Abstract
Increasing evidence suggests that apoptosis may be the underlying cell death mechanism in the selective loss of dopaminergic neurons in Parkinson's disease. Because the inhibition of caspases provides only partial protection in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/1-methyl-4-phenylpyridinium (MPTP/MPP+) model of Parkinson's disease, we investigated the role of the proapoptotic c-Jun N-terminal kinase (JNK) signaling cascade in SH-SY5Y human neuroblastoma cells in vitro and in mice in vivo. MPTP/MPP+ led to the sequential phosphorylation and activation of JNK kinase (MKK4), JNK, and c-Jun, the activation of caspases, and apoptosis. In mice, adenoviral gene transfer of the JNK binding domain of JNK-interacting protein-1 (a scaffold protein and inhibitor of JNK) inhibited this cascade downstream of MKK4 phosphorylation, blocked JNK, c-Jun, and caspase activation, the death of dopaminergic neurons, and the loss of catecholamines in the striatum. Furthermore, the gene transfer resulted in behavioral benefit. Therefore, inhibition of the JNK pathway offers a new treatment strategy for Parkinson's disease that blocks the death signaling pathway upstream of the execution of apoptosis in dopaminergic neurons, providing a therapeutic advantage over the direct inhibition of caspases.
Today, only symptomatic and no neuroprotective therapies are available for the treatment of Parkinson's disease (PD). Pathologically, the hallmark of PD is loss of dopaminergic neurons in the substantia nigra, leading to the major clinical and pharmacological abnormalities that characterize the disease. In the recent years, mutations in four genes have been identified to cause either autosomal-dominant or autosomal-recessive PD in rare families (1, 2). Although the genetic contribution to the etiology of PD is indisputable in some cases, the majority of PD is sporadic, and the cause of neuronal loss in the substantia nigra is not known. Some advances have been made in defining morphological and biochemical events in the pathogenesis of the disease. Inhibition of oxidative phosphorylation, excitotoxicity, and generation of reactive oxygen species are considered important mediators of neuronal death in PD (3, 4). Although evidence implicating morphological characteristics of apoptosis in PD has remained controversial (5, 6), the major executioners of apoptosis, caspases, are activated in dopaminergic substantia nigra neurons (7).
Insights into the pathogenesis of PD have been achieved experimentally by using the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Chronic administration of MPTP activated caspases (8) and induced apoptosis in the substantia nigra pars compacta (SNpc) of mice (9), whereas apoptosis was not found in a more acute dosing regimen (10). Apoptosis is regulated by a number of proapoptotic and antiapoptotic genes (11, 12). We have shown recently that peptide and protein inhibitors of caspases prevent loss of dopaminergic neurons in MPTP/1-methyl-4-phenylpyridinium (MPP+) toxicity but do not preserve functional integrity of these neurons (8). We now asked whether interfering with the signals leading to apoptosis would result in better outcome than inhibiting the final execution of cell death.
Persistent activation of c-Jun N-terminal kinases (JNKs) has been demonstrated in various apoptotic cell death paradigms (13–15). Increased striatal JNK activity induced by exposure to high concentrations of dopamine was accompanied by apoptosis (16). Recently, two cytoplasmic proteins that bind selectively to JNK, known as JNK interacting proteins 1 and 2 (JIP-1 and JIP-2) (17, 18), were isolated. JIP-1/2 are scaffold proteins that bind to several components of the JNK signaling cascade including the JNK group of mitogen-activated protein kinases (MAPKs), the MAPK kinase isoform MKK7, and members of the mixed-lineage kinase group of MAPK kinase kinases (19). Overexpression of JIP-1 causes the cytoplasmic retention of JNK and thereby inhibits gene expression mediated by the JNK signaling pathway (17). However, interpretation of these results is complicated by the large number of proteins with which JIP-1 interacts. JIP-1 contains two well described domains: the C-terminal PTB domain of JIP-1 interacts with RhoGEF and may be involved in growth cone remodeling (20), consistent with the location of JIP-1 in nascent growth cones, whereas the JNK binding domain (JBD) of JIP-1 is located at the N terminus (17, 19). Expression of the JBD has been shown to be an effective inhibitor of JNK in PC12 cells (17).
Here, we report that MPTP/MPP+ activates the JNK/c-Jun pathway in vivo and in vitro, and that adenovirus-mediated gene transfer of JBD protects dopaminergic cells from MPTP/MPP+ toxicity downstream from MKK4 phosphorylation.
Materials and Methods
Viral Vector Construction and Virus Purification.
Recombinant, E1-deleted, adenoviral constructs (AdV) were produced according to standard techniques. The flag-tagged JBD of JIP-1 (residues 127–281) was cloned into the AdV transfer vector pXCXCMV under the control of the human cytomegalovirus major IE promoter enhancer fragment (663 bp of pcDNA 1; Invitrogen) followed by a bovine growth hormone polyA tail. Recombinant virus (AdV-JBD) was generated by homologous recombination with pJM17 in 293 cells (American Type Culture Collection), grown to high titer and purified by CsCl density-gradient ultracentrifugation. The AdV-EGFP vector was constructed as described previously (21).
Cell Culture and Infection with Viral Vectors.
Human neuroblastoma SH-SY5Y cells were cultured as described (8). At 80% confluency (2 days after plating), the volume of conditioned medium was reduced to one-third, and recombinant adenovirus was added at 150 multiplicity of infection. After allowing the virus to be absorbed for 30 min, the conditioned medium was returned to the culture. After 48 h, the cells were treated with 5 mM MPP+ or vehicle.
Cell lysates of SH-SY5Y cells were harvested at 12 h, and cell viability was measured by using the trypan blue-exclusion assay at 30 h after MPP+ treatment as described (22). The cell viability was expressed as percentages of surviving cells versus sum of surviving and dead cells. For immunohistochemical analysis of flag-tagged JPD expression, the cells were washed in PBS twice and then fixed in 4% paraformaldehyde (PFA) in PBS (pH = 7.4) for 10 min. After washing in PBS for three times, the cultures were used for immunohistochemical analysis.
Adenovirally Mediated Gene Transfer in Vivo.
Animal use was in accordance with the European Convention for Animal Care and Use of Laboratory Animals and was approved by the local Animal Care Committee. Twelve-week-old male C57BL/6 mice were anesthetized with methohexital (50 mg/kg i.p., Lilly, Bad Homburg, Germany) in PBS, and 2.6 × 107 plaque-forming units of the recombinant adenovirus in a volume of 2 μl or 2 μl of PBS (pH = 7.4) were stereotaxically injected into the left striatum (coordinates: flat skull position, bregma, 2.5 mm laterally, 3 mm below dura). For immunohistochemical detection of transgene expression 7 days after AdV-JBD delivery, four mice were anesthetized with 4% chloral hydrate and were cardiacally perfused with ice-cold 4% PFA in PBS (pH = 7.4). The brains were removed and placed in 4% PFA overnight at 4°C, then changed to 30% sucrose for 2 days and then stored at −80°C. At the same time point, the mice were killed under deep anesthesia, and their striatum and substantia nigra were rapidly removed, frozen, and stored at −80°C until Western blot analysis.
MPTP Mouse Studies.
One week after adenovirus delivery to the left striatum, the mice were treated with either PBS or MPTP hydrochloride (Research Biochemicals, Natick, MA). MPTP was administered in 0.1 ml of PBS at a dose of 30 mg/kg i.p. at 24-h intervals for five doses (8, 9). Ten animals were used in each group for the detection of striatal catecholamine concentrations and for counting SNpc dopaminergic neurons. At 5 days after the last MPTP injection, the mice were tested for amphetamine-induced motor asymmetry (23, 24). The mice were given D-amphetamine (2 mg/kg, i.p.) and were placed in small (diameter = 20 cm) plastic bowls. Ipsiversive and contraversive rotations to the intrastriatal injection side were monitored visually between 5 and 24 min postamphetamine injection. For analysis, a net turning score was calculated by subtracting the mean number of full turns (rotating more than 360°) per minute to the left from the number of full turns to the right (24). The animals were killed at day 7 after the last MPTP injection. Both the right and left striata were rapidly dissected, frozen, and stored at −80°C until the catecholamine concentrations were measured by HPLC with electrochemical detection (8). The posterior parts of the same brains containing the midbrain were removed, immersed for 24 h in 4% PFA, and cryoprotected in 30% sucrose in 0.1 M PBS for 2 days at 4°C. These brains were then frozen on dry ice-cooled isopentane and stored at −80°C.
Western Blot Analysis.
MPTP treatment of C57BL/6 mice started at 7 days after transfection with AdV-JBD or AdV-EGFP. Three mice of each group and at each time point were killed 6 h after the second, third, and fifth administration of MPTP. Their substantia nigra was rapidly removed, frozen, and stored at −80°C until analysis. The preparation of lysates and the Western blots were performed as described (8). All primary antibodies were used at a 1:1,000 dilution. Primary antibodies used were the polyclonal phosphospecific MKK4 (Thr-223, Ab 9151; New England Biolabs), monoclonal phosphospecific JNK1/2 (Thr-183, Tyr-185, V73A; Promega), and a polyclonal phosphospecific c-Jun antibody (9164S, New England Biolabs). Activated caspase-3 was detected by using the CM1 antibody (IDUN Pharmaceuticals, La Jolla, CA), and caspase-9 processing was detected by an antibody directed against the large subunit (IDUN Pharmaceuticals). Bound secondary antibodies were visualized by using enhanced chemiluminescence. Blots were repeated at least three times for every condition.
Immunohistochemistry.
For tyrosine hydroxylase (TH) immunohistochemistry (n = 7 per group), brains were serially sectioned (50 μm) through the entire midbrain. Neurons containing TH were visualized by incubating the tissue sections successively with a mouse monoclonal antibody to rat TH (1:200, Boehringer, Mannheim, Germany), biotinylated horse anti-mouse IgG (1:200, Vector Laboratories), followed by the staining procedure by using Vectastain ABC kit (Vector Laboratories) in combination with DAB reagents.
Histochemistry for flag-tagged JBD expression was performed on 16-μm cryosections of mouse brains (n = 4) and on 3,3-diaminobenzidene cultured cells. A polyclonal mouse anti-flag antibody (Stratagene) and a carbocyanine-2-labeled goat anti-mouse IgG (Biotrend, Cologne, Germany) were used to detect flag immunoreactivity.
For the cellular detection of activated caspase-3 and phospho-c-Jun (p-c-Jun), the mice (n = 4 per group) were anesthetized and perfused at 6 h after the third MPTP injection. The mouse brains were fixed in 4% PFA for 2 h at room temperature, then in 100% isopropanol overnight at 4°C. The samples were paraffin-embedded and cut into 10-μm coronal sections. To allow double-labeling experiments, we used rabbit anti-CM1, rabbit anti-p-c-Jun, and mouse anti-TH antibodies as primary antibodies and goat anti-mouse IgG (carbocyanine-2 labeled) and anti-rabbit IgG (carbocyanine-3 labeled) as secondary antibodies. The CM1 antibody raised against the C terminus of the human p17 caspase-3 fragment, specifically recognizing activated caspase-3 (25), was used to detect activated caspase-3. Sections were washed, mounted on coverslips, and then analyzed by confocal laser scanning microscopy (LSM510, Zeiss). The specificity of immune reactions was confirmed by substituting the primary antisera or monoclonal antibodies with nonimmune IgG.
Stereology.
The total number of TH-positive SNpc neurons was counted from seven mice per group by using the optical fractionator, an unbiased stereological technique of cell counting (26) described previously (8). Counts were performed manually and blinded for treatment.
Statistical Analysis.
Data are expressed as means ± SEM values. Tests of variance homogeneity, normality, and distribution were performed to ensure that the assumptions required for standard parametric analysis of variance were satisfied. Statistical analysis was performed by ANOVA followed by Tukey's post hoc test to compare group means. In all analyses, the null hypothesis was rejected at the 0.05 level.
Results
In Vitro Inhibition of MPP+-Induced JNK and c-Jun Phosphorylation, Caspase Activation, and Cell Death Downstream from MKK4 by AdV-JBD Gene Transfer.
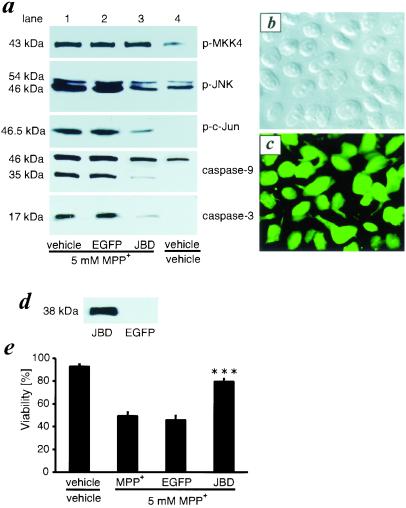
It has been reported recently that MPTP induces sequential phosphorylation of JNK kinase (MKK4) and JNK in the substantia nigra of mice (27). A putative inhibitor of MKK4, CEP1347/KT-7515, protected against MPTP toxicity (28). To confirm that these effects were caused by the active metabolite of MPTP, MPP+, we treated the neuroblastoma cell line SH-SY5Y with MPP+. In vitro treatment of SH-SY5Y cells with 5 mM MPP+ resulted in increased phosphorylation of MKK4, JNK, and c-Jun at 12 h (Fig. 1a, lane 1) without affecting the total expression of JNK or c-Jun (data not shown). To test whether MPP+ toxicity involves the sequential activation of caspases 9 and 3, we studied the cleavage of these caspases. We detected a cleavage of 46-kDa procaspase-9 to its 35-kDa intermediate and the p17 cleavage product of procaspase-3, indicating that caspases are processed and activated.
Figure 1.
JBD inhibits MPP+-induced JNK, c-Jun, and caspase activation and blocks cell death in vitro. (a) SH-SY5Y cells were infected with AdV-EGFP or AdV-JBD or were left untreated. After 48 h, these cells were treated with MPP+. Lysates of SH-SY5Y cells were collected for Western blotting 12 h later and exposed to antibodies recognizing p-MKK4, p-JNK, p-c-Jun, caspase-9, or activated caspase-3. (b) The phase contrast image was taken from AdV-JBD-infected SH-SY5Y cells. (c) Flag immunohistochemistry shows JBD-positive cells in the same culture as b. Comparing b and c, almost 100% of the cells express JBD. (d) Western blot detection of JBD expression in SH-SY5Y cells. (e) JBD overexpression rescues SH-SY5Y cells from MPP+ toxicity. ***, P < 0.001 compared with MPP+-treated or AdV-EGFP- and MPP+-treated cells (n = 6).
Adenoviral vectors infect SH-SY5Y cells with high efficacy (22). To study the effects of JNK inhibition on MPP+-induced cell death, we used adenoviral vectors encoding the JBD of JIP-1 (AdV-JBD). Almost 100% of SH-SY5Y cells expressed flag-tagged JBD at 48 h after infection (Fig. 1 b and c). As expected, JBD migrated at an apparent molecular mass of 38 kDa (Fig. 1d). Expression of the JBD inhibited the MPP+-induced phosphorylation of JNK (p-JNK) and c-Jun (p-c-Jun) but did not affect the phosphorylation of MKK4 (Fig. 1a, lane 3). EGFP gene transfection had no such effect (Fig. 1a, lane 2). Further, ectopic JBD expression attenuated the cleavage of caspases 9 and 3 (Fig. 1a, lane 3). Treatment with 5 mM MPP+ for 30 h resulted in the death of 50% of SH-SY5Y cells as measured by trypan blue staining. Ectopic expression of JBD, but not of EGFP, resulted in protection from MPP+-induced cell death (Fig. 1e).
MPTP Treatment of Mice Results in JBD-Insensitive MKK4 and JBD-Sensitive JNK, c-Jun, and Caspase Activation in SNpc.
Delivery of adenoviral vectors to the striatum of mice infects a high percentage (>90%) of dopaminergic neurons in the SNpc and leads to strong transgene expression in the SNpc as well as in the striatum (8). Accordingly, delivery of AdV-JBD to the left striatum led to expression of the JBD in neuronal and glial cells in the striatum (Fig. 2a) at 1 week after infection. A high percentage of dopaminergic neurons in SNpc expressed the flag-tagged JBD (Fig. 2b), presumably as a result of retrograde axonal transport. Double labeling for TH confirmed that ≈90% of the dopaminergic neurons were transfected. There was no JBD expression in the contralateral, noninjected hemisphere (Fig. 2c).
Figure 2.
JBD expression inhibits MPTP-induced JNK and c-Jun but not MKK4 phosphorylation in SNpc. Detection of flag-tagged JBD expression in the striatum (a) and the SNpc (b) by immunohistochemistry or Western Blot (c) at 7 days after stereotaxic AdV-JBD injection into the striatum. Seven days after virus transfection with AdV-EGFP and AdV-JBD, mice were treated with injections of MPTP at 24-h intervals. Lysates of SNpc were collected after varying doses of MPTP and analyzed for p-MKK4, p-JNK, or p-c-Jun (d).
Morphologic studies suggest that “chronic” MPTP treatment of mice leads to apoptosis of dopaminergic SNpc neurons (29–31), whereas no apoptosis was found in an acute dosing regimen (10). Therefore, by using the paradigm of Tatton and Kish (9), we injected five times 30 mg/kg i.p. at 24-h intervals. MPTP treatment resulted in a robust increase of MKK4, JNK, and c-Jun phosphorylation in the substantia nigra of mice at 6 h after the second and third injection of 30 mg/kg MPTP. The increase was smaller after five MPTP injections (Fig. 2d). Corresponding to the in vitro observations in SH-SY5Y cells, AdV-JBD blocked JNK and c-Jun phosphorylation but, as expected, did not block MKK4 phosphorylation (Fig. 2d).
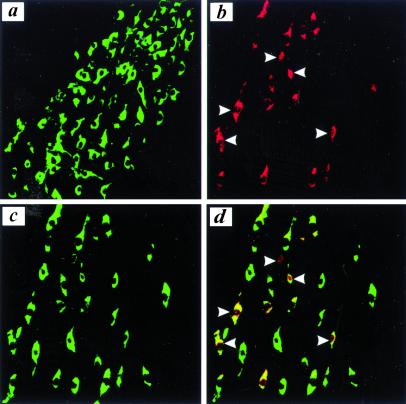
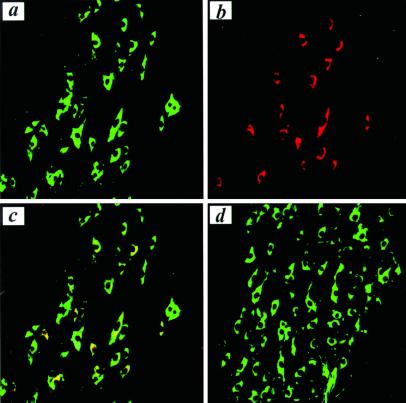
Next, we studied the cellular expression of p-c-Jun in MPTP toxicity by immunohistochemistry. We did not detect p-c-Jun expression in dopaminergic neurons of untreated control animals (Fig. 3a). Because the Western blot showed the peak of c-Jun phosphorylation at 6 h after the third MPTP injection, we used this time point for further immunohistochemical analysis. MPTP treatment led to a substantial increase in the number of p-c-Jun-positive cells in SNpc (Fig. 3b). Most of these cells showed coexpression of TH (Fig. 3 c and d). In several cells, p-c-Jun expression was detected in the nucleus. At the same time point after MPTP treatment, we detected expression of activated caspase-3 in the cytosol of a substantial number of dopaminergic neurons, by using an antibody specifically recognizing active caspase-3 (Fig. 4 a–-c). AdV-JBD, but not AdV-EGFP, completely prevented MPTP-induced caspase-3 activation (Fig. 4d).
Figure 3.
MPTP induces phosphorylation of c-Jun and nuclear translocation of p-c-Jun. (a) We did not detect p-c-Jun expression in SNpc dopaminergic neurons of untreated controls (green for TH, red for p-c-Jun, yellow for double staining). (b) MPTP treatment led to the induction of p-c-Jun (red) and its translocation into the nucleus (white arrow). Double staining for TH (c) revealed that the vast majority of p-c-Jun-positive cells colocalized (yellow) with dopaminergic neurons (d).
Figure 4.
JBD blocks MPTP-induced caspase-3 activation. In TH-positive SNpc neurons (a, green) MPTP activates caspase-3 (b, red) as demonstrated by colocalization using confocal microscopy (c, yellow). JBD expression inhibited the activation of caspase-3 and the loss of TH-positive neurons (d).
AdV-JBD Gene Transfer Protects Dopaminergic SNpc Neurons and Striatal Catecholamine Concentrations from MPTP Toxicity in Mice.
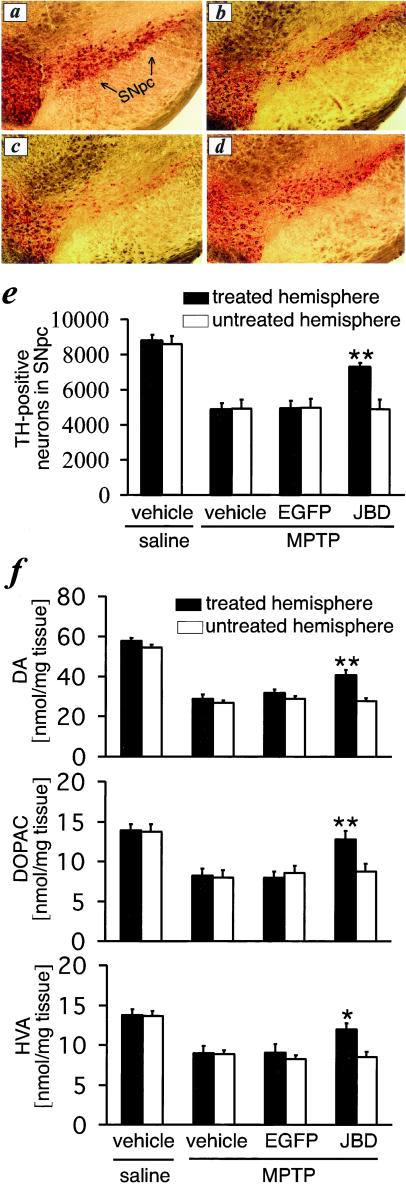
When analyzed at 7 days after the last MPTP administration, treatment with 30 mg/kg MPTP i.p. at 24-h intervals for five doses significantly reduced the number of TH-positive neurons in SNpc (Fig. 5 a, b, and e) and the concentrations of striatal dopamine, dihydrophenylacetic acid, and homovanillic acid (Fig. 5f). Treatment with a control vector, AdV-EGFP, did not affect the survival of SNpc dopaminergic neurons (Fig. 5 c and e) and striatal dopamine, dihydrophenylacetic acid, and homovanillic acid concentrations (Fig. 5f). In contrast, AdV-JBD was protected from the MPTP-induced loss of TH-positive cells in the SNpc (Fig. 5 d and e) and striatal concentrations of dopamine, dihydrophenylacetic acid, and homovanillic acid (Fig. 5f). The protective effects were restricted to the hemisphere of adenovirus injection and transgene expression and did not extend to the contralateral hemisphere (Fig. 5 e and f).
Figure 5.
JBD prevents MPTP-induced loss of TH-positive cells in SNpc and catecholamines in the striatum. Compared with controls (a), MPTP induces a loss of TH-positive neurons (b), which is not affected by prior treatment with AdV-EGFP (c). AdV-JBD prevents the loss of TH-positive cells in SNpc (d and e) and the reduction of catecholamine concentrations in the striatum (f). *, P < 0.05 and **, P < 0.01, as compared with MPTP-treated mice that previously received vehicle or AdV-EGFP injections into the striatum (n = 7–10).
AdV-JBD Gene Transfer Results in Behavioral Changes.
Following administration of D-amphetamine, all mice became more activated. Over the 20-min test period, MPTP-treated mice with prior intrastriatal injection of either PBS (n = 7) or AdV-EGFP (n = 7) were less active (22 ± 2.5 and 29 ± 4.6 turns in any direction, respectively) compared with control mice that did not receive MPTP (45 ± 2.9). In contrast, mice with prior transfer of AdV-JBD (n = 8) turned significantly more (55 ± 6.3; P < 0.05) than mice with intrastriatal injection of either AdV-EGFP or PBS. The net motor asymmetry was calculated by subtracting the number of full body turns per minute ipsilateral from the number of turns contralateral to the intrastriatal injection side. AdV-EGFP/MPTP (−0.2 ± 0.3 turns/min), PBS/MPTP (0.2 ± 0.3 turns/min), and PBS/PBS (0.2 ± 0.2 turns/min) treated mice did not show a side bias in motor behavior. In contrast, the unilateral AdV-JBD transfected mice showed significant motor asymmetry with preferential turning away from the transfected side (2.5 ± 0.3 turns/min, P < 0.01 as compared with any of the other three control groups).
Discussion
Apoptosis and caspase-mediated cell death are thought to be important mediators of neuronal death in acute and chronic neurodegenerative diseases, including Parkinson's disease (7, 11, 12, 32, 33). JNKs are a subfamily of mitogen-activated protein kinases. Although the functions of JNKs under physiological conditions are largely unknown, there is increasing evidence that JNKs are potent effectors of apoptosis or degeneration of neurons in vitro and in vivo (34–36). Here, we show that JNK activation is a proapoptotic signal that triggers caspase-mediated death of dopaminergic neurons in an in vitro and in vivo model of neurodegeneration. Because JIP-1 can bind to other kinases involved in the JNK cascade, we used a fragment of the JIP-1 molecule (residues 127–281), which encompasses a binding domain (JBD) that interacts with JNK-1, -2, and -3 but is not involved in the binding of JIP-1 to upstream effectors such as MKK7, DLK, MLK3, and HPK1 (18, 19). In line with these data, we show that the MPTP-induced activation of JNK and c-Jun but not the activation of MKK4 was inhibited by JBD overexpression. We demonstrate that inhibition of JNK by overexpression of the JBD protects neurons from apoptosis in vivo. Because apoptosis has not only been implicated in Parkinson's disease but also in several other neurodegenerative diseases including Alzheimer's disease, Huntington's disease, and amyotrophic lateral sclerosis, inhibition of JNK may offer a treatment strategy for several other neurological disorders. In contrast to a systemic treatment with inhibitors of JNK, a gene therapeutic approach will allow to target the inhibitor specifically to cells at risk by choosing neuron- or even neuron subtype-specific promoters.
The small molecule CEP1347/KT-7515, a putative inhibitor of the JNK pathway, is protective against MPTP toxicity (28). However, because CEP1347/KT-7515 prevents already the activation of MKK4 (27) and has neurotrophin-like properties,§ the exact mechanism by which it provides protection against MPTP toxicity remains unclear. In our experiments, the expression of the JBD did not block the activation of the upstream kinase MKK4 but specifically blocked the phosphorylation of JNK. We present in vivo evidence that the inhibition of JNK by overexpression of the JBD blocks the activation of caspases and the death of dopaminergic neurons induced by MPTP/MPP+. In addition, JBD expression also attenuated the decrease of striatal catecholamine concentrations and resulted in behavioral changes indicating improved dopaminergic synaptic function. This is of importance as adenovirus-mediated overexpression of the caspase inhibitor XIAP only protected dopaminergic somata in the substantia nigra and not their terminals from degeneration, resulting in a failure to rescue the depletion of catecholamines in the striatum (37–39). In terms of functional restoration, the inhibition of an upstream apoptotic signaling cascade seems to be superior to an inhibition of the execution of apoptosis. Using the same MPTP treatment paradigm, we have shown recently that the loss of TH-positive SNpc neurons correlates well with the loss of fluorogold-labeled SNpc cells with a nigrostriatal projection (37–39). Therefore, the MPTP-induced decrease of TH-positive cells does not merely signify a decrease of TH expression but truly cell death of dopaminergic neurons.
In our study and in experiments reported recently (27), MPTP did not alter the levels of total JNK and MKK4, but specifically induced changes in the phosphorylation state of the proteins (27). The specific role of the JNK signaling pathway depends on the cellular context. Activation of the JNK signaling pathway results in inactivation of antiapoptotic factors or activation of proapoptotic factors by phosphorylation (13). As reviewed by Davis (13), potential mechanisms for the induction of apoptosis through the JNK signaling pathway include the induction of CD95/Fas-L, the phosphorylation of p53, c-myc, Bcl-2, and Bcl-xL. The functional significance of these targets has remained controversial. In addition, JNK activation has been reported to induce cytochrome c release (40) and to activate the cytochrome c-mediated death pathway independent from death receptor signaling (41). We show here that MPTP/MPP+ induced activation of caspases, and death of dopaminergic cells is mediated by JNK signaling. Inhibition of JNK activation by JBD blocks not only cell death but also the cleavage of caspase-9 and caspase-3.
We find that the JNK signaling cascade converges on the phosphorylation of c-Jun. We further demonstrate the translocation of p-c-Jun from the cytosol to the nucleus, which is in agreement with its function as a nuclear transcription factor. Ectopic expression of c-Jun leads to apoptosis, and antibodies or dominant-negative mutants of c-Jun protect neuronal cells from trophic factor withdrawal-induced apoptosis (37–39). Primary neurons from mice with mutations at the phosphorylation sites of c-Jun (serines 63 and 73 mutated to alanines) are resistant to kainate-induced neuronal apoptosis (42). The activation of c-Jun is mediated by JNK signaling. Dominant-negative JNK mutants and MKK4 mutants block dopamine-induced apoptosis in striatal cultures (16). In vivo, activated JNK has been implicated as a mediator of dopaminergic neuronal death in a developmental model of target deprivation-induced apoptosis (43). Mice lacking MKK4 or both JNK1 and JNK2 genes exhibit defects in developmental neuronal apoptosis (34, 36), whereas targeted disruption of the JNK3 gene causes defects in kainate-induced apoptosis (35). The antibody used in this study detects the phosphorylation of JNKs with a molecular size of 54 and 46 kDa corresponding to JNK2 and JNK1, respectively. This does not rule out that JNK3 is activated. Unfortunately, none of the antibodies we used proved to be specific for the detection of JNK3 (data not shown).
Because JBD overexpression attenuated not only the loss of dopaminergic neurons in SNpc but also the loss of dopaminergic synaptic markers in the striatum induced by MPTP, inhibition of the JNK transduction pathway may provide a promising opportunity to treat the progressive cell loss and the functional impairment in PD.
Acknowledgments
We thank Sibylle Haid for excellent technical assistance. The construct of the Flag-tagged JNK binding domain (JBD) was a generous gift of Dr. Martin Dickens (University of Leicester). This study was supported by Deutsche Forschungsgemeinschaft Grant Schu 932/2-2 (to J.B.S.) and grants from the Wellcome Trust and South and West National Health Service Research Development directorate (to J.B.U.).
Abbreviations
- AdV
adenoviral constructs
- JBD
JNK binding domain
- JIP-1
JNK interacting protein-1
- JNK
c-Jun N-terminal kinase
- MPP+
1-methyl-4-phenylpyridinium
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- PD
Parkinson's disease
- MAPK
mitogen-activated protein kinase
- PFA
paraformaldehyde
- TH
tyrosine hydroxylase
- p-c-Jun
phospho-c-Jun
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Harris, C. A., Deshmukh, M., Maroney, A. & Johnson, E. M., Jr. (2000) Soc. Neurosci. Abstr. 26, 601 (abstr.).
References
- 1.Zhang Y, Dawson V L, Dawson T M. Neurobiol Dis. 2000;7:240–250. doi: 10.1006/nbdi.2000.0319. [DOI] [PubMed] [Google Scholar]
- 2.de Silva H R, Khan N L, Wood N W. Curr Opin Genet Dev. 2000;10:292–298. doi: 10.1016/s0959-437x(00)00082-4. [DOI] [PubMed] [Google Scholar]
- 3.Beal M F. Ann Neurol. 1995;38:357–366. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- 4.Jenner P, Olanow C W. Ann Neurol. 1998;44:S72–S84. doi: 10.1002/ana.410440712. [DOI] [PubMed] [Google Scholar]
- 5.Honig L S, Rosenberg R N. Am J Med. 2000;108:317–330. doi: 10.1016/s0002-9343(00)00291-6. [DOI] [PubMed] [Google Scholar]
- 6.Burke R E, Kholodilov N G. Ann Neurol. 1998;44:S126–S133. doi: 10.1002/ana.410440719. [DOI] [PubMed] [Google Scholar]
- 7.Hartmann A, Hunot S, Michel P P, Muriel M P, Vyas S, Faucheux B A, Mouatt-Prigent A, Turmel H, Srinivasan A, Ruberg M, et al. Proc Natl Acad Sci USA. 2000;97:2875–2880. doi: 10.1073/pnas.040556597. . (First Published February 25, 2000; 10.1073/pnas.040556597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eberhardt O, von Coelln R, Kügler S, Lindenau J, Rathke-Hartlieb S, Gerhardt E, Haid S, Isenmann S, Gravel C, Srinivasan A, et al. J Neurosci. 2000;20:9126–9134. doi: 10.1523/JNEUROSCI.20-24-09126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tatton N A, Kish S J. Neuroscience. 1997;77:1037–1048. doi: 10.1016/s0306-4522(96)00545-3. [DOI] [PubMed] [Google Scholar]
- 10.Jackson-Lewis V, Jakowec M, Burke R E, Przedborski S. Neurodegeneration. 1995;4:257–269. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 11.Yuan J, Yankner B A. Nature (London) 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- 12.Mattson M P. Nat Rev Mol Cell Biol. 2000;1:120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- 13.Davis R J. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 14.Mielke K, Herdegen T. Prog Neurobiol. 2000;61:45–60. doi: 10.1016/s0301-0082(99)00042-8. [DOI] [PubMed] [Google Scholar]
- 15.Basu S, Kolesnick R. Oncogene. 1998;17:3277–3285. doi: 10.1038/sj.onc.1202570. [DOI] [PubMed] [Google Scholar]
- 16.Luo Y, Umegaki H, Wang X, Abe R, Roth G S. J Biol Chem. 1998;273:3756–3764. doi: 10.1074/jbc.273.6.3756. [DOI] [PubMed] [Google Scholar]
- 17.Dickens M, Rogers J S, Cavanagh J, Raitano A, Xia Z, Halpern J R, Greenberg M E, Sawyers C L, Davis R J. Science. 1997;277:693–696. doi: 10.1126/science.277.5326.693. [DOI] [PubMed] [Google Scholar]
- 18.Yasuda J, Whitmarsh A J, Cavanagh J, Sharma M, Davis R J. Mol Cell Biol. 1999;19:7245–7254. doi: 10.1128/mcb.19.10.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitmarsh A J, Cavanagh J, Tournier C, Yasuda J, Davis R J. Science. 1998;281:1671–1674. doi: 10.1126/science.281.5383.1671. [DOI] [PubMed] [Google Scholar]
- 20.Meyer D, Liu A, Margolis B. J Biol Chem. 1999;274:35113–35118. doi: 10.1074/jbc.274.49.35113. [DOI] [PubMed] [Google Scholar]
- 21.Harding T C, Geddes B J, Murphy D, Knight D, Uney J B. Nat Biotechnol. 1998;16:553–555. doi: 10.1038/nbt0698-553. [DOI] [PubMed] [Google Scholar]
- 22.von Coelln R, Kügler S, Bähr M, Weller M, Dichgans J, Schulz J B. J Neurochem. 2001;77:263–273. doi: 10.1046/j.1471-4159.2001.t01-1-00236.x. [DOI] [PubMed] [Google Scholar]
- 23.Brundin P, Isacson O, Gage F H, Prochiantz A, Björklund A. Brain Res. 1986;366:346–349. doi: 10.1016/0006-8993(86)91316-8. [DOI] [PubMed] [Google Scholar]
- 24.Doucet G, Brundin P, Seth S, Murata Y, Strecker R E, Triarhou L C, Ghetti B, Björklund A. Exp Brain Res. 1989;77:552–568. doi: 10.1007/BF00249608. [DOI] [PubMed] [Google Scholar]
- 25.Srinivasan A, Roth K A, Sayers R O, Shindler K S, Wong A M, Fritz L C, Tomaselli K J. Cell Death Differ. 1998;5:1004–1016. doi: 10.1038/sj.cdd.4400449. [DOI] [PubMed] [Google Scholar]
- 26.West M J. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- 27.Saporito M S, Thomas B A, Scott R W. J Neurochem. 2000;75:1200–1208. doi: 10.1046/j.1471-4159.2000.0751200.x. [DOI] [PubMed] [Google Scholar]
- 28.Saporito M S, Brown E M, Miller M S, Carswell S. J Pharmacol Exp Ther. 1999;288:421–427. [PubMed] [Google Scholar]
- 29.Polymeropoulos M H, Lavedan C, Leroy E, Ide S E, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 30.Gasser T, Müller-Myhsok B, Wszolek Z K, Oehlmann R, Calne D B, Bonifati V, Bereznai B, Fabrizio E, Vieregge P, Horstmann R D. Nat Genet. 1998;18:262–265. doi: 10.1038/ng0398-262. [DOI] [PubMed] [Google Scholar]
- 31.Leroy E, Boyer R, Auburger G, Leube B, Ulm G, Mezey E, Harta G, Brownstein M J, Jonnalagada S, Chernova T, et al. Nature (London) 1998;395:451–452. doi: 10.1038/26652. [DOI] [PubMed] [Google Scholar]
- 32.Schulz J B, Weller M, Moskowitz M A. Ann Neurol. 1999;45:421–429. doi: 10.1002/1531-8249(199904)45:4<421::aid-ana2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 33.Robertson G S, Crocker S J, Nicholson D W, Schulz J B. Brain Pathol. 2000;10:283–292. doi: 10.1111/j.1750-3639.2000.tb00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang D, Tournier C, Wysk M, Lu H T, Xu J, Davis R J, Flavell R A. Proc Natl Acad Sci USA. 1997;94:3004–3009. doi: 10.1073/pnas.94.7.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang D D, Kuan C Y, Whitmarsh A J, Rincon M, Zheng T S, Davis R J, Rakic P, Flavell R A. Nature (London) 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- 36.Kuan C Y, Yang D D, Samanta Roy D R, Davis R J, Rakic P, Flavell R A. Neuron. 1999;22:667–676. doi: 10.1016/s0896-6273(00)80727-8. [DOI] [PubMed] [Google Scholar]
- 37.Estus S, Zaks W J, Freeman R S, Gruda M, Bravo R, Johnson E M., Jr J Cell Biol. 1994;127:1717–1727. doi: 10.1083/jcb.127.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ham J, Babij C, Whitfield J, Pfarr C M, Lallemand D, Yaniv M, Rubin L L. Neuron. 1995;14:927–939. doi: 10.1016/0896-6273(95)90331-3. [DOI] [PubMed] [Google Scholar]
- 39.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 40.Hatai T, Matsuzawa A, Inoshita S, Mochida Y, Kuroda T, Sakamaki K, Kuida K, Yonehara S, Ichijo H, Takeda K. J Biol Chem. 2000;275:26576–26581. doi: 10.1074/jbc.M003412200. [DOI] [PubMed] [Google Scholar]
- 41.Tournier C, Hess P, Yang D D, Xu J, Turner T K, Nimnual A, Bar-Sagi D, Jones S N, Flavell R A, Davis R J. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 42.Behrens A, Sibilia M, Wagner E F. Nat Genet. 1999;21:326–329. doi: 10.1038/6854. [DOI] [PubMed] [Google Scholar]
- 43.Oo T F, Henchcliffe C, James D, Burke R E. J Neurochem. 1999;72:557–564. doi: 10.1046/j.1471-4159.1999.0720557.x. [DOI] [PubMed] [Google Scholar]