [Google Scholar]
- 2.Lipinski M M, Jacks T. Oncogene. 1999;18:7873–7882. doi: 10.1038/sj.onc.1203244. [DOI] [PubMed] [Google Scholar]
- 3.Akli S, Zhan S, Abdellatif M, Schneider M D. Circ Res. 1999;85:319–328. doi: 10.1161/01.res.85.4.319. [DOI] [PubMed] [Google Scholar]
- 4.Soonpaa M H, Koh G Y, Pajak L, Jing S L, Wang H, Franklin M T, Kim K K, Field L J. J Clin Invest. 1997;99:2644–2654. doi: 10.1172/JCI119453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodnar A G, Ouellette M, Frolkis M, Holt S E, Chiu C P, Morin G B, Harley C B, Shay J W, Lichtsteiner S, Wright W E. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 6.Hahn W C, Counter C M, Lundberg A S, Beijersbergen R L, Brooks M W, Weinberg R A. Nature (London) 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 7.McEachern M J, Krauskopf A, Blackburn E H. Annu Rev Genet. 2000;34:331–358. doi: 10.1146/annurev.genet.34.1.331. [DOI] [PubMed] [Google Scholar]
- 8.Shay J W, Wright W E. Science. 2001;291:839–840. doi: 10.1126/science.1058546. [DOI] [PubMed] [Google Scholar]
- 9.Griffith J D, Comeau L, Rosenfield S, Stansel R M, Bianchi A, Moss H, de Lange T. Cell. 1999;97:503–514. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 10.Karlseder J, Broccoli D, Dai Y, Hardy S, de Lange T. Science. 1999;283:1321–1325. doi: 10.1126/science.283.5406.1321. [DOI] [PubMed] [Google Scholar]
- 11.Prowse K R, Greider C W. Proc Natl Acad Sci USA. 1995;92:4818–4822. doi: 10.1073/pnas.92.11.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickson M A, Hahn W C, Ino Y, Ronfard V, Wu J Y, Weinberg R A, Louis D N, Li F P, Rheinwald J G. Mol Cell Biol. 2000;20:1436–1447. doi: 10.1128/mcb.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Snow B E, Hande M P, Yeung D, Erdmann N J, Wakeham A, Itie A, Siderovski D P, Lansdorp P M, Robinson M O, Harrington L. Curr Biol. 2000;10:1459–1462. doi: 10.1016/s0960-9822(00)00805-8. [DOI] [PubMed] [Google Scholar]
- 14.Artandi S E, DePinho R A. Nat Med. 2000;6:852–855. doi: 10.1038/78595. [DOI] [PubMed] [Google Scholar]
- 15.Goytisolo F A, Samper E, Martin-Caballero J, Finnon P, Herrera E, Flores J M, Bouffler S D, Blasco M A. J Exp Med. 2000;192:1625–1636. doi: 10.1084/jem.192.11.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbert B, Pitts A E, Baker S I, Hamilton S E, Wright W E, Shay J W, Corey D R. Proc Natl Acad Sci USA. 1999;96:14276–14281. doi: 10.1073/pnas.96.25.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borges A, Liew C C. J Mol Cell Cardiol. 1997;29:2717–2724. doi: 10.1006/jmcc.1997.0503. [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi Y, Nozawa K, Savoysky E, Hayakawa N, Nimura Y, Yoshida S. Exp Cell Res. 1998;242:120–127. doi: 10.1006/excr.1998.4102. [DOI] [PubMed] [Google Scholar]
- 19.Martin-Rivera L, Herrera E, Albar J P, Blasco M A. Proc Natl Acad Sci USA. 1998;95:10471–10476. doi: 10.1073/pnas.95.18.10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gidh-Jain M, Huang B Y, Jain P, Gick G, El-Sherif N. J Mol Cell Cardiol. 1998;30:627–637. doi: 10.1006/jmcc.1997.0628. [DOI] [PubMed] [Google Scholar]
- 21.Kiaris H, Schally A V. Proc Natl Acad Sci USA. 1999;96:226–231. doi: 10.1073/pnas.96.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim N W, Wu F. Nucleic Acids Res. 1997;25:2595–2597. doi: 10.1093/nar/25.13.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Broccoli D, Godley L A, Donehower L A, Varmus H E, de Lange T. Mol Cell Biol. 1996;16:3765–3772. doi: 10.1128/mcb.16.7.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Counter C M, Avilion A A, LeFeuvre C E, Stewart N G, Greider C W, Harley C B, Bacchetti S. EMBO J. 1992;11:1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinrich S L, Pruzan R, Ma L, Ouellette M, Tesmer V M, Holt S E, Bodnar A G, Lichtsteiner S, Kim N W, Trager J B, et al. Nat Genet. 1997;17:498–502. doi: 10.1038/ng1297-498. [DOI] [PubMed] [Google Scholar]
- 26.Subramaniam A, Jones W K, Gulick J, Wert S, Neumann J, Robbins J. J Biol Chem. 1991;266:24613–24620. [PubMed] [Google Scholar]
- 27.Zhang D, Gaussin V, Taffet G E, Belaguli N S, Yamada M, Schwartz R J, Michael L H, Overbeek P A, Schneider M D. Nat Med. 2000;6:556–563. doi: 10.1038/75037. [DOI] [PubMed] [Google Scholar]
- 28.Kurrelmeyer K M, Michael L H, Baumgarten G, Taffet G E, Peschon J J, Sivasubramanian N, Entman M L, Mann D L. Proc Natl Acad Sci USA. 2000;97:5456–5461. doi: 10.1073/pnas.070036297. . (First Published April 25, 2000; 10.1073/pnas.070036297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choukroun G, Hajjar R, Fry S, del Monte F, Haq S, Guerrero J L, Picard M, Rosenzweig A, Force T. J Clin Invest. 1999;104:391–398. doi: 10.1172/JCI6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei Y, Mizzen C A, Cook R G, Gorovsky M A, Allis C D. Proc Natl Acad Sci USA. 1998;95:7480–7484. doi: 10.1073/pnas.95.13.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Negoro S, Oh H, Tone E, Kunisada K, Fujio Y, Walsh K, Kishimoto T, Yamauchi-Takihara K. Circulation. 2001;103:555–561. doi: 10.1161/01.cir.103.4.555. [DOI] [PubMed] [Google Scholar]
- 32.Didenko V V, Hornsby P J. J Cell Biol. 1996;135:1369–1376. doi: 10.1083/jcb.135.5.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson T, Allard M F, Sreenan C M, Doss L K, Bishop S P, Swain J L. Mol Cell Biol. 1990;10:3709–3716. doi: 10.1128/mcb.10.7.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molkentin J D, Lu J R, Antos C L, Markham B, Richardson J, Robbins J, Grant S R, Olson E N. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Hannon G J, Beach D H. Nature (London) 2000;405:755–756. doi: 10.1038/35015674. [DOI] [PubMed] [Google Scholar]
- 36.González-Suárez E, Samper E, Ramírez A, Flores J M, Martín-Caballero J, Jorcano J L, Blasco M A. EMBO J. 2001;20:2619–2630. doi: 10.1093/emboj/20.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadoshima J, Izumo S. Circ Res. 1995;77:1040–1052. doi: 10.1161/01.res.77.6.1040. [DOI] [PubMed] [Google Scholar]
- 38.Oh H, Fujio Y, Kunisada K, Hirota H, Matsui H, Kishimoto T, Yamauchi-Takihara K. J Biol Chem. 1998;273:9703–9710. doi: 10.1074/jbc.273.16.9703. [DOI] [PubMed] [Google Scholar]
- 39.Volarevic S, Thomas G. Prog Nucleic Acid Res Mol Biol. 2000;65:101–127. doi: 10.1016/s0079-6603(00)65003-1. [DOI] [PubMed] [Google Scholar]
- 40.Matushansky I, Radparvar F, Skoultchi A I. Proc Natl Acad Sci USA. 2000;97:14317–14322. doi: 10.1073/pnas.250488697. . (First Published December 12, 2000; 10.1073/pnas.250488697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiyono T, Foster S A, Koop J I, McDougall J K, Galloway D A, Klingelhutz A J. Nature (London) 1998;396:84–88. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]