A 9-month-old boy with migrating partial seizures of infancy due to a de novo KCNT1 mutation c.2278A>T (p.Ile760Phe) developed bluish discoloration of the hands, feet, and lips (figure) during a 9-month trial of quinidine (40 mg/kg/d; level 3.4 μg/mL).1 There was no exposure to other medications that cause pigmentary changes. Given minimal improvement in seizures and development, quinidine was stopped. Discoloration persisted at 3 months but markedly improved by the 6-month follow-up. Though common with other potassium channel blockers (ezogabine and quinine), such discoloration has only rarely been reported with quinidine, all in adults.2 Epileptologists should be aware of this potential complication of quinidine therapy.
Figure. Skin discoloration after 9 months of quinidine use.
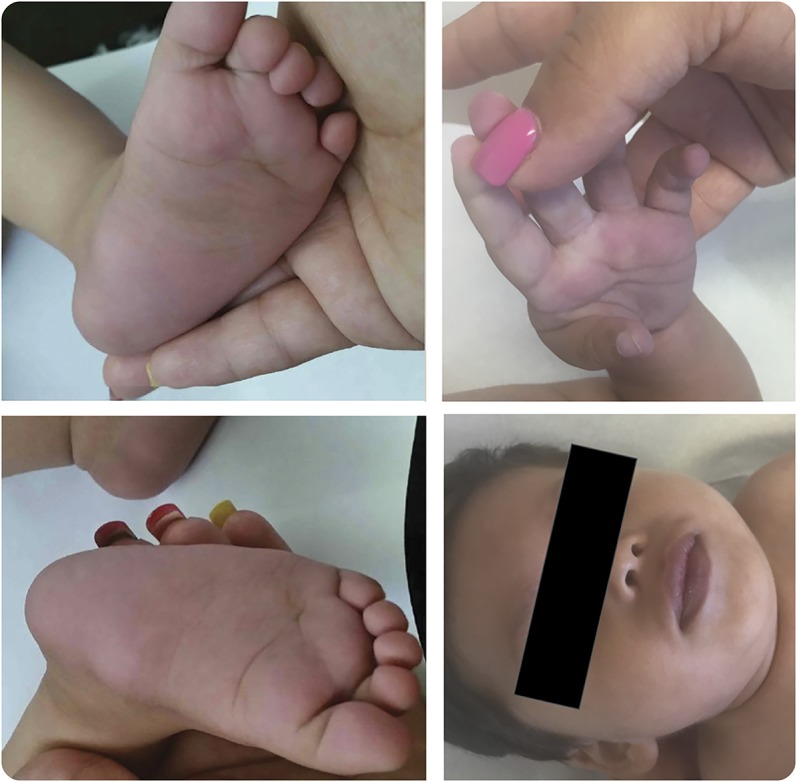
The patient developed bluish discoloration of the hands, feet, and lips in a similar distribution to that seen in ezogabine-related skin discoloration. The sclerae were not affected. A dilated retinal examination has not been performed due to the severity of the patient's illness.
Footnotes
Author contributions: Fiona Baumer: prepared manuscript and took photographs. Maureen Sheehan: critically revised manuscript.
Study funding: Fiona Baumer is supported by a KL2 Mentored Career Development Award of the Stanford Clinical and Translational Science Award to Spectrum (NIH KL2 TR 001083) and UL1 TR 001085.
Disclosure: The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
References
- 1.Bearden D, Strong A, Ehnot J, DiGiovine M, Dlugos D, Goldberg EM. Targeted treatment of migrating partial seizures of infancy with quinidine. Ann Neurol 2014;76:457–461. [DOI] [PubMed] [Google Scholar]
- 2.Conroy EA, Liranzo MO, McMahon J, Steck WD, Tuthill RJ. Quinidine-induced pigmentation. Cutis 1996;57:425–427. [PubMed] [Google Scholar]


