Abstract
The effect of a kinase inhibitor Go6796 on growth of epidermal growth factor (EGF)-stimulated estrogen receptor negative (ER−) breast cancer cells in vivo and role of nuclear factor kappa B (NF-κB) on tumorogenesis have been investigated. This was studied in an animal model by implanting ER− mouse mammary epithelial tumor cells (CSMLO) in syngeneic A-J mice. (i) Local administration of Go6976 an inhibitor of protein kinases C alpha and beta inhibited growth of tumors and caused extensive necrotic degeneration and regression of the tumors without causing any microscopically detectable damage to the vital organs liver and lung. (ii) Stable expression of dominant-negative mutants of the beta subunit (dnIkkβ) of the inhibitory kappa B (IκB) kinase (dnIkk) that selectively blocked activation of NF-κB caused loss of tumorigenic potential of CSMLO cells. Stable expression of dnIkkβ also blocked phorbol 12-myristate 13-acetate (PMA)-induced activation of NF-κB and overexpression of cyclin D1, concomitantly with the loss or reduced tumorigenic potential of these cells. Thus, results from in vivo and in vitro experiments strongly suggest the involvement of NF-κB in ER− mammary epithelial cell-mediated tumorigenesis. We propose that blocking NF-κB activation not only inhibits cell proliferation, but also antagonizes the antiapoptotic role of this transcription factor in ER− breast cancer cells. Thus, NF-κB is a potential target for therapy of EGFR family receptor-overexpressing ER− breast cancers.
The current therapeutic approach with antihormones, targeted at hormone receptors, is effective only in a fraction of breast cancer patients. All estrogen receptor negative (ER−) and also a fraction of ER positive (ER+) tumors do not respond to antihormone treatment (1, 2). Thus, alternative treatment protocols aimed at different targets for these classes of antihormone nonresponsive breast cancers need to be explored.
The level of nuclear factor kappa-B (NF-κB) has been shown to be elevated in ER− human breast cancers, as compared with ER+ cells (3–7). This could be correlated with the increased level of epidermal growth factor family receptors (EGFR) in ER− cells (3, 7, 8–10). Our previous results demonstrated that activation of NF-κB is a downstream consequence of EGF–EGFR interaction (7). A pathway has been proposed for the EGF–EGFR-mediated cell proliferation signal that involves activation of phosphatidylinositol 3-kinase (PI3-kinase), protein kinase C, and NF-κB with overexpression of the downstream cell cycle regulatory protein cyclin D1 (ccD1) and retinoblastoma (Rb) phosphorylation (7). These results, along with its antiapoptotic action, strongly suggest the involvement of activated NF-κB in ER− breast cancers (5, 7–10). This role is examined here with a mouse tumor model generated with an ER− mouse mammary epithelial carcinoma cell line, CSMLO (11, 12).
The role of the NF-κB family of proteins in immune, inflammatory, and apoptotic responses is well documented (6, 13, 14). These are activated by growth factors, cytokines, and mitogens that control cell proliferation, differentiation, and morphogenesis and are transcription factors that activate several cell cycle regulatory proteins (13–17). The role of NF-κB in tumorigenesis is circumstantial, such as higher levels of activated NF-κB in ER− tumor cells. NF-κB exits in an inactive state in most cell types, except B lymphocytes (16). Activation of NF-κB involves phosphorylation of two conserved serines in the N-terminal domain of IκB, which is then degraded by the ubiquitin pathway (18). Signaling of NF-κB activation is a multistep process transmitted by a cascade of kinases leading to activation of the ultimate kinase complex, Ikk, composed of Ikk-α, Ikk-β, and the regulatory protein Ikk-γ (also known as NEMO) (19–21). Different NF-κB activating agents generate diverging signals that ultimately activate Ikk by regulating the function of one of these components. In general, Ikk-β has a much higher level of kinase activity than Ikk-α and plays a critical role for the degradation of IκB and consequently the activation of NF-κB (22, 23). Thus, the Ikk complex is a potential target for controlling NF-κB activation and its functions.
Mice deficient in either Ikk-α, Ikk-β, or both exhibit multiple developmental and morphological defects and enhanced apoptosis leading to embryonic lethality or death at birth that could be correlated to lack of NF-κB activation (24, 25). Enhanced apoptosis in liver causing embryonic lethality observed in Ikk-β-deficient mice could be related to tumor necrosis factor (TNF) signals, because it is overcome in progeny of mating to TNF-null mice (26).
Although a substantial amount of work is done with genetically altered animals leading to stable loss of activation of NF-κB and its consequences, very limited experiments have been done with externally introduced agents that selectively inhibit NF-κB activation. The antiinflammatory activity of a peptide that specifically inhibited the interaction of Ikk-γ with Ikk complex and selectively blocked activation of NF-κB is demonstrated in an animal model (27). We demonstrate here in a mouse tumor model the antitumorigenic activity of a compound that inhibits activation of NF-κB without causing significant detectable cellular damage of vital organs. Furthermore, selective activation of NF-κB by stable expression of a dnIkkβ mutant plasmid induced loss of tumorigenic potential of the parent CSMLO cells, thus strongly suggesting a role of this transcription factor in ER− mammary epithelial cell carcinogenesis.
Materials and Methods
Materials.
The mouse mammary adenocarcinoma cells in culture (CSMLO) were grown in complete medium supplemented with 10% FBS and growth factors as described (7, 11, 12). Anti-human ER antibody (SC543), anti-mouse ccD1 antibody, anti-p50, and anti-p65 antibodies for the NF-κB subunits were obtained from Santa Cruz Biotechnology. Rabbit polyclonal IgG raised against the conserved region of actin and anti-Flag (M2) monoclonal antibody were obtained from Sigma–Aldrich. The fluorescein-conjugated goat anti-mouse IgG was from Oncogene Science. Complementary strands of the oligonucleotide (5′-TCGACAGGGACTTTCCGAGAG-3′) containing the NF-κB motif (bold faced) were custom synthesized by Integrated DNA Technologies (Coralville, IA). The double-stranded NF-κB-oligonucleotide was end-labeled with [γ-32P]ATP (NEN) and T4 kinase (New England Biolabs) as described (7), and was used for electrophoretic mobility shift assay (EMSA). Hydrocortisone, insulin, DTT, dimethyl sulfoxide (DMSO), and phenylmethylsulfonyl fluoride were obtained from Sigma. Hybond nitrocellulose membrane and ECL (enhanced chemiluminescence) immunodetection kits were obtained from Amersham Pharmacia. Go6976, a nonglyosidic indolcarbazole, and an inhibitor of protein kinase C alpha and beta was purchased from Calbiochem–Novabiochem (28, 29).
Plasmid constructs.
cDNA of the dominant-negative Ikk-β activity mutant k44 M (K → M) tagged with amino-terminal Flag sequences was cloned downstream of the cytomegalovirus (CMV) promoter in pCMV5 (22–23). These expression plasmids were provided by Richard B. Gaynor of the University of Texas Southwestern Medical Center, Dallas. For selection of G418-resistant clones the cells were cotransfected with the expression plasmid pcDNA 3.1 (Invitrogen), containing G418-resistant cDNA driven by CMV promoter.
Methods.
Measurements of the level of active NF-κβ by EMSA.
The level of active NF-κβ in the nuclear extracts of control and treated cells was determined by its DNA binding activity by EMSA as described (7, 30, 31). The [32P]DNA–protein complex was identified as a retarded radioactive band in EMSA detected by autoradiography. It was characterized by (i) competition with nonradioactive NF-κB oligonucleotides, (ii) comparative direct binding studies with [γ-32P]-labeled mutant and wild-type NF-κB-oligonucleotide, and (iii) interaction with antibodies to p65 and anti-p50 subunits, as reported (7).
Isolation of G418-resistant transfectants.
To establish stable dominant-negative dnIkkβ-expressing transfectants, the CSMLO cells were transfected with the plasmid K44-M (K → M; refs. 22 and 23) along with the selection plasmid pcDNA 3.1 by using Superfect following the protocol provided by the supplier (Qiagen, Chatsworth, CA). As a negative control, separate batches of cells were transfected with empty vector (vector control) and the pcDNA 3.1. After 48 h, the transfected cells were harvested and plated into four 100-mm dishes and incubated for another 24 h in complete medium. The transfected cells were then grown for several generations in selection medium (complete medium plus 500 μg/ml G418) and incubated under the standard tissue culture conditions. Six individual clones from K44-M (K → M) dnIkkβ-transfected CSMLO cells designated as dnIkkβ1-1 through Ikkβ1-6 and from empty vector control plasmid transfected CSMLO cells designated as vect1-1 through vect1-6 were isolated, maintained in selection medium, and stored in liquid N2. The dnIkk clones containing dnIkkβ-conjugated Flag protein was detected by immunofluorescence technique.
Immunofluorescence detection of Flag-tagged dnIkkβ.
Cells (0.5 × 105) were plated in 8-well chamber slides (Nalge Nunc) and grown in stock medium for 48 h. Cells were then fixed in 4% paraformaldehyde for 10 min followed by incubation in 0.5% Triton X-100 in PBS for 10 min for permeabilization, and were blocked with 10% goat serum in PBS for 1 h. Cells were then incubated for 1h in 1:100 diluted solution of anti-Flag-antibody (M2) in 10% goat serum followed by several washes and incubation in 1:100 solution of the secondary antibody fluorescein-goat-anti-mouse IgG (Oncogene) for the detection of Flag conjugated to dnIkkβ protein. Washed cells were mounted in Vecta Shield (Vector Laboratories) containing 0.1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI) to counter stain the nuclei. To determine the background signal a negative control was performed with the secondary antibody only.
Animal model.
The tumorigenic potential of CSMLO and dnIkkβ-expressing stable transfectants was examined in female A-J mice 2–3 weeks old. For each mouse, 1.0 s× 106 cells were implanted under the dorsal skin. Palpable tumors were consistently detected within 10–15 days following implantation of the cells. Body weight and tumor volumes (32) were monitored weekly. Go6976 (0.5 ml of 0.05 mM stock solution in 0.1% DMSO) was administered to each animal locally two times a week. The control group received the same volume of the solvent (0.5 ml of 0.1%).
Results
Inhibition of Tumor Growth by Go6976.
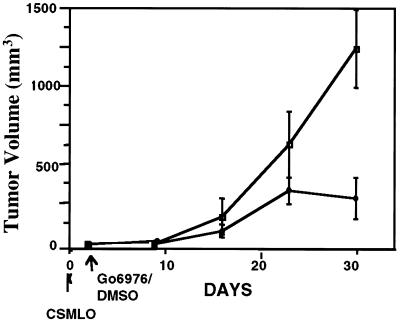
The inhibitory influence of Go6976 on tumor growth was studied by administering the drug within 48 h of implantation of CSMLO cells when no palpable tumors were detected. Periodical measurement of tumor volume (32) revealed a substantial inhibition of tumor growth in Go6976-treated mice in comparison to the untreated controls (DMSO-treated group, five animals in each group, Fig. 1). These results demonstrate that Go6976 inhibited the growth of CSMLO-induced tumors when administered within a short period after implantation of the cells.
Figure 1.
Inhibition of growth of tumors by Go6976. Tumors were generated in female A-J mice by implantation of CSMLO cells (106) on day 0 (indicated by the arrow). Go6976 (0.5 ml of 0.05 mM solution in 0.1% DMSO) was administered 48 h later to a group of five animals, twice a week locally at the site of implantation of cells (♦). Control group (five animals) received 0.5 ml of 0.1% DMSO similarly (□). Growth of tumors was monitored by measurements of tumor volume at regular intervals (32).
Regression of Tumor Growth by Go6976.
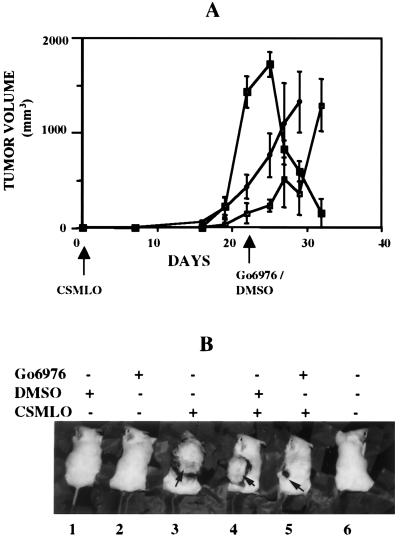
Go6976 not only inhibited tumor growth, but also regression of the full-grown tumors. Tumors in all of the five animals treated with Go6976 21 days after implantation of CSMLO cells regressed sharply (Fig. 2 A and B, animal 5). The second group of tumor-bearing mice (five animals) received nothing (Fig. 2 A and B, animal 3) and the third group (five animals) received the solvent DMSO (Fig. 2 A and B, animal 4). The tumor volumes in the second and the third group of animals continued to increase (Fig. 2 A and B, animals 3 and 4). Mice without tumor treated either with DMSO or with Go6976 (Fig. 2B, animals 1 and 2) did not show any apparent physical defects as judged by body weight and agility, and these parameters were not different from the animals without any treatments (Fig. 2, animal 6).
Figure 2.
Regression of tumors by Go6976. (A) CSMLO cells were implanted on day 0 (indicated by the arrow) as for Fig. 1. Go6976 was administered on day 21 (indicated by the arrow), locally under the tumors of the five animals with comparatively larger tumors (■). Another five animals received the same volume of 0.1% DMSO (♦) on the same day, and the control group of five animals received nothing (□). (B) One representative of five animals from each group. Animals 1 and 2 are DMSO- and Go6976-treated non-tumor-bearing animals, respectively. Animal 3 is tumor-bearing and without treatment. Animals 4 and 5 represent tumor-bearing groups treated with DMSO and Go6976, respectively. Animal 6 is an A-J mouse that received nothing.
Inhibition of PMA-Induced Activation of NF-κB by Go6976 in CSMLO Cells.
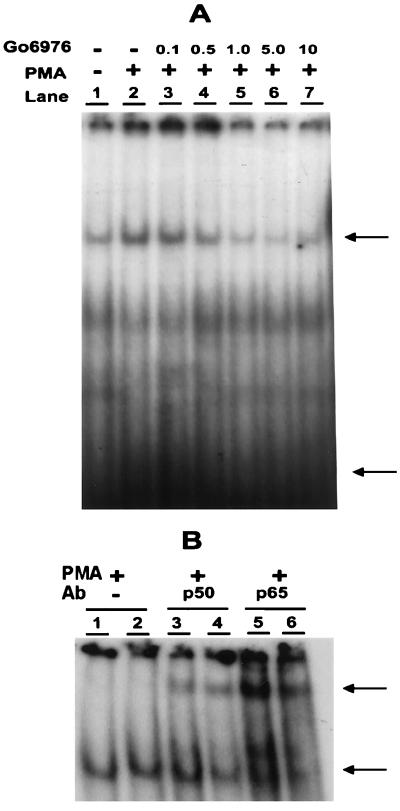
The previously reported NF-κB inhibitory effect of Go6976 in human breast cancer (7) and Jurkat T lymphocytic (33) cell lines is confirmed also in CSMLO cells. CSMLO cells are ER− as determined by Western blot analysis. A low basal level activity of NF-κB was detected in nuclear extracts from CSMLO cells as determined by EMSA (Fig. 3A, lane 1), which was stimulated by PMA (20 ng/ml; lane 2). Activation could be detected at 2–4 h of PMA treatment, was maximal at 6 h, and remained the same for 24 h (data not shown). By simultaneous treatment with Go6976 for 48 h, the PMA-induced elevation of NF-κB activation was inhibited in a concentration-dependent manner (lanes 3–7). The growth of cells under culture conditions was not affected by this short-term drug treatment with 10 μM Go6976 (7). The retarded radioactive DNA–protein complex was supershifted with either anti-p50 (Fig. 3B, lanes 3 and 4) or anti-p65 (lanes 5 and 6) antibody, but not with anti-cRel antibody (data not shown), thus characterizing the active NF-κB in CSMLO cells as a p50/p65 heterodimer.
Figure 3.
Active NF-κB complex in CSMLO cells: Stimulation by PMA and inhibition by Go6976. (A) Nuclear extracts (5 μg protein) from control and PMA-treated (20 ng/ml for 18 h) CSMLO cells were incubated in a standard EMSA reaction mixture containing [γ-32P]-labeled double-stranded oligonucleotide plus Go6976 at the indicated μM concentrations for 48 h and subjected to nondenaturing PAGE (7, 31). The autoradiographic signals of the retarded NF-κB–[32P]DNA complex is indicated by the upper arrow and the free [γ-32P]-labeled probe by the lower arrow. (B) The NF-κB–[32P]DNA complex was characterized by supershift assay with anti-p50 (lanes 3 and 4) or p65 (B, lanes 5 and 6) antibodies. Nuclear extracts were incubated with specific antibodies for 15 min at room temperature, followed by incubation for an additional 30 min in the presence of [γ-32P] double-stranded NF-κB oligonucleotide, and subjected to EMSA as described (7, 31). The supershifted complexes are indicated by the upper arrow.
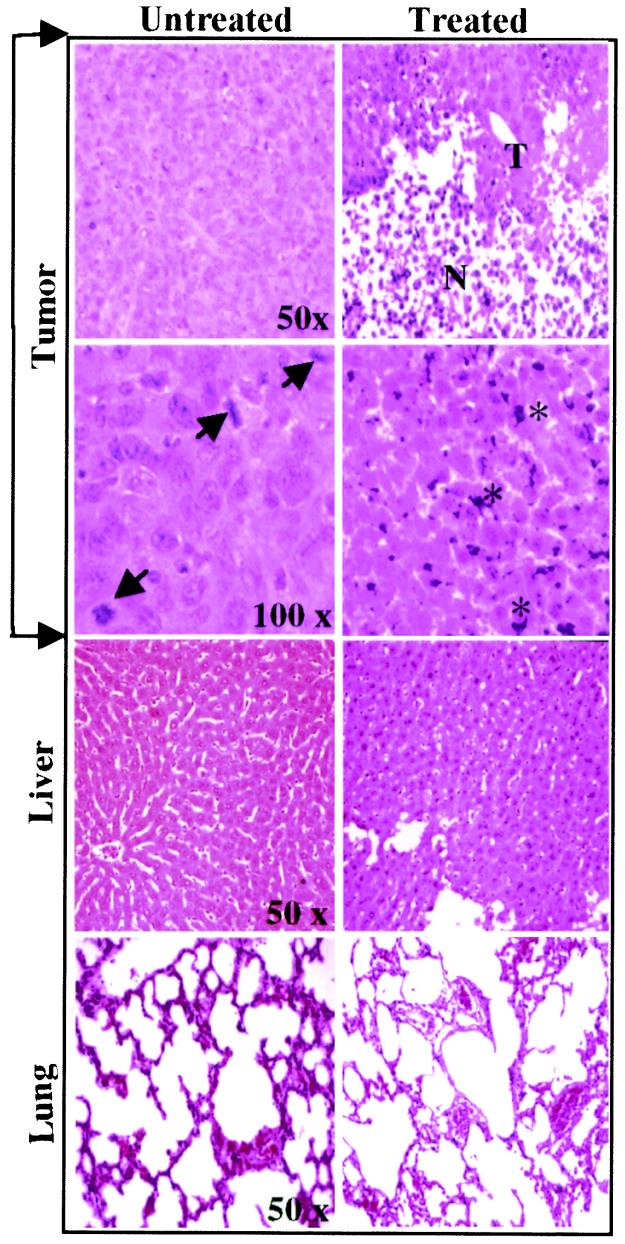
Fig. 4 shows histological changes in tumor, liver, and lung tissues from untreated (Left) and Go6976-treated animals (Right). Tumor tissues from untreated and DMSO-treated (data not shown) animals demonstrated characteristic tumor cell phenotypes, such as enlarged nuclei and increased mitotic index (indicated by arrowheads). In contrast, in Go6976-treated animals extensive necrosis (indicated by N) of the tumor tissues was detected with few residual tumor cells (indicated by T). Similar histological examination of liver and lung tissue (Fig. 4) sections from tumor bearing animals (Fig. 2B, animals 3, 4, and 5) or non-tumor-bearing animals (data not shown) did not show any microscopically detectable pathological changes. Because CSMLO cells are characterized as noninvasive, these observations were expected (11, 12). Examination of residual tumor tissues in treated animal at higher magnification (100×) revealed a large number of pycnotic cells with nuclear clumping suggestive of DNA fragmentation and apoptosis (indicated by stars in Right), whereas this was not observed in tumor tissues from untreated animals (Left). These results demonstrated that localized Go6976 treatment selectively killed the target tumor cells at a concentration not toxic to the vital organs, liver, and lung. These results (Figs. 1 and 2) and prior data (7) suggest that Go6976 is a selective killer of ER− mouse mammary epithelial tumor cells.
Figure 4.
Histology of tissues from untreated and Go6976-treated tumor-bearing animals. Tumor growth and treatment conditions are the same as described for Fig. 1. Tumor, liver, and lung tissues from untreated and treated tumor bearing animals were dissected 11 days after the initiation of treatment and 32 days after implantation of the cells. Tissues were processed for hematoxylin/eosin (H&E) staining, examined under a light microscope, and photographed at the indicated magnifications. Arrows show mitotic cells in untreated tumor tissue. Residual tumor (T) and necrotic cells (N) of the treated tumor are shown. Stars in the treated block indicate pycnotic cells with apparent fragmentation and clumping of nuclear DNA. Treated and untreated liver and lung tissues did not show significant microscopically detectable damages and were not different from liver and lung tissues of normal mice without tumors (not shown).
Selective Inhibition of NF-κB Activation by Stable Expression of Dominant-Negative Ikk-β (dnIKKβ) Mutants.
Our second experimental approach for examining the role of NF-κB in the CSMLO cell-induced tumorigenesis was to selectively block activation of the NF-κB–IκB complex and thereby retain it in its inactive state in the cytoplasm. This was accomplished by the stable expression of a dominant-negative mutant of one of the subunits of Ikk. Because Ikk-β is more potent in activating NF-κB than is Ikk-α, we focused our initial studies on dnIkkβ-expressing transfectants and compared them with parental CSMLO cells and transfectants expressing the vector control plasmid. The dnIkkβ-expressing transfectants grew comparatively slower than the parent CSMLO cells.
Immunofluorescent Detection of Flag-Tagged dnIkkβ in Transfectants.
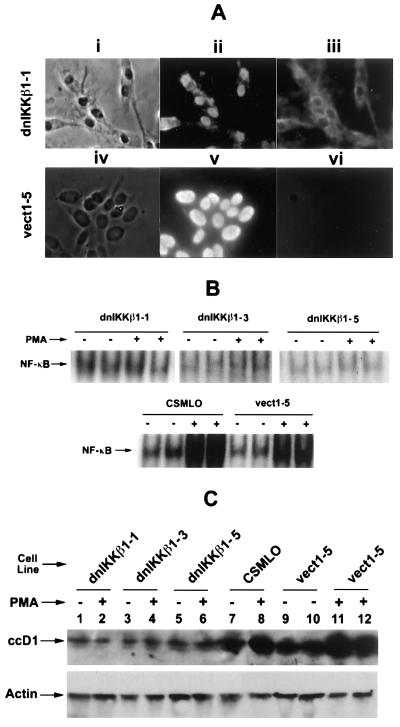
The dnIkkβ transfectants were characterized by identifying the Flag protein tagged to it by an immunofluorescence technique using Flag-specific antibody. Fig. 5A demonstrates the immunofluorescence signal of one dnIkkβ transfectant (Upper, iii) that represents the exogenously introduced Flag-tagged dominant-negative mutant dnIkkβ protein in the cytoplasm. A G418 resistant transfectant with empty vector plasmid did not show this fluorescence (Lower, vi), suggesting that the positive signal observed in this transfectant is specific for Flag-tagged dnIkkβ protein.
Figure 5.
Inhibition of NF-κB activation and down stream events by stable expression of dominant-negative IκB-kinase β (dnIkkβ). (A Upper) dnIkkβ-Expressing transfectant cells by light microscopy (i), 4′,6-diamidino-2-phenylindole (DAPI)-stained nuclei (ii), and immunofluorescence with anti-FLAG antibody in the presence of the secondary antibody (iii). The positive signals show dnIkkβ-conjugated FLAG protein in the cytoplasm. (Lower) Processed vector-control plasmid-transfected CSMLO cells in which no FLAG protein could be detected (vi). (B Upper) Active NF-κB was determined by its [γ-32P]DNA binding activity by EMSA, in three dnIkkβ-expressing stable transfectants (dnIkkβ1-1, dnIkkβ1-3, and dnIkk-β1-5) and (Lower) in parent CSMLO cells and vector control plasmid expressing transfectant (vect1-5). (C Upper) The level of ccD1 in the same three-dnIkkβ-expressing CSMLO and vector control transfected cells, as measured by Western blot analysis. (Lower) Actin analyzed similarly by immunodetection with anti-β-actin antibody that serves as a loading control.
The Level of Active NF-κB in dnIkkβ-Expressing Transfectants.
The functional state of NF-κB in the dnIkkβ-expressing transfectants was established by measuring its DNA-binding activity with EMSA. The basal level of active NF-κB was not further stimulated by PMA in three different clones of dnIkkβ-expressing transfectants as judged by its unaltered [32P]DNA binding activity (Fig. 5B Upper). In contrast, the basal level of active NF-κB was strongly elevated by PMA in parent CSMLO cells and in transfectants with vector control plasmid vect-1–5 (Fig. 5B Lower). These results demonstrated that PMA activation above the basal level of NF-κB is blocked by externally introduced dnIkkβ, thereby establishing the experimental goal of selective inhibition of NF-κB activation by specifically targeting the Ikk with dnIkkβ.
The Level of ccD1 in dnIkkβ-Expressing Transfectants.
Up-regulation of the ccD1 is a downstream consequence of NF-κB activation (7, 34). Similar to the results obtained in human breast cancer cells (7), Go6976 also blocked transactivation of ccD1 in CSMLO cells transfected with dnIkkβ. The elevated level of ccD1 following PMA treatment measures activation of the NF-κB–IκB complex. Fig. 5C demonstrates that ccD1 was not altered by PMA treatment of the three clones of dnIkkβ-expressing transfectants (5C, lanes 1–6) in which NF-κB activation was effectively blocked (5B, Upper), whereas ccD1 level was substantially elevated by PMA in parental and transfectants carrying the control plasmid vect-1-5 (5C, lanes 7–12). These levels of ccD1 in CSMLO cells and transfectants correlates well with activation of NF-κB–IκB complex (Fig. 5B). The basal level of ccD1 in the dnIkkβ-expressing transfectants was lower than that in the parent and vector-control-expressing cells. This may be a reflection of the short half-life of ccD1. Thus, ability to up-regulate downstream ccD1 gene expression following PMA treatment in the parent CSMLO and in transfectant expressing vector control plasmid, and the inability to do the same in dnIkkβ-expressing transfectants can be correlated to the level of activation of inactive NF-κB–IκB complex in these cells.
Tumorigenic Potential of CSMLO Cell-Expressing dnIKKβ Mutants.
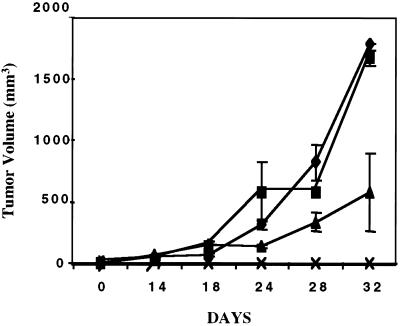
The consequence of blocked NF-κB activation in dnIkkβ-expressing transfectants on tumor growth was examined in the mouse tumor model. Fig. 6 shows volumes of tumors induced by CSMLO cells (five animals), two transfectants with vector control plasmid (six animals, three per clone), and two transfectants expressing dnIkkβ (six animals, three per clone). The CSMLO cells and transfectants carrying the vector control plasmid generated tumors similarly. One dnIkkβ-expressing clone (dnIkkβ1-1) did not form tumor in any of the three animals even after 32 days. The other dnIkkβ-expressing clone (dnIkkβ1-3) formed tumors at a substantially reduced rate and of smaller sizes compared with those formed by the CSMLO or the vector control plasmid expressing transfectants (Fig. 6). The loss or decreased tumorigenic potential of the dnIkkβ-expressing transfectants, compared with parent and the vector controls was in agreement with the reduced level of NF-κB activation and cellular level of ccD1. All of these results, especially those with dnIkkβ expression that selectively blocked NF-κβ–IκB activation and ccD1 transactivation, strongly suggest a role of NF-κB in tumorigenesis in ER− mammary epithelial cells.
Figure 6.
Loss of tumorigenic potential of CSMLO cells by the stable expression of pdnIkkβ in A-J mice. Tumors were generated in female A-J mice by implanting either CSMLO cells (five animals), or two vector control plasmid transfected clones (vect1-3 and vect1-5, three animals per clone) or two pdnIkkβ-expressing clones (pdnIkkβ1-1 and pdnIkkβ-1-3, three animals per clone). Average with standard deviations of CSMLO-administered animals (♦), vector-control-administered animals (■, six animals), and one of the two pdnIkkβ-expressing-clones (pdnIkkβ1-3)-administered animals (▵, three animals) are plotted. The other pdnIkkβ1-1 clone did not form any tumor even 32 days after implantation of the cells in any one of the three animals (ρ).
Discussion
In this study the role of NF-κB on the tumorigenic potential of ER− breast cancer cells was examined in an animal model. The rationale for selecting NF-κB as a therapeutic target is based on (i) the increased level of activated NF-κB observed in many human breast tumors and (ii) on our previous results on its role in enhanced proliferation and cell cycle progression in ER− human breast cancer cells (7).
The requirement of active NF-κB for tumor growth was demonstrated first by blocking its activation with Go6976, a PKC inhibitor, and more specifically by expression of dnIkkβ. Go6976 blocked ER− tumor growth in mice and caused regression of established tumors that could be correlated with the drug's inhibition of NF-κB activation. Anti-cell-proliferation activity of Go6976 may be caused by decreased NF-κB activation and down-regulation of ccD1 and the subsequent cell cycle progression (7). Although the inhibitory influence of Go6976 on PKCα and -β has been well characterized (28, 29), its influences on other kinases has not been documented. Thus, the inhibition by Go6976 of any one of these other kinases that are involved in the activation of NF-κB, in addition to PKC, is not eliminated.
The specific role of active NF-κB for tumor growth in mice was demonstrated with stable expression of dnIkkβ mutant plasmid that selectively blocks NF-κB activation (22, 23) and thereby the downstream event of transactivation of ccD1 (Fig. 5B). Although the growth rate of dnIkkβ-expressing transfectants was reduced by a factor of about 1.25 (doubling time about 60 h) in comparison to the CSMLO cells and vector-control-expressing transfectants (doubling time about 48 h), the vast difference in tumor growth between the former and the latter cannot be attributed only to this retarded cell growth rate. The antitumorigenic effect of NF-κB is a net outcome of its multiple influences on key cellular events such as apoptosis (14), angiogenesis (35), and cell proliferation (14). Both the pharmacological and genetic manipulations provided support for a direct role of this transcription factor in tumorigenesis by ER− breast cancer.
Furthermore, Go6976 caused established tumors to regress rapidly, and their microscopic examination demonstrated DNA clumping and pycnotic cells, suggestive of activation of apoptosis. This drug blocked the kinase-dependent NF-κB activation, but did not affect the basal level of NF-κB. The drug-treated cells maintained limited cell proliferation and induced apoptosis that is consistent with the reported antiapoptotic activity of NF-κB. Thus, Go6976 blocked the antiapoptotic action of NF-κB by selectively blocking its activation in CSMLO cells. Thus, the drug has a dual effect against tumors—one is inhibition of NF-κB-mediated cell proliferation, and its more novel activity is to decrease the antiapoptotic activity specific to tumor cells.
The major effect of Go6976 against the tumor was seen without mouse lethality or affecting gross physical damage to the mice or to their vital organs. As its activation is a downstream consequence of the signal initiated by the EGF–EGFR family receptor interaction (7), NF-κB might provide a target for therapy of ER− breast cancer patients with elevated EGFR family receptors. A combination of the current treatment protocol with Herceptin and inhibitors of NF-κB activation, such as Go6976, might provide more effective therapy for this class of human breast cancers. Go6976 might also be useful against the 2/3 of ER− breast cancers that do not express EGFR, and which are not responsive to either Herceptin or classical antihormone treatments. Activation in these cells of NF-κB by means of signal transduction pathways other than that initiated by EGF–EGFR interaction and its role in tumorigenesis may be postulated. For example, in contrast to EGF, transforming growth factor β (TGF-β) inhibits proliferation of breast cancer cells, and this antiproliferation effect is regulated by the NF-κB/Rel family of transcription factors (36, 37). The observed low TGF-β in ER− cells may be associated with the loss of this suppression mediated through TGF-β/Smads signaling for NF-κB/Rel A activation in this type of breast cancer cell (5, 36). NF-κB is also a target of platelet-derived growth factor (PDGF) and this signal is transmitted by the ras/phosphatidylinositol 3-kinase (PI3-kinase)/AKT/Ikk/NF-κB pathway (38).
Toxicity has been one of the major hurdles for defining the role of NF-κB in animals, more specifically those genetically modulated. It is anticipated that inhibition of activation of NF-κB will adversely affect major cellular functions, such as immune responses of B lymphocytes. The up-regulation of this transcription factor in inflammatory diseases is documented, as is the fact that an inhibitor of Ikk reversed inflammatory reactions in an animal model without any adverse effects (27). Similarly, inhibition of the activation of NF-κB by adenoviral delivery of the inhibitory protein IκBα (39) or by inhibition of proteosome-mediated degradation of IκB (40) increased apoptosis and made tumor cells more sensitive to chemotherapy. Our results and the results of other investigators (27, 39, 40) suggest that therapeutic strategies targeted at the inhibition of NF-κB activation are feasible. The residual basal activity of NF-κB remaining after drug treatment may suffice to preserve normal cell viability.
We hypothesize that a basis for the selective toxicity of the drug to ER− breast cancer cells (7) is due to their development of an anti-antiapoptotic response. Initially, normal cells are transformed to activate molecular proliferation signals. These signals then also “clash” with normally growth-regulating signals to activate apoptosis (41). Antiapoptotic mutants that permit some cells to survive are then selected during tumor progression; such antiapoptotic mutations as those of p53, Bcl-2, and NF-κB are frequent in advanced cancers. Thus, a basis for the therapeutic index of inhibitors of NF-κB activation is their activity against a specific antiapoptotic function developed in tumor vs. normal cells.
Acknowledgments
We are thankful to Dr. Richard B. Gaynor of the University of Texas Southwestern Medical Center, Dallas, for providing the recombinant plasmids expressing the dominant negative IKK subunits. This work is supported by a research grant from the Breast Cancer Research Program of Massachusetts Department of Public Health, Boston. E.G. is supported by Fundaçao de Amparo à Pesquisa do Estado de Sao Paulo (Grant 99/08179-5).
Abbreviations
- NF-κB
nuclear factor kappa B
- ER
estrogen receptor
- PMA
phorbol 12-myristate 13-acetate
- EMSA
electrophoretic mobility shift assay
- ccD1
cyclin D1
- dnIkkβ
dominant-negative mutants of the beta subunit
- Rb
retinoblastoma
References
- 1.Jordan V C. Breast Cancer Res Treat. 1995;36:267–285. doi: 10.1007/BF00713399. [DOI] [PubMed] [Google Scholar]
- 2.Hedden A, Muller V, Jensen E V. Ann NY Acad Sci. 1995;761:109–120. doi: 10.1111/j.1749-6632.1995.tb31373.x. [DOI] [PubMed] [Google Scholar]
- 3.Nakshatri H, Bhat-Nakshatri P, Martin D A, Goulet R J, Jr, Sledge G W. Mol Cell Biol. 1997;17:3629–3639. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhat-Nakshatri P, Newton T R, Goulet R, Jr, Nakshatri H. Proc Natl Acad Sci USA. 1998;95:6971–6976. doi: 10.1073/pnas.95.12.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sovak M A, Bellas R E, Kim D W, Zaneiski G J, Rogers A E, Traish A M, Sonenshein G E. J Clin Invest. 1997;100:2952–2960. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rayet B, Gelinas C. Oncogene. 1999;18:6938–6947. doi: 10.1038/sj.onc.1203221. [DOI] [PubMed] [Google Scholar]
- 7.Biswas D K, Cruz A P, Gansberger E, Pardee A B. Proc Natl Acad Sci USA. 2000;97:8542–8547. doi: 10.1073/pnas.97.15.8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun L, Carpenter G. Oncogene. 1998;16:2095–2102. doi: 10.1038/sj.onc.1201731. [DOI] [PubMed] [Google Scholar]
- 9.Eithier S P. J Natl Cancer Inst. 1995;87:964–973. doi: 10.1093/jnci/87.13.964. [DOI] [PubMed] [Google Scholar]
- 10.Galang C K, Garcia-Ramirez J J, Solski P A, Westwick J K, Der C J, Neznanov N N, Oshima R G, Hauser C A. J Biol Chem. 1996;271:7992–7998. doi: 10.1074/jbc.271.14.7992. [DOI] [PubMed] [Google Scholar]
- 11.Senin V M, Buntsevich A M, Afanasyeva A V, Kiseleva N S. Exp Oncol USSR. 1983;5:35–38. [Google Scholar]
- 12.Ebralidze A, Tulchinsky E, Grigorian M S, Afanasyeva A, Senin V, Revazova E, Lukanidin E. Genes Dev. 1989;3:1086–1093. doi: 10.1101/gad.3.7.1086. [DOI] [PubMed] [Google Scholar]
- 13.Baeurle P A, Baltimore D. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 14.Baldwin A S. Annu Rev Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 15.Pahl H L. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 16.Sen R, Baltimore D. Cell. 1986;46:705–716. [PubMed] [Google Scholar]
- 17.Verma I M, Stevensen J K, Schwarz E M, VanAntwarp D, Miyamoto S. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 18.Karin M. Oncogene. 1999;18:6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 19.Rothwarf D M, Zandi E, Natoli G, Karin M. Nature (London) 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 20.Yamaoka S, Courtuis G, Bessia C, Whiteside S T, Weil R, Agon F, Kirk H E, Kay R J, Israel A. Cell. 1998;93:1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 21.Mercurio F, Murry B W, Shevchenko A, Bennett B L, Young D B, Li J W, Pascual G, Motiwala A, Zhu H, Mann M, Manning A M. Mol Cell Biol. 1999;19:1526–1538. doi: 10.1128/mcb.19.2.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin M-J, Christenson L B, Yamamoto Y, Kwak Y-T, Xu S, Mercurio F, Burlossa M, Cobb M H, Gaynor R B. Cell. 1998;93:875–884. doi: 10.1016/s0092-8674(00)81447-6. [DOI] [PubMed] [Google Scholar]
- 23.Yin M-J, Yamamoto Y, Gaynor R B. Nature (London) 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 24.Li Q, Estepa G, Memet S, Israel A, Verma I M. Genes Dev. 2000;14:1729–1733. [PMC free article] [PubMed] [Google Scholar]
- 25.Li Q, Lu Q, Hwang J Y, Buscher D, Lee K F, Izpisua-Belmonte I C, Verma I M. Genes Dev. 1999;13:1322–1328. doi: 10.1101/gad.13.10.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Q, Van Antwarp D, Mercurio F, Lee K F, Verma I M. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- 27.May M J, D'Acquisto F, Madge L A, Glockner J, Pober J S, Ghosh S. Science. 2000;289:1550–1553. doi: 10.1126/science.289.5484.1550. [DOI] [PubMed] [Google Scholar]
- 28.Qatasha K A, Rudolph C, Marme D, Schachtele C, May W S. Proc Natl Acad Sci USA. 1993;90:4674–4678. doi: 10.1073/pnas.90.10.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martiny-Bbaron G, Kazaniertz M G, Blumberg P M, Kochs G, Hug H, Marme D, Schachtele C. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- 30.Dignam J D, Lebovitz R M, Roeder R D. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biswas D K, Reddy P M, Pickard M, Makkad M, Pettit N, Pardee A B. J Biol Chem. 1998;273:33817–33824. doi: 10.1074/jbc.273.50.33817. [DOI] [PubMed] [Google Scholar]
- 32.Grigorian M, Ambartsumian N, Lykkesfeldt A E, Bastholm L, Elling F, Georgiev G, Lukanidin E. Int J Cancer. 1996;67:831–841. doi: 10.1002/(SICI)1097-0215(19960917)67:6<831::AID-IJC13>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 33.Biswas D K, Ahlers C M, Dezube B J, Pardee A B. Proc Natl Acad Sci USA. 1993;90:11044–11048. doi: 10.1073/pnas.90.23.11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hinz M, Krappmann D A, Eichten A, Heder C, Scheidreit C, Strauss M. Mol Cell Biol. 1999;20:1448–1459. doi: 10.1128/mcb.19.4.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dikov M M, Oyama T, Cheng P, Takashi T, Takahashi K, Sepetavec T, Edwards B, Adachi Y, Nadaf S, Daniel T, Carbon D P. Cancer Res. 2001;61:2015–2021. [PubMed] [Google Scholar]
- 36.Sovak M A, Arsura M, Zanieski G, Kavanagh K T, Soenenshein G E. Cell Growth Differ. 1999;10:537–544. [PubMed] [Google Scholar]
- 37.Lopez-Rovira T, Chjalaux E, Rosa J L, Bartrons R, Ventura F. J Biol Chem. 2000;275:28937–28946. doi: 10.1074/jbc.M909923199. [DOI] [PubMed] [Google Scholar]
- 38.Ramashokova J A, Makarov S S. Nature (London) 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 39.Wang C-Y, Cusack J C, Liu R, Baldwin A S., Jr Nat Med. 1999;5:412–417. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- 40.Cusack J C, Jr, Liu R, Houston M, Abendroth K, Elliott P J, Adams J, Baldwin A S., Jr Cancer Res. 2001;61:3535–3540. [PubMed] [Google Scholar]
- 41.Evan G, Littlewood T. Science. 1998;281:1317–1322. doi: 10.1126/science.281.5381.1317. [DOI] [PubMed] [Google Scholar]