Abstract
Obesity, sedentary lifestyle and aging are associated with mitochondrial dysfunction and impaired insulin sensitivity. Acute exercise increases insulin sensitivity in skeletal muscle; however, whether mitochondria are involved in these processes remains unclear. The aim of this study was to investigate the effects of insulin stimulation at rest and after acute exercise on skeletal muscle mitochondrial respiratory function (JO2) and hydrogen peroxide emission (JH2O2), and the associations with insulin sensitivity in obese, sedentary men. Nine men (means ± SD: 57 ± 6 years; BMI 33 ± 5 kg.m2) underwent hyperinsulinemic-euglycemic clamps in two separate trials 1–3 weeks apart: one under resting conditions, and another 1 hour after high-intensity exercise (4x4 min cycling at 95% HRpeak). Muscle biopsies were obtained at baseline, and pre/post clamp to measure JO2 with high-resolution respirometry and JH2O2 via Amplex UltraRed from permeabilized fibers. Post-exercise, both JO2 and JH2O2 during ADP stimulated state-3/OXPHOS respiration were lower compared to baseline (P<0.05), but not after subsequent insulin stimulation. JH2O2 was lower post-exercise and after subsequent insulin stimulation compared to insulin stimulation in the rest trial during succinate supported state-4/leak respiration (P<0.05). In contrast, JH2O2 increased during complex-I supported leak respiration with insulin after exercise compared with resting conditions (P<0.05). Resting insulin sensitivity and JH2O2 during complex-I leak respiration were positively correlated (r = 0.77, P<0.05). We conclude that in obese, older and sedentary men, acute exercise modifies skeletal muscle mitochondrial respiration and H2O2 emission responses to hyperinsulinemia in a respiratory state-specific manner, which may have implications for metabolic diseases involving insulin resistance.
Introduction
More than one-third of the adult population worldwide are overweight or obese [1, 2]. Obesity increases the risk of developing insulin resistance, and this may be exacerbated by aging and sedentary lifestyle [3]. To counter this, regular exercise is a primary intervention for the prevention and management of metabolic diseases [4, 5]. The beneficial effects of exercise may occur in part by preventing or alleviating mitochondrial dysfunction which is thought to cause, or at least contribute to these pathophysiologic states [6–8]. Of note, even a single bout of exercise increases whole body insulin sensitivity, primarily in skeletal muscle, for up to 48-h post-exercise [9, 10]. However, whether skeletal muscle mitochondria are involved in mediating these effects after an acute bout of exercise currently remain unclear.
Exercise elicits disturbances to the skeletal muscle cellular environment, and this may transiently alter mitochondrial electron transport system (ETS) enzyme activity and mitochondrial membrane permeability in the hours post-exercise [11–13]. Mitochondrial ETS enzymes intrinsically generate reactive oxygen species (ROS), primarily in the form of superoxide which is rapidly dismutated to the membrane permeable hydrogen peroxide (H2O2). A number of sites within the ETS have been shown to generate ROS, and their rates of generation are largely determined by the mitochondrial respiratory state, which is linked to the bioenergetic demands of the cellular environment [14, 15]. During exercise mitochondrial ROS generation is decreased, but net muscle ROS generation is increased via non-mitochondrial sources such as NADPH and xanthine oxidases [16]. However, the sudden decrease in post-exercise energetic demand may have major impact on mitochondrial bioenergetics and therefore alter ROS generation, which could be also be amplified by numerous post-translational modifications to mitochondrial enzymes [15, 17]. Transient changes in ROS generation may have important retrograde signaling effects. For example, sustained or chronically elevated levels of ROS (i.e.: oxidative stress) have been implicated in insulin resistance via activation of stress activated protein kinases (SAPKs), which leads to insulin receptor substrate-1/2 (IRS1/2) serine phosphorylation, to negatively regulate phosphatidylinositol 3-kinase (PI3K) activity and its downstream signaling for GLUT-4 translocation and glucose uptake [18]. On the other hand, acute transient increases in ROS generation may oxidize and inhibit negative regulators of insulin signaling such as protein tyrosine phosphatase-1B (PTP1B) and phosphatase tensin homolog (PTEN), thus allowing PI3K phosphorylation [19–22].
In skeletal muscle mitochondria, nearly 100 mitochondrial proteins are known to be phosphorylated in response to a hyperinsulinemic-euglycemic clamp [23]. Consistent with the notion that mitochondrial dysfunction is associated with insulin resistance and likelihood of prediabetes [24], mitochondrial respiratory function has been shown to be elevated after a hyperinsulinemic-euglycemic clamp in insulin-sensitive individuals, but not in patients with type-2 diabetes [25–27]. Whether a similar mitochondrial-insensitivity to the insulin clamp is also characteristic of obese individuals is currently unclear. It was recently shown that obese women had unchanged JH2O2 after high fat feeding in the untrained state; yet following 12 weeks of exercise training, JH2O2 was elevated after the same meal, [28]. This suggests that greater mitochondrial sensitivity to nutrient intake and improved metabolic flexibility is an important component of exercise adaptation. Despite this, it remains unknown whether a single bout of exercise can similarly affect mitochondrial responses in an obese, older and sedentary population.
Therefore, the aims of this study were to test the hypothesis that in obese, older and sedentary individuals, mitochondrial respiration and H2O2 emission in permeabilized skeletal muscle fibers would be perturbed in a respiratory state-dependent manner in response to a hyperinsulinemic-euglycemic clamp to a greater extent when preceded by a single session of high intensity interval exercise (HIIE) compared to resting conditions. We also sought to explore the hypothesis that there would be correlation between rates of mitochondrial H2O2 emission and insulin sensitivity.
Methods
Participants
Nine obese and sedentary men (mean ± SD; age: 57.3 ± 6.5 years, body mass: 100.1 ± 12.1 kg, BMI: 32.7 ± 5.0 kg.m-2, : 21.4 ± 5.4 ml.kg-1.min-1) without diabetes (fasting glucose: 5.3 ± 0.8 mmol.L-1; HBa1c: 5.6 ± 0.2%) participated in this study. These participants were recruited as part of a larger study and as such, detailed participant characteristics and exclusion criteria are described elsewhere [29, 30]. Briefly, participants were excluded if they took medications known to affect insulin secretion and/or insulin sensitivity; had musculoskeletal or other conditions which prevented daily activity; or symptomatic or uncontrolled metabolic or cardiovascular disease. Each participant was given written and verbal explanations about the study before providing written informed consent as per the declaration of Helsinki. This study protocol was approved by Victoria University Human Research Ethics Committee.
Experimental design
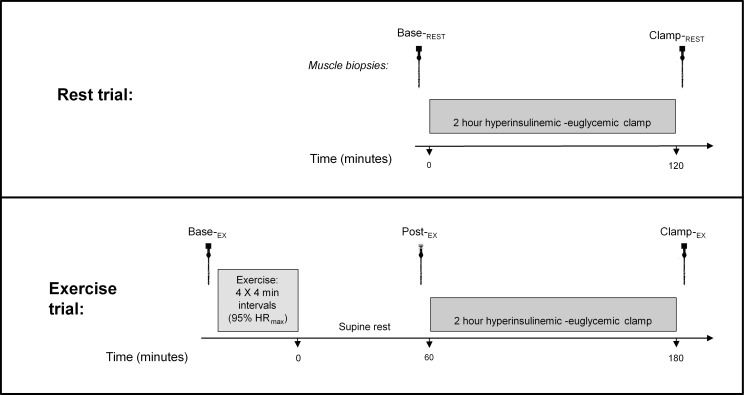
Participants visited the Victoria University Exercise Physiology laboratory on three separate occasions. In the first visit, participants underwent a screening and characterization session which included a graded exercise test and familiarization with the experimental procedures. At least one week following this, the first of the two experimental trials were conducted. As shown in Fig 1, the first experimental trial was a ‘rest trial’ (i.e. no exercise) and consisted of a 2 h hyperinsulinemic-euglycemic clamp (insulin clamp, see below). During this rest trial, muscle biopsies were obtained at baseline (Base-REST) and after the insulin clamp (Clamp-REST). The final visit was 1–3 weeks later for the ‘exercise trial’. In this, participants performed an acute HIIE exercise session (see below) then 1 hour later underwent another 2 h insulin clamp. In this exercise trial, biopsies were obtained at baseline (Base-EX), 1 h post exercise (Post-EX; which was immediately prior to the commencement of the insulin clamp), and then post insulin clamp (Clamp-EX). Prior to both trial days, participants performed an overnight fast, and abstained from physical activity for at least 72 h and alcohol and caffeine consumption for 24 h. Dietary information was provided and participants were asked to consume approximately 300 g of carbohydrate in the 24 h prior to the rest trial, which was recorded in a diet diary and replicated for the exercise trial.
Fig 1. Overview of study design.
Middle-aged obese men each performed two separate trials (rest and exercise) 1–3 weeks apart. Rest trial muscle biopsies were taken at baseline (Base-REST) and post 2 hour hyperinsulinemic-euglycemic insulin clamp (Clamp-REST); exercise trial biopsies were obtained at baseline (Base-EX), 1 hour post-exercise (Post-EX) and after a subsequent 2 hour hyperinsulinemic-euglycemic insulin clamp (Clamp-EX).
Graded exercise test
After pre-screening, participants underwent a sign and symptom-limited graded exercise test for characterization of aerobic fitness, as described elsewhere [31]. Oxygen uptake (VO2) was measured in 15 sec intervals by gas analysis (Medgraphics, Cardio2 and CPX/D System with Breezeex Software, 142090–001, Revia, MN, USA) calibrated with gases of known concentrations, and a 3 L volume Hans-Rudolph syringe prior to each test.
High-intensity interval exercise protocol
At least a week after the graded exercise test, participants performed a bout of high-intensity intermittent exercise (HIIE) on a cycle ergometer (Corvial, Lode B.V., The Netherlands). The exercise consisted of 4 minutes warm-up at a workload corresponding to 50% of the peak heart rate (HRpeak) obtained during the graded exercise test, followed by 4 x 4 min at 95% HRpeak. HIIE bouts were separated by 2 min active recovery (cycling at a low intensity at 50–60% HRpeak). All participants successfully completed the exercise session at the desired intensity.
Hyperinsulinemic-euglycemic clamp
The insulin clamp was performed after an overnight fast, as previously described [29]. Briefly, insulin (Actrapid; Novo Nordisk, Denmark) was infused intravenously at 40 mU.m-2 per minute for 120 minutes leading to an elevated yet stable plasma insulin concentration from 10–120 min. Blood glucose concentration was assessed at 5 min intervals during the clamp (YSI 2300 STAT plus; YSI Inc., USA) and the glucose infusion rate (GIR; mg kg-1 min-1) was adjusted accordingly to meet a target blood glucose of 5 mmol L-1. Insulin sensitivity was determined as the mean GIR per unit plasma insulin (mIU ml-1) during the last 30 min of the clamp (M/I index) [32].
Muscle biopsy procedure
Overall, five muscle biopsies were obtained from each participant (Fig 1). Local anesthetic (1% Xylocaine, AstraZeneca, Australia) was injected into skin, subcutaneous tissue and fascia overlying the vastus lateralis muscle. After small incisions were made into the skin and fascia (one per biopsy), muscle was excised using a Borgström needle with suction [29, 30, 33]. Each biopsy was taken from a separate incision ~1 cm proximal from the previous biopsy. Samples of approximately 50–100 mg were obtained and aliquoted for separate analysis including ~10 mg which was immediately placed in an ice-cold preservation medium (BIOPS, see below) for mitochondrial respiration analysis on the same day, while the remaining portion was blotted to remove blood and rapidly frozen in liquid nitrogen and stored at -80°C for later analysis.
Muscle fiber preparation for mitochondrial respiratory function analysis
The ~10 mg aliquot of muscle was placed in ice-cold preserving solution (BIOPS; in mM: 7.23 K2EGTA, 2.77 CaK2EGTA, 5.77 Na2ATP, 6.56 MgCl2-6H2O, 20 taurine, 15 phosphocreatine, 20 imidazole, 0.5 dithiothreitol, 50 K+-MES; pH 7.1) until analysis, typically within 3 hours after sampling [34–36]. Using a dissecting microscope, muscle fibers were mechanically separated using fine-point forceps for no more than 3 min while submerged in ice-cold BIOPS. Separated fibers were permeabilized (saponin 50 μg/mL in BIOPS) for 30 min with agitation followed by 3 x 5 min washes in ice-cold respiration buffer (MiR05, see below). Fiber bundles of approximately 2–3 mg were blotted on filter paper for 5 s, then exact sample mass (wet-weight) was recorded using a microbalance (Cubis MSE3.6P-0TR-DM, Sartorius, Goettingen, Germany).
Mitochondrial respiration and hydrogen peroxide emission assay
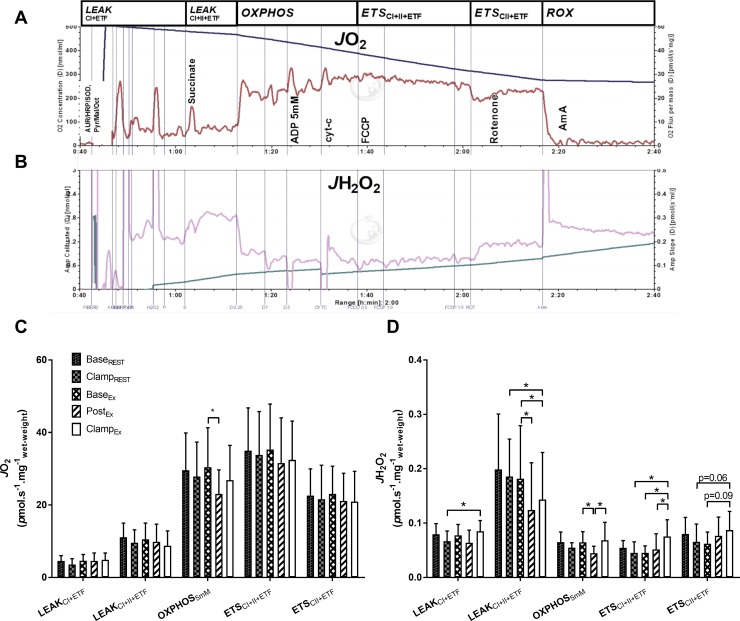
Mitochondrial oxygen flux (JO2) from permeabilized muscle fibers was determined using high resolution respirometry at 37°C, high oxygen concentration (300–450 nmol.ml-1; to avoid oxygen diffusion limitation) and continuous stirring (Oxygraph O2k, Oroboros Instruments, Innsbruck, Austria), and mitochondrial hydrogen peroxide emission (JH2O2) was measured simultaneously via fluorimetry (O2k-Fluorescence LED-2 Module; Oroboros Instruments, Innsbruck, Austria) as previously described [36–39]. Briefly, permeabilized fiber bundles were analysed in duplicate, in chambers containing MiR05 respiration buffer (in mM: 0.5 EGTA, 10 KH2PO4, 3 MgCl2-6H2O, 60 lactobionic acid, 20 taurine, 20 HEPES, 110 D-sucrose, 1 mg/mL bovine serum albumin; pH 7.1). Amplex UltraRed (25 μM; Molecular Probes, Invitrogen), horseradish peroxidase (5 U/mL) and superoxide dismutase (SOD; 5 U/mL) were added for simultaneous fluorimetric measurement of H2O2 at 525/590 nm excitation/emission wavelengths, calibrated with known amounts of H2O2 as described previously [36, 37, 39]. A range of respiratory states were induced using a substrate, uncoupler, and inhibitor titration (SUIT) protocol, added sequentially as follows: malate (2 mM), pyruvate (5 mM) and octanoylcarnitine (0.02 mM) to assess complex I + electron transfer flavoprotein (ETF) supported state-4 leak respiration (LEAKCI+ETF); succinate (10 mM) was then added for convergent complex II electron input during leak respiration (LEAKCI+II+ETF); ADP (1 & 5 mM) was then added to induce state-3 oxidative phosphorylation (OXPHOS); cytochrome-c (10 μM) was added to confirm outer mitochondrial membrane integrity; stepwise 0.05 μM titrations of carbonyl cyanide p-trifloromethoxyphenylhydrazone (FCCP) were then added to uncouple the inner mitochondrial membrane to assess electron transfer system capacity (ETSCI+II+ETF). The complex I specific inhibitor rotenone (0.5 μM) was subsequently added to assess electron transfer from complex II (ETSCII+ETF). Finally, antimycin-A (2.5 μM) was added to inhibit complex III to determine residual oxygen flux rates which were subtracted from all prior JO2 measures.
Muscle protein extraction and western blotting
Abundance of specific proteins in muscle samples were determined without centrifugation (i.e. all cellular fractions present) using methods described previously [40]. Specifically, frozen muscle was cut into approximately 20 x 20 μm sections (Cryostat HM550, Thermo Scientific, Australia), and dissolved in 200 μL homogenizing buffer (0.125 M Tris-HCl, 4% SDS, 10% Glycerol, 10 mM EGTA, 0.1 M DTT, with 0.1 μL.mL-1 of protease and phosphatase inhibitor cocktail [#P8340 and #P5726, Sigma Aldrich, Castle Hill, NSW, Australia]), vortexed and freeze-thawed. Protein concentration was then determined using a commercially available assay (Red 660, G-Biosciences, Astral Scientific, Gymea NSW, Australia). Samples were diluted to equivalent concentrations (1 μg.μL-1) in homogenizing buffer and bromophenol blue added (1% v/v) before heating at 95°C for 5 min. Samples were loaded onto 26 well, stain-free, precast 4–20% gradient gels (Criterion™ TGX Stain-Free™ Precast, BioRad, Gladesville NSW, Australia) at a concentration of 6–8 μg protein per lane. Molecular weight marker (PageRuler® Plus, Thermo Scientific, Australia) and a five-point standard curve was also loaded on each gel using a pooled sample allowing quantification of blot intensities within and between gels via linear regression. Optimal protein loading was determined to ensure that subsequent blot intensities were within the linear range of the standard curve [40]. After separation by SDS PAGE, stain-free gels were activated by UV light (ChemiDoc™ MP, BioRad, Gladesville NSW, Australia) and imaged to visualize the total protein of each lane then the proteins were transferred to PVDF membranes (Trans-Blot® Turbo™, BioRad, Gladesville NSW, Australia). Membranes were then blocked in 20 mM Tris, 150 mM NaCl, and 0.1% Tween 20 (TBST) containing 5% nonfat milk for 1 h at room temperature, washed, then incubated with primary antibody overnight at 4°C. Membranes were incubated with the following primary antibodies diluted 1:1000 in TBST containing 5% BSA and 0.1% sodium azide: mitochondrial complexes I-V cocktail (MitoSciences #MS601), PRX pathway cocktail (Abcam #184868), and UCP3 (Abcam #10985). Membranes were washed with TBST, then probed with appropriate horseradish peroxidase-conjugated secondary antibody (PerkinElmer, Australia) diluted 1:50,000 in 5% non-fat milk/TBST for 1 hour at RT. Protein-antibody-HRP conjugates were visualized by chemiluminescence using ECL detection (SuperSignal® West Femto, Thermo Scientific, Australia), imaged (ChemiDoc™ MP, BioRad, Australia) and then analysed (ImageLab v5.1, BioRad, Australia). Specifically, immunoblots for proteins of interest and associated stain-free total protein loading were quantified relative to their respective standard curves, and these values were then used to report protein of interest relative to total protein [40]. In the absence of stain-free loading control data for mitochondrial complexes due to equipment fault. Blot intensities derived from the mitochondrial cocktail antibody served as their own loading control since these were imaged on the same membrane. This was achieved by first normalizing each samples blot intensity to a pooled sample loaded on all membranes, then, were expressed relative to complex-V within the same image, and complex-V was expressed relative to complex-III.
Citrate synthase activity assay
Citrate synthase activity was performed as per Srere [41] modified for a 96 well plate format [34, 35]. Muscle was mechanically homogenized 1:20 w/v in buffer (0.175 M KCl; 2 mM EDTA; pH 7.4) then freeze-thawed. Sample (10 μL) was added to 190 μL of working solution (final concentrations in mM: 72.5 tris, 0.1 DTNB, 0.45 acetyl co-A, 0.25% v/v Triton X-100) followed by 10 μL of oxaloacetic acid (0.5 mM) to initiate the reaction. Citrate synthase (CS) activity at 30°C was determined by the change in absorbance at 412 nm over 4 min in a spectrophotometer (x-Mark, Bio-Rad laboratories, USA).
Reduced and oxidized glutathione assay
Reduced (GSH) and oxidized (GSSG) muscle glutathione content was determined using a commercially available kit (Bioxytech GSH/GSSG-412, Oxis Health Products, Portland, OR, USA) described previously [42]. Briefly, freeze dried muscle was dissected free of connective tissue, divided into two aliquots then homogenized in 80 μl.mg-1 (dry weight) ice-cold 5% metaphosphoric acid, one aliquot containing the reduced-glutathione scavenger 1-methyl-2-vinyl-pyridinium trifluoromethane sulphonate (10% v/v) for GSSG, and the other aliquot without for GSH. Homogenate was centrifuged at 23,000 g for 15 min at 4°C. Samples, standards and blanks (50 μl) were added to a 96 well plate in triplicate, followed by 50 μl each of chromogen, glutathione reductase and just prior to measurement, NADPH. Change in absorbance at 412 nm due to the reduction of DTNB was measured for 4 min in a spectrophotometer (xMark; Bio-Rad Laboratories, USA).
Statistical analysis
Owing to the study design with two time points in the rest trial, and three in the exercise trial, between-trial effects at baseline and post-insulin stimulation were analysed by two-way (intervention x time point) ANOVA with repeated measures; whereas effects within the exercise trial were assessed by repeated measures one-way ANOVA with Fisher’s LSD post-hoc tests using statistical software (IBM SPSS statistics version 22). Significance was accepted at P<0.05. Associations between insulin sensitivity and JH2O2 after the rest and post-exercise insulin clamp were determined using Pearson’s correlation coefficient. All data are presented as mean ± SD for n = 9 unless otherwise stated.
Results
Mitochondrial respiration
LEAK respiration: Throughout the SUIT protocol (Fig 2A), there were no significant effects of exercise and/or insulin on JO2 during LEAKCI+ETF respiration state; however, in the presence of complex II substrate succinate (LEAKCI+II+ETF), JO2 trended lower in the rest trial after insulin compared to baseline (P = 0.09; Fig 2C).
Fig 2. Oxygen flux (JO2) and mitochondrial hydrogen peroxide emission (JH2O2) from permeabilized skeletal muscle fibers during various mitochondrial respiratory states, pre- and post-exercise and/or insulin clamp.
Representative traces of JO2 (A) and JH2O2 (B) are shown from one subject, and overall data are quantified (C and D). See Methods for abbreviations and details of the SUIT protocol. Values are mean ± SD for n = 9. *P<0.05 significantly different.
OXPHOS respiration: There was no effect of insulin on JO2 under maximal (5 mM ADP) OXPHOS respiratory state in the rest trial. Compared to baseline, JO2 during OXPHOS respiration was lower 1-hr post exercise (P<0.01; Fig 2C), which then returned to baseline levels with subsequent insulin stimulation. Titration of cytochrome-C increased OXPHOS respiration JO2 by an average of 7.6% across the study. Respiratory control ratios were not significantly affected for OXPHOS / LEAKCI+ETF (mean±SD): Base-REST 7.6 ± 5.1; Clamp-REST 8.9 ± 4.1; Base-EX 8.1 ± 5.4; Post-EX 5.7 ± 2.1; Clamp-EX 6.2 ± 2.8; or for OXPHOS / LEAKCI+II+ETF: Base-REST 2.7 ± 0.7; Clamp-REST 3.0 ± 0.9; Base-EX 3.0 ± 0.7; Post-EX 2.6 ± 0.7; Clamp-EX 3.4 ± 1.4.
ETS uncoupled respiration: There were no significant effects of insulin and/or exercise on JO2 during uncoupled respiration when supported by convergent complex I, II and ETF electron input (ETSCI+II+ETF) or with electron input in the absence of complex I after rotenone inhibition (ETSCII+ETF; Fig 2C).
Mitochondrial H2O2 emission
LEAK respiration: Throughout the SUIT protocol (Fig 2B), mitochondrial hydrogen peroxide emission (JH2O2) during LEAKCI+ETF respiration was greater after insulin in the exercise trial compared to the resting trial (P<0.01; Fig 2D). During LEAKCI+II+ETF respiration (with added succinate), JH2O2 was lower at both Post-EX (P = 0.01; Fig 2D) and Clamp-EX (P = 0.05; Fig 2D) compared to Base-EX. In addition, during LEAKCI+II+ETF, there was lower JH2O2 with insulin in the exercise trial compared to the resting trial (P = 0.02; Fig 2D).
OXPHOS respiration: Under 5 mM ADP stimulated OXPHOS respiration, JH2O2 was lower after exercise compared to baseline (P = 0.03; Fig 2D) and also compared to subsequent insulin (P = 0.02; Fig 2D). There was no effect of the subsequent cytochrome-c titration on JH2O2, as determined from combined data of all nine participants across five conditions (pre v.s. post cytochrome-c titration JH2O2: 0.058 ± 0.020 v.s. 0.058 ± 0.029 pmol.s-1.mg-1 tissue wet weight, P = 0.95, n = 45).
ETS uncoupled respiration: In the uncoupled respiration state (ETSCI+II+ETF) in the exercise trial, JH2O2 was greater with insulin compared to both baseline (P = 0.02) and post-exercise (P = 0.04; Fig 2D). Furthermore, JH2O2 was greater in this respiratory state with insulin in the exercise trial compared to the resting trial (P = 0.01; Fig 2D). In the presence of rotenone to inhibit complex-I electron input (ETSCII+ETF), in the exercise trial there was a trend for elevated JH2O2 after insulin stimulation compared with both baseline (P = 0.09) and with insulin in the rest trial (P = 0.06; Fig 2D).
Glucose infusion rate
GIR was 26% greater after exercise compared with the resting trial (4.21 ± 2.41 v.s. 3.33 ± 2.16 mg.kg-1.min-1; P = 0.03; S1A Fig). Plasma hyperinsulinemia induced by the clamp was stable in the last 30 min of the clamp and equivalent between trials (Clamp-REST: 73.6 ± 24.5 mIU L-1 and Clamp-EX: 67.1 ± 20.5 mIU L-1; P = 0.37; S1B Fig). GIR per unit plasma insulin was 27% greater post-exercise compared to rest (6.26 ± 3.6 v.s. 4.95 ± 3.77 M/I index; P = 0.04; S1C Fig). Blood glucose concentration was well maintained at ~5 mmol.L-1 in the final 30 minutes of the clamps (coefficient of variation: 2.1%, S1D Fig).
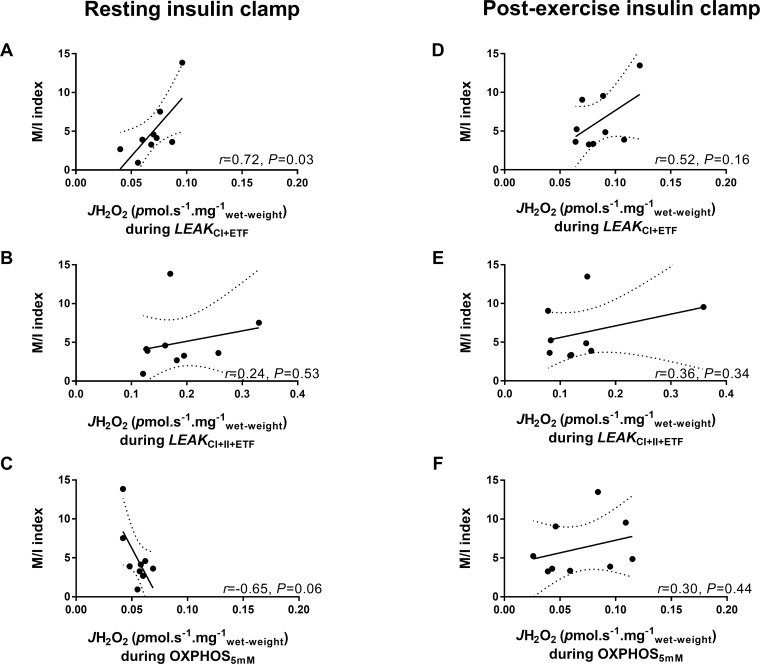
Correlations between insulin sensitivity and mitochondrial H2O2
There was a significant correlation between whole body insulin sensitivity after the resting clamp and JH2O2 during LEAKCI+ETF (r = 0.72; P = 0.03; Fig 3A), however, this association was not significant post-exercise (r = 0.52; P = 0.16; Fig 3D). There was a trend for a negative correlation during OXPHOS5mM at rest (r = -0.65, P = 0.06; Fig 3C). There were no correlations between JH2O2 and insulin sensitivity during LEAKCI+II+ETF (Fig 3B and 3E), or either uncoupled ETS respiratory states (S1 File).
Fig 3. Correlations between insulin sensitivity (M/I index) and mitochondrial H2O2 emission.
Comparisons are made after the resting insulin clamp (A-D) and the post-exercise insulin clamp (E-G) under specific respiratory states: LEAKCI+ETF (A & D), LEAKCI+II+ETF (B & E), and OXPHOS5mM (C & F). M/I index is insulin sensitivity: glucose infused (mg.kg-1.min-1) per unit plasma insulin (mIU ml-1). Dotted lines represent 95% confidence bands of the best-fit line for n = 9.
Muscle glutathione content
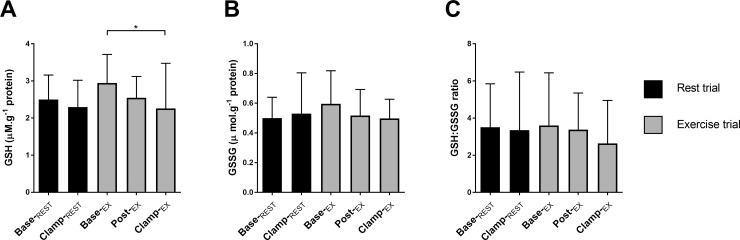
Reduced-glutathione (GSH) was lower after insulin compared with baseline in the exercise trial (P = 0.04; Fig 4A). There was no significant effect of insulin or exercise on oxidized-glutathione (GSSG; Fig 4B), or on the ratio of GSH to GSSG (Fig 4C).
Fig 4. Muscle glutathione status.
Reduced glutathione (A, GSH), oxidized glutathione (B, GSSG) content was normalized to total protein of whole muscle homogenate and expressed as a ratio (C). Values are mean ± SD for n = 9. *P<0.05 significantly different.
Mitochondrial protein abundance and citrate synthase activity
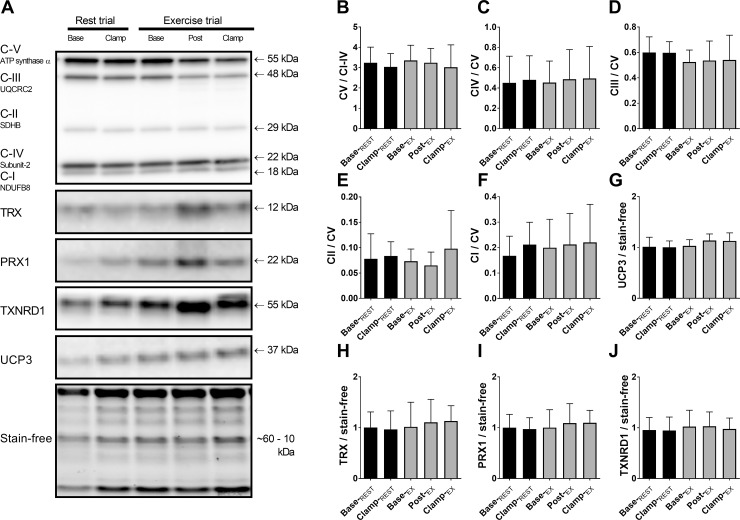
Mitochondrial ETS proteins were assessed to confirm that acute changes in mitochondrial respiration were independent of changes in their abundance. There were no significant changes in any of the measured ETS subunits, or uncoupling protein-3 (Fig 5B–5G). Citrate synthase enzyme activity, an additional marker of mitochondrial abundance, was unchanged with exercise or insulin (means±SD: Base-REST: 4.6 ± 2.5; Clamp-REST: 5.0 ± 1.7; Base-EX: 5.3 ± 3.0; Post-EX: 4.3 ± 3.4; Clamp-EX: 4.6 ± 2.3 μmol.min-1.g-protein-1; n.s.).
Fig 5. Mitochondrial and antioxidant proteins from whole muscle homogenate.
Representative blots depict typical immunoblots from one subject (A). Immunoblots of mitochondrial complex V (CV) are expressed relative to average intensities of CI to IV of the same lane (B); complexes IV, III, II and I are normalized to CV of each lane (C-F). Uncoupling protein-3 (G), antioxidant enzymes TRX (thioredoxin, H), PRX1 (peroxiredoxin-1, I), and TXNRD1 (thioredoxin reductase-1, J) normalized to whole sample protein determined from stain-free image. Values are mean ± SD for n = 9.
Muscle endogenous antioxidant protein abundance
To investigate possible reasons for the transient shifts in mitochondrial H2O2 emission observed, muscle antioxidant enzyme abundance were measured at corresponding time points. There was no significant change in muscle protein content of thioredoxin (TRX), peroxiredoxin-1 (PRX1) or thrioredoxin reductase-1 (TXNRD1) at any time points (Fig 5H–5J).
Discussion
The main findings of this study were that following acute exercise, but not under resting conditions, mitochondrial H2O2 emission was altered in a respiratory state-specific manner in response to hyperinsulinemic-euglycemic clamp in obese, middle-aged and sedentary men. In addition, there was a post-exercise decrease in rates of ADP stimulated mitochondrial respiration and H2O2 emission prior to insulin stimulation. There was also a significant correlation between whole body insulin sensitivity and skeletal muscle mitochondrial H2O2 emission during LEAKCI+ETF respiration at rest; however, in contrast to our hypothesis, this relationship was not observed post-exercise.
Our finding that mitochondrial JH2O2 was lower one hour post-exercise in the physiologically relevant OXPHOS/state-3 respiration, is important since oxidative stress (i.e. chronically elevated ROS levels) is involved in the pathophysiology of various chronic diseases [43]. Indeed, exercise training is known to attenuate chronic oxidative stress [44, 45], and our data suggest that this improvement may begin even with a single bout of exercise. It should be noted that although ROS formation is highly dependent on membrane potential and the subsequent redox status of electron carriers in the ETS, some ROS formation is expected even under low membrane potential respiratory states (i.e. OXPHOS and uncoupled ETS) [14, 15].
To the best of our knowledge, only one study has measured H2O2 emission permeabilized skeletal muscle mitochondrial following acute exercise [46]. While that study was performed with rodents, their data indicated a trend for pyruvate+malate+succinate supported H2O2 emission to be lower in white gastrocnemius muscle 18 hours post exercise [46], and this is in line with the decreased post-exercise LEAKCI+II+ETF JH2O2 (supported by malate+pyruvate+octanoylcarnitine and succinate) in the present study. The decrease in post-exercise/insulin clamp JH2O2 and JO2 during succinate-driven state-4/leak respiration is also consistent with a recent finding that ROS generation from complex I (via reverse electron flow) is inhibitory to complex II function [47]. Specifically, in that study, small molecule inhibitors of the complex-I ubiquinone binding site were shown to attenuate superoxide/H2O2 generation, which attenuated pathophysiological responses [47]. Taken together with our findings of decreased post-exercise H2O2 emissions during LEAKCI+II+ETF respiration, acute exercise may elicit similar beneficial effects in obese, middle-aged and sedentary men via similar mechanisms which warrants further investigation.
On the other hand, we observed elevated mitochondrial H2O2 in response to the post-exercise insulin clamp during LEAKCI+ETF and uncoupled ETSCI+II+ETF respiratory states (Fig 2D) which may be pertinent, since ROS generation in specific spatial and temporal patterns at physiological levels is known to have an important role in cell signal transduction [21, 48]. Our findings are consistent with a recent study in obese women, which demonstrated a lack of response of JH2O2 after a high fat meal in the untrained state, yet after 12 weeks of exercise training there was an acute increase in JH2O2 in response to the same meal [28]. In line with this, in the present study individuals with greater insulin sensitivity at rest tended to have lower mitochondrial H2O2 emission during the ADP stimulated oxidative phosphorylation respiration state, but significantly greater mitochondrial H2O2 emission during conditions of high membrane potential (LEAKCI+ETF). This may allow a greater dynamic range of ROS generation, which could be important for signal transduction purposes, such as post-exercise insulin signaling. Notably, AS160 an essential regulatory protein in the distal insulin signaling pathway, has been demonstrated to be sensitive to H2O2 in rodents [20] while infusion of the antioxidant N-acetylcysteine was shown to attenuate post-exercise whole-body insulin sensitivity in young healthy humans [42]. Exercise is known to an additive effect on insulin induced phosphorylation of AS160 which is a key mediator of the post-exercise enhancement of skeletal muscle insulin sensitivity [49–51]. Indeed, in the present study, phosphorylation of both Ser588 and Ser318 of AS160, were greater after the post-exercise insulin clamp compared to the resting insulin clamp [30]. Despite this, in the present study, we were unable to detect significant correlations between H2O2 emission and post-exercise insulin sensitivity in our obese subjects. Further investigation is required to better understand these potential molecular interactions.
Several potential mechanisms may explain the altered H2O2 emission with post-exercise insulin clamp observed in the present study. The lower muscle GSH content following the post-exercise insulin clamp (Fig 4) suggests that some perturbation to muscle redox homeostasis occurred. Even small changes in concentration of the reduced glutathione (GSH), a key thiol antioxidant in vivo and substrate for glutathione peroxidase, has been shown to have significant effects on redox homeostasis [52]. Therefore, decreased GSH may permit the elevated rates of mitochondrial H2O2 emission during LEAKCI+ETF and ETSCI+II+ETF. The lack of a corresponding increase in post-exercise oxidized glutathione (GSSG) reported in young healthy adults [42] could be related to the specific population studied presently, although the minor difference in baseline values between trials should also be considered when interpreting these data. In the present study, six out of the nice participants showed a post-exercise increase the total abundance of thioredoxin (Trx) and peroxiredoxin-1 (Prx1) and thioredoxin reductase (TXNRD1), but overall this was not significantly changed with exercise and/or insulin. These proteins play a key role in the modulation of low concentrations of ROS, due to their low KM for H2O2 [52], and therefore in spatio-temporal redox-signaling [53, 54]. Although our data are inconclusive, some of the change in mitochondrial JH2O2 may be partly explained due to acute changes in the total antioxidant capacity conferred by the Trx/Prx system. In addition, it is possible that various post-translational protein modifications not measured in the present study could acutely alter the post-exercise enzymatic activity of these and other mitochondrial antioxidant enzymes such as SOD1 and GPx1 [53]. Taken together, these findings may suggest that even in an aging and obese population there remains a ‘functional reserve’ in antioxidant capacity to allow for spatio-temporal redox-mediated cell signaling without inducing a state of oxidative stress [54].
Our findings of a transient decrease in JO2 during OXPHOS after exercise are in line with previous studies which report impaired post-exercise respiratory complex enzyme function [12, 55], and decreased OXPHOS JO2 in permeabilized muscle fibers following high intensity exercise in horses [13, 46, 56]. In permeabilized skeletal muscle mitochondria of young healthy humans, Tonkonogi et al. [57] have reported that state-3 (i.e. ‘OXPHOS’) respiratory rates are increased immediately and 2 hours post-exercise, while Perry et al. [58] reported no change immediately or 3 hours post exercise on maximal OXPHOS JO2. However, direct comparisons to their findings are difficult to make with our subjects due to the much lower OXPHOS JO2 rates and respiratory control ratios, as expected [59], and in these previous studies state-3 respiratory measures were supported by pyruvate+malate (complex-I) alone, whereas in the present study there was convergent substrate input from pyruvate, malate, octanoylcarnitine as well as succinate (complex-II). Interestingly, in our obese, older and sedentary subjects, there was no increase of JO2 in response to insulin, which appears to be consistent with what has been previously demonstrated in patients with type-2 diabetes when compared with young, healthy individuals [26, 60, 61]. The lack of response in JO2 to insulin in our subjects with obesity could therefore be interpreted as the early signs of impaired metabolic flexibility which is observed in type-2 diabetes [62]. An alternative explanation is that the aforementioned studies co-infused amino acids to avoid low plasma amino acid concentrations which can occur during longer durations (4–8 hours) of insulin infusion, which may have provided sufficient time and stimulus for the synthesis of new mitochondrial proteins. Indeed, more recent studies have reported that in the absence of amino acid co-infusion, insulin did not appear to increase mitochondrial ATP synthesis rates, even in young healthy individuals [25, 63].
There are some potential limitations to the present study. The small sample size, while typical of similar invasive human studies, may preclude the possibility of detecting potentially small effects between insulin sensitivity and mitochondrial H2O2 emission. Despite this, post hoc power analysis demonstrated that the reported reduction (~30%) of JH2O2 during OXPHOS after exercise with n = 9 and α = 0.05 that the study was adequately powered (93%). It is acknowledged that the lack of a non-obese control group limits the ability to specifically ascribe the present findings to that of a pathophysiological response. The present study design would be strengthened with an additional exercise session with a 3 hour post exercise muscle sample in the absence of hyperinsulinemia, to allow for a more robust comparison of the specific effects of insulin on transient changes in mitochondrial function after exercise. Also, it cannot be excluded that a longer rest period between the cessation of exercise and the start of the insulin clamp may be required to measure the effects of insulin-stimulated glucose uptake independent of any residual effects of contraction-mediated glucose uptake [10]. The study design could potentially be influenced by the different number of muscle biopsies obtained in each trial since repeated muscle biopsies can impact glycogen resynthesis [64, 65]. However, this seems unlikely as these effects were observed over a longer period of time (48 h) and another study reported no effect of repeated biopsies within a shorter 5–6 hour window [66]. Furthermore, it has been shown that repeated biopsies 1 hour apart from adjacent sites of the same muscle had no effect on ERK1/2 phosphorylation [67]. Cytochrome-c added to the mitochondrial respiratory assay is used to assess potential damage to the outer mitochondrial membrane during sample preparation and it should be acknowledged that the redox-active heme group in cytochrome-c may scavenge unpaired electrons and thereby potentially influence the subsequent detection of H2O2. Therefore, although in the present study cytochrome-c was added only after OXPHOS respiration states and at the same concentration in all experiments, our JH2O2 data in the presence of cytochrome-c should nonetheless be interpreted accordingly. The present method for measuring JO2 and JH2O2 has been well characterized previously [37–39], although some caution should be used when interpreting these findings in the context of in vivo physiology due to the use of hyperoxygenation (to avoid oxygen diffusion limitations) and saturating substrate concentrations in the permeabilized muscle fiber mitochondrial respiration assay. It is also acknowledged, due to practical limitations, we did not assess malalate+pyruvate and octanoylcarnitine supported JO2 and JH2O2 separately. These limitations should therefore be considered in future investigations.
In conclusion, this study provides novel evidence that a single bout of aerobic exercise acutely modifies skeletal muscle mitochondrial respiration and H2O2 emission and responses to insulin stimulation in obese, middle-aged and sedentary males, and this may have implications for metabolic diseases featuring insulin resistance.
Supporting information
(A) Mean glucose infusion rate, (B) plasma insulin concentration and (C) M/I index data plotted for individual subjects for the final 30 min of each clamp. (D) Blood glucose concentrations for the final 30 min of the clamps, data are mean ± SD for n = 9.
(TIF)
(XLSX)
Acknowledgments
We are grateful to the participants for their enthusiasm, time, and effort, as well as the laboratory staff and students for their invaluable assistance conducting the exercise trials.
Data Availability
Summary de-identified data pertinent to the manuscript has been provided in a supplementary file. The Victoria University Human Research Ethics Committee restricted access to the raw data from this study because the study involves human participants. Per national regulations for confidential information in Australia and in compliance with the Victoria University Human Research Ethics Committee, the authors will handle data access requests. The full data are available from the authors (Nigel.Stepto@vu.edu.au or Itamar.Levinger@vu.edu.au) for researchers who meet criteria for access to confidential data as determined by the Victoria University Human Research Ethics Committee (Senior Ethics Officer (Secretary), Ms Elizabeth Hill +61 3 9919 4781, elizabeth.hill@vu.edu.au).
Funding Statement
This research was funded by the Victoria University Research Development Grant Scheme (I.L., D.L.H. and G.K.M.) This funding was from the authors institution and they had no input into study design and data analysis. They have no embargo on publication of results. Two authors received funding to support their salaries to undertake this investigator initiated research. I.L. is a Heart Foundation Future Leader Fellow (ID No. 100040). N.K.S. was supported by the Australian Government through the National Collaborative Research Network Grant Scheme.
References
- 1.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2014;384(9945):766–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmet P, Alberti K, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–7. doi: 10.1038/414782a [DOI] [PubMed] [Google Scholar]
- 3.Amati F, Dubé JJ, Coen PM, Stefanovic-Racic M, Toledo FG, Goodpaster BH. Physical inactivity and obesity underlie the insulin resistance of aging. Diabetes care. 2009;32(8):1547–9. doi: 10.2337/dc09-0267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hordern MD, Dunstan DW, Prins JB, Baker MK, Singh MAF, Coombes JS. Exercise prescription for patients with type 2 diabetes and pre-diabetes: a position statement from Exercise and Sport Science Australia. Journal of Science and Medicine in Sport. 2012;15(1):25–31. doi: 10.1016/j.jsams.2011.04.005 [DOI] [PubMed] [Google Scholar]
- 5.Chow LS, Odegaard AO, Bosch TA, Bantle AE, Wang Q, Hughes J, et al. Twenty year fitness trends in young adults and incidence of prediabetes and diabetes: the CARDIA study. Diabetologia. 2016:1–7. doi: 10.1007/s00125-016-4046-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montgomery MK, Turner N. Mitochondrial dysfunction and insulin resistance: an update. Endocrine connections. 2015;4(1):R1–R15. doi: 10.1530/EC-14-0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sivitz WI, Yorek MA. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxidants & Redox Signaling. 2010;12(4):537–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher-Wellman KH, Weber TM, Cathey BL, Brophy PM, Gilliam LA, Kane CL, et al. Mitochondrial respiratory capacity and content are normal in young insulin-resistant obese humans. Diabetes. 2014;63(1):132–41. doi: 10.2337/db13-0940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mikines KJ, Sonne B, Peter A, Farrell BT, Galbo H. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol Endo Metab. 1988;254:248–59. [DOI] [PubMed] [Google Scholar]
- 10.Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Rev Physiol. 2013;93(3):993–1017. [DOI] [PubMed] [Google Scholar]
- 11.Tonkonogi M, Harris B, Sahlin K. Mitochondrial oxidative function in human saponin‐skinned muscle fibres: effects of prolonged exercise. J Physiol. 1998;510(1):279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green H, Bombardier EB, Duhamel T, Holloway GP, Tupling AR, Ouyang J. Acute responses in muscle mitochondrial and cytosolic enzyme activities during heavy intermittent exercise. J Appl Physiol. 2008;104(4):931 doi: 10.1152/japplphysiol.01151.2007 [DOI] [PubMed] [Google Scholar]
- 13.Votion DM, Fraipont A, Goachet A-G, Robert C, van Erck E, Amory H, et al. Alterations in mitochondrial respiratory function in response to endurance training and endurance racing. Equine Veterinary Journal. 2010;42(s38):268–74. [DOI] [PubMed] [Google Scholar]
- 14.Murphy MP. How mitochondria produce reactive oxygen species. Biochemical Journal. 2009;417(Pt 1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goncalves RL, Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Brand MD. Sites of superoxide and hydrogen peroxide production by muscle mitochondria assessed ex vivo under conditions mimicking rest and exercise. J Biol Chem. 2015;290(1):209–27. doi: 10.1074/jbc.M114.619072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakellariou GK, Vasilaki A, Palomero J, Kayani A, Zibrik L, McArdle A, et al. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxidants & Redox Signaling. 2013;18(6):603–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kramer PA, Duan J, Qian W-J, Marcinek DJ. The Measurement of Reversible Redox Dependent Post-translational Modifications and Their Regulation of Mitochondrial and Skeletal Muscle Function. Frontiers in physiology. 2015;6:347 doi: 10.3389/fphys.2015.00347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiganis T. Reactive oxygen species and insulin resistance: the good, the bad and the ugly. Trends in Pharmacological Sciences. 2011;32:82–9. doi: 10.1016/j.tips.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 19.Powers SK, Talbert EE, Adhihetty PJ. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J Physiol. 2011;589(9):2129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S, et al. Reactive oxygen species enhance insulin sensitivity. Cell Metabolism. 2009;10(4):260–72. doi: 10.1016/j.cmet.2009.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher-Wellman KH, Neufer PD. Linking mitochondrial bioenergetics to insulin resistance via redox biology. Tr Endocr & Metab. 2012;23(3):142–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parker L, Shaw C, Stepto NK, Levinger I. Exercise and Glycemic Control: Focus on Redox Homeostasis and Redox-Sensitive Protein Signaling. Frontiers in Endocrinology. 2017;8:87 doi: 10.3389/fendo.2017.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao X, Bak S, Pedersen AJ, Jensen ON, Højlund K. Insulin increases phosphorylation of mitochondrial proteins in human skeletal muscle in vivo. Journal of proteome research. 2014;13(5):2359–69. doi: 10.1021/pr401163t [DOI] [PubMed] [Google Scholar]
- 24.Fabbri E, Chia CW, Spencer RG, Fishbein KW, Reiter DA, Cameron D, et al. Insulin Resistance is Associated with Reduced Mitochondrial Oxidative Capacity Measured by 31P Magnetic Resonance Spectroscopy in Non-Diabetic Participants from the Baltimore Longitudinal Study of Aging. Diabetes. 2016:db160754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szendroedi J, Schmid AI, Chmelik M, Toth C, Brehm A, Krssak M, et al. Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLoS medicine. 2007;4(5):e154 doi: 10.1371/journal.pmed.0040154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proceedings of the National Academy of Sciences. 2003;100(13):7996–8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen KF, Dufour S, Shulman GI. Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med. 2005;2(9):e233 doi: 10.1371/journal.pmed.0020233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konopka AR, Asante A, Lanza IR, Robinson MM, Johnson ML, Dalla Man C, et al. Defects in mitochondrial efficiency and H2O2 emissions in obese women are restored to a lean phenotype with aerobic exercise training. Diabetes. 2015;64(6):2104–15. doi: 10.2337/db14-1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levinger I, Jerums G, Stepto NK, Parker L, Serpiello FR, McConell GK, et al. The Effect of Acute Exercise on Undercarboxylated Osteocalcin and Insulin Sensitivity in Obese Men. Journal of Bone and Mineral Research. 2014;29(12):2571–6. doi: 10.1002/jbmr.2285 [DOI] [PubMed] [Google Scholar]
- 30.Parker L, Stepto NK, Shaw CS, Serpiello FR, Anderson M, Hare DL, et al. Acute High-intensity Interval Exercise-induced Redox Signaling is Associated with Enhanced Insulin Sensitivity in Obese Middle-aged Men. Frontiers in Physiology. 2016;7:411 doi: 10.3389/fphys.2016.00411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levinger I, Goodman C, Hare DL, Jerums G, Selig S. The effect of resistance training on functional capacity and quality of life in individuals with high and low numbers of metabolic risk factors. Diabetes care. 2007;30(9):2205–10. doi: 10.2337/dc07-0841 [DOI] [PubMed] [Google Scholar]
- 32.Howlett KF, Mathews A, Garnham A, Sakamoto K. The effect of exercise and insulin on AS160 phosphorylation and 14-3-3 binding capacity in human skeletal muscle. Am J Physiol Endo Metab. 2008;294(2):E401–E7. [DOI] [PubMed] [Google Scholar]
- 33.Parker L, Trewin AJ, Levinger I, Shaw CS, Stepto NK. The effect of exercise-intensity on skeletal muscle stress kinase and insulin protein signaling. PLoS One. 2017;12(2):e0171613 doi: 10.1371/journal.pone.0171613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stephenson EJ, Camera DM, Jenkins TA, Kosari S, Lee JS, Hawley JA, et al. Skeletal muscle respiratory capacity is enhanced in rats consuming an obesogenic Western diet. Am J Physiol Endo Metab. 2012;302(12):E1541–E9. [DOI] [PubMed] [Google Scholar]
- 35.Stephenson EJ, Stepto NK, Koch LG, Britton SL, Hawley JA. Divergent skeletal muscle respiratory capacities in rats artificially selected for high and low running ability: a role for Nor1? J Appl Physiol. 2012;113(9):1403–12. doi: 10.1152/japplphysiol.00788.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Timpani CA, Trewin AJ, Stojanovska V, Robinson A, Goodman CA, Nurgali K, et al. Attempting to Compensate for Reduced Neuronal Nitric Oxide Synthase Protein with Nitrate Supplementation Cannot Overcome Metabolic Dysfunction but Rather Has Detrimental Effects in Dystrophin-Deficient mdx Muscle. Neurotherapeutics. 2016:1–18. doi: 10.1007/s13311-015-0414-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krumschnabel G, Fontana-Ayoub M, Sumbalova Z, Heidler J, Gauper K, Fasching M, et al. Simultaneous high-resolution measurement of mitochondrial respiration and hydrogen peroxide production. Mitochondrial Medicine: Volume I, Probing Mitochondrial Function. 2015:245–61. [DOI] [PubMed] [Google Scholar]
- 38.Makrecka-Kuka M, Krumschnabel G, Gnaiger E. High-resolution respirometry for simultaneous measurement of oxygen and hydrogen peroxide fluxes in permeabilized cells, tissue homogenate and isolated mitochondria. Biomolecules. 2015;5(3):1319–38. doi: 10.3390/biom5031319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hickey AJR, Renshaw GMC, Speers-Roesch B, Richards JG, Wang Y, Farrell AP, et al. A radical approach to beating hypoxia: depressed free radical release from heart fibres of the hypoxia-tolerant epaulette shark (Hemiscyllum ocellatum). Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 2011;182(1):91–100. doi: 10.1007/s00360-011-0599-6 [DOI] [PubMed] [Google Scholar]
- 40.Murphy RM, Lamb GD. Important considerations for protein analyses using antibody based techniques: down-sizing Western blotting up-sizes outcomes. J Physiol. 2013;591(23):5823–31. doi: 10.1113/jphysiol.2013.263251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srere P. [1] Citrate synthase:[EC 4.1. 3.7. Citrate oxaloacetate-lyase (CoA-acetylating)]. Methods in enzymology. 1969;13:3–11. [Google Scholar]
- 42.Trewin AJ, Lundell LS, Perry BD, Patil KV, Chibalin AV, Levinger I, et al. Effect of N-acetylcysteine infusion on exercise induced modulation of insulin sensitivity, and signaling pathways in human skeletal muscle. Am J Physiol Endo Metab. 2015;309(4):E388–97. [DOI] [PubMed] [Google Scholar]
- 43.Henriksen EJ, Diamond-Stanic MK, Marchionne EM. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med. 2011;51(5):993–9. doi: 10.1016/j.freeradbiomed.2010.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campbell PT, Gross MD, Potter JD, Schmitz KH, Duggan C, McTiernan A, et al. Effect of exercise on oxidative stress: a 12-month randomized, controlled trial. Medicine and science in sports and exercise. 2010;42(8):1448 doi: 10.1249/MSS.0b013e3181cfc908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fisher-Wellman K, Bloomer RJ. Acute exercise and oxidative stress: a 30 year history. Dynamic Medicine. 2009;8(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson EJ, Yamazaki H, Neufer PD. Induction of endogenous uncoupling protein 3 suppresses mitochondrial oxidant emission during fatty acid-supported respiration. J Biol Chem. 2007;282(43):31257–66. doi: 10.1074/jbc.M706129200 [DOI] [PubMed] [Google Scholar]
- 47.Brand MD, Goncalves RL, Orr AL, Vargas L, Gerencser AA, Jensen MB, et al. Suppressors of Superoxide-H 2 O 2 Production at Site I Q of Mitochondrial Complex I Protect against Stem Cell Hyperplasia and Ischemia-Reperfusion Injury. Cell Metabolism. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Powers S, Duarte J, Kavazis A, Talbert E. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Experimental Physiology. 2010;95(1):1 doi: 10.1113/expphysiol.2009.050526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Funai K, Schweitzer GG, Sharma N, Kanzaki M, Cartee GD. Increased AS160 phosphorylation, but not TBC1D1 phosphorylation, with increased postexercise insulin sensitivity in rat skeletal muscle. Am J Physiol Endo Metab. 2009;297(1):E242–E51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Treebak JT, Frøsig C, Pehmøller C, Chen S, Maarbjerg SJ, Brandt N, et al. Potential role of TBC1D4 in enhanced post-exercise insulin action in human skeletal muscle. Diabetologia. 2009;52(5):891–900. doi: 10.1007/s00125-009-1294-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jessen N, An D, Lihn AS, Nygren J, Hirshman MF, Thorell A, et al. Exercise increases TBC1D1 phosphorylation in human skeletal muscle. Am J Physiol Endo Metab. 2011;301(1):E164–E71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aon MA, Stanley BA, Sivakumaran V, Kembro JM, O'Rourke B, Paolocci N, et al. Glutathione/thioredoxin systems modulate mitochondrial H2O2 emission: an experimental-computational study. The Journal of general physiology. 2012;139(6):479–91. doi: 10.1085/jgp.201210772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woo HA, Yim SH, Shin DH, Kang D, Yu D-Y, Rhee SG. Inactivation of peroxiredoxin I by phosphorylation allows localized H 2 O 2 accumulation for cell signaling. Cell. 2010;140(4):517–28. doi: 10.1016/j.cell.2010.01.009 [DOI] [PubMed] [Google Scholar]
- 54.Rhee SG, Kang SW, Jeong W, Chang T-S, Yang K-S, Woo HA. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Current opinion in cell biology. 2005;17(2):183–9. doi: 10.1016/j.ceb.2005.02.004 [DOI] [PubMed] [Google Scholar]
- 55.Stepto NK, Benziane B, Wadley GD, Chibalin AV, Canny BJ, Eynon N, et al. Short-Term Intensified Cycle Training Alters Acute and Chronic Responses of PGC1α and Cytochrome C Oxidase IV to Exercise in Human Skeletal Muscle. PLoS One. 2012;7(12):e53080 doi: 10.1371/journal.pone.0053080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gollnick PD, Bertocci LA, Kelso TB, Witt EH, Hodgson DR. The effect of high-intensity exercise on the respiratory capacity of skeletal muscle. Pflügers Archiv. 1990;415(4):407–13. [DOI] [PubMed] [Google Scholar]
- 57.Tonkonogi M, Walsh B, Tiivel T, Saks V, Sahlin K. Mitochondrial function in human skeletal muscle is not impaired by high intensity exercise. Pflügers Archiv. 1999;437(4):562–8. doi: 10.1007/s004240050818 [DOI] [PubMed] [Google Scholar]
- 58.Perry CG, Kane DA, Herbst EA, Mukai K, Lark DS, Wright DC, et al. Mitochondrial creatine kinase activity and phosphate shuttling are acutely regulated by exercise in human skeletal muscle. J Physiol. 2012;590(21):5475–86. doi: 10.1113/jphysiol.2012.234682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gnaiger E. Capacity of oxidative phosphorylation in human skeletal muscle: new perspectives of mitochondrial physiology. The international journal of biochemistry & cell biology. 2009;41(10):1837–45. [DOI] [PubMed] [Google Scholar]
- 60.Phielix E, Schrauwen-Hinderling VB, Mensink M, Lenaers E, Meex R, Hoeks J, et al. Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes. 2008;57(11):2943–9. doi: 10.2337/db08-0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Asmann YW, Stump CS, Short KR, Coenen-Schimke JM, Guo Z, Bigelow ML, et al. Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes. 2006;55(12):3309–19. doi: 10.2337/db05-1230 [DOI] [PubMed] [Google Scholar]
- 62.Hawley J, Lessard S. Mitochondrial function: use it or lose it. Diabetologia. 2007;50(4):699–702. doi: 10.1007/s00125-007-0595-2 [DOI] [PubMed] [Google Scholar]
- 63.Barazzoni R, Short KR, Asmann Y, Coenen-Schimke JM, Robinson MM, Nair KS. Insulin fails to enhance mTOR phosphorylation, mitochondrial protein synthesis, and ATP production in human skeletal muscle without amino acid replacement. Am J Physiol Endo Metab. 2012;303(9):E1117–E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Costill D, Pearson D, Fink W. Impaired muscle glycogen storage after muscle biopsy. J Appl Physiol. 1988;64(5):2245–8. [DOI] [PubMed] [Google Scholar]
- 65.Constantin-Teodosiu D, Casey A, Short A, Hultman E, Greenhaff P. The effect of repeated muscle biopsy sampling on ATP and glycogen resynthesis following exercise in man. Eur J Appl Physiol Occup Physiol. 1996;73(1):186–90. [DOI] [PubMed] [Google Scholar]
- 66.Lundby C, Nordsborg N, Kusuhara K, Kristensen KM, Neufer PD, Pilegaard H. Gene expression in human skeletal muscle: alternative normalization method and effect of repeated biopsies. Eur J Appl Physiol. 2005;95(4):351–60. doi: 10.1007/s00421-005-0022-7 [DOI] [PubMed] [Google Scholar]
- 67.Widegren U, Jiang XJ, Krook A, Chibalin AV, Björnholm M, Tally M, et al. Divergent effects of exercise on metabolic and mitogenic signaling pathways in human skeletal muscle. The FASEB Journal. 1998;12(13):1379–89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Mean glucose infusion rate, (B) plasma insulin concentration and (C) M/I index data plotted for individual subjects for the final 30 min of each clamp. (D) Blood glucose concentrations for the final 30 min of the clamps, data are mean ± SD for n = 9.
(TIF)
(XLSX)
Data Availability Statement
Summary de-identified data pertinent to the manuscript has been provided in a supplementary file. The Victoria University Human Research Ethics Committee restricted access to the raw data from this study because the study involves human participants. Per national regulations for confidential information in Australia and in compliance with the Victoria University Human Research Ethics Committee, the authors will handle data access requests. The full data are available from the authors (Nigel.Stepto@vu.edu.au or Itamar.Levinger@vu.edu.au) for researchers who meet criteria for access to confidential data as determined by the Victoria University Human Research Ethics Committee (Senior Ethics Officer (Secretary), Ms Elizabeth Hill +61 3 9919 4781, elizabeth.hill@vu.edu.au).