Abstract
Activated microglial cells produce the pro-inflammatory mediators such as nitric oxide (NO) and cytokines. The excessive release of these mediators can lead to neurodegenerative diseases, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD). Inhibition of the release of these pro-inflammatory molecules may prevent or halt the progression of these diseases. Plumbagin (PL), a naphthoquinone compound in the roots of the traditional medicinal plant Plumbago zeylanica L., showed anti-inflammatory effects on macrophages. However, PL effects on activated microglia remain unknown. In the present study, PL has been examined for its anti-inflammatory effect on LPS – activated microglial BV-2 cells. In this study, NO and iNOS expression were investigated in BV-2 microglial cells in the presence of PL or the selective iNOS inhibitor L-N6-(1-iminoethyl) lysine (L-NIL). The results obtained indicate that PL was > 30-fold potent than L-NIL in inhibiting NO production with an IC50 of 0.39 μM. Our immunofluorescence study confirmed the ability of PL to significantly inhibit iNOS expression in the activated microglia. Furthermore, the extracellular microglial pro-inflammatory cytokine expression in the presence of 2 μM of PL was detected, quantified, and validated using cytokine antibody protein arrays and quantitative ELISA. The results obtained showed that PL significantly downregulated the expression of many cytokines including IL-1α, G-CSF, IL-12 p40/p70, MCP-5, MCP-1, and IL-6. In conclusion, PL potency in attenuating multiple pro-inflammatory agents indicates its potential to be used for neurodegenerative diseases.
Keywords: Plumbagin, Cytokines, Nitric oxide, Microglial cells, Neuroprotection, Anti-inflammatory
1. Introduction
In the central nervous system, the immune system plays a critical role in sustaining tissues homeostasis in response to external influences such as infection and injury (Glass et al., 2010). Microglial cells are the major resident immune cells in the CNS, with a resting phenotype under normal conditions (Streit, 2002). However, upon infection or neuronal injury, microglial cells become active, proliferate, and move to the injured area (Kettenmann et al., 2011). The activated microglia produce cytotoxic pro-inflammatory molecules such as nitric oxide (NO) and cytokines/chemokines. These events are common in many chronic inflammatory neurodegenerative diseases. The most common of these diseases include Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), and Huntington’s disease (HD) (Block and Hong, 2007; Lull and Block, 2010).
Cellular expression of several pro-inflammatory molecules is stimulated by nuclear factor κB (NF-κB) (Kielian, 2006). Hence, the inhibition of active NF-κB is considered to be a significant target for anti-inflammatory drugs (Tak and Firestein, 2001). Moreover, numerous studies examined the anti-inflammatory and neuroprotective effects of the bicyclic naphthoquinone plumbagin (PL) (5-hydroxy-2-methyl-1, 4-naphthoquinone). Several investigations showed that PL inhibits NF-κB (Checker et al., 2009; Luo et al., 2010; T. Wang et al., 2014; Zhang et al., 2015) and the pro-inflammatory cytokines stimulated by NF-κB activation in the in vivo models of osteoporosis (Zheng et al., 2017). Furthermore, the protective effects of PL through inhibition of LPS-induced NO, cytokines and NF-κB release in mice and RAW 264.7 macrophage cells were reported (Checker et al., 2014; T. Wang et al., 2014).
The natural compound PL is the main active constituent of the medicinal plant Plumbago zeylanica that showed anti-inflammatory effects (Sheeja et al., 2010). Plumbago zeylanica roots had been used in India for many centuries in treating skin diseases, diarrhea, dyspepsia, piles, anasarca, plague, leprosy, urinary tract infections, scabies and ulcers (Jetty et al., 2010). The plant extract was found to have neuroprotective, hepatoprotective antiatherogenic, and cardiotonic properties (Tilak et al., 2004). PL is found in other medicinal plants belonging to the Plumbaginaceae, Droseraceae, and Ebenaceae families (Khaw et al., 2015). Recently, many studies evaluated the medicinal significance of PL and indicated its possible use in treating depression (Dhingra and Bansal, 2015), rheumatoid arthritis (Poosarla et al., 2011), and diabetes (Sunil et al., 2012). Moreover, PL was found to have anticancer properties against breast cancer (Ahmad et al., 2008; Kuo et al., 2006), prostate cancer (Aziz et al., 2008; Nair et al., 2015; F. Wang et al., 2015), ovarian (Thasni et al., 2008), pancreatic (Chen et al., 2009; Hafeez et al., 2012), lung cancer (Gomathinayagam et al., 2008), cervical cancer (Appadurai and Rathinasamy, 2015; Srinivas et al., 2004), brain cancer (Khaw et al., 2015), in addition to melanoma and myeloma (Sandur et al., 2010; Wang et al., 2008).
Based on previous reports and our research for natural compounds with anti-inflammatory effects, we hypothesized that PL would have an anti-inflammatory effect in LPS-activated microglial cells. Therefore, the present study is designed to examine the ability of PL to reduce microglial pro-inflammatory molecules such as nitric oxide (NO), iNOS, and corresponding cytokine expressions in the LPS-activated microglial BV-2 cell line.
2. Experimental sections
2.1. Materials and reagents
Chemicals purchased from Sigma-Aldrich (St. Louis, MO, USA) include plumbagin (PL) (purity 99%), L-N6 – (1-Iminoethyl) lysine (L-NIL), dimethyl sulfoxide (DMSO), Trypsin–EDTA solution, Lipopolysaccharides from Escherichia coli O111: B4 (LPS), and propidium iodide. Cell culture flasks and plates, Dulbecco’s Modified Eagle Medium (DMEM) and fetal bovine serum (FBS) were purchased from VWR International (Radnor, PA, USA). Penicillin/streptomycin and DPBS were obtained from Atlanta Biologicals (Atlanta, GA, USA). Mouse Cytokine Antibody Array Kit (Cat # AAM-CYT-1000) and ELISA kits were purchased from RayBiotech (Norcross GA, USA). Anti-iNOS antibody (Ab 49999) and goat anti-mouse Alexa Fluor® 488 (ab 150117) were obtained from Abcam (Cambridge, MA, USA).
2.2. Cell culture
BV-2 microglial cells were generously provided by Dr. Elisabetta Blasi (Blasi et al., 1990) and were cultured at 37 °C in humidified 5% CO2 incubator and were subcultured as needed with trypsin/EDTA. The DMEM growing medium was supplemented with 5% heat-inactivated FBS (v/v), 4 mM L-glutamine, and 1% penicillin/streptomycin (100 U/mL penicillin G sodium and 0.1 mg/mL streptomycin sulfate). DMEM experimental medium was phenol free and supplemented with 2.5% heat-inactivated FBS.
2.3. Cell viability assay
In this experiment, cells were incubated overnight in the experimental media at density 5 × 104 cells/well in 96-well plates. The redox dye resazurin was used for determining the viability of BV-2 cells after 24 h treatment with PL (0–5 μM) or L-NIL (0–100 μM) in experimental media. The tested compound plumbagin was dissolved in DMSO before dilution in the media, and the final concentration of DMSO in all experiments did not exceed 0.1% in the control wells (Elsisi et al., 2005). Also, wells without cells were used as a blank. In this assay, resazurin solution of 0.5 mg/mL in a sterile phenol red free HBSS was used at a concentration level of 10% v/v. The reduced resazurin was measured at an excitation/emission of 530/590 nm using Synergy HTX Multi-Reader (BioTek, USA). The percentage of BV-2 cells survival compared to the control was calculated.
2.4. Nitric oxide assay
In this study, the effect of PL on nitric oxide (NO) production in LPS-activated BV-2 microglia cells was examined. Based on a previously conducted optimization study in our lab, 1 μg/mL of LPS was found to be the optimum concentration for BV-2 microglial cells activation. (Taka et al., 2015). Others also indicated similar LPS dose (Pinho et al., 2011). In the current study, cells were incubated overnight in experimental media at density 5 × 104 cells/well in 96-well plates. Next day, cells were first stimulated for 1 h with 1 μg/mL LPS, then treated with concentrations range of 0–2.0 μM of PL or 0–100.00 μM of L-NIL. Control wells were treated with DMSO alone at the highest used concentration (0.1%), and equivalent wells without cells were used as blanks. After a 24 h exposure period, NO concentrations were measured using Griess reagent. 50 μL each of both the cell-free supernatant and 2× Griess reagent were mixed for a Griess reagent final concentration of 2.5% phosphoric acid, 0.1% naphthyl ethylenediamine dihydrochloride, and 1% sulfanilamide. The mixture was incubated for 10 min. at room temperature, and the absorbance was measured at 550 nm using Synergy HTX Multi-Reader (BioTek, USA).
2.5. Immunofluorescence assay
Briefly, four sets of cells were treated as follows: LPS-treated (1 μg/mL), PL-treated (2 μM), co-treated cells (1 μg/mL LPS + 2 μM PL), and DMSO-treated resting cells. After 24 h exposure period, cells were washed twice with DPBS and were fixed for 10 min with freshly prepared 4% paraformaldehyde. After that, cells were washed again with DPBS and premetallized for 2 min with 0.2% Tween followed by another wash. Cells were then placed on a shaker for 2 h. at RT with anti-iNOS antibody-diluted TBST (1:150), washed again with DPBS and re-incubated in the dark at RT for another 2 h. with goat anti-mouse Alexa Fluor® 488 (1:500). Finally, cells were counterstained for 15 min with 5.0 μg/mL propidium iodide and washed with DPBS. Images were taken using fluorescence microscope Olympus IX71 (Hunt Optics and Imaging Inc. Pittsburgh, PA, USA).
2.6. Mouse cytokine/chemokine protein microarray
For cytokines microarray analysis, four flasks of BV-2 cells were grown to confluence in 75-cm2 TC flasks, using the same cell culture media. On the day of the experiment, the media were discarded, and cells were washed with phenol-free experimental media. Immediately, cells were treated with 1 μg/mL LPS, 2 μM PL, and 1 μg/mL LPS + 2 μM PL, and control samples were exposed to only DMSO. After a 24 h exposure period, the cell-free supernatant of each sample was collected, aliquoted, and stored at −80 °C for later use. The experiment was repeated three times. A semiquantitative method was established to evaluate chemokines/cytokines expression in BV-2 cell culture supernatants using antibody-coated array membranes. The assay was conducted following the protocol of the used kits from Ray Biotech. Briefly, membranes were placed carefully in the incubation tray and blocked with the provided buffer on a shaker for 30 min. at RT. Thereafter, the blocking buffer was decanted, and the membranes were treated with 1.0 mL cell-free supernatant from resting cells, PL-treated, LPS-stimulated or co-treated cells and placed overnight on a low-speed shaker at 4 °C. Next day, the media were decanted from each chamber and the membranes were washed with wash buffers indicated in the kit. Next, 1 mL of freshly constituted biotinylated antibody cocktail was pipetted to each membrane and again incubated at RT for 2 h. followed by the same previously applied buffer washing. The membranes were incubated again for another 2 h with 2 mL of diluted horseradish peroxidase – conjugated streptavidin (HRP-Streptavidin) followed by the final washes. Spots intensity on the blots were detected using chemiluminescence detection. Blots images were captured using a Flour-S Max Multiimager (Bio-Rad Laboratories, Hercules, CA) and analyzed to obtain the spots density with Quantity – One Software (Bio-Rad Laboratories, Hercules, CA), followed by Excel-based data analysis software specific for Mouse Cytokine Array C1000 (CODE: S02-AAM-CYT-1000) to subtract the background and normalize the density of the cytokines on the blot relative to the positive control.
2.7. Cytokine ELISA studies
Enzyme-Linked Immunosorbent Assay (ELISA) kits were used to confirm the effect of PL on cytokines/chemokines expressions detected by the microarray investigation. The assays used were antibody specific for mouse as follows; MCP-1 (Cat # ELM-MCP1); MCP-5 (Cat # ELM –MCP5); IL-6 (Cat # ELM – IL-6); IL-1α (Cat # ELM-IL1a); IL-12p40/p70 (Cat # ELM-IL12p40/p70); and G-CSF (Cat # ELM – GCSF). Briefly, standard curve, samples, and reagents were prepared at RT following the protocol instructions. Standards and samples of 100 μL were incubated in the antibody pre-coated 96-well plates provided for 2.5 h. The supernatant was replaced by 100 μL of freshly constituted biotinylated antibody for another hour and then decanted. Streptavidin solution (100 μL) was added for 45 min followed by addition of 100 μL of the substrate reagent for 30 min incubation. The reaction was terminated by the addition of 50 μL of a stop solution, and the intensity was measured at 450 nm using Synergy HTX Multi-Reader (BioTek, USA).
2.8. Statistical analysis
Data were analyzed using the Graph Pad Prism 6.2 Software (San Diego, CA, USA). All data points were obtained from the average of at least two independent studies and expressed as mean ± SEM. Inhibitory concentrations (IC50s) for NO studies were determined by nonlinear regression with lowest 95% confidence interval and R2 best fit. The significance of the difference between control and treated groups was determined using one-way ANOVA followed by Bonferroni’s multiple comparison’s tests. Significance of the difference between the control and treated groups is considered at * P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001. For blots and ELISA studies, student t-test was used to verify the significance of the difference between control and LPS groups, and between LPS and LPS + PL groups.
3. Results
In this study, the optimum concentration of PL that used with BV-2 cells for its anti-inflammatory effects without interfering with cell viability was evaluated. The obtained data are presented in Fig. 1A and show the non-significant effect on cells treated with different PL concentrations up to 2 μM. A significant linear effect of PL was found at concentrations higher than 2 μM. Using the iNOS inhibitor (Fig. 1B), the relationship obtained was not significant when BV-2 cells were treated with different L-NIL concentrations (0–100 μM).
Fig. 1.
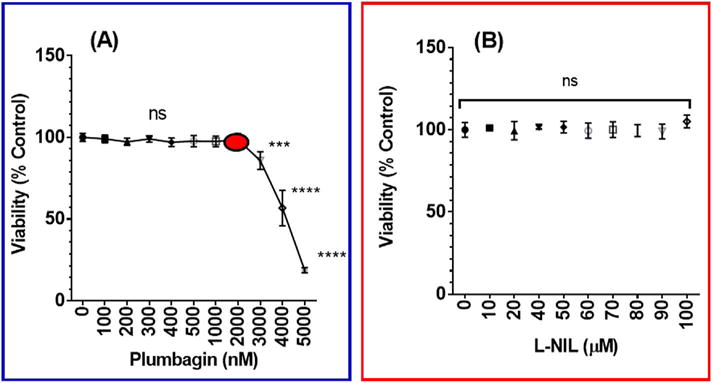
The effect of plumbagin or L-NIL on LPS – activated BV-2 microglial cells viability. Microglial cells (5 × 104 cells/well) were treated for 24 h with PL (0–5 μM) (A) vs. L-NIL (0–100 μM) (B). Control wells were treated with DMSO at the highest used concentration. The viability of BV-2 cells was determined using the redox dye resazurin and the percentage of BV-2 cells survival compared to the control was calculated. Data points were expressed as the mean ± SEM, n = 4. The significance of the difference between control and treatments was determined using one-way ANOVA followed by Bonferroni’s multiple comparisons. *** p < 0.001, **** p < 0.0001, and non-significant (ns).
The obtained data presented in Fig. 2A showed a significant negative relationship between PL concentrations and NO inhibition. At the highest tested PL concentration of 2 μM, 90% inhibition of NO production was obtained with an IC50 of 386.1 ± 16.17 nM. On the other hand, the selective iNOS inhibitor L-NIL showed an IC50 of 11.77 ± 0.56 μM (Fig. 2B). Noticeably, PL was 30-fold more potent in NO inhibition than L-NIL.
Fig. 2.
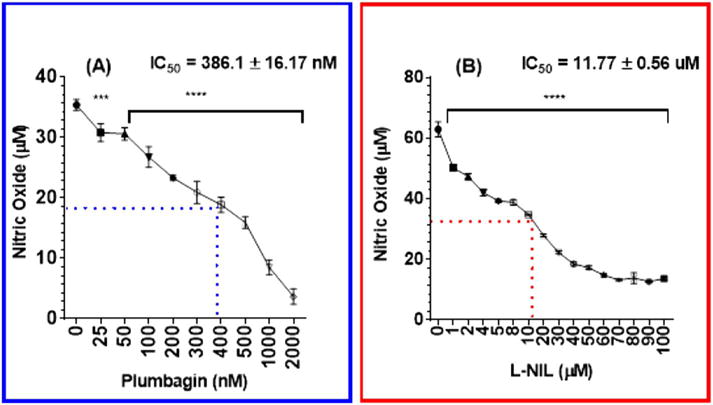
The effect of plumbagin or L-NIL on nitric oxide production in LPS-activated BV-2 microglial cells. Microglial cells (5 × 104 cells/well) were first stimulated for 1 h with LPS (1 μg/mL), then treated with concentrations range 0–2 μM of PL (A) or 0–100 μM of L-NIL (B) and control wells were treated with DMSO. After 24 h exposure period, NO concentrations were measured using Griess reagent, and the absorbance was measured at 550 nm. Data points were expressed as the mean ± SEM, n = 4. IC50 ± SEM is the average of at least two data sets. The significance of the difference between control and treatments was determined using one-way ANOVA followed by Bonferroni’s multiple comparisons. *** p < 0.001, and **** p < 0.0001.
Using the immunocytochemical assay, iNOS expression changes were visualized in resting cells, in the presence of LPS and LPS + PL-treated cells (Fig. 3). PL-treated cells (Fig. 3B) have no expression of iNOS when compared to the control cells (Fig. 3A). The images show LPS ability to induce iNOS as indicated by the intense green fluorescence (Fig. 3C) as well as the ability of PL to attenuate iNOS expression (Fig. 3D).
Fig. 3.
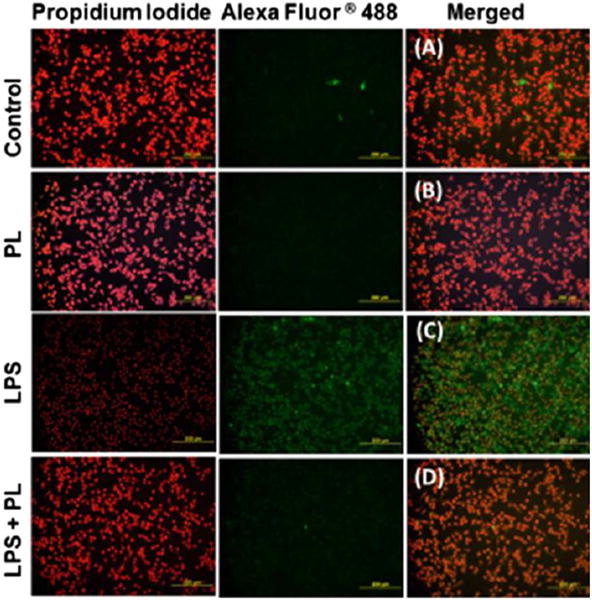
The effect of plumbagin on iNOS expression in LPS-activated BV-2 microglial cells. Four sets of cells were treated as follows: LPS-treated (1 μg/mL), PL-treated (2 μM), co-treated cells (1 μg/mL of LPS + 2 μM of PL), and resting DMSO-treated cells. After 24 h experimental period the cells were fixed and premetallized. Next, the treated cells were incubated with Anti-iNOS antibody, followed by another incubation period with goat anti-mouse Alexa Fluor® 488 and counterstained with propidium iodide. Samples were photographed at 20× magnification using fluorescence microscope. The images showed that in the absence of LPS, iNOS expression was not visually observed in resting cells (A) and PL-treated cells (B). In the presence of LPS in (C), iNOS expression was indicated by the intense green fluorescence color observed. The effect was attenuated in co-treated cells (D). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
To study the anti-inflammatory effects of PL in activated BV-2 cells, we examined 96 cytokines expression changes in the presence and absence of LPS and PL (Fig. 4A and B). Cytokines tested were divided into two different sets of AAM-CYT; AAM-CYT-3 (Fig. 4A) and AAM-CYT-4 blots. The cytokine/chemokine arrays were presented in four sets of blots; resting cells, LPS – activated cells, PL – treated cells, and LPS + PL treated cells (Fig. 4B). Data obtained from the AAM-CYT-3 blots imaging (Fig. 5) where supernatant of cells pre-exposed to 1 μg/mL of LPS showed a significant increase in some cytokine expression (**** p < 0.0001–* p < 0.05). Six cytokines/chemokines were highly expressed with granulocyte-colony stimulating factor (G-CSF) > Interleukin 12 (IL-12 p40/p70) > Interleukin 1 alpha (IL-1α) > monocyte chemoattractant protein 5 (MCP-5) > Interleukin 6 (IL-6) > monocyte chemoattractant protein 1 (MCP-1), giving the highest increase of 12.19-fold and the lowest of 2.33-fold. PL treatment of activated BV-2 cells resulted in significant cytokine inhibition of G-CSF (### p < 0.0001), IL-1α (## p < 0.001), IL-12 (# p < 0.01), MCP-5 (###p < 0.001) while there were no significant changes in IL-6 or MCP-1. Meanwhile, there were no significant differences between cytokines produced by resting cells vs. PL-treated cells.
Fig. 4.
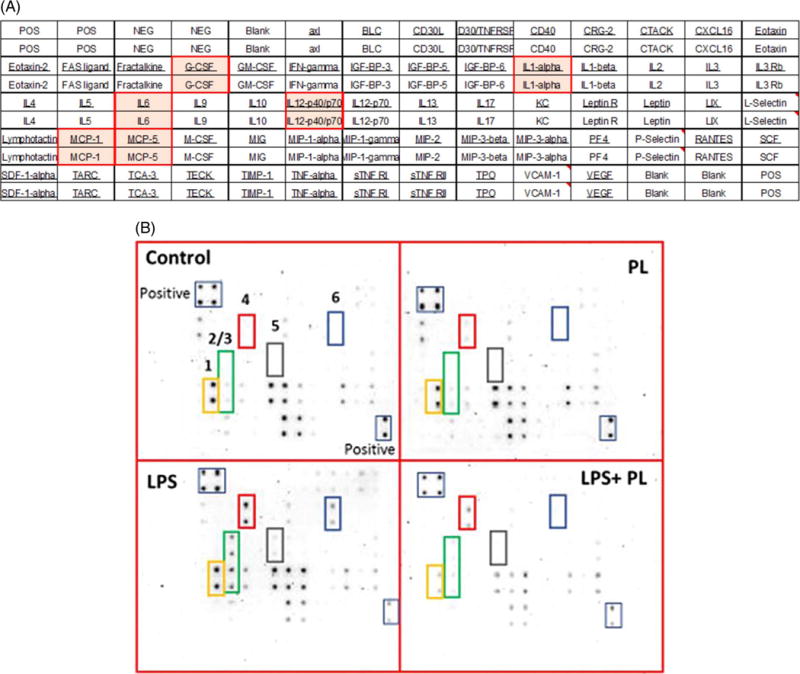
Plumbagin effect on different cytokine expression in LPS-activated BV-2 microglial cells. (A). Microarray layout AAM-CYT-3 was used to assess chemokines/cytokines expression in cell-free supernatant. (B). Microarray chemiluminescence detection. Four blots represented the supernatants of the following: control resting cells treated with DMSO, cells treated with 2 μM of PL, cell treated with LPS (1 μg/mL) and finally supernatant of LPS-activated cells after exposure to PL. The most affected cytokines are designated as follows: 1, MCP-1; 2, IL-6; 3, MCP-5; 4, G-CSF; 5, IL-12 p40/p70; 6, IL-1α.
Fig. 5.
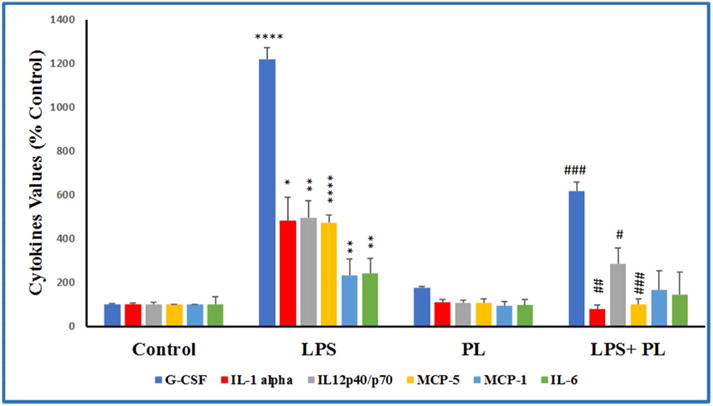
The effect of plumbagin on cytokines release in LPS-activated microglial cells. The normalized data showed cytokines expression in four sets of experimental cells supernatants; DMSO-treated control cell, PL-treated cells (2 μM), LPS-stimulated cells (1 μg/mL), and LPS + PL-treated cells (1 μg/mL + 2 μM, respectively). Three independent studies were performed with n = 6, and the intensities were expressed as % control. The significance of the difference was determined by unpaired t-test between resting vs. LPS-activated cells (*), as well as LPS-treated vs. LPS + PL-treated cells (#). Significance is considered at * p < 0.05, ** p < 0.01, **** p < 0.0001, # p < 0.05, ## p < 0.01 ### p < 0.001.
To further validate the obtained array results, six independent ELISA studies were conducted to quantify the six cytokines inhibited by PL (Fig. 6). Overall, the data showed consistency with that of the array observation, and a significant relationship was obtained between resting vs. LPS-treated as well as between LPS vs. LPS + PL treated cells. Among all studied cytokines/chemokines, IL-1α was highly increased in the supernatant of LPS –activated cells followed by 80% inhibition in LPS-PL co-treated cells supernatant. The other cytokines were inhibited as following; G-CSF (58%) > IL-12 p40/p70 (55%) > MCP-5 (48%) > MCP-1 (34%) > IL-6 (23%).
Fig. 6.
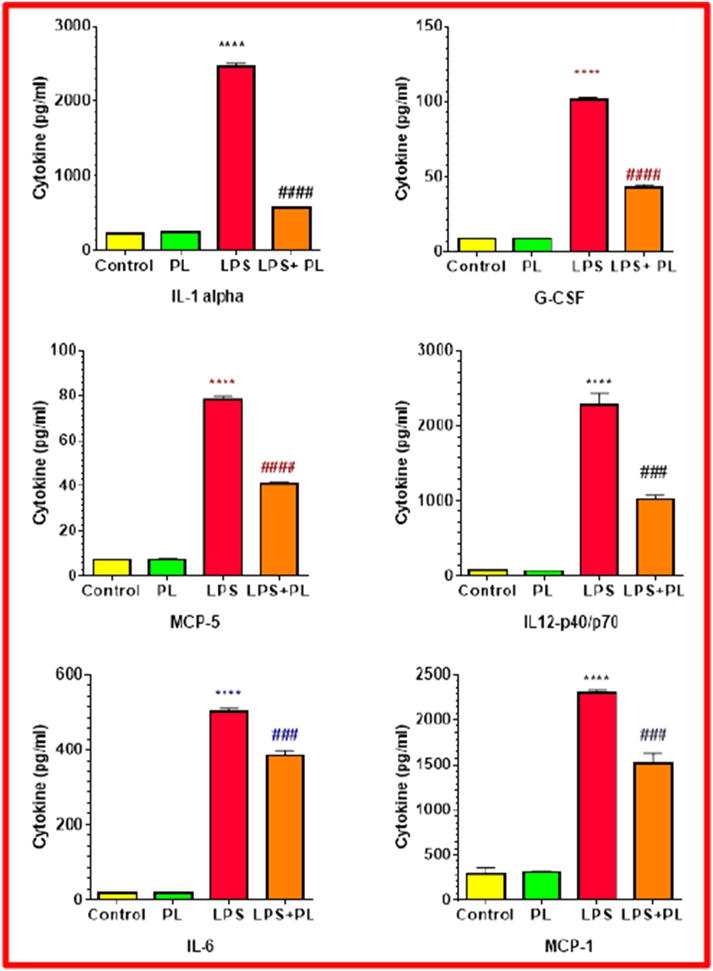
The effect of plumbagin on cytokine release levels in LPS-activated BV-2 microglial cells. Cytokines quantification was determined (pg/mL) in the supernatant of DMSO treated control cell, PL–treated cells (2 μM), LPS–stimulated cells (1 μg/mL), and LPS-PL co-treated cells. Two independent studies were performed with n = 6, and the significance of the difference was determined by unpaired t-test between resting vs. LPS-activated cells (*), as well as LPS-treated vs. LPS + PL-treated cells (#). Significance is considered at **** p < 0.0001, ### p < 0.001, and #### p < 0.0001.
4. Discussion
Inflammation associated with neurodegeneration can occur in many progressive brain diseases, such as AD, PD, HD, ALS, and multiple sclerosis (MS) (Jellinger and Stadelmann, 2001). In AD, for example, activated microglia and inflammatory cytokines are critical contributors to the pathogenesis of this disease. Similarly, in animal models of PD, the exposure to the toxic agent in the brain may lead to chronic microglial cell activation that continuously releases cytokines even after the exposure is stopped, leading to progressive dopaminergic (DA) neurons loss (Block and Hong, 2007).
Microglial cell activation is a hallmark of inflammation and pathology in the brain (Dheen et al., 2007). The microglial cells are encompassing 12% of the brain cells, and their unique morphology makes them different from other glia and neurons (Ginhoux et al., 2013). Microglial cells can act both as neuroprotective and neurotoxic (Block and Hong, 2005). It performs pivotal functions in response to immunological stimuli and brain injury, which also contribute to inflammation-mediated neurodegeneration. When microglial cells are activated, they release cytotoxic pro-inflammatory factors such as nitric oxide (NO), cytokines/chemokines, and reactive oxygen species (ROS) (Kreutzberg, 1996). The BV-2 microglial model is well established one for neurodegeneration research (Henn et al., 2009). The BV-2 cells can be activated by lipopolysaccharides, LPS, and the enzyme iNOS, in particular, is a microglial activation indicator (Jellinger and Stadelmann, 2001). Activated microglia cells induce iNOS synthesis that generates NO, which is essential for host defenses in response to external foreign bodies or tissue damage (Asiimwe et al., 2016; Moncada et al., 1991). The increasing levels of NO production lead to neuronal respiration inhibition and glutamate release, which might lead to the excitotoxic death of neurons and tissue damage (Bal-Price and Brown, 2001). Also, excessive cytokines production results in collateral damage to proteins, lipids/membranes and DNA of the host tissue (Sandur et al., 2010). Thus, inhibiting the iNOS, NO, and consequential pro-inflammatory cytokines is essential for neuroprotection and halting the neurodegeneration progression.
The obtained data showed the ability of PL to inhibit iNOS synthesis as well as its NO product in a dose-dependent manner. PL did not affect the viability of the microglial cells up to 2 μM concentration. Although we did not examine the mechanism of the toxic effect of plumbagin, we have previously reported that most of natural compounds in higher concentrations can lead to cell death through apoptosis (Elsisi et al., 2005). Meanwhile, the current investigation indicates that L-NIL has no toxic effect using concentrations up to 100 μM since L-NIL is a very specific inhibitor for iNOS with minimum cytotoxicity. Consistently, previous studies on lymphocytes indicated that PL was safe up to 5 μM (Checker et al., 2009). On the other hand, the inhibitory potency of the natural compound PL was higher than the selective iNOS inhibitor, L-NIL. Our findings are consistent with a recent study on PL as an inhibitor of NO and iNOS expression in human osteoarthritis chondrocytes (Zheng et al., 2017). The inhibition of NO production by suppressing iNOS is one of the major pathways for anti-inflammatory effect of many drugs (Luo et al., 2010).
Many studies reported that cytokines are associated with increased cognitive decline and dementia (van Exel et al., 2003; J. D. Weaver et al., 2002). In AD, the most potent inflammatory cytokines, IL-1α, IL-1β and IL-6 produced by activated microglia and astrocytes are up-regulated (McGeer et al., 2002) and play a significant role in the disease progression via Aβ accumulation (Reale et al., 2009). Likewise, IL-1β, IL-6, and TNF-α are found to be high in the basal ganglia and cerebrospinal fluid (CSF) in PD. Aggravated concentrations of these cytokines can be the leading cause of neurodegeneration. Therefore, downregulating these pro-inflammatory mediators are crucial in managing these diseases. As an indicator of inflammation, elevated levels of IL-1α, G-CSF, IL-12 p40/p70, MCP-5, MCP-1, and IL-6 were detected in LPS-activated BV-2 cells (Figs. 4–6). Strikingly, PL attenuated the expression of these cytokines with IL-1α being the most inhibited in both array and ELISA. Previous in-vivo and in-vitro studies have shown the effectiveness of PL to significantly inhibit cytokines release (Checker et al., 2009) and presumably inhibiting inflammation and collagen production (H. Wang et al., 2016) Therefore, down-regulating these pro-inflammatory mediators by PL could be a promising approach in managing neurodegenerative diseases.
Meanwhile, the pro-inflammatory cytokine IL-1α is critical in regulating the immune response, and it plays a major role in elderly neurodegenerative diseases (Wu et al., 2007). In our investigation, the natural compound PL could attenuate IL-1α expression by at least 80% (Fig. 6). Both IL-1α and IL-1β are IL-1 isoforms that cause both neurotoxic and neuroprotective effects (Wu et al., 2007), stimulating the expression of genes associated with inflammation and autoimmune diseases (Di Paolo and Shayakhmetov, 2016; Dinarello, 2002, 2009). Moreover, IL-1α can trigger early onset of AD, the most prevalent neurodegenerative disease (Combarros et al., 2007; T. Wang et al., 2014) and initiate inflammation. Nevertheless, inflammation can be sustained by both IL-1α and IL-1β isoforms (Rider et al., 2011). Furthermore, extensive studies on AD emphasized the role of cytokine genes and DNA polymorphism (Grimaldi et al., 2000; Sheng et al., 1996; Yildiz et al., 2015). Therefore, individuals with IL-1α TT–889 genotypes and a second polymorphism in IL-1β – 511 genes were at high risk of developing AD ten times compared with those who did not have either of these polymorphisms (Grimaldi et al., 2000). Likewise in PD, IL-1α with IL-1β acted synergistically in the disease susceptibility (Wu et al., 2007; Zhou et al., 2008).
Also, cytokines IL-6, MCP-1, and MCP-5 are highly expressed in many neurodegenerative disorders (Bose and Cho, 2013; J. D. Weaver et al., 2002). A previous in-vivo study showed elevated levels of MCP-1 and MCP-5 mRNA in focal brain damage (Sun et al., 2000). In the current study, PL significantly inhibited both cytokines (Figs. 5 and 6). MCP-5 was found to have an indirect role in AD tauopathies (Garwood et al., 2010). Elevated MCP-1 levels were identified in the senile plaques of AD patients (Conductier et al., 2010; Sokolova et al., 2009), PD patients, and in severe depression (Reale et al., 2009). On the other hand, IL-6 cytokine, a glycoprotein produced by TH17 cells triggered inflammatory cellular responses, neurogenesis, and gliogenesis in the CNS (Spooren et al., 2011; C. T. Weaver and Murphy, 2007). Our data showed that PL significantly attenuated IL-6 (Figs. 5 and 6). Consistently, a previous in-vivo study proved the significant anti-in-flammatory effect of PL through IL-6 inhibition (Checker et al., 2009). Accordingly, the findings supported our hypothesis by confirming PL anti-inflammatory role through inhibiting multiple cytokines expressions.
Evidence was provided to indicate that IL-12 is involved in the pathogenesis of AD (Zhu et al., 2014) and MS (Nicoletti et al., 1996). Parallel to our finding (Figs. 5 and 6), LPS strongly induced primary microglial production of interleukin-12 (IL-12) (Xu et al., 2007; Zhu et al., 2014). In the brain, microglia produced IL-12 that can initiate Th1-type cytokines and further aggravate pro-inflammatory response in the Th1 cells (Sonobe et al., 2005). Although it is controversy, iNOS inhibitor was found to decrease the production of IL-12 (Hogaboam et al., 1997), the finding that might explain the effect of PL on IL-12 p40/p70 inhibition in the current investigation. Likewise, PL significantly inhibited G-CSF expression (Figs. 5 and 6). High level of the cytokine was found to play a role in the pathogenesis of inflammatory arthritis through elevating neutrophil levels (Eyles et al., 2008), where it was correlated with disease activity and severity (Nakamura et al., 2000). Interestingly, mice deficient in G-CSF are highly resistant to collagen–induced arthritis, whereas the administration of G-CSF antibodies to mice after the onset of the disease prevents its progression. (Lawlor et al., 2004).
Selective NO biosynthesis inhibitors and synthetic arginine analogs are used for the treatment of NO-induced inflammation (Sharma et al., 2007). However, the currently available therapy does not prevent the progression of neurodegenerative disease. PL is a natural compound that was investigated for its neuroprotective and anti-inflammatory effects. The neuroprotective effect of PL has been explained by its potential to activate Nrf2 (Chu et al., 2016; Son et al., 2010). Up-regulated Nrf2 protects the brain against blood-brain barrier breakdown (Alfieri et al., 2011), spinal cord injury (Zhang et al., 2015) and cerebral ischemia (Son et al., 2010). Therefore, Nrf2 may have a protective role in neurodegenerative diseases, including PD (Choi et al., 2012; Cuadrado et al., 2009), AD (Kanninen et al., 2008), and HD (Stack et al., 2010) as well as traumatic brain injury (Yan et al., 2008). Also, in-vivo and in-vitro studies indicated the potent anti-inflammatory and protective effects of PL by inhibiting nitric oxide production, iNOS expression, prostaglandin-E2 production, TNF-α, IL-1β, IL-6, as well as an NF-κB release in mice and RAW 264.7 macrophage cells (Checker et al., 2014; Luo et al., 2010; T. Wang et al., 2014),
In conclusion, the results of this study indicate that PL proves to be effective in reducing, nitric oxide, MCP-1, IL-6, MCP-5, G-CSF, IL-12 p40/p70; and IL-1α levels. These results indicate that PL reduces microglia – pro-inflammatory agents and may be useful in delaying the onset or attenuating the progression of microglia-derived neurodegeneration such as AD. The current study points out to the need for further investigation of the efficacy of PL in AD animal models as a possible therapeutic agent.
Acknowledgments
This research was supported by NIH-national Institute of Minority Health and Health Disparity Grants G12 MD007582 and P20 MD 006738.
Footnotes
Competing interest
Authors have declared that no competing interests exist.
References
- Ahmad A, Banerjee S, Wang Z, Kong D, Sarkar FH. Plumbagin-induced apoptosis of human breast cancer cells is mediated by inactivation of NF-kappaB and Bcl-2. J Cell Biochem. 2008;105:1461–1471. doi: 10.1002/jcb.21966. [DOI] [PubMed] [Google Scholar]
- Alfieri A, Srivastava S, Siow RC, Modo M, Fraser PA, Mann GE. Targeting the Nrf2-Keap1 antioxidant defence pathway for neurovascular protection in stroke. J Physiol. 2011;589:4125–4136. doi: 10.1113/jphysiol.2011.210294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appadurai P, Rathinasamy K. Plumbagin-silver nanoparticle formulations enhance the cellular uptake of plumbagin and its antiproliferative activities. IET Nanobiotechnol. 2015;9:264–272. doi: 10.1049/iet-nbt.2015.0008. [DOI] [PubMed] [Google Scholar]
- Asiimwe N, Yeo SG, Kim MS, Jung J, Jeong NY. Nitric oxide: exploring the contextual link with Alzheimer’s disease. Oxidative Med Cell Longev. 2016;2016:7205747. doi: 10.1155/2016/7205747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz MH, Dreckschmidt NE, Verma AK. Plumbagin, a medicinal plant-derived naphthoquinone, is a novel inhibitor of the growth and invasion of hormone-refractory prostate cancer. Cancer Res. 2008;68:9024–9032. doi: 10.1158/0008-5472.CAN-08-2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal-Price A, Brown GC. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J Neurosci. 2001;21:6480–6491. doi: 10.1523/JNEUROSCI.21-17-06480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27:229–237. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Block ML, Hong JS. Chronic microglial activation and progressive dopaminergic neurotoxicity. Biochem Soc Trans. 2007;35:1127–1132. doi: 10.1042/BST0351127. [DOI] [PubMed] [Google Scholar]
- Bose S, Cho J. Role of chemokine CCL2 and its receptor CCR2 in neurodegenerative diseases. Arch Pharm Res. 2013;36:1039–1050. doi: 10.1007/s12272-013-0161-z. [DOI] [PubMed] [Google Scholar]
- Checker R, Sharma D, Sandur SK, Khanam S, Poduval TB. Anti-in-flammatory effects of plumbagin are mediated by inhibition of NF-kappaB activation in lymphocytes. Int Immunopharmacol. 2009;9:949–958. doi: 10.1016/j.intimp.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Checker R, Patwardhan RS, Sharma D, Menon J, Thoh M, Sandur SK, Sainis KB, Poduval TB. Plumbagin, a vitamin K3 analogue, abrogates lipopolysaccharide-induced oxidative stress, inflammation and endotoxic shock via NF-kappaB suppression. Inflammation. 2014;37:542–554. doi: 10.1007/s10753-013-9768-y. [DOI] [PubMed] [Google Scholar]
- Chen CA, Chang HH, Kao CY, Tsai TH, Chen YJ. Plumbagin, isolated from Plumbago zeylanica, induces cell death through apoptosis in human pancreatic cancer cells. Pancreatology. 2009;9:797–809. doi: 10.1159/000210028. [DOI] [PubMed] [Google Scholar]
- Choi SY, Son TG, Park HR, Jang YJ, Oh SB, Jin B, Lee J. Naphthazarin has a protective effect on the 1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine-induced Parkinson’s disease model. J Neurosci Res. 2012;90:1842–1849. doi: 10.1002/jnr.23061. [DOI] [PubMed] [Google Scholar]
- Chu H, Yu H, Ren D, Zhu K, Huang H. Plumbagin exerts protective effects in nucleus pulposus cells by attenuating hydrogen peroxide-induced oxidative stress, inflammation and apoptosis through NF-kappaB and Nrf-2. Int J Mol Med. 2016;37:1669–1676. doi: 10.3892/ijmm.2016.2564. [DOI] [PubMed] [Google Scholar]
- Combarros O, Llorca J, Sanchez-Juan P, Mateo I, Infante J, Rodriguez E, Sanchez-Quintana C, Berciano J. Interaction between prion protein and interleukin-1A genes increases early-onset Alzheimer’s disease risk. J Neurol. 2007;254:115–117. doi: 10.1007/s00415-006-0291-z. [DOI] [PubMed] [Google Scholar]
- Conductier G, Blondeau N, Guyon A, Nahon JL, Rovere C. The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. J Neuroimmunol. 2010;224:93–100. doi: 10.1016/j.jneuroim.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Cuadrado A, Moreno-Murciano P, Pedraza-Chaverri J. The transcription factor Nrf2 as a new therapeutic target in Parkinson’s disease. Expert Opin Ther Targets. 2009;13:319–329. doi: 10.1517/13543780802716501. [DOI] [PubMed] [Google Scholar]
- Dheen ST, Kaur C, Ling EA. Microglial activation and its implications in the brain diseases. Curr Med Chem. 2007;14:1189–1197. doi: 10.2174/092986707780597961. [DOI] [PubMed] [Google Scholar]
- Dhingra D, Bansal S. Antidepressant-like activity of plumbagin in unstressed and stressed mice. Pharmacol Rep. 2015;67:1024–1032. doi: 10.1016/j.pharep.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Di Paolo NC, Shayakhmetov DM. Interleukin 1alpha and the inflammatory process. Nat Immunol. 2016;17:906–913. doi: 10.1038/ni.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. The IL-1 family and inflammatory diseases. Clin Exp Rheumatol. 2002;20:S1–13. [PubMed] [Google Scholar]
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Elsisi NS, Darling-Reed S, Lee EY, Oriaku ET, Soliman KF. Ibuprofen and apigenin induce apoptosis and cell cycle arrest in activated microglia. Neurosci Lett. 2005;375:91–96. doi: 10.1016/j.neulet.2004.10.087. [DOI] [PubMed] [Google Scholar]
- van Exel E, de Craen AJ, Remarque EJ, Gussekloo J, Houx P, Bootsma-van der Wiel A, Frolich M, Macfarlane PW, Blauw GJ, Westendorp RG. Interaction of atherosclerosis and inflammation in elderly subjects with poor cognitive function. Neurology. 2003;61:1695–1701. doi: 10.1212/01.wnl.0000098877.07653.7c. [DOI] [PubMed] [Google Scholar]
- Eyles JL, Hickey MJ, Norman MU, Croker BA, Roberts AW, Drake SF, James WG, Metcalf D, Campbell IK, Wicks IP. A key role for G-CSF-induced neutrophil production and trafficking during inflammatory arthritis. Blood. 2008;112:5193–5201. doi: 10.1182/blood-2008-02-139535. [DOI] [PubMed] [Google Scholar]
- Garwood CJ, Cooper JD, Hanger DP, Noble W. Anti-inflammatory impact of minocycline in a mouse model of tauopathy. Front Psychiatry. 2010;1:136. doi: 10.3389/fpsyt.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Lim S, Hoeffel G, Low D, Huber T. Origin and differentiation of microglia. Front Cell Neurosci. 2013;7:45. doi: 10.3389/fncel.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomathinayagam R, Sowmyalakshmi S, Mardhatillah F, Kumar R, Akbarsha MA, Damodaran C. Anticancer mechanism of plumbagin, a natural compound, on non-small cell lung cancer cells. Anticancer Res. 2008;28:785–792. [PubMed] [Google Scholar]
- Grimaldi LM, Casadei VM, Ferri C, Veglia F, Licastro F, Annoni G, Biunno I, De Bellis G, Sorbi S, Mariani C, Canal N, Griffin WS, Franceschi M. Association of early-onset Alzheimer’s disease with an interleukin-1alpha gene polymorphism. Ann Neurol. 2000;47:361–365. [PubMed] [Google Scholar]
- Hafeez BB, Jamal MS, Fischer JW, Mustafa A, Verma AK. Plumbagin, a plant derived natural agent inhibits the growth of pancreatic cancer cells in in vitro and in vivo via targeting EGFR, Stat3 and NF-kappaB signaling pathways. Int J Cancer. 2012;131:2175–2186. doi: 10.1002/ijc.27478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn A, Lund S, Hedtjarn M, Schrattenholz A, Porzgen P, Leist M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX. 2009;26:83–94. doi: 10.14573/altex.2009.2.83. [DOI] [PubMed] [Google Scholar]
- Hogaboam CM, Chensue SW, Steinhauser ML, Huffnagle GB, Lukacs NW, Strieter RM, Kunkel SL. Alteration of the cytokine phenotype in an experimental lung granuloma model by inhibiting nitric oxide. J Immunol. 1997;159:5585–5593. [PubMed] [Google Scholar]
- Jellinger KA, Stadelmann C. Problems of cell death in neurodegeneration and Alzheimer’s Disease. J Alzheimers Dis. 2001;3:31–40. doi: 10.3233/jad-2001-3106. [DOI] [PubMed] [Google Scholar]
- Jetty A, Subhakar C, Rajagopal D, Jetty M, Subramanyam M, Marthanda Murthy M. Antimicrobial activities of neo- and 1-epineo-isoshinanolones from Plumbago zeylanica roots. Pharm Biol. 2010;48:1007–1011. doi: 10.3109/13880200903433760. [DOI] [PubMed] [Google Scholar]
- Kanninen K, Malm TM, Jyrkkanen HK, Goldsteins G, Keksa-Goldsteine V, Tanila H, Yamamoto M, Yla-Herttuala S, Levonen AL, Koistinaho J. Nuclear factor erythroid 2-related factor 2 protects against beta amyloid. Mol Cell Neurosci. 2008;39:302–313. doi: 10.1016/j.mcn.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Khaw AK, Sameni S, Venkatesan S, Kalthur G, Hande MP. Plumbagin alters telomere dynamics, induces DNA damage and cell death in human brain tumour cells. Mutat Res Genet Toxicol Environ Mutagen. 2015;793:86–95. doi: 10.1016/j.mrgentox.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Kielian T. Toll-like receptors in central nervous system glial inflammation and homeostasis. J Neurosci Res. 2006;83:711–730. doi: 10.1002/jnr.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Kuo PL, Hsu YL, Cho CY. Plumbagin induces G2-M arrest and autophagy by inhibiting the AKT/mammalian target of rapamycin pathway in breast cancer cells. Mol Cancer Ther. 2006;5:3209–3221. doi: 10.1158/1535-7163.MCT-06-0478. [DOI] [PubMed] [Google Scholar]
- Lawlor KE, Campbell IK, Metcalf D, O’Donnell K, van Nieuwenhuijze A, Roberts AW, Wicks IP. Critical role for granulocyte colony-stimulating factor in inflammatory arthritis. Proc Natl Acad Sci U S A. 2004;101:11398–11403. doi: 10.1073/pnas.0404328101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lull ME, Block ML. Microglial activation and chronic neurodegeneration. Neurotherapeutics. 2010;7:354–365. doi: 10.1016/j.nurt.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P, Wong YF, Ge L, Zhang ZF, Liu Y, Liu L, Zhou H. Anti-inflammatory and analgesic effect of plumbagin through inhibition of nuclear factor-kappaB activation. J Pharmacol Exp Ther. 2010;335:735–742. doi: 10.1124/jpet.110.170852. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Yasojima K, McGeer EG. Association of interleukin-1 beta polymorphisms with idiopathic Parkinson’s disease. Neurosci Lett. 2002;326:67–69. doi: 10.1016/s0304-3940(02)00300-2. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Nair HA, Snima KS, Kamath RC, Nair SV, Lakshmanan VK. Plumbagin nanoparticles induce dose and pH dependent toxicity on prostate cancer cells. Curr Drug Deliv. 2015;12:709–716. doi: 10.2174/1567201812666150316150033. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Ueki Y, Sakito S, Matsumoto K, Yano M, Miyake S, Tominaga T, Tominaga M, Eguchi K. High serum and synovial fluid granulocyte colony stimulating factor (G-CSF) concentrations in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2000;18:713–718. [PubMed] [Google Scholar]
- Nicoletti F, Patti F, Cocuzza C, Zaccone P, Nicoletti A, Di Marco R, Reggio A. Elevated serum levels of interleukin-12 in chronic progressive multiple sclerosis. J Neuroimmunol. 1996;70:87–90. doi: 10.1016/s0165-5728(96)00101-4. [DOI] [PubMed] [Google Scholar]
- Pinho BR, Sousa C, Valentao P, Andrade PB. Is nitric oxide decrease observed with naphthoquinones in LPS stimulated RAW 264.7 macrophages a beneficial property? PLoS One. 2011;6:e24098. doi: 10.1371/journal.pone.0024098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poosarla ADNR, Athota RR, Sunkara VG. Modulation of T cell proliferation and cytokine response by Plumbagin, extracted from Plumbago zeylanica in collagen induced arthritis. BMC Complement Altern Med. 2011;11:114. doi: 10.1186/1472-6882-11-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reale M, Greig NH, Kamal MA. Peripheral chemocytokine profiles in Alzheimer’s and Parkinson’s diseases. Mini Rev Med Chem. 2009;9:1229–1241. doi: 10.2174/138955709789055199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider P, Carmi Y, Guttman O, Braiman A, Cohen I, Voronov E, White MR, Dinarello CA, Apte RN. IL-1alpha and IL-1beta recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol. 2011;187:4835–4843. doi: 10.4049/jimmunol.1102048. [DOI] [PubMed] [Google Scholar]
- Sandur SK, Pandey MK, Sung B, Aggarwal BB. 5-hydroxy-2-methyl-1,4-naphthoquinone, a vitamin K3 analogue, suppresses STAT3 activation pathway through induction of protein tyrosine phosphatase, SHP-1: potential role in chemosensitization. Mol Cancer Res. 2010;8:107–118. doi: 10.1158/1541-7786.MCR-09-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 2007;15:252–259. doi: 10.1007/s10787-007-0013-x. [DOI] [PubMed] [Google Scholar]
- Sheeja E, Joshi SB, Jain DC. Bioassay-guided isolation of anti-inflammatory and antinociceptive compound from Plumbago zeylanica leaf. Pharm Biol. 2010;48:381–387. doi: 10.3109/13880200903156424. [DOI] [PubMed] [Google Scholar]
- Sheng JG, Ito K, Skinner RD, Mrak RE, Rovnaghi CR, Van Eldik LJ, Griffin WS. In vivo and in vitro evidence supporting a role for the inflammatory cytokine interleukin-1 as a driving force in Alzheimer pathogenesis. Neurobiol Aging. 1996;17:761–766. doi: 10.1016/0197-4580(96)00104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolova A, Hill MD, Rahimi F, Warden LA, Halliday GM, Shepherd CE. Monocyte chemoattractant protein-1 plays a dominant role in the chronic inflammation observed in Alzheimer’s disease. Brain Pathol. 2009;19:392–398. doi: 10.1111/j.1750-3639.2008.00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son TG, Camandola S, Arumugam TV, Cutler RG, Telljohann RS, Mughal MR, Moore TA, Luo W, Yu QS, Johnson DA, Johnson JA, Greig NH, Mattson MP. Plumbagin, a novel Nrf2/ARE activator, protects against cerebral ischemia. J Neurochem. 2010;112:1316–1326. doi: 10.1111/j.1471-4159.2009.06552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonobe Y, Yawata I, Kawanokuchi J, Takeuchi H, Mizuno T, Suzumura A. Production of IL-27 and other IL-12 family cytokines by microglia and their sub-populations. Brain Res. 2005;1040:202–207. doi: 10.1016/j.brainres.2005.01.100. [DOI] [PubMed] [Google Scholar]
- Spooren A, Kolmus K, Laureys G, Clinckers R, De Keyser J, Haegeman G, Gerlo S. Interleukin-6, a mental cytokine. Brain Res Rev. 2011;67:157–183. doi: 10.1016/j.brainresrev.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Srinivas P, Gopinath G, Banerji A, Dinakar A, Srinivas G. Plumbagin induces reactive oxygen species, which mediate apoptosis in human cervical cancer cells. Mol Carcinog. 2004;40:201–211. doi: 10.1002/mc.20031. [DOI] [PubMed] [Google Scholar]
- Stack C, Ho D, Wille E, Calingasan NY, Williams C, Liby K, Sporn M, Dumont M, Beal MF. Triterpenoids CDDO-ethyl amide and CDDO-trifluoroethyl amide improve the behavioral phenotype and brain pathology in a transgenic mouse model of Huntington’s disease. Free Radic Biol Med. 2010;49:147–158. doi: 10.1016/j.freeradbiomed.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40:133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- Sun D, Tani M, Newman TA, Krivacic K, Phillips M, Chernosky A, Gill P, Wei T, Griswold KJ, Ransohoff RM, Weller RO. Role of chemokines, neuronal projections, and the blood-brain barrier in the enhancement of cerebral EAE following focal brain damage. J Neuropathol Exp Neurol. 2000;59:1031–1043. doi: 10.1093/jnen/59.12.1031. [DOI] [PubMed] [Google Scholar]
- Sunil C, Duraipandiyan V, Agastian P, Ignacimuthu S. Antidiabetic effect of plumbagin isolated from Plumbago zeylanica L. root and its effect on GLUT4 translocation in streptozotocin-induced diabetic rats. Food Chem Toxicol. 2012;50:4356–4363. doi: 10.1016/j.fct.2012.08.046. [DOI] [PubMed] [Google Scholar]
- Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taka E, Mazzio EA, Goodman CB, Redmon N, Flores-Rozas H, Reams R, Darling-Reed S, Soliman KF. Anti-inflammatory effects of thymoquinone in activated BV-2 microglial cells. J Neuroimmunol. 2015;286:5–12. doi: 10.1016/j.jneuroim.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thasni KA, Rakesh S, Rojini G, Ratheeshkumar T, Srinivas G, Priya S. Estrogen-dependent cell signaling and apoptosis in BRCA1-blocked BG1 ovarian cancer cells in response to plumbagin and other chemotherapeutic agents. Ann Oncol. 2008;19:696–705. doi: 10.1093/annonc/mdm557. [DOI] [PubMed] [Google Scholar]
- Tilak JC, Adhikari S, Devasagayam TP. Antioxidant properties of Plumbago zeylanica, an Indian medicinal plant and its active ingredient, plumbagin. Redox Rep. 2004;9:219–227. doi: 10.1179/135100004225005976. [DOI] [PubMed] [Google Scholar]
- Wang CC, Chiang YM, Sung SC, Hsu YL, Chang JK, Kuo PL. Plumbagin induces cell cycle arrest and apoptosis through reactive oxygen species/c-Jun N-terminal kinase pathways in human melanoma A375.S2 cells. Cancer Lett. 2008;18:82–98. doi: 10.1016/j.canlet.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Wang T, Wu F, Jin Z, Zhai Z, Wang Y, Tu B, Yan W, Tang T. Plumbagin inhibits LPS-induced inflammation through the inactivation of the nuclear factor-kappa B and mitogen activated protein kinase signaling pathways in RAW 264.7 cells. Food Chem Toxicol. 2014;64:177–183. doi: 10.1016/j.fct.2013.11.027. [DOI] [PubMed] [Google Scholar]
- Wang F, Wang Q, Zhou ZW, Yu SN, Pan ST, He ZX, Zhang X, Wang D, Yang YX, Yang T, Sun T, Li M, Qiu JX, Zhou SF. Plumbagin induces cell cycle arrest and autophagy and suppresses epithelial to mesenchymal transition involving PI3K/Akt/mTOR-mediated pathway in human pancreatic cancer cells. Drug Des Dev Ther. 2015;9:537–560. doi: 10.2147/DDDT.S73689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang H, Zhang Y, Wang D, Cheng X, Yang F, Zhang Q, Xue Z, Li Y, Zhang L, Yang L, Miao G, Li D, Guan Z, Da Y, Yao Z, Gao F, Qiao L, Kong L, Zhang R. Plumbagin protects liver against fulminant hepatic failure and chronic liver fibrosis via inhibiting inflammation and collagen production. Oncotarget. 2016;13:82864–82875. doi: 10.18632/oncotarget.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CT, Murphy KM. The central role of the Th17 lineage in regulating the inflammatory/autoimmune axis. Semin Immunol. 2007;19:351–352. doi: 10.1016/j.smim.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59:371–378. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- Wu YR, Chen CM, Hwang JC, Chen ST, Feng IH, Hsu HC, Liu CN, Liu YT, Lai YY, Huang HJ, Lee-Chen GJ. Interleukin-1 alpha polymorphism has an influence on late-onset sporadic Parkinson’s disease in Taiwan. J Neural Transm. 2007;114:1173–1177. doi: 10.1007/s00702-007-0726-4. [DOI] [PubMed] [Google Scholar]
- Xu J, Racke MK, Drew PD. Peroxisome proliferator-activated receptor-alpha agonist fenofibrate regulates IL-12 family cytokine expression in the CNS: relevance to multiple sclerosis. J Neurochem. 2007;103:1801–1810. doi: 10.1111/j.1471-4159.2007.04875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Wang HD, Hu ZG, Wang QF, Yin HX. Activation of the Nrf2-ARE pathway in the brain after traumatic brain injury. Neurosci Lett. 2008;431:150–154. doi: 10.1016/j.neulet.2007.11.060. [DOI] [PubMed] [Google Scholar]
- Yildiz SH, Erdogan MO, Artan S, Solak M, Yaman M, Ozbabalik BD, Terzi ES. Association of Alzheimer’s disease with APOE and IL-1alpha gene polymorphisms. Am J Alzheimers Dis Other Dement. 2015;30:756–761. doi: 10.1177/1533317512461557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Cheng L, Hou Y, Si M, Zhao YP, Nie L. Plumbagin protects against spinal cord injury-induced oxidative stress and inflammation in wistar rats through Nrf-2 upregulation. Drug Res (Stuttgart) 2015;65:495–499. doi: 10.1055/s-0034-1389950. [DOI] [PubMed] [Google Scholar]
- Zheng W, Tao Z, Chen C, Zhang C, Zhang H, Ying X, Chen H. Plumbagin prevents IL-1beta-induced inflammatory response in human osteoarthritis chondrocytes and prevents the progression of osteoarthritis in mice. Inflammation. 2017;40:849–860. doi: 10.1007/s10753-017-0530-8. [DOI] [PubMed] [Google Scholar]
- Zhou YT, Yang JF, Zhang YL, Wang XY, Chan P. Protective role of interleukin-1 alpha gene polymorphism in Chinese Han population with sporadic Parkinson’s disease. Neurosci Lett. 2008;445:23–25. doi: 10.1016/j.neulet.2008.08.054. [DOI] [PubMed] [Google Scholar]
- Zhu XC, Tan L, Jiang T, Tan MS, Zhang W, Yu JT. Association of IL-12A and IL-12B polymorphisms with Alzheimer’s disease susceptibility in a Han Chinese population. J Neuroimmunol. 2014;274:180–184. doi: 10.1016/j.jneuroim.2014.06.026. [DOI] [PubMed] [Google Scholar]


