Abstract
Background
Hypertriglyceridemia increases risk for atherosclerotic cardiovascular disease and may contribute to atherosclerosis by changing circulating monocyte phenotypes. High-dose n-3 polyunsaturated fatty acids (PUFAs) reduce blood triglyceride levels. Effects of triglyceride-lowering therapy on monocyte phenotypes are not well known.
Objective
We examined effects of n-3 PUFA treatments (eicosapentaenoic acid [EPA] plus docosapentaenoic acid [DPA] [MAT9001] versus EPA ethyl esters [EPA-EE]) on monocyte phenotypes in individuals with hypertriglyceridemia.
Methods
Individuals with triglycerides 200–400 mg/dL were recruited. Subjects received two treatments in randomized order for 14 days each: MAT9001 and EPA-EE, at 4 g/day. At 2 days before the start of, and on the last day of, each treatment, nile red staining for lipids and phenotypes of each monocyte subset were examined by flow cytometry after an overnight fast and postprandially after a high-fat meal.
Results
Treatment with MAT9001 or EPA-EE reduced fasting triglyceride levels and decreased proportions of intermediate monocytes. Only MAT9001 decreased postprandial blood triglyceride levels, lowered fasting nile red levels, indicating less lipid in classical and intermediate monocytes, and reduced postprandial CD11c levels on nonclassical monocytes. MAT9001 and EPA-EE each reduced fasting and postprandial CD11c and CD36 levels on classical and intermediate monocytes and postprandial CCR5 levels on intermediate and nonclassical monocytes, with no significant differences between the two treatments.
Conclusions
Treatment with MAT9001 in individuals with hypertriglyceridemia reduced fasting nile red staining for lipids in classical and intermediate monocytes. MAT9001 and EPA-EE each improved fasting and postprandial monocyte phenotypes, which could potentially help to protect against atherosclerosis.
Keywords: Hypertriglyceridemia, Eicosapentaenoic acid, Docosapentaenoic acid, n-3 polyunsaturated fatty acids, Monocytes, Atherosclerosis
Introduction
Hypertriglyceridemia has been identified as an independent causal risk factor for atherosclerotic cardiovascular disease (ASCVD) (1–4). However, the mechanisms whereby hypertriglyceridemia contributes to ASCVD remain incompletely understood.
Atherosclerosis is an inflammatory disease characterized by accumulation of lipid-laden macrophages (foam cells) in arterial walls (5–8). Monocyte infiltration from the circulation into arterial walls and differentiation into macrophages, which take up modified lipoproteins and become foam cells, are important steps in the development of atherosclerosis (5,8,9). Monocytes are heterogeneous and include several subsets, which may exert differential roles in inflammation and atherosclerosis. Based on surface markers CD14 and CD16, human monocytes have been classified into CD14+/CD16− classical, CD14+/CD16+ intermediate, and CD14dim/CD16+ nonclassical monocytes (10–13). Under healthy conditions, classical monocytes are the dominant monocyte population, accounting for ~80–90% of total monocytes, while intermediate and nonclassical monocytes each account for 5–10% of total monocytes (11,14). Nevertheless, results from most studies show that compared to CD16− classical monocytes, CD16+, particularly intermediate, monocytes play more important roles in inflammation (10,14).
While the traditional paradigm in atherosclerosis has focused on macrophage foam cell formation in arterial walls (9), our studies and others have shown that hyperlipidemia also causes formation of foamy monocytes, monocytes with intracellular lipid droplets, in the circulation in both mice and humans (15–22). Foamy monocytes infiltrate into arterial walls and contribute to atherosclerosis (19,23). Hypertriglyceridemia in humans with obesity and metabolic syndrome is also associated with increased proportions of CD16+ intermediate or nonclassical monocytes (12,24) and with phenotypic changes in monocytes and subsets, including upregulation of adhesion molecules, toll-like receptors, inflammatory molecules, and oxidative stress (15,20,25–28). Moreover, postprandial elevations in triglyceride (TG) levels after a single high-fat meal promote monocyte (subset) phenotypic changes, particularly in subjects with elevated fasting TGs and metabolic syndrome (20). Formation of foamy monocytes with phenotypic changes in hyperlipidemia may accelerate monocyte (subset) contributions to atherosclerosis and therefore serve as an important link between hypertriglyceridemia and the development of ASCVD (19,21,23).
Because of the causal role of hypertriglyceridemia in ASCVD, reducing TG levels may be an important strategy for ASCVD prevention (4,29). Treatment with large doses of n-3 polyunsaturated fatty acids (PUFAs) (generally 2–4 g/d) reduces blood TG levels, including fasting and postprandial TG levels (30,31). Eicosapentaenoic acid (EPA) and docosahexaenoic acid have been the most commonly used n-3 PUFAs for TG lowering (32). More recently, supplementation with n-3 docosapentaenoic acid (DPA) revealed incorporation of DPA in various plasma lipid fractions, further reducing plasma TG levels (33). Compared to EPA alone, EPA plus DPA resulted in greater reductions in fasting blood TG levels in subjects with hypertriglyceridemia (34). Moreover, n-3 PUFAs may exert direct anti-inflammatory effects through several pathways independent of changes in plasma TG levels (35). However, potential effects of n-3 PUFA treatment and reduced TG levels on monocyte (subset) lipid accumulation and phenotypes have not been well studied, particularly in individuals with hypertriglyceridemia. In the current study, we examined effects of treatment with EPA plus DPA, as compared to EPA alone, on fasting and postprandial monocyte subset lipid accumulation and phenotypes in subjects with hypertriglyceridemia.
Subjects and Methods
Study population and design
This was a substudy of a clinical trial (ClinicalTrials.gov identifier: NCT02310022) examining lipid effects of EPA plus DPA (MAT9001, Matinas BioPharma, Inc., Bedminster, NJ) versus EPA ethyl esters (EPA-EE) alone (Vascepa, icosapent ethyl, Amarin Pharma, Inc., Bedminster, NJ) in subjects with hypertriglyceridemia (34). The study was performed in a clinical research unit (Pharma Medica Research, Inc.) located in Missisauga, Ontario, Canada, and has been detailed previously (34). Briefly, the study included men and women aged 18–70 years, each with body mass index (BMI) of 19.0–40.0 kg/m2 and fasting TG levels of 200–400 mg/dL. Exclusion criteria included total cholesterol levels >300 mg/dL; nonstable use of statin therapy within 8 weeks before study drug; use of the highest recommended dose of any statin; use of TG-targeted drugs, or nonsteroidal anti-inflammatory drugs within 30 days before study drug; known history or presence of diabetes, CVD, impaired cardiovascular function, clinically significant gastrointestinal disease, or malabsorption; regular consumption of more than one meal containing fish or shellfish per week for 6 months prior to drug administration; or following a special diet within 30 days prior to the study.
The study had an open-label crossover design with two 14-day treatment periods (4 g/day MAT9001 versus 4 g/day EPA-EE) in randomized order, separated by a ≥35-day washout period. At 2 days prior to the start of each treatment and on the last day of each treatment, a postprandial study with a high-fat high-calorie breakfast was performed after a ≥12 hour fasting time. The total calorie content of the breakfast was 1241 kcal, of which 45% was from fat (Supplemental Table 1). Blood samples were collected by venipuncture before the breakfast (after fasting ≥12 hours) and at 4 and 6 hours after the breakfast.
The study protocol was approved by an ethics review board (Optimum Clinical Research Inc., Oshawa, Ontario, Canada). All subjects signed informed consent forms to participate in the study.
Blood collection and experimental procedures
Blood samples for monocyte phenotyping were collected while fasting and at 4 and 6 hours postprandially into Cyto-Chex® BCT Vacutainer Tubes (Streck, Omaha, NE) (36) and mixed with anticoagulant by inverting gently 8 times (37). After collection, the blood samples were shipped overnight in a temperature-stabilizing container with temperature of 20–25°C (around 22°C) (37) to t he investigator’s lab in Baylor College of Medicine. Samples usually arrived in the lab within 24 hours after collection. Upon receipt, samples were processed immediately for monocyte phenotype analysis as described below. In addition, separate tubes of blood were collected by Alpha Laboratories Inc. (Toronto, Ontario, Canada) for analysis of a complete blood count and lipoprotein lipids, including TG levels, which were measured using a Cobas 6000 analyzer series module c501 (Roche Diagnostics, Indianapolis, IN).
Experimental protocol for monocyte phenotyping
Two 100-µl aliquots of whole blood were incubated at room temperature with combinations of multiple fluorescent antibodies for the following molecules or nile red in 2 protocols: 1) CD14 + CD16 + CD36 + scavenger receptor type A (SRA) + CD11c + CCR2 + CX3CR1; 2) CD14 + CD16 + CCR5 + nile red. All the antibodies were purchased commercially: anti-human CD14 (clone: RMO52, Krome Orange-conjugated, Beckman Coulter, Brea, CA); anti-human CD16 (clone: 3G8, PE-Cy7-conjugated, Beckman Coulter); anti-human CD36 (clone: FA6.152, FITC-conjugated, Beckman Coulter); anti-human SRA (clone: 351615, PE-conjugated, R & D Systems, Minneapolis, MN); anti-human CD11c (clone: B-ly6, V450-conjugated, BD Biosciences, San Jose, CA); anti-human CCR2 (clone: K036C2, PerCP/Cy5.5-conjugated, Biolegend, San Diego, CA); anti-human CX3CR1 (clone: 2A9-1, APC-conjugated, Biolegend); anti-human CCR5 (clone: J418F1, APC/Cy7-conjugated, Biolegend).
For protocol 1, whole blood was incubated with the above combinations of antibodies for 30 minutes in the dark. Stained blood was processed in a Coulter TQ-prep Workstation (Beckman Coulter) using ImmunoPrep Reagent (Beckman Coulter), which lysed red blood cells and fixed the samples. The processed samples were then washed twice with PBS, and resuspended in 300 µl PBS for analysis of monocyte phenotypes (see below). For protocol 2, blood was first incubated with the above antibodies for 30 minutes. Stained blood was processed in the TQ-prep Workstation and washed twice with PBS. The sample was then stained with nile red for lipids using Lipid Droplets Fluorescence Assay Kit (Cayman Chemical, Ann Arbor, MI) following the manufacturer’s instructions. After washing twice with PBS, the sample was resuspended in 300 µl PBS for analysis of monocyte lipid staining and phenotypes. A Coulter Gallios flow cytometer (Beckman Coulter) was used to collect data.
Kaluza software (Beckman Coulter) was used to analyze data. Total leukocytes were first gated based on forward and side scatter patterns. Then, monocytes were defined within the gated leukocytes by CD14 expression in combination with the side scatter pattern. Based on surface expression levels of CD14 and CD16, monocytes were categorized into 3 subsets: CD14+/CD16− classical, CD14+/CD16+ intermediate, and CD14dim/CD16+ nonclassical monocytes. Each subset was examined for expression levels indicated by mean fluorescence intensity (MFI) of the markers as described in the above protocols (Supplemental Figure 1).
The proportion of each monocyte subset was calculated based on the flow cytometric data and presented as percentage in total monocytes. The count of each monocyte subset was also calculated based on the percentage of each monocyte subset and total monocyte count obtained by a complete blood count.
Statistical analysis
SAS, version 9.3 (SAS Institute, Cary, NC), was used for statistical analyses. Data analyses of monocyte phenotypes were blinded and exploratory. Descriptive statistics were generated for TG levels, proportions of each monocyte subset, and MFI levels of various markers on each monocyte subset while fasting and at 4 hours and 6 hours postprandially before and after each treatment. Total area under the curve (AUC) for TG levels from 0 to 6 hours was calculated using the trapezoidal rule before and after each treatment (38). Data are presented as mean ± standard error of the mean unless stated otherwise. Differences between treatments were analyzed by repeated measures analysis of covariance with treatment as a factor and baseline (pretreatment) as a covariate in each model. Differences were considered significant at P≤0.05. The distribution of residuals for each statistical model was tested for normality with the Shapiro–Wilk test (39). Parametric analyses were used for data that were normally distributed, and rank-transformed analyses were used for data that were not normally distributed (Shapiro–Wilk P<0.01). Pairwise comparisons were completed using linear contrasts with Bonferroni adjustments of the alpha levels. Within-treatment comparisons (pre- to post-treatment) for TG levels and monocyte phenotypes were analyzed before the meal and at 4 or 6 hours after the meal using paired t-test or Wilcoxon signed-rank test.
Possible carryover was assessed by evaluating the responses in each sequence group and by assessing treatment-by-sequence interactions in statistical models. No marked differences in responses were present between the two treatment sequence groups. No treatment-by-sequence interaction terms had P-values <0.02, and the number with P-values <0.05 was well within the range expected given the number of comparisons, so values for both treatment sequences were pooled.
Results
Effects of treatments on postprandial TG levels
Twenty-seven subjects were included in this substudy. The baseline demographic data and pretreatment fasting lipid levels are shown in Supplemental Tables 2 and 3.
As reported in the parent study, treatment with either MAT9001 or EPA-EE lowered fasting blood TG levels in subjects with hypertriglyceridemia, with greater reductions by MAT9001 than by EPA-EE alone (34).
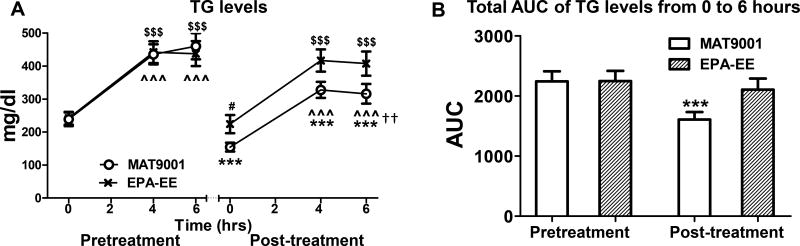
As expected, a high-fat meal increased TG levels before and after each treatment (Figure 1A). Only MAT9001 significantly reduced postprandial TG levels and TG AUC from pre- to post-treatment (Figures 1A and 1B). The post- versus pretreatment changes (reductions) in postprandial TG levels and TG AUC were significantly greater for MAT9001 than EPA-EE (P<0.01 for TG levels at 6 hours after the high-fat meal, and P<0.05 for percent changes in TG AUC for MAT9001 vs EPA-EE).
Figure 1. Effects of MAT9001 or EPA-EE treatment on postprandial TG levels.
A, Blood triglyceride (TG) levels were measured fasting (0 hour) and postprandially (4 and 6 hours after the high-fat meal) just prior to (pretreatment) and at the end of (post-treatment) each treatment. B, For each intervention, total area under the curve (AUC) of TG levels from 0 to 6 hours was compared between pre- and post-treatment values. ***P<0.001 for post- versus pretreatment with MAT9001; #P<0.05 for post- versus pretreatment with EPA-EE; ^^^P<0.001 for 4 or 6 hours versus 0 hour of MAT9001 treatment; $$$P<0.001 for 4 or 6 hours versus 0 hour of EPA-EE treatment. ††P<0.01 for MAT9001 versus EPA-EE for post- versus pretreatment.
Effects of treatments on counts and proportions of monocyte subsets
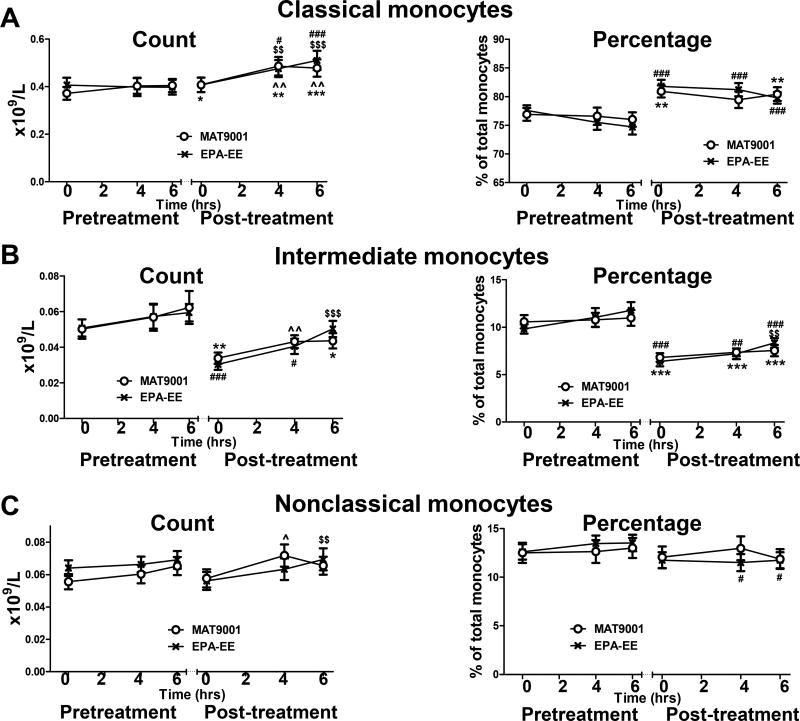
Analyses of monocyte subset counts showed that compared to the fasting state, a high-fat meal increased or tended to increase the count of each monocyte subset (Figures 2A–2C). However, the percentage of most monocyte subsets were not significantly altered by a high-fat meal either before or after each treatment (Figures 2A–2C). Nevertheless, compared to pretreatment, treatment with MAT9001 or EPA-EE decreased the percentage and count of intermediate monocytes and increased the percentage and count of classical monocytes in the fasting and postprandial states (Figures 2A and 2B). EPA-EE treatment also slightly, but significantly, lowered the percentage of nonclassical monocytes in the postprandial state (Figure 2C).
Figure 2. Effects of MAT9001 or EPA-EE treatment on monocyte subset counts and proportions.
Counts and percentages of each monocyte subset (classical [A], intermediate [B], and nonclassical [C]) were examined by flow cytometry in combination with a complete blood count in the fasting (0 hour) and postprandial (4 and 6 hours after the highfat meal) states just prior to and at the end of each treatment. *P<0.05, **P<0.01, ***P<0.001 for post- versus pretreatment of MAT9001; #P<0.05, ##P<0.01, ###P<0.001 for post- versus pretreatment of EPA-EE; ^P<0.05, ^^P<0.01 for 4 or 6 hours versus 0 hour of MAT9001 treatment; $$P<0.01, $$$P<0.001 for 4 or 6 hours versus 0 hour of EPA-EE treatment.
Effects of treatments on nile red staining of lipids in monocytes (subsets)
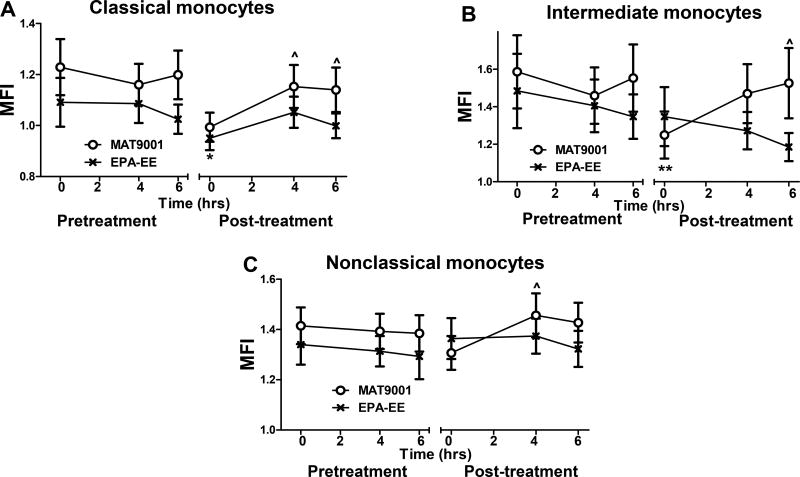
Fasting and postprandial hypertriglyceridemia is associated with increased lipid content in monocytes and with formation of foamy monocytes in the circulation (15–17,20), which may contribute to ASCVD (19). Reductions in TG levels in subjects with hypertriglyceridemia by MAT9001 were associated with significantly reduced nile red levels, indicating less lipid, in both classical and intermediate monocytes in the fasting state compared to pretreatment (Figures 3A and 3B). Compared to the fasting state, a high-fat meal increased nile staining of lipids in all monocyte subsets after, but not before, MAT9001 treatment, and nile red staining of lipids in monocytes (subsets) in the postprandial state was similar before and after MAT9001 treatment (Figures 3A–3C). These findings were consistent with our previous observations that compared to obese subjects with hypertriglyceridemia and metabolic syndrome, control subjects with normal fasting TG levels had lower proportions of foamy monocytes in the fasting state but showed greater increases in postprandial nile red staining of lipids in monocytes (20). In contrast to MAT9001 treatment, treatment with EPA-EE did not significantly alter nile red levels in any monocyte subsets in either the fasting or postprandial state (Figures 3A–3C).
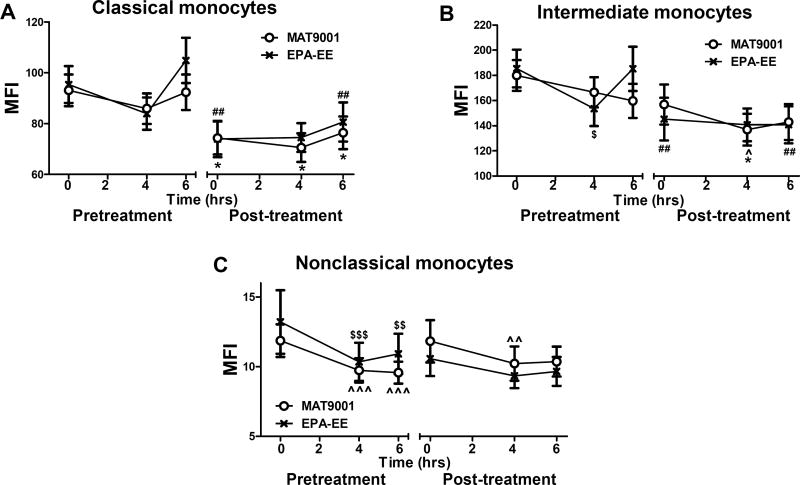
Figure 3. Effects of MAT9001 or EPA-EE treatment on monocyte nile red staining of lipids.
Nile red staining of lipids was performed in monocytes (subsets) by flow cytometry in the fasting (0 hour) and postprandial (4 and 6 hours after the high-fat meal) states and presented as mean fluorescence intensity (MFI) of nile red staining on each monocyte subset: classical (A), intermediate (B), and nonclassical (C). *P<0.05, **P<0.01 for post- versus pretreatment of MAT9001; ^P<0.05 for 4 or 6 hours versus 0 hour of MAT9001 treatment.
Effects of treatments on monocyte (subset) phenotypes
Effects on CD11c expression
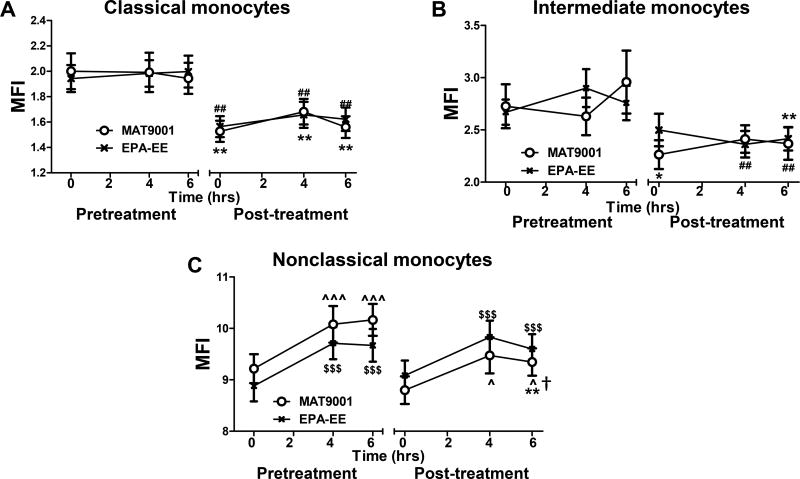
Consistent with previous studies (20), levels of CD11c, a β2 integrin that mediates monocyte adhesion to endothelial cells and contributes to the development of atherosclerosis (15,20,26,27,40), were higher on nonclassical and intermediate monocytes than on classical monocytes within individuals, and a high-fat meal increased CD11c levels on nonclassical, but not classical or intermediate, monocytes in subjects with hypertriglyceridemia (Figures 4A–4C). Compared to pretreatment, treatment with MAT9001 or EPA-EE reduced fasting and postprandial CD11c levels on classical and intermediate monocytes (Figures 4A and 4B), with no significant differences between MAT9001 and EPA-EE treatments. However, while EPA-EE treatment did not significantly change fasting or postprandial CD11c levels on nonclassical monocytes, MAT9001 treatment significantly reduced nonclassical CD11c levels at 6 hours after a high-fat meal (Figure 4C). The change (reduction) in postprandial CD11c levels on nonclassical monocytes was greater with MAT900l treatment than with EPA-EE (P<0.05).
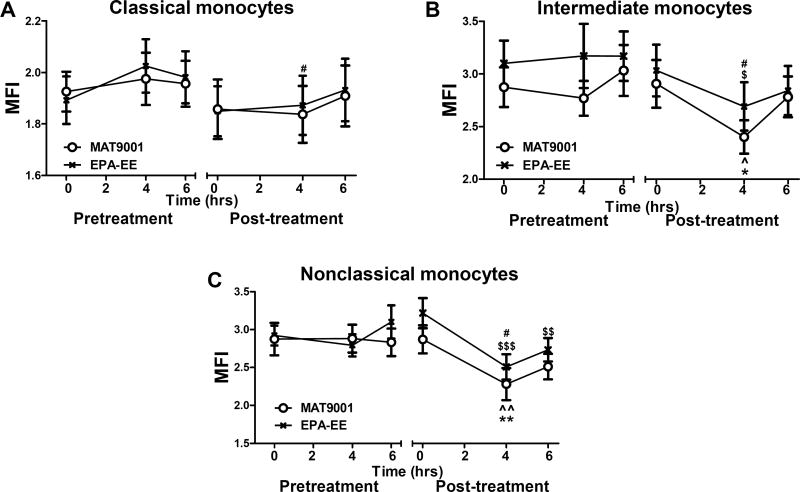
Figure 4. Effects of MAT9001 or EPA-EE treatment on monocyte CD11c.
CD11c levels were examined on monocytes (subsets) by flow cytometry in the fasting (0 hour) and postprandial (4 and 6 hours after the high-fat meal) states and presented as MFI levels on each monocyte subset: classical (A), intermediate (B), and nonclassical (C). *P<0.05, **P<0.01 for post- versus pretreatment of MAT9001; ##P<0.01 for post- versus pretreatment of EPA-EE; ^P<0.05, ^^^P<0.001 for 4 or 6 hours versus 0 hour of MAT9001 treatment; $$$P<0.001 for 4 or 6 hours versus 0 hour of EPA-EE treatment; †P<0.05 for MAT9001 versus EPA-EE for post- versus pretreatment.
Effects on scavenger receptors
Levels of CD36, a scavenger receptor implicated in macrophage or monocyte uptake of modified lipoproteins and foamy cell formation (19,41), were higher on classical and intermediate monocytes than on nonclassical monocytes within individuals (Figures 5A–5C). Compared to fasting, a high-fat meal either lowered or did not change CD36 levels on monocytes (subsets) (Figures 5A–5C). Treatment with MAT9001 or EPA-EE reduced or tended to reduce fasting and postprandial CD36 levels on classical and intermediate, but not nonclassical, monocytes (Figures 5A–5C). Treatment with MAT9001 or EPA-EE either tended to increase or did not change monocyte (subset) levels of SRA (Supplemental Figure 2), another scavenger receptor implicated in macrophage foamy cell formation (41). However, our data indicated that SRA levels were generally low on all 3 monocyte subsets as examined by flow cytometry.
Figure 5. Effects of MAT9001 or EPA-EE treatment on monocyte CD36.
CD36 MFI levels were examined on monocytes (subsets: classical [A], intermediate [B], and nonclassical [C]) by flow cytometry in the fasting (0 hour) and postprandial (4 and 6 hours after the high-fat meal) states. *P<0.05 for post- versus pretreatment of MAT9001; ##P<0.01 for post- versus pretreatment of EPA-EE; ^P<0.05, ^^P<0.01, ^^^P<0.001 for 4 or 6 hours versus 0 hour of MAT9001 treatment; $$P<0.01, $$$P<0.001 for 4 or 6 hours versus 0 hour of EPA-EE treatment.
Effects on chemokine receptors
CCR5, a receptor for CCL3, CCL4, and CCL5, was expressed at higher levels on intermediate and nonclassical monocytes than on classical monocytes within individuals (Figures 6A–6C). Treatment with MAT9001 or EPA-EE lowered or tended to lower postprandial levels of CCR5 on monocytes, particularly on intermediate and nonclassical monocyte subsets (Figures 6A–6C), with no significant differences between MAT9001 and EPA-EE. CCR2, a receptor for CCL2, was expressed at higher levels on classical and intermediate monocytes than on nonclassical monocytes within individuals. Treatment with MAT9001 or EPA-EE increased or tended to increase fasting and postprandial CCR2 levels on classical monocytes (Supplemental Figure 3A). While EPA-EE decreased fasting CCR2 levels on nonclassical monocytes, MAT9001 or EPA-EE did not reduce CCR2 levels on intermediate monocytes (Supplemental Figures 3B and 3C). CX3CR1, a receptor for CX3CL1, was expressed at higher levels on nonclassical monocytes than on classical and intermediate monocytes in the fasting state within individuals and increased or tended to increase after a high-fat meal, particularly on nonclassical monocytes (Supplemental Figure 4). However, treatment with MAT9001 or EPA-EE did not significantly alter fasting or postprandial levels of CX3CR1 on any monocyte subsets (Supplemental Figure 4).
Figure 6. Effects of MAT9001 or EPA-EE treatment on monocyte CCR5.
CCR5 MFI levels were examined on monocytes (subsets: classical [A], intermediate [B], and nonclassical [C]) by flow cytometry in the fasting (0 hour) and postprandial (4 and 6 hours after the high-fat meal) states. *P<0.05, **P<0.01 for post- versus pretreatment of MAT9001; #P<0.05 for post- versus pretreatment of EPA-EE; ^P<0.05, ^^P<0.01 for 4 or 6 hours versus 0 hour of MAT9001 treatment; $P<0.05, $$P<0.01, $$$P<0.001 for 4 or 6 hours versus 0 hour of EPA-EE treatment.
Discussion
Although MAT9001 and EPA-EE both reduced fasting TG levels in subjects with hypertriglyceridemia (34), this substudy showed that MAT9001, but not EPA-EE, significantly lowered postprandial TG levels and TG AUC. MAT9001 also reduced fasting nile red levels for lipids in classical and intermediate monocytes. MAT9001 and EPA-EE each decreased the counts and the relative ratio of intermediate monocytes in total monocytes, fasting and postprandial levels of CD11c and CD36 on classical and intermediate monocytes, and postprandial levels of CCR5 on intermediate and nonclassical monocytes, whereas only MAT9001 significantly reduced postprandial levels of CD11c on nonclassical monocytes.
Increased proportions and counts of CD16+ monocytes, particularly intermediate monocytes, and elevated monocyte (subset) levels of lipids and other markers such as CD11c, CD36, and CCR5 have been associated with hyperlipidemia and may be a link to the development of atherosclerosis (10,12,14–20,24). Therefore, reductions in the proportions and counts of intermediate monocytes and in the monocyte (subset) markers as described in subjects with hypertriglyceridemia by treatment with n-3 PUFAs may help reduce risk for ASCVD.
In mouse models, initial studies indicated a preferential increase in Ly-6Chigh classical monocytes in apoE−/− mice on western diet (42,43). More recent studies including ours also showed a significant increase of Ly-6Clow monocytes or a similar ratio of Ly-6Clow and Ly-6Chigh monocytes in mice with hypercholesterolemia and/or obesity (18,19,26,44,45). Importantly, Ly-6Clow, but few Ly-6Chigh, monocytes express CD11c and become foamy (and positive for nile red and oil red O staining) in the circulation of apoE−/− or LDLR−/− mice with hypercholesterolemia (18,19). CD11c+ foamy monocytes infiltrate into arterial walls and contribute to atherosclerosis (19). A study showed that dietary supplementation with n-3 PUFAs (fish oil or echium oil) reduced atherosclerosis in LDLR−/−, but not apoE−/−, mice, although fish oil decreased the proportion of Ly-6Chigh monocytes in the circulation of apoE−/−, but not LDLR−/−, mice. Interestingly, the reduced atherosclerosis in LDLR−/− mice with n-3 PUFAs was associated with decreased Ly-6Chigh monocytes in spleens and reduced Ly-6Clow monocyte infiltration into atherosclerotic lesions (46).
Our previous study showed that in the fasting state, nile red staining of monocytes for lipids was elevated in human subjects with hypertriglyceridemia and metabolic syndrome compared to control subjects with normal TG levels and was correlated with TG levels. However, compared to subjects with hypertriglyceridemia, control subjects with normal TG levels showed greater postprandial increases in nile red staining for lipids in monocytes (20), supporting a hypothesis that high lipid content in monocytes in the fasting state in subjects with hypertriglyceridemia may prevent further increases in postprandial lipid accumulation within circulating monocytes. This may also explain our current observations that reductions in TGs by MAT9001 in subjects with hypertriglyceridemia were associated with reduced nile red staining of lipids in monocytes in the fasting state and that a high-fat meal increased nile red staining of lipids in monocytes (subsets) after (with lower TG levels), but not before (with elevated TG levels), MAT9001 treatment. The nonsignificant effects of EPA-EE on fasting monocyte nile red levels may be because of smaller reductions in blood levels of lipids, particularly TG, by EPA-EE alone as shown in the parent study (34). Our current observation also indicated that monocyte accumulation of lipids may be reversible with lipid-lowering therapy (by MAT9001). The same is true for CD11c levels on monocyte subsets. Obesity with metabolic syndrome and hypertriglyceridemia increased CD11c on monocyte subsets (20), and, in the present study, n-3 PUFA treatment in subjects with hypertriglyceridemia decreased CD11c levels on monocyte subsets.
We previously observed that hypertriglyceridemia in obesity significantly increased fasting CD11c levels on classical and intermediate, but not nonclassical, monocytes and that a high-fat diet increased CD11c levels on nonclassical monocytes in subjects with hypertriglyceridemia, but not in control subjects (20). These observations pointed to the potential that long-term “chronic” hypertriglyceridemia increases CD11c levels on classical and intermediate monocytes in the fasting state and may “prime” nonclassical monocytes for greater CD11c response to an acute TG increase induced by a high-fat challenge. Consistent with this hypothesis, reductions in fasting TG levels by n-3 PUFAs were associated with decreased CD11c levels on classical and intermediate monocytes in the fasting (and postprandial) state, while the greater reduction in fasting TG by MAT9001 was associated with a smaller response in nonclassical monocyte CD11c to a high-fat meal challenge.
CD36 has been associated with hyperlipidemia and may contribute to atherogenesis by mediating monocyte/macrophage uptake of modified low-density lipoprotein and foam cell formation (19,41). Reductions in monocyte CD36 levels with n-3 PUFA treatment are expected to decrease monocyte uptake of atherogenic lipoproteins, thereby reducing foam cell formation and protecting against atherogenesis. CCR5 levels increase on monocytes (subsets) with hyperlipidemia and may contribute to atherosclerosis by mediating monocyte recruitment (20,42). Therefore, reductions of CCR5 levels on monocytes by n-3 PUFA treatment would also be expected to protect against atherogenesis by reducing monocyte recruitment.
In contrast to our current report, Schirmer et al reported that n-3 PUFA treatment did not change monocyte subset proportions and total monocyte levels of CD11b, CD14, and CCR2 in subjects with coronary artery disease (47). The reasons for the discrepancy are not known, but may include that the subjects in Schirmer’s study had relatively normal baseline TG levels and that the investigators examined markers on total monocytes instead of monocyte subsets (47). Indeed, as discussed above, baseline TG levels significantly impact monocyte phenotypes and monocyte response to a high-fat diet challenge. Furthermore, monocyte subsets differ in numerous markers (10,20). It would be ideal to compare markers on each monocyte subset rather than on total monocytes.
We have focused on the lipid-lowering effect of n-3 PUFAs as the main mediator for improvements in monocyte inflammatory phenotypes in subjects with hypertriglyceridemia. Our finding that EPA-EE reduced several monocyte markers in the postprandial state without a significant impact on postprandial TG levels may be because EPA-EE had already reduced fasting TG levels. Indeed, fasting TG levels influence monocyte response to a high-fat meal (20,27). However, n-3 PUFAs may also exert anti-inflammatory effects through several additional pathways: 1) n-3 PUFAs reduce the generation of intracellular secondary inflammatory messengers such as diacylglycerol and ceramide, thereby decreasing the downstream cell inflammatory response (48); 2) incorporation of n-3 PUFAs into cell membrane phospholipids alters lipid rafts, suppressing protein kinase C (PKC) recruitment into lipid rafts and downregulating PKC-mediated signaling inflammatory cascades, including suppression of NF-κB activation and inhibition of inflammatory gene transcription (48); 3) n-3 PUFAs bind to and activate peroxisome proliferator–activated receptors, with subsequent anti-inflammatory effects (35,49). These mechanisms may all contribute to “TG-independent” effects of EPA alone on postprandial monocyte phenotypes in these subjects with hypertriglyceridemia. In addition, besides the greater reductions in TG levels, the DPA content of MAT9001 may also help improve monocyte phenotypes given the potential direct anti-inflammatory effects of DPA (50). While our current study focused on effects of n-3 PUFAs on circulating monocyte phenotypes, previous studies showed that n-3 PUFAs may promote macrophage polarization from M1-like proinflammatory to M2-like phenotypes (51–53), which may also provide benefit on the prevention and treatment of ASCVD.
Limitations of this study include descriptive phenotyping of monocytes as part of an exploratory substudy of a clinical trial. However, our work provides more careful characterization of phenotypic changes in monocyte subsets in the fasting and postprandial states than was previously available. The current data, in conjunction with our prior work (20), highlight the complexity of phenotypic changes in each monocyte subset in patients with hypertriglyceridemia and the effects of interventions with different n-3 PUFA formulations on both fasting and postprandial TG levels as well as on monocyte subset phenotypic changes. These data are hypothesis generating but may be useful in designing future studies to understand better how hypertriglyceridemia alters monocyte subset phenotypes and how therapies that alter TG metabolism improve monocyte subset phenotypes and function, thereby benefiting prevention and treatment of ASCVD.
Conclusions
Treatment with both MAT9001 and EPA-EE in individuals with hypertriglyceridemia was associated with reductions in the relative ratio of intermediate monocytes, in fasting and postprandial levels of CD11c and CD36 on classical and intermediate monocytes, and in postprandial levels of CCR5 on intermediate and nonclassical monocytes. MAT9001, but not EPA-EE, significantly reduced fasting nile red staining for lipids in classical and intermediate monocytes and postprandial CD11c on nonclassical monocytes. These changes in monocyte phenotypes with n-3 PUFA treatment may benefit subjects with hypertriglyceridemia by potentially reducing development and/or progression of ASCVD.
Supplementary Material
Highlights.
MAT9001 contains eicosapentaenoic acid (EPA) and docosapentaenoic acid.
Effects of MAT9001 vs EPA on monocyte phenotype were tested in hypertriglyceridemia.
Only MAT9001 lowered fasting nile red staining for lipids in monocytes.
Only MAT9001 reduced postprandial CD11c levels on nonclassical monocytes.
MAT9001 and EPA each reduced CD11c, CD36, and CCR5 on certain monocyte subsets.
Acknowledgments
This work was sponsored by Matinas BioPharma, Inc., and partially supported by an NIH grant (R01 HL098839), an American Heart Association award (AHA16GRNT30410012), and an American Diabetes Association award (1-17-IBS-082) to H.W.
The authors thank Pharma Medica Research, Inc (Ontario, Canada) for support of the clinical study. We also thank Kerrie Jara, Baylor College of Medicine, for editorial assistance on the manuscript.
H.W. received a contract of support from Matinas BioPharma, Inc. (paid to Baylor College of Medicine). K.C.M. received research funding from and consulted for Matinas BioPharma, Inc., Sancilio & Co., AstraZeneca, and Pharmavite; received research funding from Global Association for EPA and DHA (GOED); and consulted for DSM. G.B. is a shareholder in Matinas BioPharma, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov identifier: NCT02310022
Authors’ contributions: Drs. Wu, Maki, and Bobotas designed the study. Drs. Dai and Wu acquired data. Drs. Dai, Lian, Bobotas, Dicklin, Maki, and Wu analyzed and interpreted the data and contributed to drafting, critical revision, and approval of the article.
Disclosure summary: X.D.P, Z.L., and M.R.D. have nothing to disclose.
References
- 1.CARDIoGRAMplusC4D Consortium. Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, Ingelsson E, Saleheen D, Erdmann J, Goldstein BA, Stirrups K, Konig IR, Cazier JB, Johansson A, Hall AS, Lee JY, Willer CJ, Chambers JC, Esko T, Folkersen L, Goel A, Grundberg E, Havulinna AS, Ho WK, Hopewell JC, Eriksson N, Kleber ME, Kristiansson K, Lundmark P, Lyytikainen LP, Rafelt S, Shungin D, Strawbridge RJ, Thorleifsson G, Tikkanen E, Van Zuydam N, Voight BF, Waite LL, Zhang W, Ziegler A, Absher D, Altshuler D, Balmforth AJ, Barroso I, Braund PS, Burgdorf C, Claudi-Boehm S, Cox D, Dimitriou M, Do R, Consortium D, Consortium C, Doney AS, El Mokhtari N, Eriksson P, Fischer K, Fontanillas P, Franco-Cereceda A, Gigante B, Groop L, Gustafsson S, Hager J, Hallmans G, Han BG, Hunt SE, Kang HM, Illig T, Kessler T, Knowles JW, Kolovou G, Kuusisto J, Langenberg C, Langford C, Leander K, Lokki ML, Lundmark A, McCarthy MI, Meisinger C, Melander O, Mihailov E, Maouche S, Morris AD, Muller-Nurasyid M, Mu TC, Nikus K, Peden JF, Rayner NW, Rasheed A, Rosinger S, Rubin D, Rumpf MP, Schafer A, Sivananthan M, Song C, Stewart AF, Tan ST, Thorgeirsson G, van der Schoot CE, Wagner PJ, Wellcome Trust Case Control C. Wells GA, Wild PS, Yang TP, Amouyel P, Arveiler D, Basart H, Boehnke M, Boerwinkle E, Brambilla P, Cambien F, Cupples AL, de Faire U, Dehghan A, Diemert P, Epstein SE, Evans A, Ferrario MM, Ferrieres J, Gauguier D, Go AS, Goodall AH, Gudnason V, Hazen SL, Holm H, Iribarren C, Jang Y, Kahonen M, Kee F, Kim HS, Klopp N, Koenig W, Kratzer W, Kuulasmaa K, Laakso M, Laaksonen R, Lee JY, Lind L, Ouwehand WH, Parish S, Park JE, Pedersen NL, Peters A, Quertermous T, Rader DJ, Salomaa V, Schadt E, Shah SH, Sinisalo J, Stark K, Stefansson K, Tregouet DA, Virtamo J, Wallentin L, Wareham N, Zimmermann ME, Nieminen MS, Hengstenberg C, Sandhu MS, Pastinen T, Syvanen AC, Hovingh GK, Dedoussis G, Franks PW, Lehtimaki T, Metspalu A, Zalloua PA, Siegbahn A, Schreiber S, Ripatti S, Blankenberg SS, Perola M, Clarke R, Boehm BO, O'Donnell C, Reilly MP, Marz W, Collins R, Kathiresan S, Hamsten A, Kooner JS, Thorsteinsdottir U, Danesh J, Palmer CN, Roberts R, Watkins H, Schunkert H, Samani NJ. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45:25–33. doi: 10.1038/ng.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371:32–41. doi: 10.1056/NEJMoa1308027. [DOI] [PubMed] [Google Scholar]
- 3.TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute. Crosby J, Peloso GM, Auer PL, Crosslin DR, Stitziel NO, Lange LA, Lu Y, Tang ZZ, Zhang H, Hindy G, Masca N, Stirrups K, Kanoni S, Do R, Jun G, Hu Y, Kang HM, Xue C, Goel A, Farrall M, Duga S, Merlini PA, Asselta R, Girelli D, Olivieri O, Martinelli N, Yin W, Reilly D, Speliotes E, Fox CS, Hveem K, Holmen OL, Nikpay M, Farlow DN, Assimes TL, Franceschini N, Robinson J, North KE, Martin LW, DePristo M, Gupta N, Escher SA, Jansson JH, Van Zuydam N, Palmer CN, Wareham N, Koch W, Meitinger T, Peters A, Lieb W, Erbel R, Konig IR, Kruppa J, Degenhardt F, Gottesman O, Bottinger EP, O'Donnell CJ, Psaty BM, Ballantyne CM, Abecasis G, Ordovas JM, Melander O, Watkins H, Orho-Melander M, Ardissino D, Loos RJ, McPherson R, Willer CJ, Erdmann J, Hall AS, Samani NJ, Deloukas P, Schunkert H, Wilson JG, Kooperberg C, Rich SS, Tracy RP, Lin DY, Altshuler D, Gabriel S, Nickerson DA, Jarvik GP, Cupples LA, Reiner AP, Boerwinkle E, Kathiresan S. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371:22–31. doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller M, Stone NJ, Ballantyne CM, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T, Pennathur S. Triglycerides and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2011;123:2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 5.Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38:1092–1104. doi: 10.1016/j.immuni.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun C, Simon SI, Foster GA, Radecke CE, Hwang HV, Zhang X, Hammock BD, Chiamvimonvat N, Knowlton AA. 11,12-Epoxyecosatrienoic acids mitigate endothelial dysfunction associated with estrogen loss and aging: Role of membrane depolarization. J Mol Cell Cardiol. 2016;94:180–188. doi: 10.1016/j.yjmcc.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong EJ, Krueger JG. Lipoprotein Metabolism and Inflammation in Patients With Psoriasis. Am J Cardiol. 2016;118:603–609. doi: 10.1016/j.amjcard.2016.05.060. [DOI] [PubMed] [Google Scholar]
- 8.Takata Y, Chu V, Collins AR, Lyon CJ, Wang W, Blaschke F, Bruemmer D, Caglayan E, Daley W, Higaki J, Fishbein MC, Tangirala RK, Law RE, Hsueh WA. Transcriptional repression of ATP-binding cassette transporter A1 gene in macrophages: a novel atherosclerotic effect of angiotensin II. Circ Res. 2005;97:e88–96. doi: 10.1161/01.RES.0000190400.46267.7e. [DOI] [PubMed] [Google Scholar]
- 9.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 11.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poitou C, Dalmas E, Renovato M, Benhamo V, Hajduch F, Abdennour M, Kahn JF, Veyrie N, Rizkalla S, Fridman WH, Sautes-Fridman C, Clement K, Cremer I. CD14dimCD16+ and CD14+CD16+ Monocytes in Obesity and During Weight Loss: Relationships With Fat Mass and Subclinical Atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:2322–2330. doi: 10.1161/ATVBAHA.111.230979. [DOI] [PubMed] [Google Scholar]
- 13.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais JP, D'Cruz D, Casanova JL, Trouillet C, Geissmann F. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gower RM, Wu H, Foster GA, Devaraj S, Jialal I, Ballantyne CM, Knowlton AA, Simon SI. CD11c/CD18 expression is upregulated on blood monocytes during hypertriglyceridemia and enhances adhesion to vascular cell adhesion molecule-1. Arterioscler Thromb Vasc Biol. 2011;31:160–166. doi: 10.1161/ATVBAHA.110.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varela LM, Ortega A, Bermudez B, Lopez S, Pacheco YM, Villar J, Abia R, Muriana FJ. A high-fat meal promotes lipid-load and apolipoprotein B-48 receptor transcriptional activity in circulating monocytes. Am J Clin Nutr. 2011;93:918–925. doi: 10.3945/ajcn.110.007765. [DOI] [PubMed] [Google Scholar]
- 17.den Hartigh LJ, Connolly-Rohrbach JE, Fore S, Huser TR, Rutledge JC. Fatty acids from very low-density lipoprotein lipolysis products induce lipid droplet accumulation in human monocytes. J Immunol. 2010;184:3927–3936. doi: 10.4049/jimmunol.0903475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu H, Gower RM, Wang H, Perrard XY, Ma R, Bullard DC, Burns AR, Paul A, Smith CW, Simon SI, Ballantyne CM. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation. 2009;119:2708–2717. doi: 10.1161/CIRCULATIONAHA.108.823740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu L, Dai Perrard X, Perrard JL, Yang D, Xiao X, Teng BB, Simon SI, Ballantyne CM, Wu H. Foamy Monocytes Form Early and Contribute to Nascent Atherosclerosis in Mice With Hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2015;35:1787–1797. doi: 10.1161/ATVBAHA.115.305609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan IM, Pokharel Y, Dadu RT, Lewis DE, Hoogeveen RC, Wu H, Ballantyne CM. Postprandial Monocyte Activation in Individuals With Metabolic Syndrome. J Clin Endocrinol Metab. 2016;101:4195–4204. doi: 10.1210/jc.2016-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernelot Moens SJ, Neele AE, Kroon J, van der Valk FM, Van den Bossche J, Hoeksema MA, Hoogeveen RM, Schnitzler JG, Baccara-Dinet MT, Manvelian G, de Winther MPJ, Stroes ESG. PCSK9 monoclonal antibodies reverse the pro-inflammatory profile of monocytes in familial hypercholesterolaemia. Eur Heart J. 2017;38:1584–1593. doi: 10.1093/eurheartj/ehx002. [DOI] [PubMed] [Google Scholar]
- 22.Bernelot Moens SJ, Verweij SL, Schnitzler JG, Stiekema LCA, Bos M, Langsted A, Kuijk C, Bekkering S, Voermans C, Verberne HJ, Nordestgaard BG, Stroes ESG, Kroon J. Remnant Cholesterol Elicits Arterial Wall Inflammation and a Multilevel Cellular Immune Response in Humans. Arterioscler Thromb Vasc Biol. 2017;37:969–975. doi: 10.1161/ATVBAHA.116.308834. [DOI] [PubMed] [Google Scholar]
- 23.Wu H, Ballantyne CM. Dyslipidaemia: PCSK9 inhibitors and foamy monocytes in familial hypercholesterolaemia. Nat Rev Cardiol. 2017;14:385–386. doi: 10.1038/nrcardio.2017.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogacev KS, Ulrich C, Blomer L, Hornof F, Oster K, Ziegelin M, Cremers B, Grenner Y, Geisel J, Schlitt A, Kohler H, Fliser D, Girndt M, Heine GH. Monocyte heterogeneity in obesity and subclinical atherosclerosis. Eur Heart J. 2010;31:369–376. doi: 10.1093/eurheartj/ehp308. [DOI] [PubMed] [Google Scholar]
- 25.Jialal I, Huet BA, Kaur H, Chien A, Devaraj S. Increased toll-like receptor activity in patients with metabolic syndrome. Diabetes Care. 2012;35:900–904. doi: 10.2337/dc11-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu H, Perrard XD, Wang Q, Perrard JL, Polsani VR, Jones PH, Smith CW, Ballantyne CM. CD11c expression in adipose tissue and blood and its role in diet-induced obesity. Arterioscler Thromb Vasc Biol. 2010;30:186–192. doi: 10.1161/ATVBAHA.109.198044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foster GA, Gower RM, Stanhope KL, Havel PJ, Simon SI, Armstrong EJ. On-chip phenotypic analysis of inflammatory monocytes in atherogenesis and myocardial infarction. Proc Natl Acad Sci U S A. 2013;110:13944–13949. doi: 10.1073/pnas.1300651110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jialal I, Devaraj S, Adams-Huet B, Chen X, Kaur H. Increased cellular and circulating biomarkers of oxidative stress in nascent metabolic syndrome. J Clin Endocrinol Metab. 2012;97:E1844–1850. doi: 10.1210/jc.2012-2498. [DOI] [PubMed] [Google Scholar]
- 29.Maki KC, Guyton JR, Orringer CE, Hamilton-Craig I, Alexander DD, Davidson MH. Triglyceride-lowering therapies reduce cardiovascular disease event risk in subjects with hypertriglyceridemia. J Clin Lipidol. 2016;10:905–914. doi: 10.1016/j.jacl.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Maki KC, Lawless AL, Kelley KM, Dicklin MR, Schild AL, Rains TM. Prescription omega-3-acid ethyl esters reduce fasting and postprandial triglycerides and modestly reduce pancreatic beta-cell response in subjects with primary hypertriglyceridemia. Prostaglandins Leukot Essent Fatty Acids. 2011;85:143–148. doi: 10.1016/j.plefa.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Kastelein JJ, Maki KC, Susekov A, Ezhov M, Nordestgaard BG, Machielse BN, Kling D, Davidson MH. Omega-3 free fatty acids for the treatment of severe hypertriglyceridemia: The EpanoVa fOr Lowering Very high triglyceridEs (EVOLVE) trial. J Clin Lipidol. 2014;8:94–106. doi: 10.1016/j.jacl.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Wei MY, Jacobson TA. Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: a systematic review and meta-analysis. Curr Atheroscler Rep. 2011;13:474–483. doi: 10.1007/s11883-011-0210-3. [DOI] [PubMed] [Google Scholar]
- 33.Miller E, Kaur G, Larsen A, Loh SP, Linderborg K, Weisinger HS, Turchini GM, Cameron-Smith D, Sinclair AJ. A short-term n-3 DPA supplementation study in humans. Eur J Nutr. 2013;52:895–904. doi: 10.1007/s00394-012-0396-3. [DOI] [PubMed] [Google Scholar]
- 34.Maki KC, Bobotas G, Dicklin MR, Huebner M, Keane WF. Effects of MAT9001 containing eicosapentaenoic acid and docosapentaenoic acid, compared to eicosapentaenoic acid ethyl esters, on triglycerides, lipoprotein cholesterol, and related variables. J Clin Lipidol. 2017;11:102–109. doi: 10.1016/j.jacl.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Ruan XZ, Powis SH, Fernando R, Mon WY, Wheeler DC, Moorhead JF, Varghese Z. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: evidence for a PPAR-γ-dependent mechanism. Kidney Int. 2005;67:867–874. doi: 10.1111/j.1523-1755.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- 36.Warrino DE, DeGennaro LJ, Hanson M, Swindells S, Pirruccello SJ, Ryan WL. Stabilization of white blood cells and immunologic markers for extended analysis using flow cytometry. J Immunol Methods. 2005;305:107–119. doi: 10.1016/j.jim.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 37.Catellier DJ, Aleksic N, Folsom AR, Boerwinkle E. Atherosclerosis Risk in Communities (ARIC) Carotid MRI flow cytometry study of monocyte and platelet markers: intraindividual variability and reliability. Clin Chem. 2008;54:1363–1371. doi: 10.1373/clinchem.2007.102202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes Care. 1995;18:245–250. doi: 10.2337/diacare.18.2.245. [DOI] [PubMed] [Google Scholar]
- 39.Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples) Biometrika. 1965;52:591–611. [Google Scholar]
- 40.Foster GA, Xu L, Chidambaram AA, Soderberg SR, Armstrong EJ, Wu H, Simon SI. CD11c/CD18 Signals Very Late Antigen-4 Activation To Initiate Foamy Monocyte Recruitment during the Onset of Hypercholesterolemia. J Immunol. 2015;195:5380–5392. doi: 10.4049/jimmunol.1501077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Z, de Beer MC, Cai L, Asmis R, de Beer FC, de Villiers WJ, van der Westhuyzen DR. Low-density lipoprotein from apolipoprotein E-deficient mice induces macrophage lipid accumulation in a CD36 and scavenger receptor class A-dependent manner. Arterioscler Thromb Vasc Biol. 2005;25:168–173. doi: 10.1161/01.ATV.0000149145.00865.d9. [DOI] [PubMed] [Google Scholar]
- 42.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, Randolph GJ. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. J Clin Invest. 2011;121:2025–2036. doi: 10.1172/JCI43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Combadière C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6Chi and Ly6Clo monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 46.Brown AL, Zhu X, Rong S, Shewale S, Seo J, Boudyguina E, Gebre AK, Alexander-Miller MA, Parks JS. Omega-3 fatty acids ameliorate atherosclerosis by favorably altering monocyte subsets and limiting monocyte recruitment to aortic lesions. Arterioscler Thromb Vasc Biol. 2012;32:2122–2130. doi: 10.1161/ATVBAHA.112.253435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schirmer SH, Werner CM, Binder SB, Faas ME, Custodis F, Bohm M, Laufs U. Effects of omega-3 fatty acids on postprandial triglycerides and monocyte activation. Atherosclerosis. 2012;225:166–172. doi: 10.1016/j.atherosclerosis.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Fan YY, Ly LH, Barhoumi R, McMurray DN, Chapkin RS. Dietary docosahexaenoic acid suppresses T cell protein kinase Cθ lipid raft recruitment and IL-2 production. J Immunol. 2004;173:6151–6160. doi: 10.4049/jimmunol.173.10.6151. [DOI] [PubMed] [Google Scholar]
- 49.Straus DS, Glass CK. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 2007;28:551–558. doi: 10.1016/j.it.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 50.Morin C, Hiram R, Rousseau E, Blier PU, Fortin S. Docosapentaenoic acid monoacylglyceride reduces inflammation and vascular remodeling in experimental pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2014;307:H574–586. doi: 10.1152/ajpheart.00814.2013. [DOI] [PubMed] [Google Scholar]
- 51.Gladine C, Zmojdzian M, Joumard-Cubizolles L, Verny MA, Comte B, Mazur A. The omega-3 fatty acid docosahexaenoic acid favorably modulates the inflammatory pathways and macrophage polarization within aorta of LDLR(−/−) mice. Genes Nutr. 2014;9:424. doi: 10.1007/s12263-014-0424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carvalho SC, Apolinario LM, Matheus SM, Santo Neto H, Marques MJ. EPA protects against muscle damage in the mdx mouse model of Duchenne muscular dystrophy by promoting a shift from the M1 to M2 macrophage phenotype. J Neuroimmunol. 2013;264:41–47. doi: 10.1016/j.jneuroim.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 53.Famenini S, Rigali EA, Olivera-Perez HM, Dang J, Chang MT, Halder R, Rao RV, Pellegrini M, Porter V, Bredesen D, Fiala M. Increased intermediate M1–M2 macrophage polarization and improved cognition in mild cognitive impairment patients on omega-3 supplementation. FASEB J. 2017;31:148–160. doi: 10.1096/fj.201600677RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.