Abstract
The endocrine-disrupting chemical bisphenol A (BPA) increases adipose tissue mass in vivo and promotes adipogenesis in vitro; however, mechanisms explaining BPA’s obesogenic effect remain unknown. We investigated the effects of gestational BPA and its analog, bisphenol S (BPS), exposure on the adipogenic differentiation ability of fetal preadipocytes and the role of endoplasmic reticulum stress in regulating this process. Pregnant sheep (n = 7 to 8 per group) mated to the same male were exposed to BPA or BPS from days 30 to 100 of gestation; pregnancies were terminated 20 days later. Adipose tissue was harvested and fetal preadipocytes isolated. Adipose tissue gene expression, adipocyte size, preadipocyte gene expression, adipogenic differentiation, and dynamic expression of genes involved in adipogenesis and endoplasmic reticulum stress were assessed. Gestational BPA enhanced adipogenic differentiation in female, but not male, preadipocytes. The unfolded protein response (UPR) pathway was upregulated in BPA-exposed female preadipocytes supportive of a higher endoplasmic reticulum stress. Increased expression of estradiol receptor 1 and glucocorticoid receptor in female preadipocytes suggests that this may be a potential cause behind the sex-specific effects observed upon BPA exposure. Gestational BPS affected adipogenic terminal differentiation gene expression in male preadipocytes, but not adipogenic differentiation potential. We demonstrate that gestational BPA exposure can modulate the differentiation ability of fetal preadipocytes. UPR upregulation in gestationally BPA-exposed female preadipocytes may contribute to the increased preadipocyte’s adipogenic ability. The marked sex-specific effect of BPA highlights higher susceptibility of females to bisphenol A and potentially, a higher risk to develop obesity in adulthood.
Gestational bisphenol A and S exposure had different effects on fetal adipogenic differentiation ability. These effects were sex-specific and altered the uncoupled protein response.
With obesity prevalence on the rise since the 1960s (1), more than one-third of the US population is currently overweight or obese (2). Importantly, obesity has been linked to a reduced life expectancy and comorbidities such as heart disease, type 2 diabetes, infertility, and cancer (3). Although genetic factors and a lack of energy balance are well-known factors contributing to the obesity epidemic (4), the upward trend suggests a multifactorial etiology, with social and environmental factors playing a role in this increased prevalence (4).
Endocrine-disrupting chemicals (EDCs) are compounds that can interfere with endocrine signaling pathways via receptor binding and/or epigenetic mechanisms, thus interfering with adipose tissue metabolism and contributing to the obesity epidemic (5). Obesogens are a class of EDC known to disrupt lipid metabolism, metabolic sensors, sex-steroid synthesis, and energy balance (6, 7). Bisphenol A (BPA), an EDC used in the manufacturing of plastics and epoxy resins, is considered an obesogen (6, 7). Epidemiological studies reveal a positive association between prenatal BPA levels and obesity in children (8, 9) and adults (10–12). Although conflicting evidence is available (13, 14), BPA’s obesogenic nature is further supported by in vitro studies (15, 16) and animal studies investigating the effects of pre-, peri-, and postnatal exposure to BPA on adipose tissue mass in the offspring (17–22). Our previous work has demonstrated that female sheep gestationally exposed to BPA develop insulin resistance, a disrupted adipokine profile in adipose tissue, adipocyte hypertrophy, and an increase in visceral to subcutaneous adipose tissue ratio upon a postnatal high-calorie diet (22). Additionally, previous work has shown sex-specific effects of perinatal BPA on adipose tissue deposition in animal studies, with females being more susceptible to BPA’s effect (18, 19, 21). However, the mechanisms by which BPA increases body weight and adipose mass and its sex-specific effect have not been elucidated yet.
Adipogenesis is the complex process by which preadipocytes transition into lipid-filled, insulin-responsive adipocytes, and it is tightly regulated by transcription factors (23). The endoplasmic reticulum (ER) is a critical site of protein synthesis and lipid metabolism. ER stress is a homeostatic response that results in unfolded and/or misfolded proteins accumulation. To restore ER homeostasis, unfolded protein response (UPR), a highly conserved defense mechanism, is activated (24). Double-stranded RNA-dependent protein kinase–like ER kinase (PERK), inositol-requiring protein 1 (IRE1), and activating transcription factor 6 (ATF6) are the three main UPR pathways. All three play a role in the regulation of lipid metabolism via lipogenesis transcription factors modulation (25) and adipogenesis (26–28) and have been gaining attention because of their involvement in the pathogenesis of obesity (29). Importantly, BPA has been shown to affect ER stress response regulation in various organs (30–32); however, it remains unknown whether BPA can affect the ER stress response to modulate adipogenesis.
Current knowledge on adipocyte biology heavily relies on the use of the preadipocyte cell line 3T3-L1 (murine origin) and in vivo mouse studies. Although rodent species offer clear advantages for the study of adipogenesis (lower cost or easy access to genetic modification) (33), other species, such as the sheep, are also considered excellent models to study the relationship of sex steroids with obesity (34, 35) and offer several advantages critical for research that focus on the effects of gestational exposures on the progeny (36, 37). First, sheep, similar to humans and unlike rodents, are precocial species and thus the majority of their organs, including the adipose tissue, mature before birth (38). Sheep, as with humans, are monovulatory, which reduces potential confounding factors seen in litter-bearing species, such as the intrauterine fetal position phenomenon (39, 40). This becomes especially important when studying EDC with steroidal activity (41), such as bisphenols. Importantly, the sheep model has also been used to predict human fetal exposure to BPA (42). All the these points highlight the advantages of using sheep as an animal model in fetal adipocyte biology research (34).
In recent years, public pressure has led to shifting away from BPA in various consumer products. Attention has begun to focus on other bisphenolic chemicals that are also used in the manufacture of plastics (43, 44). Among these bisphenols, bisphenol S (BPS), an organic compound with a similar biochemical structure to that of BPA, has been widely used in beverage and food cans and in thermal receipt papers (45, 46) and can now be detected in up to 70% of human urine samples (43, 47) and fetal cord blood (48). Recent evidence has demonstrated that BPS may also act as an obesogen because it can induce adipogenesis in vitro (49). However, whether these effects are reproducible in vivo remains unknown.
Although prenatal exposure to BPA is associated with enhanced adipogenesis and increased adipose tissue accumulation in adulthood (17–22), to date, the mechanisms by which they occur remain unclear. The objectives of this study were to assess (1) whether gestational exposure to bisphenolic compounds BPA and/or BPS will affect the adipogenic ability of fetal preadipocytes, (2) whether these effects are sex-specific, and (3) the role of ER stress in modulating the adipogenic potential of gestationally exposed preadipocytes to these bisphenolic compounds. To address these questions, we have used a monovulatory, precocial species that has enabled us to investigate the effects of these EDC during adipose tissue fetal development in midpregnancy.
Materials and Methods
Animal experimentation
All procedures used in this study were approved by the Michigan State University Institutional Animal Care and Use Committee and are consistent with the National Research Council’s Guide for the Care and Use of Laboratory Animals and the current Animal Welfare Act. This work meets the Animals in Research: Reporting In Vivo Experiments guidelines for reporting animal research (50). All reagents were from Sigma-Aldrich (St. Louis, MO) unless stated otherwise. The study was conducted at the Michigan State University Sheep Research Facility (East Lansing, MI) using an in-house sheep flock of multiparous Polypay Dorsett crossbred. Healthy and primiparous female sheep were bred using a time-mated pregnancy strategy. Three vasectomized rams were raddled on their chests, and estrus was detected by the presence of paint on the backs of the females as a result of mounting. In the morning, all mounted females were moved with a fertile ram (a single fertile ram was used to reduce confounding factors of paternal origin). Ram fertility was tested by a breeding soundness examination including inspection of the genital organs and assessment of sperm production and quality. Once mated, the females were randomly assigned to the treatment groups, ensuring similar body score condition and body weight across groups. Animals were fed a standard diet meeting National Research Council requirements, as previously described (51), and all feeding regimens met the nutrient requirements for sheep. Potential phytoestrogen load present in feed occurred across all groups. Because animals were kept group-housed to reduce isolation stress, all animals were exposed to the same phytoestrogen load from their food source.
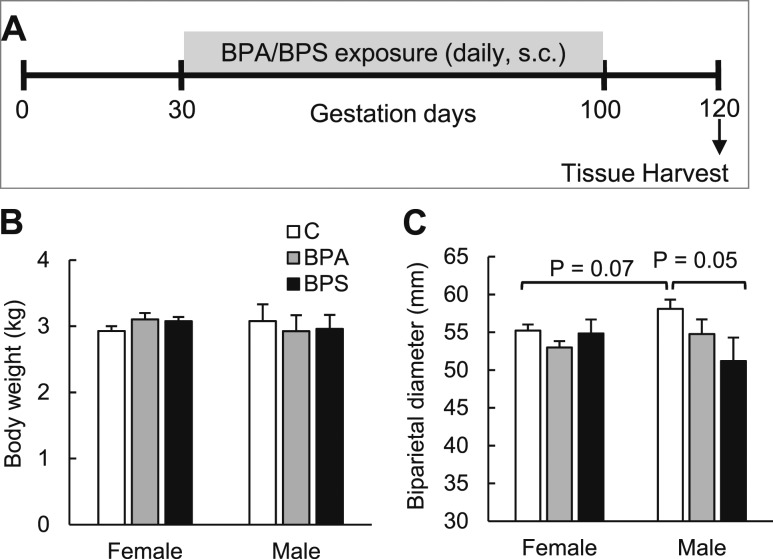
Gestational BPA and BPS treatment consisted of daily subcutaneous injections of BPA (0.5 mg/kg/d; purity ≥99%, catalog no. 239658, Sigma-Aldrich) or BPS (purity: 99.7%; catalog no. 146915000, Acros Organics, Geel, Belgium) in corn oil from days 30 through 100 of gestation (term: ∼147 days; Fig. 1A). Control mothers received corn oil only (vehicle). The internal dose of BPA achieved in umbilical arterial samples using the 0.5 mg/kg/d dose has already been published (52) and is targeted to produce maternal blood levels of BPA (∼2.6 ng/mL) approaching the median concentration of BPA measured in maternal circulation in the United States (53). The same dose of BPS was used to match the BPA exposure dose used in the study. The number of breeders used was eight, eight, and seven, in control, BPA, and BPS groups, respectively. Breeding resulted in six control, six BPA, and four BPS female fetuses and four control, five BPA, and four BPS male fetuses. All pregnancies (n = 23) but six were singletons. Twinning was included in the statistical analysis as a covariate. To understand persistent effects of gestational bisphenol exposure, treatment was discontinued at gestational day 100. After a washout of 20 days, perirenal fetal adipose tissue (largest adipose depot at this fetal age) was harvested at day 120 of gestation upon pregnancy termination with a barbiturate overdose [in travenous pentobarbital sodium (Fatal Plus; Henry Schein, Melville, NY)]. A midline incision was performed, the uterus exposed, and the fetus quickly removed. Fetal body weight and biparietal diameter were assessed with calipers by a single operator. The perirenal adipose tissue was immediately harvested and either fixed in a 10% neutral buffered formalin solution for histological processing, flash frozen for gene expression studies, or freshly collected for cellular studies.
Figure 1.
Effect of gestational BPA or BPS exposure on fetal body weight and biparietal diameter. (A) Experimental design. (B) Body weight and (C) biparietal diameter in females and males of control (open bars), BPA-exposed (gray bars), and BPS-exposed (closed bars) fetuses. n = 4 to 6 per group per sex. Different letters denote statistical differences among groups at P < 0.05. For biparietal diameter, a trend for treatment × sex effect (F, 2.874; P = 0.078) was observed. C, control; s.c., subcutaneous.
Tissue histology
Fixed fetal perirenal adipose tissue (n = 4 to 6 per group per sex) was embedded in paraffin. Sections (5 µm) were cut with a microtome, stained with hematoxylin and eosin, and mounted with acrytol mounting medium. To evaluate adipocyte size, three nonoverlapping images per section were taken using a bright-field microscope and the perimeter of 300 to 500 cells per animal measured.
Primary cultured cell isolation and proliferation
Fetal primary preadipocytes were isolated following our standardized and validated protocol (54), similar to that used in the isolation of human primary preadipocytes (55). In brief, freshly collected fetal perirenal adipose tissue (1 to 2 g) was placed in prewarmed Dulbecco's phosphate-buffered saline with antibiotic-antimycotic (Invitrogen, Carlsbad, CA). After removing any visible blood clots or connective tissue, the tissue was minced into small pieces. Collagenase-I (1 mg/mL) was used to digest the tissue for 40 minutes in a 37°C water bath, filtered through a mesh filter (250 μm), and centrifuged at 1200g for 5 minutes. The cell pellet was washed with fresh omental preadipocyte medium (Zen Bio, Research Triangle Park, Durham, NC) and seeded into six-well plates. After six days of culture, cells were frozen and stored in liquid nitrogen until further use. Primary cultured cells (n = 3 per group per sex) were harvested and trypsin-digested (0.05%) for subculture at 90% confluency. Proliferation ability for each cell lineage (passage 3) was assessed using a growth curve analysis. Cells were seeded at a density of 10,000 cells per well in 24-well plates and counted in hemocytometer chambers every 24 hours for eight days. Triplicate wells for each time point per primary cell culture were used.
Cell culture and adipocyte differentiation
Before differentiation induction, fetal preadipocytes (n = 3 primary cultured cells per group per sex; passage 3) were cultured in Dulbecco’s modified Eagle medium/F12 (Invitrogen) supplemented with 1% penicillin-streptomycin (Invitrogen), 1% l-glutamine, 10 mM HEPES, and 10% fetal bovine serum (Fisher Scientific, Wilmington, NC) to confluency and allowed to grow for two additional days. Thereafter, adipocyte differentiation was induced as previously described (54). In brief, differentiation medium consisted of growth medium supplemented with 33 μM biotin, 17 mM pantothenate, 10 μg/mL insulin, 1 μM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine, and the peroxisome proliferator–activated receptor gamma (PPARγ) agonist, rosiglitazone (20 μM). We have recently demonstrated that in vitro differentiation of sheep preadipocytes requires the supplementation of a PPARγ agonist (54), similar to human primary preadipocytes (56). Adipogenic differentiation was determined by Oil Red O (ORO) stain, as a marker of lipid accumulation as previously described (54). ORO absorbance at 500 nm was measured in a microplate reader (Gemini; Molecular Devices, Sunnyvale, CA) and expressed as optical density (OD). ORO-positive area was also calculated using Fiji (imagej.nih.gov) (57). ORO-stained cells were further stained with 4′,6-diamidino-2-phenylindole to assess the number of cells. Nine fields were imaged and counted. No significant differences were observed in number of cells among treatment groups or sex (data not shown). Because phenol red may exert estrogenic action (58), a pilot study was conducted to assess if the presence of phenol red affected the adipogenic differentiation outcomes (Supplemental Fig. 1 (736.7KB, pdf) ).
Quantitative real-time polymerase chain reaction
Total RNA was extracted using an RNeasy Mini kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol from cell or adipose tissue. RNA quality and concentration were measured by Nanodrop (Thermo Fisher Scientific, Wilmington, NC). A total of 1 µg RNA (A260/A280: 2.0 ± 0.05, RNA concentration: 300 ± 50 ng/μL) was reverse transcribed into complementary DNA (cDNA) using a High Capacity cDNA Reverse Transcription Kit (Promega, Madison, WI) in 10-µL reaction volumes. Quantitative real-time polymerase chain reaction (qRT-PCR; ABI-Quant Studio 7 Flex Real-Time PCR System, Thermo Fisher, Carlsbad, CA) was performed to examine the messenger RNA (mRNA) expression of the genes encoding sheep adiponectin (ADIPOQ), caspase-8 (CASP8), CCAAT/enhancer-binding protein α (C/EBPα), C/EBP homologous protein 10 (CHOP10), delta-like noncanonical Notch ligand 1 (DLK1), estrogen receptor 1/2 (ESR1/ESR2), estrogen-related receptor α (ERRα), fatty acid–binding protein 4 (FABP4), glucocorticoid receptor (GR), glucose transporter type 4 (GLUT4), heat shock protein family A member 5 (HSPA5), IRE1α, leptin, lipoprotein lipase (LPL), mitogen-activated protein kinase 8 (MAPK8), PPARγ that detects both PPARγ1 and PPARγ2 transcripts, PERK, sterol regulatory element binding transcription factor 1 (SREBF1), sex determining region-box 6 (SOX6), spliced X-box binding protein 1 (XBP1-s), and Wnt family member 10B (WNT10B). Primer sequences are provided in Supplemental Table 1 (736.7KB, pdf) . mRNA levels encoding the indicated genes were normalized against GAPDH and presented as relative fold change to that of the control and calculated using the ΔΔCT method (59). Two additional housekeeping genes (RPL27 and β-ACTIN) were used to validate this work (not shown). All experiments and quantitative real-time PCR was run in triplicate. cDNA amplification reaction (50 ng) consisted of template denaturation and polymerase activation at 95°C for 30 seconds, followed by 40 cycles of denaturation at 95°C for 15 seconds, annealing at 60°C for 30 seconds, and extension at 72°C for 30 seconds. Melt curve analyses were performed for all genes, and the specificity as well as integrity of the PCR products was confirmed by the presence of a single peak of melt curve and PCR products by agarose gel electrophoresis.
Statistical analysis
All data are presented as mean ± standard error (SE). Appropriate transformations were applied, as needed, to account for normality of data. Comparisons among the three treatment groups and two sexes were analyzed by two-way analysis of variance with Tukey post hoc tests with twinning as a covariate. Comparisons among groups without sex interaction were tested using a one-way analysis of variance with Tukey post hoc tests with twinning as a covariate or a t test between groups and within time points. All previously mentioned analyses were run using PASW Statistics for Windows, release 18.0.1. To assess the impact of gestational bisphenol exposure on adipocyte size, an empirical cumulative distribution function was also calculated for each measurement, and the difference among groups tested using a Kolmogorov-Smirnov test. The significance of the difference between the distributions of cell perimeter was tested using a permutation test with 10,000 iterations using R Statistical Computing, release 3.3.0. Differences were considered significant at P < 0.05.
Results
Gestational BPA and BPS effects on adipose tissue
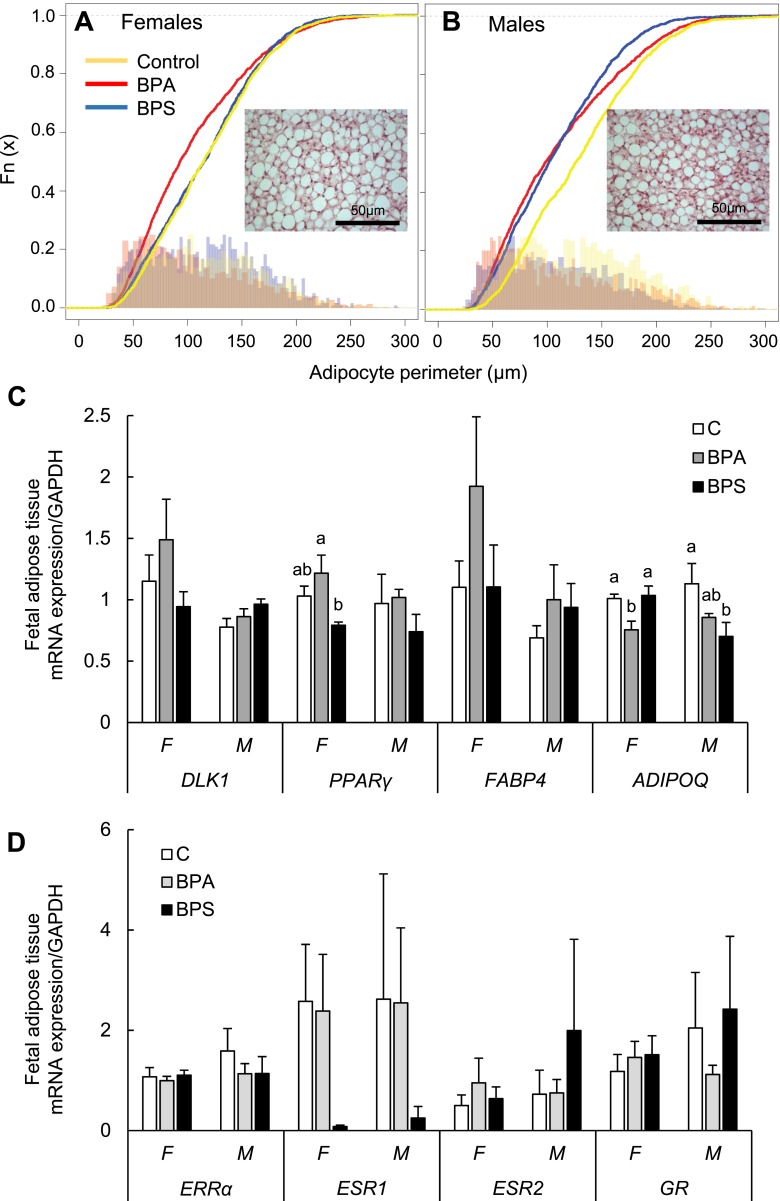
Prenatal exposure to BPA or BPS did not affect fetal body weight in female or male (Fig. 1B) fetuses at gestational day 120 (before fast growth occurs in late gestation), but tended to lower biparietal diameter in male BPS-exposed fetuses (P = 0.05, Fig. 1C). Perirenal adipose tissue was not weighed to enable quick collection for cell culture work, but no gross differences were noticed among groups. Histological examination of the perirenal adipose pad revealed that gestational BPA or BPS did not significantly affect adipocyte size of unilocular adipocytes (white adipocytes), but cell size plot evidenced a left shift (smaller size) in BPA females (Fig. 2A) and both BPA and BPS males compared with their respective controls (Fig. 2B). No sex-specific differences were observed in adipocyte size. Gene expression in fetal adipose tissue revealed that no substantial differences were observed in BPA- or BPS-treated groups in adipogenesis initial (DLK1), early (C/EBPα), and late (LPL, FABP4, GLUT4) stage marker genes expression in females or males compared with their respective control groups (Fig. 2C; Supplemental Fig. 2 (736.7KB, pdf) ). PPARγ mRNA expression was significantly higher in fetal adipose tissue in BPA-exposed compared with BPS-exposed female fetuses (Fig. 2C). ADIPOQ expression was significantly lower in BPA-exposed females and BPS-exposed males compared with their respective controls (Fig. 2C). mRNA expression was also similar among groups in the cholesterol regulators SREBF1, leptin, (Supplemental Fig. 2 (736.7KB, pdf) ) and steroid receptors (ERRα, ESR1, ESR2, and GR) (Fig. 2D).
Figure 2.
Effect of gestational BPA and BPS exposure on adipocyte size and mRNA expression (mean ± SE) in fetal perirenal adipose tissue. Adipocyte perimeter (μm) plots in (A) females and (B) males of control (yellow), BPA-exposed (red), and BPS-exposed (blue) fetuses. Representative fetal adipose tissue section (hematoxylin and eosin) from control female and male is shown within cell perimeter plots. (C, D) mRNA expression (mean ± SE) in fetal perirenal adipose tissue of preadipocyte (DLK1), early (PPARγ), and late (FABP4, ADIPOQ) adipocyte markers, and steroid receptors (ERRα, ESR1, ESR2, and GR) in females (F) and males (M) of control (open bars), BPA-exposed (gray bars), and BPS-exposed (closed bars) fetuses. Different letters denote differences among treatments within sex: a ≠ b, P < 0.05; a′ ≠ b′, 0.06 < P < 0.08. n = 4 to 6 per group per sex. Horizontal bars denote differences between sexes within gene. For PPARγ, a treatment effect (F, 3.351; P < 0.048) was observed. For ADIPOQ, a treatment × sex effect (F, 4.107; P < 0.033) and a treatment effect (F, 5.262; P < 0.015) were observed. C, control; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Gestational BPA and BPS on fetal preadipocyte adipogenic differentiation and proliferation
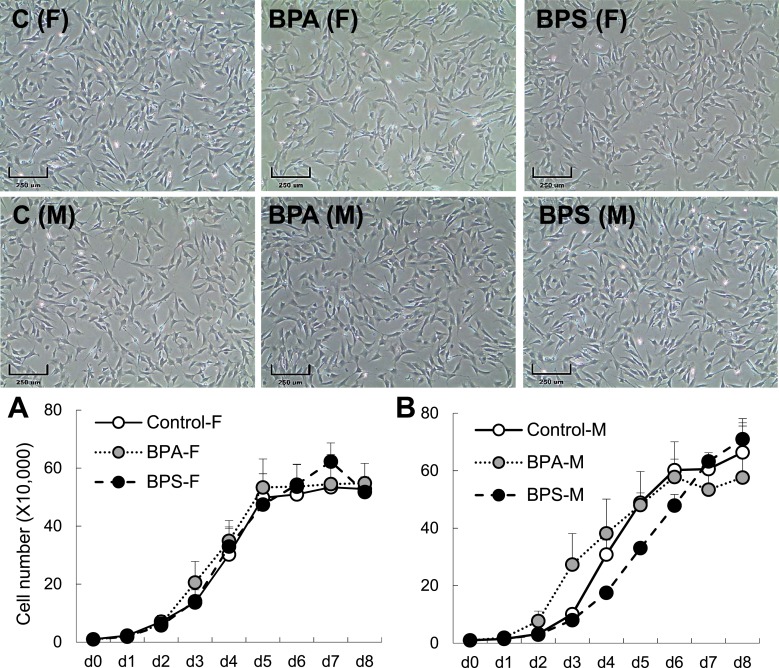
We investigated the effects of prenatal BPA and BPS exposure on adipogenic differentiation by evaluating the differentiation ability of primary cultured fetal preadipocytes. After 8 days of adipogenic differentiation, regardless of treatment group, all female primary cells had higher differentiation ability compared with their male counterparts (Fig. 3C). Gestational BPA-exposed female preadipocytes had increased adipogenic differentiation compared with both control and BPS groups (Fig. 3A and 3B). This finding was supported by an upregulation in gene expression of early (PPARγ, C/EBPα) and late (LPL, FABP4, ADIPOQ) adipogenic markers at terminal differentiation stage (day 8 of differentiation; Fig. 3C). Gestational BPS exposure had no effect on adipogenic ability or gene expression in female primary preadipocytes (Fig. 3C). Quantification of the adipogenic differentiation in gestationally BPA-exposed male preadipocytes revealed a substantial reduction when quantified using the ORO area method (Fig. 3E), but not when quantified using the OD value (Fig. 3D). This discrepancy is likely due to the lower sensitivity of the OD measurement in wells with very low differentiation ability. Gestational BPA reduced gene expression of early (PPARγ, C/EBPα) and late (LPL, FABP4, and ADIPOQ) adipogenic markers in male primary cells at differentiation day 8 (Fig. 3F). Gestational BPS did not affect adipogenic differentiation in male primary cells (Fig. 3D), but increased late (FABP4 and ADIPOQ) adipogenic markers (Fig. 3F). To assess if differences observed in differentiation potential were driven by an increased preadipocyte proliferation ability, a cell proliferation assay was conducted. Preadipocyte proliferation rate was not different by sex or treatment after 8 days of culture (Fig. 4).
Figure 3.
Effect of gestational BPA and BPS exposure on fetal adipogenic differentiation. Representative images of ORO-stained differentiated adipocytes (day 8) on females (top) and males (bottom) of control (left; C), BPA (middle), and BPS (right) gestationally exposed fetuses. Adipocyte differentiation quantification by OD value and ORO stain–positive area in control and gestationally BPA- and BPS-exposed (A, B) female and (D, E) male primary preadipocytes (n = 3 per group), respectively. (C, F) mRNA expression (mean ± SE) of preadipocyte (DLK1), early- (PPARγ, C/EBPα) and late- (LPL, FABP4, ADIPOQ) stage adipocyte markers at terminal differentiation in control (open), BPA (gray), and BPS (closed) (C) females and (F) males. Scale bar: 250 μm. Different letters denote statistical differences among treatment groups at P < 0.05. Given the large difference in adipogenic differentiation between sexes, gene expression normalization was performed relative to the control group of each sex.
Figure 4.
Effect of gestational BPA and BPS exposure on fetal preadipocyte proliferation. Representative images of preadipocytes at day (d) 0 of culture in control, BPA, and BPS gestationally exposed female (F; top) and male (M; bottom) cultured primary cells (n = 3 per group). Growth curves (mean ± SE) of (A) female and (B) male primary preadipocytes. Scale bar: 250 μm. C, control.
Gestational BPA and BPS on preadipocyte gene expression
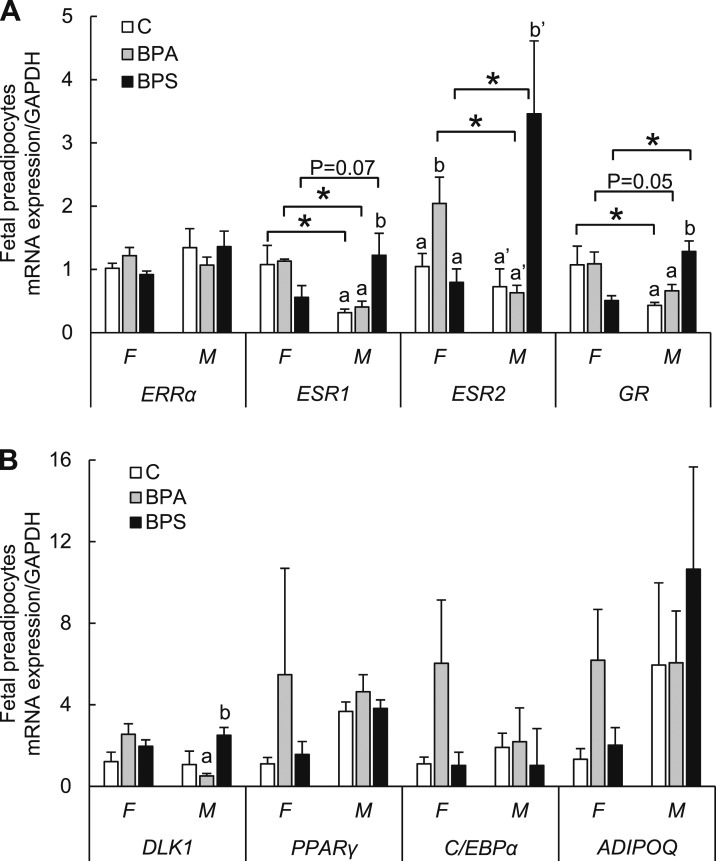
To understand if the sex-specific difference in the adipogenic differentiation was partially due to differential expression in sex-steroid receptors, we evaluated gene expression on GR, ESR1, ESR2, and ERRα. To avoid the confounder of other cells present in the adipose tissue (endothelial, differentiated adipocytes, macrophages, or stromal cells), we investigated the steroid receptor expression in isolated preadipocytes. In fetal preadipocytes (Fig. 5A), ESR1 and GR expression was higher in females than control (P < 0.05) and BPA (P < 0.05 and P = 0.05, respectively) groups compared with their male counterparts. ESR2 expression was also higher in BPA females compared with their male counterparts (P < 0.05). The opposite expression pattern was observed in BPS-exposed male preadipocytes with higher ESR1 (P = 0.07), ESR2 (P < 0.05), and GR (P < 0.05) expression compared with their female counterparts. In females, BPA-exposed preadipocytes had higher ESR2 expression compared with control females (P < 0.05). In males, BPS-exposed preadipocytes had increased ESR1, ESR2, and GR (P < 0.05) expression compared with control males. BPA-exposed male preadipocytes had no differences in steroid receptor expression. Expression of ERRα was not significantly different among treatments or sex.
Figure 5.
(A, B) Effect of gestational BPA exposure on fetal preadipocyte steroid receptor expression. mRNA expression (mean ± SE) of steroid receptor expression of ESR1 and ESR2, ERRα, GR, preadipocyte DLK1, and early (PPARγ, C/EBPα) and late (ADIPOQ) adipocyte markers, in undifferentiated preadipocytes in females (F) and males (M) of control (open bars; C), BPA-exposed (gray bars), and BPS-exposed (closed bars) fetuses. n = 4 to 6 per group per sex. Treatment × sex effects were observed for ESR1 (F, 6.686; P < 0.05), ESR2 (F, 6.042; P < 0.013), GR (F, 12.092; P < 0.001), and DLK1 (F, 4.827; P < 0.03). Treatment effect was observed for DLK1 (F, 3.539; P = 0.057). *P < 0.05.
No important differences were observed in BPA- or BPS-treated groups in adipogenesis early (C/EBPα and PPARγ) and late (ADIPOQ) stage marker genes expression in female or male preadipocytes compared with their respective control groups (Fig. 5B). DLK1 mRNA expression was higher in male preadipocytes in BPS- vs BPA-exposed fetuses (Fig. 5B).
Gestational BPA accelerates fetal adipogenic differentiation in females
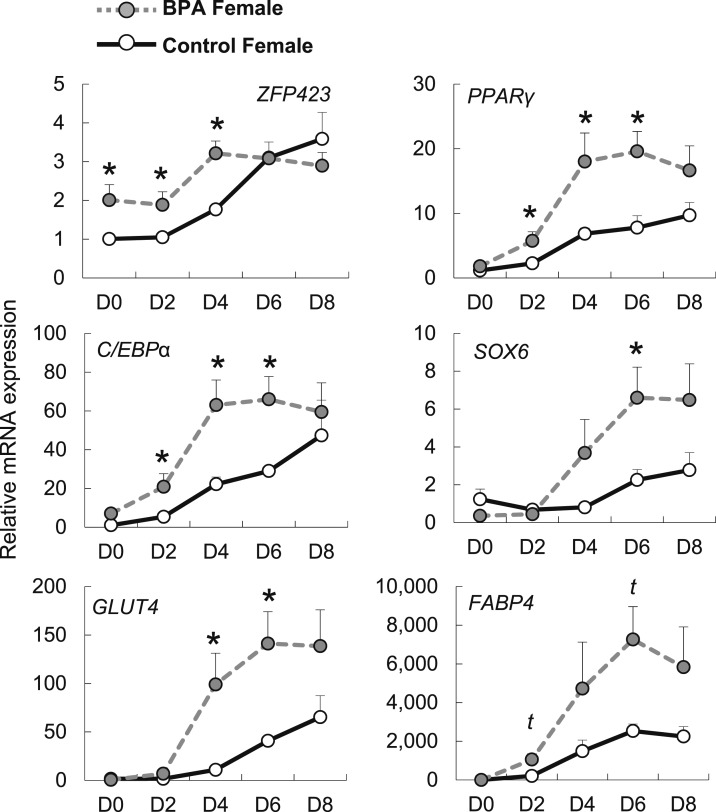
To further explore the underlying mechanism of BPA on enhanced adipogenic differentiation, the remainder of the study focused on female preadipocytes. To test whether prenatal exposure to BPA affects the differentiation process, gene expression time course on differentiation days 0, 2, 4, 6, and 8 was evaluated in female preadipocytes (Fig. 6). ZFP423, a transcriptional regulator of PPARγ, and an indicator of the ability of adipogenic differentiation, was upregulated from day 0 to day 4 in prenatal BPA exposed female primary preadipocytes. Gene expression of C/EBPα and PPARγ, early adipogenic genes, were upregulated in gestationally BPA exposed preadipocytes after day 2 of differentiation. FABP4, GLUT4, and SOX6 began to diverge later in the differentiation process (day 4) in gestationally BPA-exposed preadipocytes. Lower differentiation in control primary cells was not due to cellular apoptosis during differentiation, as shown by lack of change in apoptotic markers throughout differentiation (CASP8, MAPK8; data not shown).
Figure 6.
Effect of gestational BPA exposure on female adipogenic gene expression during adipogenic differentiation. mRNA (mean ± SE) expression of ZFP423, C/EBPα, GLUT4, PPARγ, SOX6, and FABP4 at differentiation days (D) 0, 2, 4, 6, and 8 in control (solid black line) and BPA (dotted gray line) gestationally exposed female primary preadipocytes (n = 3 per group). *Significance at P < 0.05; t, statistical trend at 0.05 > P > 0.06.
Gestational BPA exposure modulates ER stress
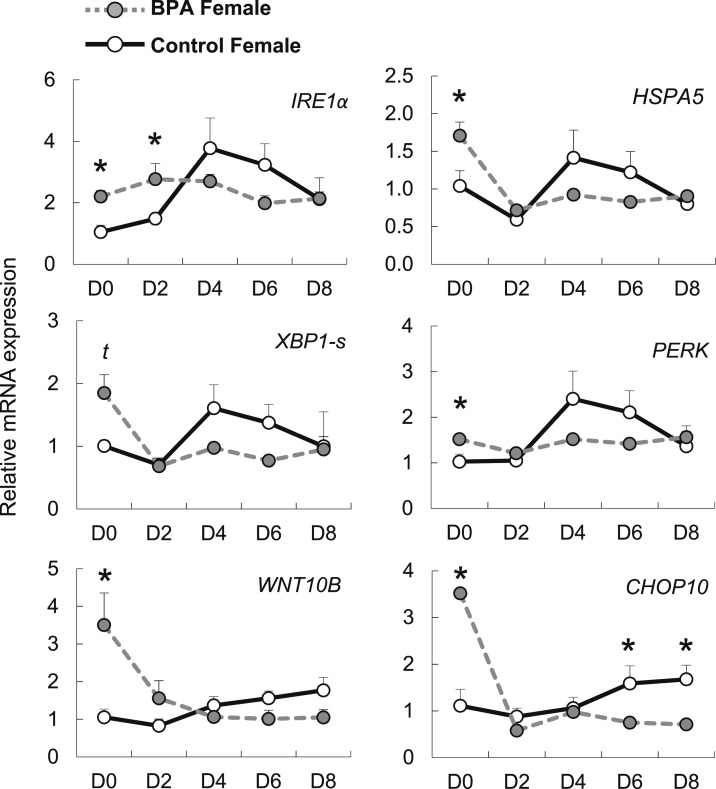
Because ER stress can modulate adipogenesis, we tested if gestational BPA could trigger a cellular stress response leading to an increased UPR. HSPA5 mRNA, induced upon an enhanced UPR, was upregulated in undifferentiated BPA-exposed female preadipocytes (Fig. 7). IRE1α and genes downstream of the IRE1α UPR pathway (XBP1-s and WNT10B) were also upregulated in undifferentiated BPA-exposed preadipocytes. Similarly, PERK and genes downstream of the PERK UPR pathway (CHOP10) were also upregulated in undifferentiated BPA exposed preadipocytes. Upon differentiation stimuli, and by day 2 and 4 of differentiation, HSPA5 and all genes downstream of the UPR pathway, except for CHOP10 were not different from control preadipocytes.
Figure 7.
Effect of gestational BPA exposure on ER stress during adipogenic differentiation. mRNA (mean ± SE) expression of ER stress pathway genes IRE1α, XBP1-s, WNT10B, HSPA5, PERK, and CHOP10 at differentiation days (D) 0, 2, 4, 6, and 8 in control (solid black line) and BPA (dotted gray line) gestationally exposed female primary preadipocytes (n = 3 per group). *Significance at P < 0.05; t, statistical trend at 0.05 < P < 0.06.
Discussion
Growing consensus supports the role of EDCs as a risk factor for metabolic diseases, including obesity (4). BPA is considered one of such EDCs with obesogenic ability (15, 16, 18, 19, 21) and can also result in programming of insulin resistance in rodents (20) and sheep (22). In this study, we have begun to dissect the mechanism by which BPA induces its obesogenic effects during fetal adipose tissue development. Our findings demonstrate that gestational exposure to BPA, at a relevant internal dose exposure [∼2.5 ng/mL unconjugated BPA in fetal blood (52)] alters the differentiation ability of fetal preadipocytes. Increased adipogenesis was only observed in female derived BPA-exposed preadipocytes, supporting previously reported sex-specific effects of BPA in programming adipose tissue (18, 19, 21), and was associated with modulation of the ER stress response during adipogenic differentiation (Fig. 8). Importantly, given the growing evidence of paternal effects on metabolic programming (60, 61), in this work, we have limited the paternal contribution by using a single male parent for all pregnancies. We have also demonstrated that gestational BPS exposure affects steroid receptor expression in adipose tissue and preadipocytes and results in impaired terminal adipogenic differentiation in male fetuses. This finding highlights critical differences between these two bisphenolic compounds on fetal adipose tissue development.
Figure 8.
Working model of the effects of gestational BPA exposure on female fetal preadipocyte adipogenesis. We hypothesize that gestational BPA stimulates ER stress, triggering the UPR in female preadipocytes. Activated UPR pathways (PERK and IRE1α) stimulate CHOP10 and WNT10B mRNA expression, in turn inhibiting adipogenic differentiation of fetal preadipocytes. The adipogenic cocktail (0.5 mM 3-isobutyl-1-methylxanthine, dexamethasone, insulin, and PPARγ agonist) counteracts BPA-induced upregulation of CHOP10 and WNT10B. This results in an acceleration and enhancement of adipogenesis in gestationally BPA-exposed female preadipocytes. Further work is required to verify this hypothesis. This second hit (adipogenic cocktail) is necessary to overcome activation of the UPR in BPA-exposed preadipocytes. We hypothesize (dotted lines) that in vivo, a second hit, such as high-fat or saturated-fat diets, may exert similar effects to those induced by the adipogenic cocktail in vitro, leading to an obesogenic phenotype.
Selection of sheep as the animal model was based on our prior work supporting a metabolic compromise upon gestational BPA exposures (22). Sheep are considered an excellent model to study obesity (34, 35) because of their shared similarities during fetal development with humans (eg, as most adipose tissue deposition is completed before birth) (35, 62, 63). Sheep also respond with increased adipose tissue deposition in sedentary conditions or high caloric diets (22, 64).
Effect of gestational BPA exposure on adipose tissue metabolism
Several animal studies have demonstrated how pre- or perinatal exposure to BPA can lead to increased adipose tissue deposition in adulthood, often accompanied by insulin resistance (19, 22, 65). In this study, we did not evaluate if gestational BPA or BPS exposure resulted in perirenal adipose tissue mass changes (only by gross observation). Although perigestational BPA exposure does not always result in increased adipose mass, in males in particular (19, 21, 66), reported increases in adipose tissue mass upon BPA exposure have only been reported during postnatal life (18–21). Particularly in sheep, prenatal BPA exposure increased adipocyte size during adulthood, but not increased visceral adipose tissue mass (22). Thus, increased adipose tissue mass observed in BPA-exposed animals (19) may occur later in life because of postnatal adipose tissue hypertrophy (67). In addition, lower ADIPOQ expression in fetal adipose tissue from BPA fetuses (this study) reflects a similar change to that observed in visceral adipose tissue from adult sheep gestationally exposed to BPA that were insulin resistance (22). ADIPOQ has been identified as a predictor of cardiovascular risk and type 2 diabetes in adults (68–70). But, whether differences in ADIPOQ during fetal life may be an early indicator of poor cardiometabolic function later in life remains to be demonstrated.
Effect of sex on adipogenic differentiation ability
Our results demonstrate a marked sex-specific effect in adipogenic differentiation ability with fetal male preadipocytes exhibiting very poor adipogenic differentiation ability compared with females. This report is among the first to demonstrate sex-specific adipogenic ability between females and males during fetal life. Whether these sex-specific differences relate to inherent differences in adipose tissue development between female and male fetuses and/or sex-specific critical windows of susceptibility to EDCs in the adipose tissue remains to be investigated. Because the critical role of ESR1 (71) and GR in adipogenesis (72), we hypothesize that the increased expression of ESR1 and GR in control female preadipocytes enables a greater response to adipogenic differentiation stimuli in vitro (adipogenic cocktail) than that of male preadipocytes. Although scarce information is available regarding sex-specific steroid receptor expression in preadipocytes and adipocytes, our results demonstrating a sex-specific effect on estrogen receptor expression (higher ESR1) in female preadipocytes are in line with human data reporting sex-specific differences in differentiated adipocytes (increased ESR2) expression in adult female adipocytes compared with that of adult males (73). To note is that our study did not evaluated protein expression and given the low abundance of ESR2 (73), ESR2 gene expression may not be a true reflection of transcript levels. It also remains unknown if a sex-specific pattern of steroid receptor expression also occurs in human preadipocytes. It remains to be tested if our sex-specific adipogenic differentiation results can be extrapolated to other species.
Gestational BPA enhances adipogenic differentiation
In vitro studies using 3T3-L1 cells, a preadipocyte cell line, have demonstrated that BPA interferes with adipogenic differentiation by increasing preadipocyte growth, altering master regulatory genes of adipogenesis, and accelerating terminal differentiation (15, 16, 74); however, the mechanism by which BPA induces increased adipose tissue mass in vivo continues to be elusive. We have demonstrated that in vivo exposure to BPA increases adipose tissue mass likely by reprogramming the ability of fetal preadipocytes to differentiate into mature adipocytes. The study was designed to discontinue BPA exposure 20 days before tissue harvest for cell isolation. This elicited a washout period to avoid confounding effects from continuous exposure to the EDC at the time of harvest and highlights the persistent nature of the observed effects. Our work is in line with evidence that in vitro BPA accelerates terminal differentiation in 3T3-L1 cells (16), and enhances human mesenchymal stem cells (MSCs) differentiation ability in vitro (74, 75). Reprogramming of MSC in favor of the adipogenic lineage occurs on exposures to other obesogenic EDC such as tributyltin (76). Although we did not investigate whether the observed effect was due to reprogramming of MSCs before adipogenic lineage commitment, it is possible that MSC were also a target in the current study. If so, it is interesting to speculate that if MSC were the direct target, other MSC-derived cell lineages (osteogenic and myogenic) may also be affected. This has not been investigated in the context of prenatal exposures to BPA, but is a much warranted field of study (77).
The need of a “second hit” upon environmental, nutritional, or stress exposures is a common finding in the developmental origins of health and disease field (78). The differentiation stimuli in vitro (adipogenic cocktail) could be viewed as a trigger (and second hit) of the pathological phenotype. Because some BPA studies report that a high-fat diet or overfeeding exacerbates BPA’s metabolic disruptions (22, 79, 80), we speculate that responses during postnatal life upon prenatal BPA exposure may be enhanced if the individual is faced with a particular adipogenic stimuli (eg, high-fat diet, saturated fat diets, high caloric intake; Fig. 8). This hypothesis would support the discrepancy in obesogenic phenotypes reported after prenatal BPA exposures (13, 14, 19, 21) and other reports where a high-fat diet or overfeeding exacerbates BPA’s metabolic disruptions (22, 79, 80). A caveat of this hypothesis is that some studies have reported that BPA, independent of a second hit, promote an adipogenic phenotype, which has contributed to the controversy about the obesogenic ability of BPA (13, 14, 17–22). It is possible that interactions with other factors, such as genetic background or species, may explain the discrepancies in the metabolic effects upon gestational BPA exposures.
Sex-specific effects of gestational BPA on adipogenic differentiation
The sex-specific effect induced by gestational BPA exposure in female (increased adipogenesis) vs male (reduced adipogenesis) preadipocytes is in support of previous sex-specific differences observed upon pre- or perinatal exposures toward increased adipose tissue mass (19) and adipose tissue infiltration (81). That BPA can interfere with ESR1 and ESR2 and modulate GR action (82, 83), coupled with our findings that female preadipocytes had higher ESR1 and GR expression compared with male preadipocytes, supports the hypothesis that female preadipocytes may be more susceptible to BPA’s effect than males. In support of sex-specific responsiveness to estrogenic compounds, estradiol-induced preadipocyte proliferation is higher in adult human (84) and rat (85) female preadipocytes compared with that of male preadipocytes. Unfortunately, similar studies assessing sex-specific responsiveness to adipogenic differentiation are lacking in humans. To our knowledge, increased adipogenic ability reported in in vitro BPA-exposed human adipose stem cells has only been reported in female primary cells (75, 86).
ESR2 was expressed in ovine fetal preadipocytes, similar to that reported in the 3T3-L1 cell line (87). Although studies have not been able to confirm ESR2 expression in human preadipocytes (73, 88), available RNA sequencing databases of human preadipocytes prove otherwise (89). When comparing steroid receptor expression between BPA-exposed preadipocytes, ESR2 expression was higher in BPA female vs control, but not ESR1 or GR. Higher ESR2 expression is associated to BPA exposure in humans in other systems (90). Because the role of ESR2 in adipogenesis can be more prominent in the absence of ESR1 (91), it is likely that steroid differences (higher ESR2) do not contribute to the increased differentiation ability of BPA-exposed female preadipocytes.
ER stress modulation of adipogenesis
ER stress occurs when the ER homeostasis is disrupted. To restore ER homeostasis, UPR is triggered through three pathways initiated with the transmembrane receptors PERK, ATF6, and IRE1α. The UPR response is highly conserved across species (24) and its activation can modulate adipogenesis (26, 27). The activation of PERK and IRE1 pathways enhance lipogenesis upregulating C/EBPα, PPARγ, and SREBP-1c, but inhibit lipogenesis under severe or prolonged ER stress (26). Our results demonstrate that both PERK and IRE1α pathways are upregulated in fetal female preadipocytes gestationally exposed to BPA. In support of previous work in other model systems, our findings demonstrate that BPA can interfere with ER homeostasis (31, 32). Independent activation of downstream effectors of both PERK and IRE1α, such as CHOP10 and WNT10B, respectively, can suppress adipogenesis (26). In this study, adipogenic induction of BPA-exposed female preadipocytes resulted in a profound downregulation of both PERK (CHOP10) and IRE1α (XBP1-s and WNT10B) pathways to levels close to that of the control preadipocytes by day 2 of differentiation. The last two effectors in these pathways (CHOP10 and WNT10B) control adipogenic differentiation by direct suppression of C/EBPβ and C/EBPα, respectively (26). Thus, we hypothesize that the downregulation of downstream effectors of both PERK and IRE1α pathways may contribute to the enhanced adipogenic differentiation observed in female preadipocytes gestationally exposed to BPA. BPA may have selectively reprogrammed female preadipocytes (higher ESR1, ESR2, and GR expression compared with males), resulting in an increased UPR response. These findings support that the UPR pathway regulates adipogenesis in ovine cells similar to that in mice and humans (26, 28). However, further research including knockdown of specific UPR pathway targets is required to demonstrate a direct role of ER stress in mediating BPA’s adipogenic effect.
Even though UPR pathway upregulation can result in cell death (92), BPA-exposed female preadipocytes did not display apoptosis gene upregulation before or during differentiation. This supports that the UPR pathway upregulation (CASP8, MAPK8) may be an adaptive event of the preadipogenic lineage upon BPA. Adaptive responses upon chronic ER stress result in cell survival in several organs (93), but such adaptive responses in adipose tissue are less understood (93). Further studies are needed to investigate how gestational BPA exposure induces UPR signaling, the mechanism that maintains chronic UPR upregulation before differentiation induction, and the mechanism controlling UPR downregulation upon adipogenic stimulation. During prenatal exposures, BPA can alter the epigenetic signature of cells and tissues leading to a disease phenotype (94); however, information on BPA-induced epigenetic modifications on adipose tissue is limited to one study using the 3T3-L1 cell line demonstrating global hypermethylation (95).
Sex-specific effects of BPS on terminal adipogenic differentiation
In our study, BPS altered gene expression and terminal differentiation in gestationally BPS-exposed male, but not female, preadipocytes. During the late adipogenic phase, FABP4 and ADIPOQ were upregulated in male BPS-exposed preadipocytes. Our findings do not support the higher obesogenicity reported for BPS in 3T3-L1 preadipocytes (96), but rather support the contention that gestational BPS exposure may result in a dysfunctional adipocytes as observed with other obesogenic EDCs such as TBT (97). Whether the differential expression of steroid receptors observed in BPS male preadipocytes (upregulation of ESR1, ESR2, and GR) contributes to this dysfunctional adipocyte phenotype requires further investigation.
Conclusion
These findings demonstrate how gestational exposure to EDCs can modify the fate of adipocyte precursors supporting the concept of environmentally mediated metabolic disruption. These results have important implications for the understanding the contributory role of EDCs to the obesity epidemic. Additional research is needed to further understand the underlying mechanisms by which BPA and BPS can modify preadipocyte adipogenic fate and result in dysfunctional adipocytes.
Acknowledgments
We thank Dr. Ehrhardt and the Michigan State University Sheep Teaching and Research Farm for animal procurement and husbandry; Lindsay Hannah, Gabriela Saldana, Igor Suguiura, and Dr. Steve Suhr for help during animal experimentation; and Janina Kavetsky for help with tissue imaging.
Financial Support: Research reported in this publication was supported by National Institute of Environmental Health Sciences, National Institutes of Health Grant 1K22ES026208 (to A.V-L.), Michigan State University (MSU) General Funds, MSU AgBioResearch, and the US Department of Agriculture National Institute of Food and Agriculture Hatch Project Grant MICL02383. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ADIPOQ
- adiponectin
- ATF6
- activating transcription factor 6
- BPA
- bisphenol A
- BPS
- bisphenol S
- CASP8
- caspase-8
- cDNA
- complementary DNA
- C/EBP
- CCAAT/enhancer-binding protein α
- CHOP10
- C/EBP homologous protein 10
- DLK1
- delta-like noncanonical Notch ligand 1
- EDC
- endocrine-disrupting chemical
- ER
- endoplasmic reticulum
- ERRα
- estrogen-related receptor α
- ESR1
- estrogen receptor 1
- ESR2
- estrogen receptor 2
- FABP4
- fatty acid–binding protein 4
- GLUT4
- glucose transporter type 4
- GR
- glucocorticoid receptor
- HSPA5
- heat shock protein family A member 5
- IRE1
- inositol-requiring protein 1
- LPL
- lipoprotein lipase
- MAPK8
- mitogen-activated protein kinase 8
- mRNA
- messenger RNA
- MSC
- mesenchymal stem cell
- OD
- optical density
- ORO
- Oil Red O
- PCR
- polymerase chain reaction
- PERK
- protein kinase–like ER kinase
- PPARγ
- peroxisome proliferator–activated receptor gamma
- SE
- standard error
- SOX6
- sex determining region-box 6
- UPR
- unfolded protein response
- XBP1-s
- spliced X-box binding protein 1.
References
- 1.CDC. Overweight and obesity. Available at: https://www.cdc.gov/obesity/data/adult.html. Accessed 14 December 2016.
- 2.Lobstein T, Jackson-Leach R, Moodie ML, Hall KD, Gortmaker SL, Swinburn BA, James WP, Wang Y, McPherson K. Child and adolescent obesity: part of a bigger picture. Lancet. 2015;385(9986):2510–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Heart Lung, and Blood Institute/National Institutes of Health. Managing overweight and obesity in adults: systematic evidence review from the obesity expert panel. Available at: https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/obesity-evidence-review. Accessed 15 September 2017.
- 4.Schwartz MW, Seeley RJ, Zeltser LM, Drewnowski A, Ravussin E, Redman LM, Leibel RL. Obesity pathogenesis: an Endocrine Society Scientific Statement. Endocr Rev. 2017;38(4):267–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swedenborg E, Rüegg J, Mäkelä S, Pongratz I. Endocrine disruptive chemicals: mechanisms of action and involvement in metabolic disorders. J Mol Endocrinol. 2009;43(1):1–10. [DOI] [PubMed] [Google Scholar]
- 6.Janesick AS, Blumberg B. Obesogens: an emerging threat to public health. Am J Obstet Gynecol. 2016;214(5):559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song Y, Chou EL, Baecker A, You NC, Song Y, Sun Q, Liu S. Endocrine-disrupting chemicals, risk of type 2 diabetes, and diabetes-related metabolic traits: a systematic review and meta-analysis. J Diabetes. 2016;8(4):516–532. [DOI] [PubMed] [Google Scholar]
- 8.Hoepner LA, Whyatt RM, Widen EM, Hassoun A, Oberfield SE, Mueller NT, Diaz D, Calafat AM, Perera FP, Rundle AG. Bisphenol A and adiposity in an inner-city birth cohort. Environ Health Perspect. 2016;124(10):1644–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vafeiadi M, Roumeliotaki T, Myridakis A, Chalkiadaki G, Fthenou E, Dermitzaki E, Karachaliou M, Sarri K, Vassilaki M, Stephanou EG, Kogevinas M, Chatzi L. Association of early life exposure to bisphenol A with obesity and cardiometabolic traits in childhood. Environ Res. 2016;146:379–387. [DOI] [PubMed] [Google Scholar]
- 10.Carwile JL, Michels KB. Urinary bisphenol A and obesity: NHANES 2003-2006. Environ Res. 2011;111(6):825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song Y, Hauser R, Hu FB, Franke AA, Liu S, Sun Q. Urinary concentrations of bisphenol A and phthalate metabolites and weight change: a prospective investigation in US women. Int J Obes. 2014;38(12):1532–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang T, Li M, Chen B, Xu M, Xu Y, Huang Y, Lu J, Chen Y, Wang W, Li X, Liu Y, Bi Y, Lai S, Ning G. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J Clin Endocrinol Metab. 2012;97(2):E223–E227. [DOI] [PubMed] [Google Scholar]
- 13.Anderson OS, Peterson KE, Sanchez BN, Zhang Z, Mancuso P, Dolinoy DC. Perinatal bisphenol A exposure promotes hyperactivity, lean body composition, and hormonal responses across the murine life course. FASEB J. 2013;27(4):1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan KK, Haller AM, Sorrell JE, Woods SC, Jandacek RJ, Seeley RJ. Perinatal exposure to bisphenol-a and the development of metabolic syndrome in CD-1 mice. Endocrinology. 2010;151(6):2603–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boucher JG, Boudreau A, Atlas E. Bisphenol A induces differentiation of human preadipocytes in the absence of glucocorticoid and is inhibited by an estrogen-receptor antagonist. Nutr Diabetes. 2014;4:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masuno H, Iwanami J, Kidani T, Sakayama K, Honda K. Bisphenol a accelerates terminal differentiation of 3T3-L1 cells into adipocytes through the phosphatidylinositol 3-kinase pathway. Toxicol Sci. 2005;84(2):319–327. [DOI] [PubMed] [Google Scholar]
- 17.Miyawaki J, Sakayama K, Kato H, Yamamoto H, Masuno H. Perinatal and postnatal exposure to bisphenol a increases adipose tissue mass and serum cholesterol level in mice. J Atheroscler Thromb. 2007;14(5):245–252. [DOI] [PubMed] [Google Scholar]
- 18.Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect. 2001;109(7):675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Somm E, Schwitzgebel VM, Toulotte A, Cederroth CR, Combescure C, Nef S, Aubert ML, Hüppi PS. Perinatal exposure to bisphenol a alters early adipogenesis in the rat. Environ Health Perspect. 2009;117(10):1549–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alonso-Magdalena P, Quesada I, Nadal Á. Prenatal exposure to BPA and offspring outcomes: the diabesogenic behavior of BPA. Dose Response. 2015;13(2):15593258– 15590395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubin BS, Paranjpe M, DaFonte T, Schaeberle C, Soto AM, Obin M, Greenberg AS. Perinatal BPA exposure alters body weight and composition in a dose specific and sex specific manner: The addition of peripubertal exposure exacerbates adverse effects in female mice. Reprod Toxicol. 2017;68:130–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veiga-Lopez A, Moeller J, Sreedharan R, Singer K, Lumeng C, Ye W, Pease A, Padmanabhan V. Developmental programming: interaction between prenatal BPA exposure and postnatal adiposity on metabolic variables in female sheep. Am J Physiol Endocrinol Metab. 2016;310(3):E238–E247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12(11):722–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollien J. Evolution of the unfolded protein response. Biochim Biophys Acta. 2013; 1833:2458–2463. [DOI] [PubMed] [Google Scholar]
- 25.Fu S, Watkins SM, Hotamisligil GS. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012;15(5):623–634. [DOI] [PubMed] [Google Scholar]
- 26.Han J, Murthy R, Wood B, Song B, Wang S, Sun B, Malhi H, Kaufman RJ. ER stress signalling through eIF2α and CHOP, but not IRE1α, attenuates adipogenesis in mice. Diabetologia. 2013;56(4):911–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowe CE, Dennis RJ, Obi U, O’Rahilly S, Rochford JJ. Investigating the involvement of the ATF6α pathway of the unfolded protein response in adipogenesis. Int J Obes. 2012;36(9):1248–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sha H, He Y, Chen H, Wang C, Zenno A, Shi H, Yang X, Zhang X, Qi L. The IRE1alpha-XBP1 pathway of the unfolded protein response is required for adipogenesis. Cell Metab. 2009;9(6):556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–461. [DOI] [PubMed] [Google Scholar]
- 30.Tabuchi Y, Kondo T. cDNA microarray analysis reveals chop-10 plays a key role in Sertoli cell injury induced by bisphenol A. Biochem Biophys Res Commun. 2003;305(1):54–61. [DOI] [PubMed] [Google Scholar]
- 31.Asahi J, Kamo H, Baba R, Doi Y, Yamashita A, Murakami D, Hanada A, Hirano T. Bisphenol A induces endoplasmic reticulum stress-associated apoptosis in mouse non-parenchymal hepatocytes. Life Sci. 2010;87(13-14):431–438. [DOI] [PubMed] [Google Scholar]
- 32.Tabuchi Y, Takasaki I, Kondo T. Identification of genetic networks involved in the cell injury accompanying endoplasmic reticulum stress induced by bisphenol A in testicular Sertoli cells. Biochem Biophys Res Commun. 2006;345(3):1044–1050. [DOI] [PubMed] [Google Scholar]
- 33.Pap A, Cuaranta-Monroy I, Peloquin M, Nagy L. Is the mouse a good model of human PPARγ-related metabolic diseases? Int J Mol Sci. 2016;17(8):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayes JS, Watson GH. Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev. 2004;5(4):197–216. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez-Bulnes A, Chavatte-Palmer P. Contribution of large animals to translational research on prenatal programming of obesity and associated diseases [published online ahead of print August 11, 2017]. Curr Pharm Biotechnol. 2017. [DOI] [PubMed]
- 36.Padmanabhan V, Veiga-Lopez A. Reproduction symposium: developmental programming of reproductive and metabolic health. J Anim Sci. 2014;92(8):3199–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fowden AL, Valenzuela OA, Vaughan OR, Jellyman JK, Forhead AJ. Glucocorticoid programming of intrauterine development. Domest Anim Endocrinol. 2016;56(Suppl):S121–S132. [DOI] [PubMed] [Google Scholar]
- 38.Ojha S, Budge H. Early origins of obesity and developmental regulation of adiposity In: Symonds ME, ed. Adipose Tissue Biology. New York, NY: Springer New York; 2012:379–408. [Google Scholar]
- 39.Drickamer LC, Vom Saal FS, Marriner LM, Mossman CA. Anogenital distance and dominance status in male house mice (Mus domes ticus). Aggress Behav. 1995;21:301–309. [Google Scholar]
- 40.Zielinski WJ, Vandenbergh JG. Effect of intrauterine position and social density on age of first reproduction in wild-type female house mice (Mus musculus). J Comp Psychol. 1991;105(2):134–139. [DOI] [PubMed] [Google Scholar]
- 41.vom Saal FS, Clark MM, Galef BG Jr, Drickamer LC, Vandenbergh JG. The intrauterine position (IUP) phenomenon In: Knobil E, Neill J, eds. Encyclopedia of Reproduction. Vol 2 New York: Academic Press; 1999:893–900. [Google Scholar]
- 42.Corbel T, Perdu E, Gayrard V, Puel S, Lacroix MZ, Viguié C, Toutain PL, Zalko D, Picard-Hagen N. Conjugation and deconjugation reactions within the fetoplacental compartment in a sheep model: a key factor determining bisphenol A fetal exposure. Drug Metab Dispos. 2015;43(4):467–476. [DOI] [PubMed] [Google Scholar]
- 43.Liao C, Liu F, Alomirah H, Loi VD, Mohd MA, Moon HB, Nakata H, Kannan K. Bisphenol S in urine from the United States and seven Asian countries: occurrence and human exposures. Environ Sci Technol. 2012;46(12):6860–6866. [DOI] [PubMed] [Google Scholar]
- 44.Eladak S, Grisin T, Moison D, Guerquin MJ, N’Tumba-Byn T, Pozzi-Gaudin S, Benachi A, Livera G, Rouiller-Fabre V, Habert R. A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil Steril. 2015;103(1):11–21. [DOI] [PubMed] [Google Scholar]
- 45.Liao C, Kannan K. Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. J Agric Food Chem. 2013;61(19):4655–4662. [DOI] [PubMed] [Google Scholar]
- 46.Liao C, Liu F, Kannan K. Bisphenol s, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol a residues. Environ Sci Technol. 2012;46(12):6515–6522. [DOI] [PubMed] [Google Scholar]
- 47.Ye X, Wong LY, Kramer J, Zhou X, Jia T, Calafat AM. Urinary concentrations of bisphenol A and three other bisphenols in convenience samples of U.S. adults during 2000-2014. Environ Sci Technol. 2015;49(19):11834–11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu J, Li J, Wu Y, Zhao Y, Luo F, Li S, Yang L, Moez EK, Dinu I, Martin JW. Bisphenol A metabolites and bisphenol S in paired maternal and cord serum. Environ Sci Technol. 2017;51(4):2456–2463. [DOI] [PubMed] [Google Scholar]
- 49.Boucher JG, Ahmed S, Atlas E. Bisphenol S induces adipogenesis in primary human preadipocytes from female donors. Endocrinology. 2016;157(4):1397–1407. [DOI] [PubMed] [Google Scholar]
- 50.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roberts JN, May KJ, Veiga-Lopez A. Time-dependent changes in pregnancy-associated glycoproteins and progesterone in commercial crossbred sheep. Theriogenology. 2017;89:271–279. [DOI] [PubMed] [Google Scholar]
- 52.Veiga-Lopez A, Luense LJ, Christenson LK, Padmanabhan V. Developmental programming: gestational bisphenol-A treatment alters trajectory of fetal ovarian gene expression. Endocrinology. 2013;154(5):1873–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Padmanabhan V, Siefert K, Ransom S, Johnson T, Pinkerton J, Anderson L, Tao L, Kannan K. Maternal bisphenol-A levels at delivery: a looming problem? J Perinatol. 2008;28(4):258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pu Y, Veiga-Lopez A. PPARγ agonist through the terminal differentiation phase is essential for adipogenic differentiation of fetal ovine preadipocytes. Cell Mol Biol Lett. 2017;22:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skurk T, Hauner H. Primary culture of human adipocyte precursor cells: expansion and differentiation In: Mitry RR, Hughes RD, eds. Human Cell Culture Protocols. Totowa, NJ: Humana Press; 2012:215–226. [DOI] [PubMed] [Google Scholar]
- 56.Patel NG, Holder JC, Smith SA, Kumar S, Eggo MC. Differential regulation of lipogenesis and leptin production by independent signaling pathways and rosiglitazone during human adipocyte differentiation. Diabetes. 2003;52(1):43–50. [DOI] [PubMed] [Google Scholar]
- 57.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Welshons WV, Wolf MF, Murphy CS, Jordan VC. Estrogenic activity of phenol red. Mol Cell Endocrinol. 1988;57(3):169–178. [DOI] [PubMed] [Google Scholar]
- 59.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. [DOI] [PubMed] [Google Scholar]
- 60.Rando OJ, Simmons RA. I’m eating for two: parental dietary effects on offspring metabolism. Cell. 2015;161(1):93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Day J, Savani S, Krempley BD, Nguyen M, Kitlinska JB. Influence of paternal preconception exposures on their offspring: through epigenetics to phenotype. Am J Stem Cells. 2016;5(1):11–18. [PMC free article] [PubMed] [Google Scholar]
- 62.Polin RA, Abman SH, Rowitch DH, Benitz WE, Fox WW. Fetal and Neonatal Physiology.5th ed. Philadelphia, PA: Elsevier; 2016. [Google Scholar]
- 63.Gemmell RT, Alexander G. Ultrastructural development of adipose tissue in foetal sheep. Aust J Biol Sci. 1978;31(5):505–515. [DOI] [PubMed] [Google Scholar]
- 64.McCann JP, Bergman EN, Beermann DH. Dynamic and static phases of severe dietary obesity in sheep: food intakes, endocrinology and carcass and organ chemical composition. J Nutr. 1992;122(3):496–505. [DOI] [PubMed] [Google Scholar]
- 65.Vom Saal FS, Nagel SC, Coe BL, Angle BM, Taylor JA. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol Cell Endocrinol. 2012;354(1-2):74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miao M, Yuan W, Zhu G, He X, Li DK. In utero exposure to bisphenol-A and its effect on birth weight of offspring. Reprod Toxicol. 2011;32(1):64–68. [DOI] [PubMed] [Google Scholar]
- 67.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Näslund E, Britton T, Concha H, Hassan M, Rydén M, Frisén J, Arner P. Dynamics of fat cell turnover in humans. Nature. 2008;453(7196):783–787. [DOI] [PubMed] [Google Scholar]
- 68.Lekva T, Michelsen AE, Aukrust P, Henriksen T, Bollerslev J, Ueland T. Leptin and adiponectin as predictors of cardiovascular risk after gestational diabetes mellitus. Cardiovasc Diabetol. 2017;16(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Snehalatha C, Mukesh B, Simon M, Viswanathan V, Haffner SM, Ramachandran A. Plasma adiponectin is an independent predictor of type 2 diabetes in Asian Indians. Diabetes Care. 2003;26(12):3226–3229. [DOI] [PubMed] [Google Scholar]
- 70.Jiang YN, Owei I, Wan J, Ebenibo S, Dagogo-Jack S. Adiponectin levels predict prediabetes risk: the Pathobiology of Prediabetes in A Biracial Cohort (POP-ABC) study. Bmj Open Diab Res Care. 2016:4(1):e000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA. 2000;97(23):12729–12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park YK, Ge K. Glucocorticoid receptor accelerates, but is dispensable for, adipogenesis. Mol Cell Biol. 2017;37(2):pii: e00260-16. [DOI] [PMC free article] [PubMed]
- 73.Dieudonné MN, Leneveu MC, Giudicelli Y, Pecquery R. Evidence for functional estrogen receptors alpha and beta in human adipose cells: regional specificities and regulation by estrogens. Am J Physiol Cell Physiol. 2004;286(3):C655–C661. [DOI] [PubMed] [Google Scholar]
- 74.Chamorro-García R, Kirchner S, Li X, Janesick A, Casey SC, Chow C, Blumberg B. Bisphenol A diglycidyl ether induces adipogenic differentiation of multipotent stromal stem cells through a peroxisome proliferator-activated receptor gamma-independent mechanism. Environ Health Perspect. 2012;120(7):984–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ohlstein JF, Strong AL, McLachlan JA, Gimble JM, Burow ME, Bunnell BA. Bisphenol A enhances adipogenic differentiation of human adipose stromal/stem cells. J Mol Endocrinol. 2014;53(3):345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chamorro-García R, Sahu M, Abbey RJ, Laude J, Pham N, Blumberg B. Transgenerational inheritance of increased fat depot size, stem cell reprogramming, and hepatic steatosis elicited by prenatal exposure to the obesogen tributyltin in mice. Environ Health Perspect. 2013;121(3):359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bateman ME, Strong AL, McLachlan JA, Burow ME, Bunnell BA. The effects of endocrine disruptors on adipogenesis and osteogenesis in mesenchymal stem cells: a review. Front Endocrinol (Lausanne). 2017;7:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heindel JJ, Balbus J, Birnbaum L, Brune-Drisse MN, Grandjean P, Gray K, Landrigan PJ, Sly PD, Suk W, Cory Slechta D, Thompson C, Hanson M. Developmental origins of health and disease: integrating environmental influences. Endocrinology. 2015;156(10):3416–3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ding S, Fan Y, Zhao N, Yang H, Ye X, He D, Jin X, Liu J, Tian C, Li H, Xu S, Ying C. High-fat diet aggravates glucose homeostasis disorder caused by chronic exposure to bisphenol A. J Endocrinol. 2014;221(1):167–179. [DOI] [PubMed] [Google Scholar]
- 80.Wei J, Sun X, Chen Y, Li Y, Song L, Zhou Z, Xu B, Lin Y, Xu S. Perinatal exposure to bisphenol A exacerbates nonalcoholic steatohepatitis-like phenotype in male rat offspring fed on a high-fat diet. J Endocrinol. 2014;222(3):313–325. [DOI] [PubMed] [Google Scholar]
- 81.Strakovsky RS, Wang H, Engeseth NJ, Flaws JA, Helferich WG, Pan YX, Lezmi S. Developmental bisphenol A (BPA) exposure leads to sex-specific modification of hepatic gene expression and epigenome at birth that may exacerbate high-fat diet-induced hepatic steatosis. Toxicol Appl Pharmacol. 2015;284(2):101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prasanth GK, Divya LM, Sadasivan C. Bisphenol-A can bind to human glucocorticoid receptor as an agonist: an in silico study. J Appl Toxicol. 2010;30(8):769–774. [DOI] [PubMed] [Google Scholar]
- 83.Kurosawa T, Hiroi H, Tsutsumi O, Ishikawa T, Osuga Y, Fujiwara T, Inoue S, Muramatsu M, Momoeda M, Taketani Y. The activity of bisphenol A depends on both the estrogen receptor subtype and the cell type. Endocr J. 2002;49(4):465–471. [DOI] [PubMed] [Google Scholar]
- 84.Anderson LA, McTernan PG, Barnett AH, Kumar S. The effects of androgens and estrogens on preadipocyte proliferation in human adipose tissue: influence of gender and site. J Clin Endocrinol Metab. 2001;86(10):5045–5051. [DOI] [PubMed] [Google Scholar]
- 85.Dieudonne MN, Pecquery R, Leneveu MC, Giudicelli Y. Opposite effects of androgens and estrogens on adipogenesis in rat preadipocytes: evidence for sex and site-related specificities and possible involvement of insulin-like growth factor 1 receptor and peroxisome proliferator-activated receptor gamma2. Endocrinology. 2000;141(2):649–656. [DOI] [PubMed] [Google Scholar]
- 86.Linehan C, Gupta S, Samali A, O’Connor L. Bisphenol A-mediated suppression of LPL gene expression inhibits triglyceride accumulation during adipogenic differentiation of human adult stem cells. PLoS One. 2012;7(5):e36109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yi KW, Shin JH, Seo HS, Lee JK, Oh MJ, Kim T, Saw HS, Kim SH, Hur JY. Role of estrogen receptor-alpha and -beta in regulating leptin expression in 3T3-L1 adipocytes. Obesity (Silver Spring). 2008;16(11):2393–2399. [DOI] [PubMed] [Google Scholar]
- 88.Joyner JM, Hutley LJ, Cameron DP. Estrogen receptors in human preadipocytes. Endocrine. 2001;15(2):225–230. [DOI] [PubMed] [Google Scholar]
- 89.Nair S, Lee YH, Rousseau E, Cam M, Tataranni PA, Baier LJ, Bogardus C, Permana PA. Increased expression of inflammation-related genes in cultured preadipocytes/stromal vascular cells from obese compared with non-obese Pima Indians. Diabetologia. 2005;48(9):1784–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Melzer D, Harries L, Cipelli R, Henley W, Money C, McCormack P, Young A, Guralnik J, Ferrucci L, Bandinelli S, Corsi AM, Galloway T. Bisphenol A exposure is associated with in vivo estrogenic gene expression in adults. Environ Health Perspect. 2011;119(12):1788–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Davis KE, Neinast MD, Sun K, Skiles WM, Bills JD, Zehr JA, Zeve D, Hahner LD, Cox DW, Gent LM, Xu Y, Wang ZV, Khan SA, Clegg DJ. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab. 2013;2(3):227–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hiramatsu N, Chiang WC, Kurt TD, Sigurdson CJ, Lin JH. Multiple mechanisms of unfolded protein response-induced cell death. Am J Pathol. 2015;185(7):1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang S, Kaufman RJ. The impact of the unfolded protein response on human disease. J Cell Biol. 2012;197(7):857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stel J, Legler J. The role of epigenetics in the latent effects of early life exposure to obesogenic endocrine disrupting chemicals. Endocrinology. 2015;156(10):3466–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bastos Sales L, Kamstra JH, Cenijn PH, van Rijt LS, Hamers T, Legler J. Effects of endocrine disrupting chemicals on in vitro global DNA methylation and adipocyte differentiation. Toxicol In Vitro. 2013;27(6):1634–1643. [DOI] [PubMed] [Google Scholar]
- 96.Ahmed S, Atlas E. Bisphenol S- and bisphenol A-induced adipogenesis of murine preadipocytes occurs through direct peroxisome proliferator-activated receptor gamma activation. Int J Obes. 2016;40(10):1566–1573. [DOI] [PubMed] [Google Scholar]
- 97.Regnier SM, El-Hashani E, Kamau W, Zhang X, Massad NL, Sargis RM. Tributyltin differentially promotes development of a phenotypically distinct adipocyte. Obesity (Silver Spring). 2015;23(9):1864–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]