Abstract
Tetrabromobisphenol-A (TBBPA) is a brominated flame retardant (BFR) commonly used in electronics to meet fire safety standards and has the largest worldwide production of any BFR. TBBPA has been detected in human breast milk and maternal/cord serum, indicating exposure to mothers, fetuses, and breastfeeding newborns although exposure to fetuses and newborns is poorly understood. Pregnant or nursing Wistar Han IGS rats were administered [14C]-TBBPA in a single dose (25 mg/kg, 2.5 μCi/kg) and euthanized between 0.5&24h post dose to determine disposition in pregnant and nursing rats and their pups. Systemic exposure was largely unchanged between 1&8h post dose in pregnant rats; [14C]-radioactivity in blood varied only slightly between 0.5&8h (2.6±0.6→2.6±0.8 nmol-eq/mL) but was below the limit of detection at 24h with an absorption half-life of 16min and elimination half-life of 17h. Cmax was observed at 30min in lactating rats and concentrations fell steadily through 8h. Plasma from pregnant rats contained a mixture of TBBPA and TBBPA-conjugates at 30min but only metabolites in subsequent samples. TBBPA was not detected in lactating dam plasma in this study. Placental concentrations increased through 8h while whole-fetus Cmax occurred at 2h post dose. In lactating animals, liver, uterus, and mammary time-concentration curves lagged slightly behind blood-concentration curves. It was clear from these studies that TBBPA is available to both the developing fetus and nursing pup following maternal exposure, and nursing pups are continuously exposed via contaminated milk produced by their mother. This research was supported in part by the Intramural Research Program of NIH/NCI.
Keywords: brominated flame retardant, toxicokinetics, disposition, perinatal exposure, postnatal exposure, pregnant and nursing dams
Introduction
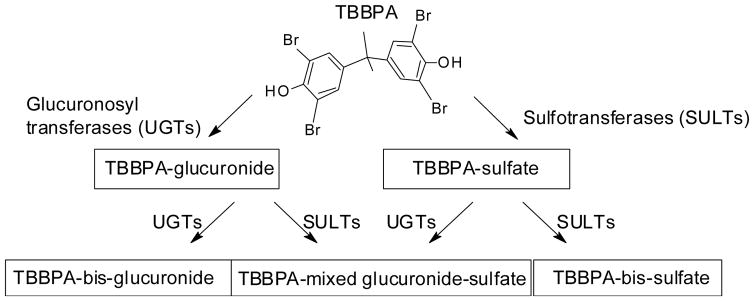
Tetrabromobisphenol A (TBBPA, CAS No. 79-94-7, Figure 1) currently represents the highest production volume brominated flame retardant (BFR) and constitutes approximately 60% of worldwide demand for BFRs. TBBPA is used in electronics to meet fire safety standards, with a global market volume of >145,000 metric tons per year (1). TBBPA is most commonly produced as a reactive flame retardant in printed circuit boards, but approximately 10–30% of applications are as an additive FR, most notably in Acrylonitrile-Butadiene-Styrene (ABS) plastic casings (2, 3). Over 90% of printed circuit boards contain TBBPA in the form of ‘reactively-bound’ flame-retardant although it has been shown that an estimated 0.06% of the total amount used remains unbound (4). TBBPA is also used in paper, textiles, as a plasticizer, and as an intermediate for the syntheses of other flame retardants (3).
Figure 1.
Chemical structure for TBBPA and metabolism scheme for its conjugative metabolites.
In single administration studies, TBBPA has an LD50 of greater than 5 g/kg when administered by gavage to rats (5). Hakk et al. demonstrated that TBBPA (2 mg/kg) is readily absorbed from the gastrointestinal tract of male Sprague Dawley (SD) rats where it undergoes biotransformation to o-glucuronide and o-sulfate conjugates (Figure 1) followed by biliary elimination to the intestine (6). Conjugated TBBPA is readily deconjugated in the gut by microflora and absorbed TBBPA is eliminated in the feces as parent chemical. Kuester et al. showed that at a 10-fold higher dose of TBBPA, the systemic bioavailability of the compound (in whole blood) remained low (1.6% available) with a terminal half-life of 95 min in male Fischer-344 (F344) rats (7). We have shown previously that female Wistar Han IGS [Crl:WI(Han)] rats (8) exhibit similar disposition and kinetic profiles as those seen for male SD and F344 rats. Shauer et al. concluded that the bioavailability of TBBPA in humans following a single exposure is expected to be low (9) but the bioavailability of TBBPA in humans after chronic exposures in pregnant and non-pregnant humans was not explored.
TBBPA is a rodent carcinogen, a suspected carcinogen in humans, and can act as an endocrine disruptor. In repeat-dose subacute and one-generation reproductive studies, TBBPA exposure resulted in decreased thyroxine levels and other endocrine effects (10, 11). TBBPA interacts with peroxisome proliferator-activated receptors (PPARs) to induce downstream obesogenic effects like adipocyte differentiation via PPAR-gamma (12). At very high doses, TBBPA causes hepatotoxicities and heme metabolism disturbances (13, 14) that are likely due to the formation of free radicals (15). Repeated and chronic exposures to orally administered TBBPA resulted in the downregulation of gene products implicated in several immunologic pathways in uterine tissues (11, 16, 17). TBBPA has also been shown to compete with estrogen for conjugation by the estrogen sulfotransferase (SULT1E1), potentially prolonging estrogen signaling in sensitive tissues (e.g., uterus) (18).
The primary routes of exposure to TBBPA are through ingestion, inhalation, or dermal contact with contaminated dust particles. Recent studies of postpartum mothers found 44–50% of breast milk samples and 30% of maternal/cord serum samples contained detectable levels of TBBPA, demonstrating significant exposure to mothers and fetuses and the risk of exposure of newborns via breastfeeding (19–21). TBBPA has been consistently detected at low levels in environmental samples (22) but these levels are expected to increase as the production and use of TBBPA in an additive mode increases (3).
Data from TBBPA chronic exposure studies showed an enhanced susceptibility of female Crl:WI(Han) rats to TBBPA toxicities, including dose-dependent increases in uterine epithelial tumors (23). The presence of TBBPA in human breast milk and serum samples and the chemical’s potential for endocrine disruption and tumorigenesis prompted this study into the disposition and kinetics of TBBPA in dams and pups after exposure in utero or during nursing. The treatment groups measured the following exposures: disposition of TBBPA in pregnant dams and fetuses immediately prior to parturition (gestation day 20; GD20); the kinetics of TBBPA in nursing pups at the age of maximal milk consumption (postnatal day 12; PND12); and the kinetics of TBBPA immediately prior to weaning (postnatal day 20; PND20). In all cases, the Crl:WI(Han) rat dam was administered a single oral bolus of TBBPA (25 mg/kg).
Materials and methods
Chemicals
[14C]-labeled TBBPA (ring-labeled; Figure 1, Lot # 3225-235, Perkin Elmer Life and Analytical Sciences [Boston, MA], re-purified in 2013 by Moravek Biochemicals [Brea, CA]) and used in these studies had a radiochemical purity of >98% (specific activity = 90.3 mCi/mmol) and a relative chemical purity of >98%, as compared to a TBBPA reference standard (Sigma-Aldrich; St. Louis, MO). Scintillation cocktails were obtained from MP Biomedicals (Ecolume; Santa Ana, CA) or Perkin-Elmer (Ultima-Flo M & PermaFluor E+; Torrance, CA). All other reagents used in these studies were high performance liquid chromatography (HPLC) or analytical grade.
Animal model
Timed-pregnant female Crl:WI(Han) rats (Charles River Laboratories, Raleigh, NC) were used in these studies. Animals were maintained in an AAALAC-approved animal care facility at NIEHS (Research Triangle Park, NC). Plug-positive dams were delivered 13 days after confirmation of pregnancy and pregnancies were timed to GD20 for in utero exposures (30 dams) or allowed to proceed through parturition (50 dams). Nursing dams were housed with their litters, and pups were sexed based on anogenital distance on postnatal day 4 (24). Litters were culled and balanced to 4 males and 4 females after sexing to control for litter size and possible sex differences in litter units. Pregnant animals were housed individually and nursing dams were housed with their litters in polycarbonate shoebox cages with Sani-Chip Hardwood Bedding (PJ Murphy, Mt. Montville, NJ) and Enviro-Dri enrichment (Shephard Specialty Papers, Watertown, TN) throughout these studies. Food [NIH #31(25)] and water (City of Durham, NC) were provided for ad libitum consumption. The NIEHS Institutional Care and Use committee approved all procedures.
Dosing
On GD20, PND12, or PND20, Crl:WI(Han) dams (N=4–5 per time-point) were administered a single dose of [14C]-labeled TBBPA in sesame oil (25 mg/kg, 2.5 μCi/kg, 4 mL/kg) by gavage and returned to their home cage. Dosing solutions were prepared on the day of administration; dosing solutions were prepared by adding appropriate volumes of [14C]-TBBPA and non-radiolabeled TBBPA dissolved in acetone to sesame oil at a dose volume of 4 mL/kg; acetone was removed by evaporation under a steady stream of nitrogen. Oral doses were administered using a #16 ball-tipped stainless steel feeding needle. Animals were weighed the morning of dosing and randomized by weight for assigning animals into the various time-point groupings. Dam weights are shown in Table S 1. Animals were euthanized by CO2 inhalation at 0.5, 1, 2, 4, 8, or 24 h post dose for sample collection.
Sample collection
Following CO2 asphyxiation, blood and tissues were collected from the dam and fetuses or nursing pups. Dam blood samples were collected via cardiac puncture immediately following euthanasia. Complete necropsies were performed on dams, with pooled adipose, adrenals, brain, heart, kidneys, large intestine & contents, liver, lung, mammary, muscle, pancreas, ovaries, skin, small intestine & contents, spleen, stomach & contents, thymus, thyroid, urinary bladder, and uterus collected and placed in labeled, pre-weighed vials, as described previously (8). In addition, pups were necropsied and selected tissues (liver, stomach, stomach contents) were collected and placed in labeled pre-weighed vials. Placentas were collected, pooled, and placed in labeled pre-weighed vials. Fetuses from pregnant dams were placed in separate labeled pre-weighed vials for individual analyses. Plasma was isolated from heparinized blood by centrifugation (5 min at 3,000 RPM).
Analytical methods
Samples were analyzed in parallel for quantitative and qualitative analyses. Quantitative analyses of total [14C]-radioactivity content were determined using a Beckman Coulter LS6500 Multi-Purpose Scintillation spectrometer. Placenta and fetuses, along with livers and stomach contents from dams and pups were analyzed for levels of radioactivity. Placentas were pooled by litter, homogenized and sampled for extraction (N=4–5/time-point). Individual fetal samples were snap frozen and homogenized using a BioPulverizer Cryogenic Tissue Crusher (N=3 per sex, 3 litters per time-point). All other samples were minced, then mixed before sampling (N=3 individuals per time-point). Triplicate aliquots (~1g) were placed in labeled pre-weighed vials and weights determined gravimetrically. The aliquots were then combusted to 14CO2 using a Packard 307 Biological Sample Oxidizer followed by quantitation of total radioactivity using a Beckman Coulter LS6500 multipurpose scintillation counter. Duplicate samples were weighed and placed in glass vials for exhaustive extraction. Whole blood and tissues containing [14C]-radioactivity were sampled and subjected to extractions using a previously described method (26). Briefly, aliquots of whole blood or minced tissue samples were placed in labeled pre-weighed glass screw-top tubes and re-suspended in 5 mL toluene and mixed by vortexing for 1 minute. Samples were then centrifuged (830 × g for 10 min) using a Sorvall RC6+ centrifuge (Thermo Scientific, Inc., Waltham, MA) with a SH-3000 swing bucket rotor to sediment solids. This process was repeated twice, with the supernatants transferred and pooled. When recoveries were less than 95% of expected values, the pellet was further extracted with ethyl acetate (3 × 5 mL) and 1% HCl in 95% ethanol (3 × 5 mL). At each step, the samples were centrifuged and the supernatants transferred and pooled. Extracts were concentrated to approx. 100 μL using a Savant SPD1010 SpeedVac (Thermo Scientific, Inc., Waltham, MA) without heating, and aliquots were analyzed by radiochemical HPLC or LC-MS (methods described below). Remaining sample pellets were air dried, weighed, and [14C]-radioactivity remaining in the sample (unextracted fraction) was quantified by combustion in a Model 307 Biological Sample Oxidizer (PerkinElmer; Torrance, CA) followed by LSC counting.
TBBPA was quantified by UV/Vis absorbance and radiochemical detection following HPLC separation. The High-Performance Liquid Chromatography (HPLC) system used for analysis of extracts was composed of an Agilent 1100 system, an Agilent Eclipse Plus C18 column (3.5μm, 4.6mm×150mm) and an IN/US βRAM-3. Mobile phases consisted of 0.1% formic acid in water (mobile phase A) and 0.1% formic acid in acetonitrile (mobile phase B). Sample separations were performed using a gradient. Initial conditions (100% A) were reduced to 60% A over 5 minutes, 60% A was reduced to 10% A over 2 minutes, then to 0% A over 5 minutes. 0% A was maintained for one minute and then returned to initial conditions over one minute. Flow rates were 1 mL/min. Instrument control and analysis software was LAURA4. Radiochemical flow cell was 500 μL and scintillant flow rate was 2 mL/min. The limit of detection for the radiochemical detector was 3 times background peak height. Qualitative analyses of extracted tissues and blood (described above) were carried out using ion-trap mass spectrometry with electrospray ionization in negative ion mode. The mass spectrometer (MS) system was Thermo Fisher Scientific LTQXL ion trap MS coupled to a Thermo Fisher Scientific (Waltham, MA) Ultimate 3000 binary UPLC. Column and HPLC conditions were the same as above. MS conditions were: sheath gas flow rate: 20; auxiliary gas flow rate: 5; sweep gas flow rate: 5; spray voltage: 5 kV; capillary temp: −50°C; capillary voltage: −33 V; tube lens voltage: −30 V.
Statistics and data visualization
The data were plotted and subjected to ANOVA statistical analyses using GraphPad Prism 7 (GraphPad Software, Inc, La Jolla, CA). Values were considered significantly different at p < 0.05.
Results
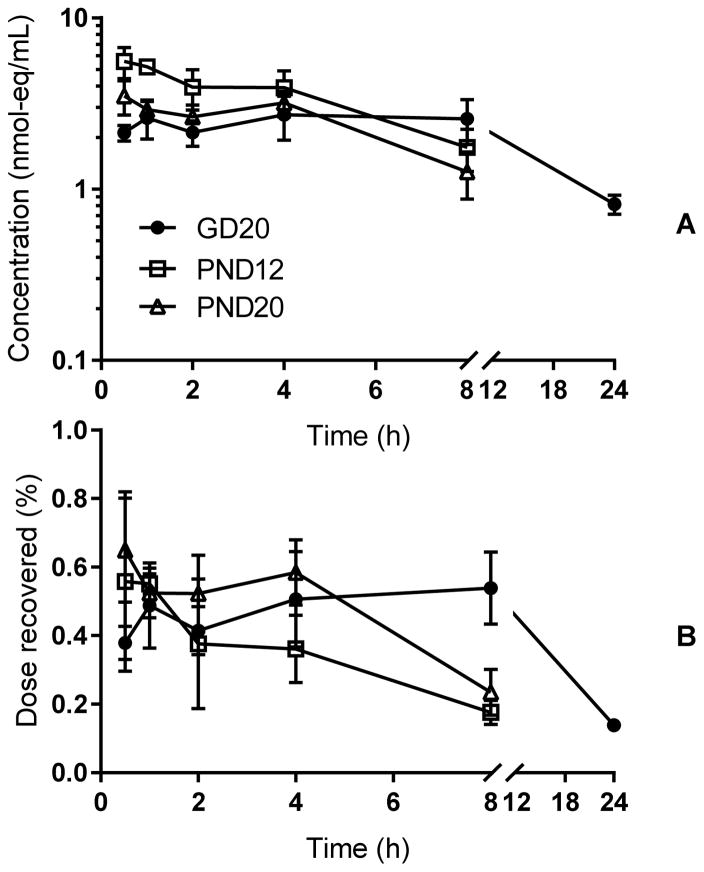
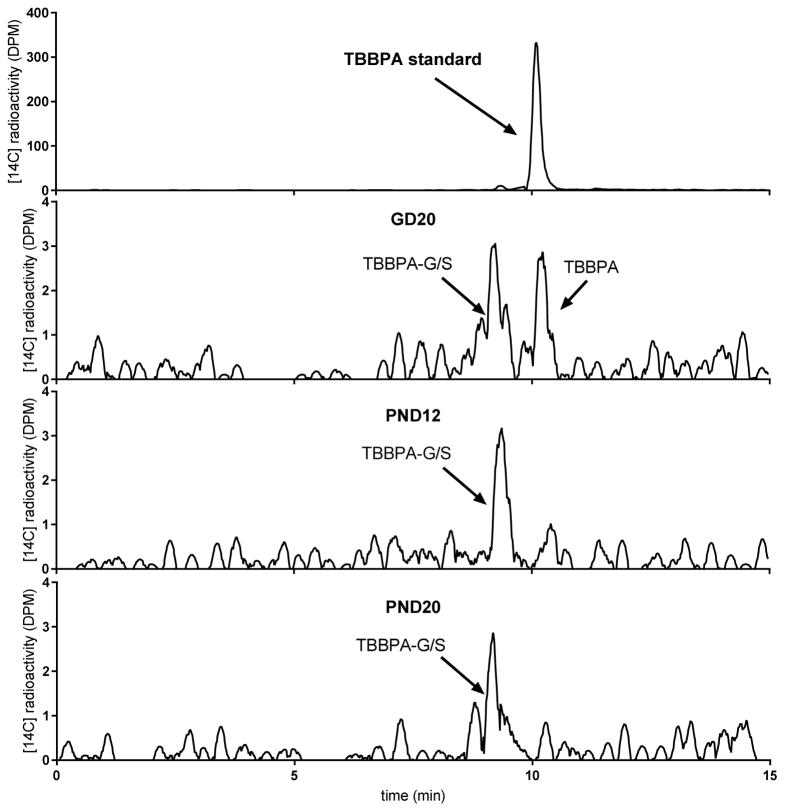
In time-concentration profiles for total TBBPA in blood (parent plus metabolites; Figure 2), [14C]-radioactivity concentrations in blood varied only slightly over the time course and systemic exposure was unchanged between 1 and 8 h post dose in pregnant rats. In nursing rats, at both PND12 and 20, concentrations in blood steadily decreased after 30 minutes, indicating time to Cmax was 30 minutes or less. Cmax was observed at 30 min in pregnant rats and concentrations fell steadily through 24 h where they reached concentrations equivalent to those seen for the nursing dams (GD20: 0.8±0.1 nmol-eq/mL; PND12: 1.0±0.2 nmol-eq/mL; PND20: 1.3±0.4 nmol-eq/mL). In GD20 dam blood extracts, parent compound and metabolite (TBBPA-glucuronide and TBBPA-sulfate, as determined by enzymatic digests and LC/MS analyses and described previously (8), data not shown) were seen at 0.5 h and 1 h but parent TBBPA was not detected in lactating dam (PND12 or PND20) plasma after 1 h. Samples from all other times contained only metabolites (Figure 3).
Figure 2.
Time-concentration curves for total chemical (nmol-eq/mL, panel A) in whole blood from rat dams collected at GD20, PND12, and PND20. Panel B: dose fraction recovered in blood. N=4–5 dams per time-point.
Figure 3.
Representative radiochromatograms of dam blood extracts collected at 30 min. Parent molecule (TBBPA) and/or a mixture of metabolites (TBBPA-glucuronide [TBBPA-G] and TBBPA-sulfate [TBBPA-S]) were detected.
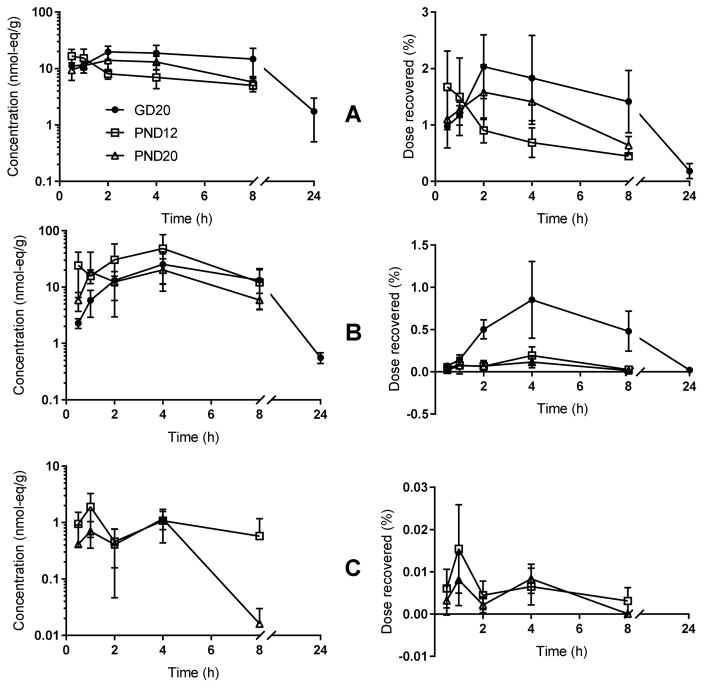
Maternal tissues retained low levels of [14C]-radioactivity throughout the time-course. Concentrations (nmol-eq/g) in liver lagged slightly behind, but clearly paralleled those observed for blood, with an observed Cmax at 2 h post dose (Figure 4). Similarly, time to maximum tissue concentrations in uterus and mammary tissues lagged Cmax seen in blood. Mammary tissue contained only a very small fraction of the total dose (<0.02%) while the amount of dose recovered in uteri from pregnant animals approached the amount of dose recovered in liver (~1–2%), indicative of the increased overall mass and blood flow to the uterus during pregnancy.
Figure 4.
Time-concentration curves for total chemical in: (A) dam liver, (B) uterus, and (C) mammary gland. N=4–5 dams per time-point. Left panels show concentration of dose in nmol-eq/g; right panels show percent of dose recovered in whole tissue.
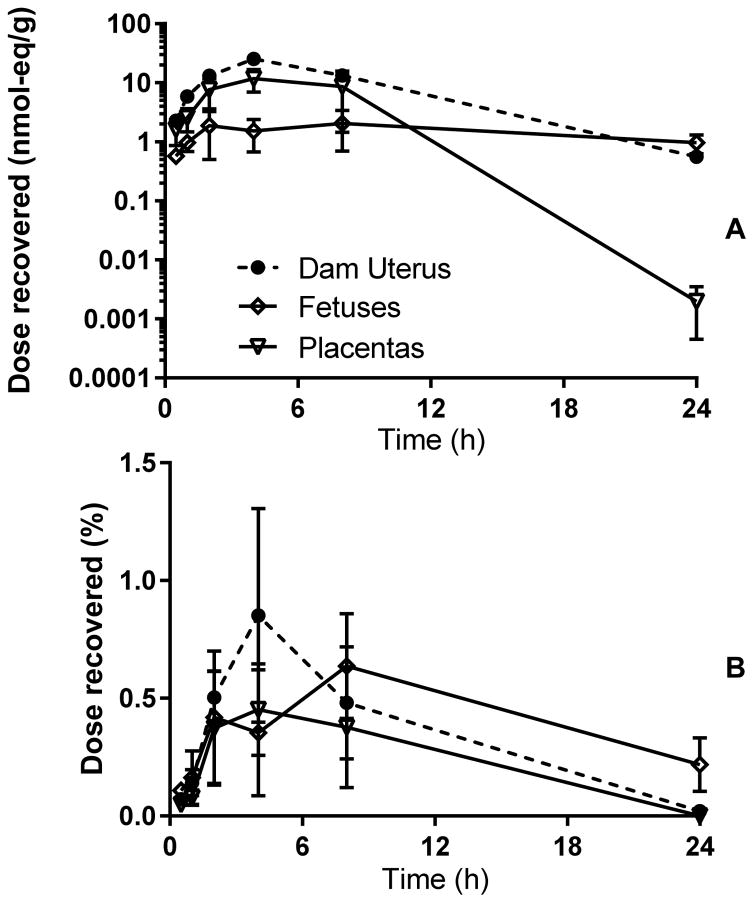
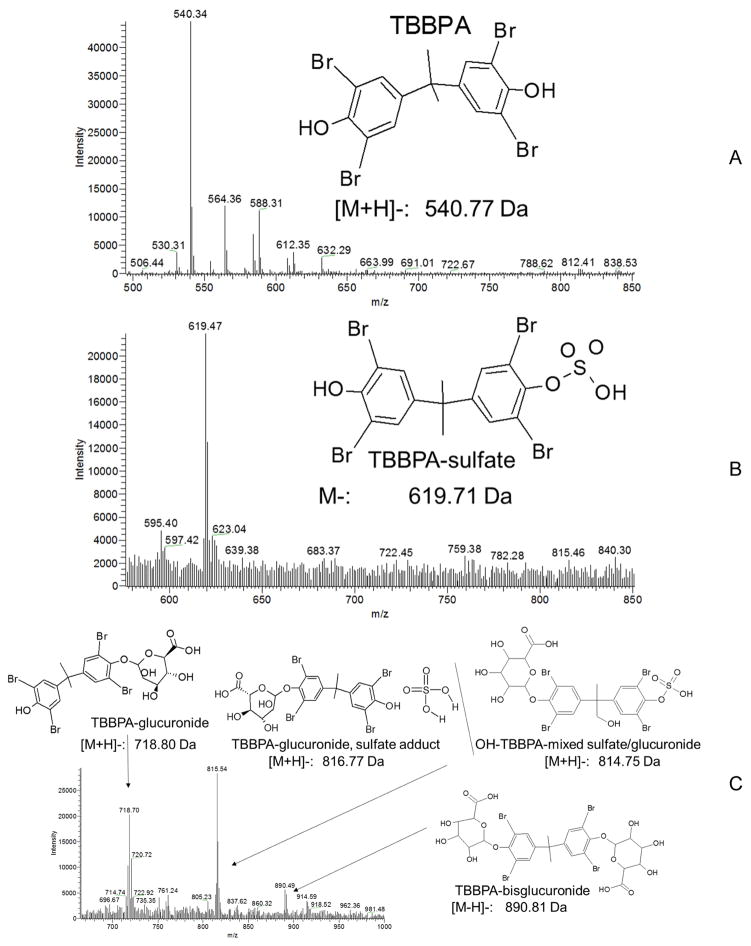
The placenta appeared to play a protective role in reducing the concentrations of TBBPA and its metabolites in fetuses between 30 minutes and 8 h after dosing (Figure 5). By 24 h, concentrations in fetus were equal to or higher than those found in uterus even as placental concentrations decreased sharply. Placental observed Cmax occurred at 4 h, while whole-fetus concentrations were level through 24 h. Fetuses and placentas collected at GD20 did not appear to be depots for TBBPA and its metabolites, with less than 1% of the dose found in placenta or fetuses at any time tested. LC-MS analyses of placenta extracts (Figure 6) found masses corresponding to those of parent TBBPA (m/z = 540.3) and two known conjugates, TBBPA-glucuronide (m/z = 718.7) and TBBPA-sulfate (m/z = 619.5). A mass consistent with the mixed glucuronide/sulfate conjugate of hydroxylated TBBPA was also tentatively identified (m/z = 815.54); this mass is similar to that of a sulfate-adducted TBBPA-glucuronide; further investigation is necessary to confirm the identity of this mass. Sample recovery (percent of dose) are presented as litter units.
Figure 5.
Time-concentration curves for dam uterus, placentas and fetuses collected at GD20 (nmol-eq/g, panel A). Panel B: dose fraction recovered in placentas or fetuses. 3 fetuses from each litter x 3 litters were analyzed at each time-point and placentas were pooled by litter.
Figure 6.
Representative LC/MS spectra for placenta extracts (4 h). A. TBBPA: RT: 2.1–2.5 min, m/z = 540.34; B. TBBPA-glucuronide: RT: 1.4–1.5 min, m/z = 718.7; OH-TBBPA-sulfate/glucuronide mixed conjugate, m/z = 815.54; C. TBBPA-sulfate: RT: 1.3–1.4 min, m/z = 619.54. In general, the mass of the [M+H]-ion was used for molecular identification.
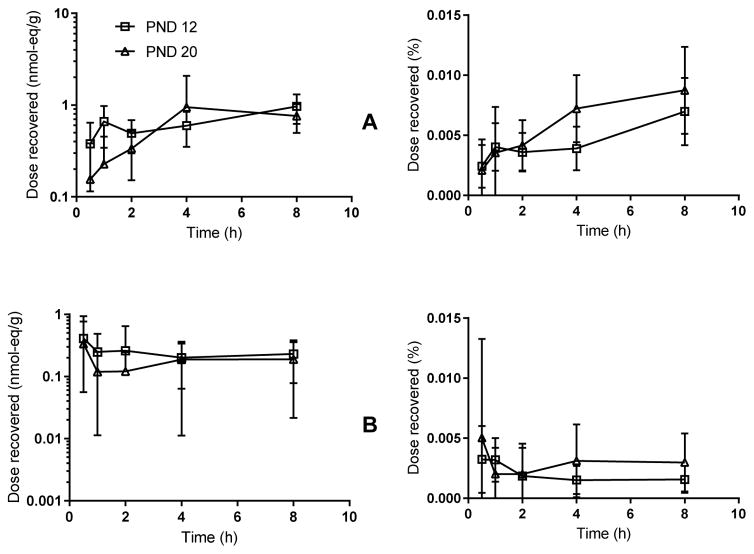
Stomach contents and liver tissue from nursing pups at PND12 and PND20 were assayed to assess TBBPA transfer from milk and internal exposure in nursing animals. Pup plasma was not evaluated. PND12 pup stomach contents contained 0.5 nmol-eq/g at 4 h and ~1.0 nmol-eq/g at 8 h. PND20 pup stomach contents followed a similar trend to the PND12 pup stomach contents, with a maximum concentration of ~1.0 nmol-eq/g occurring at 4 and 8 h, indicating nursing pups were continuously ingesting chemical from their mother’s milk. [14C]-radioactivity in liver collected from PND12 pups decreased with time from 0.6 nmol-eq/g at 30 min post dose to less than 0.2 nmol-eq/g at 8 h post dose. The levels of TBBPA in the PND20 pup livers were low (<0.2 nmol-eq/g) but fairly level over the 8 h exposure window, indicating the pups were consistently absorbing TBBPA from their mother’s milk. Inter-litter and inter-sex differences were compared using a two-way ANOVA test for liver and stomach contents at all time points collected. No statistically significant sex or litter differences were observed in these studies (data not shown).
Discussion
Placental and fetal exposure to endocrine active chemicals like TBBPA are cause for concern (27). In these studies, [14C]-radioactivity was detected in placenta and fetuses following a single gestational exposure through 24 h, indicating persistent pollution of the fetal compartment. Interestingly, by 24 h chemical was not detectable in the placental compartment, indicating an initial trapping of TBBPA or its metabolites after they crossed the placenta into the developing fetus. These data mirror cord blood studies in humans showing detectable levels of TBBPA and its metabolites available to the fetus. TBBPA has been detected in human umbilical cord tissue (28), although it was not always detectable in maternal or neonatal cord blood. Given TBBPA’s ability to dysregulate endocrine signaling through a variety of pathways, this information raises concern.
Exposure to endocrine active chemicals early in postnatal development is a recognized hazard for development of disease later in life (29, 30). We show here that nursing dams exposed on PND12, the time of maximal milk consumption per unit pup weight, or PND20, the time of theoretical absolute maximal milk consumption (due to body size) immediately before weaning, transferred TBBPA to the pups via nursing, as both stomach contents and livers collected from the pups contained appreciable amounts of chemical. We therefore concluded that nursing human infants are likely to experience lactational exposure following maternal exposure to the chemical, as has been shown for other toxicants (31–33).
Physiological changes during pregnancy include the expansion of the central compartment, increases in cardiac output, and alterations in liver and kidney function (34). Liver size does not change although enzyme expression and activity has been observed to change during pregnancy (35). Hepatic metabolism of xenobiotics is altered in pregnancy, with observed increases in sulfotransferase expression and activities and decreases in uridine diphosphate glucuronosyltransferases (36). In addition, the placenta and fetus present additional compartments with unique metabolic and depot functions for the sequestration and prolonged exposure to endocrine-active xenobiotics like TBBPA (37).
Following parturition, blood volume decreases but total body water remains elevated to support the production of milk by the mammary gland (34, 38). Pregnancy also alters bile flow (39). An increase in total body water generally increases the volume of distribution for xenobiotics along with increased clearance (34). The expansion in the lipid portion of the mammary gland increases the likelihood of lipophilic chemicals (e.g., TBBPA) partitioning into this new lipid-rich depot, leading to lactational transfer of the chemical (40).
Conclusions
Placental and lactational transfer of xenobiotics has not been described for TBBPA except for a few studies that detected total TBBPA in nursing mothers’ milk (19–21, 41) and a one- and two-generation study investigating overt developmental effects of TBBPA (10, 42). While these studies are informative, they either did not perform qualitative and quantitative assessments of chemical transferred from the mother to the fetus or nursing pup, or subject the analytes in milk to conditions that degrade the conjugates to parent molecule or are not amenable to metabolite detection. This study was able to demonstrate that systemic exposure was unchanged between 1 & 8 h post dose in pregnant rats after a single oral dose and maximal blood concentrations were observed at 30 min in nursing rats then and fell steadily through 8 h. We found that TBBPA is available to both the developing fetus and nursing pup following maternal exposure (average recovery per litter is shown in Table 1). In addition, it was apparent that nursing pups are continuously exposed to TBBPA via contaminated milk and those exposures can arise from a single oral exposure to TBBPA by the mother.
Table 1.
Average dose (nmol-eq) recovered in each litter (N=3–5). n.d.: not determined
| Time (h) | GD20 | PND12 | PND20 |
|---|---|---|---|
| 0.5 | 13 ± 1 | 6 ± 5 | 6 ± 0.4 |
| 1 | 20 ± 14 | 6 ± 2 | 5 ± 4 |
| 2 | 51 ± 35 | 5 ± 2 | 5 ± 3 |
| 4 | 43 ± 32 | 5 ± 3 | 9 ± 4 |
| 8 | 71 ± 39 | 8 ± 2 | 11 ± 3 |
| 24 | 27 ± 14 | n.d. | n.d. |
Although we were unable to continuously monitor blood profiles, it appeared that parent TBBPA was more rapidly conjugated by both pregnant and nursing dams than non-pregnant animals (8) with nursing dams exhibiting the greatest apparent conjugative efficiency, as no parent TBBPA was ever detected in nursing dam blood. We speculated that the detection of parent TBBPA in blood from pregnant but not nursing dams could also be the result of increased overall blood volume and the downregulation of drug metabolizing enzymes during pregnancy or increased cardiac output, increased liver perfusion, and upregulated hepatic enzymes during lactation. These possibilities were not further investigated in these studies. The presence of metabolite at early time-points in the pregnant and nursing animals supports studies that have shown efficient metabolism of TBBPA in the liver. More research is needed to explore at what levels we see adverse effects in offspring and what mechanisms are affected. Research is also needed to determine why TBBPA is less efficiently metabolized in pregnant than in nursing animals. Ongoing research is investigating the mechanisms underlying the accumulation of the mixture of TBBPA and its conjugates in the placenta and the possible involvement of altered activity of p-glycoprotein and other efflux transporters expressed in the placenta following TBBPA exposure.
Supplementary Material
Table S 1. Dam weights
Figure 7.
Time-concentration curves for: (A) pup stomach contents and (B) pup liver. Left: sample concentrations in nmol-eq/g, Right: fraction of total dose recovered in the sample relative to dose administered to the dam. Pups nursed freely up until the time of tissue collection. N=3 litters per time-point, 2–3 males, 2–3 females per litter. No differences were seen between males and females.
Highlights.
Systemic exposure was unchanged between 1 & 8h post dose in pregnant rats after a single oral dose.
Maximal blood concentrations were observed at 30 min in nursing rats and fell steadily through 8 h.
TBBPA is available to both the developing fetus and nursing pup following maternal exposure.
Nursing pups are continuously exposed via contaminated milk produced by their mother.
Acknowledgments
The authors would like to thank Drs. June Dunnick, Adam Filgo, Suzanne Fenton, Suramya Waidyanatha, and Ms. Sagi Guillera (National Toxicology Program) for advice as well as Dr. J. Michael Sanders and Ms. Sherry Coulter for technical assistance. This research was supported in part by the Intramural Research Program of NIH/NCI (Project ZIA BC 011476).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Wit CA, Herzke D, Vorkamp K. Brominated flame retardants in the Arctic environment — trends and new candidates. Science of The Total Environment. 2010;408(15):2885–918. doi: 10.1016/j.scitotenv.2009.08.037. doi: http://dx.doi.org/10.1016/j.scitotenv.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 2.Canada; Dept. Of the Environment DoH, editor. Risk Management Scope for Phenol, 4,4′-(1-methylethylidene) bis[2,6-dibromo- (Tetrabromobisphenol A) Chemical Abstract Service Registry Number (CAS RN): 79-94-7. 2012. [Google Scholar]
- 3.BSEF. TBBPA Factsheet. Bromine Science and Environment Forum; 2012. http://wwwbsefcom/uploads/Factsheet_TBBPA_25-10-2012pdf. [Google Scholar]
- 4.USEPA. Report 744-R-15-001: Flame Retardants in Printed Circuit Boards. Design for the Environment, Office of Pollution Prevention; 2013. https://www.epa.gov/sites/production/files/2015-08/documents/pcb_final_report.pdf. [Google Scholar]
- 5.IPCS/WHO; World Health Organization G, editor. Tetrabromobisphenol A and Derivatives. 1995. [Google Scholar]
- 6.Hakk H, Larsen G, Bergman A, Orn U. Metabolism, excretion and distribution of the flame retardant tetrabromobisphenol-A in conventional and bile-duct cannulated rats. Xenobiotica; the fate of foreign compounds in biological systems. 2000;30(9):881–90. doi: 10.1080/004982500433309. Epub 2000/10/31. [DOI] [PubMed] [Google Scholar]
- 7.Kuester RK, Solyom AM, Rodriguez VP, Sipes IG. The effects of dose, route, and repeated dosing on the disposition and kinetics of tetrabromobisphenol A in male F-344 rats. Toxicol Sci. 2007;96(2):237– 45. doi: 10.1093/toxsci/kfm006. Epub 2007/01/20. [DOI] [PubMed] [Google Scholar]
- 8.Knudsen GA, Sanders JM, Sadik AM, Birnbaum LS. Disposition and kinetics of Tetrabromobisphenol A in female Wistar Han rats. Toxicol Rep. 2014;1(0):214–23. doi: 10.1016/j.toxrep.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schauer UM, Volkel W, Dekant W. Toxicokinetics of tetrabromobisphenol a in humans and rats after oral administration. Toxicol Sci. 2006;91(1):49–58. doi: 10.1093/toxsci/kfj132. Epub 2006/02/17. [DOI] [PubMed] [Google Scholar]
- 10.Van der Ven LT, Van de Kuil T, Verhoef A, Verwer CM, Lilienthal H, Leonards PE, Schauer UM, Canton RF, Litens S, De Jong FH, Visser TJ, Dekant W, Stern N, Hakansson H, Slob W, Van den Berg M, Vos JG, Piersma AH. Endocrine effects of tetrabromobisphenol-A (TBBPA) in Wistar rats as tested in a one-generation reproduction study and a subacute toxicity study. Toxicology. 2008;245(1–2):76–89. doi: 10.1016/j.tox.2007.12.009. Epub 2008/02/08. [DOI] [PubMed] [Google Scholar]
- 11.Sanders JM, Coulter SJ, Knudsen GA, Dunnick JK, Kissling GE, Birnbaum LS. Disruption of estrogen homeostasis as a mechanism for uterine toxicity in Wistar Han rats treated with tetrabromobisphenol A. Toxicology and applied pharmacology. 2016 doi: 10.1016/j.taap.2016.03.007. Epub 2016/03/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riu A, Grimaldi M, le Maire A, Bey G, Phillips K, Boulahtouf A, Perdu E, Zalko D, Bourguet W, Balaguer P. Peroxisome proliferator-activated receptor gamma is a target for halogenated analogs of bisphenol A. Environmental health perspectives. 2011;119(9):1227–32. doi: 10.1289/ehp.1003328. Epub 2011/05/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szymanska JA, Piotrowski JK, Frydrych B. Hepatotoxicity of tetrabromobisphenol-A: effects of repeated dosage in rats. Toxicology. 2000;142(2):87–95. doi: 10.1016/s0300-483x(99)00108-0. Epub 2000/02/24. [DOI] [PubMed] [Google Scholar]
- 14.Szymanska JA, Sapota A, Frydrych B. The disposition and metabolism of tetrabromobisphenol-A after a single i.p. dose in the rat. Chemosphere. 2001;45(4–5):693–700. doi: 10.1016/s0045-6535(01)00015-7. Epub 2001/10/30. [DOI] [PubMed] [Google Scholar]
- 15.Chignell CF, Han SK, Mouithys-Mickalad A, Sik RH, Stadler K, Kadiiska MB. EPR studies of in vivo radical production by 3,3′,5,5′-tetrabromobisphenol A (TBBPA) in the Sprague-Dawley rat. Toxicology and applied pharmacology. 2008;230(1):17–22. doi: 10.1016/j.taap.2008.01.035. Epub 2008/03/18. [DOI] [PubMed] [Google Scholar]
- 16.Dunnick JK, Sanders JM, Kissling GE, Johnson C, Boyle MH, Elmore SA. Environmental Chemical Exposure May Contribute to Uterine Cancer Development: Studies with Tetrabromobisphenol A. Toxicologic pathology. 2014 doi: 10.1177/0192623314557335. Epub 2014/12/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall SM, Coulter SJ, Knudsen GA, Sanders JM, Birnbaum LS. Gene expression changes in immune response pathways following oral administration of tetrabromobisphenol A (TBBPA) in female Wistar Han rats. Toxicology letters. 2017 doi: 10.1016/j.toxlet.2017.03.008. Epub 2017/03/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gosavi RA, Knudsen GA, Birnbaum LS, Pedersen LC. Mimicking of estradiol binding by flame retardants and their metabolites: a crystallographic analysis. Environmental health perspectives. 2013;121(10):1194–9. doi: 10.1289/ehp.1306902. Epub 2013/08/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cariou R, Antignac JP, Zalko D, Berrebi A, Cravedi JP, Maume D, Marchand P, Monteau F, Riu A, Andre F, Le Bizec B. Exposure assessment of French women and their newborns to tetrabromobisphenol-A: occurrence measurements in maternal adipose tissue, serum, breast milk and cord serum. Chemosphere. 2008;73(7):1036–41. doi: 10.1016/j.chemosphere.2008.07.084. Epub 2008/09/16. [DOI] [PubMed] [Google Scholar]
- 20.Fujii Y, Nishimura E, Kato Y, Harada KH, Koizumi A, Haraguchi K. Dietary exposure to phenolic and methoxylated organohalogen contaminants in relation to their concentrations in breast milk and serum in Japan. Environment international. 2014;63:19–25. doi: 10.1016/j.envint.2013.10.016. Epub 2013/11/23. [DOI] [PubMed] [Google Scholar]
- 21.Shi Z, Jiao Y, Hu Y, Sun Z, Zhou X, Feng J, Li J, Wu Y. Levels of tetrabromobisphenol A, hexabromocyclododecanes and polybrominated diphenyl ethers in human milk from the general population in Beijing, China. The Science of the total environment. 2013;452–453:10–8. doi: 10.1016/j.scitotenv.2013.02.038. Epub 2013/03/19. [DOI] [PubMed] [Google Scholar]
- 22.Covaci A, Harrad S, Abdallah MA, Ali N, Law RJ, Herzke D, de Wit CA. Novel brominated flame retardants: a review of their analysis, environmental fate and behaviour. Environment international. 2011;37(2):532–56. doi: 10.1016/j.envint.2010.11.007. Epub 2010/12/21. [DOI] [PubMed] [Google Scholar]
- 23.NTP. Toxicology Studies of Tetrabromobisphenol A (CASRN 79-94-7) in F344/NTac Rats and B6C3F1/N Mice and Toxicology and Carcinogenesis Studies of Tetrabromobisphenol A in Wistar Han [Crl:WI(Han)] Rats and B6C3F1/N Mice (Gavage Studies) National Toxicology Program, Health and Human Services; 2014. Found at: https://ntp.niehs.nih.gov/results/pubs/longterm/reports/longterm/tr500580/listedreports/tr587/index.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenham LW, Greenham V. Sexing mouse pups. Laboratory Animals. 1977;11(3):181–4. doi: 10.1258/002367777780936620. [DOI] [PubMed] [Google Scholar]
- 25.Ziegler Brothers I. Rodent NIH-31 Open Formula Auto. http://www.zeiglerfeed.com/product_literature/lab%20research%20literature_Rodent/Rodent%20NIH-31%20Open.pdf2010.
- 26.Knudsen GA, Sanders JM, Birnbaum LS. Disposition of the emerging brominated flame retardant, bis(2-ethylhexyl) tetrabromophthalate, in female Sprague Dawley rats: effects of dose, route and repeated administration. Xenobiotica; the fate of foreign compounds in biological systems. 2016:1–10. doi: 10.1080/00498254.2016.1174793. Epub April 21, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLachlan JA. Environmental signaling: from environmental estrogens to endocrine-disrupting chemicals and beyond. Andrology. 2016;4(4):684–94. doi: 10.1111/andr.12206. Epub 2016/05/28. [DOI] [PubMed] [Google Scholar]
- 28.Kawashiro Y, Fukata H, Omori-Inoue M, Kubonoya K, Jotaki T, Takigami H, Sakai S, Mori C. Perinatal exposure to brominated flame retardants and polychlorinated biphenyls in Japan. Endocrine journal. 2008;55(6):1071–84. doi: 10.1507/endocrj.k08e-155. Epub 2008/08/23. [DOI] [PubMed] [Google Scholar]
- 29.Vaiserman A. Early-life Exposure to Endocrine Disrupting Chemicals and Later-life Health Outcomes: An Epigenetic Bridge? Aging and disease. 2014;5(6):419–29. doi: 10.14336/ad.2014.0500419. Epub 2014/12/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharpe RM. Hormones and testis development and the possible adverse effects of environmental chemicals. Toxicology letters. 2001;120(1–3):221–32. doi: 10.1016/s0378-4274(01)00298-3. Epub 2001/04/27. [DOI] [PubMed] [Google Scholar]
- 31.Andersson M, Ersson L, Brandt I, Bergstrom U. Potential transfer of neurotoxic amino acid beta- N-methylamino-alanine (BMAA) from mother to infant during breast-feeding: Predictions from human cell lines. Toxicology and applied pharmacology. 2017;320:40–50. doi: 10.1016/j.taap.2017.02.004. Epub 2017/02/09. [DOI] [PubMed] [Google Scholar]
- 32.Lee S, Kim S, Park J, Kim HJ, Choi G, Choi S, Kim S, Kim SY, Kim S, Choi K, Moon HB. Perfluoroalkyl substances (PFASs) in breast milk from Korea: Time-course trends, influencing factors, and infant exposure. The Science of the total environment. 2017;612:286–92. doi: 10.1016/j.scitotenv.2017.08.094. Epub 2017/09/03. [DOI] [PubMed] [Google Scholar]
- 33.Limon-Miro AT, Aldana-Madrid ML, Alvarez-Hernandez G, Antunez-Roman LE, Rodriguez-Olibarria G, Valencia Juillerat ME. Breast milk intake and mother to infant pesticide transfer measured by deuterium oxide dilution in agricultural and urban areas of Mexico. Chemosphere. 2017;181:682–9. doi: 10.1016/j.chemosphere.2017.04.110. Epub 2017/05/06. [DOI] [PubMed] [Google Scholar]
- 34.Ansari J, Carvalho B, Shafer SL, Flood P. Pharmacokinetics and Pharmacodynamics of Drugs Commonly Used in Pregnancy and Parturition. Anesthesia and analgesia. 2016;122(3):786–804. doi: 10.1213/ane.0000000000001143. Epub 2016/02/20. [DOI] [PubMed] [Google Scholar]
- 35.Bacq Y. The Liver in Normal Pregnancy. Austin (TX): Landes Bioscience; 2000–2013. [cited 2017 4/24/2017]. Available from: Available from: https://www.ncbi.nlm.nih.gov/books/NBK6005/ [Google Scholar]
- 36.Wen X, Donepudi AC, Thomas PE, Slitt AL, King RS, Aleksunes LM. Regulation of Hepatic Phase II Metabolism in Pregnant Mice. The Journal of pharmacology and experimental therapeutics. 2013;344(1):244–52. doi: 10.1124/jpet.112.199034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garland M, Myers MM, Szeto HH, Stark RI. MATERNAL-FETAL PHARMACOKINETICS: THE TWO-COMPARTMENT MODEL REVISITED.[bull] 422. Pediatr Res. 1996;39(S4):73. [Google Scholar]
- 38.Pipe NGJ, Smith T, Halliday D, Edmonds CJ, Williams C, Coltart TM. CHANGES IN FAT, FAT-FREE MASS AND BODY WATER IN HUMAN NORMAL PREGNANCY. BJOG: An International Journal of Obstetrics & Gynaecology. 1979;86(12):929–40. doi: 10.1111/j.1471-0528.1979.tb11240.x. [DOI] [PubMed] [Google Scholar]
- 39.Reyes H, Kern F., Jr Effect of pregnancy on bile flow and biliary lipids in the hamster. Gastroenterology. 1979;76(1):144–50. Epub 1979/01/01. [PubMed] [Google Scholar]
- 40.Fleishaker JC, McNamara PJ. In vivo evaluation in the lactating rabbit of a model for xenobiotic distribution into breast milk. Journal of Pharmacology and Experimental Therapeutics. 1988;244(3):919– 24. [PubMed] [Google Scholar]
- 41.Carignan CC, Abdallah MA, Wu N, Heiger-Bernays W, McClean MD, Harrad S, Webster TF. Predictors of tetrabromobisphenol-A (TBBP-A) and hexabromocyclododecanes (HBCD) in milk from Boston mothers. Environmental science & technology. 2012;46(21):12146–53. doi: 10.1021/es302638d. Epub 2012/09/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colnot T, Kacew S, Dekant W. Mammalian toxicology and human exposures to the flame retardant 2,2′,6,6′-tetrabromo-4,4′-isopropylidenediphenol (TBBPA): implications for risk assessment. Archives of toxicology. 2014;88(3):553–73. doi: 10.1007/s00204-013-1180-8. Epub 2013/12/20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S 1. Dam weights