Abstract
It is current belief that numbers of CD8+ memory T lymphocytes in the memory phase of an immune response are maintained by homeostatic proliferation. Here, we compare the proliferation of CD8+ memory T lymphocytes, generated by natural infections and by intentional immunization, in spleen and bone marrow (BM). Fifty percent of CD8+ memory T lymphocytes in the spleen are eliminated by cyclophosphamide within 14 days, indicating that numbers of at least 50% of splenic CD8+ memory T lymphocytes are maintained by proliferation. The numbers of CD8+ memory T lymphocytes in the BM, however, were not affected by cyclophosphamide. This stability was independent of circulating CD8+ memory T cells, blocked by FTY720, showing that BM is a privileged site for the maintenance of memory T lymphocytes, as resident cells, resting in terms of proliferation.
Keywords: Bone marrow, CD8+ memory T lymphocytes, Cyclophosphamide, Homeostatic proliferation, Tissue resident memory
Introduction
At present, it is unclear how numbers of CD8+ memory T lymphocytes are maintained over time in the memory phase of an immune response, i.e. in the apparent absence of antigen. The current view is that numbers of CD8+ memory T cells are maintained by homeostatic proliferation, a proliferation induced by cytokines like interleukin‐15 (IL‐15) 1, 2, 3 compensating a gradual loss of memory cells by apoptosis 4, 5, 6. This view is based on analysis of the proliferation of CD8+ memory T cells, isolated from the spleen, labeled with CFSE and adoptively transferred from one mouse to another. The cells home to a variety of organs, and 15 days later, about 30 to 50% of the cells have divided once or twice, with 50% in the bone marrow (BM) 7. This corresponds well to the frequencies of endogenous CD8+ memory T cells incorporating BrdU into their DNA, as a measure of proliferation, which is also about 50% in 14 days 4. From these analyses, it has been concluded that CD8+ memory T lymphocytes in spleen and BM are maintained by homeostatic proliferation 7, 8. Conflicting evidence comes from the analysis of Ki‐67 expression in CD8+ memory T cells. Ki‐67 is expressed by cycling cells, in the G1 to M phases of cell cycle. Cells resting in the G0 phase of cell cycle do not express Ki‐67 9. According to Ki‐67 staining, more than 95%, in spleen and BM of mice 10, and 98 to 99.5% of CD8+ memory T cells, in blood and BM of humans 11 are in G0 of cell cycle at any given time point. In line with this, more than 99% of the cells are not in the S/G2/M phases of cell cycle, according to PI staining of their DNA, discriminating cells in G0 or G1 from cells in the S/G2/M phases of cell cycle 8, 10. With respect to the reported proliferation of CD8+ memory T cells, as measured by BrdU incorporation, it has been shown that BrdU induces proliferation of CD8+ memory T cells, as reflected by Ki‐67 expression and entry of cells into S/G2/M phases of the cell cycle, according to PI staining 10.
Here, we use cyclophosphamide (CyP) to analyze proliferation of CD8+ memory T cells in the spleen and in the BM, directly. CyP is nitrogen mustard that adds alkyl groups to DNA, resulting in DNA cross‐linking. Cross‐linked DNA cannot be replicated efficiently and cells attempting to divide die by apoptosis 12.
Results and discussion
Activated CD8+ T lymphocytes of acute immune reactions are eliminated by cyclophosphamide
To show that proliferating CD8+ T lymphocytes are eliminated by CyP, we induced a secondary immune reaction to ovalbumin (Ova) in C57BL/6 mice, and treated them with CyP on days 0 and 4 of the reaction, then enumerated antigen‐specific CD8+ T lymphocytes on day 7 (Fig. 1A). CyP decreased the absolute numbers of B220+ B cells by 60% in the spleen and 80% in the BM (Fig. 1B), demonstrating that CyP was delivered to both organs efficiently 13, 14, 15. CD8+CD44+ memory T cells as such were reduced by 50% in the spleen, but not in the BM (Fig. 1C). CD8+ memory T cells of the immune response to Ova were labeled by fluorescent H2Kb‐SIINFEKL pentamers. The frequencies of SIINFEKL‐binding CD8+CD44+ T lymphocytes were reduced in the spleen and BM, in the example displayed by 78% in the spleen and 58.2% in the BM (Fig. 1D), although less cells were detectable in the BM than in the spleen at this time point. Absolute numbers of SIINFEKL‐specific CD8+CD44+ T lymphocytes were reduced from 10.97 × 103 (± 3.58 × 103 SEM) to 1.01 × 103 (± 0.17 × 103 SEM), i.e. by 90.79% in the spleen, and from 4.29 × 103 (± 0.73 × 103 SEM) to 2.20 × 103 (± 0.41 × 103 SEM), i.e. by 48.73% in the BM (Fig. 1E). As expected, SIINFEKL‐specific CD8+ memory T cells in cell cycle, expressing Ki‐67, were eliminated entirely (Fig. 1F).
Figure 1.
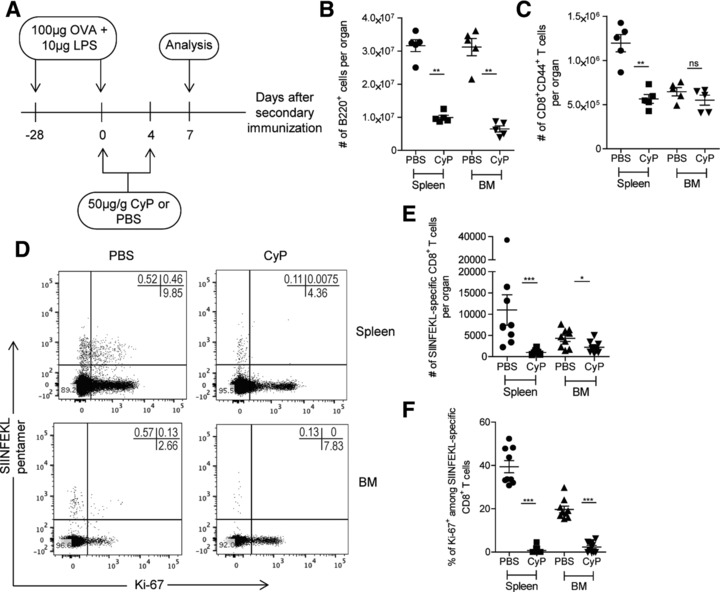
Proliferating CD8+ T lymphocytes are eliminated by CyP in acute immune reactions. (A) Experimental setup: CyP was applied on days 0 and 4 of a secondary immune response to Ova. Numbers of specific CD8+ T cells were determined in spleen and BM on day 7 of the secondary immune response. (B) Absolute numbers of B220+ cells in spleen and BM upon administration of CyP or vehicle. (C) Absolute numbers of CD8+CD44+ T cells in spleen and BM upon administration of CyP or vehicle. (D) Representative dot plots of SIINFEKL‐pentamer versus Ki‐67 gated on CD4−CD8+CD44+ viable cells. (E) Absolute numbers of SIINFEKL‐specific CD8+ T cells in spleen and BM upon administration of CyP or vehicle. (F) Frequencies of Ki‐67+ among SIINFEKL‐specific CD8+ T cells in spleen and BM upon administration of CyP or vehicle. Data in (B) and (C) are representative of two independent experiments, each with four to five mice per group. Data in (E) and (F) represent pooled results from two independent experiments, each with four to five mice per group. Data are presented as mean ± SEM. *p < 0.05, ** p < 0.01, *** p < 0.001, as determined by two‐tailed Student's t test.
CD8+ memory T cells of the BM are not eliminated by cyclophosphamide
Next, we investigated the proliferation of CD8+ memory T cells in the memory phase of an immune response. To this extent, we treated C57BL/6 mice immunized twice with Ova, 3 months after onset of the secondary immune reaction, on days 90, 94, 98, and 102 with CyP, and enumerated their CD8+ memory T cells on day 105 (Fig. 2A). Again, we confirmed delivery of CyP to the BM by analyzing the ablation of B220+ B cells, which were significantly reduced by 70% in spleen and 50% in BM (Fig. 2B). Numbers of SIINFEKL‐specific CD8+ memory T cells in the BM were not significantly affected by CyP, with 1921 (± 234.8 SEM) in CyP treated versus 2113 (± 336.5 SEM) in untreated mice. On the other hand, in the spleen, the numbers of SIINFEKL‐specific T cells were significantly reduced by 70%, from 1352 (± 210.2 SEM) to 411.5 (± 44.14 SEM) (Fig. 2C). In the BM, the numbers of CD8+CD44+ memory T lymphocytes as such were also not affected by the CyP treatment, with 1.31 × 106 (± 0.92 × 105 SEM) versus 1.23 × 106 (± 1.12 × 105 SEM) in treated versus untreated animals, respectively. In contrast, in the spleen numbers of CD8+CD44+ memory T cells were significantly reduced by 46% from 1.10 × 106 (± 0.59 × 105 SEM) to 0.59 × 106 (± 0.41 × 105 SEM) (Fig. 2D). In the spleen, CyP did reduce the frequency of CD8+CD44+Ki‐67+ memory T cells from 13.7 (± 1.14 SEM) to 5.7% (± 0.77 SEM) (Fig. 2E and F). Since CyP eliminates about 50% of the cells within 14 days, apparently 80% of the cells eliminated by CyP, i.e. 40% of all cells, had switched from proliferative rest (Ki‐67−) to proliferation within these 14 days. Interestingly, in the BM even cells expressing Ki‐67 were not CyP sensitive, i.e. their frequency did not change significantly (Fig. 2F). The DNA of CD8+CD44+ memory T cells had efficiently been alkylated by CyP, since stimulating them with anti‐CD3/anti‐CD28 revealed that they were no longer able to expand (Fig. 2G).
Figure 2.
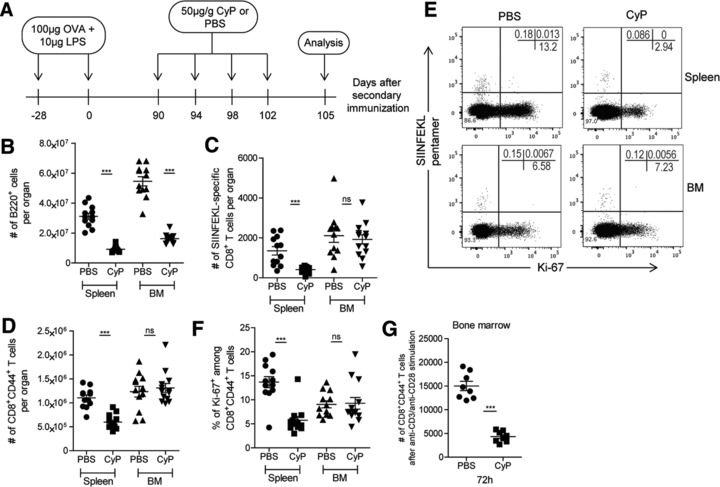
CD8+ memory T cells of the spleen but not those of BM are eliminated by CyP in the memory phase of immune responses. (A) Experimental setup: CyP was applied on days 90, 94, 98, and 102 after induction of a secondary immune response to Ova. Numbers of specific and total CD8+ T cells were determined in spleen and BM on day 105, i.e. on day 15 after the start of treatment with CyP. (B) Absolute numbers of B220+ cells in spleen and BM upon administration of CyP or vehicle. (C) Absolute numbers of SIINFEKL‐specific CD8+ T cells in spleen and BM upon administration of CyP or vehicle. (D) Absolute numbers of CD8+CD44+ T cells in spleen and BM upon administration of CyP or vehicle. (E) Representative dot plots of SIINFEKL‐pentamer versus Ki‐67 gated on CD4−CD8+CD44+ viable cells. (F) Frequencies of Ki‐67+ among CD8+CD44+ T cells in spleen and BM upon administration of CyP or vehicle. (G) Numbers of CD8+CD44+ T cells sorted from vehicle‐ or CyP‐treated mice after in vitro anti‐CD3/anti‐CD28 stimulation at 72 h. Data from (A) to (F) represent pooled results from two independent experiments, each with five to eight mice per group. Data in (G) represent one independent experiment with four mice per group with technical duplicates. Data are presented as mean ± SEM. *** p < 0.001, as determined by two‐tailed Student's t test.
Maintenance of CD8+ memory T cells in the BM is independent of their circulating counterpart
To rule out that, in the BM and in the presence of CyP, numbers of proliferating and dying CD8+ memory T cells were compensated by immigrating CD8+ memory T cells, we blocked circulation by FTY720 16, 17, a sphingosine‐1‐phosphate analog (Fig. 3A). FTY720 reduced the frequency of CD3+CD8+ T lymphocytes in the blood from 38.7 to 1.78% in the example given in 3B (left panel), and the absolute numbers of circulating CD3+CD8+ T lymphocytes to 2 cells/μL of blood (± 1.26 SEM for FTY720 + CyP or ± 1.06 SEM for FTY720 + PBS) as opposed to 190 (± 52.84 SEM) or 166 (± 45.39 SEM) cells in the controls (Fig. 3B, right panel). In FTY720‐treated animals, CyP significantly reduced the numbers of CD8+CD44+ memory T cells of the spleen by 48% from 0.6 × 106 (± 1.07 × 105) to 0.27 × 106 (± 0,29 × 105), while the numbers of CD8+CD44+ memory T cells in the BM did not significantly differ (0.9 × 106 ± 49.79 × 103 SEM versus 1.05 × 106 ± 83.98 × 103 SEM) (Fig. 3C). We also did not observe significant differences in the absolute numbers of SIINFEKL‐specific CD8+ memory T cells, with and without FTY720 and with and without CyP (Fig. 3D). These results suggest that CD8+ memory T cells of an intentional immune response, but also the global population of CD8+ memory T cells of the BM, as generated by natural infections, are resting in terms of proliferation and residents of the BM, at least for the time of observation. In the BM, around 30% of the CD8+ memory cells express CD69 10 and around 70% do not express CCR7 (data not shown). It has been proposed that CD69 marks “tissue‐resident” memory T cells 18, 19, and that CCR7− CD8+ memory T cells are maintained by IL‐15 induced proliferation 3. The present analysis shows that both CD69+ and CD69− and also CCR7+ and CCR7− CD8+ memory T cells of the BM are resting and resident.
Figure 3.
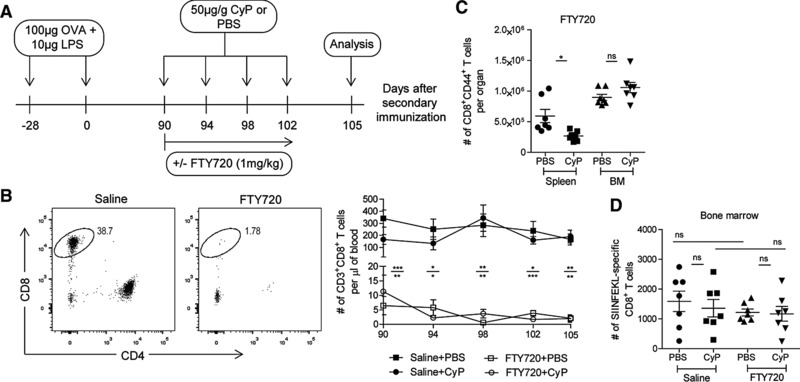
Maintenance of resting CD8+ memory T cells in the BM is independent of circulating CD8+ memory T cells. (A) Experimental setup: CyP was applied on days 90, 94, 98, and 102 after induction of a secondary immune response to Ova. At the same time, mice were administered FTY720 during the window of CyP treatment. Numbers of CD8+ memory T cells were determined in blood, spleen, and BM on day 105, i.e. on day 15 after the start of the cotreatment with CyP and FTY720. (B) Left: representative dot plots of CD8 versus CD4 gated on CD3+ viable cells, blood. Right: absolute numbers of CD3+CD8+ T cells in the blood of FTY720‐ or saline‐treated animals upon administration of CyP or vehicle. Upper * refers to saline + PBS versus FTY720 + PBS, lower * refers to saline‐CyP versus FTY720‐CyP. (C) Absolute numbers of CD8+CD44+ T cells in spleen and BM upon administration of CyP or vehicle in FTY720‐treated animals. (D) Absolute numbers of SIINFEKL‐specific CD8+ T cells in BM upon administration of CyP or vehicle in FTY720‐ or saline‐treated animals. Data represent pooled results from two independent experiments, each with three to four mice per group. Data are presented as mean ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001, as determined by one‐way ANOVA (D) or two‐tailed Student's t test.
Becker et al. had observed prominent proliferation of CD8+ memory T cells in all tissues, including spleen and BM, after adoptive transfer of splenic CD8+ memory T cells, labeled with CFSE, into naïve mice 7. It is interesting to note that some of these splenic CD8+ memory T cells after transfer efficiently homed to all tissues analyzed, and were readily detectable in lung, liver, lymph nodes, BM, and spleen. The continued proliferation of these adoptively transferred splenic CD8+ memory T cells over 15 to 25 days in spleen and BM of the recipients is in line with the present finding that some splenic CD8+ memory T cells are maintained by proliferation in vivo. The present data also indicate that only 50% of the splenic CD8+ memory T cells are maintained by proliferation. Treatment of mice with CyP for 7 or 14 days both reduced the numbers of splenic CD8+ memory T cells by 50% (Figs. 1C and 2D), suggesting that the other 50% are resistant to CyP, i.e. resting in terms of proliferation.
Concluding remarks
In summary, the present data show that the BM is a privileged site for the maintenance of CD8+ memory T lymphocytes as cells resting in terms of proliferation. Blocking circulation of memory CD8+ T cells with FTY720 also suggests that most if not all BM CD8+ memory T cells are resident, at least for the time of observation. The data support the concept of “resting and resident” memory T cells rather than the concept of “cycling and circulating” memory T cells. The data also suggest that the reactive immunological memory of patients treated with CyP can be severely impaired. Like long‐lived memory plasma cells 20, 21, CD8+ memory T cells of the BM are resistant to CyP, in their resting state. If reactivated, however, they will not be able to mount an efficient secondary immune response.
Materials and methods
Mice
All mice were purchased from Charles River, Germany and maintained under SPF conditions at the mouse facility of German Rheumatism Research Centre, Berlin. Experiments were performed according to institutional guidelines and German Federal laws on animal protection. Eight‐week‐old C57BL/6 mice were subcutaneously immunized twice at 4‐week intervals with 100 μg OVA + 10 μg LPS (Salmonella minnesota, Invivogen). Mice were then intravenously administered with CyP (50 mg/kg) or PBS either for 1 week at 4‐day intervals starting on the day of the challenge or for 2 weeks at 4‐day intervals, starting 90 days after the challenge. Where indicated, CyP‐ or PBS‐treated mice were fed with 1 mg/kg FTY720 (Cayman Chemical) or saline control dissolved in drinking water. Water was changed every 3 days. Analysis was carried out 3 days after the last CyP or PBS injection.
Flow cytometry
Single‐cell suspensions were obtained from spleen and BM. After lysis of erythrocytes, cell counts were performed by MACSQuant (Miltenyi, Bergisch‐Gladbach). For cell staining, cells were first incubated with 10 μg/mL anti‐FCγRII/III (2.4G2) in FACS buffer (PBS/0.1% BSA/2mM EDTA) for 10 minutes at 4°C. Cells were then incubated in PBS for 10 minutes at room temperature with H‐2Kb‐SIINFEKL pentamer (Proimmune, Oxford). Cells were stained for 15 minutes at 4°C with anti‐CD3 (145‐2C11), anti‐CD4 (RM4.4), anti‐CD44 (IM7), anti‐B220 (RA3.6B2), and anti‐CD8α (53‐6.72). Cells were then fixed overnight in Foxp3 fixation/permeabilization working solution according to the manufacturer's instructions (eBioscience, San Diego, CA) and finally stained intracellularly at room temperature for 45 minutes with anti‐Ki67 (B56). Viability of cells was assessed by LIVE/DEAD Fixable Dead Cell Stain (Thermo Fisher Scientific, Waltham, MA). Stained samples were analyzed on a BD Fortessa flow cytometer (BD Biosciences, San Jose, CA) or MACSQuant (Miltenyi, Bergisch‐Gladbach, Germany). Flow cytometric data were analyzed by FlowJo software (FlowJo LLC, Ashland).
In vitro stimulation assay
CD8+CD44+ memory T cells were immunomagnetically sorted from the BM of mice treated with CyP or PBS, according to manufacturer´s instructions (Miltenyi). A total of 5 × 103 CD8+CD44+ memory T cells were then plated in technical duplicates and stimulated with plate‐bound anti‐CD3 and anti‐CD28 (3 μg/mL) antibodies (BD Biosciences). Shortly before 48 h, cells were taken out from the stimuli and transferred to a new plate. Analysis was carried out at 72 h from the initial stimulation.
Absolute cell number calculation per organ
For mouse spleen, the whole organs were processed and the total numbers of T cells were calculated based on the cell numbers in a defined volume determined by flow cytometry (MACSQuant, Miltenyi). For mouse BM, individual femurs were individually processed and the total numbers of T cells were determined in the same way. A single femur was estimated to harbor 6.3% of total BM, therefore the conversion factor 15.87 was used to enumerate total cell numbers in the BM of an individual mouse 22.
Conflict of interest
The authors declare no commercial or financial conflict of interest.
Abbreviation
- CyP
cyclophosphamide
Supporting information
Peer review correspondence
Supporting Information
Acknowledgments
We want to thank Tuula Geske, Heidi Hecker‐Kia, and Heidi Schliemann for expert technical help, Toralf Kaiser and Jenny Kirsch as operators of the flow cytometry core facility (FCCF), Patrick Thiemann and Manuela Ohde for assistance with animal care, and Qingyu Cheng for providing cyclophosphamide. This work was supported by European Research Council Advanced Grant IMMEMO (ERC‐2010‐AdG.20100317 Grant 268987; to A.R.) and by DFG Priority Program Immunobone 1468 to A.R. and H.D.C. F.S. was supported by Osteoimmune, a FP7 Marie Curie Initial Training Network (FP7‐PEOPLE‐2011‐ITN‐289150). M‐F.M, P.M., and M.M. are supported by the state of Berlin and the "European Regional Development Fund (ERDF 2014–2020, EFRE 1.8/11, Deutsches Rheuma‐Forschungszentrum). P.M. was supported by EUTRAIN, a FP7 Marie Curie Initial Training Network for Early Stage Researchers funded by the European Union (FP7‐PEOPLE‐2011‐ITN‐289903). The DRFZ is a Leibniz Institute. F.S. and Ö.S.A. equally contributed to the work, designed and performed the experiments, analyzed and interpreted the data and wrote the manuscript. M.M., P.M., M‐F.M, S.H., H‐D.C. and K.T. provided scientific suggestions. A.R. designed the experiments, conceived the study, and wrote the manuscript.
See accompanying commentary by Nolte et al. https://doi.org/10.1002/eji.201747249
References
- 1. Becker, T. C. et al, Interleukin 15 is required for proliferative renewal of virus‐specific memory CD8 T cells. J. Exp. Med. 2002. 195: 1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Surh, C. D. and Sprent, J. , Homeostasis of naive and memory T cells. Immunity 2008. 29: 848–862. [DOI] [PubMed] [Google Scholar]
- 3. Jung, Y. W. , Kim, H. G. , Perry, C. J. and Kaech, S. M. , CCR7 expression alters memory CD8 T‐cell homeostasis by regulating occupancy in IL‐7‐ and IL‐15‐dependent niches. Proc. Natl. Acad. Sci. U. S. A. 2016. 113: 8278–8283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parretta, E. et al, Kinetics of in vivo proliferation and death of memory and naive CD8 T cells: parameter estimation based on 5‐bromo‐2'‐deoxyuridine incorporation in spleen, lymph nodes, and bone marrow. J. Immunol. 2008. 180: 7230–7239. [DOI] [PubMed] [Google Scholar]
- 5. Boyman, O. , Létourneau, S. , Krieg, C. and Sprent, J. , Homeostatic proliferation and survival of naïve and memory T cells. Eur. J. Immunol. 2009. 39: 2088–2094. [DOI] [PubMed] [Google Scholar]
- 6. Nolz, J. C. , Rai, D. , Badovinac, V. P. and Harty, J. T. , Division‐linked generation of death‐intermediates regulates the numerical stability of memory CD8 T cells. Proc. Natl. Acad. Sci. U. S. A. 2012. 109: 6199–6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Becker, T. C. , Coley, S. M. , Wherry, E. J. and Ahmed, R. , Bone marrow is a preferred site for homeostatic proliferation of memory CD8 T cells. J. Immunol. 2005. 174: 1269–1273. [DOI] [PubMed] [Google Scholar]
- 8. Parretta, E. et al, CD8 cell division maintaining cytotoxic memory occurs predominantly in the bone marrow. J. Immunol. 2005. 174: 7654–7664. [DOI] [PubMed] [Google Scholar]
- 9. Gerdes, J. et al, Cell‐cycle analysis of a cell proliferation‐associated human nuclear antigen defined by the monoclonal antibody Ki‐67. J. Immunol. 1984. 133: 1710–1715. [PubMed] [Google Scholar]
- 10. Sercan Alp, Ö. et al, Memory CD8(+) T cells colocalize with IL‐7(+) stromal cells in bone marrow and rest in terms of proliferation and transcription. Eur. J. Immunol. 2015. 45: 975–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Okhrimenko, A. et al, Human memory T cells from the bone marrow are resting and maintain long‐lasting systemic memory. Proc. Natl. Acad. Sci. U. S. A. 2014. 111: 9229–9234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hall, A. G. and Tilby, M. J. , Mechanisms of action of, and modes of resistance to, alkylating agents used in the treatment of haematological malignancies. Blood Rev. 1992. 6: 163–173. [DOI] [PubMed] [Google Scholar]
- 13. Freitas, A. A. , Rocha, B. , Forni, L. and Coutinho, A. , Population dynamics of B lymphocytes and their precursors: demonstration of high turnover in the central and peripheral lymphoid organs. J. Immunol. 1982. 128:54–60. [PubMed] [Google Scholar]
- 14. Hao, Z. and Rajewsky, K. , Homeostasis of peripheral B cells in the absence of B cell influx from the bone marrow. J. Exp. Med. 2001. 194:1151–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mumtaz, I. M. et al, Bone marrow of NZB/W mice is the major site for plasma cells resistant to dexamethasone and cyclophosphamide: implications for the treatment of autoimmunity. J. Autoimmun. 2012. 39:180–188. [DOI] [PubMed] [Google Scholar]
- 16. Graler, M. H. and Goetzl, E. J. , The immunosuppressant FTY720 down‐regulates sphingosine 1‐phosphate G‐protein‐coupled receptors. FASEB J. 2004. 18, 551–553. [DOI] [PubMed] [Google Scholar]
- 17. Brinkmann, V. et al, The immune modulator FTY720 targets sphingosine 1‐phosphate receptors. J. Biol. Chem. 2002. 277, 21453–21457. [DOI] [PubMed] [Google Scholar]
- 18. Mackay, L. K. et al, Cutting edge: CD69 interference with sphingosine‐1‐phosphate receptor function regulates peripheral T cell retention. J. Immunol. 2015. 194: 2059–2063. [DOI] [PubMed] [Google Scholar]
- 19. Bankovich, A. J. , Shiow, L. R. and Cyster, J. G. , CD69 suppresses sphingosine 1‐phosophate receptor‐1 (S1P1) function through interaction with membrane helix 4. J. Biol. Chem. 2010. 285: 22328–22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoyer, B. F. et al, Short‐lived plasmablasts and long‐lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J. Exp. Med. 2004. 199: 1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taddeo, A. et al, Long‐lived plasma cells are early and constantly generated in New Zealand Black/New Zealand White F1 mice and their therapeutic depletion requires a combined targeting of autoreactive plasma cells and their precursors. Arthritis Res. Ther. 2015. 17:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Benner, R. , van Oudenaaren, A. and Koch, G. , Induction of antibody formation in mouse bone marrow. Immunological methods, Volume II. Lefkovits I. and Pernis B. eds. Academic Press, New York, 1981, pp. 247–261. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Peer review correspondence
Supporting Information


