Abstract
Proliferation of glomerular epithelial cells, including the podocytes, is a key histologic feature of crescentic glomerulonephritis. We previously found that retinoic acid (RA) inhibits proliferation and induces differentiation of podocytes by activating RA receptor-α (RARα) in a murine model of HIV-associated nephropathy. Here, we examined whether RA would similarly protect podocytes against nephrotoxic serum-induced crescentic glomerulonephritis and whether this effect was mediated by podocyte RARα. RA treatment markedly improved renal function and reduced the number of crescentic lesions in nephritic wildtype mice, while this protection was largely lost in mice with podocyte-specific ablation of Rara (Pod-Rara knockout). At a cellular level, RA significantly restored the expression of podocyte differentiation markers in nephritic wildtype mice, but not in nephritic Pod-Rara knockout mice. Furthermore, RA suppressed the expression of cell injury, proliferation, and parietal epithelial cell markers in nephritic wildtype mice, all of which were significantly dampened in nephritic Pod-Rara knockout mice. Interestingly, RA treatment led to coexpression of podocyte and parietal epithelial cell markers in a small subset of glomerular cells in nephritic mice, suggesting that RA may induce transdifferentiation of parietal epithelial cells towards a podocyte phenotype. In vitro, RA directly inhibited the proliferation of parietal epithelial cells and enhanced the expression of podocyte markers. In vivo lineage-tracing of labeled parietal epithelial cells confirmed that RA increased the number of parietal epithelial cells expressing podocyte markers in nephritic glomeruli. Thus, RA attenuates crescentic glomerulonephritis primarily through RARα-mediated protection of podocytes and in part through the inhibition of parietal epithelial cell proliferation and induction of their transdifferentiation into podocytes.
Keywords: Crescentic glomerulonephritis, retinoic acid receptor-alpha, podocytes, parietal epithelial cells, proliferation, transdifferentiation
INTRODUCTION
Crescentic glomerulonephritis (GN), characterized by multilayered accumulation of proliferating cells in the Bowman’s space, is a rapidly progressive disease leading to end stage renal failure. Immunosuppressive therapy, which carries a significant side effect profile, is the mainstay of treatment for GN. Thus, there is a need to identify more specific therapy with less significant side effects for GN. While podocytes are terminally differentiated quiescent cells in normal kidneys, proliferative cells in the crescents were shown to express podocyte-specific markers in the murine model of anti-glomerular basement membrane (GBM) GN 1,2 and in human crescentic GN, where the cells also co-expressed cell proliferation markers 3, suggesting that proliferative podocytes are a component of cellular crescents in GN. A more convincing evidence for podocyte involvement in crescents is that mice with podocyte-specific deletion of the von Hippel-Lindau gene specifically develop crescentic GN with proliferative podocytes 4, suggesting that podocyte proliferation caused by genetic manipulation can result in kidney disease similar to crescentic GN. Indeed, inhibition of podocyte proliferation improves renal function in the murine model of crescentic GN 5.
However, recent lineage-tracing experiments demonstrated that glomerular parietal epithelial cells (PECs) are a major component of crescents in GN and pseudo-crescents in collapsing glomerulosclerosis 6. Studies also suggest that PEC injury leads to cellular activation and proliferation of the remaining PECs, which ultimately forms cellular crescents 7. In addition, PECs were found to express stem cell markers and have the ability to transdifferentiate into podocytes 8. Recent data further revealed that while podocyte generation from PECs is mainly confined to the glomerular development period and does not occur in aging kidneys or in response to nephron loss, it may occur after acute glomerular injury 9,10. Although the exact mechanism of cell injury in crescentic GN requires further clarification, it is likely that podocyte injury triggers glomerular cell proliferation and subsequent crescent formation.
Retinoic acids (RA) are derivatives of vitamin A and have multiple cellular functions including inhibition of proliferation, induction of cell differentiation, and inhibition of inflammation 11. In addition to their established benefits in treating a variety of cancers, RA also provides protection in multiple experimental models of kidney disease with podocyte injury 12,13,14,15,16. We previously found that RA reduces proteinuria and glomerulosclerosis in a murine model of HIV-associated nephropathy (HIVAN) where pseudocrescent formation is present 17. RA also inhibits HIV-induced glomerular cell proliferation and preserves the expression of podocyte differentiation markers 17,18. These studies suggest that RA may be an effective therapeutic option against proliferative glomerular diseases, such as crescentic GN and HIVAN.
Retinoids exert their biological effects by binding two families of nuclear receptors, the retinoic acid receptors (RAR) and the retinoid X receptors (RXR), which have distinct cellular functions and are expressed in a variety of tissues including the kidney 12. While all-trans-retinoic acid (atRA) binds to and activates all RAR subtypes (RAR-α, -β, and -γ) 19, our recent data suggest that protective effects atRA against podocyte injury and in improving glomerular disease requires retinoic acid receptor-α (RARα) and that RARα is the most predominant RAR subtype expressed in podocytes 20,21. However, as previous studies were performed using a global deletion of Rara, it was unclear how much of the renoprotection conferred by atRA was mediated directly through the podocytes. In addition, many studies have also shown an important role of regulatory T cells in the pathogenesis of crescentic GN 22, and since atRA can mediate the induction regulatory T cells and inhibition of the differentiation of Th17 cells 23, the beneficial effects of atRA or RAR agonists in vivo may also be in part due to the modulation of immune responses. Therefore, in the current study we sought to delineate the contribution of RA signaling directly on podocytes through podocyte RARα. Using podocyte-specific knockout mice of Rara, our results now show that the marked amelioration kidney injury by RA in the setting of nephrotoxic serum-induced GN (NTS-GN) is largely dependent on the podocyte-specific expression of RARα, underlining the importance of RA’s direct protective effects on podocytes. These renoprotective effects were also associated with inhibition of glomerular cell proliferation and with restoration of differentiated podocyte marker expression. Intriguingly, in addition to sparing the podocytes from injury, RA also induced a simultaneous expression of podocyte and PEC markers in a small subset of the glomerular cells in NTS-GN kidneys, suggesting that these may be transitional cells undergoing transdifferentiation from PECs into podocytes. Taking in vitro and in vivo lineage-tracing approaches, we confirmed that RA indeed enhances the transdifferentiation of PECs into podocytes. Taken together, our study indicates that RA attenuates crescentic GN primarily through RARα-mediated protection of podocytes and in part through inhibiting the proliferation of PECs and enhancing their transdifferentiation into podocytes.
RESULTS
Generation of podocyte-specific RARα-null mice
To generate podocyte-specific RARα-null mice, mice carrying homozygous floxed Rara allele (RARαL2/L2) 24 were crossed with transgenic mice expressing Cre recombinase driven by the podocin promoter (podocin-Cre) 25. PCR analysis of genomic DNA obtained from isolated glomeruli showed a presence of both floxed L2 allele and an excised L- allele (Supplementary Figure 1A and 1B) in the podocyte-specific RARα-null (Pod-Rara−/−) mice, which was not present in the Rara wildtype (Pod-Rara+/+) mice. To further confirm the specific deletion of RARα in podocytes, we isolated primary podocytes from Pod-Rara−/− and Pod-Rara+/+ mice and cultured them in vitro, as previously described 26. Western blot analysis showed the loss of RARα expression in primary podocytes isolated from Pod-Rara−/− compared to Pod-Rara+/+ (Supplementary Figure 1C). Weak residual expression of RARα observed in some primary podocyte samples of Pod-Rara−/− mice may be due to contamination of other glomerular cells during podocyte isolation and/or incomplete excision of floxed Rara in podocytes by podocin-Cre transgene. There were no gross defects observed in Pod-Rara−/− mice, nor changes in their kidney function when observed up to 8 months of age (data not shown).
Podocyte RARα is required for improvement in renal function and cellular crescents in NTS-GN mice following RA treatment
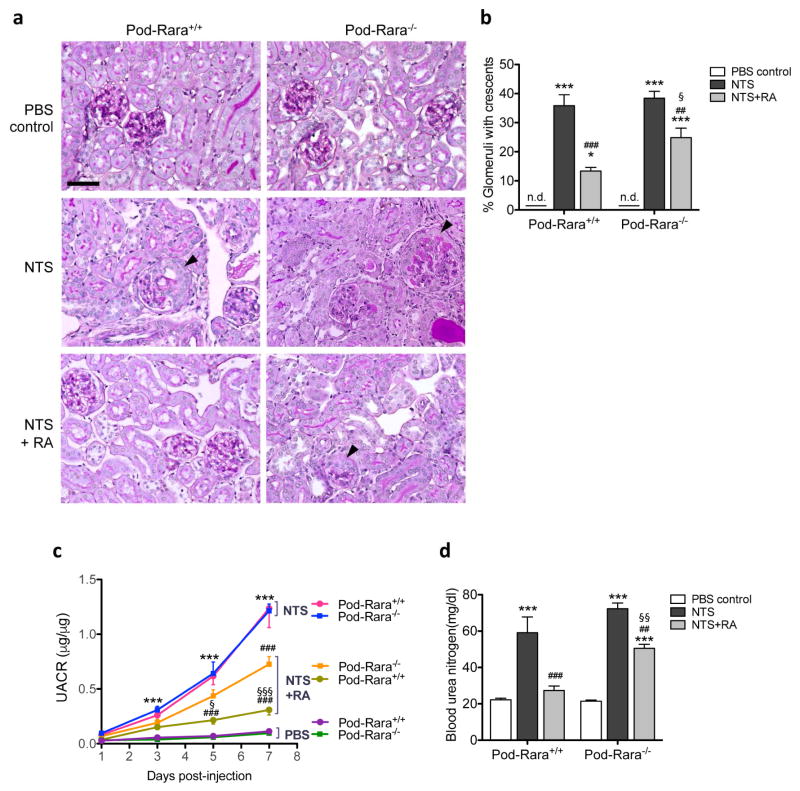
To address the role of RA and RARα in podocytes in glomerular injury, we employed a murine model of NTS-induced glomerulonephritis (NTS-GN). NTS-GN was established in mice at 7 weeks of age by injection of the anti-glomerular basement membrane (anti-GBM) antibody, following the preimmunization with sheep immunoglobulin (IgG) as described previously 27, 28. Control mice were administered phosphate-buffered saline (PBS) vehicle control following preimmunization. To assess whether the administration of RA would be protective against NTS-GN, mice were treated daily with either RA or vehicle, starting one day prior to NTS injection for a total of 7 days. Representative image of their kidney histology is shown in Figure 1A. As anticipated, NTS administration led to glomerular crescent formation in both Pod-Rara+/+ and Pod-Rara−/− mice (Figure 1A, arrowheads; Figure 1B). RA treatment markedly reduced the number of crescents in Pod-Rara+/+ kidneys, but to a much less extent in Pod-Rara−/− kidneys. NTS also induced significant albuminuria (Figure 1C, Supplementary Figure 2) and resulted in declining kidney function, as measured by blood urea nitrogen levels in both Pod-Rara−/− and Pod-Rara+/+ mice (Figure 1D). RA treatment markedly diminished albuminuria and improved renal function in nephritic Pod-Rara+/+ mice, whereas RA had little effects in Pod-Rara−/− mice. We further verified that beneficial effects of RA are not mediated through the suppression of the immune complex deposition, as NTS-induced immune complex deposition (mouse IgG, sheep IgG, and C3) in glomeruli was not different between NTS-injected mice with or without RA treatment (Supplementary Figure 3). Together, these results suggest that RA protects from NTS-induced glomerular injury, which is conferred directly through the activation of podocyte RARα. While much of this protection is lost in Pod-Rara−/− GN mice, nevertheless a small partial protection remained with RA treatment. This may be through a small residual podocyte RARα as observed in some of the cultured primary podocytes (Supplementary Figure 1C), potentially through incomplete ablation of Rara by podocin-Cre. We also cannot exclude the possibility that RA may affect other glomerular cell types, such as PECs, that may contribute to the observed partial protection in Pod-Rara−/− mice. In addition, we also observed that RA administration led to a marked decrease in the presence of infiltrating inflammatory cells in nephritic mice of both genotypes (Supplementary Figures 4–6), suggesting that the immunomodulatory effects of RA also contribute to the partial protection observed in nephritic Pod-Rara−/− mice.
Figure 1. Role of RA and RARα in NTS-induced GN.
(a) Representative images of Periodic acid-Schiff (PAS)-stained kidney sections are shown for Pod-Rara+/+ and Pod-Rara−/− mice injected with vehicle (PBS), NTS only (NTS) or NTS with RA treatment (NTS+RA). Arrowheads show NTS-induced glomerular crescents (magnification: x200, scale bar: 50μm). (b) Quantification of percentage of glomeruli with crescents. (c) Development of proteinuria was assessed by urinary albumin to creatinine ratio (UACR). (d) Measurement of blood urea nitrogen levels. (*P<0.05 and ***P<0.001, compared to respective PBS-injected control; ##P<0.01 and ###P<0.001 compared to respective NTS-injected mice; §P<0.05 and §§P<0.01 compared to NTS+RA Pod-Rara+/+; n=6 in each group, n.d., not detected.)
RA restores the expression of podocyte markers and protects against cell injury in NTS-GN
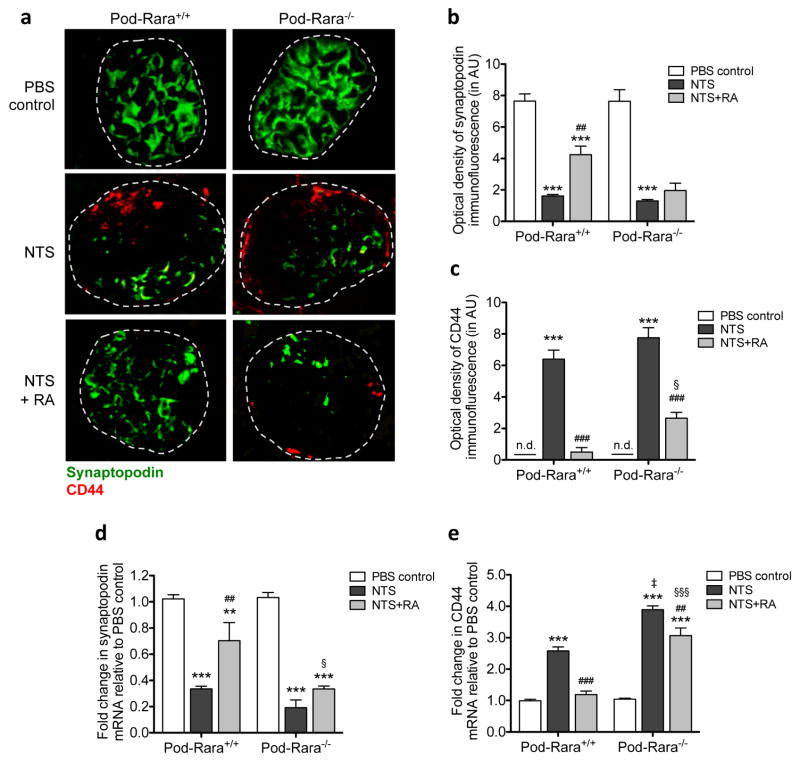
We and others have previously shown that RA reduces markers of proliferation and preserves podocyte-specific differentiation markers both in vitro and in vivo 17,18. Therefore, we next examined whether RA affected the expression of podocyte differentiation markers in NTS-GN and if so, whether this was mediated directly through podocyte RARα. NTS led to a marked reduction in both protein and mRNA expression of synaptopodin in mice of both genotypes (Figure 2A–B, 2D). While RA treatment significantly restored its expression in nephritic Pod-Rara+/+ mice, this restoration was largely lost in nephritic Pod-Rara−/− glomeruli. We next examined the expression of CD44, which is markedly enhanced in renal injury, including crescentic GN 29–31. There was an apparent increase in both protein and mRNA levels of CD44 in nephritic mice of both genotypes (2A, C, and E). Interestingly, in nephritic Pod-Rara−/− glomeruli mRNA expression of CD44 was significantly greater than in nephritic Pod-Rara+/+ (there was also an observable increase in the immunostaining of CD44, although not statistically significant, which may be due to the limitation of semi-quantitative measurement of immunofluorescent signals). RA treatment significantly suppressed the CD44 mRNA expression in glomeruli of nephritic Pod-Rara+/+ mice, but to a much lesser extent in nephritic Pod-Rara−/− mice, further indicating that podocyte RARα is necessary for the full protective effects of RA against NTS-induced injury.
Figure 2. Role of RA and RARα in the expression of podocyte differentiation and injury markers.
(a) Representative images of synaptopodin (green) and CD44 (red) immunofluorescence are shown (magnification: x400, scale bar: 50μm). (b, c) Quantification of immunofluorescence optical density for synaptopodin (b) and CD44 (c) are shown. AU, arbitrary units. n.d., not detected. (d, e) Real-time PCR analysis for synaptopodin (d) and CD44 (e) from isolated glomeruli are shown. (**P<0.01 and ***P<0.001 compared to respective PBS-injected control; ##P<0.01 and ###P<0.001 compared to respective NTS-injected mice; ‡P<0.001 compared to NTS-injected Pod-Rara+/+; §P<0.05 and §§§<0.001 compared to NTS+RA Pod-Rara+/+. n.d., not detected, n=6 in each group).
RA inhibits cell proliferation in NTS-GN
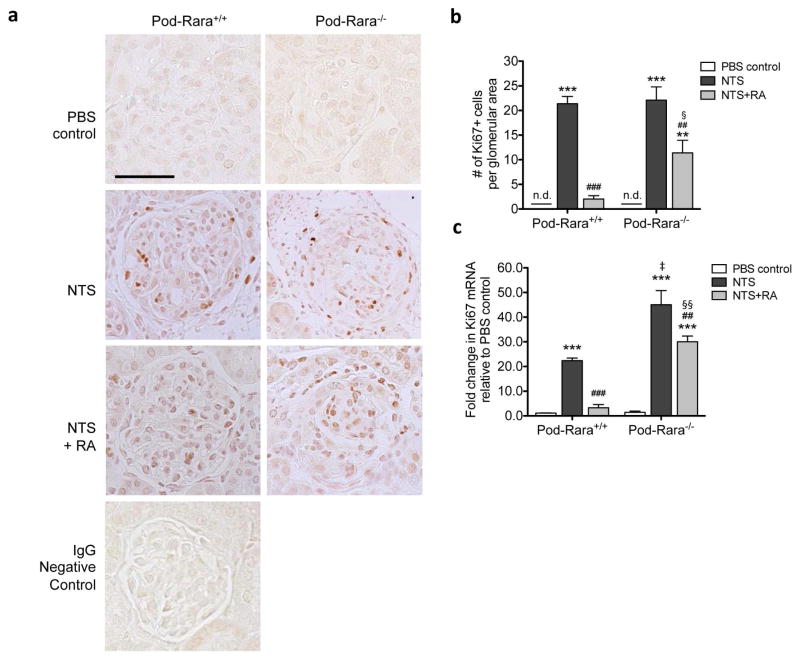
We next sought to determine the effects of RA on cell proliferation that accompanies NTS-GN and its requirement for podocyte RARα. Immunohistological staining revealed numerous Ki-67-positive nuclei in glomeruli of NTS-injected mice compared to vehicle-injected mice (Figure 3A–B). Ki-67 mRNA expression also considerably increased in nephritic glomeruli (Figure 3C). RA treatment led to a near complete repression of glomerular Ki-67 expression in nephritic Pod-Rara+/+ mice, whereas it had only a modest effect in nephritic Pod-Rara−/− mice, suggesting that RA inhibits glomerular cell proliferation through mechanisms that are both dependent and independent on the activation of podocyte RARα. While we cannot conclude which glomerular cell types were Ki-67-positive, the data nonetheless suggest that decreased podocyte injury mediated by RA impacts glomerular cell proliferation in NTS-GN. Consistent with this notion, similar to what was observed with CD44 expression above, Ki-67 mRNA expression in nephritic Pod-Rara−/− glomeruli was significantly greater than in nephritic Pod-Rara+/+, suggesting a potential association between degree of injury and the degree of cell proliferation in GN. In addition, this increase may also be attributable to the endogenous RA ligands that may limit proliferation through binding to RARα in nephritic Pod-Rara+/+ mice, but not in Pod-Rara−/− mice, consistent with earlier reports showing significant role of endogenous RA in glomerular disease 32,33.
Figure 3. Role of RA and RARα in glomerular cell proliferation in NTS-GN.
(a) Immunohistochemical staining of kidney sections for Ki-67 are shown. Representative images of kidneys of mice in each group are shown (magnification: x400, scale bar: 50μm). (b) Quantification of Ki-67+ cells per glomerular cross section. (c) Real-time PCR analysis of Ki-67 from isolated glomeruli is shown. (***P<0.001 compared to respective PBS-injected control; ##P<0.01 and ###P<0.001 compared to respective NTS-injected mice; ‡P<0.001 compared to NTS-injected Pod-Rara+/+; §§P<0.01 compared to NTS+RA Pod-Rara+/+. n.d., not detected, n=6 in each group).
RA reduces the expression of parietal epithelial cell markers in NTS-GN
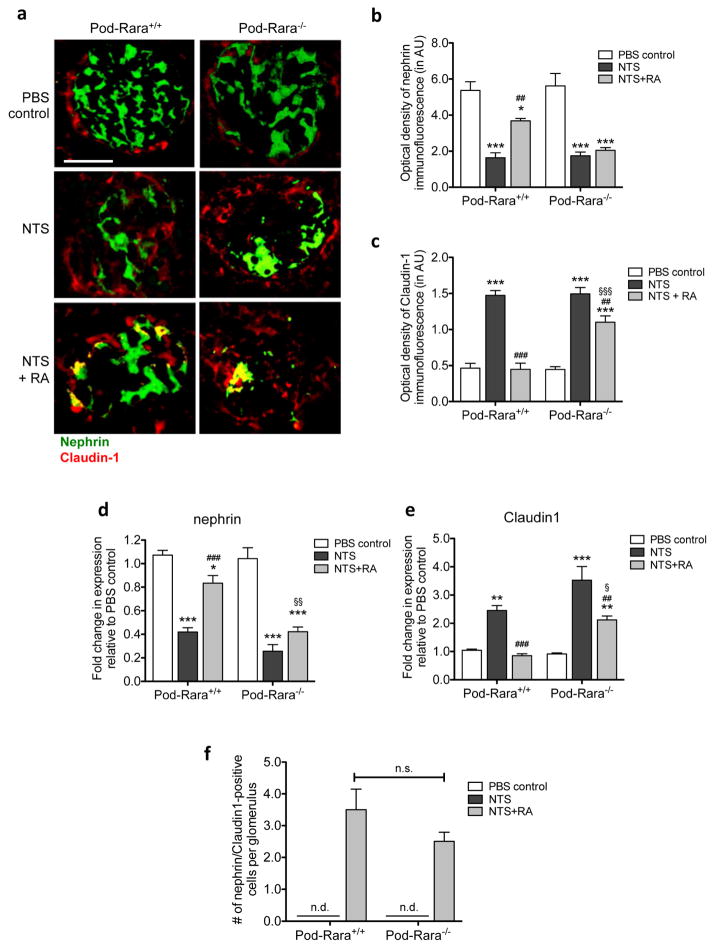
As PECs are shown to be a major component of proliferating cells in crescentic GN, we sought to examine whether PECs contributed to the RA-mediated inhibition of cell proliferation by comparing the expression of Claudin-1 and nephrin, PEC and podocyte markers respectively, in control and nephritic mice. As expected, there was a marked decrease in the expression of nephrin, consistent with the above observation of synaptopodin expression in Figure 2, and conversely a notable increase in Claudin-1 expression in nephritic mice of both genotypes (Figure 4A–C). RA treatment in nephritic Pod-Rara+/+ mice largely restored nephrin expression and effectively prevented the NTS-induced Claudin-1 upregulation. However, RA had no effect on nephrin expression and led to a partial inhibition of Claudin-1 in nephritic Pod-Rara−/− mice. Real-time PCR analyses of nephrin and Claudin-1 in isolated glomeruli showed similar results (Figure 4D–E). These results indicate that RA/RARα signaling on podocytes to prevent its cellular injury, which in turn might prevent PEC proliferation in NTS-GN. However, since RA treatment did partially reduce the expression of Claudin-1 in nephritic Pod-Rara−/− mice, even in ongoing podocyte injury (as observed by lack of recovery in nephrin expression), the data also suggest that there may be a direct effect of RA on PEC proliferation. Interestingly, we observed that a small number of Claudin-1-positive cells were also nephrin-positive, which were found only in the RA-treated GN glomeruli (Figure 4F), whose numbers were not significantly different between RA-treated Pod-Rara+/+ and Pod-Rara−/− mice. We speculated that this might be due to RA’s ability to transdifferentiate PECs towards a podocyte phenotype, independent from RARα function in pre-existing podocytes, as previous findings by Zhang et al. also showed that RA could increase the expression of podocyte markers in PECs in the setting of experimental glomerular disease 34.
Figure 4. Role of RA and RARα in the expression PECs and podocyte markers.
(a) Immunofluorescence staining of nephrin and Claudin-1. Representative images from mice in each group are shown (magnification: x400, scale bar: 50μm). (b, c) Semi-quantification of immunofluorescence optical density for nephrin (b) and Claudin-1 (c) are shown. AU, arbitrary units. (d, e) Real-time PCR analysis was performed for nephrin and Claudin-1. (f) Quantification of nephrin/Claudin-1-double positive cells in glomeruli of NTS and vehicle-injected mice. Nephrin and Claudin-1-double positive cells were detected only in the nephritic mice treated with RA. (*P<0.05, **P<0.01, and ***P<0.001 compared to respective PBS-injected control; ##P<0.01 and ###P<0.001 compared to respective NTS-injected mice; §P<0.05 and §§P<0.01 compared to NTS+RA Pod-Rara+/+. n.d., not detected, n.s. not significant, n=6 in each group).
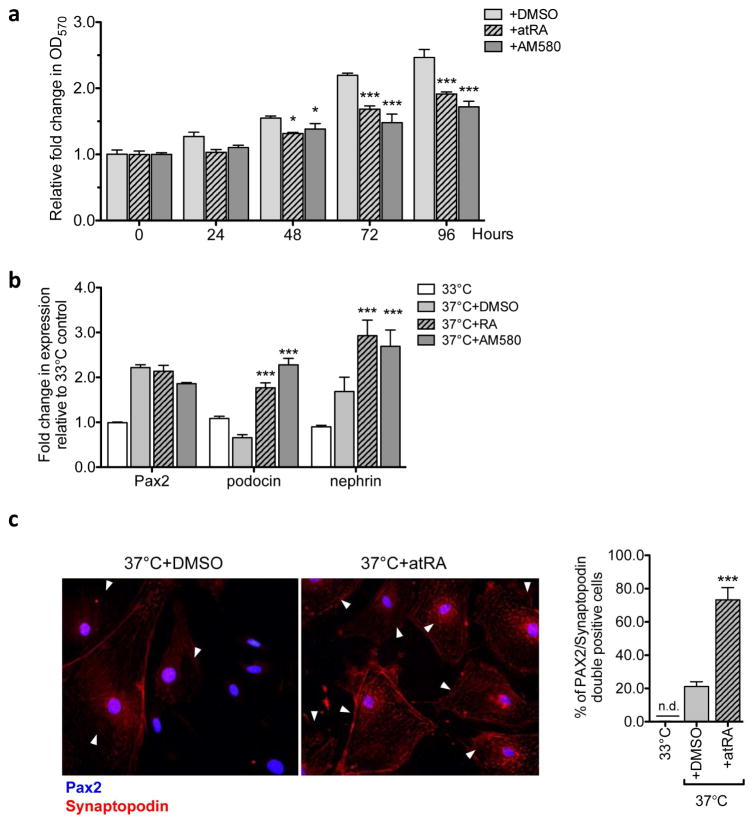
RA inhibits the proliferation of PECs and induces their transdifferentiation in vitro
Since we observed that RA administration in vivo led to a reduced expression of PEC marker Claudin-1 and to a dual expression of Claudin-1 and nephrin in a small subset of glomerular cells, we sought to validate these observations in vitro. We obtained conditionally immortalized mouse PECs (from laboratory of Dr. Stuart Shankland) and cultured them as described in Ohse et al. 35. Cultured mPECs were allowed to differentiate by incubation at 37°C without IFN-γ to inactivate the T-antigen for 14 days. To test whether RA can directly inhibit PEC proliferation through RARα, differentiated mPECs were treated with either vehicle (DMSO), atRA (5μM), or RARα-specific agonist AM580 (200nM), and their proliferation was assessed using a crystal violet staining method 36. Addition of either atRA or AM580 significantly decreased their proliferation, which was detectable at 48 hours and more pronounced by 72 to 96 hours in culture (Figure 5A). Furthermore, by 72 hours of incubation with atRA or AM580, there was a marked enhancement in the mRNA expression levels of podocin, nephrin, and synaptopodin in comparison to vehicle treated cells, while the PEC marker Pax2 remained unchanged (Figure 5B). We further confirmed that atRA enhanced the podocyte marker expression in mPECs by immunofluorescence. While there is a small percentage of differentiated mPECs that express both Pax2 and synaptopodin at basal level (21.25±1.38%) in vitro, atRA treatment led to a substantial increase in number of cells that co-express Pax2 and synapotodin (73.25±3.68%) (Figure 5C, arrowheads). Together, these results support the above in vivo finding that RA can directly inhibit proliferation of PECs and enhance their transdifferentiation into podocytes.
Figure 5. RA inhibits proliferation of PECs and enhances expression of podocyte marker synaptopodin in vitro.
(a) Immortalized mouse PECs were cultured in 37°C without IFN-γ for 14 days to induce their differentiation and were incubated with either vehicle (DMSO), atRA (5μM), or AM580 (200nM) for 96 hours. Crystal violet cell proliferation assay was performed to determine the effect of RA on PECs. Growth curve of differentiated PECs are shown as relative fold change in crystal violet stain (OD570nm) compared to 0h (no factors added) (*P<0.05 and ***P<0.001 compared to DMSO control; n=6). (b) Immortalized mouse PECs were cultured either at 33°C with IFN- γ or 37°C without IFN- γ to induce differentiation for 14 days. Following 14 days, cells were additionally treated with either vehicle (DMSO) atRA (5μM), or AM580 (200nM) for 72 hours. Real-time PCR analysis for PEC marker, Pax2, and podocyte markers, podocin, nephrin and synaptodopodin, are shown (*P<0.05, **P<0.01 and ***P<0.001 compared to 33°C-grown undifferentiated controls; ##P<0.01 and ###P<0.001 compared to DMSO-treated differentiated PECs. n=6). (c) Representative image of Pax2/synaptopodin immunofluorescence in differentiated PECs treated with vehicle or atRA (5μM) for 72 hours. Quantification of Pax2/synaptopodin co-expressing PECs/field is shown on the right (***P<0.001 compared to DMSO control).
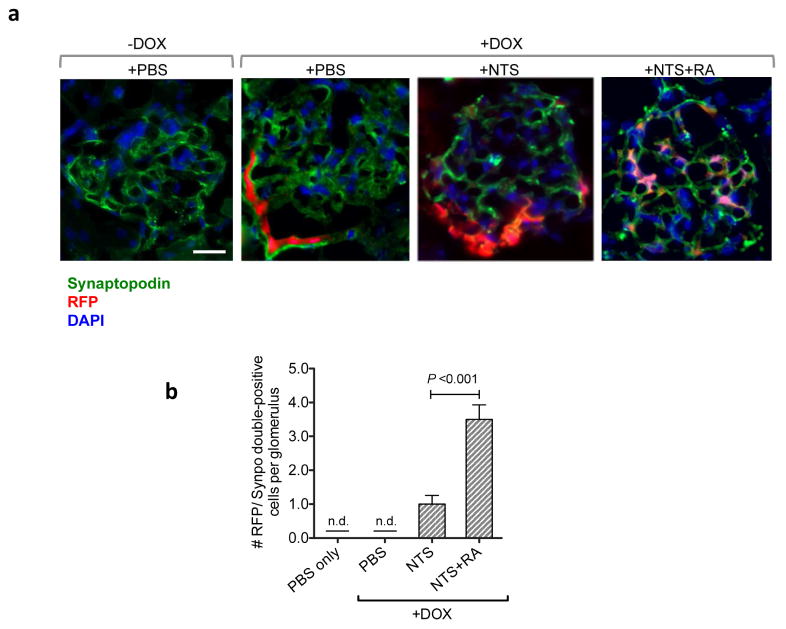
RA induces transdifferentiation of PECs into podocytes in vivo
To further validate the in vitro findings, we performed the lineage-tracing of PECs in vivo in the setting of NTS-GN. Cre reporter mice that express red fluorescent protein (RFP), tdTomato, upon recombination 37 were crossed with mice carrying PEC-rtTA 6 and TetO-Cre transgenes. In the resulting triple transgenic mice (PEC-rtTA; TetO-Cre; tdTomato) PECs were first fluorescently labeled with by feeding mice with doxycycline (DOX)-supplemented chow (625mg/kg of chow) for 4 weeks prior to NTS injection. DOX feeding induced a robust tdTomato RFP expression in PECs (Figure 6A). RA treatment in NTS-injected mice induced migration of RFP-labelled PECs into the glomerular tufts, where they exhibited podocyte morphology and concomitantly expressed synaptopodin. We observed an average of three to four RFP/synaptopodin double-positive cells per glomerulus in RA-treated GN mice, while such cells were not present in the glomeruli of nephritic mice without RA treatment (Figure 6B), further lending credence to the above findings that RA induces PEC transdifferentiation. Taken together, our data indicate that RA confers protection against NTS-GN largely through direct activation of RARα in podocytes and in part through the inhibition of PEC proliferation and induction of their transdifferentiation into podocytes.
Figure 6. Lineage tracing of PECs shows increased PECs expressing podocyte marker synaptodopodin in vivo following RA admnistration in NTS-GN mice.
PEC-rtTA; TetO-Cre; CAGs-tdTomato transgenic mice were fed with DOX for 4 weeks before injection of NTS to label PECs with tdTomato RFP. (a) Representative images of glomeruli in mice with or without DOX induction show RFP-labeled PECs only with the DOX induction (magnification: x400, scale bar 20μM). RA treatment induced migration of PECs into glomerular tufts in NTS-treated mice. Co-localization of RFP with synaptopodin (in green) confirms the transdifferentiation of PECs into podocytes in NTS+RA glomeruli. (b) Quantification of RFP/synaptopodin (synpo)-double positive cells/glomerular cross section (n.d., not detected, n=6 per group).
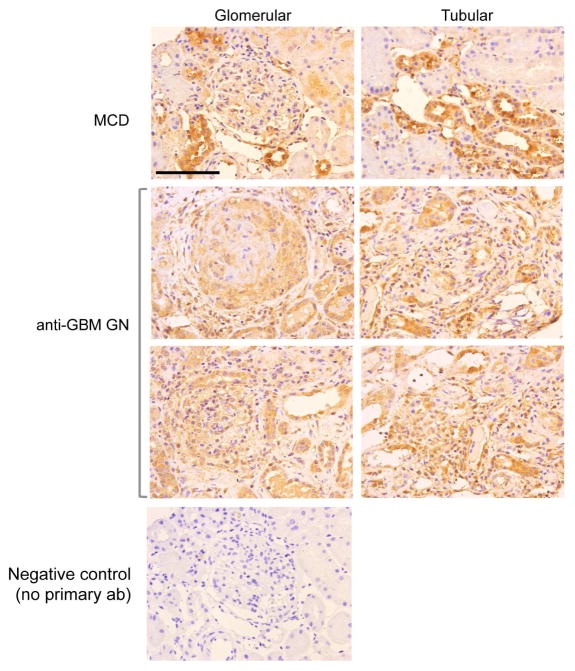
RARα expression in human crescentic GN
Since our data suggested that RA might be an effective therapeutic approach against renal injury in crescentic GN, we wanted to confirm that there were no alterations in the expression pattern of RARα in human crescentic GN. We were able to obtain patient biopsy samples from 4 patients with minimal change disease (MCD) and 5 patients with crescentic GN (clinical information of the patients is summarized in Supplementary Table 1). Immunostaining for RARα showed that its expression is broadly distributed in all glomerular and tubular cells in both MCD and in crescentic GN patient kidneys, suggesting that the expression level of RARα is not significantly altered in crescentic GN in comparison to normal kidneys (Figure 7). These findings are consistent with our previous studies showing that RARα expression was not different between human HIVAN kidneys and control kidneys 20. Thus, as our data demonstrates that RARα is a key molecule mediating the beneficial effects of RA and its expression is not altered in the diseased kidney, development of RARα agonists may be a novel therapeutic approach to treat patients with crescentic GN.
Figure 7. Expression of RARα in human crescentic GN kidneys.
Immunohistochemical analysis of RARα in biopsy samples of human crescentic GN and minimal change disease (MCD) kidneys. Representative image from crescentic GN biopsy samples (n=5, 3 IgA nephropathy, 2 anti-GBM patients) in comparison to those of MCD (n=3). There is no significant change in RARα expression pattern between crescentic GN compared to MCD kidneys (Magnification 400x, scale bar: 50μm).
DISCUSSION
In addition to the critical role that RA plays during kidney development, RA has been demonstrated to preserve differentiation markers in cellular injury as well as induce the differentiation of kidney progenitor cells 38. Furthermore, RA has also been shown to attenuate inflammation and apoptosis in models of podocyte injury 39. In this current study we sought to address whether RA can improve renal function and ameliorate kidney injury in the context of NTS-GN and whether this would be mediated directly through the inhibition of podocyte injury. Our data indeed demonstrates that RA confers renoprotection in NTS-GN and that much of the beneficial effects of RA are directly mediated by engagement of podocyte RARα. Our data also indicate that reduced podocyte injury is associated with reduced number of proliferative cells in NTS-GN. Furthermore, in addition to podocytes, our data show that RA can directly reduce proliferation of PECs and induce their transdifferentiation into podocytes. Lineage tracing of PECs in vivo lends further support that RA can induce the number of transitional PECs co-expressing PEC and podocyte maker expression in the setting of glomerular injury. To our knowledge, this is the first study to show a specific role of RARα in podocytes against pathogenesis of crescentic GN and also a first to demonstrate in vivo by lineage tracing that RA can induce PECs to generate new podocytes. Our previous studies also suggest that PECs also express RARα 20. Therefore, future studies are required using PEC-specific deletion of RARα in vivo to further confirm our findings.
Recent data suggest that podocyte generation occurs mainly during glomerular development and may occur only after acute glomerular injury, and that the regeneration capacity of the podocytes in aging kidneys is very limited. In fact, in response to nephron loss podocytes undergo hypertrophy rather than regeneration 9,10. Therefore, it is unlikely that podocyte regeneration, such as through transdifferentiation of PECs, is sufficient for full recovery of injury in kidney disease such as FSGS or GN. Our data suggest that RA is able to generate podocytes from PECs, albeit a small number per glomeruli, and that this may contribute partially to the beneficial effects of RA. However, the majority of the renoprotective effects of RA are shown to be mediated directly by RARα in podocytes and that there is a significant, but limited contribution of RA-induced podocyte regeneration from PECs, which is consistent with the previous findings 9,10.
Recent data demonstrated that PECs are the sources of proliferative cells in early crescents 40. Our data suggests that initiation of podocyte injury is a critical step leading to crescentic GN, as protective effects of RA were markedly diminished in Pod-Rara−/− mice. However, we did observe some renoprotection by RA in nephritic Pod-Rara−/− mice, albeit significantly less than in nephritic Pod-Rara+/+ mice. This may be due to a combination of factors, which include: 1) an incomplete knockout of RARα in podocytes driven by podocin-Cre, resulting in small residual level of RARα signaling in podocytes; 2) retinoic acid receptors other than RARα that may mediate the renoprotective effects of RA in podocytes (however, our previous in vitro and in vivo studies clearly showed that RARα is the key RA receptor in podocytes 17,20); 3) effects of RA on glomerular cells other than podocytes to confer protection. Our findings suggest that RA has directly affects PECs, as shown by RA-mediated inhibition of PEC proliferation in vitro and in vivo. In addition, we observed that RA induced the transdifferentiation of PECs into podocytes; and 4) immunomodulatory effects of RA 22 that may contribute to the renoprotection. Indeed, our data on reduced expression of infiltrating cells in both Pod-Rara+/+ and Pod-Rara−/− mice support the important immuomodulatory role of RA in NTS-GN.
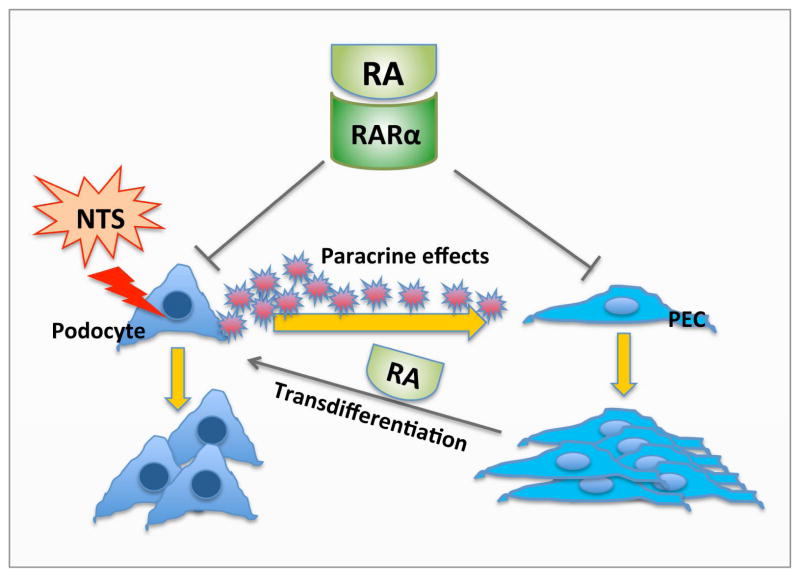
Treatment of GN is currently limited to immunosuppressive therapy. Our data indicate that RA may be a potential treatment of crescentic GN by directly minimizing podocyte injury. Since RARα is a key receptor mediating the effects of RA and its expression does not appear to be altered in human crescentic GN, specific agonists of RARα could be developed as a novel therapeutic option. It is known that RARα agonists have lower toxicity than RA, since many adverse effects of RA are mediated through activation of RARγ. As such, RARα agonists do not have much toxicity in skin cells, which is mediated mainly by RARγ 41,42. Novel RARα agonists with less toxicity have also been developed recently and show efficacy in experimental model of HIV-associated nephropathy 21. In sum, we have demonstrated that RA improves renal function and reduces the number of crescents in NTS-GN mice primarily by reducing podocyte injury and by inhibiting proliferation of glomerular cells, and to a small extent, by inducing transdifferentiation of PECs into podocytes (illustrated in schematics in Figure 8). RARα is a key molecule mediating the beneficial effects of RA, and RARα agonists may be further developed as new therapeutic approach to treat patients with crescentic GN.
Figure 8. Schema of RA effects in crescentic GN.
Podocyte injury is the initial event leading to the development of GN. Podocytes may undergo proliferation and/or stimulate PEC proliferation through paracrine effects. RA improves renal function and reduces the number of crescents in NTS-GN mice primarily by protecting podocytes from initial injury and inhibiting proliferation of glomerular cells (podocytes and/or PECs), and possibly by inducing transdifferentiation of PECs into podocytes.
METHODS
Human archival kidney samples
Human biopsy samples of crescentic GN and minimal change were obtained from archival biopsy specimens collected at Icahn School of Medicine at Mount Sinai (ISMMS) under a protocol approved by the Institutional Review Board at ISMMS and at Zhongshan Hospital in Shanghai, China under a protocol approved by the Ethics Committee at Zhongshan Hospital, Fudan University, China.
Generation of mouse lines
All animal studies were performed according to the protocols approved by Institutional Animal Care and Use Committee at Icahn School of Medicine at Mount Sinai. The conditional floxed RARα−/− mice were provided by Dr. Pierre Chambon (Strasbourg, France) 43. Podocin-Cre transgenic mice in C57BL/6 were obtained from Jackson laboratory (Bar Harbor, ME). These mice were crossed to generate the experimental Pod-Rara−/− mice in the mixed background of 129/Sv and C57BL/6, and Pod-Rara+/+ wildtype littermates were used as controls. Genomic DNA was isolated from tail, glomerular, no glomerular fraction (NGF), liver, or cortex for genotyping by PCR using the following three primers: 1: 5′-CTC CCT GTG ACC ACC AGA AGC TC-3′, 2: 5′-GGA AGG AAC TAG GGC AGA GG-3′ and 3: 5′-TAT CCT GTT GAC CCC AGC TC-3′. These primers amplify three different Rara alleles: wildtype (400bp), floxed allele (450bp), and excised allele (330bp). PEC-rtTA mice were kindly provided by Dr. M. J. Moeller. Cre reporter strain [B6.129S6-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J, Stock# 007905] that expresses red fluorescent protein (RFP) variant, tdTomato, following Cre-mediated recombination was purchased from The Jackson Laboratory (Bar Harbor, ME). Induction of transgene expression in PEC-rtTA/TetO-Cre/CAG-tdTomato mice was achieved by giving 625mg/kg doxycycline-supplemented chow (Bio-Serv, French Town, NJ) for 4 weeks prior to NTS injection. After 4 weeks of supplementation, the chow was replaced with normal chow.
Induction of anti-GBM nephritis and RA treatment
Antiglomerular basement membrane (anti-GBM) nephritis was induced with injection nephrotoxic serum (NTS) as described in Salant et al. 27. Briefly, Pod-Rara+/+ and Pod-Rara−/− mice between 7 weeks of age were immunized with a single intraperitoneal injection of 0.5 mg of sheep IgG (Jackson Immunoresearch Laboratories, West Grove, PA) in 0.2 ml of a 1:1 emulsion with complete Freund’s adjuvant (Sigma Chemical Co., St. Louis, MO). Six days later, glomerulonephritis was induced with an intravenous injection of 2.5ul/g NTS (66.7g/L in sterile PBS) or sterile PBS as control through the tail vein (anti-GBM antibody NTS was kindly provided by Dr. David J. Salant). To assess the effect of RA treatment in NTS-GN, Pod-Rara+/+ and Pod-Rara−/− were treated daily with intraperitoneal injection of atRA (16 mg/kg) or vehicle (corn oil) beginning one day before NTS injection. Seven days after NTS injection, serum samples were collected and kidneys were harvested for histology and glomeruli isolation. There were 6 mice in each group of PBS-injected, NTS-injected, or NTS-injected with RA treatment per genotype.
Measurement of urine albumin and creatinine
Urine albumin was quantified by ELISA using a kit from Bethyl Laboratories, Inc. (Houston, TX). Urine creatinine levels were measured in the same samples using QuantiChromTM creatinine assay kit (DICT-500) (BioAssay Systems) according to the manufacturer’s instruction. The urine albumin excretion was expressed as the ratio of albumin to creatinine.
Measurement of BUN
Blood urea nitrogen (BUN) was measured by using a commercially available kit (Bioassay Systems, Hayward, CA).
Histopathology and immunohistochemistry
Kidney samples were either frozen in OCT embedding compound or fixed in 10% formalin then embedded in paraffin. Periodic Acid Schiff’s (PAS) staining of paraffin-sections was used for quantification of crescents. The proportion of glomeruli with crescents was determined by an examiner blinded to the experimental condition. At least 30 glomeruli per animal were counted. Each biological group had 6 animals. Paraffin-embedded sections were used for Ki-67 staining by using a rabbit anti-Ki67 antibody (Vector Laboratories, Burlingame, CA). For quantification of Ki-67-positive nuclei, more than 10 glomeruli per animal and three animals from each biologic group were examined to calculate the number of Ki-67 stained nuclei.
Immunofluorescence
Frozen section of OCT-embedded tissue was used for synaptopodin, CD44, nephrin, Claudin-1, mouse IgG, sheep IgG, and C3 complement immunofluorescent labeling. Cultured mPECs were fixed with 2% formaldehyde containing 4% sucrose for 10 min, then permeabilized with 0.3% Triton-X100 in PBS for 10 min. The following primary antibodies were used: rabbit-anti-Claudin-1, goat-anti-RARα, rabbit-anti-RARβ from Abcam (Cambridge, MA); rabbit anti-synaptopodin antibody from Dr. Peter Mundel (Massachusetts General Hospital, Boston, MA); mouse anti-synaptopodin antibody from Fitzgerald Industries International. Inc. (Acton, MA); rabbit anti-nephrin antibody from Dr. Larry Holzman (University of Pennsylvania, Philadelphia, PA); rabbit anti-Pax2 antibody from Invitrogen (Waltham, MA); rat anti-CD44 from eBioscience (San Diego, CA); anti-mouse IgG and anti-sheep IgG from Jackson ImmunoResearch Laboratories (West Grove, PA); After mounting, slides were examined by Zeiss Axioplan2IE microscope.
Quantification of Immunostaining
After sections were immunostained, negatives were digitized, and images with a final magnification of X400 were obtained. ImageJ 1.26t software was used to measure the level of immunostaining in the glomeruli. First, the images were converted to 8-bit grayscale. Next, the glomerular region was selected for measurement of area and integrated density. Next, the background intensity was measured by selecting three distinct areas in the background with no staining. The corrected optical density (COD) was determined as shown below:
where ID is the integrated density of the selected glomerular region, A is the area of the selected glomerular region, and MGV is the mean gray value of the background readings) 44.
Glomerular isolation
Mouse glomeruli were isolated as described 45. Briefly, animals were perfused with Hank’s Buffered Salt Solution (HBSS) containing 2.5mg/ml iron oxide and 1% bovine serum albumin. At the end of perfusion, kidneys were removed, decapsulated, minced into 1-mm3 pieces, and digested in HBSS containing 1mg/ml collagenase A and 100 U/ml deoxyribonuclease I. Digested tissue was then passed through 100-micron cell strainer and collected by centrifugation. The pellet was resuspended in 2 ml of HBSS and glomeruli were collected using magnetic beads.
Real-time PCR
Total RNA was isolated from either isolated glomeruli using RNeasy Mini Kit (Qiagen, Valencia, CA). Real-time PCR was performed using complementary DNA reverse transcribed from RNA with SYBR green PCR master mix (Applied Biosystems, Foster city, CA) and PCR reactions were carried out using the Applied Biosystems 7900HT Fast Real-Time PCR system. Primer sets were obtained from Sigma Aldrich (St. Louis, MO) for Ki67, nephrin, synaptopodin, CD44, Claudin-1, and GAPDH. Sequences of the PCR primers are provided in Supplementary Table 2. SDS2.2.1 software was used to quantitatively analyze CT values of target genes. Data were normalized to GAPDH presented as fold increase compared to the reference experimental group using the 2−ΔΔCT method.
Mouse PEC culture and cell proliferation assay
Immortalized mouse PEC cells were a generous gift of Dr. Stuart Shankland. mPECs were cultured as described by Ohse et al.35. Briefly, mPECs were expanded under growth-permissive conditions at 33°C with 50U/ml IFN-γ in RPMI1640 media containing 2% fetal bovine serum (FBS), penicillin (100U/ml), streptomycin (100mg/ml), sodium pyruvate (1mmol/L). Differentiation was induced by switching to growth-restrictive conditions (37°C without IFN- γ) for 14 to 16 days, at which either vehicle (DMSO), atRA (5μM), or AM580 (200nM) was added for 72 hours (for real-time PCR analysis and immunocytochemistry) or 96 hours (cell proliferation assay). Cell proliferation assay was conducted using the crystal violet staining method, as described previously 36. Briefly, each 12-well plate of differentiated mPECs (incubated with vehicle, atRA or AM580) were fixed every 24 hours, until 96 hours, using 4% PFA. After the last collection of the 12-well plate at 96 hours, fixed cells were incubated with crystal violet staining solution (0.1% crystal violet powder in 10% ethanol) for 20 minutes in room temperature. Incorporated crystal violet dye was solubilized with 1% SDS and quantified using absorbance measurement at 570nm. The assay was performed using 6 replicates per group and repeated twice.
Statistical Analysis
Data are expressed as mean ± SEM. The unpaired t-test was used to comparison between groups or two-way ANOVA followed by Bonferroni correction was used when comparing between groups for treatment conditions using the GraphPad Prism software. P-value<0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
JCH is supported by NIH 1R01DK078897, 1R01DK088541, 1R01DK109683 and VA Merit Award IBX000345C; PYC is supported by NIH 5R01DK098126; KL is supported by NIH 5R01DK98126 and P30DK079307. DJS is supported by NIH 1R01DK090029.
Footnotes
DISCLOSURE: The authors declare that they have no competing financial interests.
AUTHOR CONTRIBUTION:
YD, RL, XD and JCH conceived and designed the experiments; YD, RL, AC, LG, SS, and WC performed the experiments; YD, RL, SS, LG, FS, PYC, KL and JCH analyzed the data; DJS, JP, SS, MJM, and NG contributed reagents and materials; YD, KL and JCH wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Le Hir M, Keller C, Eschmann V, et al. Podocyte bridges between the tuft and Bowman’s capsule: an early event in experimental crescentic glomerulonephritis. J Am Soc Nephrol. 2001;12:2060–2071. doi: 10.1681/ASN.V12102060. [DOI] [PubMed] [Google Scholar]
- 2.Moeller MJ, Soofi A, Hartmann I, et al. Podocytes populate cellular crescents in a murine model of inflammatory glomerulonephritis. J Am Soc Nephrol. 2004;15:61–67. doi: 10.1097/01.asn.0000102468.37809.c6. [DOI] [PubMed] [Google Scholar]
- 3.Bariety J, Bruneval P, Meyrier A, et al. Podocyte involvement in human immune crescentic glomerulonephritis. Kidney Int. 2005;68:1109–1119. doi: 10.1111/j.1523-1755.2005.00503.x. [DOI] [PubMed] [Google Scholar]
- 4.Ding M, Cui S, Li C, et al. Loss of the tumor suppressor Vhlh leads to upregulation of Cxcr4 and rapidly progressive glomerulonephritis in mice. Nat Med. 2006;12:1081–1087. doi: 10.1038/nm1460. [DOI] [PubMed] [Google Scholar]
- 5.Griffin SV, Krofft RD, Pippin JW, et al. Limitation of podocyte proliferation improves renal function in experimental crescentic glomerulonephritis. Kidney Int. 2005;67:977–986. doi: 10.1111/j.1523-1755.2005.00161.x. [DOI] [PubMed] [Google Scholar]
- 6.Smeets B, Uhlig S, Fuss A, et al. Tracing the origin of glomerular extracapillary lesions from parietal epithelial cells. J Am Soc Nephrol. 2009;20:2604–2615. doi: 10.1681/ASN.2009010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sicking EM, Fuss A, Uhlig S, et al. Subtotal ablation of parietal epithelial cells induces crescent formation. J Am Soc Nephrol. 2012;23:629–640. doi: 10.1681/ASN.2011050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ronconi E, Sagrinati C, Angelotti ML, et al. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol. 2009;20:322–332. doi: 10.1681/ASN.2008070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger K, Schulte K, Boor P, et al. The regenerative potential of parietal epithelial cells in adult mice. J Am Soc Nephrol. 2014;25:693–705. doi: 10.1681/ASN.2013050481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wanner N, Hartleben B, Herbach N, et al. Unraveling the role of podocyte turnover in glomerular aging and injury. J Am Soc Nephrol. 2014;25:707–716. doi: 10.1681/ASN.2013050452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans TR, Kaye SB. Retinoids: present role and future potential. Br J Cancer. 1999;80:1–8. doi: 10.1038/sj.bjc.6690312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Q, Lucio-Cazana J, Kitamura M, et al. Retinoids in nephrology: promises and pitfalls. Kidney Int. 2004;66:2119–2131. doi: 10.1111/j.1523-1755.2004.66002.x. [DOI] [PubMed] [Google Scholar]
- 13.Lehrke I, Schaier M, Schade K, et al. Retinoid receptor-specific agonists alleviate experimental glomerulonephritis. Am J Physiol Renal Physiol. 2002;282:F741–751. doi: 10.1152/ajprenal.00026.2001. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki A, Ito T, Imai E, et al. Retinoids regulate the repairing process of the podocytes in puromycin aminonucleoside-induced nephrotic rats. J Am Soc Nephrol. 2003;14:981–991. [Google Scholar]
- 15.Perez de Lema G, Lucio-Cazana FJ, Molina A, et al. Retinoic acid treatment protects MRL/lpr lupus mice from the development of glomerular disease. Kidney Int. 2004;66:1018–1028. doi: 10.1111/j.1523-1755.2004.00850.x. [DOI] [PubMed] [Google Scholar]
- 16.Wagner J, Dechow C, Morath C, et al. Retinoic acid reduces glomerular injury in a rat model of glomerular damage. J Am Soc Nephrol. 2000;11:1479–1487. doi: 10.1681/ASN.V1181479. [DOI] [PubMed] [Google Scholar]
- 17.He JC, Lu TC, Fleet M, et al. Retinoic acid inhibits HIV-1-induced podocyte proliferation through the cAMP pathway. J Am Soc Nephrol. 2007;18:93–102. doi: 10.1681/ASN.2006070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaughan MR, Pippin JW, Griffin SV, et al. ATRA induces podocyte differentiation and alters nephrin and podocin expression in vitro and in vivo. Kidney Int. 2005;68:133–144. doi: 10.1111/j.1523-1755.2005.00387.x. [DOI] [PubMed] [Google Scholar]
- 19.Allenby G, Bocquel MT, Saunders M, et al. Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc Natl Acad Sci U S A. 1993;90:30–34. doi: 10.1073/pnas.90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratnam KK, Feng X, Chuang PY, et al. Role of the retinoic acid receptor-alpha in HIV-associated nephropathy. Kidney Int. 79:624–634. doi: 10.1038/ki.2010.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong Y, Wu Y, Liu R, et al. Novel retinoic acid receptor alpha agonists for treatment of kidney disease. PLoS One. 6:e27945. doi: 10.1371/journal.pone.0027945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tipping PG, Holdsworth SR. T cells in crescentic glomerulonephritis. J Am Soc Nephrol. 2006;17:1253–1263. doi: 10.1681/ASN.2005091013. [DOI] [PubMed] [Google Scholar]
- 23.Raverdeau M, Mills KH. Modulation of T cell and innate immune responses by retinoic Acid. J Immunol. 2014;192:2953–2958. doi: 10.4049/jimmunol.1303245. [DOI] [PubMed] [Google Scholar]
- 24.Chapellier B, Mark M, Garnier JM, et al. A conditional floxed (loxP-flanked) allele for the retinoic acid receptor alpha (RARalpha) gene. Genesis. 2002;32:87–90. doi: 10.1002/gene.10071. [DOI] [PubMed] [Google Scholar]
- 25.Moeller MJ, Sanden SK, Soofi A, et al. Podocyte-specific expression of cre recombinase in transgenic mice. Genesis. 2003;35:39–42. doi: 10.1002/gene.10164. [DOI] [PubMed] [Google Scholar]
- 26.Chuang PY, Xu J, Dai Y, et al. In vivo RNA interference models of inducible and reversible Sirt1 knockdown in kidney cells. Am J Pathol. 2014;184:1940–1956. doi: 10.1016/j.ajpath.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salant DJ, Cybulsky AV. Experimental glomerulonephritis. Methods Enzymol. 1988;162:421–461. doi: 10.1016/0076-6879(88)62096-9. [DOI] [PubMed] [Google Scholar]
- 28.Dai Y, Gu L, Yuan W, et al. Podocyte-specific deletion of signal transducer and activator of transcription 3 attenuates nephrotoxic serum-induced glomerulonephritis. Kidney Int. 2013;84:950–961. doi: 10.1038/ki.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura H, Kitazawa K, Honda H, et al. Roles of and correlation between alpha-smooth muscle actin, CD44, hyaluronic acid and osteopontin in crescent formation in human glomerulonephritis. Clin Nephrol. 2005;64:401–411. doi: 10.5414/cnp64401. [DOI] [PubMed] [Google Scholar]
- 30.Sano N, Kitazawa K, Sugisaki T. Localization and roles of CD44, hyaluronic acid and osteopontin in IgA nephropathy. Nephron. 2001;89:416–421. doi: 10.1159/000046113. [DOI] [PubMed] [Google Scholar]
- 31.Roy-Chaudhury P, Khong TF, Williams JH, et al. CD44 in glomerulonephritis: expression in human renal biopsies, the Thy 1.1 model, and by cultured mesangial cells. Kidney Int. 1996;50:272–281. doi: 10.1038/ki.1996.312. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Dai Y, Chuang PY, et al. Induction of Retinol Dehydrogenase 9 Expression in Podocytes Attenuates Kidney Injury. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2013111150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peired A, Angelotti ML, Ronconi E, et al. Proteinuria impairs podocyte regeneration by sequestering retinoic acid. J Am Soc Nephrol. 2013;24:1756–1768. doi: 10.1681/ASN.2012090950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Pippin JW, Vaughan MR, et al. Retinoids augment the expression of podocyte proteins by glomerular parietal epithelial cells in experimental glomerular disease. Nephron Exp Nephrol. 2012;121:e23–37. doi: 10.1159/000342808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohse T, Pippin JW, Vaughan MR, et al. Establishment of conditionally immortalized mouse glomerular parietal epithelial cells in culture. J Am Soc Nephrol. 2008;19:1879–1890. doi: 10.1681/ASN.2007101087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feoktistova M, Geserick P, Leverkus M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb Protoc. 2016;2016 doi: 10.1101/pdb.prot087379. pdb prot087379. [DOI] [PubMed] [Google Scholar]
- 37.Madisen L, Zwingman TA, Sunkin SM, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merlet-Benichou C, Vilar J, Lelievre-Pegorier M, et al. Role of retinoids in renal development: pathophysiological implication. Curr Opin Nephrol Hypertens. 1999;8:39–43. doi: 10.1097/00041552-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Mallipattu SK, He JC. The beneficial role of retinoids in glomerular disease. Front Med (Lausanne) 2015;2:16. doi: 10.3389/fmed.2015.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh SK, Jeansson M, Quaggin SE. New insights into the pathogenesis of cellular crescents. Curr Opin Nephrol Hypertens. 20:258–262. doi: 10.1097/MNH.0b013e32834583ec. [DOI] [PubMed] [Google Scholar]
- 41.Fisher GJ, Talwar HS, Xiao JH, et al. Immunological identification and functional quantitation of retinoic acid and retinoid X receptor proteins in human skin. J Biol Chem. 1994;269:20629–20635. [PubMed] [Google Scholar]
- 42.Nagpal S, Chandraratna RA. Recent developments in receptor-selective retinoids. Curr Pharm Des. 2000;6:919–931. doi: 10.2174/1381612003400146. [DOI] [PubMed] [Google Scholar]
- 43.Chapellier B, Mark M, Messaddeq N, et al. Physiological and retinoid-induced proliferations of epidermis basal keratinocytes are differently controlled. EMBO J. 2002;21:3402–3413. doi: 10.1093/emboj/cdf331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Potapova TA, Sivakumar S, Flynn JN, et al. Mitotic progression becomes irreversible in prometaphase and collapses when Wee1 and Cdc25 are inhibited. Mol Biol Cell. 2011;22:1191–1206. doi: 10.1091/mbc.E10-07-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takemoto M, Asker N, Gerhardt H, et al. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol. 2002;161:799–805. doi: 10.1016/S0002-9440(10)64239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.