Abstract
Heritable symbionts are common in insects with many contributing to host defence. Hamiltonella defensa is a facultative, bacterial symbiont of the pea aphid, Acyrthosiphon pisum that provides protection against the endoparasitoid wasp Aphidius ervi. Protection levels vary among strains of H. defensa that are differentially infected by bacteriophages named APSEs. By contrast, little is known about mechanism(s) of resistance owing to the intractability of host-restricted microbes for functional study. Here, we developed methods for culturing strains of H. defensa that varied in the presence and type of APSE. Most H. defensa strains proliferated at 27°C in co-cultures with the TN5 cell line or as pure cultures with no insect cells. The strain infected by APSE3, which provides high levels of protection in vivo, produced a soluble factor(s) that disabled development of A. ervi embryos independent of any aphid factors. Experimental transfer of APSE3 also conferred the ability to disable A. ervi development to a phage-free strain of H. defensa. Altogether, these results provide a critical foundation for characterizing symbiont-derived factor(s) involved in host protection and other functions. Our results also demonstrate that phage-mediated transfer of traits provides a mechanism for innovation in host restricted symbionts.
Keywords: bacteria, endosymbiont, mutualism, mobile element, horizontal transfer
1. Introduction
Insects, including species of medical and agricultural importance, frequently host maternally transmitted bacterial symbionts [1]. By providing novel genes that expand the functional repertoire of the host insect, heritable symbionts often play key roles in mediating host ecology [2,3]. Phloem-feeding aphids (Hemiptera: Aphidoidea), for example, depend on the obligate (=primary) symbiont Buchnera aphidicola, which lives in specialized cells called bacteriocytes and supplies nutrients deficient in the aphid diet [4]. Aphids also commonly host one or more species of facultative (=secondary) symbionts that can live either extracellularly in the hemocoel or intracellularly in bacteriocytes or neighbouring sheath cells [5,6]. Facultative symbionts provide several conditional benefits to hosts including resistance to parasitoid wasps and pathogens, protection against thermal stress and facilitation of host plant usage [7–16]. Some facultative symbionts are also infected by bacteriophages [17–19] that enhance host fitness by provisioning novel traits and altering within-host bacterial abundance [18,20,21].
The γ-proteobacterium Hamiltonella defensa is among the most common aphid facultative symbionts, occurring in 34% of sampled species [22,23]. Hamiltonella defensa confers protection against parasitoids in at least three aphid species, including the pea aphid, Acyrthosiphon pisum [8,14,24]. Field and laboratory surveys identify multiple strains of H. defensa in A. pisum with most persistently infected by a podovirus-like bacteriophage named APSE [25–27]. Four APSE variants (APSE1, 2, 3 and 8) are currently known. The viral genome of each shares strong conservation in regulatory and structural genes, but varies in a 3.7–5.8 kb domain containing predicted eukaryotic-like toxin genes with hypothesized functions in parasitoid defence [18,19,28,29]. A major mortality agent of A. pisum is the endoparasitoid wasp Aphidius ervi [30–33]. In examined strains, H. defensa confers protection against this wasp only when infected with APSE [34]. In APSE3-associated strains, the spontaneous loss of phage eliminates protection against A. ervi and increases titres of H. defensa, which adversely affect aphid fitness [20,21]. Protection levels against A. ervi and the timing of wasp mortality in aphids differ among H. defensa strains and APSE variants [20,24,29,35–37]. Recent results indicate that some genotypes of A. pisum exhibit resistance towards A. ervi in the absence of H. defensa or other facultative symbionts [38]. Endogenous and symbiont defences also target only specific parasitoid species [29,39]. These findings collectively suggest A. pisum defence against parasitoids is multifactorial with APSE-encoded toxins or other gene products interacting additively or synergistically with factors produced by aphids.
Asexual reproduction and the ability to manipulate symbiont infections make aphids excellent models for studying the phenotypic effects of facultative symbionts [6,7]. By contrast, in vivo studies have provided few insights into the mechanisms underlying symbiont-mediated phenotypes. The ability to culture normally host-restricted symbionts and their bacteriophages outside of aphids and other insects would open new avenues for studying function. While most heritable symbionts of insects remain unculturable, a few species have been propagated in co-cultures with insect cells [40–44] or as pure cultures [43,45]. The approximately 2.1 Mb genome of H. defensa is significantly smaller than its closest free-living relatives and lacks a number of genes with metabolic functions [46]. Nonetheless, Darby et al. [40] reported that one strain of H. defensa (called T type in this study) from black bean aphids (Aphis fabae) and a strain of the closely related species Regiella insecticola (U type) from A. pisum persisted in vitro when co-cultured with the C6/36 cell line from the mosquito Aedes albopictus or the S2 cell line from Drosophila melanogaster. The growth and abundance of H. defensa and R. insecticola in these cultures were not quantified but descriptions of their maintenance suggest both primarily persisted intracellularly at low abundance [40]. By contrast, H. defensa grows to high titres as A. pisum develop from first instar nymphs to adults [21,36].
Here, we report the in vitro culture of four H. defensa strains that exhibit variable protection against parasitoids in vivo owing to differences in APSE infections (table 1). We then demonstrate that one strain of APSE-infected H. defensa kills A. ervi in the absence of any aphid factor, and that horizontal transfer of this APSE to a non-infected strain of H. defensa confers the same killing activity.
Table 1.
Characteristics of H. defensa strains cultured.
| H. defensa strain | APSE variant | primary phage toxin | protection in aphids |
|---|---|---|---|
| A2C | no phage | no phage | none |
| NY26 | APSE2 | CdtB | moderate |
| AS3 | APSE3 | YDR | high |
| ZA17 | APSE8 | CdtB | moderate |
2. Material and methods
(a). Insects and cell lines
Clonal A. pisum lines carrying the A2C, AS3, NY26 and ZA17 strains of H. defensa were maintained on fava bean plants at 20°C and a 16 L : 8 D photoperiod as previously described [20,35–37]. Aphidius ervi used in the study were reared in the laboratory as a large population founded from commercially purchased (Arbico) and field-collected wasps on an A. pisum line with no facultative symbionts; adult wasps were provided continuous access to honey and water at 20°C under a 16 L : 8 D photoperiod [36]. Culture media and insect cell lines used in the study are summarized in the electronic supplementary material, table S1.
(b). Hamiltonella defensa cultures
Acyrthosiphon pisum adults containing the A2C, AS3, NY26 or ZA17 strain of H. defensa were surface sterilized by submerging in 5% bleach, 1% Tween20 and 0.1% ROCCAL-D (Pfizer) (v/v in water) for 30 s followed by rinsing in sterile water for 30 s. Each aphid was then transferred to a 25 µl drop of medium and pierced using sterile forceps to release haemolymph from the body cavity into the medium. Each drop was then transferred to an individual culture well in a 24-well culture plate (Corning) containing 1 ml of medium with or without fetal bovine serum (FBS) and particular insect cell lines (electronic supplementary material, table S1). Primary cultures were maintained under ambient atmosphere at 20° or 27°C. Established cultures were passaged weekly or biweekly by adding 1 × 106 bacteria to 25 cm culture flasks (Falcon) containing 4.0 ml of TC100 medium plus 10% FBS with or without 1 × 106 TN5 cells (electronic supplementary material, table S1).
(c). Estimating Hamiltonella defensa titres
Cohorts (n = 5) of two-day old adult aphids infected with the A2C, AS3, NY26 or ZA17 strains of H. defensa were placed in 500 µl of phosphate buffered saline (PBS). The cuticle of each aphid was then pierced using forceps as described above. After removing the aphids, each drop was transferred to 1.5 ml microfuge tube and gently centrifuged at 50g to pellet aphid cells and debris. The supernatant was then transferred to a new tube and spun at 8000g for 10 min at 4°C to pellet H. defensa. After decanting the supernatant, DNA was extracted as described [21] and stored at −20°C. DNA was similarly isolated from H. defensa in established cultures by collecting 500 µl of medium 3, 6, 9 or 15 days after new cultures were passaged. Hamiltonella defensa titres were estimated by qPCR using specific primers for the single-copy H. defensa dnaK gene (electronic supplementary material, table S2) [21,35,47]. Ten microlitres of qPCR reactions contained 0.5 µl of 5 µM of each primer, 5 µl of the Rotor-Gene SYBR Green Master Mix and 1 µl of a 1 : 100 dilution of template and 3 µl of ultrapure water. Four technical replicates were run per sample using a Rotor-Gene Q cycler (Qiagen) with data acquired during the extension step. In vivo titre data were analysed by analysis of variance (ANOVA) while in vitro data were analysed by repeated-measures ANOVA.
(d). Diagnostic PCR
Diagnostic PCR reactions were run using specific primers that amplified unique domains in: (i) the dnaK gene present in all strains of H. defensa, (ii) the head protein gene (p24) present in all APSE variants, (iii) the ydr gene in APSE3 that persistently infects AS3, (iv) the cdtB gene in APSE2 and APSE8 that persistently infect NY26 and ZA17 respectively, (v) a hypothetical protein unique to ZA17, (vi) an rtx-toxin gene unique to NY26, and (vii) the dnak gene of B. aphidicola (electronic supplementary material, table S2). PCR reactions (25 µl) contained 2.5 µl of 25 mM Mg2+, 0.5 µl of mixed dNTPs (2.5 µM each), 0.5 µl of each primer (5 µM), 1 µl of a 1/100 dilution of template DNA, 0.2 µl of HotMaster Taq DNA polymerase and 19.6 µl of ultrapure water. Samples were run using a T100 thermal cycler (Bio-Rad). Eight microlitres of each PCR reaction were then run on a 1% agar gel stained with ethidium bromide and visualized using a BioDoc-iT imaging system.
(e). Microscopy
Hamiltonella defensa were slide mounted and examined with a Leica DMXRXE upright epifluorescent microscope with differential interference optics and a Leica digital camera. Established cultures in flasks containing TN5 cells were also examined after staining with 20 µg ml−1 acridine orange (Sigma) or 1 µg ml−1 Hoechst 33342 (Sigma) in water using a Leica DM IRE2 inverted epifluorescent microscope with phase-contrast optics fitted with a Hamamatsu digital camera. Aphidius ervi eggs were stained with acridine orange and 1 µg ml−1 propidium iodide (Sigma) followed by examination as described above. Images were captured using Leica or SimplePCI software (Compix). For transmission electron microscopy (TEM), H. defensa and TN5 cells from established cultures were pelleted 4 days post-passage by centrifugation at 2000g at 4°C. After decanting the supernatant, the pellet consisting of bacteria and insect cells was fixed for 2 h in 0.1 M cacodylate–HCl buffer (pH 7.0) containing 2% glutaraldehyde (v/v), 2% paraformaldehyde (v/v) and 0.2% picric acid (v/v). After rinsing in 0.1 M cacodylate–HCl buffer and repelleting, cells were resuspended in 2% agar at 50°C followed by centrifugation for 10 min and storage at 4°C until the agar hardened. Agar-embedded samples were then post-fixed in 1% OsO4 in 0.1 M cacodylate–HCl buffer for 1 h, rinsed in deionized water, and dehydrated in graded ethanols (30–100%) with 15 min wash steps. Samples were then rinsed 1× in acetone and 2× in propylene oxide for 15 min before infiltrating in proplyene oxide: Epon-Araldite (1 : 1, 1 : 3) for 2 h. Samples were embedded in 100% Epon-Araldite, which polymerized over 24 h in a 75°C oven. Sections were cut with a Reichert Ultracut S ultramicrotome and stained with uranyl acetate and lead citrate. Sections were then examined and photographed using a JEOL JEM1011 (JEOL, Inc., Peabody, MA) TEM at 100 kV.
(f). Aphidius ervi assays
Acyrthosiphon pisum without facultative symbionts were individually parasitized by A. ervi. Aphids were surface sterilized as previously described 2 or 36 h post-parasitism and then dissected to isolate either newly laid A. ervi eggs or 1st instar larvae. One egg or larva was placed per well of a 96-well culture plate that contained 100 µl TC100 + 30% FBS. A2C, AS3 or ZA17 from 3-day-old established cultures (greater than 50 generations old and capable of growing without TN5 cells) were then added to wells at a density of 1 × 104 to 1 × 106 bacteria ml−1. Aphidius ervi eggs were allowed to develop for 72 h at 27°C while first instars were maintained for 48 h. Embryonic stages of A. ervi were recorded every 24 h with the treatment effects assessed by Fisher exact tests.
(g). APSE infectivity experiments
One millilitre of medium from 3-day old established cultures of the AS3 strain was collected and centrifuged at 8000g. Cell pellet template DNA was extracted as described above. The supernatant was collected and filtered through an 0.2 µm syringe filter to eliminate all bacterial cells. Two microlitres of TURBO DNase (Ambion) and 5 µl of buffer was then added to 50 µl of this supernatant for 40 min at 37°C. This treatment degraded any naked DNA in the medium but cannot degrade DNA that is packaged inside of a virus particle. After adding EDTA (10 mM) to inactivate the DNase, supernatant template DNA was then extracted [21]. APSE-specific primers (electronic supplementary material, table S2) were then used to amplify viral DNA using qPCR as above. APSE3 copy number ml−1 of medium was calculated by multiplying the qPCR estimate by the dilution factor and elution volume. Two independently acquired biological replicates were performed for each treatment with samples internally replicated four times. A2C titre was determined by qPCR as described above. Six new A2C cultures were initiated by adding 1 × 107 bacteria to 4 ml of fresh TC100 + 10% FBS and 1 × 104 TN5 cells in a six-well culture plate (Corning). APSE3 in medium from the AS3 strain was then added to three A2C cultures (n = 3) at a multiplicity of infection (MOI) of 0.1 while three other A2C cultures were grown without APSE3. After 9 days at 27°C, 1 ml of medium was centrifuged followed by qPCR to determine APSE3 and H. defensa copy number in the cell pellet and APSE3 copy in the DNAse pretreated supernatant. The effects of APSE3-infected A2C on development of A. ervi eggs were then compared with non-infected (phage-free) A2C by adding 1 × 106 bacteria per well and assessing the proportion of eggs that developed to the 128 cell stage as described above.
(h). Figure assembly and statistical analyses
Micrographs were assembled using Adobe Illustrator and Photoshop while figures were generated using R or GraphPad Prism 5.0. Statistical analyses were performed using R (www.r-project.org) or JMP Pro version 11 (SAS Institute Inc., Cary, NC).
3. Results
(a). Hamiltonella defensa grows extracellularly in vivo and in vitro
Previous qPCR estimates indicated that titres of APSE- and non-infected H. defensa increase in A. pisum from approximately 1 × 107 per first instar nymph to more than approximately 1 × 109 per 2-day old adult [21]. In the current study, we determined that the titres of A2C, AS3, NY26 and ZA17 specifically in the haemolymph of 2-day old adults exceeded 1 × 109 bacteria (electronic supplementary material, figure S1). Most H. defensa were also non-motile pleomorphic rods in plasma with no bacteria in haemocytes [48] (electronic supplementary material, figure S1). Collectively, these findings supported that most H. defensa persist and probably grow extracellularly in A. pisum. We, therefore, used adult haemolymph as the source of H. defensa for establishing primary cultures. We initially focused on the phage-free A2C strain and tested several different types of insect cell culture media with or without FBS and different insect cell lines (electronic supplementary material, table S1). At 27°C and ambient atmosphere we observed bacteria with identical morphology to H. defensa in all primary cultures including those containing C6/36 and S2 cells used by Darby et al. [40]. However, after 3 days bacteria only noticeably increased in abundance in culture wells containing TC100 medium plus 10% FBS and the TN5 cell line (electronic supplementary material, table S1). We, therefore, used these conditions to initiate primary cultures for the APSE-infected AS3, NY26 and ZA17 strains (electronic supplementary material, table S1). Similar to A2C, AS3 and ZA17 increased in abundance under ambient atmosphere at 27°C, whereas NY26 showed no increase in abundance at 27°C but did so when maintained at 18°C. Approximately 1 × 106 bacteria from primary cultures were transferred after 7 (A2C, AS3, ZA17) or 14 days (NY26) to 25 cm2 cell culture flasks containing 4 ml of TC100 plus 10% FBS and 1 × 104 TN5 cells. Each strain was thereafter passaged weekly (A2C, AS3, ZA17) or biweekly (NY26) under the same conditions and henceforth called established cultures. Diagnostic PCR assays confirmed that each established culture contained only the strain of H. defensa that was present in the donor aphid (electronic supplementary material, figure S2).
(b). Established cultures grow as extracellular bacteria
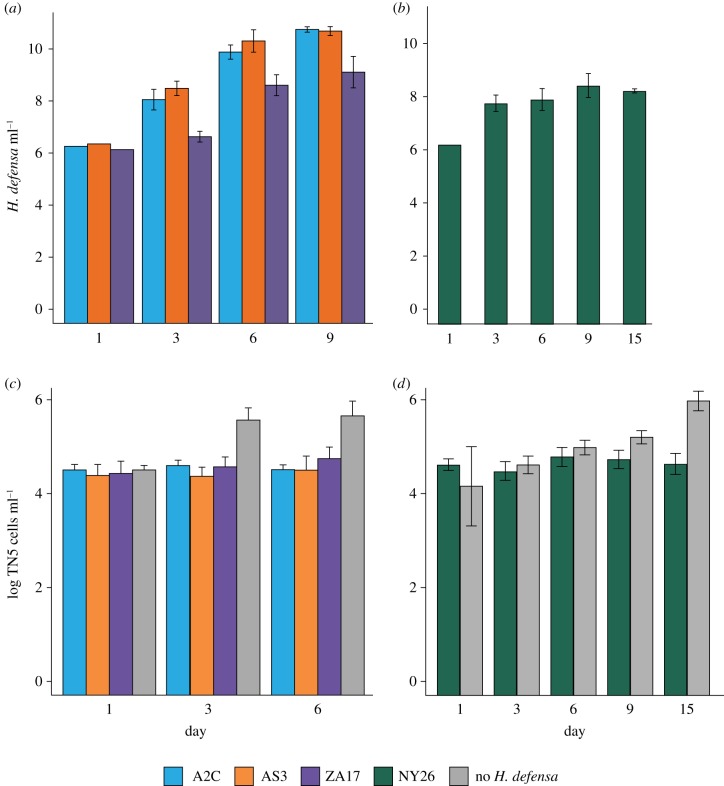
In established cultures, each H. defensa strain increased from 1 × 106 to a maximum of approximately 1 × 1010 bacteria ml−1 in 6 (A2C, AS3, ZA17) or 15 (NY26) days (figure 1a,b). TN5 cells co-cultured with each strain H. defensa exhibited little increase in abundance relative to TN5 cells cultured without bacteria (figure 1c,d). However, almost no TN5 cells with and without bacteria were stained by propidium iodide, which indicated most were viable. TN5 cells co-cultured with each strain of H. defensa also remained attached to culture plates and morphologically looked identical to TN5 cells without bacteria. Hamiltonella defensa in established cultures were morphologically similar to bacteria in aphids, but commonly formed pairs or short chains of cells not observed in A. pisum (electronic supplementary material, figure S3a–d). Staining with the DNA-specific dye Hoechst 33342 also indicated that H. defensa in established cultures predominantly grew extracellularly (electronic supplementary material, figure S3e,f). This conclusion was further supported by TEM analysis of the A2C, AS3 and ZA17 strains. Ultrastructure at low magnification showed an abundance of variably shaped H. defensa that were extracellular (electronic supplementary material, figure S4a,b). Higher magnification images further showed that the cell envelope of H. defensa consisted of an inner and outer membrane but lacked a symbiosome membrane [49] present in some intracellular symbionts including Buchnera (electronic supplementary material, figure S3c). Inspection of hundreds of TN5 cells identified only one that contained an H. defensa cell in the cytoplasm, which indicated bacteria rarely enter this cell type (electronic supplementary material, figure S3d). We stored established cultures of H. defensa by adding 15% glycerol (vol/vol) to culture medium. After thawing on ice, aliquots stored for more than 1 month grew similarly to continuously propagated cultures when re-inoculated into medium indicating all strains can be cryopreserved.
Figure 1.
Growth of the A2C, AS3, NY26 and ZA17 strains and TN5 cells in established cultures. (a) Genome copy number (mean ± 95% confidence intervals) ml−1 of medium for the A2C, AS3 and ZA17 strains over 9 days at 27°C as measured by qPCR. Each strain grew but not at the same rate as indicated by significant strain (F = 212.8; p < 0.0001) and day (F = 1364.9; p < 0.0001) effects (repeated-measures ANOVA). (b) Genome copy number ml−1 of medium for the NY26 strain over 15 days at 18°C. Copy number significantly increased during this period (F = 88.1; p < 0.0001). (c) TN5 cells ml−1 of medium over 9 days at 27°C when co-cultured with the A2C, AS3 and ZA17 strains or no H. defensa. Treatment (F = 65.1; p < 0.0001) and day (F = 16.4; p < 0.0001) are both significant (repeated-measures ANOVA) owing primarily to growth of TN5 cells in the no H. defensa treatment. (d) TN5 cells ml−1 of medium over 15 days at 18°C when co-cultured with the NY26 strain or no H. defensa. Treatment (F = 53.6; p < 0.0001) and day (F = 26.4; p < 0.0001) are both significant (repeated-measures ANOVA) owing to growth of cells with no bacteria.
(c). Some strains of Hamiltonella defensa grow as pure cultures in liquid medium
After one year and more than 50 passages, we tested each strain for the ability to grow in TC100 plus 10% FBS but no TN5 cells. Results showed that the A2C, AS3 and ZA17 strains grew at similar rates and to similar densities (approx. 1 × 1010 ml−1) at 27°C as bacteria co-cultured with TN5 cells. Each strain could also be routinely passaged and maintained as a pure culture in TC100 plus 10% FBS but would not grow in TC100 in the absence of FBS. The NY26 strain in contrast could not be cultured in the absence of TN5 cells. Given these results, we reassessed whether primary cultures of A2C, AS3 and ZA17 could be established from adult aphids by adding haemolymph to TC100 plus 10% FBS and no TN5 cells. Repeated efforts failed to generate any primary cultures that grew. Attempts to propagate H. defensa from established cultures on TC100 plus 10% FBS agar plates under ambient or a microaerobic atmosphere also failed to produce colonies for any strain.
(d). AS3 disables development of Aphidius ervi
Similar to symbionts, most endoparasitoid wasps require specialized conditions for development that make in vitro culture difficult [50–52]. While no wasps in the genus Aphidius have been successfully reared to adulthood in vitro, prior studies established that A. ervi eggs develop to the first instar in TC100 plus 10% FBS [53]. Aphidius ervi eggs have a thin chorion (egg shell) and develop in the haemolymph of aphid hosts. Eggs undergo complete cleavage to initially form a 128 cell embryo that is enveloped by an extraembryonic membrane of polar body origin within the chorion [53]. The embryo then ruptures from the chorion as a spherical morula that completes development into a first instar wasp larva [53]. Previous in vivo assays further showed that A. ervi eggs: (i) usually die during embryogenesis in A. pisum containing APSE3-infected H. defensa, (ii) exhibit lower levels of mortality that can occur before or after completion of embryogenesis in A. pisum containing APSE2- or APSE8-infected H. defensa, and (iii) exhibit no mortality in A. pisum containing phage-free H. defensa [36]. We thus assessed whether established cultures of H. defensa affected development of A. ervi eggs by focusing on the APSE3-infected AS3, APSE8-infected ZA17 and phage-free A2C strains. We did not bioassay APSE2-infected NY26 because of its low-temperature requirements and APSE2 contains a similar toxin gene cassette, including cdtB, as APSE8 [36].
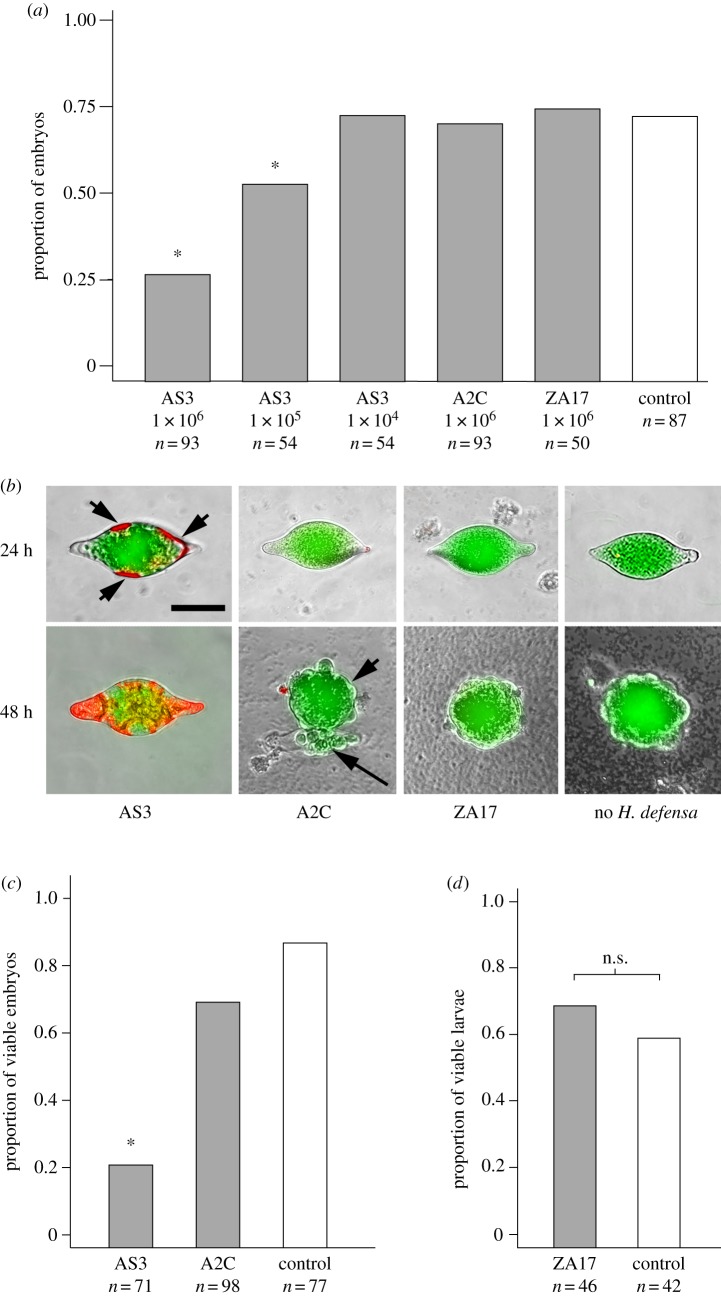
At a starting density of 1 × 106 bacteria ml−1, AS3 significantly reduced the proportion of newly laid eggs that developed into 128 cell stage embryos, whereas A2C and ZA17 did not (figure 2a). AS3 at 1 × 105 ml−1 also significantly reduced the proportion of A. ervi eggs that developed to the 128 cell stage but 1 × 104 bacteria ml−1 did not (figure 2a). Staining with propidium iodide showed that eggs co-cultured with AS3 initially exhibited reduced viability of cells forming the extraembryonic membrane at 24 h post-culture (figure 2b). This was followed by mortality of most cells in the extraembryonic membrane plus many embryonic cells by 48 h (figure 2b). By contrast, most embryos showed no loss of viability at 24 or 48 h when co-cultured with ZA17, A2C or no H. defensa (figure 2b). We next cultured AS3 or A2C (negative control) at a starting density of 1 × 106 ml−1 for 6 h in TC100 plus 10% FBS. We then removed the bacteria and added newly laid A. ervi eggs to the conditioned medium. Most A. ervi eggs in AS3-conditioned medium failed to develop to the 128 cell stage, while most eggs in A2C-conditioned or non-conditioned (control) medium developed to the 128 cell stage (figure 2c). To assess whether ZA17 affected A. ervi larvae, bacteria were co-cultured with first instars followed by assessment of larval viability after 24 and 48 h. Results revealed no reduction in viability when compared with larvae co-cultured with A2C or no bacteria (figure 2d).
Figure 2.
The AS3 strain of H. defensa disrupts development of A. ervi during early embryogenesis. (a) Proportion of A. ervi eggs that developed to the 128 cell stage after 36 h when co-cultured with the AS3, A2C or ZA17 strains with no H. defensa (Control). Starting densities ml−1 are indicated on the x-axis. Asterisks (*) indicate treatments that significantly differed from the no bacteria control (χ25 = 62.7, p < 0.0001 followed by post hoc pairwise Fisher exact tests). (b) Epiflourescent micrographs of A. ervi eggs co-cultured with AS3, A2C, ZA17 or no bacteria for 24 or 48 h followed by staining with propidium iodide (red) and acridine orange (green). Several cells in the extraembryonic membrane of eggs co-cultured with AS3 are stained by propidium iodide at 24 h (arrowheads) while many embryonic cells are stained at 48 h. By contrast, eggs cultured with A2C, Za17 or no H. defensa are only stained by acridine orange indicating no loss of viability. Eggs cultured with A2C, ZA17 or no bacteria exhibit little or no staining by propidium iodide. The morula (arrowhead) that ruptured out of the chorion (arrow) is visible at 48 h for the image shown under A2C, while only a morula is shown at 48 h under ZA17 and no H. defensa. Scale bar equals 200 µm. (c) Asterisk (*) indicates that the proportion of A. ervi eggs that developed to the 128 cell stage at 36 h was significantly lower in medium conditioned by AS3 than medium conditioned by A2C or medium with no H. defensa (χ22 = 61.2, p ≤ 0.0001 followed by post hoc pairwise Fisher exact tests). (d) Proportion of viable first instar A. ervi after 48 h did not differ (n.s.) when cultured with ZA17 or no H. defensa (χ21 = 0, p = 1).
(e). APSE3 confers anti-Aphidius ervi defence to the A2C strain
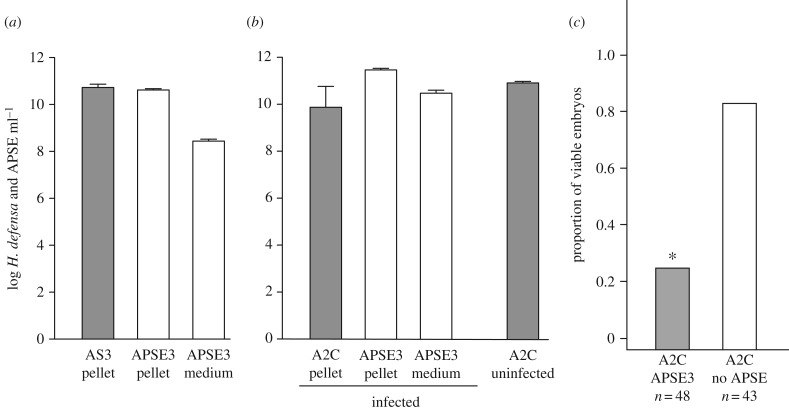
Comparative genomic data indicate that all APSEs persist as integrated proviruses in H. defensa genomes, while other results indicate that some APSE variants replicate [19,21,28]. Phylogenetic evidence also suggests that APSEs have horizontally moved among H. defensa strains [25]. However, no experimental evidence supports that any APSE is infectious or that phage acquisition alters the phenotype of strains in defence against parasitoids. As APSE3-infected AS3 disabled development of A. ervi, we assessed whether established cultures produced APSE3 that could infect phage-free A2C. First, we measured the abundance of bacteria and APSE3 in a 5-day old culture of AS3 by centrifuging 1 ml of medium to produce a bacterial pellet and supernatant. qPCR assays using DNA isolated from the pellet showed that copy number of APSE3 and AS3 were similar, which supported that approximately 1 copy of the APSE3 genome was present per bacterial cell (figure 3a). However, assays also showed that DNAse pretreated supernatants contained 2.7 × 108 copies of the APSE3 genome, which supported that established cultures of AS3 produced APSE3 particles (figure 3a). We, therefore, used this supernatant to inoculate cultures containing 1 × 107 A2C at an MOI of 0.1 to assess whether APSE3 could infect a phage-free strain of H. defensa. qPCR analysis 9 days post-infection detected a 10-fold higher copy number of the APSE3 versus A2C genome in DNA templates from pelleted bacterial cells, and 4 × 1010 copies of the APSE3 genome ml−1 in DNAse pre-treated supernatants (figure 3b). This outcome strongly supported that APSE3 infected A2C, which also resulted in production of APSE3 particles. However, it also indicated that infection was not fully fatal to A2C as large numbers of bacteria still survived. We, therefore, compared the effects of APSE3-infected A2C to non-infected A2C on development of A. ervi by adding 1 × 106 bacteria to culture wells containing 1 ml of medium and newly laid wasp eggs. Results showed that APSE3-infected A2C reduced the proportion of eggs that developed to the 128 cell stage relative to uninfected A2C (figure 3c). This reduction was also nearly identical to the effects of APSE3-infected AS3 (figure 3a).
Figure 3.
AS3 produces APSE3 that infects A2C and alters its ability to kill A. ervi embryos. (a) Genome copy number (mean ± 95% confidence intervals) of AS3 and APSE3 in 1 ml of an established 5-day old culture. Data generated from analysis of two independent cultures after centrifuging medium and isolating DNA from the bacterial pellet and DNase pretreated supernatant. (b) Genome copy of A2C and APSE3 in 1 ml of an A2C culture 9 days post-infection with virus from an AS3 culture. Data generated from analysis of three independent cultures after centrifuging medium and isolating DNA from the bacterial pellet and DNase pretreated supernatant. The left side of the graph shows mean copy number for A2C and APSE3 in the bacterial pellet, and APSE3 in medium pretreated with DNAse. The right side of the graph shows genome copy number for A2C in 1 ml of medium from 9-day old cultures that were not infected with APSE3. (c) Proportion of A. ervi eggs that developed to the 128 cell stage after 36 h when co-cultured with A2C that were infected 9 days earlier by APSE3 or that were uninfected. Both treatments were bioassayed by adding 1 × 106 bacteria to 1 ml of medium containing newly laid A. ervi eggs. The asterisk (*) above the bar for APSE3-infected A2C indicates this treatment significantly differed from the uninfected A2C control (χ21 = 66.5, p < 0.0001).
4. Discussion
No obligate and only a few facultative heritable symbionts of insects have been cultured outside of hosts. One difficulty in culturing these organisms is that heritable symbionts are generally restricted to host tissues and genome reduction results in many species having specialized requirements for growth that are difficult to mimic in vitro [43]. Another challenge is that the slow growth of many heritable symbionts elevates the risk of contamination when trying to establish primary cultures [43]. Most successes in culturing heritable insect symbionts have used insect cells that could serve as hosts for intracellular growth along with media developed for culture of insect cells rather than bacteria [40–42,44].
Given these precedents, we took a similar approach to culturing H. defensa. However, our priorities focused on identifying conditions in which strains of H. defensa that vary in levels of in vivo protection against A. ervi grow to comparable titres as in the aphid A. pisum. This allowed us to conduct experiments in vitro that would indicate whether symbiont products from different strains disable development of A. ervi. Consistent with in vivo results [20], in vitro assays indicate that APSE3-infected AS3 disable embryonic development of A. ervi. However, our in vitro data differ from prior in vivo studies by, to our knowledge showing for the first time that: (i) AS3 kills A. ervi eggs in the absence of any aphid-produced product, and (ii) mortality is owing to a soluble factor released from H. defensa/APSE that can enter wasp eggs prior to rupturing from the chorion as a morula. We also found that while cytotoxic to A. ervi this factor does not reduce the viability of TN5 cells, although TN5 cell growth was lower when co-cultured with H. defensa when compared to growth without bacteria. This finding is consistent with observations that wasps are killed in vivo, but aphids suffer relatively minor costs to infection with H. defensa [31].
Also consistent with prior in vivo studies [20], we found that the phage-free strain A2C did not affect A. ervi development. However, our results show that APSE3 from AS3 infects A2C, which then gains the ability to disable A. ervi development. This indicates that some APSE variants retain the capacity to infect H. defensa and horizontally transfer the factor(s) that kill A. ervi embryos. While host restriction inevitably leads to heritable symbiont decay over time [54], phage-mediated horizontal transfer of ecologically important traits between heritable symbionts provides a mechanism to maintain symbiont innovation. By contrast, our finding that ZA17 infected by APSE8 did not affect development of A. ervi eggs or larvae in vitro suggests interactions with aphid factors are required for disabling wasp development in vivo. Consistent with this, H. defensa infected with APSE8 confer little protection against A. ervi in some aphid genotypes but moderate protection in others [29].
Our results identified TC100 plus 10% FBS as the preferred medium for H. defensa growth. However, we could not establish primary cultures from aphids in this medium without TN5 cells, although the A2C, AS3 and ZA17 strains could be propagated as pure liquid cultures after multiple passages in vitro. We observed lower survival and/or growth of H. defensa in the other media and cell lines we tested including C6/36 and S2 cells. Thus, culture conditions probably account for the slow in vitro growth and intracellular persistence reported previously for H. defensa [40]. No cell lines have been established from aphids but our results are consistent with other studies showing that cells from non-host insect species benefit the culture of some heritable symbionts [40–44]. The near absence of intracellular H. defensa in TN5 cells strongly argues that infection of this cell line is not essential for persistence or growth of H. defensa. What TN5 cells provide that is required for initiating primary cultures and maintenance of the NY26 strain remains unclear. Unknown adaptations by A2C, AS3 and ZA17 after multiple passages in vitro could play a role in why these strains could be shifted to growth in pure liquid cultures. However, this shift did not alter the ability of the AS3 strain with APSE3 to kill A. ervi eggs or for APSE3 transfer to confer A. ervi-killing activity to the A2C strain. The factors that underlie the requirement of NY26 for culture at lower temperatures with TN5 cells will also require further study.
In summary, our results provide an essential foundation for identifying the H. defensa-associated molecules that provide protection to aphids against parasitoids. They also provide new opportunities for understanding APSE-H. defensa interactions, which are of interest given our results show that APSE3 replicates but does not cause high level mortality of H. defensa. The ability to propagate particular strains of H. defensa in pure cultures also provides opportunities for characterizing genomic and transcriptome strain variation and potentially manipulating this symbiont genetically.
Supplementary Material
Acknowledgements
We thank Mary Ard at the University of Georgia Microscopy facility for assistance with transmission electron microscopy.
Ethics
The authors declare that this research (i) has not been published previously elsewhere, (ii) was not misconducted, (iii) did not involve animal treatment, and (iv) did not involve plagiarism in any form.
Data accessibility
In vitro culture media tested, PCR primers used in the study, diagnostic PCR results and microscopy images are provided as the electronic supplementary material. Datasets used to generate figures 1a–d, 2a,c,d and 3a–c are also provided as electronic supplementary material.
Authors' contributions
M.R.S. and K.M.O. conceived the study; M.R.S., J.W.B., G.C. and K.M.O. designed the study; J.W.B, G.C. and M.R.S. collected data; J.W.B, G.C. and M.R.S analysed data. M.R.S. drafted the manuscript with J.W.B, G.C. and K.M.O. contributing to writing of the paper.
Competing interests
The authors declare no competing financial interests.
Funding
This research was supported by a grant from the US National Science Foundation (IOS 1256794) to K.M.O and M.R.S.
References
- 1.Moran NA, McCutcheon JP, Nakabachi A. 2008. Genomics and evolution of heritable bacterial symbionts. Annu. Rev. Genet. 42, 165–190. ( 10.1146/Annurev.Genet.41.110306.130119) [DOI] [PubMed] [Google Scholar]
- 2.Moran NA. 2007. Symbiosis as an adaptive process and source of phenotypic complexity. Proc. Natl Acad. Sci. USA 104, 8627–8633. ( 10.1073/pnas.0611659104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliver KM, Martinez AJ. 2014. How resident microbes modulate ecologically-important traits of insects. Curr. Opin. Insect Sci. 4, 1–7. ( 10.1016/j.cois.2014.08.001) [DOI] [PubMed] [Google Scholar]
- 4.Douglas A. 1998. Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annu. Rev. Entomol. 43, 17–37. ( 10.1146/annurev.ento.43.1.17) [DOI] [PubMed] [Google Scholar]
- 5.Moran NA, Russell JA, Koga R, Fukatsu T. 2005. Evolutionary relationships of three new species of Enterobacteriaceae living as symbionts of aphids and other insects. Appl. Environ. Microbiol. 71, 3302–3310. ( 10.1128/AEM.71.6.3302-3310.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliver KM, Degnan PH, Burke GR, Moran NA. 2010. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu. Rev. Entomol., 55, 247–266. ( 10.1146/annurev-ento-112408-085305) [DOI] [PubMed] [Google Scholar]
- 7.Brisson JA, Stern DL. 2006. The pea aphid, Acyrthosiphon pisum: an emerging genomic model system for ecological, developmental and evolutionary studies. Bioessays 28, 747–755. ( 10.1002/bies.20436) [DOI] [PubMed] [Google Scholar]
- 8.Asplen MK, Bano N, Brady CM, Desneux N, Hopper KR, Malouines C, Oliver KM, White JA, Heimpel GE. 2014. Specialisation of bacterial endosymbionts that protect aphids from parasitoids. Ecol. Entomol. 39, 736–739. ( 10.1111/een.12153) [DOI] [Google Scholar]
- 9.Heyworth E, Ferrari J. 2015. A facultative endosymbiont in aphids can provide diverse ecological benefits. J. Evol. Biol. 28, 1753–1760. ( 10.1111/jeb.12705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Łukasik P, van Asch M, Guo H, Ferrari J, Godfray CJ. 2013. Unrelated facultative endosymbionts protect aphids against a fungal pathogen. Ecol. Lett. 16, 214–218. ( 10.111/ele.12031) [DOI] [PubMed] [Google Scholar]
- 11.Montllor CB, Maxmen A, Purcell AH. 2002. Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol. Entomol. 27, 189–195. ( 10.1046/j.1365-2311.2002.00393.x) [DOI] [Google Scholar]
- 12.Oliver KM, Russell JA, Moran NA, Hunter MS. 2003. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl Acad. Sci. USA 100, 1803–1807. ( 10.1073/pnas.0335320100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scarborough CL, Ferrari J, Godfray HC.J. 2005. Aphid protected from pathogen by endosymbiont. Science 310, 1781 ( 10.1126/science.1120180) [DOI] [PubMed] [Google Scholar]
- 14.Schmid M, Sieber R, Zimmermann YS, Vorburger C. 2012. Development, specificity and sublethal effects of symbiont-conferred resistance to parasitoids in aphids. Funct. Ecol. 26, 207–215. ( 10.1111/j.1365-2435.2011.01904.x) [DOI] [Google Scholar]
- 15.Vorburger C, Gehrer L, Rodriguez P. 2010. A strain of the bacterial symbiont Regiella insecticola protects aphids against parasitoids. Biol. Lett. 6, 109–111. ( 10.1098/rsbl.2009.0642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner SM, Martinez AJ, Ruan YM, Kim KL, Lenhart PA, Dehnel AC, Oliver KM, White JA, Carroll S. 2015. Facultative endosymbionts mediate dietary breadth in a polyphagous herbivore. Funct. Ecol. 29, 1402–1410. ( 10.1111/1365-2435.12459) [DOI] [Google Scholar]
- 17.Metcalf JA, Bordenstein SR. 2012. The complexity of virus systems: the case of endosymbionts. Curr. Opin. Microbiol. 15, 546–552. ( 10.1016/j.mib.2012.04.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moran NA, Degnan PH, Santos SR, Dunbar HE, Ochman H. 2005. The players in a mutualistic symbiosis: insects, bacteria, viruses, and virulence genes. Proc. Natl Acad. Sci. USA 102, 16 919–16 926. ( 10.1073/pnas.0507029102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Wilk F, Dullemans AM, Verbeek M, van den Heuvel J. 1999. Isolation and characterization of APSE-1, a bacteriophage infecting the secondary endosymbiont of Acyrthosiphon pisum. Virology 262, 104–113. ( 10.1006/viro.1999.9902) [DOI] [PubMed] [Google Scholar]
- 20.Oliver KM, Degnan PH, Hunter MS, Moran NA. 2009. Bacteriophages encode factors required for protection in a symbiotic mutualism. Science 325, 992–994. ( 10.1126/science.1174463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weldon S, Strand M, Oliver K. 2013. Phage loss and the breakdown of a defensive symbiosis in aphids. Proc. R. Soc. B 280, 20122103 ( 10.1098/rspb.2012.2103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henry LM, Maiden MCJ, Ferrari J, Godfray HCJ, Bourke A. 2015. Insect life history and the evolution of bacterial mutualism. Ecol. Lett. 18, 516–525. ( 10.1111/ele.12425) [DOI] [PubMed] [Google Scholar]
- 23.Zytynska SE, Weisser WW. 2016. The natural occurrence of secondary bacterial symbionts in aphids. Ecol. Entomol. 41, 13–26. ( 10.1111/een.12281) [DOI] [Google Scholar]
- 24.Oliver KM, Moran NA, Hunter MS. 2005. Variation in resistance to parasitism in aphids is due to symbionts not host genotype. Proc. Natl Acad. Sci. USA 102, 12 795–12 800. ( 10.1073/pnas.0506131102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Degnan PH, Moran NA. 2008. Evolutionary genetics of a defensive facultative symbiont of insects: exchange of toxin-encoding bacteriophage. Mol. Ecol. 17, 916–929. ( 10.1111/j.1365-294X.2007.03616.x) [DOI] [PubMed] [Google Scholar]
- 26.Henry LM, Peccoud J, Simon J-C, Hadfield JD, Maiden MJ, Ferrari J, Godfray HCJ. 2013. Horizontally transmitted symbionts and host colonization of ecological niches. Curr. Biol. 23, 1713–1717. ( 10.1016/j.cub.2013.07.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell JA, et al. 2013. Uncovering symbiont-driven genetic diversity across North American pea aphids. Mol. Ecol. 22, 2045–2059. ( 10.1111/mec.12211) [DOI] [PubMed] [Google Scholar]
- 28.Degnan PH, Moran NA. 2008. Diverse phage-encoded toxins in a protective insect endosymbiont. Appl. Environ. Microbiol. 74, 6782–6791. ( 10.1128/aem.01285-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez AJ, Doremus MR, Kraft LJ, Kim KL, Oliver KM, Fenton A. 2017. Multi-modal defenses in aphids offer redundant protection and increased costs likely impeding a protective mutualism. J. Anim. Ecol. 70, 87 ( 10.1111/1365-2656.12675) [DOI] [PubMed] [Google Scholar]
- 30.Hufbauer RA. 2002. Aphid population dynamics: does resistance to parasitism influence population size? Ecol. Entomol. 27, 25–32. ( 10.1046/j.1365-2311.2002.0379a.x) [DOI] [Google Scholar]
- 31.Oliver KM, Campos J, Moran NA, Hunter MS. 2008. Population dynamics of defensive symbionts in aphids. Proc. R. Soc. B 275, 293–299. ( 10.1098/rspb.2007.1192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schellhorn NA, Kuhman TR, Olson AC, Ives AR. 2002. Competition between native and introduced parasitoids of aphids: nontarget effects and biological control. Ecology 83, 2745–2757. ( 10.2307/3072012) [DOI] [Google Scholar]
- 33.Smith AH, et al. 2015. Patterns, causes and consequences of defensive microbiome dynamics across multiple scales. Mol. Ecol. 24, 1135–1149. ( 10.1111/mec.13095) [DOI] [PubMed] [Google Scholar]
- 34.Oliver KM, Smith AH, Russell JA, Clay K. 2014. Defensive symbiosis in the real world: advancing ecological studies of heritable, protective bacteria in aphids and beyond. Funct. Ecol. 28, 341–355. ( 10.1111/1365-2435.12133) [DOI] [Google Scholar]
- 35.Doremus MR, Oliver KM. 2017. Aphid heritable symbiont exploits defensive mutualism. Appl. Environ. Microbiol. 83, e03276-16 ( 10.1128/aem.03276-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez AJ, Weldon SR, Oliver KM. 2014. Effects of parasitism on aphid nutritional and protective symbioses. Mol. Ecol. 23, 1594–1607. ( 10.1111/mec.12550) [DOI] [PubMed] [Google Scholar]
- 37.Oliver KM, Noge K, Huang EM, Campos JM, Becerra JX, Hunter MS. 2012. Parasitic wasp responses to symbiont-based defense in aphids. BMC Biol. 10, 11 ( 10.1186/1741-7007-10-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez AJ, Ritter SG, Doremus MR, Russell JA, Oliver KM. 2014. Aphid-encoded variability in susceptibility to a parasitoid. BMC Evol. Biol. 14, 127 ( 10.1186/1471-2148-14-127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLean AHC, Godfray HCJ. 2015. Evidence for specificity in symbiont-conferred protection against parasitoids. Proc. R. Soc. B 282, 20150977 ( 10.1098/rspb.2015.0977) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Darby AC, Chandler SM, Welburn SC, Douglas AE. 2005. Aphid-symbiotic bacteria cultured in insect cell lines. Appl. Environ. Microbiol. 71, 4833–4839. ( 10.1128/aem.71.8.4833-4839.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hypsa V, Dale C. 1997. In vitro culture and phylogenetic analysis of ‘Candidatus Arsenophonus triatominarum,’ an intracellular bacterium from the triatomine bug, Triatoma infestans. Int. J. Syst. Bacteriol. 47, 1140–1144. ( 10.1099/00207713-47-4-1140) [DOI] [PubMed] [Google Scholar]
- 42.Matthew CZ, Darby AC, Young SA, Hume LH, Welburn SC. 2005. The rapid isolation and growth dynamics of the tsetse symbiont Sodalis glossinidius. FEMS Microbiol. Lett. 248, 69–74. ( 10.1016/j.femsle.2005.05.024) [DOI] [PubMed] [Google Scholar]
- 43.Pontes MH, Dale C. 2006. Culture and manipulation of insect facultative symbionts. Trends Microbiol. 14, 406–412. ( 10.1016/j.tim.2006.07.004) [DOI] [PubMed] [Google Scholar]
- 44.Welburn SC, Maudlin I, Ellis DS. 1987. In vitro cultivation of Rickettsia-like-organisms from Glossina spp. Ann. Trop. Med. Parasitol. 81, 331–335. ( 10.1080/00034983.1987.11812127) [DOI] [PubMed] [Google Scholar]
- 45.Sabri A, Leroy P, Haubruge E, Hance T, Frere I, Destain J, Thonart P. 2011. Isolation, pure culture and characterization of Serratia symbiotica sp nov., the R-type of secondary endosymbiont of the black bean aphid Aphis fabae. Int. J. Syst. Evol. Microbiol. 61, 2081–2088. ( 10.1099/ijs.0.024133-0) [DOI] [PubMed] [Google Scholar]
- 46.Degnan PH, Yu Y, Sisneros N, Wing RA, Moran NA. 2009. Hamiltonella defensa, genome evolution of protective bacterial endosymbiont from pathogenic ancestors. Proc. Natl Acad. Sci. USA 106, 9063–9068. ( 10.1073/pnas.0900194106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burke GR, Thomas SA, Eum JH, Strand MR, Strand MR. 2013. Mutualistic polydnaviruses share essential replication gene functions with pathogenic ancestors. PLoS Pathog. 9, e1003348 ( 10.1371/journal.ppat.1003348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laughton AM, Garcia JR, Altincicek B, Strand MR, Gerardo NM. 2011. Characterisation of immune responses in the pea aphid, Acyrthosiphon pisum. J. Insect. Physiol. 57, 830–839. ( 10.1016/j.jinsphys.2011.03.015) [DOI] [PubMed] [Google Scholar]
- 49.Baumann P. 2005. Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu. Rev. Microbiol. 59, 155–189. ( 10.1146/annurev.micro.59.030804.121041) [DOI] [PubMed] [Google Scholar]
- 50.Grenier S. 2012. Artificial rearing of entomophagous insects, with emphasis on nutrition and parasitoids: general outlines from personal experience. Karaelmas Sci. Eng. J. 2, 1–12. ( 10.7212/zkufbd.v2i2.97) [DOI] [Google Scholar]
- 51.Pennacchio F, Strand MR. 2006. Evolution of developmental strategies in parasitic hymenoptera. Annu. Rev. Entomol. 51, 233–258. ( 10.1146/annurev.ento.51.110104.151029) [DOI] [PubMed] [Google Scholar]
- 52.Thompson SN. 1999. Nutrition and culture of entomophagous insects. Annu. Rev. Entomol. 44, 561–592. ( 10.1146/annurev.ento.44.1.561) [DOI] [PubMed] [Google Scholar]
- 53.Grbic M, Strand MR. 1998. Shifts in the life history of parasitic wasps correlate with pronounced alterations in early development. Proc. Natl Acad. Sci. USA 95, 1097–1101. ( 10.1073/pnas.95.3.1097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bennett GM, Moran NA. 2015. Heritable symbiosis: the advantages and perils of an evolutionary rabbit hole. Proc. Natl Acad. Sci. USA 112, 10 169–10 176. ( 10.1073/pnas.1421388112) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
In vitro culture media tested, PCR primers used in the study, diagnostic PCR results and microscopy images are provided as the electronic supplementary material. Datasets used to generate figures 1a–d, 2a,c,d and 3a–c are also provided as electronic supplementary material.