Abstract
Linuron is a widely used herbicide in agriculture; its endocrine disruptive toxicity has recently received public attention. This study was designed to examine the developmental toxicity of linuron on the reproductive system of male offspring following maternal exposure. Mother rats received oral gavages of linuron, once daily, at the dose of 0, 50, 100, 150 or 200 mg/kg, from gestational day (GD)13 to GD18; gonadal organs from GD20 fetuses were examined. Data indicated that exposed male offspring had a significantly shortened anogenital distance. Pathological examination further revealed a lack of fusion in the urogenital fold in treated fetuses, the damaged seminiferous tubules, and the injured Leydig cell ultrastructure. Analysis of serum testosterone concentrations at postnatal day (PND)2 showed a significant dose-related reduction (about 33.7–58.75%, r = −0.838, p < 0.05) as compared to controls. Immunohistochemical results demonstrated a significantly reduced expression of enzymes pertinent to the testosterone production including P450scc, 3β-HSD, and PCNA in Leydig cells (p < 0.05). qPCR studies confirmed decreased levels of mRNAs encoding P450scc, 3β-HSD and PCNA (p < 0.05). Taken together, these data suggest that maternal exposure to linuron hampers the male gonadal organ development; this appears to be due to linuron’s direct action on the production of testosterone in fetal and postnatal offspring.
Keywords: Linuron, Testosterone, Offspring, Reproductive toxicity, Developmental toxicity, Fetus, Leydig cells
1. Introduction
Reproductive disorders due to environmental exposure to antiandrogenic pesticides, fungicides, and herbicides have been well recognized in literature (Auger et al., 2013; Klot et al., 2014; Monosson et al., 1999). Particularly alarming is the increased incidence of developmental disorders in male reproductive system due to exposure to these toxicants (Sharpe and Skakkebaek, 1993; Storgaard et al., 2006). Toxic chemicals can interact with the androgen receptor (AR), alter the production and metabolism of testosterone, and/or directly damage the formation of male reproductive organs in the fetal stage. While studies on the reproductive toxicity of antiandrogenic substances are extensive, the mechanisms underlying these toxic actions in most cases remain elusive.
Linuron (also called methoxydiuron or afalon; CAS#330-55-2) is a widely used herbicide in the production of soybeans, corn, cotton, carrots, wheat, peanuts, sugar cane, fruit and other vegetables. Reports in literature suggest that linuron acts as an environmental endocrine disruptor; short-term exposures to linuron in rats result in a reduced production of testosterone (Lambright et al., 2000; Santos et al., 2014; Wilson et al., 2009). Exposure to linuron in mothers also causes the abnormal sexual differentiation and development in male offspring, such as hypospadias, cryptorchidism, prostate hyperplasia, and testicular atrophy (McIntyre et al., 2000; Hotchkiss et al., 2004; Sultan et al., 2001). The deformity of the male reproductive system is irreversible and persistent, and may last for lifetime. Limited data from in vivo and in vitro experiments suggest that linuron may compete with androgen for the AR binding (Gray et al., 2001; Lambright et al., 2000). Yet, the current understanding on how maternal exposure to this chemical may lead to the altered development of male reproductive system and what mechanism underlies linuron toxicity is incomplete.
The purpose of this study was to explore and confirm the developmental toxicity of linuron on male reproductive system following maternal exposure by examining the changes in male sex hormone levels (testosterone) as well as in male reproductive organs in fetal and postnatal stages. It is known that the production of testosterone is regulated by P450scc, which catalyzes the formation of pregnenolone from cholesterol, and by 3β-HSD, which converts pregnenolone to progesterone (Arukwe, 2008; Rone et al., 2009; Issop et al., 2013). To understand the mechanism of linuron toxicity, we further investigated the changes of P450scc, 3β-HSD and other male sex hormone-related enzymes in male offspring after maternal exposure. The study, by offering a better understanding of linuron-induced reproductive toxicity, may provide the useful information for better prevention and intervention.
2. Materials and methods
2.1. Chemicals and reagents
Linuron was purchased from Chem Service Inc. (West Chester, USA); the enzyme-linked immunosorbent assay (ELISA) kit to quantify serum total testosterone from Diagnostic Products Corp. (Los Angeles, CA); all primary and secondary antibodies from Santa Cruz Biotechnology (Santa Cruz, CA); and formaldehyde and other routinely used reagents from Sigma (St. Louis, MO). All reagents were of analytical grade, HPLC grade or the best available pharmaceutical grade.
2.2. Animals and treatments
Sprague-Dawley rats (4–8 week) of both sexes were obtained from the Animal Center of the Third Military Medical University (Chongqing, China). Upon arrival, the animals were housed in a temperature (21±1 °C)- and humidity (55±5%)-controlled room under a 12-h light/dark cycle and allowed for acclimatization for two weeks prior to experimentation. At the time of experiment, rats were 9 weeks old weighing 240±10 g for males (n = 60) and 180±10 g for females (n = 60). Rats had access to food and tap water at libitum. The study was conducted in compliance with the Animal Care and Use Guidelines in China and approved by the Animal Care and Use Committee of Zunyi Medical College.
After acclimatization, each male rat was caged with two females. Vaginal smears were performed daily; the sperm-positive smear in female rats was considered as Gestational Day (GD) 0. The pregnant dams were housed individually and were randomly assigned to one of the following exposure conditions. Linuron was dissolved in groundnut oil. At GD13, mother rats received the oral gavage (2 mL/kg body weight), once daily, at the dose of 0 (control), 50, 100, 150 or 200 mg/kg, for 5 consecutive days. The daily equivalent volume of oil vehicle was given to the animals in the control group. These dosage levels were selected based on the previous report in literature (Lambright et al., 2000). Each exposure group had 10 dams. At the designated time, mother and/or fetal male rats were dissected to determine reproductive toxicity.
Daily oral gavage caused the minimal stress, as animals were gradually accustomed to the procedure. The dams were weighed daily before and after dose administration. After parturition (PND0), the pups were counted and weighed. Pups were then caged with their biological mother thereafter.
2.3. Pathological examination
At 20 days into pregnancy (GD20), four mother rats from each group were sacrificed to collect fetuses; the genital tubercle and testis of male fetal rats were isolated and prepared for pathological examination. Each sample was fixed in 4% formaldehyde for 24 h; the samples were then dehydrated and embedded in paraffin according to the routine pathological sample preparation procedures. The tissues were cut into 5-mm section with a microtome and stained with haematoxylin and eosin (H&E). Each of tissue samples was made in triplicates. During the tissue dissection, the position of testis and the development of prostate were also observed.
The tissue samples from the same dams described above were used for electron microscopic examination. The testes were fixed in 3% glutaraldehyde solution for 6 h and treated with 1% osmium tetroxide for 45 min for tissue preparation prior to electron microscopic analysis.
2.4. Determination of serum testosterone levels in postnatal male rats
Blood samples were collected from 2 to 3 PND2 male pups from 4–5 l in each treatment group by decapitation and allowed to coagulate on ice. Serum samples were prepared by centrifuging the whole blood samples at approximately 1000 × g at 4 °C for 30 min and stored at −20 °C until analysis. Serum total testosterone was determined in duplicates by using an enzyme-linked immunosorbent assay (ELISA) kit (Diagnostic Products Corp, Los Angeles, CA).
2.5. Immunohistochemical examination
Bilateral testes from male fetuses of GD20 rats (2 pups from 4 l in each treatment group) were extracted as described above and prepared in 4% formaldehyde solution for 48 h. Conventional immunohistochemical procedure (including dehydration in gradient ethanol, xylene clearance, and paraffin embedding) was followed to prepare the sections. Sections were heated to 62 °C to remove wax and then rehydrated prior to treatment with blocking serum (phosphate-buffered saline/0.5% Triton X-100 with 5% serum) for 1 h at room temperature. Primary antibodies, i.e., mouse Mab against 3β-HSD (1:300 dilution), mouse Mab against P450scc (1:200 dilution), anti-proliferating cell nuclear antigen (PCNA) (1:100 dilution) and mouse Mab against AR (1:200 dilution) were incubated overnight at 4 °C. After washes, the sections were incubated at room temperature with the secondary antibodies for 1 h. Secondary antibodies included mouse anti-biotin (1:75 dilution) and goat anti-rabbit immunoglobulin G (IgG) rhodamine (1:300 dilution). Samples were examined under a light microscope; an IPWIN60 software was used to analyze the optical density (OD) value for the signals from Leydig cells. Each group had 8 sections and each section had 3 visual fields.
2.6. Real-time RT-qPCR analysis
The expression levels of mRNAs encoding P450c17, 17β-HSD and AR in bilateral testes collected from male GD20 fetuses were quantified using qPCR. The total RNA was isolated by using TRIzol reagent. An aliquot of 0.5 μg RNA was reverse-transcribed into cDNA. The iTaq Universal SYBR Green Supermix (Bio-Rad, CA) was used for qPCR analyses. The amplification was run in the FTC-2000A Real-Time PCR Detection system (Funglyn Biotech, CHN). After initial 2-min denaturation at 94 °C, the amplification program was set at 45 cycles of 20 s denaturation at 94 °C, 30 s gradient 55.0–60.0 °C and 40 s extension at 72 °C. Each real-time RT-PCR reaction was run in triplicates. The forward and reverse primers for tested genes were designed by Takara Biotechnology (Shiga, Japan). Primers sequences for these genes are listed in Table 1. The rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. To compare the interest gene expression levels in different groups, the comparative 2−ΔΔCt method was used (Livak and Schmittgen, 2001).
Table 1.
Forward and reverse primer sequence for selected genes in qPCR study.
| Gene | Forward primer (5′–3′) | Reverse primer (5′–3′) | |
|---|---|---|---|
| PCNA | AAGAGGAAGCTGTGTCCATAGA | CTTCATCTTCGATCTTGGGA | 204bp |
| P450c17 | GGAGAAGCTAATCTGTCAGGAA | GCATCCACGATACCCTCAGT | 198bp |
| 17β-HSD | CAGAAGAGATTGAGAGGACCA | CAGGAAATGACTTGGGAGCA | 158bp |
| AR | GGACATGCGTTTGGACAGTA | ACTTCTGTTTCCCTTCCGCA | 173bp |
| GAPDH | TGGGTGTGAACCACGAGAA | GGCATGGACTGTGGTCATGA | 141bp |
2.7. Statistical analysis
The statistical analyses were performed with SPSS software version 21.0 for Windows (SPSS Inc., Chicago, IL). Values of all variables are presented as mean ± standard deviation. One-way analysis of variance (ANOVA) with Tukey’s HSD as post-hoc test and LSD-t test were used to determine the differences between different treatment groups. The differences between two means were considered significant if p values were equal or less than 0.05.
3. Results
3.1. Systemic effects of linuron on postnatal male rats following maternal exposure
To understand the developmental toxicity of linuron on male reproductive system, the mother rats were orally exposed to various doses of linuron from GD13 to GD18; the male offspring were then subjected to testing serum testosterone level at postnatal day 2 (PND2), and to measurement of body weight and anogenital distance (AGD) at PND28. Linuron exposure in mothers caused a dose-related reduction in male offspring in their serum testosterone concentrations, about 33.7%, 46.0%, and 58.8% reduction in 100-mg/kg, 150-mg/kg and 200-mg/kg groups, respectively, as compared to controls (p < 0.05) (Table 2). A linear regression analysis of this dose-effect relationship established a correlation coefficient of r = −0.838 (p < 0.05).
Table 2.
Comparison of testosterone (Tes), anogenital distance (AGD) and body weight.
| Group (mg/kg) | PND2
|
PND28
|
|||
|---|---|---|---|---|---|
| n | Tes (ng/ml) | n | AGD (mm) | Body weight (g) | |
| 0 (control) | 10 | 4.63 ± 0.72 | 20 | 20.3 ± 0.65 | 77.5 ± 0.65 |
| 50 | 10 | 4.43 ± 0.62 | 20 | 20.3 ± 0.78 | 77.3 ± 0.56 |
| 100 | 10 | 3.07 ± 1.22* | 20 | 16.7 ± 1.11* | 77.3 ± 0.44 |
| 150 | 10 | 2.50 ± 0.95* | 20 | 16.5 ± 0.69* | 77.3 ± 0.52 |
| 200 | 10 | 1.91 ± 0.82* | 20 | 15.3 ± 0.84* | 76.3 ± 0.42 |
For PND2 experiments, male pups (2–3) from 4–5 l in each treatment group underwent experimentation. Animal numbers were doubled for PND28 study. Data represent mean ± SD.
: p < 0.05 as compared to controls. PND: postnatal day.
At PND28, while the body weights were not significantly changed in male offspring, the AGD, as measured between the anus and penis, were significantly shortened in male pups of the linuron-exposed groups, about 17.7–24.6% reduction in 100–200 mg/kg groups, as compared to controls (Table 2). Further linear regression analysis revealed a dose-related correlation efficient of r = −0.873 (p < 0.05). Clearly, the maternal exposure to linuron caused the damage to offspring male reproductive functions.
3.2. Pathological changes of fetal male reproductive system following maternal linuron exposure
Oral exposure to linuron in maternal rats for five consecutive days caused a significant pathological damage to offspring male reproductive structure (Fig. 1). In control fetuses, the HE staining showed that the genital tubercles possessed a well-developed urethra; the urogenital fold became confluent; and no epithelial gaps were observed (Fig. 1A). In contrast, the samples from the linuron-exposed fetuses showed that the genital tubercles were opened with continuous urethral groove; there was no fusion in the urogenital fold, but the epithelial gaps were evident (Fig. 1B). Noticeably also, the location of urethral opening was unusual. It is known that an incompletely developed urogenital fold will ultimately lead to hypospadias (Baskin and Ebbers, 2006).
Fig. 1.
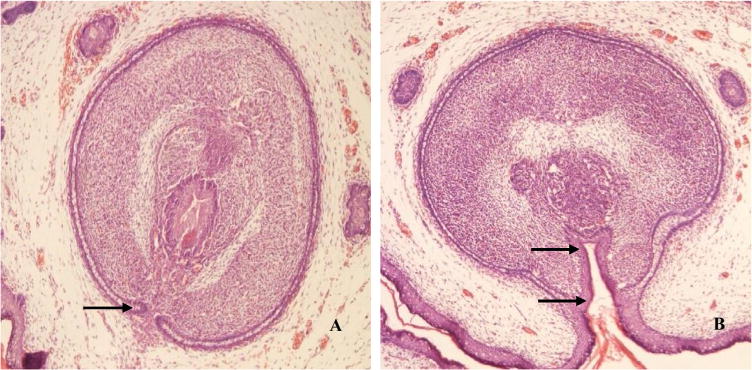
Pathological changes of the genital tubercle following maternal exposure to linuron. Mother rats received oral gavage at 150 mg/kg from GD13 to GD18; fetal male gonadal organs at GD20 were dissected for examination. (A). A typical genital tubercle from a control fetus. Arrowhead indicates a normal urogenital fold. (B). A typical genital tubercle from a linuron-exposed fetus. Arrowhead indicates no fusion in the urogenital fold and the epithelial gaps. (×100).
Further examination of testicular cord under microscope revealed four typical cell types in the seminiferous tubules, including Leydig cells, spermatogonia, supporting Sertoli cells and peritubular myoid cells. In controls, spermatogonia had clear nuclear staining and lined against the basement membrane; there were abundant Sertoli cells in lumen of tubules (Fig. 2A). Treatment with linuron appeared to reduce spermatogonia, disrupt the normal arrangement of cell layers in tubule lumens, and cause karyopyknosis (Fig. 2B). There were obvious vacuoles in nearly all of the cell types. With the increase of linuron dose, these morphological changes became more severe (Fig. 2C and D) and significant cell loss could be seen in Fig. 2D.
Fig. 2.
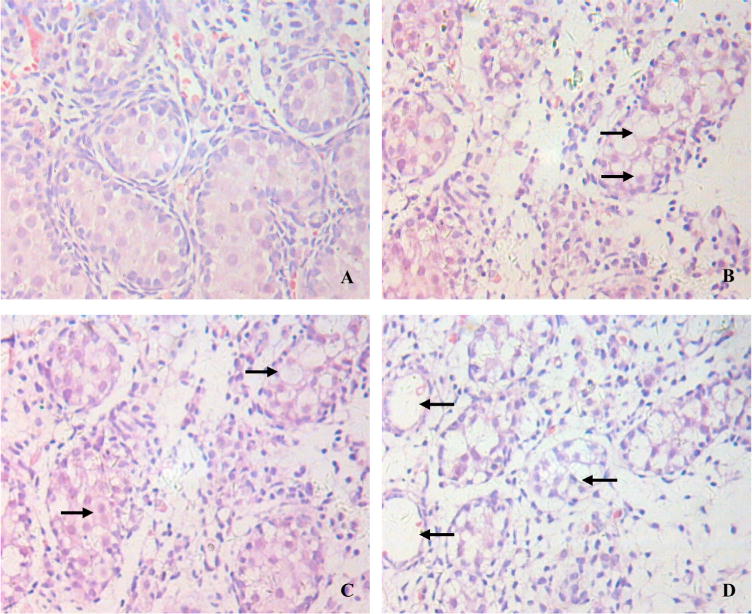
Pathological changes in seminiferous tubules following maternal exposure to linuron. Mother rats received oral gavage at 0 (A), 100 mg/kg (B), 150 mg/kg (C), or 200 mg/kg (D) from GD13 to GD18. Fetal male dams at GD20 were dissected for examination. Arrowheads indicate karyopyknosis and vacuoles. (×400).
The Leydig cells distributing in interstitial space function to secrete male sex hormone. Under the electron microscope, a distension of rough endoplasmic reticulum in Leydig cells became evident (Fig. 3B). At the high dose (200 mg/kg), the swollen mitochondria in Leydig cells could be observed in most cases (Fig. 3C).
Fig. 3.
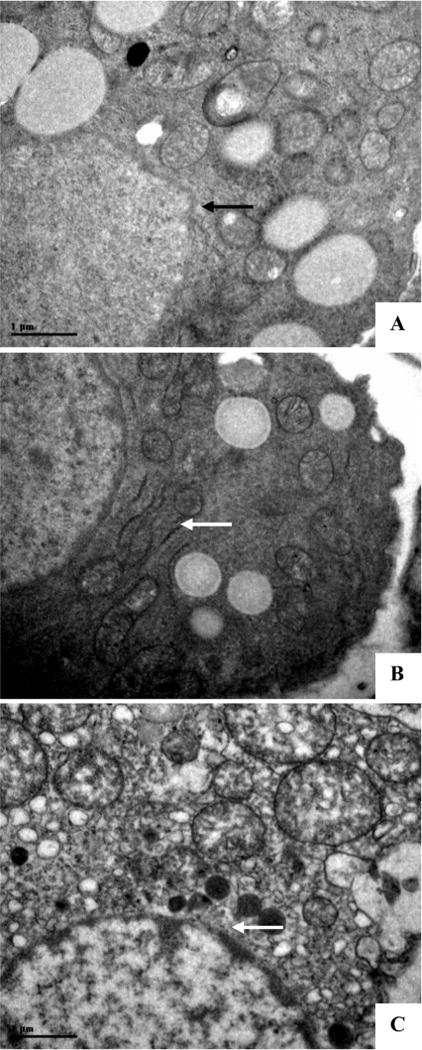
Ultrastructure of Leydig cells. Mother rats received oral gavage from GD13 to GD18; fetal male gonadal organ at GD20 were dissected for electron microscopic study. (A) A typical sample from a control rat. Arrowhead indicates a normal mitochondrion. (B) A typical sample from a rat treated with 150 mg/kg linuron. Arrowheads indicate a dilated endoplasmic reticulum. (C) A typical sample from a rat treated with 200 mg/kg linuron. Arrowhead indicates swollen mitochondrion. (×12,500).
3.3. Effect of maternal linuron exposure on enzymes and proteins participating in male reproduction
The apparent reproductive toxicity following maternal linuron exposure could be due to the altered production of male sex hormones or androgen receptor (AR) in fetuses. To test this hypothesis, we used the immunohistochemistry to examine the expression of a host of selected enzymes and proteins in fetal Leydig cells from GD20 dams. Data presented in Fig. 4 showed that PCNA, a marker for DNA synthesis during cell proliferation, was apparently reduced, so were the enzymes involving in synthesis of testosterone, i.e., P450scc and 3β-HSD. By quantitation of expression signals, there were 18.3%, 15.5%, and 16.9% reductions in PCNA, P450scc and 3β-HSD, respectively, as compared to controls (p < 0.05) (Table 3), while the signal levels for AR in Leydig cells were not changed (Fig. 4 and Table 3).
Fig. 4.
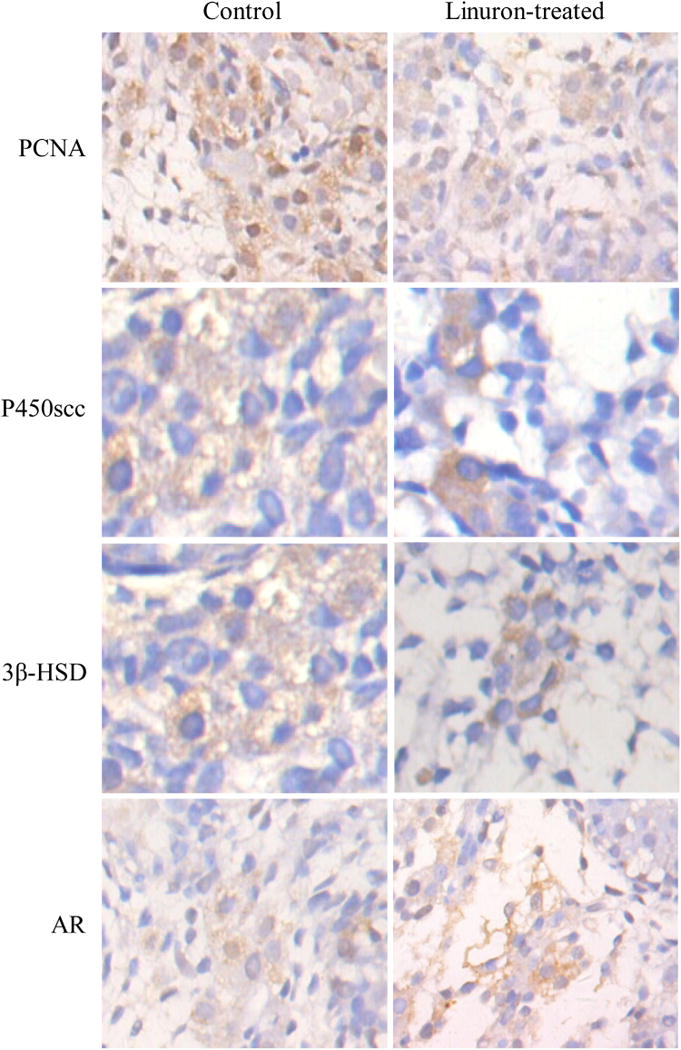
Immunohistochemical analysis of PCNA, P450scc, 3β-HSD and AR expression in Leydig cells. Mother rats received oral gavage at 200 mg/kg from GD13 to GD18; fetal male gonadal organ at GD20 were dissected for IHC study. (×400).
Table 3.
Quantitation of signal density of PCNA, P450scc, 3β-HSD and AR expression in GD20 testicular tissue by immunohistochemical analysis.
| Group (mg/kg/day) | n | Optical Density
|
|||
|---|---|---|---|---|---|
| PCNA | P450scc | 3β-HSD | AR | ||
| control | 8 | 1931 ± 39 | 1683 ± 21 | 1714 ± 21 | 1238 ± 68 |
| 200 | 8 | 1578 ± 18* | 1422 ± 14* | 1424 ± 28* | 1277 ± 62 |
Mother rats received oral gavage at 200 mg/kg from GD13 to GD18. Fetal male pups (2 from 4 l in each treatment group) at GD20 were dissected for IHC examination. The optical density for each protein was analyzed using IPWIN60 software. Data represent mean ± SD, n = 8 different fetal rats.
: p < 0.05 compared to controls.
To verify the results from immunohistochemical studies, we further employed qPCR to quantify the mRNAs encoding these proteins. Data presented in Table 4 demonstrated that the gene expressions of PCNA, P450c17 and 17β-HSD in fetal testes at GD20 were significantly lower, about 47.0%, 50.8% and 39.9%, respectively, than those in controls (p < 0.05), while the gene expression of AR was not changed (Table 4).
Table 4.
Relative mRNA expression of PCNA, 17β-HSD, P450c17 and AR in GD20 testes.
| group | n | 2−ΔΔCt
|
|||
|---|---|---|---|---|---|
| PCNA | 17β-HSD | P450c17 | AR | ||
| control | 10 | 1.64 ± 0.98 | 1.87 ± 0.26 | 1.53 ± 0.50 | 1.51 ± 0.43 |
| exposure | 10 | 0.87 ± 0.32* | 0.92 ± 0.26* | 0.92 ± 0.42* | 1.35 ± 0.56 |
Mother rats received oral gavage at 200 mg/kg from GD13 to GD18. Fetal gonadal organs (2 from 5 l in each treatment group) at GD20 were dissected for qPCR analysis. Data represent mean ± S.D.
: p < 0.05 compared to controls.
4. Discussion
Results of these experiments demonstrate that oral exposure to linuron in mother rats can profoundly alter the reproductive organ development in male fetal and neonatal rats. The alteration can be observed at morphological, biochemical, and molecular levels. Under normal physiological condition, the urogenital folds have two fusions; one is on the surface of the genital tubercle and the other is positioned in the middle. Upon the formation of complete urethral, the chamber is absorbed to form the epidermis. Urogenital groove and urogenital fold can integrate together to form a cavernous body of urethra tube (Lambright et al., 2000). Following maternal exposure to linuron, however, the fusion of urogenital groove and urogenital fold was incomplete, resulting in an abnormal location of the urethral orifice. The failure to form a complete urogenital groove may lead to a shortened anogenital distance in postnatal rats. Our evidence supports the view that maternal linuron exposure hampers sexual differentiation and development in male offspring.
The formation and differentiation of the gonadal organs during the embryonic development are regulated by a host of hormones through the hypothalamus-pituitary-gonad axis in mother as well as in fetus (Hotchkiss et al., 2004; Kroupova et al., 2014); the process is highly sensitive to variation of hormone levels in the fetal stage (Vukusic et al., 2013). Reports by Fitch et al. (1990) show that from the embryonic day GD17 to the postnatal day 6–8, the secretion of testosterone in offspring testes reaches the peak, which determines the early differentiation and development of testis (Sinha and Swerdloff, 1999; Russell et al., 1993). Other reports also show that impaired testosterone secretion during this stage usually causes the irreversible and permanent damage to testicular development, which in turn directly affects the reproductive function in adulthood (Han et al., 2004; Wu et al., 2010; Esteves et al., 2011). Our data clearly show that exposure to linuron in mother rats caused a dose-related decline of testosterone in offspring testes. Thus, it appears that linuron’s reproductive toxicity is directly associated with the production of testosterone.
What is the targeted cell type underlying linuron developmental, reproductive and toxicity? Results from electron microscopic studies demonstrated that at GD20, the Leydig cells in fetal rats appeared to be significantly injured by linuron treatment. The rough endoplasmic reticulum in Leydig cells became dilated and the mitochondria were swollen. Since the endoplasmic reticulum in Leydig cells is a known intracellular location where testosterone is synthesized, it is likely that the damage on endoplasmic reticula in Leydig cells, in combination with the distorted energy supply in malfunctioned mitochondria, may reduce the production of testosterone from the fetal to postnatal stage.
Synthesis of testosterone requires numerous enzymes that transport cholesterol across the mitochondrial membrane and convert cholesterol to pregnenolone; the latter reaction is catalyzed by mitochondrial P450scc. Pregnenolone is then further catalyzed by 3β-HSD to form progesterone. These reactions are the rate-limiting steps in the synthesis of testosterone (Arukwe, 2008; Rone et al., 2009; Issop et al., 2013). It is also known that Leydig cells in fetal mouse differentiate rapidly during GD12–GD14, followed by synthesis of testosterone. To investigate the mechanism of linuron toxicity, we determined a number of key enzymes and proteins involving testosterone production in fetal GD20 rats. Our IHC data demonstrated a significant reduced expression of P450scc and 3β-HSD in Leydig cells of fetal GD20 rats. The qPCR experiments confirmed these findings and further indicated a 40–51% reduction of these two critical enzymes in fetal gonad organs. It is interesting to notice that linuron exposure had no any significant effect on AR gene expression. Recent data in literature have also suggested that exposure to other gonadotoxicants such as polychlorinated biphenyls or acteoside can inhibit P450scc activity and decrease testosterone synthesis, leading to hypospadias and cryptorchidism (McGlynn et al., 2009; Liu et al., 2015). Thus, our results establish that linuron exposure in mothers inhibits the key enzymes indispensable to male sex hormone production. The exact mechanism on how linuron interacts with these enzymes at molecular and/or genetic levels remains unknown and deserves further in-depth investigation.
In summary, the present study confirms the developmental toxicity of linuron on male reproductive system. Our data show that maternal exposure to linuron results in an altered development of male gonadal organs, damaged seminiferous tubules, and abnormal Leydig cell ultrastructure. The mechanism underlying linuron toxicity appears to be associated with the direct action of the chemical on the production of testosterone in fetal and postnatal offspring.
Supplementary Material
HIGHLIGHTS.
Linuron-induced developmental toxicity in male offspring gonadal organs was studied.
Maternal exposure to linuron hampers male gonadal organ development in fetus.
Maternal exposure to linuron harms offspring seminiferous tubules and Leydig cells.
Linuron toxicity is due to its direct effect on the production of testosterone.
Acknowledgments
This study was partly supported by the Natural Science Foundation of Guizhou Provincial Scientific and Technology Department Grant (J[2007]2125) (Li Yan), Education Department of Guizhou Province ([2008]033) (Li Yan), International Scientific and Technology cooperation project of Guizhou Province (G[2014] 7012) (Li Yan), Innovative talent team training project of Zunyi city ([2015]42) (Li Yan) and the Scientific and Technology Foundation of Health and Family Planning Commission of Guizhou Provincial (D-424) (Li Yan).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.toxlet.2016.12.013.
Footnotes
Conflict of interest
None.
References
- Arukwe A. Steroidogenic acute regulatory (StAR) protein and cholesterol side-chain cleavage (P450scc)-regulated steroidogenesis as an organ-specific molecular and cellular target for endocrine disrupting chemicals in fish. Cell Biol Toxicol. 2008;24(6):527–540. doi: 10.1007/s10565-008-9069-7. doi: http://dx.doi.org/10.1007/s10565-008-9069-7. [DOI] [PubMed] [Google Scholar]
- Auger J, Le Denmat D, Berges R, Doridot L, Salmon B, Canivenc-Lavier MC, Eustache F. Environmental levels of oestrogenic and antiandrogenic compounds feminize digit ratios in male rats and their unexposed male progeny. Proc Biol Sci. 2013;280(1768):1532. doi: 10.1098/rspb.2013.1532. doi: http://dx.doi.org/10.1098/rspb.2013.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin LS, Ebbers MB. Hypospadias: anatomy, etiology, and technique. J Pediatr Surg. 2006;41(3):463–472. doi: 10.1016/j.jpedsurg.2005.11.059. doi: http://dx.doi.org/10.1016/j.jpedsurg.2005.11.059. [DOI] [PubMed] [Google Scholar]
- Esteves SC, Miyaoka R, Agarwal A. An update on the clinical assessment of the infertile male. Clinics. 2011;66(4):691–700. doi: 10.1590/S1807-59322011000400026. doi: http://dx.doi.org/10.1590/s1807-59322011000400026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch RH, Berrebi AS, Cowell PE, Schrott LM, Denenberg VH. Corpus callosum: effects of neonatal hormones on sexual dimorphism in the rat. Brain Res. 1990;515(1–2):111–116. doi: 10.1016/0006-8993(90)90584-x. doi: http://dx.doi.org/10.1016/0006-8993(90)90584-x. [DOI] [PubMed] [Google Scholar]
- Gray LE, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, Guillette L. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update. 2001;7(3):248–264. doi: 10.1093/humupd/7.3.248. doi: http://dx.doi.org/10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- Han XD, Tu ZG, Gong Y, Shen SN, Wang XY, Kang LN, Chen JX. The toxic effects of nonylphenol on the reproductive system of male rats. Reprod Toxicol. 2004;19(2):215–221. doi: 10.1016/j.reprotox.2004.06.014. doi: http://dx.doi.org/10.1016/j.reprotox.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Parks-Saldutti LG, Ostby JS, Lambright C, Furr J, Vandenbergh JG, Gray LJ. A mixture of the antiandrogens linuron and butyl benzyl phthalate alters sexual differentiation of the male rat in a cumulative fashion. Biol Reprod. 2004;71(6):1852–1861. doi: 10.1095/biolreprod.104.031674. doi: http://dx.doi.org/10.1095/biolreprod.104.031674. [DOI] [PubMed] [Google Scholar]
- Issop L, Rone MB, Papadopoulos V. Organelle plasticity and interactions in cholesterol transport and steroid biosynthesis. Mol Cell Endocrinol. 2013;371(1–2):34–46. doi: 10.1016/j.mce.2012.12.003. doi: http://dx.doi.org/10.1016/j.mce.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Klot CAJV, Kramer MW, Böker A, Herrmann TR, Peters I, Kuczyk MA, Merseburger AS. Is there an anti-androgen withdrawal syndrome for enzalutamide? World J Urol. 2014;32(5):1171–1176. doi: 10.1007/s00345-014-1288-3. doi: http://dx.doi.org/10.1007/s00345-014-1288-3. [DOI] [PubMed] [Google Scholar]
- Kroupova HK, Trubiroha A, Lorenz C, Contardo-Jara V, Lutz I, Grabic R, Kloas W. The progestin levonorgestrel affects hypothalamus–pituitary–gonad axis in pubertal roach (Rutilus rutilus) Toxicol Lett. 2014;229:S114–S115. doi: 10.1016/j.aquatox.2014.05.008. doi: http://dx.doi.org/10.1016/j.toxlet.2014.06.413. [DOI] [PubMed] [Google Scholar]
- Lambright C, Ostby J, Bobseine K, Wilson V, Hotchkiss AK, Mann PC, Gray LJ. Cellular and molecular mechanisms of action of linuron: an antiandrogenic herbicide that produces reproductive malformations in male rats. Toxicol Sci. 2000;56(2):389–399. doi: 10.1093/toxsci/56.2.389. doi: http://dx.doi.org/10.1093/toxsci/56.2.389. [DOI] [PubMed] [Google Scholar]
- Liu S, Zhang J, Li W, Zhang T, Hu D. Acteoside reduces testosterone by inhibiting cAMP, p450scc, and StAR in rat Leydig cells. Mol Cell Toxicol. 2015;11(1):11–17. doi: http://dx.doi.org/10.1007/s13273-015-0002-x. [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. doi: http://dx.doi.org/10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McGlynn KA, Guo X, Graubard BI, Brock JW, Klebanoff MA, Longnecker MP. Maternal pregnancy levels of polychlorinated biphenyls and risk of hypospadias and cryptorchidism in male offspring. Environ Health Perspect. 2009;117(9):1472–1476. doi: 10.1289/ehp.0800389. doi: http://dx.doi.org/10.1289/ehp.0800389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre BS, Barlow NJ, Wallace DG, Maness SC, Gaido KW, Foster PM. Effects of in utero exposure to linuron on androgen-dependent reproductive development in the male Crl:CD(SD)BR rat. Toxicol Appl Pharm. 2000;167(2):87–99. doi: 10.1006/taap.2000.8998. doi: http://dx.doi.org/10.1006/taap.2000.8998. [DOI] [PubMed] [Google Scholar]
- Monosson E, Kelce WR, Lambright C, Ostby J, Gray LJ. Peripubertal exposure to the antiandrogenic fungicide, vinclozolin, delays puberty, inhibits the development of androgen-dependent tissues, and alters androgen receptor function in the male rat. Toxicol Ind Health. 1999;15(1–2):65–79. doi: 10.1177/074823379901500107. doi: http://dx.doi.org/10.1191/074823399678846600. [DOI] [PubMed] [Google Scholar]
- Rone MB, Fan J, Papadopoulos V. Cholesterol transport in steroid biosynthesis: role of protein-protein interactions and implications in disease states. Biochim Biophys Acta. 2009;1791(7):646–658. doi: 10.1016/j.bbalip.2009.03.001. doi: http://dx.doi.org/10.1016/j.bbalip.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell LD, Ettlin RA, Hikim APS, Clegg ED. Histological and histopathological evaluation of the testis. Int J Androl. 1993;16(1):83. doi: http://dx.doi.org/10.1111/j.1365-2605.1993.tb01156.x. [Google Scholar]
- Santos SM, Videira RA, Fernandes MA, Santos MS, Moreno AJ, Vicente JA, Jurado AS. Toxicity of the herbicide linuron as assessed by bacterial and mitochondrial model systems. Toxicol In Vitro. 2014;28(5):932–939. doi: 10.1016/j.tiv.2014.04.004. doi: http://dx.doi.org/10.1016/j.tiv.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Skakkebaek NE. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet. 1993;341(8857):1392–1396. doi: 10.1016/0140-6736(93)90953-e. doi: http://dx.doi.org/10.1016/0140-6736(93)90953-e. [DOI] [PubMed] [Google Scholar]
- Sinha HA, Swerdloff RS. Hormonal and genetic control of germ cell apoptosis in the testis. Rev Reprod. 1999;4(1):38–47. doi: 10.1530/ror.0.0040038. doi: http://dx.doi.org/10.1530/ror.0.0040038. [DOI] [PubMed] [Google Scholar]
- Storgaard L, Bonde JP, Olsen J. Male reproductive disorders in humans and prenatal indicators of estrogen exposure: a review of published epidemiological studies. Reprod Toxicol. 2006;21(1):4–15. doi: 10.1016/j.reprotox.2005.05.006. doi: http://dx.doi.org/10.1016/j.reprotox.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Sultan C, Balaguer P, Terouanne B, Georget V, Paris F, Jeandel C, Nicolas J. Environmental xenoestrogens, antiandrogens and disorders of male sexual differentiation. Mol Cell Endocrinol. 2001;178(1–2):99–105. doi: 10.1016/s0303-7207(01)00430-0. doi: http://dx.doi.org/10.1016/s0303-7207(01)00430-0. [DOI] [PubMed] [Google Scholar]
- Vukusic PT, Janjic T, Dujmovic I, Poljicanin A, Soljic V, Saraga-Babic M, Vukojevic K. The involvement of proliferation and apoptosis in the early human gonad development. J Mol Histol. 2013;44(1):55–63. doi: 10.1007/s10735-012-9455-6. doi: http://dx.doi.org/10.1007/s10735-012-9455-6. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Lambright CR, Furr JR, Howdeshell KL, Earl GLJ. The herbicide linuron reduces testosterone production from the fetal rat testis during both in utero and in vitro exposures. Toxicol Lett. 2009;186(2):73–77. doi: 10.1016/j.toxlet.2008.12.017. doi: http://dx.doi.org/10.1016/j.toxlet.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Wu JJ, Wang KL, Wang SW, Hwang GS, Mao IF, Chen ML, Wang PS. Differential effects of nonylphenol on testosterone secretion in rat Leydig cells. Toxicology. 2010;268(1–2):1–7. doi: 10.1016/j.tox.2009.10.030. doi: http://dx.doi.org/10.1016/j.tox.2009.10.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


