Abstract
Background
Socioeconomic status (SES) is associated with asthma morbidity in observational studies but the factors underlying this association are uncertain.
Objective
We investigated whether three SES correlates—low income, low education, and high perceived stress— were independent risk factors for treatment failure and asthma exacerbations in the context of a randomized controlled trial (RCT).
Methods
The effect of low SES [household income (defined as <$50,000/year), household educational level (defined as less than a bachelor’s degree) and high perceived stress (defined as a score of ≥20 on a perceived stress scale)] on asthma morbidity was analyzed in 381 participants utilizing Poisson regression models. The primary outcome was treatment failure (defined in the trial protocol as a significant clinical or airflow deterioration) and the secondary outcome was asthma exacerbations requiring systemic corticosteroids.
Results
54% of participants had a low income, 40% had a low educational level, and 17% had high perceived stress levels. Even after adjusting for race and other important confounders, participants with lower income had higher rates of both treatment failures [RR=1.6, 95%CI 1.1–2.3, p=0.03] and exacerbations [RR=1.9, 95%CI 1.1–3.3, p=0.02]. Adherence with inhaled corticosteroids was similarly high for both income categories. Education and perceived stress were not significantly associated with either outcome.
Conclusions
In the context of a RCT, participants with lower income were more likely to experience adverse asthma outcomes independent of education, perceived stress, race, and medication adherence.
Keywords: disparities, socioeconomic status, education, psychological stress, asthma exacerbation, VIDA, low income
Introduction
Socioeconomic status (SES) is a variably-defined concept that correlates with distinct factors such as income, education, and perceived stress, and relates to sociodemographic characteristics such as race and ethnicity1. Lower SES broadly associates with health disparities2–6, including greater asthma morbidity7,8–14. The mechanism for the association between lower SES and greater asthma morbidity is unclear, but several underlying factors have been hypothesized to causally link them3, 15–17. One such factor is vitamin D insufficiency, which has been associated with both greater asthma morbidity16–19 and lower SES20, 21. Another is the lower access and adherence to asthma controller therapy related to lower SES22–26.
The NHLBI-sponsored ‘Vitamin D Add-On Therapy Enhances Corticosteroid Responsiveness in Asthma (VIDA)’ trial examined whether vitamin D supplementation could improve asthma control when added to inhaled corticosteroid (ICS) therapy in participants with low serum vitamin D27. Although vitamin D supplementation did not significantly reduce treatment failures compared with placebo overall, it did so in the subgroup who achieved vitamin D sufficiency with supplementation. We collected detailed information on three SES correlates—income, education, and perceived stress—in the setting of reliable and equal access to controller asthma therapy and monitored adherence. We hypothesized that low SES would be associated with poor asthma outcomes due to lower vitamin D levels at baseline, an inability to achieve vitamin D sufficiency with supplementation, or a differential effectiveness of vitamin D supplementation.
Methods
A detailed description of the recruitment, design, study visit structure and procedures including spirometry, and statistical analysis for the VIDA trial has been reported previously27.
Participants
We conducted a secondary analysis of a multicenter randomized double-blinded placebo-controlled trial (VIDA). Eligible participants were aged ≥18 years with asthma confirmed by beta-agonist reversibility or methacholine responsiveness. Participants were vitamin D insufficient (baseline serum 25-(OH)D3 <30ng/mL), had uncontrolled symptoms despite low dose-ICS therapy, and predicted FEV1 ≥50%. 408 participants were randomized to placebo or cholecalciferol for 28 weeks as add-on therapy in the setting of a tapering ICS regimen. Participants were recruited from 16 academic U.S. medical centers and excluded if they did not meet entry or run-in criteria27.
Procedures and outcomes
Study protocol was approved by the institutional review board at each participating institution27. The a priori primary outcome was ‘treatment failure’, defined as ≥1 of the following: peak expiratory flow ≤65% of baseline (2 of 3 consecutive measurements); FEV1 ≤80% of baseline (2 consecutive measurements); levalbuterol dose increase by ≥8 puffs/day for 48 hours (vs. baseline); additional ICS or systemic corticosteroid treatment; asthma-related emergency department visit or hospitalization with systemic corticosteroid treatment; participant dissatisfaction with treatment; and physician clinical safety judgment. The secondary outcome was ‘asthma exacerbation’, defined as meeting treatment failure criteria and ≥1 of the following: failure to respond to rescue algorithm within 48 hours; FEV1 ≤50% of baseline or <40% of predicted (2 consecutive measurements); levalbuterol use of ≥16 puffs/day for 48 hours; exacerbation per physician opinion, and systemic corticosteroid treatment for asthma.
SES data were collected from questionnaires standardized across study sites and administered by centrally-trained clinical staff. Race, ethnicity, household size were self-reported. Combined household annual income level was collected as ‘<$25,000’, ‘$25,000–$49,999’, ‘$50,000–$99,999’, or ‘≥$100,000’, and defined as ‘low’ if <$50,000 28, 29, 30. Highest household education level was collected as ‘No high school diploma’, ‘GED’, ‘High school diploma’, ‘Technical training’, ‘Some college but no degree’, ‘Associate’s degree’, ‘Bachelor’s degree’, ‘Master’s degree’, or ‘MD/PhD/JD/PharmD’, and defined as ‘low’ if less than Bachelor’s degree31, 32. Perceived stress was measured by the Perceived Stress Scale (PSS), and defined as ‘high’ if PSS score ≥2033–35. Adherence to the study drugs was measured as follows: ciclesonide was monitored with the MediTrack DOSER device, vitamin D and placebo capsules were monitored with the Aardex MEMS 6 cap, and oral corticosteroids were measured with a pill count. Study drugs were supplied free of charge to subjects. Sensitization to aeroallergens was determined by skin prick testing with standard allergen preparations from Greer Laboratories® (Lenoir, NC).
Statistical methods
Comparisons were made between placebo and vitamin D treatment groups for categorical variables, such as race and income using Pearson chi-square or Fisher’s exact test; and continuous or ordinal variables, such as age and vitamin D levels using two-sample t-tests or Wilcoxon rank sum tests, as appropriate. Poisson regression models were chosen to model the number of treatment failures and exacerbations, as appropriate for modeling count data while considering the duration of follow-up for each participant included as the offset on the natural log scale36, 37. A log-linear relationship was assumed between the mean number of events and the factors included in the model. Overdispersion of each model was assessed by evaluating the Deviance statistic and ensuring that the Deviance/DF value was close to 1. These models were first evaluated individually to determine the effect of each SES factor on individual event outcomes adjusting for study site, race (Black vs. non-Black), BMI (<= 25 vs. >25), and treatment (vitamin D vs. placebo) as per the primary VIDA trial analysis: , where Yi is the number of events, ti is the length of time in the study, xi is the vector of covariates included in the model, and β is the vector of regression parameters. Subsequently, additional Poisson models evaluated the effects of all three SES correlates simultaneously, adjusting for the above plus additional relevant covariates (age, sex, ethnicity, BMI, household size and education, perceived stress, baseline FEV1% predicted, bronchodilator response, hospitalization rate, and second-hand smoke exposure). Collinearity among the covariates was evaluated by the variance inflation factor (VIF)38, and only covariates with a VIF <10 were included in the models 39. Interaction terms between SES correlates and treatment assignment, and between SES correlates and race, were created to determine whether vitamin D treatment and race modified associations with asthma outcomes. Results for individual predictors are presented in terms of Wald chi-square statistics and p-values from the Poisson regression models, as well as rate ratios and 95% confidence intervals to compare event rates between groups. A sample size of 408 participants was determined based on the primary hypothesis of the main clinical trial. All analyses were performed using SAS 9.4 software (SAS Institute Inc., Cary, NC).
Results
We collected information on 381 (93.4%) of the 408 participants in the VIDA trial who contributed information on income, as well education or stress. Table 1 describes the baseline characteristics of these subjects, stratified by treatment assignment. Mean age was 40 years, with the majority composed of females and Whites. Most participants were overweight or obese. As per inclusion criteria, all participants had mild-to-moderate asthma and were maintained on ≥1 asthma controller medication prior to enrollment. 54% of participants had a household income <$50,000, 40% had a household educational level of less than a bachelor’s degree, and 17% reported high stress levels.
Table 1.
Demographic and clinical baseline characteristics
| CHARACTERISTICS | Placebo (n=193) | Vitamin D (n=188) |
|---|---|---|
| Age | 39.6 (12.8) | 40.2 (12.5) |
| Gender (M) | 62 (32%) | 61 (32%) |
| Race | ||
| American Indian/Alaskan Native | 0 (0%) | 1 (0.5%) |
| Asian and Pacific Islander | 7 (3.6%) | 6 (3.2%) |
| Black | 64 (33.2%) | 59 (31.4%) |
| White | 103 (53.4%) | 100 (53.2%) |
| Hispanic | 17 (8.8%) | 19 (10.1%) |
| Other | 2 (1.0%) | 3 (1.6%) |
| Ethnicity (Hispanic or Latino) | 20 (10.4%) | 22 (11.7%) |
| Household income (<$50,000/year) | 107 (55.4%) | 100 (53.2%) |
| Household Educational level (< Bachelor’s Degree) | 77 (40.1%) | 75 (40.1%) |
| Perceived Stress Level (≥20) | 30 (15.5%) | 35 (18.6%) |
| BMI (kg/m2) | 31.6 (9.5) | 32.3 (8.3) |
| Baseline Vitamin D level | 18.4 (7.0) | 19.1 (6.7) |
| Attained Vitamin D level sufficiency | 16 (8.9%) | 146 (81.1%) |
| Clinical history in the year prior to enrollment: | ||
| ED/unscheduled office visit | 60 (31.1%) | 72 (38.3%) |
| Hospitalizations | 12 (6.2%) | 7 (3.7%) |
| Missed work/school (0/1–7/>7 days) | 116 (60%)/50 (26%)/27 (14%) | 108 (58%)/56 (30%)/23 (12%) |
| Controller medications | ||
| LTRA or 5-LO inhibitor use | 49 (25.5%) | 44 (23.4%) |
| Oral corticosteroids | 56 (29.0%) | 65 (34.6%) |
| Inhaled corticosteroids | 75 (38.9%) | 86 (45.7%) |
| Inhaled corticosteroids + long acting bronchodilator | 123 (63.7%) | 115 (61.5%) |
| Asthma control | ||
| Asthma Symptom Utility Index score | 0.82 (0.12) | 0.83 (0.11) |
| ACT score | 19.1 (3.1) | 19.0 (3.4) |
Table 2 demonstrates the relationships between SES correlates and asthma outcomes, tested individually in separate Poisson regression models. Only a household income <$50,000 was significantly associated with poor asthma outcomes (p=0.01 for both treatment failures and exacerbations). The rate of treatment failures was 1.6-fold, (95% CI 1.1–2.3) higher, and the rate of exacerbations was 2.0-fold (95% CI 1.2–3.3) higher in participants with a household income <$50,000 income compared to those with higher income. In contrast, neither household educational level nor perceived stress were associated with treatment failures (p=0.06 and p=0.39) or exacerbations (p=0.34 and p=0.54), respectively.
Table 2.
Individual relationships between SES correlates and asthma outcomes. Demographic characteristics associated with SES and their relationship with asthma outcomes are also shown. SES: Socioeconomic status
| Unadjusted associations (Rate Ratio, 95%CI, p-value) | |||
|---|---|---|---|
| SES correlates | Frequency | Treatment Failures | Exacerbations |
| Household income (<$50,000/year) | 207/381 | 1.6 (1.1, 2.3), p=0.01 | 2.0 (1.2, 3.3), p=0.01 |
| Household educational level (< Bachelor’s Degree) | 241/381 | 1.4 (1.0, 1.9), p=0.06 | 1.3 (0.8, 2.0), p=0.34 |
| Perceived Stress Level (≥20) | 68/381 | 1.2 (0.8, 1.8), p=0.39 | 1.2 (0.7, 2.1), p=0.54 |
| Demographic characteristics associated with SES | |||
| Race (Black) | 131/381 | 1.7 (1.2, 2.4), p=0.001 | 1.7 (1.0, 2.6), p=0.03 |
| Ethnicity (Hispanic or Latino) | 46/381 | 0.6 (0.3, 1.1), p=0.09 | 0.9 (0.2, 1.4), p=0.23 |
Due to the link between race, ethnicity and other SES correlates, we also tested the relationship between race and ethnicity and asthma outcomes in individual models (Table 2). Of these demographic characteristics, only race was significantly associated with poor asthma outcomes (p=0.001 for treatment failures and p=0.03 for exacerbations). The rate of both treatment failures (95% CI 1.2–2.4) and exacerbations (95% CI 1.0–2.6) in Blacks was 1.7 fold higher than in non-Blacks. Hispanic/Latino ethnicity was not significantly associated with treatment failures (p=0.09) or exacerbations (p=0.23) (RR=0.6 for both). Blacks were more likely to have an income <$50,000 (74% of Blacks vs. 45% of non-Blacks; p<0.0001). We tested whether race and other relevant covariates confounded the associations between household income and asthma outcomes; these covariates did not weaken the association between household income and asthma outcomes (see Supplementary Table 1 for seven models analyzing this association). These relevant covariates were evaluated for collinearity before inclusion in the models (all VIF between 1.0–1.3).
In our final multivariate model, participants with low income experienced a 1.5-fold (95%CI 1.0–2.2, p=0.034) higher rate of treatment failures per person-year than those with high income, with adjustment for study site, race, BMI, and treatment (Table 3), Similarly, those with low income experienced a 1.8-fold (95%CI 1.1–3.1, p=0.025) greater rate of exacerbations per person-year than those with high income (Table 3). We retested this model using a four-category definition of income (‘<$25,000’, ‘$25,000–$49,999’, ‘$50,000–$99,999’, and ‘≥$100,000’) to examine whether the association was ordinal. We found that lower income levels associated with progressively poorer asthma outcomes, but the income groups did not demonstrate a statistically significant ordinal association (p=0.07 for both treatment failures and exacerbations), with adjustment for study site, race, BMI and treatment.
Table 3.
Rate ratios for treatment failures and exacerbations for low vs. high income groups, with adjustment for study site, race, BMI, and treatment
| Outcome | Income Group | Event rate per person-year (95%CI) | Poisson Regression Rate Ratio (95%CI) | P-value |
|---|---|---|---|---|
| Treatment Failures | Low | 0.89 (0.69, 1.15) | 1.5 (1.0, 2.2) | 0.034 |
| High | 0.60 (0.42, 0.85) | |||
| Exacerbations | Low | 0.44 (0.31, 0.63) | 1.8 (1.1, 3.1) | 0.025 |
| High | 0.24 (0.14, 0.40) |
To further address whether the effect of household income on asthma outcomes is independent of race, we stratified our analyses by race. For both Black and non-Black subgroups, participants with low income had poorer asthma outcomes, compared to those with high income but this was not statistically significant. This test of ‘race by income interaction’ was not statistically significant for either treatment failures (interaction p=0.72) or exacerbations (interaction p=0.99) (Supplementary Table 2), suggesting that race does not potentiate the relationship between income and asthma outcomes.
Adherence to ICS therapy and dose of ICS therapy was not statistically significantly different between income groups. Low income participants were adherent 94.85% of days and high income participants were adherent 94.15% of days (p=0.23). Similarly, low income participants used a median 2.57 ICS puffs/day while those with high income used a median 2.58 puffs/day (p=0.27).
Individuals with low incomes were more likely to be female (p<0.01), Black (p <0.01), have a higher BMI (p<0.01), higher perceived stress levels (p=0.04), and a lower household educational level (p<0.0001) compared to those with higher income. Participants with lower incomes also had a lower baseline ACT score (18.8 vs. 19.5, p=0.03), and a greater bronchodilator response compared to the higher income group (%change FEV1=15.5% vs. 13.2%, p=0.03). Participants with low incomes were more likely to be hospitalized for asthma exacerbations (7.2% vs. 2.3%; p=0.03). Both income groups were similar in terms of other covariates (Supplementary Table 3).
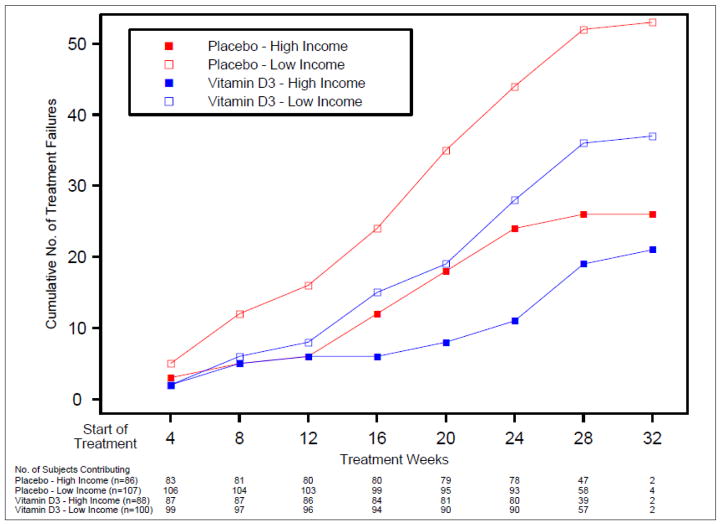
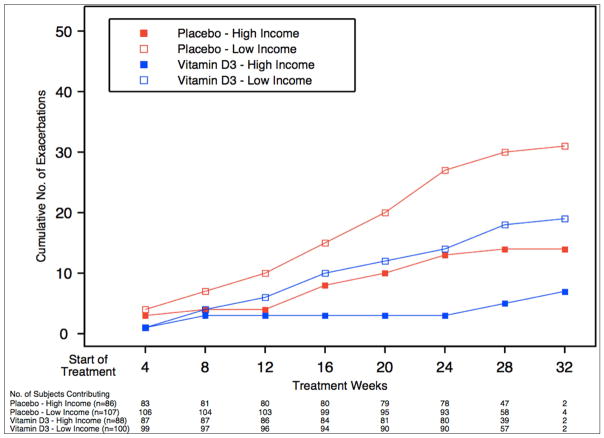
Having identified low income as an independent risk factor for worse asthma outcomes, we addressed whether this risk was mediated by issues related to vitamin D insufficiency (e.g. baseline levels, ability to achieve sufficiency, and response to treatment). Participants with low income had a mean vitamin D level of 18.2ng/mL while those with high income had 19.4ng/mL (p=0.09). Since Blacks were overrepresented in the low income group (Blacks represent 44% of the low income and 18% of the high income subgroup, p<0.01), and Blacks had lower baseline vitamin D levels (15.6 vs. 20.3ng/mL, p<0.001), we compared baseline vitamin D levels between non-Blacks across income categories. Non-Black low income subgroup had a baseline vitamin D level of 20.2ng/mL while the high income subgroup had a level of 20.3ng/mL, which was not statistically different (p=0.93). Supplementation resulted in vitamin D sufficiency in 82% of participants in VIDA, and low income was not a predictor for achieving vitamin D sufficiency (p=0.84). Lastly, we considered whether treatment with vitamin D was differentially effective in the low income subgroup. The low income subgroup had poorer asthma outcomes than the high income subgroup independent of treatment assignment (Table 4, Figures 1 and 2), however this was not statistically significant. The test for effect modification by treatment assignment on the relationship between income and asthma outcomes was not statistically significant for either treatment failures (interaction p=0.83) or exacerbations (interaction p=0.59) (Table 4), suggesting that vitamin D supplementation does not attenuate the relationship between income and asthma outcomes.
Table 4.
Treatment failure rate by treatment assignment, stratified by income level, with adjustment for study site, race, BMI, and treatment
| Outcome | Treatment | Income Level | Adverse asthma outcomes (per person/year) | Test of treatment assignment by income interaction (P-value) |
|---|---|---|---|---|
| Treatment failures | Placebo | Low | 0.92 | 0.83 |
| High | 0.56 | |||
| Vitamin D | Low | 0.69 | ||
| High | 0.44 | |||
| Exacerbation | Placebo | Low | 0.54 | 0.59 |
| High | 0.30 | |||
| Vitamin D | Low | 0.35 | ||
| High | 0.15 |
Figure 1.
Cumulative number of treatment failures by income level group, stratified by treatment
Figure 2.
Cumulative number of asthma exacerbations by income group, stratified by treatment assignment
Discussion
In this randomized controlled trial of adults with mild-to-moderate asthma, we identified income as an independent risk factor for poor asthma outcomes. The treatment failure rate in participants with a household income of <$50,000 was 1.5-fold higher, and the exacerbation rate was 1.8-fold higher compared to participants with a household income ≥$50,000. These results were robust to adjustment by age, gender, race, BMI, study site, treatment, ethnicity, household size and education, perceived stress, baseline lung function and hospitalization rate, and second-hand smoke exposure.
Income as an independent risk factor for asthma morbidity has been well described in the asthma disparities literature9, 14, 40. A significant limitation of these studies is that many important patient characteristics and asthma-related outcomes (such as access or adherence to asthma controller therapy, asthma severity, and asthma exacerbations) are ascertained via self-report. Our study is novel in that our data derive from a randomized controlled trial, with extensive, centrally-regulated clinical characterization and longitudinal follow-up. In this trial, participants were provided with and were highly adherent to ICS therapy, with similar amounts of ICS use across income categories. In this trial, participants were provided with and were highly adherent to ICS therapy, with similar amounts of ICS across income categories. Our finding thus challenges the commonly-posited hypothesis that greater morbidity is seen in low-income patients with asthma, solely because of lack of access or adherence to ICS – although these findings need to be confirmed in a real-world setting 22–26. We hypothesize that there are additional unknown factors contributing to low income being associated asthma morbidity. Second-hand smoke is unlikely to explain the income-asthma outcome association because only a minority of participants reported exposure to it (<10% for both income categories); our results were also robust to adjustment by second-hand smoke exposure. Another commonly-held notion is that cockroach and dust mite sensitization can be related to exposure in some inner-city asthmatic populations41. These allergens are thought to be important in the genesis or maintenance of asthma, and possible driver of worse outcomes42–44. Surprisingly, our results show similar sensitization patterns to perennial aeroallergens across income categories (Supplementary Table 3). This may be due to our study population being composed only of participants aged ≥18 years, and not a pediatric population which may be more susceptible to the pro-asthmatic effects of exposure and sensitization to perennial aeroallergens.
It remains unclear from our results what is the pro-asthmatic factor associated with low incomes. We hypothesized that this pro-asthmatic factor in low SES participants would relate to vitamin D insufficiency, given its association with both asthma and lower SES. However, our results suggest the contrary, since participants had comparable baseline vitamin D levels and similarly achieved vitamin D sufficiency with supplementation across income categories. Further, treatment with vitamin D did not modify the association between income and asthma outcomes.
The low income subgroup had a significantly greater reversibility with albuterol at (15.5% vs. 13.2% increase in FEV1; p = 0.03), and a higher frequency of asthma-related hospitalizations in the year prior to enrollment (7.2% vs. 2.3%; p = 0.03), suggesting that the low income subgroup may have had worse disease at enrollment (Supplementary Table 3). Nonetheless, clinical and spirometric differences are unlikely to explain our results since their inclusion into our models did not weaken the income-asthma outcomes association (Supplementary Table 1).
The correlation between SES factors and race is frequently raised as a limitation in disparities studies due to historical issues in the United States regarding residential segregation32. However, our results suggest that income is an independent risk factor for adverse asthma outcomes irrespective of race. We tested for interactions between these two characteristics and found that race did not modify the income-adverse asthma outcomes association. A limited sample size may explain this test’s lack of statistical significance, but participants with low incomes had higher rates of adverse asthma outcomes regardless of race, which supports our results. We were similarly limited and underpowered to test for effect modification by treatment on the income-adverse asthma outcomes association. Again, participants with low incomes had higher rates of adverse asthma outcomes regardless of treatment assignment. Another limitation was our categorical definition for household income with setting a threshold at $50,000/year. The distribution of the household income categorical variable had the most powerful, balanced comparison between those with income <$50,000 vs. those with income ≥$50,000. Additionally, this cutoff has been used in the disparities literature as the lowest income sufficient to cover basic needs of an average family28–30. Further, although we did not use federal poverty levels to define income in our models we approximated these levels by adjusting by household size, which impacts federal poverty levels. We evaluated whether an ordinal association existed between income and asthma outcomes when applying a four-category income definition (‘<$25,000’, ‘$25,000–$49,999’, ‘$50,000–$99,999’, and ‘≥$100,000’), due to the imbalance in the sample size for these categories. Although these tests suggested an ordinal relationship between income and asthma outcomes, limited sample sizes likely explain their lack of statistical significance (p=0.07 for both outcomes). Another limitation of this analysis is the fact that the sample size for the clinical trial was determined by the primary research hypothesis, and not by the measures included in the secondary analysis. Moreover, as this analysis was conducted on clinical trial participants there is limited generalizability as subjects were closely monitored in regards to medication usage, were given free medications, had regular study visits and had low vitamin D levels. Finally, we did not find household education or perceived stress to be associated with asthma outcomes. We caution against concluding that these two SES correlates are unrelated to asthma outcomes; these negative findings may be specific to our cohort or ascertainment methods.
In summary, we found that in the setting of a randomized clinical trial, lower household income is associated with poor asthma outcomes. The factors underlying lower income as a risk factor for worse asthma are unknown and warrant further research. Clinicians should be aware of the higher morbidity associated with low income for reasons independent of asthma severity, lung function, and access and compliance with controller therapy. Clinical researchers may want to ensure that randomization of trial participants, account for household income to adequately balance risk factors.
Supplementary Material
Key Messages.
Observational studies have limitations in their ability to examine disparities in asthma, as these studies have relied on self-reported measures of medication use, asthma diagnosis, severity, outcomes, and access to care.
Using data collected from a randomized controlled trial, we found that subjects with lower income had a significantly higher number of asthma treatment failures and asthma exacerbations, independent of race, BMI, education, perceived stress, baseline lung function, hospitalizations, inhaled corticosteroid adherence, inhaled corticosteroid dose, environmental allergen sensitization, and second-hand smoke exposure.
Acknowledgments
Funding information: this study was conducted with the support of grants HL098102, U10HL098096, UL1TR000150, UL1TR000430, UL1TR000050, HL098075, UL1TR001082, HL098090, HL098177, UL1TR000439, HL098098, UL1TR000448, HL098107, HL098112, HL098103, UL1TR000454, and HL098115 that were awarded by the National Heart, Lung, and Blood Institute. In addition, Dr. Cardet received funds from a NIAID diversity supplement 3U19AI095219-04S1. Dr. Louisias was supported by NIH grant K12HS022986 and 5T32 HS00063-21. Dr. Phipatanakul received funds from NIAID K24AI 106822.
The authors would like to thank the VIDA trial participants, the AsthmaNet clinical research coordinators, Ms. Michelle Smith and the rest of the data coordinating center at Penn State University for making this study possible. This project was supported in part by grant number K12HS022986 from the AHRQ. The content is solely the responsibility of the authors and does not necessarily represent the official views of the AHRQ.
Abbreviations list
- ICS
inhaled corticosteroid
- PSS
Perceived Stress Scale
- RCT
randomized clinical trial
- SES
socioeconomic status
- VIDA
the ‘Vitamin D Add-On Therapy Enhances Corticosteroid Responsiveness in Asthma’ trial
Footnotes
Summary conflict of interest statements for each author (or a statement indicating no conflicts exist for the specified author[s]): MEW has received honoraria from Boston Scientific, Teva, Novartis, Boehringer Ingelheim, Sanofi, Regeneron Vectura, Sunovion, Ambit Bioscience, Meda, Mylan, Tunitas, Genentech, Theravance, and AstraZeneca. Other authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chong S, Lobb E, Khan R, Abu-Rayya H, Byun R, Jalaludin B. Neighbourhood safety and area deprivation modify the associations between parkland and psychological distress in Sydney, Australia. BMC Public Health. 2013;13:422. doi: 10.1186/1471-2458-13-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen E, Paterson LQ. Neighborhood, family, and subjective socioeconomic status: How do they relate to adolescent health? Health Psychol. 2006;25:704–14. doi: 10.1037/0278-6133.25.6.704. [DOI] [PubMed] [Google Scholar]
- 3.Chen E, Miller GE. Socioeconomic status and health: mediating and moderating factors. Annu Rev Clin Psychol. 2013;9:723–49. doi: 10.1146/annurev-clinpsy-050212-185634. [DOI] [PubMed] [Google Scholar]
- 4.Chen E, Martin AD, Matthews KA. Socioeconomic status and health: do gradients differ within childhood and adolescence? Soc Sci Med. 2006;62:2161–70. doi: 10.1016/j.socscimed.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 5.Chen E, Martin AD, Matthews KA. Understanding health disparities: the role of race and socioeconomic status in children’s health. Am J Public Health. 2006;96:702–8. doi: 10.2105/AJPH.2004.048124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chetty R, Stepner M, Abraham S, Lin S, Scuderi B, Turner N, et al. The Association Between Income and Life Expectancy in the United States, 2001–2014. JAMA. 2016;315:1750–66. doi: 10.1001/jama.2016.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moorman JE, Person CJ, Zahran HS. Asthma attacks among persons with current asthma - United States, 2001–2010. MMWR Surveill Summ. 2013;62(Suppl 3):93–8. [PubMed] [Google Scholar]
- 8.Ernst P, Demissie K, Joseph L, Locher U, Becklake MR. Socioeconomic status and indicators of asthma in children. Am J Respir Crit Care Med. 1995;152:570–5. doi: 10.1164/ajrccm.152.2.7633709. [DOI] [PubMed] [Google Scholar]
- 9.Litonjua AA, Carey VJ, Weiss ST, Gold DR. Race, socioeconomic factors, and area of residence are associated with asthma prevalence. Pediatr Pulmonol. 1999;28:394–401. doi: 10.1002/(sici)1099-0496(199912)28:6<394::aid-ppul2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Kopel LS, Gaffin JM, Ozonoff A, Rao DR, Sheehan WJ, Friedlander JL, et al. Perceived neighborhood safety and asthma morbidity in the School Inner-City Asthma Study. Pediatr Pulmonol. 2015;50:17–24. doi: 10.1002/ppul.22986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen E, Hanson MD, Paterson LQ, Griffin MJ, Walker HA, Miller GE. Socioeconomic status and inflammatory processes in childhood asthma: the role of psychological stress. J Allergy Clin Immunol. 2006;117:1014–20. doi: 10.1016/j.jaci.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 12.Chen E, Fisher EB, Bacharier LB, Strunk RC. Socioeconomic status, stress, and immune markers in adolescents with asthma. Psychosom Med. 2003;65:984–92. doi: 10.1097/01.psy.0000097340.54195.3c. [DOI] [PubMed] [Google Scholar]
- 13.Wright RJ, Subramanian SV. Advancing a multilevel framework for epidemiologic research on asthma disparities. Chest. 2007;132:757S–69S. doi: 10.1378/chest.07-1904. [DOI] [PubMed] [Google Scholar]
- 14.Forno E, Celedon JC. Asthma and ethnic minorities: socioeconomic status and beyond. Curr Opin Allergy Clin Immunol. 2009;9:154–60. doi: 10.1097/aci.0b013e3283292207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans GW, Kim P. Multiple risk exposure as a potential explanatory mechanism for the socioeconomic status-health gradient. Ann N Y Acad Sci. 2010;1186:174–89. doi: 10.1111/j.1749-6632.2009.05336.x. [DOI] [PubMed] [Google Scholar]
- 16.Brehm JM, Acosta-Perez E, Klei L, Roeder K, Barmada M, Boutaoui N, et al. Vitamin D insufficiency and severe asthma exacerbations in Puerto Rican children. Am J Respir Crit Care Med. 2012;186:140–6. doi: 10.1164/rccm.201203-0431OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosser F, Brehm JM, Forno E, Acosta-Perez E, Kurland K, Canino G, et al. Proximity to a major road, vitamin D insufficiency, and severe asthma exacerbations in Puerto Rican children. Am J Respir Crit Care Med. 2014;190:1190–3. doi: 10.1164/rccm.201408-1568LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120:1031–5. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 19.Brehm JM, Schuemann B, Fuhlbrigge AL, Hollis BW, Strunk RC, Zeiger RS, et al. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol. 2010;126:52–8. e5. doi: 10.1016/j.jaci.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manicourt DH, Devogelaer JP. Urban tropospheric ozone increases the prevalence of vitamin D deficiency among Belgian postmenopausal women with outdoor activities during summer. J Clin Endocrinol Metab. 2008;93:3893–9. doi: 10.1210/jc.2007-2663. [DOI] [PubMed] [Google Scholar]
- 21.Wallace TC, Reider C, Fulgoni VL., 3rd Calcium and vitamin D disparities are related to gender, age, race, household income level, and weight classification but not vegetarian status in the United States: Analysis of the NHANES 2001–2008 data set. J Am Coll Nutr. 2013;32:321–30. doi: 10.1080/07315724.2013.839905. [DOI] [PubMed] [Google Scholar]
- 22.Apter AJ, Boston RC, George M, Norfleet AL, Tenhave T, Coyne JC, et al. Modifiable barriers to adherence to inhaled steroids among adults with asthma: it’s not just black and white. J Allergy Clin Immunol. 2003;111:1219–26. doi: 10.1067/mai.2003.1479. [DOI] [PubMed] [Google Scholar]
- 23.Boulet LP, Vervloet D, Magar Y, Foster JM. Adherence: the goal to control asthma. Clin Chest Med. 2012;33:405–17. doi: 10.1016/j.ccm.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Krishnan JA, Riekert KA, McCoy JV, Stewart DY, Schmidt S, Chanmugam A, et al. Corticosteroid use after hospital discharge among high-risk adults with asthma. Am J Respir Crit Care Med. 2004;170:1281–5. doi: 10.1164/rccm.200403-409OC. [DOI] [PubMed] [Google Scholar]
- 25.Foster JM, Lavoie KL, Boulet L-P. Treatment adherence and psychosocial factors in severe asthma. In: Chung KFBEH, Wenzel SE, editors. Difficult-to-Treat Severe Asthma. Sheffield, UK: European Respiratory Society Journals Ltd; 2011. pp. 29–49. [Google Scholar]
- 26.Ponieman D, Wisnivesky JP, Leventhal H, Musumeci-Szabo TJ, Halm EA. Impact of positive and negative beliefs about inhaled corticosteroids on adherence in inner-city asthmatic patients. Ann Allergy Asthma Immunol. 2009;103:38–42. doi: 10.1016/S1081-1206(10)60141-X. [DOI] [PubMed] [Google Scholar]
- 27.Castro M, King TS, Kunselman SJ, Cabana MD, Denlinger L, Holguin F, et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA. 2014;311:2083–91. doi: 10.1001/jama.2014.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cauthen NK, Fass S. Measuring Poverty in the United States. National Center for Children in Poverty, Mailman School of Public Health Columbia University; 2008. pp. 1–4. [Google Scholar]
- 29.Roberts B, Povich D, Mather M. Low-income working families: The growing economic gap. The Working Poor Families Project. 2013;301:657–1480. [Google Scholar]
- 30.Massey DSF, Mary J. Does Rising Income Bring Integration? New Results for Blacks, Hispanics, and Asians in 1990. Soc Sci Res. 1999;28:316–26. [Google Scholar]
- 31.Kallem S, Carroll-Scott A, Rosenthal L, Chen E, Peters SM, McCaslin C, et al. Shift-and-persist: a protective factor for elevated BMI among low-socioeconomic-status children. Obesity (Silver Spring) 2013;21:1759–63. doi: 10.1002/oby.20195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Massey DS. Segregation and Stratification. Du Bois Review. 2004;1:7–25. [Google Scholar]
- 33.Cohen S, Williamson G. The Social Psychology of Health. Newbury Park, CA: Sage; 1988. Perceived Stress in a Probability Sample of the United States; pp. 31–67. [Google Scholar]
- 34.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 35.Gonzalez-Ramirez MT, Rodriguez-Ayan MN, Hernandez RL. The perceived stress scale (PSS): normative data and factor structure for a large-scale sample in Mexico. Span J Psychol. 2013;16:E47. doi: 10.1017/sjp.2013.35. [DOI] [PubMed] [Google Scholar]
- 36.Stokes ME, Davis CS, Koch GG. Categorical data analysis using SAS. SAS institute; 2000. [Google Scholar]
- 37.Agresti A. Analysis of Ordinal Categorical Data. 2. 2002. Wiley series in probability and statistics; pp. 397–405. [Google Scholar]
- 38.Belsley DA, Kuh E, Welsch RE. Regression diagnostics: Identifying influential data and sources of collinearity. John Wiley & Sons; 1980. [Google Scholar]
- 39.Kutner MH, Nachtsheim C, Neter J. Applied linear regression models. McGraw-Hill/Irwin; 2004. [Google Scholar]
- 40.Moorman JE, Person CJ, Zahran HS. Asthma attacks among persons with current asthma - United States, 2001–2010. MMWR Suppl. 2013;62:93–8. [PubMed] [Google Scholar]
- 41.Gruchalla RS, Pongracic J, Plaut M, Evans R, Visness CM, Walter M, et al. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. Journal of Allergy and Clinical Immunology. 2005;115:478–85. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Ahluwalia SK, Peng RD, Breysse PN, Diette GB, Curtin-Brosnan J, Aloe C, et al. Mouse allergen is the major allergen of public health relevance in Baltimore City. J Allergy Clin Immunol. 2013;132:830–5. e1–2. doi: 10.1016/j.jaci.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sedaghat AR, Matsui EC, Baxi SN, Bollinger ME, Miller R, Perzanowski M, et al. Mouse Sensitivity is an Independent Risk Factor for Rhinitis in Children with Asthma. J Allergy Clin Immunol Pract. 2016;4:82–8. e1. doi: 10.1016/j.jaip.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheehan WJ, Rangsithienchai PA, Wood RA, Rivard D, Chinratanapisit S, Perzanowski MS, et al. Pest and allergen exposure and abatement in inner-city asthma: a work group report of the American Academy of Allergy, Asthma & Immunology Indoor Allergy/Air Pollution Committee. J Allergy Clin Immunol. 2010;125:575–81. doi: 10.1016/j.jaci.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.