Abstract
Dysfunction of cardiac cells under hypoxia has been identified as an essential event leading to myocytes functional failure. MiRNAs are importantly regulatory small-noncoding RNAs that negatively regulate gene expression through the direct binding of 3′-UTR region of their target mRNAs. Recent studies have demonstrated that miRNAs are aberrantly expressed in the cardiovascular system under pathological conditions.Pyruvate dehydrogenase kinase 1 (PDK1) is a kinase which phosphorylates pyruvate dehydrogenase to inactivate it, leading to elevated anaerobic glycolysis and decreased cellular respiration. In the present study, we report that miR-138 expressions were significantly suppressed under long exposure to hypoxia. In addition, overexpression of miR-138 protects human cardiac cells against hypoxia. We observed miR-138 inhibits glycolysis but promotes mitochondrial respiration through directly targetting PDK1. Moreover, we demonstrate that hypoxia induces cardiac cell death through increased glycolysis and decreased mitochondrial respiration. Inhibition of glycolysis by either glycolysis inhibitor or knockdown glycolysis enzymes, Glucose transportor 1 (Glut1) or PDK1 contributes to cardiac cells’ survival. The cell sentivity to hypoxia was recovered when the PDK1 level was restored in miR-138 overexpressing cardiac cells. The present study leads to the intervention of novel therapeutic strategies against cardiac cells dysfunction during surgery or ischemia.
Keywords: cardiac cells, hypoxia-induced apoptosis, MiR-138, Pyruvate Dehydrogenase Kinase 1
Introduction
Heart failure, a heart disease caused by myocardial injury, results in high mortality [1]. Multiple factors are involved in the etiology of heart failure such as oxidative stress [2], hypoxic condition [3], stimulation by inflammation factor or cytokines [4], and injury of cardiac tissues [5]. Amongst them, apoptosis of cardiac muscle cells under hypoxia has been identified as an essential event leading to myocytes functional failure, fibrosis, and ensuing ventricular remodeling [6]. During the myocardical infraction,occusion of coronary arties deprives the oxygen of myocardium. Moreover, hypoxia may persist within the infarct for days or weeks, exacerbating the injury [7]. Therefore, understanding the molecular mechanisms of the hypoxia-induced cardiomyocytes’ death contributes to the development of therapeutic approaches for the treatment of ischemia-induced myocardial injury.
Under normal conditions, heart generates energy for fuel contractile function and viability oxidation through fatty acids oxidation [8]. However, the impaired myocardial tissues switch to other metabolic pathways by accelerated utilization of glucose, lactate, ketones, and amino acids [9]. Pyruvate dehydrogenase complex (PDC) is a complex of three enzymes that convert pyruvate into acetyl-CoA [10], which will be used in the citric acid cycle for the processes of oxidative phosphorylation [10]. Pyruvate dehydrogenase kinase (PDK) is a kinase which phosphorylates PDC to inactivate it [11]. Thus, increased PDK expression or activity will prevent the incorporation of pyruvate into oxidative phosphorylation process, leading to elevated anaerobic glycolysis and decreased cellular respiration.
MiRNAs have been recognized as an important regulatory small-noncoding RNAs that negatively regulate gene expression through the direct binding of 3′-UTR region of their target mRNAs [12]. Recent studies have demonstrated that miRNAs are aberrantly expressed in the cardiovascular system under pathological conditions and play essential roles in cardiovascular diseases including cardiomyopathy [13], cardiac fibrosis [14], cardiomyocytes dysfunction [15], cardiac ischemia [16], and arrhythmia [17]. MiR-138 has been reported as one of the miRNAs that functions in heart diseases [18]. Interestingly, previous studies reported that miR-138 expression was induced in hypoxic cardiomyocytes and regulated the hypoxia-induced cell apoptosis [19]. However, the mechanisms underlying that miR-138 protects cardiomyocytes from hypoxia-induced apoptosis were not completely understood. In the present study, we will study the roles of miR-138 in regulating cardiac cells death during hypoxia. The functions of miR-138 in cellular glucose metabolism will be investigated.
Materials and methods
Cell culture and low oxygen treatments
The AC16 human cardiomyocyte cell line was purchased from EMD Millipore and the human renal epithelial derived 293T cells were purchased from American Type Culture Collection (Bethesda, MD, U.S.A.). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 12% FBS, and 1% antibiotics (streptomycin and penicillin). AC16 cells were incubated at 37°C in a humid atmosphere with 5% CO2 and 95% air. Cells were placed in an Invivo200 cultivator (Ruskin Technology Ltd, U.K.) containing a gaseous mixture of 94% N2, 5% CO2, and 1% O2 at 37°C for durations of 8, 16, 24, 48, and 72 h, respectively. Cells in normoxia group were incubated under the same conditions.
MiRNA, siRNA, and plasmid DNA transfection
The miR-138 mimics, miR-138 inhibitor, and negative control miRNAs were purchased from manufacturer GenePharma (Shanghai, China). AC16 cells were seeded in a six-well plate at 2 × 105/well overnight. Then, the cells were transfected with miRNA mimics, inhibitor, or miR-NC (50 nM) using Lipofectamine RNAiMAX transfection reagent (Invitrogen, U.S.A.) in accordance with the manufacturer’s instructions. Glut1 siRNA, pyruvate dehydrogenase kinase 1 (PDK1) siRNA, or control siRNA was transfected at 100 nM. Overexpression vector of PDK1 or control vector was transfected at 4 µg. Seventy-two hours after transfection, cells were collected for following assays.
Real-time Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from AC16 cells using TRIzol® reagent (Invitrogen, U.S.A.) according to the manufacturer’s instructions. The isolated RNA was treated with DNase I (Invitrogen, U.S.A.) to eliminate genomic DNA. cDNA was synthesized using the TaqMan Advanced miRNA cDNA Synthesis Kit (Thermo Fisher Scientific. Inc, U.S.A.). cDNA product was used for the amplification procedure in a 20-µl reaction mixture containing 10-µl SYBR Green PCR Master Mix (Invitrogen, U.S.A.), 7 µl diethylpyrocarbonate-H2O, and 1 µl miR-138 primer and universal primer. The amplification was conducted using the 7900HT Fast Real-Time PCR system (Applied Biosystems, U.S.A.). The protocol consisted of an initial denaturation and enzyme activation at 95°C for 10 min, followed by 35 cycles of 30 s denaturation at 95°C, an attachment of primers for 1 min at 60°C, and extension at 72°C for 30 s, and finally one cycle at 72°C for 10 min for final elongation. U6 was used as an internal control for miR-138 expression. Relative expression levels of miR-138 were calculated using the 2−ΔΔCt method.
Luciferase assay
The luciferase assay was performed according to the previous report [19]. The 3′-UTR of PDK1 harboring either the wild-type miR-138-binding site or a mutant miR-138-binding site was cloned into the psiCHECK-2 vector (Promega, U.S.A.) immediately downstream of the stop codon of the luciferase gene to generate the psiCHECK-PDK1-3′-UTR luciferase reporter plasmid. Plasmid DNA and miR-138 mimics or control miRNAs were cotransfected into 293T or AC16 cells using Lipofectamine 2000 (Invitrogen Inc., U.S.A.) for 72 h. Luciferase activities were measured with a Dual-Glo Luciferase Assay System (Promega, U.S.A.). Firefly luciferase activity was normalized to Renilla luciferase activity. All experiments were performed in triplicate.
Cell survival
Cell survival rate was determined by MTT (Sigma, U.S.A.) colorimetric assay according to the previous report [19]. Briefly, AC16 cells were seeded in 96-well tissue culture plates at 2 × 104 cells per well and incubated in hypoxic or normal conditions for various hours. Cells were washed with PBS and then incubated in 100 ml of 5 mg/ml MTT solution (Invitrogen Inc., U.S.A.) for 3 h. After incubation, DMSO (Invitrogen Inc., U.S.A.), was added into each well. MTT was converted into purple-colored formazan in living cells and absorbance of solution was taken at 450 nm using the microplate reader Thermo Plate (Rayto Life and Analytical Science Co. Ltd, Germany). All the experiments were performed in triplicate.
Measurements of glycolysis rate
The glucose uptake assay was performed using the Glucose Uptake Colorimetric Assay Kit (#K676) from BioVision (Milpitas, CA, U.S.A.) according to the manufacturer’s instructions. The lactate production was analyzed by the Lactate Colorimetric/Fluorometric Assay Kit (#K607) from BioVision (Milpitas, CA, U.S.A.) according to the manufacturer’s instructions. The extracellular acidification rate (ECAR) and oxygen consumption rate were measured using the Seahorse XFp Extracellular Flux Analyzer from Agilent (Santa Clara, CA, U.S.A.). Results were repeated three times and normalized by protein concentrations of each test.
Measurements of intracellular ATP
The intracellular ATP assay was performed using the ATP Assay Kit (colorimetric/fluorometric) from Abcam (#ab83355, Cambridge, U.K.) according to the manufacturer’s instructions. Results were repeated three times and normalized by protein concentrations of each test.
Measurements of mitochondrial respiration chain activity
The mitochondrial respiration chain activity (complexes I, II, III, IV, and V) was measured using the MitoTox™ Complete OXPHOS Activity Assay Kit (5 Assays) (ab110419) from Abcam, Inc. (Cambridge, MA, U.S.A.) according to the manufacturer’s instructions. Results were repeated three times and normalized by protein concentrations of each test.
Western blot
Rabbit monoclonal anti-Glut1 (#12939), rabbit monoclonal anti-LDHA (#3582), rabbit monoclonal anti-PDK1 (#13037), and mouse monoclonal anti-β-actin (#3700) were purchased from Cell Signaling Technology (Danvers, MA, U.S.A.). Proteins from cells were extracted using RIPA lysis buffer (Beyotime, China) and separated by SDS/PAGE (10% gel). Subsequently, proteins from gel were transferred on to PVDF membranes. After blocking by 5% nonfat milk for 1 h at room temperature, membranes were probed with primary antibodies at 4°C overnight, followed by incubation with horseradish peroxidase conjugated secondary antibodies (Beyotime, China). After washing completely, membranes were detected by ECL method (Beyotime, China).
Statistical analysis
Data are expressed as mean ± S.D. Statistical differences amongst different groups were assessed by Student’s t test using Prism version 5.0 (GraphPad Software, Inc., La Jolla, CA, U.S.A.). P<0.05 was considered to be of statistical significance.
Results
Expressions of miR-138 in cardiac cells are modulated by hypoxia
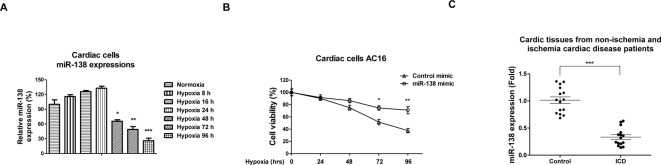
We investigated the roles of miR-138 in cardiac cells under hypoxia. First, we detected expressions of several miRNAs under hypoxia in human cardiac cell line AC16, of which miR-138 was one of the most sensitive miRNAs to hypoxia (results not shown). Consistently, qRT-PCR analysis revealed that miR-138 was slightly induced under hypoxic conditions (1% oxygen concentration) at 8, 16, and 24 h (Figure 1A). Moreover, at 48, 72, and 96 h, we detected that miR-138 was significantly suppressed under hypoxia (Figure 1A), suggesting that miR-138 may be involved in the cellular adaptation processes in response to oxygen supply. Expression of miR-138 was found to be down-regulated in a time-dependent manner and decreased by 5.2-fold at 72-h exposure to hypoxia (Figure 1A). To analyze the potential roles of miR-138 in cardiac cells, we overexpressed miR-138 in AC16 cells by transfection with miR-138 mimics or control miRNAs (Supplementary Figure S1). As we expected, overexpression of miR-138 contributed to the AC16 cell survival under hypoxic condition at 72 and 96 h (Figure 1B). To test whether miR-138 changes in cardiomyocytes during ischemia in vivo, we analyzed the expressions of miR-138 from 15 noncardiac ischemia patients and 15 ischemia cardiac disease (ICD) patients. Consistent with in vitro results, we found expressions of miR-138 were significantly decreased in cardiac tissues of ICD patients (Figure 1C), suggesting that miR-138 plays important functions in human ICD. Taken together, these results demonstrated that miR-138 protects cardiac cells after long hypoxic exposure time.
Figure 1. MiR-138 is down-regulated by long-time hypoxia and negatively correlated with ICD.
(A) Human cardiac cells AC16 were treated with normoxia or hypoxia (1% oxygen) for 8, 16, 24, 48, 72, or 96 h, followed by the measurements of miR-138 expressions by qRT-PCR. (B) AC16 cells were transfected with control mimic or miR-138 mimic at 50 nM for 48 h, cells were exposed to hypoxia at 0, 24, 48, 72, and 96 h. The cell viability was measured by MTT assay. (C) Expressions of miR-138 were analyzed in cardiac tissues from 15 nonischemia and 15 ICD patients by qRT-PCR. All experiments were performed in triplicate. Data are presented with the indication of mean ± S.D. *: P<0.05; **: P<0.01; ***: P<0.001.
MiR-138 inhibits glycolysis and promotes mitochondrial respiration
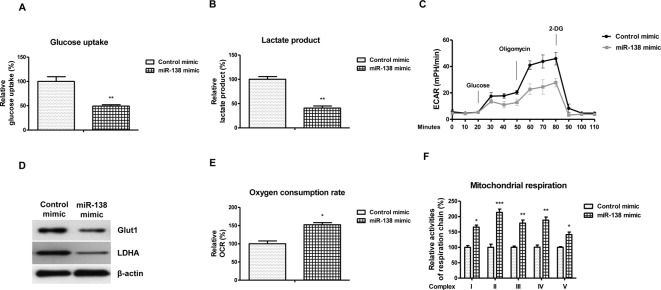
Previous studies demonstrated the cellular glycolysis and mitochondrial respiration were regulated by hypoxia, contributing to cell survival with low oxygen supply [10,11]. To explore the potential functions of miR-138 in the cellular metabolism, we measured the glucose metabolism of AC16 cells under normoxia or hypoxia. We observed that the glucose uptake (Figure 2A), lactate product (Figure 2B), and ECAR (Figure 2C) were significantly inhibited by miR-138 overexpression. Moreover, glycolysis key enzymes, Glut1 and LDHA were found to be suppressed by miR-138 (Figure 2D), suggesting that miR-138 targets the hypoxia-modulated cellular glycolysis in AC16 cells. According to previous report, cells experienced metabolic switch from mitochondrial respiration to glycolysis under hypoxia, we therefore hypothesized that the mitochondrial respiration of human cardiac cells was regulated by miR-138 [10,11]. the oxygen consumption of AC16 cells was significantly decreased with the increase of miR-138 (Figure 2E). Consistently, we detected the activities of complexes I, II, III, IV, and V from mitochondrial respiration chain that were significantly decreased by overexpressing miR-138 (Figure 2F). Taken together, these results suggest that miR-138 inhibits glycolysis but promotes mitochondrial respiration in human cardiac cells.
Figure 2. MiR-138 suppresses glycolysis and promotes mitochondrial respiration of cardiac cells.
AC16 cells were transfected with control mimic or miR-138 mimic at 50 nM for 48 h, then (A) glucose uptake, (B) lactate product, and (C) ECAR were analyzed. (D) Western blot analysis of Glut-1, LDHA protein expression in AC16 cells with control mimic or miR-138 mimic transfection. A representative image is presented using β-actin as the loading reference. (E) AC16 cells were transfected with control mimic or miR-138 mimic at 50 nM for 48 h, then the oxygen consumption rate and (F) activities of complexes I, II, III, IV, and V from mitochondrial respiration chain were analyzed. All experiments were performed in triplicate. Data are presented with the indication of mean ± S.D. *: P<0.05; **: P<0.01; ***: P<0.001.
PDK1 is a direct target of miR-138
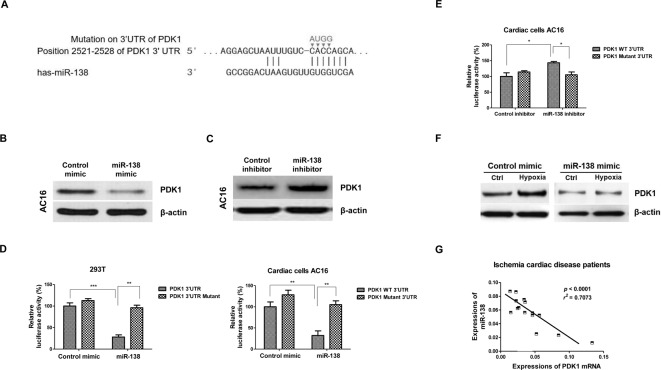
To elucidate the underlying molecular mechanisms for the metabolic switch driven by miR-138, we performed a bioinformatics analysis using three softwares: TargetScan, microrna.org, and Exiqon to predict the putative miR-138 target genes. We observed that the PDK1 contained a conservative miR-13-binding site in its 3′-UTR predicted by all three softwares (Figure 3A and Supplementary Figure S2). Moreover, the miR-138-binding sites on 3′-UTR of PDK1 is conserved in multiple species (Supplementary Figures S3 and S4). PDK1 is a mitochondrial enzyme which inhibits the activity of pyruvate dehydrogenase, a complex of enzymes that converts cytosolic pyruvate into mitochondrial acetyl-CoA [11]. It has been known that PDK1 plays important roles in maintaining cellular glucose metabolism [11]. In addition, inhibition of PDK1 with either siRNAs or inhibitor shifts the metabolism of cancer cells from glycolysis to mitochondrial respiration [20]. Given that miR-138 inhibited glycolysis and promoted mitochondrial respiration (Figure 2), it was strongly supported that PDK1 may be targetted by miR-138. To confirm this prediction, we transfected miR-138 mimics or control mimics into AC16 cells, then measured the protein expression of PDK1. Our results from Western blot showed overexpression of miR-138 suppressed PDK1 expression in AC16 cells (Figure 3B). Moreover, AC16 cells with transfection of miR-138 inhibitor showed up-regulated PDK1 expression compared with control inhibitor transfection (Supplementary Figure 5 and Figure 3C). To test whether PDK1 is a direct target of miR-138, we cloned the wild-type or binding site mutant 3′-UTR of PDK1 (Figure 3A) into pmiR-report vector and cotransfected miR-138 mimics or negative control with vectors in 293T and AC16 cells. The luciferase activity was significantly reduced in miR-138 and wild-type 3′-UTR cotransfection cells compared with that of the negative control and wild-type 3′-UTR cotransfection (Figure 3D). Importantly, the luciferase activity was not reduced in miR-138 and binding site mutant 3′-UTR cotransfection cells (Figure 3D). Consistently, luciferase activity was significantly increased in miR-138 inhibitor and wild-type 3′-UTR cotransfection cells compared with that of the control inhibitor and wild-type 3′-UTR cotransfection (Figure 3E). To investigate whether miR-138 could target PDK1 under hypoxia, we treated AC16 cells without or with miR-138 overexpression with hypoxia. Results in Figure 3F demonstrated that under hypoxia, PDK1 expression was induced. AC16 cells with miR-138 transfection significantly suppressed PDK1 expression under both normoxia and hypoxia conditions (Figure 3F). In addition, we observed a significant negative correlation between PDK1 mRNA expressions and endogenous miR-138 levels in ICD patient samples, indicating that miR-138 could target PDK1 in vivo (Figure 3G). All these findings suggested that miR-138 inhibited PDK1 protein expression by direct binding to its 3′-UTR.
Figure 3. MiR-138 directly targets PDK1.
(A) Target prediction from TargetScan.org, microrna.org, and Exiqon. The 3′-UTR region of PDK1 contains binding sites for miR-138. (B) AC16 cells were transfected with control mimic or miR-138 mimic at 50 nM for 48 h, Western blot analysis of PDK1 protein expression was performed. (C) AC16 cells were transfected with control inhibitor or miR-138 inhibitor at 50 nM for 48 h, Western blot analysis of PDK1 protein expression was performed. A representative image is presented using β-actin as the loading reference. (D) Luciferase assay demonstrated miR-138 mimic bond to 3′-UTR of PDK1 to attenuate luciferase activity but did not affect the luciferase activity of mutant 3′-UTR of PDK1 in 293T (left) and AC16 (right) cells. (E) Luciferase assay demonstrated inhibition of miR-138 increased luciferase activity of the vector containing wild-type 3′-UTR of PDK1 but did not affect the luciferase activity of mutant 3′-UTR of PDK1 in AC16 cells. (F) AC16 cells were transfected with control mimic or miR-138 mimic for 48 h, cells were treated with or without hypoxia. Western blot analysis of PDK1 protein expression was performed. (G) Reverse correlation between miR-138 expressions and PDK1 mRNA was analyzed in cardiac tissues from ICD patients. All experiments were performed in triplicate. Data are presented with the indication of mean ± S.D. **: P<0.01; ***: P<0.001.
Hypoxia suppresses cardiac cells’ viability through increased glycolysis and decreased mitochondrial respiration
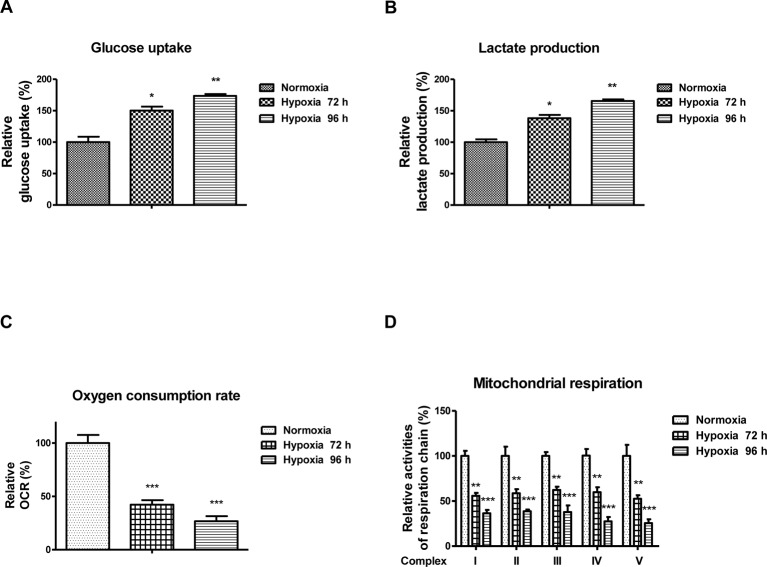
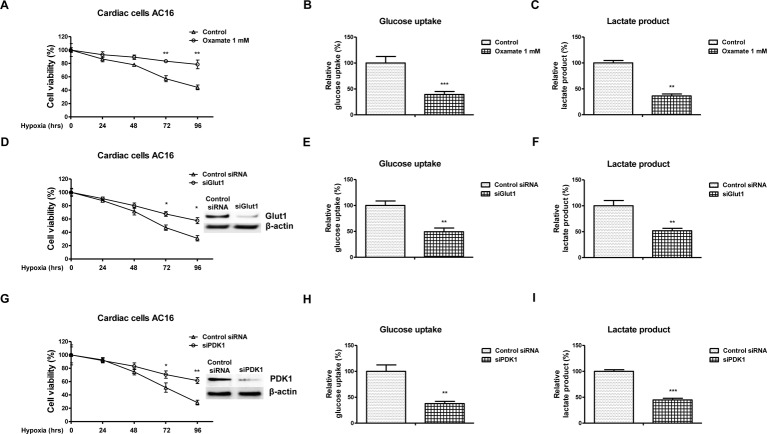
The above results demonstrated roles of miR-138 in regulating cellular metabolism under hypoxia, we next assessed whether the hypoxia-induced miR-138 led to decreased viability of cardiac cells through regulation of glucose metabolism. Consistent with previous reports, cells exhibited up-regulation of glycolysis rate and decreased mitochondria respiration under hypoxia at 72 and 96 h (Figure 4A–D), indicating inhibition of glycolysis might protect cardiac cells from hypoxia-induced dysfunction [7]. To test this, we treated AC16 cells with glycolysis inhibitor, oxamate under normoxia and hypoxia. Cardiac cells displayed attenuated cell viability and glycolysis rate with glycolysis inhibitor treatments under hypoxia (Figure 5A–C). Moreover, we observed similar results with knockdown Glut1 or PDK1 by siRNA. AC16 cells showed decreased cell viability and glycolysis rate with knocking down of Glut1 or PDK1 (Figure 5D–I). Taken together, these results demonstrated that inhibition of glucose metabolism under hypoxia could protect cardiac cells.
Figure 4. Hypoxia induces glycolysis and suppresses mitochondrial respiration.
AC16 cells were treated with normoxia, hypoxia at 72 or 96 h. The (A) glucose uptake, (B) lactate product, (C) oxygen consumption rate, and (D) mitochondrial respiration were analyzed. All experiments were performed in triplicate. Data are presented with the indication of mean ± S.D. *: P<0.05; **: P<0.01; ***: P<0.001.
Figure 5. Inhibition of glycolysis contributes to cardiac cells’ survival under hypoxia.
(A) AC16 cells were treated with or without oxamate at 1 mM for 48 h. Cells were exposed to hypoxia at 0, 24, 48, 72, and 96 h. Cell viability was analyzed by MTT assay, (B) glucose uptake and (C) lactate product were measured. (D) AC16 cells were transfected with control siRNA or siGlut1 for 48 h. Cells were exposed to hypoxia at 0, 24, 48, 72, and 96 h, followed by measurement of cell viability by MTT assay, (E) glucose uptake, and (F) lactate product were measured. The expression of Glut1 was analyzed by Western blot. (G) AC16 cells were transfected with control siRNA or siPDK1 for 48 h. Cells were exposed to hypoxia at 0, 24, 48, 72, and 96 h, followed by measurement of cell viability by MTT assay, (H) glucose uptake and (I) lactate product were measured. The expression of PDK1 was analyzed by Western blot. All experiments were performed in triplicate. Data are presented with the indication of mean ± S.D. *: P<0.05; **: P<0.01. ***: P<0.001.
Overexpression of miR-138 increases the cardiac cells’ viability through targetting PDK1 under hypoxia
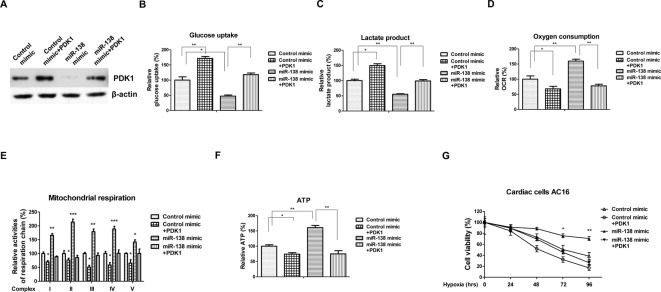
Our results demonstrate overexpression of miR-138 contributed to the AC16 cell survival under hypoxia (Figure 1C). To verify whether the miR-138-mediated anti-apoptotic effects under hypoxia were through the modulation of glucose metabolism, we overexpressed PDK1 in miR-138 overexpressing AC16 cells by cotransfection of control vector or PDK1 overexpression vector with miR-138 (Figure 6A). Western blot results in Figure 6A demonstrated transfection of PKD1 into miR-138 overexpressing cells could efficiently restore the original protein levels of PDK1. Overexpression of miR-138 promoted glucose uptake and lactate product (Figure 6B,C), decreased oxygen consumption, mitochondrial complex activity, and intracellular ATP (Figure 6D–F). Restoration of PDK1 recovered glucose uptake (Figure 6B) and lactate product (Figure 6C) in AC16 cells. Consistently, the oxygen consumption rate (Figure 6D), mitochondrial respiration (Figure 6E), and intracellular ATP (Figure 6F) were decreased by PDK1 restoration in miR-138 overexpressing cells. We next assessed the cell viability of AC16 cells with or without PDK1 restoration under hypoxia. As we expected, restoration of PDK1 in miR-138 overexpressing cells resensitized cells to low oxygen (Figure 6G), indicating that the miR-138-mediated anti-apoptotic effects under hypoxia was through directly targetting PDK1.
Figure 6. Overexpression of miR-138 protects cardiac cells against hypoxia through targetting PDK1.
(A) AC16 cells were transfected with control mimic, control mimic plus PDK1, miR-138 mimic alone, or cotransfection of miR-138 and PDK1 for 48 h. Western blot analysis of PDK1 protein expression. A representative image is presented using β-actin as the loading reference. (B) AC16 cells were transfected with control mimic, control mimic plus PDK1, miR-138 mimic alone, or cotransfection of miR-138 mimic and PDK1 overexpression plasmid for 48 h. The glucose uptake, (C) lactate product, (D) oxygen consumption, (E) mitochondrial respiration, and (F) intracellular ATP were measured. (G) AC16 cells were transfected with control mimic, control mimic plus PDK1, miR-138 mimic alone, or cotransfection of miR-138 and PDK1 for 48 h. Cells were exposed to hypoxia at 0, 24, 48, 72, and 96 h, followed by the detection of cell viability by MTT assay. All experiments were performed in triplicate. Data are presented with the indication of mean ± S.D. *: P<0.05; **: P<0.01; ***: P<0.001.
Discussion
During cardiac surgery, the heart is continually exposed to ischemia and myocardial infarction due to lack of oxygen supply from blood flow. These ischemic episodes cause apoptosis of cardiomyocytes, resulting in dysfunction of heart. To prevent the expansion of these apoptotic regions, the in vitro hypoxic cardiac cells model contributes to understand the potential mechanisms of the hypoxia-induced cardiomyocytes dysfunction. In the present study, we investigated the roles of miR-138 during low oxygen exposure of cardiac cells. qRT-PCR analysis revealed that miR-138 was slightly induced under hypoxic conditions at early time point, suggesting miR-138 may be involved in the cellular adaptation processes in response to oxygen supply. Moreover, miR-138 was significantly suppressed by hypoxia at 48, 72, and 96 h, suggesting that targetting miR-138 might contribute to the prevention of the myocardial infarction mediated heart failure because hypoxia may persist within the infarct for days or weeks, exacerbating the injury. Recent studies demonstrated similar functions of miR-138 in pulmonary artery smooth muscle cells (PASMCs) during hypoxia as our observations [21]. They reported expression of exogenous miR-138 suppressed the PASMC apoptosis and prevented caspase activation through targetting serine/threonine kinase (Mst1).
Previous study demonstrated that overexpression of miR-138 significantly benefited cardiomyocytes from hypoxia-induced cell apoptosis [19]. They indicated that the hypoxia could induce the cardiomyocyte apoptosis, which is the main reason for the cardiac diseases. In the present study, we established the cardiac cell death model under hypoxia. Under conditions of hypoxia, oxygen and nutrients supply are severely hampered, leading to energy deprivation. In response to hypoxia, the ischemic myocardium switches from respiration to glycolytic energy metabolism, with increased glucose consumption, lactic acid production, and lower intracellular pH [22]. Our results showed that overexpression of miR-138 significantly suppressed PDK1 expression through directly targetting 3′-UTR of PDK1 mRNA, resulting in a metabolic switch from the hypoxia-induced glycolysis to mitochondrial respiration. This metabolic switch contributes to protect cardiac cells from hypoxia, presenting local delivery of miR-138 as an approach against cardiac cells dysfunction during surgery or ischemia.
In conclusion, we illustrated that overexpression of miR-138 inhibits the hypoxia-induced cardiac cells death via suppression of the dysregulated glycolysis and recovery of mitochondrial respiration. We report that miR-138 directly targets PDK1, an enzyme which promotes glycolysis. The present study leads to intervention of novel therapeutic strategies against cardiac cells dysfunction during surgery or ischemia.
Supporting information
Figure 1.
Figure 2.
Figure 3.
Figure 4.
Figure 5.
Abbreviations
- ECAR
extracellular acidification rate
- Glut1
Glucose transportor 1
- ICD
ischemia cardiac disease
- PASMC
pulmonary artery smooth muscle cells
- PDC
pyruvate dehydrogenase complex
- PDK1
pyruvate dehydrogenase kinase 1
- qRT-PCR
Real-time Reverse Transcription Polymerase Chain Reaction
Funding
This work was supported by the Scientific Research Fund Project of Chinese Cardiovascular Physicians [grant number 2013QY06-30].
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Author contribution
H.Z. designed the experiment. H.X. prepared the manuscript and carried out the analysis. Q.-H.J., J.G., and Y.-D.C. collected the samples. All the authors read and approved the final manuscript.
References
- 1.Minicucci M.F., Azevedo P.S., Polegato B.F., Paiva S.A. and Zornoff L.A. (2011) Heart failure after myocardial infarction: clinical implications and treatment. Clin. Cardiol. 34, 410–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsutsui H., Kinugawa S. and Matsushima S. (2011) Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 301, H2181–H2190 [DOI] [PubMed] [Google Scholar]
- 3.Li R., Geng H.H., Xiao J., Qin X.T., Wang F., Xing J.H. et al. (2016) miR-7a/b attenuates post-myocardial infarction remodeling and protects H9c2 cardiomyoblast against hypoxia-induced apoptosis involving Sp1 and PARP-1. Sci. Rep. 6, 29082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gullestad L., Ueland T., Vinge L.E., Finsen A., Yndestad A. and Aukrust P. (2012) Inflammatory cytokines in heart failure: mediators and markers. Cardiology 122, 23–35 [DOI] [PubMed] [Google Scholar]
- 5.Kalogeris T., Baines C.P., Krenz M. and Korthuis R.J. (2012) Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 298, 229–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiong M., Wang Z.V., Pedrozo Z., Cao D.J., Troncoso R., Ibacache M. et al. (2011) Cardiomyocyte death: mechanisms and translational implications. Cell Death Dis. 2, e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong S.G., Lee W.H., Theodorou L., Kodo K., Lim S.Y., Shukla D.H. et al. (2014) HIF-1 reduces ischaemia-reperfusion injury in the heart by targeting the mitochondrial permeability transition pore. Cardiovasc. Res. 104, 24–36 [DOI] [PubMed] [Google Scholar]
- 8.Lopaschuk G.D. (2016) Metabolic modulators in heart disease: past, present, and future. Can. J. Cardiol. 33, 838–849 [DOI] [PubMed] [Google Scholar]
- 9.Marín-García J. and Goldenthal M.J. (2002) Fatty acid metabolism in cardiac failure: biochemical, genetic and cellular analysis. Cardiovasc. Res. 54, 516–527 [DOI] [PubMed] [Google Scholar]
- 10.Shao D. and Tian R. (2015) Glucose transporters in cardiac metabolism and hypertrophy. Compr. Physiol. 6, 331–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutendra G. and Michelakis E.D. (2013) Pyruvate dehydrogenase kinase as a novel therapeutic target in oncology. Front. Oncol. 3, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yates L.A., Norbury C.J. and Gilbert R.J. (2013) The long and short of microRNA. Cell 153, 516–519 [DOI] [PubMed] [Google Scholar]
- 13.Zhang H., Liu S., Dong T., Yang J., Xie Y., Wu Y. et al. (2016) Profiling of differentially expressed microRNAs in arrhythmogenic right ventricular cardiomyopathy. Sci. Rep. 6, 28101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thum T. and Lorenzen J.M. (2012) Cardiac fibrosis revisited by microRNA therapeutics. Circulation 126, 800–802 [DOI] [PubMed] [Google Scholar]
- 15.Tian Y., Liu Y., Wang T., Zhou N., Kong J., Chen L. et al. (2015) A microRNA-Hippo pathway that promotes cardiomyocyte proliferation and cardiac regeneration in mice. Sci. Transl. Med. 7, 279ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan Z.X. and Yang J. (2015) The role of microRNAs in regulating myocardial ischemia reperfusion injury. Saudi Med. J. 36, 787–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim G.H. (2013) MicroRNA regulation of cardiac conduction and arrhythmias. Transl. Res. 161, 381–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morton S.U., Scherz P.J., Cordes K.R., Ivey K.N., Stainier D.Y. and Srivastava D. (2008) MicroRNA-138 modulates cardiac patterning during embryonic development. Proc. Natl. Acad. Sci. U.S.A. 105, 17830–17835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He S., Liu P., Jian Z., Li J., Zhu Y., Feng Z. et al. (2013) miR-138 protects cardiomyocytes from hypoxia-induced apoptosis via MLK3/JNK/c-jun pathway. Biochem. Biophys. Res. Commun. 441, 763–769 [DOI] [PubMed] [Google Scholar]
- 20.Zhang S., Hulver M.W., McMillan R.P., Cline M.A. and Gilbert E.R. (2014) The pivotal role of pyruvate dehydrogenase kinases in metabolic flexibility. Nutr. Metab. (Lond.) 11, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li S., Ran Y., Zhang D., Chen J., Li S. and Zhu D. (2013) MicroRNA-138 plays a role in hypoxic pulmonary vascular remodelling by targeting Mst1. Biochem. J. 452, 281–291 [DOI] [PubMed] [Google Scholar]
- 22.Doenst T., Nguyen T.D. and Abel E.D. (2013) Cardiac metabolism in heart failure: implications beyond ATP production. Circ. Res. 113, 709–724 [DOI] [PMC free article] [PubMed] [Google Scholar]