Abstract
The oviductal microenvironment is a site for key events that involve gamete maturation, fertilization and early embryo development. Secretions into the oviductal lumen by either the lining epithelium or by transudation of plasma constituents are known to contain elements conducive for reproductive success. Although previous studies have identified some of these factors involved in reproduction, knowledge of secreted proteins in the oviductal fluid remains rudimentary with limited definition of function even in extensively studied species like cattle. In this study, we used a shotgun proteomics approach followed by bioinformatics sequence prediction to identify secreted proteins present in the bovine oviductal fluid (ex vivo) and secretions from the bovine oviductal epithelial cells (in vitro). From a total of 2087 proteins identified, 266 proteins could be classified as secreted, 109 (41%) of which were common for both in vivo and in vitro conditions. Pathway analysis indicated different classes of proteins that included growth factors, metabolic regulators, immune modulators, enzymes, and extracellular matrix components. Functional analysis revealed mechanisms in the oviductal lumen linked to immune homeostasis, gamete maturation, fertilization and early embryo development. These results point to several novel components that work together with known elements mediating functional homeostasis, and highlight the diversity of machinery associated with oviductal physiology and early events in cattle fertility.
Introduction
The oviductal microenvironment is a site for key events that involve gamete maturation, fertilization and early embryo development, processes that ultimately determine reproductive success. The oviductal epithelium has long been known to secrete specific proteins and metabolic elements, which in addition to components derived from blood plasma forms the oviductal fluid [1, 2]. In recent years, there has been accumulating evidence that several of these protein components might contribute to developmental events that occur in this microenvironment [3, 4]. In support, supplementing oviductal fluid components during in vitro fertilization (IVF) has demonstrated improved fertilization success and development rates [5–7]; co-culture with bovine oviductal epithelial cells (OECs) during IVF has indicated positive effects on early development of embryos [8–12]. These effects have been particularly linked to regulation of metabolic pathways [10, 13, 14], and in some cases epigenetic modulation of the developing embryo [11, 15]. Nevertheless, a comprehensive evaluation of secreted proteins in the oviductal fluid remains to be conducted, and data exist only from targeted studies with limited definition of function even in extensively studied species like cattle [16, 17]
Early embryonic loss is a major basis for reduced fertility in cattle [18]. Following fertilization, the embryo resides in the oviductal microenvironment for the first 3–4 days of development, during which sequential cleavage leading up to the 16-cell stage occurs before the embryo enters the uterus [19]. Efforts to study bovine oviductal fluid components started in the late 1950s [20], with initial focus on total protein content and free amino acid levels [21–23], and concentrations of metabolic components [21, 24]. Subsequent studies examining specific proteins in bovine oviductal fluid have largely taken topical or focused approaches, for example, visualizing proteins that associate with gametes [25–27], immuno-identification of glycoproteins synthesized at estrus [28, 29], insulin-like growth factors and binding proteins [30]. Proteomic profiling of components in the oviductal fluid and uterine fluid have been performed in other farm animal species like pigs [31, 32], and this has led to improvement of in vitro embryo production methods [33]. However, potential proteins that could be present in the bovine oviductal fluid have only been extrapolated from gene expression studies on the oviductal epithelium [34–36].
Knowledge of the bovine oviductal microenvironment and its effect on physiology of early embryo development would be important for improving in vitro embryo production methods and perhaps identifying unique bovine pluripotency mediators. In the present investigation, we use a shotgun proteomics approach to identify and compare secreted proteins in the bovine oviductal fluid, and secretions from OECs in culture with and without stimulation. Our results reveal several novel components that highlight the diversity of functions associated with the oviductal microenvironment. These findings represent the first step towards improved understanding of factors that could influence early events in cattle fertility.
Materials and methods
Animals and reagents
Samples from Holstein cows (Bos taurus) were collected from the slaughterhouse (Cargill®, Wyalusing, PA). Healthy reproductive tracts in both follicular and luteal phases of the estrous cycle were included in this study. All reagents were purchased from Sigma-Aldrich (St Louis, MO), unless otherwise noted.
Collection of oviductal fluid
Reproductive tracts were removed immediately after slaughter and both oviducts were isolated for collection at random stages of the estrous cycle. Using a fire-polished glass Pasteur pipette, fluid from the ampulla and isthmus of 28 cows (10–30 μl per animal) was collected by gentle aspiration. Fluid collected were combined and centrifuged at 2000 x g for 5 minutes, supernatant then removed and filtered using a low protein binding 0.2 μm polyethersulfone syringe filter to remove any cells or debris. Samples were snap-frozen in liquid nitrogen and held at -80°C until further processing. The sample collection procedure was completed within 30 minutes after slaughter of the animal.
Culture and characterization of OECs
Intact oviducts together with the tip of the uterine horn and the ovary were dissected immediately after slaughter and transported on ice in Dulbecco’s modified eagle medium (DMEM) containing 10 mM 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (HEPES) and penicillin-streptomycin supplement. Chilled oviducts were dissected from the surrounding connective tissue and washed several times using phosphate buffered saline (PBS). The region of ampulla and isthmus were trimmed and retained in petridishes with M199 medium containing penicillin streptomycin. The oviductal mucosa that contains the epithelial layer was gently extruded by mechanical pressure using atraumatic forceps and collected in a separate tissue culture dish containing the same medium in pairs (S1 Movie). The cell aggregates were then gently dispersed using a fire-polished Pasteur pipette and centrifuged at 500 x g for 5 minutes in a swinging bucket centrifuge. The cell clusters were suspended in complete culture medium M199 containing 10% fetal bovine serum, 1% non-essential amino acids supplement and 1% penicillin-streptomycin and plated in tissue culture dishes and incubated at 37°C under an atmosphere of 5% CO2 to allow attachment and proliferation. Cell morphology and growth was assessed visually, and subsequently OECs were evaluated for expression of the epithelial marker cytokeratin (Fig 1).
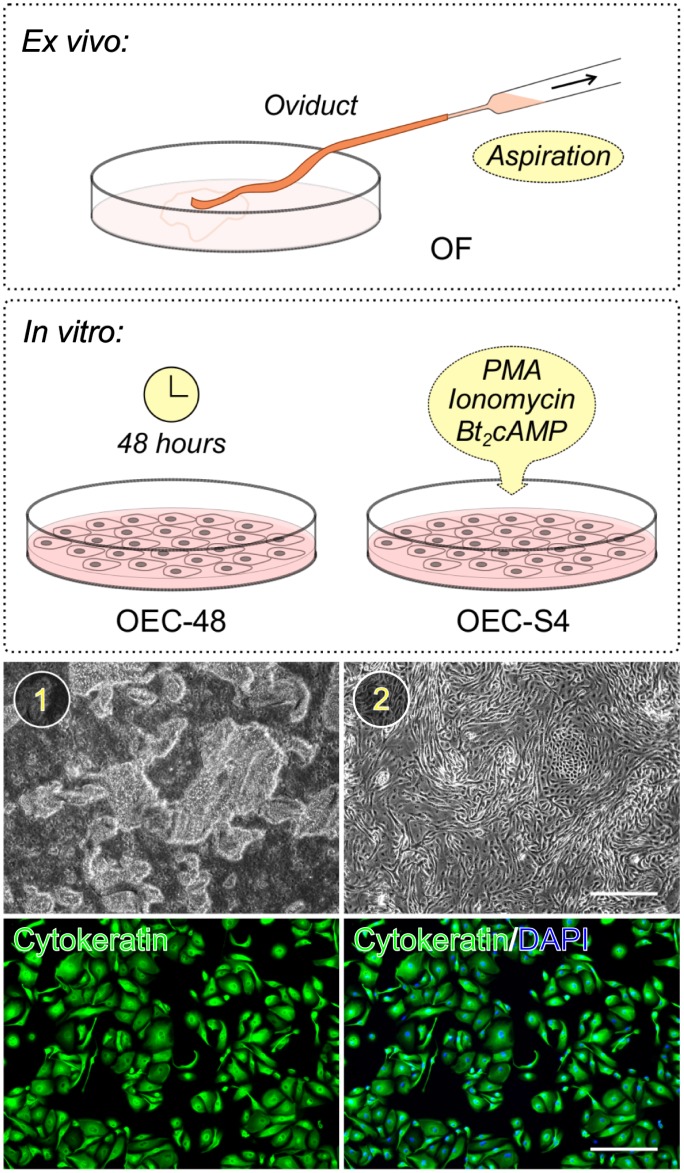
Fig 1. Collection of oviductal cell secretions.
Oviducts were dissected and removed from the mesosalphinx and fluid was collected either by direct aspiration (ex vivo) or after the culture of the oviductal epithelium (in vitro). Media used for culturing oviductal epithelial cells (OECs) were collected after passive conditioning for 48 hours or after a 4-hour stimulation with phorbol myristate acetate (PMA), ionomycin and dibutyryl cyclic adenosine monophosphate (Bt2cAMP). (1) Extruded oviductal mucosa containing intact epithelial sheets. (2) Representative image showing attachment and growth of the oviductal epithelial cells after 2–3 days in culture (Scale bar 300 μm). Immunohistochemistry for cytokeratin as a marker for OECs showed that the cultures established by this method were of high purity (Scale bar 200 μm).
Immunocytochemistry
Primary bovine OECs were grown on coverslips coated with 0.2% gelatin and fixed with 4% formaldehyde. Cells were then permeabilized with 0.1% Triton X-100 in PBS for 1 minute and blocked using 5% normal goat serum for 30 minutes. Coverslips were subsequently incubated with a mouse monoclonal anti-cytokeratin antibody (1:200 dilution; Cell Signaling Technology) for 1 hour. Coverslips were then washed three times using PBS and incubated with Alexa Fluor conjugated anti-mouse Fab’ fragments for 30 minutes, washed again with PBS, counterstained/mounted with 4’,6-diamidino-2-phenylindole (DAPI) containing Prolong Gold reagent (Life Technologies, Carlsbad, CA). Images were acquired using an inverted microscope (DMI 3000, Leica) using a cooled monochromatic camera (DFC365FX, Leica).
Collection of OEC conditioned media
Confluent OEC mixed cultures from at least 10 different animals were used for generating two types of OEC conditioned media mainly from apical secretions. First, adherent cells were washed with two repeated changes of PBS followed by two repeated changes of serum free M199 medium. For the 48-hour collection period (OEC-48), plates were returned to the incubator and kept undisturbed for that duration. For the stimulated secretions (OEC-S4), fresh serum free M199 medium supplemented with 5 ng/ml phorbol myristate acetate (PMA), 500 ng/ml of ionomycin and 0.5 mM of dibutyryl cyclic adenosine monophosphate (Bt2cAMP) was added to the cells and incubated for 4 hours. Use of these secretagogues were to enhance protein secretions mimicking cell activation signals that induce protein kinase A, protein kinase C, and increase intracellular Ca2+ levels. At the end of the incubation period, media were collected from the dishes, centrifuged at 500 x g for 5 minutes, filtered using a 0.2 μm PES syringe filter, snap frozen in liquid nitrogen and stored at -80°C until further processing.
Sample preparation and digestion
Protein concentrations were determined using BCA assay kit following manufacturer directions. Samples were then precipitated using the ProteoExtract Protein Precipitation Kit (CalBiochem). Resulting protein pellet was solubilized in 6 M urea in 50mM ammonium bicarbonate. Dithiothreitol (DTT) was added to a final concentration of 5 mM and samples were incubated for 30 min at 37°C. Subsequently, 20 mM iodoacetamide (IAA) was added to a final concentration of 15 mM and incubated for 30 min at room temperature, followed by the addition of 20 μL DTT to quench the IAA reaction. Lys-C/trypsin (Promega) was used at a 1:25 ratio (enzyme:protein) and incubated at 37°C for four hours. Samples were then diluted to <1 M urea by the addition of 50 mM ammonium bicarbonate and digested overnight at 37°C. The following day, samples were desalted using C18 Macro Spin columns (Nest Group) and dried down by vacuum centrifugation.
LC-MS/MS analysis
LC separation was done on a Proxeon Easy-nLC II HPLC (Thermo Scientific) with a Proxeon nanospray source. The digested peptides were reconstituted in 2% acetonitrile/0.1% trifluoroacetic acid and 10 μl of each sample was loaded onto a 100 μm x 25 mm Magic C18 100Å 5U reverse phase trap where they were desalted online before being separated on a 75 μm x 150 mm Magic C18 200Å 3U reverse phase column. Peptides were eluted using a gradient of 0.1% formic acid and 100% acetonitrile with a flow rate of 300 nL/min. A 120-min gradient was run with 5% to 35% acetonitrile over 100 min, 35% to 80% acetonitrile over 10 min, 80% acetonitrile for 2 min, 80% to 5% acetonitrile over 5 min, and finally held at 5% acetonitrile for 5 min.
Mass spectra was collected on an Orbitrap Q Exactive mass spectrometer (Thermo Fisher Scientific) in a data-dependent mode with one MS precursor scan followed by 15 MS/MS scans. A dynamic exclusion of 5 sec was used. MS spectra were acquired with a resolution of 70,000 and a target of 1 × 106 ions or a maximum injection time of 20 msec. MS/MS spectra were acquired with a resolution of 17,500 and a target of 5 × 104 ions or a maximum injection time of 250 msec. Peptide fragmentation was performed using higher-energy collision dissociation (HCD) with a normalized collision energy (NCE) value of 27. Unassigned charge states as well as +1 and ions > +5 were excluded from MS/MS fragmentation.
Database searching
Tandem mass spectra were extracted by Proteome Discoverer v1.2. Charge state deconvolution and deisotoping were not performed. All MS/MS samples were analyzed using X! Tandem (The GPM, www.thegpm.org; version CYCLONE 2013.02.01.1). X! Tandem was set up to search the Uniprot bovine proteome (23,942 entries) and 116 common laboratory contaminants (www.thegpm.org/crap) with an equal number of reverse decoy sequences assuming the digestion enzyme trypsin. X! Tandem was searched with a fragment ion mass tolerance of 20 PPM and a parent ion tolerance of 20 PPM. Carbamidomethyl of cysteine was specified in X! Tandem as a fixed modification. Glu->pyro-Glu of the n-terminus, ammonia-loss of the n-terminus, gln->pyro-Glu of the n-terminus, deamidated of asparagine and glutamine, oxidation of methionine and tryptophan, dioxidation of methionine and tryptophan and acetyl of the n-terminus were specified in X! Tandem as variable modifications.
Criteria for protein identification
Scaffold (version 4.2.0, Proteome Software Inc., Portland, OR) was used to validate MS/MS based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 79.0% probability by the Scaffold Local FDR algorithm. Protein identifications were accepted if they could be established at greater than 95.0% probability to achieve an FDR less than 5.0% and contained at least 2 unique peptides. This resulted in a spectra decoy FDR of 0.35% and a protein decoy FDR of 4.9%. Protein probabilities were assigned by the Protein Prophet algorithm [37]. Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony. Proteins sharing significant peptide evidence were grouped into clusters.
Analysis of identified proteins
To predict proteins secreted via the classical secretory pathway in the resulting dataset, we used SignalP v4.1 (http://www.cbs.dtu.dk/services/SignalP/) [38] to identify N-terminal sequence motifs directing proteins to the secretory pathway, in tandem we used TargetP v1.01 (www.cbs.dtu.dk/services/TargetP) [39] to refine this dataset by removing proteins destined for the mitochondria [40]. This predicted dataset was further refined using Phobius (http://phobius.sbc.su.se/) [41] to remove proteins that contained transmembrane regions. In this overall analysis, proteins were considered secreted if they contained an N-terminal secretory sequence, did not traffic to the mitochondria, and lacked transmembrane regions. In parallel, to predict proteins secreted via the non-classical secretory pathway in the same dataset, we used SecretomeP v2.0 (http://www.cbs.dtu.dk/services/SecretomeP/) [42] for feature-based analysis and identification of secreted proteins that do not contain an N-terminal signal sequence motif. Results from SecretomeP were further filtered using Phobius as described above. In this pipeline, glycosylphosphatidylinositol anchored surface proteins would also be identified as secreted. The resulting protein lists were classified by using gene ontology (GO) terms using PANTHER (protein analysis through evolutionary relationships tool [43]. For integrated functional evaluation, the proteins identified were also analyzed using Ingenuity® pathway analysis (IPA) to model and interpret biological significance of identified components. Common candidates in the proteomics dataset and from reanalysis of two published transcriptomics datasets from Bos taurus (NCBI GEO: GSE74612 [44]), and Bos indicus (GEO GSE65681 [36]), were identified and visualized as Circos plots [45], together with the classification based on GO terms.
Data availability
Raw data, mzML and Scaffold results are available from the MassIVE proteomics repository (MSV000081192) and Proteome Exchange (PXD006794). Complete protein lists are provided in supporting information (S1 Dataset).
Results and discussion
Secreted proteins in the bovine oviductal fluid
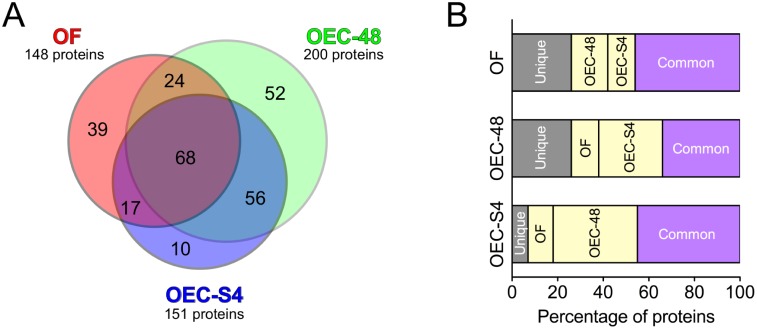
Experimental groups in this study enabled the identification of proteins that were secreted under ex vivo (OF) and in vitro (OEC-48 and OEC-S4) conditions (Fig 1). Cytokeratin expression evaluated in the in vitro cultures showed that OECs were of high purity without any contaminating fibroblasts (Fig 1). Protein mass spectrometric analysis identified a total of 2087 proteins combined for the three groups: 1289 proteins in OF, 1148 proteins in OEC-48, and 1391 proteins in OEC-S4. Within this combined list that would include proteins present within exosomes or released from damaged cells, 266 proteins passed the SignalP, TargetP, SecretomeP and Phobius filters indicating the putative number of secreted proteins identified: 148 proteins in OF, 200 proteins in OEC-48, and 151 proteins in OEC-S4. Of these, 68 proteins (26%) were common for all three groups (S1 Table), 109 proteins (41%) were common between in vivo and in vitro conditions, and 165 proteins (62%) were common for at least two of the groups (Fig 2A and 2B, S1 Dataset). We did not detect immunoglobulins in any of the samples demonstrating that our sampling method was without plasma/serum contamination.
Fig 2. Comparison of proteins identified under different collection methods for oviductal cell secretion conditions.
A total of 266 secreted proteins were identified. (A) Distribution of proteins that were unique or common between the three different groups: oviductal fluid (OF) and conditioned media obtained from oviductal epithelial cell cultures without (OEC-48) or after stimulation (OEC-S4). A subset of 68 proteins was common for the three groups. OEC-S4 had the least number of unique proteins that were not represented in OF or OEC-48. (B) Distribution of the percentage of unique and common proteins within each of the three collection groups.
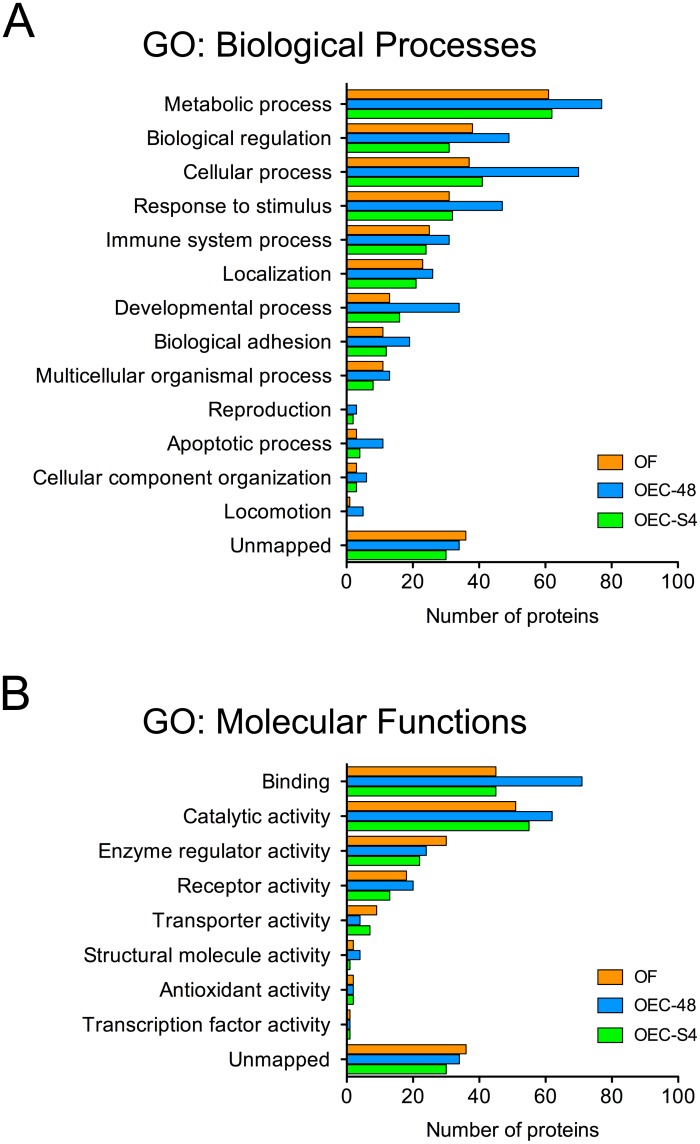
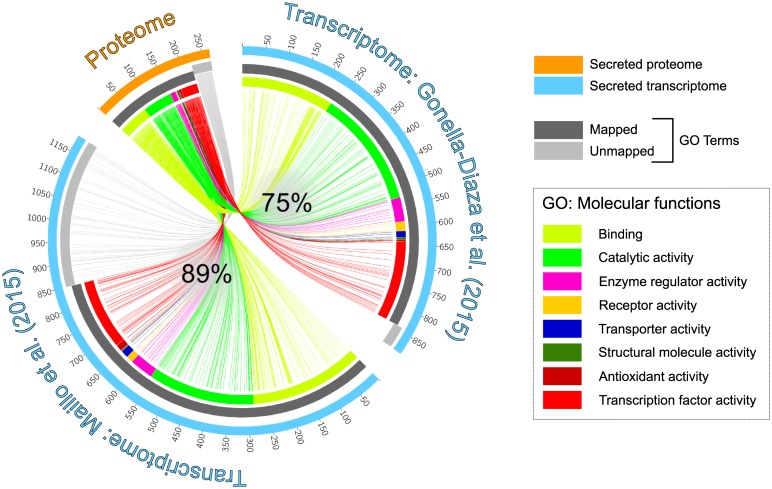
General categories of function for proteins identified in the oviductal secretome based on biological process or molecular function (Fig 3) were aligned to the major physiological processes that occur in the oviductal lumen. When the list of secreted proteins identified were compared to oviduct transcriptomics data from two different studies, we identified 236 (89%) of the proteins identified also represented as transcripts in Maillo et al. [35], and 200 (75%) in Gonella-Diaza et al. [36] (Fig 4). This also highlighted an important finding that most of the proteins detected were synthesized and secreted by the oviductal epithelium, and that there was little contribution of plasma protein components to the oviductal fluid (S2 Table). This is in contrast to a previous observation made in rabbits in which immunoglobulins and albumin were identified as major components [46]. We can only speculate the reason underlying this distinction, and one possibility is the species differences that are known to impact events that occur in the oviduct.
Fig 3. Gene ontology (GO) classification for all proteins identified in oviductal cell secretions.
Distribution of GO terms describing (A) biological processes, and (B) molecular functions for the number of identified secreted proteins.
Fig 4. Comparison of oviductal cell secreted proteins to transcriptome of the bovine oviduct from two published datasets.
Of the 266 secreted proteins identified in oviductal cells, 236 (89%) and 200 (75%) of the proteins were detected as transcripts in the oviduct by Maillo et al. [35], and the Gonella-Diaza et al. [36] respectively. This comparison indicates that almost 90% of the proteins identified in this study are synthesized by the oviductal epithelium, with only 30 (11%) of proteins putatively derived by plasma protein transudation.
The list of proteins and the experimental group in which they were detected are presented based on broad categorizations as, growth factors and cytokines integral to this functional niche (Table 1), homeostasis maintained by protease and protease inhibitors (Table 2), other enzymes involved in a variety of functions (Table 3), other proteins associated with gamete maturation fertilization and preimplantation embryo development (Table 4). Although, a subset of these proteins identified are already supported by functional evidence in the literature from different species, we identified several proteins and associated physiological pathways that have not been previously reported for this microenvironment. In sections below, we focus in brief on the relevance of these results and discuss their importance in understanding fertility in cattle.
Table 1. Growth factors and cytokines in the oviductal cell secretions.
| Protein name | Accession | OF | OEC-48 | OEC-S4 |
|---|---|---|---|---|
| Growth factors | ||||
| Bone morphogenetic protein 7* | F1MLT0 | ● | ||
| Connective tissue growth factor | O18739 | ● | ● | |
| C type lectin domain family 11, member A | A5D7L1 | ● | ||
| Endothelial cell specific molecule 1 | A5D7V3 | ● | ● | ● |
| Fibroblast growth factor 18 | Q0VCA0 | ● | ||
| Fibroblast growth factor 21* | E1BDA6 | ● | ||
| Glia maturation factor beta | P60984 | ● | ● | ● |
| Granulin | E1BHY6 | ● | ● | |
| Growth arrest specific 6 | F1MZ40 | ● | ● | ● |
| Growth differentiation factor 15 | E1BBL5 | ● | ||
| Hepatocyte growth factor | Q76BS1 | ● | ||
| Hepatoma derived growth factor | Q9XSK7 | ● | ● | ● |
| Inhibin, beta A chain | P07995 | ● | ||
| Kit ligand | Q28132 | ● | ||
| Macrophage colony stimulating factor 1 | F1MGS9 | ● | ● | |
| Midkinea | Q9N0E6 | ● | ||
| Nephroblastoma overexpressed | Q2HJ34 | ● | ||
| Nicotinamide phosphoribosyltransferase* | F1MJ80 | ● | ||
| Pigment epithelium derived factor | Q95121 | ● | ||
| Platelet derived growth factor C | E1BJY4 | ● | ● | |
| Transforming growth factor beta 1 | P18341 | ● | ● | |
| Transforming growth factor beta 2 | P21214 | ● | ||
| Vascular endothelial growth factor A | P15691 | ● | ● | |
| Cytokines | ||||
| Complement component 5a | F1MY85 | ● | ||
| C-X-C motif chemokine 16 | Q29RT9 | ● | ● | |
| C-X-C motif chemokine 6 | P80221 | ● | ||
| Dickkopf wnt signaling pathway inhibitor 3 | A6QL81 | ● | ● | |
| Family with sequence similarity 3, member B | E1BQ21 | ● | ● | |
| Family with sequence similarity 3, member C | A5PKI3 | ● | ● | |
| Family with sequence similarity 3, member D | E1BDN9 | ● | ||
| Growth regulated protein homolog gamma | O46675 | ● | ||
| Interleukin 8 | P79255 | ● | ||
| Leukemia inhibitory factor | Q27956 | ● | ● | |
| Macrophage migration inhibitory factor | P80177 | ● | ● | |
| Myeloid derived growth factor | P62248 | ● | ● | |
| Osteopontin | P31096 | ● | ● | |
| Small inducible cytokine subfamily E, member 1 | Q3ZBX5 | ● | ● | ● |
| Tumor necrosis factor ligand 1B | E1BF06 | ● |
* Exceptions: Proteins identified with only one unique peptide
Table 2. Proteases and protease inhibitors in the oviductal cell secretions.
| Protein name | Accession | OF | OEC-48 | OEC-S4 |
|---|---|---|---|---|
| Proteases | ||||
| Cathepsin A | Q3MI05 | ● | ● | |
| Cathepsin B | P07688 | ● | ● | ● |
| Cathepsin C | F1N455 | ● | ● | |
| Cathepsin D | F1MMR6 | ● | ● | |
| Cathepsin V | P25975 | ● | ● | ● |
| Cathepsin Z | P05689 | ● | ● | |
| Coagulation factor II | P00735 | ● | ● | |
| Complement C1S | Q0VCX1 | ● | ||
| Complement C2 | Q0V7N2 | ● | ● | |
| Complement C3 | Q2UVX4 | ● | ● | ● |
| Complement factor B | P81187 | ● | ● | ● |
| Complement factor D | Q3T0A3 | ● | ● | |
| Furin | Q28193 | ● | ||
| Gamma glutamyl hydrolase | A7YWG4 | ● | ||
| Granzyme A | F6QZF5 | ● | ||
| Haptoglobin | Q2TBU0 | ● | ||
| Serine protease HTRA 1 | F1N152 | ● | ● | ● |
| Kallikrein related peptidase 10 | Q0VCZ4 | ● | ● | |
| Lactotransferrin | P24627 | ● | ● | ● |
| Legumain | Q95M12 | ● | ||
| Matrix metalloproteinase 1 | F1MT97 | ● | ||
| Matrix metalloproteinase 2 | Q9GLE5 | ● | ||
| Plasminogen | E1B726 | ● | ● | ● |
| Protein disulfide isomerase | A5D7E8 | ● | ● | ● |
| Tissue type plasminogen activator | Q28198 | ● | ● | ● |
| Tripeptidyl peptidase I | Q0V8B6 | ● | ||
| Urokinase type plasminogen activator | Q05589 | ● | ● | |
| Protease Inhibitors | ||||
| Alpha-1-antiproteinase | P34955 | ● | ● | ● |
| Alpha-2-antiplasmin | P28800 | ● | ● | |
| Alpha-2-HS-glycoprotein | P12763 | ● | ● | ● |
| Alpha-2-macroglobulin | Q7SIH1 | ● | ● | ● |
| Angiotensinogen | Q3SZH5 | ● | ||
| Antithrombin III | P41361 | ● | ● | ● |
| Cystatin B | F6QEL0 | ● | ● | ● |
| Cystatin C | P01035 | ● | ● | ● |
| Metalloproteinase inhibitor 1 | P20414 | ● | ● | ● |
| Metalloproteinase inhibitor 2 | F1N430 | ● | ● | ● |
| Metalloproteinase inhibitor 3 | P79121 | ● | ● | |
| Serpin A3-1 | Q9TTE1 | ● | ||
| Serpin A3-7 | A2I7N3 | ● | ● | ● |
| Serpin A3-8 | A6QPQ2 | ● | ||
| Serpin D1 | A6QPP2 | ● | ||
| Serpin H1 | Q2KJH6 | ● | ● | ● |
Table 3. Other enzymes identified in the oviductal cell secretions.
| Protein name | Accession | OF | OEC-48 | OEC-S4 |
|---|---|---|---|---|
| Acid ceramidase | Q17QB3 | ● | ● | |
| Alpha amylase | F1MJQ3 | ● | ||
| Alpha lactalbumin | P00711 | ● | ||
| Alpha-N-acetyl-galactosaminidase | Q1RMM9 | ● | ● | |
| Alpha-N-acetyl-glucosaminidase | A6QM01 | ● | ||
| Angiogenin 1 | P10152 | ● | ||
| Apolipoprotein A-1 binding protein | Q6QRN6 | ● | ● | |
| Arylsulfatase A | Q08DD1 | ● | ● | |
| Arylsulfatase B | A6QLZ3 | ● | ● | |
| Beta galactosidase | Q58D55 | ● | ||
| Beta hexosaminidase | H7BWW2 | ● | ● | |
| Beta hexosaminidase subunit alpha | Q0V8R6 | ● | ● | |
| Beta mannosidase | Q29444 | ● | ||
| Biotinidase | A6QQ07 | ● | ● | |
| Carbonic anhydrase IV | Q95323 | ● | ● | |
| Chitinase 3 like protein 1 | G3X7D2 | ● | ● | |
| Coagulation factor V | Q28107 | ● | ● | ● |
| Egf containing fibulin like extracellular matrix protein 1 | A2VE41 | ● | ● | ● |
| Egf domain-specific O-linked N-acetyl-glucosamine transferase | A0JND3 | ● | ||
| Endoplasmic reticulum protein 44 | Q3T0L2 | ● | ● | ● |
| Ero1 like protein | A5PJN2 | ● | ● | |
| Gamma interferon inducible lysosomal thiol reductase | F1MAU3 | ● | ● | |
| Glucose regulated protein 78 | Q0VCX2 | ● | ● | ● |
| Glucosidase 2 subunit beta | Q28034 | ● | ● | ● |
| Glutaminyl peptide cyclotransferase | Q28120 | ● | ||
| Heparanase | F1N1G1 | ● | ||
| Interferon, gamma inducible protein 30 | A6QPN6 | ● | ● | |
| Lysyl oxidase homolog 4 | Q8MJ24 | ● | ● | |
| Peptidylprolyl isomerase C | Q08E11 | ● | ● | |
| Peroxiredoxin 4 | Q9BGI2 | ● | ● | ● |
| Phospholipid transfer protein | Q58DL9 | ● | ||
| Procollagen lysine, 2-oxoglutarate 5-dioxygenase 1 | O77588 | ● | ● | |
| Procollagen lysine, 2-oxoglutarate 5-dioxygenase 2 | A7MB83 | ● | ● | |
| Prolyl 4-hydroxylase subunit alpha 1 | A6QL77 | ● | ||
| Prostaglandin-H2 D-isomerase | O02853 | ● | ● | |
| Protein disulfide isomerase | A6H7J6 | ● | ● | ● |
| Protein disulfide isomerase A4 | F1MEN8 | ● | ● | ● |
| Protein O-fucosyltransferase 1 | Q7YRE7 | ● | ||
| Protein O-glucosyltransferase 1 | Q5E9Q1 | ● | ||
| Ribonuclease 4 | Q58DP6 | ● | ● | |
| Serpin peptidase inhibitor, clade C, member 1 | P41361 | ● | ● | ● |
| Stromal cell derived factor 2 | Q3SZ45 | ● | ||
| Sulfhydryl oxidase | F1MM32 | ● | ● | ● |
| Superoxide dismutase | A3KLR9 | ● | ● | ● |
| Tissue alpha-L-fucosidase | Q2KIM0 | ● | ||
| Vanin 1 | Q58CQ9 | ● | ● |
Table 4. Other proteins associated with gamete maturation, fertilization and preimplantation embryonic development in the oviductal cell secretions.
| Protein name | Accession | OF | OEC-48 | OEC-S4 |
|---|---|---|---|---|
| Alpha-1-acid glycoprotein | Q3SZR3 | ● | ● | |
| Apolipoprotein A-I | P15497 | ● | ● | ● |
| Apolipoprotein A-IV | F1N3Q7 | ● | ||
| Apolipoprotein C-III | P19035 | ● | ||
| Apolipoprotein D | Q32KY0 | ● | ||
| Apolipoprotein H | P17690 | ● | ● | |
| Calreticulin | P52193 | ● | ● | ● |
| Endoplasmin | Q95M18 | ● | ● | ● |
| Fetuin B | Q58D62 | ● | ● | |
| Fibronectin | P07589 | ● | ● | ● |
| Oviduct specific glycoprotein | Q28042 | ● | ● | ● |
| Prosaposin | P26779 | ● | ● | ● |
| Serum Albumin | P02769 | ● | ● | ● |
| Zinc alpha-2-glycoprotein | Q3ZCH5 | ● | ● | ● |
| Complement C5a | F1MY85 | ● | ● | |
| Follistatin | P50291 | ● | ● | |
| Gelsolin | F1N1I6 | ● | ● | ● |
Growth factors and cytokines
Developmental functions that occur in the oviductal microenvironment are known to be supported by factors that signal to gametes or embryo in the lumen. Among the proteins classified as growth factors and cytokines (Table 1), only 15 have been linked in previous reports to potential functions in the oviduct. The remaining 24 proteins were candidates detected in this microenviroment for the first time, the functional significance of which remains to be uncovered. Expression of macrophage colony stimulating factor 1 (CSF1) has been reported to increase after lipopolysaccharide exposure in the bovine oviductal epithelium [47]. CSF1 has been shown to accelerate development in bovine embryos [48, 49]. Although function remains unclear, hepatocyte growth factor has been reported in human oviductal fluid [50]. Activin/inhibin subunits have been identified in the murine oviduct as responsible for stimulating early embryogenesis [51]. During bovine embryogenesis, platelet derived growth factor (PDGF) is known to stimulate development during the fourth cell cycle [52]. Transforming growth factor β and vascular endothelial growth factor have been previously identified in the bovine oviduct [53], and may play a role in the developmental competence of bovine oocytes [54], and promote early embryonic development [55]. It has been suggested that BMP signaling is involved in crosstalk between the oviduct and the embryo during early stages of development [56].
Cytokines that included several inflammation-associated candidates were found to be expressed by oviductal cells (Table 1). Only few of these have been previously reported in the oviduct for any species. Early studies have demonstrated synthesis of leukemia inhibitory factor (LIF) by bovine oviductal cells [57]. LIF expression is known to have beneficial effects on sheep oocytes [58], and early embryos [59]; similar results have been recently reported for cattle [49, 60]. Interleukin 8 expression, often connected to inflammation, has been reported in human fallopian tubes [61]. Macrophage migration inhibitory factor has been identified in bovine oviducts with higher levels detected in the postovulatory phase, but its function remains unclear [62]. Previous studies have detected and linked osteopontin expression in the bovine oviduct [63], with a role in sperm-egg binding and fertilization [64]. Conserved functions for osteopontin have also been reported in porcine [65], and murine oviducts [66]. The tumor necrosis factor α system has been suggested to be responsible for local contractions modulating transport of the gametes and embryos [67].
Proteases and protease inhibitors
Protease activity has been reported in oviducts in several species, and some of their functions have been linked to sperm capacitation [68, 69]. We identified 27 proteases and 16 protease inhibitors expressed by oviductal cells (Table 2). Cathepsins are considered to be involved in gamete maturation leading to fertilization, and we identified several cathepsins (A, B, C, D, V and Z) produced by the oviductal cells. Previous studies have reported cathepsins in the oviducts of domestic cats [70], hamsters [71], and llamas [72]. Components of the complement pathway were also identified (S1 Fig). Complements have been suggested to be important for sperm-oocyte interaction [73, 74]; complement components are also known to be activated by spermatozoa and could cause acrosome loss in rabbit spermatozoa [75]. Complements have also been demonstrated to stimulate embryo development [76, 77]. Although furin has not been reported in the oviductal secretions, its role in protein processing in the epididymal fluid has been suggested [78]. Haptoglobin mRNA has been previously reported in the oviduct of cycling cows [79]. Kallikrien-related peptidases have been linked to a role in host defence in cervical mucus [80]. Lactotransferrin has been reported in human oviducts as a modulator of gamete interaction [81].
The proteases plasminogen, tissue type plasminogen activator, urokinase type plasminogen activator, and the protease inhibitor α-2-antiplasmin were identified in the oviductal secretions. Plasminogen/plasmin system has been suggested to regulate sperm entry into the oocyte in multiple species [82, 83]. In addition, α2 macroglobulin known to inhibit proteases from all catalytic classes was also identified in the oviductal cell secretions for the first time. An immunoprotective role for placenta-sourced α2 macroglobulin has been suggested for this acute phase protein in rats [84]. Other candidates that regulate extracellular matrix remodeling were also present in the oviductal secretions (S2 Fig). Matrix metalloproteinases (MMP 1 and 2), and corresponding tissue inhibitors of metalloproteinases (TIMP 1, 2 and 3) were identified in the oviductal secretome. Differential expression for MMPs and TIMPs have been reported in the bovine oviduct during the estrous cycle [85], with potential effects across fertilization and early embryonic development.
Inhibitors of acrosomal and other lysosomal proteases like α-1-antiproteinase/antitrypsin and different serpins (A3-1, A3-7, A3-8, D1 and H1), cystatins (B and C) were identified. Similar inhibitors of acrosomal proteases has been reported in oviductal fluid collected from the rhesus monkey [86] and the rabbit [87].
Other enzymes
In addition to the proteases indicated above, the oviductal cell secretions contained numerous enzymes (Table 3). Of these, only a few have been previously identified in the oviduct in different species. Ceramide metabolism mediated by acid ceramidase has been demonstrated to be critical for early embryo survival in mice [88]. Glycosidase activities in bovine oviductal fluid have been reported in previous studies [89]. These enzymes have been associated with modifications to the oocyte zona pellucida and capacitation of spermatozoa. In addition to glucosidase 2 reported in sheep [90] and cows [23], this study also identified 6 specific enzymes that may be linked to specific carbohydrate modifications previously measured in the oviductal fluid [89]. Arylsulfatases (A and B) have been reported in the rabbit oviduct [91], and indicate potential for glycoconjugate formation in this microenvironment [92]. Carbonic anhydrase IV has been demonstrated to provide an essential role in bicarbonate mediated activation of human and murine sperm [93, 94]. Chitinase-like proteins have been previously reported in the sheep oviduct [95]. Chitinase 3 like protein 1 has been reported to regulate inflammation and tissue remodeling [96]. Glucose-regulated protein 78 secreted in the human oviduct has been demonstrated to decrease sperm zona pellucida binding [97]. Heparan sulfate proteoglycans and their binding proteins have been found to be important in the bovine reproductive physiology. Heparanase has not been previously reported in the oviduct, but its function in the uterus during implantation has been well studied in murine models [98, 99]. Phospholipid transfer protein expression is known to be stimulated in response to embryos in the murine oviduct [100]. Superoxide dismutase expression was also detected in the oviductal cell secretions indicating antioxidant defense by reducing superoxide radicals in this microenvironment. Superoxide dismutases have been previously reported in the bovine oviduct [101], and its importance in redox regulation has been emphasized in several species.
Other proteins associated with gamete maturation, fertilization and preimplantation embryo development
Among proteins that did not fall into one of the above categories, we identified several that are of functional importance for gamete regulation, fertilization and preimplantation embryonic development (Table 4). In male fertility, apolipoprotein A-1 has been associated with sperm motility [102]. Apolipoproteins can act as cholesterol acceptors that facilitate cholesterol efflux from plasma membrane of spermatozoa [103], a necessary event for capacitation/hyperactivation. Specific association of apolipoprotein A-1 to bovine seminal plasma proteins [104] and modulation of sperm capacitation [105] have been previously demonstrated. Expression of apolipoproteins in the oviduct has been reported in different mammalian model systems: Apolipoprotein D has been detected in guineapig oviducts [106]. Apolipoprotein A-1 has been detected in rat oviducts [107]. An apolipoprotein H-like protein has been purified from human follicular fluid [108]. In addition to apolipoproteins A-1 and D, apolipoproteins A-IV, C-III, and H that were detected in this study have not been previously reported in mammalian oviducts.
The oviductal secretome also contained factors that have been associated with sperm survival, transport, and signal transduction during fertilization. α-1-acid glycoprotein has been reported to inhibit neutrophil phagocytosis of sperm in the bovine oviduct [109]. Fibronectin has been demonstrated to stimulate human sperm capacitation by activating the protein kinase A pathway [110]. Organization of the extracellular matrix and paracrine communication by fibronectin has been identified to be important for early embryogenesis [111, 112]. Zinc-α-2-glycoprotein has been demonstrated to induce cAMP signaling and modulate motility in human sperm [113]. Calreticulin has been suggested to interact with the murine oocyte and mediate signaling linked to cell cycle resumption [114]. Phosphorylation of endoplasmin bound to murine spermatozoa has been associated zona pellucida interactions preceding fertilization [115]. Fetuin B is vital for maintaining fertility of murine ovulated oocytes by blocking ovastacin, a cortical granule protease known to trigger zona pellucida hardening [116]. Albumin has been found to increase blastocyst development in individual culture of bovine embryos [117]. Follistatin has been shown to be important for bovine early embryo development [118, 119]. Complement C5a has been shown to support human embryonic stem cell pluripotency even in the absence of other growth factors [120] (S1 Fig).
Conclusions
The oviduct presents a crucial site for gamete regulation including sperm capacitation, oocyte maturation, fertilization and preimplantation development of the early embryo. This study represents a comprehensive documentation of the bovine oviductal secretions comparing both ex vivo intact oviducts and in vitro oviductal epithelial cells. It is of interest to note that transudation from plasma contributes little to the defining characteristics of this luminal microenvironment in cattle. The secreted protein profile established for the oviductal fluid in this manuscript forms the foundation for future functional studies for both advancing basic understanding and making improvements to reproduction technologies in cattle. Our proteomics database will also serve as a long-term reference for addressing a variety of questions regarding the bovine oviduct, and seed new discoveries and linkages over time; perhaps aspects that we failed to appreciate given the current state of understanding will manifest with parallel advancements to bovine reproductive function. Some areas not distinguished by this study are the changes that may occur to the oviductal microenvironment with the estrous cycle [16, 121] and microvesicles/exosomes present in the oviductal fluid that may deliver proteins to sperm and regulate its functional activation [122, 123]. These remain important topics for future investigations towards refining understanding of bovine oviductal physiology.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(XLSX)
(MP4)
Acknowledgments
We thank Cargill Beef, specifically Mr. John Couture and Ms. Lisa Kerr-House for arranging our bovine oviduct collections. We also thank the Cornell Center for Vertebrate Genomics (VERGE) for providing tools for data analysis, and Dr. Susan Suarez and Dr. Florencia Ardon for providing materials for our pilot investigations.
Data Availability
Raw data, mzML and Scaffold results are available from the MassIVE proteomics repository (MSV000081192) and Proteome Exchange (PXD006794).
Funding Statement
This study was supported by grants USDA-NIFA 2013-00986 to Vimal Selvaraj and USDA Multi State NE1227 NYC-127806 to Vimal Selvaraj. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Leese HJ. The formation and function of oviduct fluid. J Reprod Fertil. 1988;82(2):843–56. Epub 1988/03/01. . [DOI] [PubMed] [Google Scholar]
- 2.Stone SL, Hamner CD. Biochemistry and physiology of oviductal secretions. Gynecol Invest. 1975;6(3–4):234–52. Epub 1975/01/01. . [DOI] [PubMed] [Google Scholar]
- 3.Gad A, Hoelker M, Besenfelder U, Havlicek V, Cinar U, Rings F, et al. Molecular mechanisms and pathways involved in bovine embryonic genome activation and their regulation by alternative in vivo and in vitro culture conditions. Biol Reprod. 2012;87(4):100 Epub 2012/07/20. doi: 10.1095/biolreprod.112.099697 . [DOI] [PubMed] [Google Scholar]
- 4.Cebrian-Serrano A, Salvador I, Garcia-Rosello E, Pericuesta E, Perez-Cerezales S, Gutierrez-Adan A, et al. Effect of the bovine oviductal fluid on in vitro fertilization, development and gene expression of in vitro-produced bovine blastocysts. Reprod Domest Anim. 2013;48(2):331–8. Epub 2012/08/23. doi: 10.1111/j.1439-0531.2012.02157.x . [DOI] [PubMed] [Google Scholar]
- 5.Martus NS, Verhage HG, Mavrogianis PA, Thibodeaux JK. Enhancement of bovine oocyte fertilization in vitro with a bovine oviductal specific glycoprotein. Journal of reproduction and fertility. 1998;113(2):323–9. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd RE, Romar R, Matas C, Gutierrez-Adan A, Holt WV, Coy P. Effects of oviductal fluid on the development, quality, and gene expression of porcine blastocysts produced in vitro. Reproduction. 2009;137(4):679–87. Epub 2009/01/21. doi: 10.1530/REP-08-0405 . [DOI] [PubMed] [Google Scholar]
- 7.Mugnier S, Kervella M, Douet C, Canepa S, Pascal G, Deleuze S, et al. The secretions of oviduct epithelial cells increase the equine in vitro fertilization rate: are osteopontin, atrial natriuretic peptide A and oviductin involved? Reprod Biol Endocrinol. 2009;7:129 Epub 2009/11/21. doi: 10.1186/1477-7827-7-129 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eyestone WH, First NL. Co-culture of early cattle embryos to the blastocyst stage with oviducal tissue or in conditioned medium. J Reprod Fertil. 1989;85(2):715–20. Epub 1989/03/01. . [DOI] [PubMed] [Google Scholar]
- 9.Rieger D, Grisart B, Semple E, Van Langendonckt A, Betteridge KJ, Dessy F. Comparison of the effects of oviductal cell co-culture and oviductal cell-conditioned medium on the development and metabolic activity of cattle embryos. J Reprod Fertil. 1995;105(1):91–8. Epub 1995/09/01. . [DOI] [PubMed] [Google Scholar]
- 10.Rief S, Sinowatz F, Stojkovic M, Einspanier R, Wolf E, Prelle K. Effects of a novel co-culture system on development, metabolism and gene expression of bovine embryos produced in vitro. Reproduction. 2002;124(4):543–56. Epub 2002/10/04. . [PubMed] [Google Scholar]
- 11.Cordova A, Perreau C, Uzbekova S, Ponsart C, Locatelli Y, Mermillod P. Development rate and gene expression of IVP bovine embryos cocultured with bovine oviduct epithelial cells at early or late stage of preimplantation development. Theriogenology. 2014;81(9):1163–73. Epub 2014/03/19. doi: 10.1016/j.theriogenology.2014.01.012 . [DOI] [PubMed] [Google Scholar]
- 12.Chen S, Palma-Vera SE, Langhammer M, Galuska SP, Braun BC, Krause E, et al. An air-liquid interphase approach for modeling the early embryo-maternal contact zone. Scientific reports. 2017;7:42298 Epub 2017/02/10. doi: 10.1038/srep42298 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedersen ME, Ozdas OB, Farstad W, Tverdal A, Olsaker I. Effects of bovine oviduct epithelial cells, fetal calf serum and bovine serum albumin on gene expression in single bovine embryos produced in the synthetic oviduct fluid culture system. Reprod Fertil Dev. 2005;17(8):751–7. Epub 2006/02/16. . [DOI] [PubMed] [Google Scholar]
- 14.Al Darwich A, Perreau C, Petit MH, Papillier P, Dupont J, Guillaume D, et al. Effect of PUFA on embryo cryoresistance, gene expression and AMPKalpha phosphorylation in IVF-derived bovine embryos. Prostaglandins Other Lipid Mediat. 2010;93(1–2):30–6. Epub 2010/07/06. doi: 10.1016/j.prostaglandins.2010.06.002 . [DOI] [PubMed] [Google Scholar]
- 15.Barrera AD, Garcia EV, Hamdi M, Sanchez-Calabuig MJ, Lopez-Cardona AP, Balvis NF, et al. Embryo culture in presence of oviductal fluid induces DNA methylation changes in bovine blastocysts. Reproduction. 2017;154(1):1–12. doi: 10.1530/REP-16-0651 . [DOI] [PubMed] [Google Scholar]
- 16.Lamy J, Labas V, Harichaux G, Tsikis G, Mermillod P, Saint-Dizier M. Regulation of the bovine oviductal fluid proteome. Reproduction. 2016;152(6):629–44. Epub 2016/11/01. doi: 10.1530/REP-16-0397 . [DOI] [PubMed] [Google Scholar]
- 17.Reyley JAM. The uterine tubal fluid: secretion, composition and biological effects. Anim Reprod. 2005;2(2):91–105. Epub April/June 2005. [Google Scholar]
- 18.Diskin MG, Parr MH, Morris DG. Embryo death in cattle: an update. Reprod Fertil Dev. 2011;24(1):244–51. Epub 2012/03/08. doi: 10.1071/RD11914 . [DOI] [PubMed] [Google Scholar]
- 19.Memili E, First NL. Zygotic and embryonic gene expression in cow: a review of timing and mechanisms of early gene expression as compared with other species. Zygote. 2000;8(1):87–96. Epub 2000/06/07. . [DOI] [PubMed] [Google Scholar]
- 20.Olds D, Vandemark NL. Composition of luminal fluids in bovine female genitalia. Fertil Steril. 1957;8(4):345–54. Epub 1957/07/01. . [DOI] [PubMed] [Google Scholar]
- 21.Carlson D, Black DL, Howe GR. Oviduct secretion in the cow. J Reprod Fertil. 1970;22(3):549–52. Epub 1970/08/01. . [DOI] [PubMed] [Google Scholar]
- 22.Stanke DF, Sikes JD, DeYoung DW, Tumbleson ME. Proteins and amino acids in bovine oviducal fluid. J Reprod Fertil. 1974;38(2):493–6. Epub 1974/06/01. . [DOI] [PubMed] [Google Scholar]
- 23.Roberts GP, Parker JM, Symonds HW. Proteins in the luminal fluid from the bovine oviduct. J Reprod Fertil. 1975;45(2):301–13. Epub 1975/11/01. . [DOI] [PubMed] [Google Scholar]
- 24.Killian GJ, Chapman DA, Kavanaugh JF, Deaver DR, Wiggin HB. Changes in phospholipids, cholesterol and protein content of oviduct fluid of cows during the oestrous cycle. J Reprod Fertil. 1989;86(2):419–26. Epub 1989/07/01. . [DOI] [PubMed] [Google Scholar]
- 25.Staros AL, Killian GJ. In vitro association of six oviductal fluid proteins with the bovine zona pellucida. Journal of reproduction and fertility. 1998;112(1):131–7. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez C, Killian G. Identification of ampullary and isthmic oviductal fluid proteins that associate with the bovine sperm membrane. Anim Reprod Sci. 1998;54(1):1–12. Epub 1999/01/07. . [DOI] [PubMed] [Google Scholar]
- 27.Gonçalves RF, Staros AL, Killian GJ. Oviductal fluid proteins associated with the bovine zona pellucida and the effect on in vitro sperm-egg binding, fertilization and embryo development. Reproduction in domestic animals = Zuchthygiene. 2008;43(6):720–9. doi: 10.1111/j.1439-0531.2007.00978.x [DOI] [PubMed] [Google Scholar]
- 28.Boice ML, Geisert RD, Blair RM, Verhage HG. Identification and characterization of bovine oviductal glycoproteins synthesized at estrus. Biol Reprod. 1990;43(3):457–65. Epub 1990/09/01. . [DOI] [PubMed] [Google Scholar]
- 29.Abe H, Sendai Y, Satoh T, Hoshi H. Bovine oviduct-specific glycoprotein: a potent factor for maintenance of viability and motility of bovine spermatozoa in vitro. Molecular reproduction and development. 1995;42(2):226–32. doi: 10.1002/mrd.1080420212 [DOI] [PubMed] [Google Scholar]
- 30.Winger QA, de los Rios P, Han VK, Armstrong DT, Hill DJ, Watson AJ. Bovine oviductal and embryonic insulin-like growth factor binding proteins: possible regulators of "embryotrophic" insulin-like growth factor circuits. Biol Reprod. 1997;56(6):1415–23. Epub 1997/06/01. . [DOI] [PubMed] [Google Scholar]
- 31.Georgiou AS, Sostaric E, Wong CH, Snijders AP, Wright PC, Moore HD, et al. Gametes alter the oviductal secretory proteome. Mol Cell Proteomics. 2005;4(11):1785–96. Epub 2005/08/18. doi: 10.1074/mcp.M500119-MCP200 . [DOI] [PubMed] [Google Scholar]
- 32.Garlow JE, Ka H, Johnson GA, Burghardt RC, Jaeger LA, Bazer FW. Analysis of osteopontin at the maternal-placental interface in pigs. Biol Reprod. 2002;66(3):718–25. Epub 2002/03/01. . [DOI] [PubMed] [Google Scholar]
- 33.Hao Y, Mathialagan N, Walters E, Mao J, Lai L, Becker D, et al. Osteopontin reduces polyspermy during in vitro fertilization of porcine oocytes. Biol Reprod. 2006;75(5):726–33. Epub 2006/07/28. doi: 10.1095/biolreprod.106.052589 . [DOI] [PubMed] [Google Scholar]
- 34.Bauersachs S, Mitko K, Blum H, Wolf E. Technical note: Bovine oviduct and endometrium array version 1: a tailored tool for studying bovine endometrium biology and pathophysiology. J Dairy Sci. 2007;90(9):4420–3. Epub 2007/08/19. doi: 10.3168/jds.2007-0132 . [DOI] [PubMed] [Google Scholar]
- 35.Maillo V, Gaora PO, Forde N, Besenfelder U, Havlicek V, Burns GW, et al. Oviduct-Embryo Interactions in Cattle: Two-Way Traffic or a One-Way Street? Biol Reprod. 2015;92(6):144 Epub 2015/05/01. doi: 10.1095/biolreprod.115.127969 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonella-Diaza AM, Andrade SC, Sponchiado M, Pugliesi G, Mesquita FS, Van Hoeck V, et al. Size of the Ovulatory Follicle Dictates Spatial Differences in the Oviductal Transcriptome in Cattle. PLoS One. 2015;10(12):e0145321 doi: 10.1371/journal.pone.0145321 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75(17):4646–58. Epub 2003/11/25. . [DOI] [PubMed] [Google Scholar]
- 38.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8(10):785–6. Epub 2011/10/01. doi: 10.1038/nmeth.1701 . [DOI] [PubMed] [Google Scholar]
- 39.Emanuelsson O, Nielsen H, Brunak S, von Heijne G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol. 2000;300(4):1005–16. Epub 2000/07/13. doi: 10.1006/jmbi.2000.3903 . [DOI] [PubMed] [Google Scholar]
- 40.Emanuelsson O, Brunak S, von Heijne G, Nielsen H. Locating proteins in the cell using TargetP, SignalP and related tools. Nat Protoc. 2007;2(4):953–71. Epub 2007/04/21. doi: 10.1038/nprot.2007.131 . [DOI] [PubMed] [Google Scholar]
- 41.Kall L, Krogh A, Sonnhammer EL. A combined transmembrane topology and signal peptide prediction method. J Mol Biol. 2004;338(5):1027–36. Epub 2004/04/28. doi: 10.1016/j.jmb.2004.03.016 . [DOI] [PubMed] [Google Scholar]
- 42.Bendtsen JD, Jensen LJ, Blom N, Von Heijne G, Brunak S. Feature-based prediction of non-classical and leaderless protein secretion. Protein Eng Des Sel. 2004;17(4):349–56. Epub 2004/04/30. doi: 10.1093/protein/gzh037 . [DOI] [PubMed] [Google Scholar]
- 43.Mi H, Muruganujan A, Casagrande JT, Thomas PD. Large-scale gene function analysis with the PANTHER classification system. Nat Protoc. 2013;8(8):1551–66. Epub 2013/07/23. doi: 10.1038/nprot.2013.092 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maillo V, Gaora PO, Forde N, Besenfelder U, Havlicek V, Burns GW, et al. Oviduct-Embryo Interactions in Cattle: Two-Way Traffic or a One-Way Street? Biol Reprod. 2015;92(6):144 doi: 10.1095/biolreprod.115.127969 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19(9):1639–45. doi: 10.1101/gr.092759.109 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oliphant G, Bowling A, Eng LA, Keen S, Randall PA. The permeability of rabbit oviduct to proteins present in the serum. Biol Reprod. 1978;18(3):516–20. Epub 1978/04/01. . [DOI] [PubMed] [Google Scholar]
- 47.Ibrahim S, Salilew-Wondim D, Rings F, Hoelker M, Neuhoff C, Tholen E, et al. Expression pattern of inflammatory response genes and their regulatory micrornas in bovine oviductal cells in response to lipopolysaccharide: implication for early embryonic development. PLoS One. 2015;10(3):e0119388 doi: 10.1371/journal.pone.0119388 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Moraes AA, Hansen PJ. Granulocyte-macrophage colony-stimulating factor promotes development of in vitro produced bovine embryos. Biol Reprod. 1997;57(5):1060–5. . [DOI] [PubMed] [Google Scholar]
- 49.Neira JA, Tainturier D, Pena MA, Martal J. Effect of the association of IGF-I, IGF-II, bFGF, TGF-beta1, GM-CSF, and LIF on the development of bovine embryos produced in vitro. Theriogenology. 2010;73(5):595–604. doi: 10.1016/j.theriogenology.2009.10.015 . [DOI] [PubMed] [Google Scholar]
- 50.Srivastava MD, Lippes J, Srivastava BI. Hepatocyte growth factor in human milk and reproductive tract fluids. Am J Reprod Immunol. 1999;42(6):347–54. . [DOI] [PubMed] [Google Scholar]
- 51.Lu R-Z, Shiota K, Tachi C, Takahashi M. Histochemical demonstration of Activin/Inhibin betaA, betaB and alpha subunits in early embryos and oviducts of different strains of mice. Journal of Reproduction and development. 1992;38(1):79–90. [Google Scholar]
- 52.Larson RC, Ignotz GG, Currie WB. Platelet derived growth factor (PDGF) stimulates development of bovine embryos during the fourth cell cycle. Development. 1992;115(3):821–6. . [DOI] [PubMed] [Google Scholar]
- 53.Gabler C, Schams D, Einspanier A, Einspanier R. VEGF, TGF-alpha, TGF-beta in the bovine oviduct and indication of their regulation during the estrous cycle. Arch Tier-zucht (spec issue). 1996;39(29):12. [Google Scholar]
- 54.Luo H, Kimura K, Aoki M, Hirako M. Vascular endothelial growth factor (VEGF) promotes the early development of bovine embryo in the presence of cumulus cells. J Vet Med Sci. 2002;64(11):967–71. . [DOI] [PubMed] [Google Scholar]
- 55.Larson RC, Ignotz GG, Currie WB. Transforming growth factor beta and basic fibroblast growth factor synergistically promote early bovine embryo development during the fourth cell cycle. Mol Reprod Dev. 1992;33(4):432–5. doi: 10.1002/mrd.1080330409 . [DOI] [PubMed] [Google Scholar]
- 56.Garcia EV, Hamdi M, Barrera AD, Sanchez-Calabuig MJ, Gutierrez-Adan A, Rizos D. Bovine embryo-oviduct interaction in vitro reveals an early cross talk mediated by BMP signaling. Reproduction. 2017;153(5):631–43. doi: 10.1530/REP-16-0654 . [DOI] [PubMed] [Google Scholar]
- 57.Reinhart KC, Dubey RK, Mummery CL, van Rooijen M, Keller PJ, Marinella R. Synthesis and regulation of leukaemia inhibitory factor in cultured bovine oviduct cells by hormones. Mol Hum Reprod. 1998;4(3):301–8. . [DOI] [PubMed] [Google Scholar]
- 58.Ptak G, Lopes F, Matsukawa K, Tischner M, Loi P. Leukaemia inhibitory factor enhances sheep fertilization in vitro via an influence on the oocyte. Theriogenology. 2006;65(9):1891–9. doi: 10.1016/j.theriogenology.2005.10.018 . [DOI] [PubMed] [Google Scholar]
- 59.Fry RC, Batt PA, Fairclough RJ, Parr RA. Human leukemia inhibitory factor improves the viability of cultured ovine embryos. Biol Reprod. 1992;46(3):470–4. . [DOI] [PubMed] [Google Scholar]
- 60.Mo X, Wu G, Yuan D, Jia B, Liu C, Zhu S, et al. Leukemia inhibitory factor enhances bovine oocyte maturation and early embryo development. Mol Reprod Dev. 2014;81(7):608–18. doi: 10.1002/mrd.22327 . [DOI] [PubMed] [Google Scholar]
- 61.Palter SF, Mulayim N, Senturk L, Arici A. Interleukin-8 in the human fallopian tube. J Clin Endocrinol Metab. 2001;86(6):2660–7. doi: 10.1210/jcem.86.6.7584 . [DOI] [PubMed] [Google Scholar]
- 62.Nahar A, Kadokawa H. Expression of macrophage migration inhibitory factor (MIF) in bovine oviducts is higher in the postovulatory phase than during the oestrus and luteal phase. Reprod Fertil Dev. 2016. doi: 10.1071/RD15546 . [DOI] [PubMed] [Google Scholar]
- 63.Gabler C, Chapman DA, Killian GJ. Expression and presence of osteopontin and integrins in the bovine oviduct during the oestrous cycle. Reproduction. 2003;126(6):721–9. . [PubMed] [Google Scholar]
- 64.Goncalves RF, Wolinetz CD, Killian GJ. Influence of arginine-glycine-aspartic acid (RGD), integrins (alphaV and alpha5) and osteopontin on bovine sperm-egg binding, and fertilization in vitro. Theriogenology. 2007;67(3):468–74. doi: 10.1016/j.theriogenology.2006.08.013 . [DOI] [PubMed] [Google Scholar]
- 65.Hao Y, Murphy CN, Spate L, Wax D, Zhong Z, Samuel M, et al. Osteopontin improves in vitro development of porcine embryos and decreases apoptosis. Mol Reprod Dev. 2008;75(2):291–8. doi: 10.1002/mrd.20794 . [DOI] [PubMed] [Google Scholar]
- 66.Liu Q, Xie QZ, Zhou Y, Yang J. Osteopontin is expressed in the oviduct and promotes fertilization and preimplantation embryo development of mouse. Zygote. 2015;23(4):622–30. doi: 10.1017/S0967199414000483 . [DOI] [PubMed] [Google Scholar]
- 67.Wijayagunawardane MP, Gabler C, Killian G, Miyamoto A. Tumor necrosis factor alpha in the bovine oviduct during the estrous cycle: messenger RNA expression and effect on secretion of prostaglandins, endothelin-1, and angiotensin II. Biol Reprod. 2003;69(4):1341–6. doi: 10.1095/biolreprod.103.017327 . [DOI] [PubMed] [Google Scholar]
- 68.Kan FW, Esperanzate PW. Surface mapping of binding of oviductin to the plasma membrane of golden hamster spermatozoa during in vitro capacitation and acrosome reaction. Mol Reprod Dev. 2006;73(6):756–66. doi: 10.1002/mrd.20459 . [DOI] [PubMed] [Google Scholar]
- 69.Kawakami E, Hirano T, Hori T, Tsutsui T. Protease-induced hyperactivation of canine spermatozoa associated with disappearance of lectin-binding glycoproteins on their surface. J Vet Med Sci. 2004;66(9):1027–31. . [DOI] [PubMed] [Google Scholar]
- 70.Jaffe RC, Donnelly KM, Mavrogianis PA, Verhage HG. Molecular cloning and characterization of a progesterone-dependent cat endometrial secretory protein complementary deoxyribonucleic acid. Mol Endocrinol. 1989;3(11):1807–14. doi: 10.1210/mend-3-11-1807 . [DOI] [PubMed] [Google Scholar]
- 71.Jimenez Diaz M, Giunta S, Valz-Gianinet J, Pereyra-Alfonso S, Flores V, Miceli D. Proteases with plasminogen activator activity in hamster oviduct. Mol Reprod Dev. 2000;55(1):47–54. doi: 10.1002/(SICI)1098-2795(200001)55:1<47∷AID-MRD7>3.0.CO;2-J . [DOI] [PubMed] [Google Scholar]
- 72.Zampini R, Arganaraz ME, Miceli DC, Apichela SA. Detection of the matrix metalloproteinases MMP-2 and MMP-9 and tissue inhibitors of metalloproteinases TIMP-1 and TIMP-2 in llama (Lama glama) oviduct. Reprod Domest Anim. 2014;49(3):492–8. doi: 10.1111/rda.12317 . [DOI] [PubMed] [Google Scholar]
- 73.Anderson DJ, Abbott AF, Jack RM. The role of complement component C3b and its receptors in sperm-oocyte interaction. Proc Natl Acad Sci U S A. 1993;90(21):10051–5. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taylor CT, Biljan MM, Kingsland CR, Johnson PM. Inhibition of human spermatozoon-oocyte interaction in vitro by monoclonal antibodies to CD46 (membrane cofactor protein). Hum Reprod. 1994;9(5):907–11. . [DOI] [PubMed] [Google Scholar]
- 75.Suarez SS, Oliphant G. Interaction of rabbit spermatozoa and serum complement components. Biol Reprod. 1982;27(2):473–83. . [DOI] [PubMed] [Google Scholar]
- 76.Lee YL, Lee KF, Xu JS, He QY, Chiu JF, Lee WM, et al. The embryotrophic activity of oviductal cell-derived complement C3b and iC3b, a novel function of complement protein in reproduction. J Biol Chem. 2004;279(13):12763–8. Epub 2003/12/31. doi: 10.1074/jbc.M311160200 . [DOI] [PubMed] [Google Scholar]
- 77.Tse PK, Lee YL, Chow WN, Luk JM, Lee KF, Yeung WS. Preimplantation embryos cooperate with oviductal cells to produce embryotrophic inactivated complement-3b. Endocrinology. 2008;149(3):1268–76. Epub 2007/11/28. doi: 10.1210/en.2007-1277 . [DOI] [PubMed] [Google Scholar]
- 78.Thimon V, Belghazi M, Dacheux JL, Gatti JL. Analysis of furin ectodomain shedding in epididymal fluid of mammals: demonstration that shedding of furin occurs in vivo. Reproduction. 2006;132(6):899–908. doi: 10.1530/REP-06-0077 . [DOI] [PubMed] [Google Scholar]
- 79.Lavery K, Gabler C, Day J, Killian G. Expression of haptoglobin mRNA in the liver and oviduct during the oestrous cycle of cows (Bos taurus). Anim Reprod Sci. 2004;84(1–2):13–26. doi: 10.1016/j.anireprosci.2003.12.010 . [DOI] [PubMed] [Google Scholar]
- 80.Shaw JL, Petraki C, Watson C, Bocking A, Diamandis EP. Role of tissue kallikrein-related peptidases in cervical mucus remodeling and host defense. Biol Chem. 2008;389(12):1513–22. doi: 10.1515/BC.2008.171 . [DOI] [PubMed] [Google Scholar]
- 81.Zumoffen CM, Gil R, Caille AM, Morente C, Munuce MJ, Ghersevich SA. A protein isolated from human oviductal tissue in vitro secretion, identified as human lactoferrin, interacts with spermatozoa and oocytes and modulates gamete interaction. Hum Reprod. 2013;28(5):1297–308. doi: 10.1093/humrep/det016 . [DOI] [PubMed] [Google Scholar]
- 82.Roldan-Olarte M, Jimenez-Diaz M, Miceli DC. Plasminogen detection in oocytes and plasminogen activator activities in the porcine oviduct during the estrous cycle. Zygote. 2005;13(2):115–23. . [DOI] [PubMed] [Google Scholar]
- 83.Coy P, Jimenez-Movilla M, Garcia-Vazquez FA, Mondejar I, Grullon L, Romar R. Oocytes use the plasminogen-plasmin system to remove supernumerary spermatozoa. Hum Reprod. 2012;27(7):1985–93. doi: 10.1093/humrep/des146 . [DOI] [PubMed] [Google Scholar]
- 84.Panrucker DE, Lai PC, Lorscheider FL. Distribution of acute-phase alpha 2-macroglobulin in rat fetomaternal compartments. Am J Physiol. 1983;245(2):E138–42. . [DOI] [PubMed] [Google Scholar]
- 85.Gabler C, Killian GJ, Einspanier R. Differential expression of extracellular matrix components in the bovine oviduct during the oestrous cycle. Reproduction. 2001;122(1):121–30. . [PubMed] [Google Scholar]
- 86.Stambaugh R, Seitz HM Jr., Mastroianni L Jr. Acrosomal proteinase inhibitors in rhesus monkey (Macaca mulatta) oviduct fluid. Fertil Steril. 1974;25(4):352–7. . [DOI] [PubMed] [Google Scholar]
- 87.McLaughlin KC, Hamner CE. Preliminary characterization of rabbit oviduct fluid trypsin inhibitors. Biol Reprod. 1975;12(5):556–65. . [DOI] [PubMed] [Google Scholar]
- 88.Eliyahu E, Park JH, Shtraizent N, He X, Schuchman EH. Acid ceramidase is a novel factor required for early embryo survival. FASEB J. 2007;21(7):1403–9. doi: 10.1096/fj.06-7016com . [DOI] [PubMed] [Google Scholar]
- 89.Carrasco LC, Coy P, Aviles M, Gadea J, Romar R. Glycosidase determination in bovine oviducal fluid at the follicular and luteal phases of the oestrous cycle. Reprod Fertil Dev. 2008;20(7):808–17. . [DOI] [PubMed] [Google Scholar]
- 90.Roberts GP, Parker JM, Symonds HW. Macromolecular components of genital tract fluids from the sheep. J Reprod Fertil. 1976;48(1):99–107. . [DOI] [PubMed] [Google Scholar]
- 91.Vitaioli L, Menghi G, Baldoni E. Arylsulphatases A and B in the oviduct of female rabbits in anestrus and estrus conditions. Acta Histochem. 1984;75(2):141–8. doi: 10.1016/S0065-1281(84)80050-1 . [DOI] [PubMed] [Google Scholar]
- 92.Vitaioli L, Baldoni E, Sanguini LC. Arylsulphatases in the rabbit oviduct: postovulatory changes tested by histochemical and biochemical procedures. Histochem J. 1985;17(8):883–90. . [DOI] [PubMed] [Google Scholar]
- 93.Wandernoth PM, Mannowetz N, Szczyrba J, Grannemann L, Wolf A, Becker HM, et al. Normal Fertility Requires the Expression of Carbonic Anhydrases II and IV in Sperm. J Biol Chem. 2015;290(49):29202–16. doi: 10.1074/jbc.M115.698597 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wandernoth PM, Raubuch M, Mannowetz N, Becker HM, Deitmer JW, Sly WS, et al. Role of carbonic anhydrase IV in the bicarbonate-mediated activation of murine and human sperm. PLoS One. 2010;5(11):e15061 doi: 10.1371/journal.pone.0015061 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.DeSouza MM, Murray MK. An estrogen-dependent secretory protein, which shares identity with chitinases, is expressed in a temporally and regionally specific manner in the sheep oviduct at the time of fertilization and embryo development. Endocrinology. 1995;136(6):2485–96. doi: 10.1210/endo.136.6.7750470 . [DOI] [PubMed] [Google Scholar]
- 96.Libreros S, Garcia-Areas R, Iragavarapu-Charyulu V. CHI3L1 plays a role in cancer through enhanced production of pro-inflammatory/pro-tumorigenic and angiogenic factors. Immunol Res. 2013;57(1–3):99–105. doi: 10.1007/s12026-013-8459-y . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marin-Briggiler CI, Gonzalez-Echeverria MF, Munuce MJ, Ghersevich S, Caille AM, Hellman U, et al. Glucose-regulated protein 78 (Grp78/BiP) is secreted by human oviduct epithelial cells and the recombinant protein modulates sperm-zona pellucida binding. Fertil Steril. 2010;93(5):1574–84. doi: 10.1016/j.fertnstert.2008.12.132 . [DOI] [PubMed] [Google Scholar]
- 98.D'Souza SS, Daikoku T, Farach-Carson MC, Carson DD. Heparanase expression and function during early pregnancy in mice. Biol Reprod. 2007;77(3):433–41. doi: 10.1095/biolreprod.107.061317 . [DOI] [PubMed] [Google Scholar]
- 99.Zcharia E, Jia J, Zhang X, Baraz L, Lindahl U, Peretz T, et al. Newly generated heparanase knock-out mice unravel co-regulation of heparanase and matrix metalloproteinases. PLoS One. 2009;4(4):e5181 doi: 10.1371/journal.pone.0005181 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee KF, Kwok KL, Chung MK, Lee YL, Chow JF, Yeung WS. Phospholipid transfer protein (PLTP) mRNA expression is stimulated by developing embryos in the oviduct. J Cell Biochem. 2005;95(4):740–9. doi: 10.1002/jcb.20444 . [DOI] [PubMed] [Google Scholar]
- 101.Roy M, Gauvreau D, Bilodeau JF. Expression of superoxide dismutases in the bovine oviduct during the estrous cycle. Theriogenology. 2008;70(5):836–42. doi: 10.1016/j.theriogenology.2008.05.042 . [DOI] [PubMed] [Google Scholar]
- 102.Akerlof E, Jornvall H, Slotte H, Pousette A. Identification of apolipoprotein A1 and immunoglobulin as components of a serum complex that mediates activation of human sperm motility. Biochemistry. 1991;30(37):8986–90. . [DOI] [PubMed] [Google Scholar]
- 103.Jha KN, Shumilin IA, Digilio LC, Chertihin O, Zheng H, Schmitz G, et al. Biochemical and structural characterization of apolipoprotein A-I binding protein, a novel phosphoprotein with a potential role in sperm capacitation. Endocrinology. 2008;149(5):2108–20. doi: 10.1210/en.2007-0582 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Manjunath P, Marcel YL, Uma J, Seidah NG, Chretien M, Chapdelaine A. Apolipoprotein A-I binds to a family of bovine seminal plasma proteins. J Biol Chem. 1989;264(28):16853–7. . [PubMed] [Google Scholar]
- 105.Therien I, Soubeyrand S, Manjunath P. Major proteins of bovine seminal plasma modulate sperm capacitation by high-density lipoprotein. Biol Reprod. 1997;57(5):1080–8. . [DOI] [PubMed] [Google Scholar]
- 106.Provost PR, Tremblay Y, el-Amine M, Belanger A. Guinea pig apolipoprotein D RNA diversity, and developmental and gestational modulation of mRNA levels. Mol Cell Endocrinol. 1995;109(2):225–36. . [DOI] [PubMed] [Google Scholar]
- 107.Argraves WS, Morales CR. Immunolocalization of cubilin, megalin, apolipoprotein J, and apolipoprotein A-I in the uterus and oviduct. Mol Reprod Dev. 2004;69(4):419–27. doi: 10.1002/mrd.20174 . [DOI] [PubMed] [Google Scholar]
- 108.Aleporou-Marinou V, Pappa H, Yalouris P, Patargias T. Purification of apolipoprotein H (beta 2-glycoprotein I)-like protein from human follicular fluid. Comp Biochem Physiol B Biochem Mol Biol. 2001;128(3):537–42. . [DOI] [PubMed] [Google Scholar]
- 109.Liu J, Marey MA, Kowsar R, Hambruch N, Shimizu T, Haneda S, et al. An acute-phase protein as a regulator of sperm survival in the bovine oviduct: alpha 1-acid-glycoprotein impairs neutrophil phagocytosis of sperm in vitro. J Reprod Dev. 2014;60(5):342–8. doi: 10.1262/jrd.2014-049 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martinez-Leon E, Osycka-Salut C, Signorelli J, Pozo P, Perez B, Kong M, et al. Fibronectin stimulates human sperm capacitation through the cyclic AMP/protein kinase A pathway. Hum Reprod. 2015;30(9):2138–51. doi: 10.1093/humrep/dev154 . [DOI] [PubMed] [Google Scholar]
- 111.Larson RC, Ignotz GG, Currie WB. Effect of fibronectin on early embryo development in cows. J Reprod Fertil. 1992;96(1):289–97. . [DOI] [PubMed] [Google Scholar]
- 112.de Almeida PG, Pinheiro GG, Nunes AM, Goncalves AB, Thorsteinsdottir S. Fibronectin assembly during early embryo development: A versatile communication system between cells and tissues. Dev Dyn. 2016;245(4):520–35. doi: 10.1002/dvdy.24391 . [DOI] [PubMed] [Google Scholar]
- 113.Qu F, Ying X, Guo W, Guo Q, Chen G, Liu Y, et al. The role of Zn-alpha2 glycoprotein in sperm motility is mediated by changes in cyclic AMP. Reproduction. 2007;134(4):569–76. doi: 10.1530/REP-07-0145 . [DOI] [PubMed] [Google Scholar]
- 114.Tutuncu L, Stein P, Ord TS, Jorgez CJ, Williams CJ. Calreticulin on the mouse egg surface mediates transmembrane signaling linked to cell cycle resumption. Dev Biol. 2004;270(1):246–60. doi: 10.1016/j.ydbio.2004.02.008 . [DOI] [PubMed] [Google Scholar]
- 115.Asquith KL, Baleato RM, McLaughlin EA, Nixon B, Aitken RJ. Tyrosine phosphorylation activates surface chaperones facilitating sperm-zona recognition. J Cell Sci. 2004;117(Pt 16):3645–57. doi: 10.1242/jcs.01214 . [DOI] [PubMed] [Google Scholar]
- 116.Dietzel E, Wessling J, Floehr J, Schafer C, Ensslen S, Denecke B, et al. Fetuin-B, a liver-derived plasma protein is essential for fertilization. Dev Cell. 2013;25(1):106–12. doi: 10.1016/j.devcel.2013.03.001 . [DOI] [PubMed] [Google Scholar]
- 117.Younis AI, Brackett BG, Fayrer-Hosken RA. Influence of serum and hormones on bovine oocyte maturation and fertilization in vitro. Gamete Res. 1989;23(2):189–201. Epub 1989/06/01. doi: 10.1002/mrd.1120230206 . [DOI] [PubMed] [Google Scholar]
- 118.Zhang K, Rajput SK, Lee KB, Wang D, Huang J, Folger JK, et al. Evidence supporting a role for SMAD2/3 in bovine early embryonic development: potential implications for embryotropic actions of follistatin. Biol Reprod. 2015;93(4):86 doi: 10.1095/biolreprod.115.130278 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhenhua G, Rajput SK, Folger JK, Di L, Knott JG, Smith GW. Pre- and Peri-/Post-Compaction Follistatin Treatment Increases In Vitro Production of Cattle Embryos. PLoS One. 2017;12(1):e0170808 doi: 10.1371/journal.pone.0170808 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hawksworth OA, Coulthard LG, Taylor SM, Wolvetang EJ, Woodruff TM. Brief report: complement C5a promotes human embryonic stem cell pluripotency in the absence of FGF2. Stem Cells. 2014;32(12):3278–84. doi: 10.1002/stem.1801 . [DOI] [PubMed] [Google Scholar]
- 121.Gerena RL, Killian GJ. Electrophoretic characterization of proteins in oviduct fluid of cows during the estrous cycle. The Journal of experimental zoology. 1990;256(1):113–20. doi: 10.1002/jez.1402560114 [DOI] [PubMed] [Google Scholar]
- 122.Al-Dossary AA, Bathala P, Caplan JL, Martin-DeLeon PA. Oviductosome-Sperm Membrane Interaction in Cargo Delivery: Detection of fusion of underlying molecular players using three-dimensional super-resolution structured illumination microscopy. J Biol Chem. 2015;290(29):17710–23. Epub 2015/05/30. doi: 10.1074/jbc.M114.633156 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Al-Dossary AA, Strehler EE, Martin-Deleon PA. Expression and secretion of plasma membrane Ca2+-ATPase 4a (PMCA4a) during murine estrus: association with oviductal exosomes and uptake in sperm. PLoS One. 2013;8(11):e80181 Epub 2013/11/19. doi: 10.1371/journal.pone.0080181 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(XLSX)
(MP4)
Data Availability Statement
Raw data, mzML and Scaffold results are available from the MassIVE proteomics repository (MSV000081192) and Proteome Exchange (PXD006794).
Raw data, mzML and Scaffold results are available from the MassIVE proteomics repository (MSV000081192) and Proteome Exchange (PXD006794). Complete protein lists are provided in supporting information (S1 Dataset).