Abstract
Objective
Alzheimer’s disease (AD) is a neurodegenerative disorder for which more than 20 genetic loci have been implicated to date. However, studies demonstrate not all genetic factors have been identified. Therefore, in this study we seek to identify additional rare variants and novel genes potentially contributing to AD.
Methods
Whole exome sequencing was performed on 23 multi-generational families with an average of eight affected subjects. Exome sequencing was filtered for rare, nonsynonymous and loss-of-function variants. Alterations predicted to have a functional consequence and located within either a previously reported AD gene, a linkage peak (LOD>2), or clustering in the same gene across multiple families, were prioritized.
Results
Rare variants were found in known AD risk genes including AKAP9, CD33, CR1, EPHA1, INPP5D, NME8, PSEN1, SORL1, TREM2 and UNC5C. Three families had five variants of interest in linkage regions with LOD>2. Genes with segregating alterations in these peaks include CD163L1 and CLECL1, two genes that have both been implicated in immunity, CTNNA1, which encodes a catenin in the cerebral cortex and MIEF1, a gene that may induce mitochondrial dysfunction and has the potential to damage neurons. Four genes were identified with alterations in more than one family include PLEKHG5, a gene that causes Charcot-Marie-Tooth disease and THBS2, which promotes synaptogenesis.
Conclusion
Utilizing large families with a heavy burden of disease allowed for the identification of rare variants co-segregating with disease. Variants were identified in both known AD risk genes and in novel genes.
Keywords: Alzheimer’s disease, dominant inheritance, linkage, multiplex, whole exome sequencing
Introduction
Alzheimer’s disease (AD) is the leading cause of dementia in the elderly [1]. The majority of individuals present with late-onset AD (≥ 65 years), but early-onset (<65 years) has also been reported in ~5% of cases. Both common genetic variants, such as the APOE ε4 allele, and rare variants, have been found to impact the risk for both early- and late-onset AD [2–5]. While more than 20 genetic loci have been connected with late-onset AD to date, the underlying genetic architecture is complex and new risk genes are still being identified [6].
While genome-wide association studies (GWAS) have been key in identifying a majority of the novel regions of genetic risk in the past ten years, by design, GWAS are unlikely to recognize risk variants with rare frequencies in the population and necessitate the use of large cohorts of hundreds or even thousands of individuals to reach statistically significant conclusions [6]. In contrast, whole exome sequencing (WES) provides an alternative and complementary method to locate rare alterations in genes which may have medium to large effects on disease risk and require far fewer participants [6–8]. WES studies have identified new mutations in both known AD genes and novel risk genes, including AKAP9, PLD3, TREM2 and UNC5C, as well as protective variants, such as those in TREML2 [7–17]. Moreover, studying families with a heavy burden of AD and searching for genetic changes that segregate with disease can provide a unique opportunity to locate rare variants in novel risk genes such as NOTCH3, PLD3 and TTC3 [9,13,18]. These large AD families can reveal how multiple genetic variants may act in concert to influence risk [19–21]. For example, the APOE ε2 allele was found to delay the age of onset by ~12 years in carriers of the E280A mutation in the PSEN1 gene in the early-onset ‘Paisa’ pedigree [19]. In addition, genetic linkage can assist in narrowing genomic regions of interest potentially related to disease in large families [22]. In an effort to discover novel genes that may contribute to late-onset AD risk, we performed WES in 23 multiplex families that present with dominant inheritance patterns and prioritized variants that were inherited from common ancestors.
Materials and Methods
Patient ascertainment of extended AD families
240 individuals (77 AD subjects, 4 individuals with mild cognitive impairment (MCI) and 159 unaffected relatives) from 23 families of European ancestry heavily affected with late-onset AD were utilized in this study (Supplementary Table 1). All family members were recruited after providing informed consent and with approval by the relevant institutional review boards. Affected individuals meet the standard NINCDS-ADRDA criteria for AD and MCI [23–25]. In addition, cognitive and neuropsychiatric data were collected on all affected indivduals using the NCRAC LOAD battery, the Geriatric Depression Scale (GDS15), the Cornell Scale for Depression in Dementia (CSDD) and the Neuropsychiatric Inventory Questionnaire (NPIQ).
Whole exome sequencing and variant detection
99 individuals (77 AD patients, 4 individuals with MCI, and 18 unaffected relatives) from 23 AD extended families underwent WES (Supplementary Table 1). Three micrograms of DNA from each sample were prepared using the SureSelect Human All Exon 50Mb Kit (Agilent Technologies) and the Paired-End Multiplexed Sequencing library kit (Illumina). Exome capture and sequence library construction was performed on a Sciclone G3 NGS Workstation (Caliper Life Sciences) and DNA was tested for uniform enrichment of targets with qPCR following established protocols provided by Agilent. Two exome sample libraries were sequenced per lane on a HiSeq 2000 Sequencing System (Illumina) in paired-end 2 × 100 bp runs. Sequencing data was processed using the Illumina RTA base calling pipeline v1.8. Reads were aligned to the human reference genome (hg19) with the Burrows- Wheeler Aligner (BWA) and variant calling performed with the Genome Analysis Toolkit (GATK) version 2.8 [26,27]. GATK parameters for variant quality control included duplicate sequence read removal, minimum read depth of 5, genotype quality (GQ) ≥ 20, variant quality score recalibration (VQSR, VQSLOD>0) and Genome Mappability Scores equal to 1 for the 35 base pair (bp) track and greater than or equal to 0.5 for the 20 bp track from the Duke Uniqueness Track [28]. The Duke uniqueness scores, generated for the ENCODE project and available as tracks in the University of California, Santa Cruz (UCSC) Genome Browser, report how unique a sequence is, where scores of 1 represent a completely unique sequence, a score of 0.5 indicates the sequence occurs exactly twice, and 0 represents the sequence occurs >4 times in the genome [29,30]. Small insertions and deletions were recognized by aligning the data with Bowtie2 and analyzing with the Pindel program [31,32].
Genotyping and variant filtering
234 individuals, including all 99 samples that had WES, were evaluated by genome-wide SNP (single nucleotide polymorphism) arrays including the Human 1Mv1 BeadChip, the 1M-DuoV3 BeadChip, the HumanOmniExpress-12 v1.0 BeadChip, and the HumanOmni2.5-4v1 BeadChip. All chips were processed using the Tecan EVO-1 robot and BeadChips were scanned with either the Illumina BeadArray Reader or iScan. Data was extracted by the Genome Studio software and a GenCall cutoff score of 0.15 was used. Samples were required to have a genotyping call rate of 98% or higher, and SNPs a call rate of 95% or greater, to pass quality control. SNPs were only included in the analysis if they were present in at least 60% of samples across all platforms. Checks for relatedness, Mendelian inconsistencies, gender based on X-chromosome heterozygosity, and concordance between the genotypes of the variants identified through exome sequencing and genotyping were evaluated with PLINK version 1.07 [33]. All samples passed the quality control metrics.
Genotyping information was further used to delineate identical by descent (IBD) regions within each multiplex AD family. IBD filtering was implemented through the extended haplotype procedure in MERLIN version 1.1.2 [34]. Regions shared across all available AD individuals within a family were used to determine the IBD sharing segments and were, therefore, unique within each family. To determine the start and stop positions of IBD sharing regions within each family, the MERLIN output was evaluated in a sliding window of ten SNPs, defining IBD as sharing at each location with a threshold >50%.
Linkage analysis
Nonparametric and parametric two-point and multipoint linkage analyses were performed using MERLIN [32]. A disease allele frequency of 0.0001 was used in an affecteds-only model for parametric analysis. PLINK was employed for LD pruning in the multipoint analysis, with CEU HapMap data as the reference population and the following settings: the indep-pairwise option with a window size of 50, a step of 5 and an r2 threshold of 0.5 [33,34].
Variant annotation and prioritization
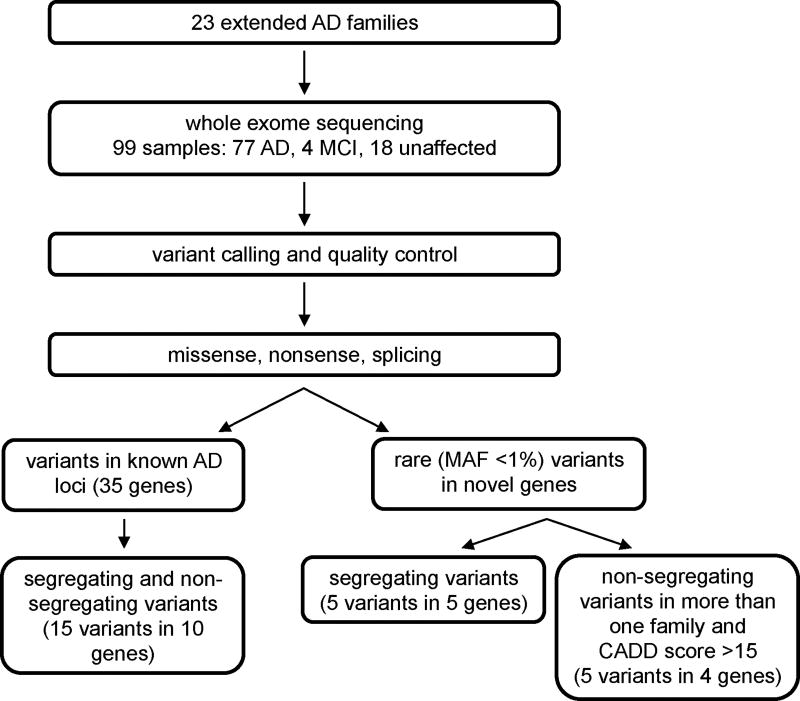
Alterations passing quality measurements were annotated with the KGGSeq and ANNOVAR programs [35,36]. Variants were normalized prior to annotation [37]. Ensembl, RefSeq, and Gencode transcripts were all annotated, and the top consequence per gene was used for prioritization. CADD v1.3 scores were downloaded from the CADD server (http://cadd.gs.washington.edu/home) [38]. Figure 1 is an overview of the filtering and prioritization strategies used in this study. Brief descriptions of our three prioritization strategies are described below.
Figure 1.
Study design. Strategy for processing the samples and prioritizing the variants that were resulting from whole exome sequencing.
Variants in reported AD genes or loci
For all of the families, we evaluated whether variants were located in known AD risk genes; this includes genes identified in both early (APP, PSEN1, PSEN2, GRN and TREM2) and late-onset AD (Supplemental Table 2) [3,4]. Variants of interest were restricted to those with a minor allele frequency (MAF) ≤ 2% in the Kaviar Genomic Variant Database (version 160204-Public, 77,781 individuals) since these genes are known loci for AD [39]. The top variants of interest were validated by traditional Sanger sequencing.
Families with LOD scores >2
For each of the families, variants that segregated in all sequenced, affected individuals within areas LOD>2 were evaluated. Variants with a global MAF ≤ 1% in the Kaviar Database were prioritized. A MAF cutoff of ≤ 1% was implemented because variants with a MAF>1% in any ethnic population are unlikely to be a highly penetrant risk variant for AD [39–41]. This stricter MAF criteria was utilized to attempt to identify novel risk genes as opposed to variants in known AD genes. Variants were also prioritized based on their potential pathogenicity with the Combined Annotation-Dependent Depletion (CADD) score; scores ≥ 15 are predicted to be more likely to contribute to a disease risk as this score represents “the median value for all possible canonical splice site changes and non-synonymous variants” [38].
Variants and genes shared across families
Analysis across all 23 families was performed to identify if there were any genes with rare, nonsynonymous or loss-of-function (LOF) variants in more than one family. Variants were selected that had a MAF ≤ 1% in the Kaviar Database and CADD scores ≥ 15 to try to identify potentially damaging alterations [39].
Association testing of top candidates
All top variants and genes from the three separate analyses described above were evaluated as potential risk variants using genome-wide association statistics for two family study cohorts (NIA-LOAD and MIRAGE) in the Alzheimer Disease Genetics Consortium [42,43]. Both gene and SNP-based tests were adjusted for age, sex and principal components (PCs). SNP-based logistic regression tests in each study were performed in the SNPTest program, and meta-analysis of these results was conducted using METAL [21,44]. Gene-based tests were conducted on meta-analysis summary statistics using VEGAS [44]. Variants tested in the gene-based analysis included all variants with a MAF<5%.
Results
Variants identified in known AD genes
Each sequenced family contained between 4 and 16 individuals diagnosed with AD. Mean age-at-onset across all families was 74.3 years. We identified 14 potentially damaging variants in 10 known AD genes and GWAS implicated loci (Table 1). Seven of the variants were observed in multiple affected individuals in the same family, while the remaining variants were observed only once. All alterations were single nucleotide changes with the exception of a four base pair deletion in CD33. This deletion is potentially the most deleterious as it is predicted to causes a frameshift that encodes two incorrect amino acids before terminating prematurely, thus failing to generate over 40% of the protein. In addition, multiple variants were observed in four genes: AKAP9, INPP5D, SORL1 and UNC5C. Each gene had at least two families with a variant identified in it, while family 191 have a single affected individual with two alterations in UNC5. One of the variants in UNC5C, Ala860Thr, was identified in two different families; this alteration has a CADD score of 33, the highest score in this category.
Table 1.
Known AD genes and loci with rare, potentially damaging variants.
| Gene | Chr | Position (hg38) |
Nucleotide | Amino acid | dbSNP | Kaviar MAF | CADD score |
Family | Affected individuals with variant/ total affected sequenced |
|---|---|---|---|---|---|---|---|---|---|
| AKAP9 | 7 | 92002147 | G>A | Glu756Lys | rs202091548 | 0.00008 | 27.8 | 191 | 1/2 |
| AKAP9 | 7 | 92017092 | G>A | Arg1288Gln | rs146797353 | 0.00822 | 6.2 | 419 | 2/2 |
| CD33 | 19 | 51225851 | CCCGG>C | Gly210Thrfs*2 | rs201074739 | 0.01339 | - | 2349 | 1/2 |
| CR1 | 1 | 207618089 | A>G | Lys2308Arg | rs41274770 | 0.01463 | 11.9 | 1893 | 2/3 |
| EPHA1 | 7 | 143398060 | C>T | Arg492Gln | rs11768549 | 0.01214 | 17.39 | 701 | 1/3 |
| INPP5D | 2 | 233125865 | G>A | Arg157Gln | rs200834931 | 0.00139 | 17.75 | 1399 | 2/2 |
| INPP5D | 2 | 233206711 | C>A | Ala994Asp | rs187622749 | 0.00433 | 22.8 | 2349 | 2/2 |
| NME8 | 7 | 37884315 | G>A | Arg336His | rs62001869 | 0.01436 | 6.08 | 1893 | 3/3 |
| PSEN1 | 14 | 73206470 | A>G | Glu318Gly | rs17125721 | 0.01423 | 16.92 | 419 | 1/2 |
| SORL1 | 11 | 121543625 | C>T | Thr588Ile | rs752726649 | 0.00001 | 32 | 191 | 2/2 |
| SORL1 | 11 | 121627591 | C>T | Thr2134Met | rs142884576 | 0.00023 | 28.6 | 1240 | 1/2 |
| TREM2 | 6 | 41161469 | C>T | Arg92His | rs143332484 | 0.00791 | 11.11 | 1893 | 1/3 |
| UNC5C | 4 | 95170263 | C>T | Ala860Thr | rs34585936 | 0.01808 | 33 | 191 | 2/2 |
| UNC5C | 4 | 95170263 | C>T | Ala860Thr | rs34585936 | 0.01808 | 33 | 2119 | 1/2 |
| UNC5C | 4 | 95202928 | G>A | Pro666Ser | rs760453427 | 0.00001 | 20.2 | 191 | 1/2 |
Segregating variants in linkage regions
Linkage scans aggregating all families identified one primary linkage region, a parametric multipoint peak on chromosome 1q23 (161.9–165.6 MB). Two families had strong linkage in this region (family specific LOD>2). However, no variants met our filtering criteria for these two families, suggesting the causal variant(s) may be non-coding changes either removed from by our filtering criteria or not present in our WES. Three of the 23 families also have family-specific parametric LOD scores >2; rare, potentially damaging alterations in five genes occurred within these regions and may potentially be the strongest novel AD candidate genes (Table 2). The five alterations were all missense changes in CD163L1, CLECL1, CTNNA1, GALR3 and MIEF1.
Table 2.
Families with segregating, rare, potentially damaging variants in high LOD regions.
| Family | Affected individuals with variant |
LOD score |
Gene | Chr | Position (hg38) |
Nucleotide | Amino acid | dbSNP | Kaviar score |
CADD score |
|---|---|---|---|---|---|---|---|---|---|---|
| 757 | 9 | 2.95 | CD163L1 | 12 | 7369477 | T>C | Thr1317Ala | rs150384982 | 0.00137 | 3.73 |
| 757 | 9 | 2.95 | CLECL1 | 12 | 9722727 | T>C | Thr135Ala | rs118152239 | 0.00769 | 0.03 |
| 911 | 7 | 2.36 | CTNNA1 | 5 | 138824559 | G>C | Gln206His | rs150893072 | 0.0043 | 23.2 |
| 1201 | 5 | 2.22 | GALR3 | 22 | 37823563 | C>G | Pro53Ala | rs78650836 | 0.00328 | 10.31 |
| 1201 | 5 | 2.22 | MIEF1 | 22 | 39512414 | C>T | Arg169Trp | rs2232088 | 0.00525 | 34 |
Genes with variants in more than one family
We identified four genes that had rare (MAF ≤ 1%), segregating, and potentially deleterious variants in at least two families (Table 3). Three of these genes had the same missense alteration identified in distinct families: MKL2, PLEKHG5 and THBS2.
Table 3.
Genes with rare, potentially damaging variants in more than one family.
| Gene | Family | Chr | Position (hg38) | Nucleotide | Amino acid | dbSNP | Kaviar score | CADD score | Affected individuals with variant/ total affecteds sequenced |
|---|---|---|---|---|---|---|---|---|---|
| DAAM2 | 191 | 6 | 39861001 | A>G | Tyr128Cys | rs201047462 | 0.00017 | 28.00 | 2/2 |
| 716 | 6 | 39884013 | C>T | Arg680Trp | rs200964833 | 0.00019 | 35.00 | 3/3 | |
| MKL2 | 1893 | 16 | 14245640 | T>C | Ser398Pro | rs113935526 | 0.00479 | 19.20 | 3/3 |
| 26044 | 16 | 14245640 | T>C | Ser398Pro | rs113935526 | 0.00479 | 19.20 | 2/3 | |
| PLEKHG5 | 803 | 1 | 6496525 | G>A | Pro40Ser | rs201669114 | 0.00148 | 27.00 | 2/2 |
| 1008 | 1 | 6496525 | G>A | Pro40Ser | rs201669114 | 0.00148 | 27.00 | 2/2 | |
| THBS2 | 1240 | 6 | 169220312 | C>A | Val1133Phe | rs112533700 | 0.00017 | 29.50 | 2/2 |
| 1893 | 6 | 169220312 | C>A | Val1133Phe | rs112533700 | 0.00017 | 29.50 | 3/3 |
Association testing of variants and genes
From our prioritized sets, a total of 9 SNPs and 14 genes were available for testing in the ADGC family-based meta-analysis datasets (NIA-LOAD and Mirage). None of the variants tested were significantly associated with disease (Supplemental Table 3). The gene MIEF1, identified as a candidate gene in a family 1201 with rare, potentially damaging segregating variants in a region with a LOD score of 2.22, reached nominal significance (p=0.049, Supplemental Table 4).
Discussion
Through WES of large families with a heavy burden of AD, variants in both known and novel loci were identified that could contribute to risk. Filtering for rare, segregating, and potentially damaging variants identified five novel candidate genes (Table 2). These genes encompass a variety of functions that are suggestive of a link to AD. For example, two of these genes are involved in regulating immunity: CD163L1 and CLECL1 [45–47]. CD163L1 is expressed in macrophages, upregulated in response to IL-10 and acts as an endocytic receptor [48]. CLECL1 is highly expressed in B cells and dendritic cells and may enhance the immune response through upregulation of IL-4 [46]. Neuroinflammation has been shown to occur in AD patients, possibly through misregulation of microglia and triggered by amyloid beta plaques [49]. Additionally, established AD risk genes, such as ABCA7, CD33 and TREM2, have also been linked to the immune system [4]. Another gene identified through this study, CTNNA1, encodes a catenin expressed at elevated levels in the nervous system [50]. GALR3 is a receptor for the neuropeptide galanin, which has been shown to modulate a variety of processes, including cognition and memory, functions disrupted in AD [51,52]. MIEF1 was nominally associated with late-onset AD in a meta-analysis of two family datasets from the ADGC, thereby suggesting that it may play a wider role in AD that extends beyond a single multiplex AD family. MIEF1 may play a role in dysfunctional mitochondria and their potential to damage neurons [53,54]. Thus, each of the genes in the peak linkage regions are connected to known AD functions or neuronal pathways.
Four genes had rare, potentially damaging variants in more than one family (Table 3). When evaluating known functions of these genes, two are of particular interest, PLEKHG5 and THBS2. PLEKHG5 has been previously implicated in both Charcot-Marie-Tooth disease and spinal muscular atrophy [55,56]. PLEKHG5 is ubiquitously expressed throughout the nervous system and murine studies demonstrated lowered expression can alter the velocity of nerve conduction [55,56]. In addition, THBS2 is an intriguing novel AD candidate gene due to its involvement in synaptogenesis in immature astrocytes [57]. Further investigation into each of these novel AD candidates and the variants identified in this study is required.
After evaluating our families for rare alterations in known AD genes and loci, variants were discovered in genes previously connected to both early and late-onset AD (Table 1). Four genes had multiple alterations: AKAP9, INPP5D, SORL1 and UNC5C. Some of these alterations have the potential to interfere with a protein’s function due to their location within specific domains. For example, a rare alteration in UNC5C identified in two distinct families, Ala860Thr, falls within the highly conserved DEATH domain, a region composed of alpha-helices and involved in apoptotic functions. Another study identified a different alteration in the same region in AD families and proposed that the alteration may increase the susceptibility of neurons to death [17]. A single affected individual in family 2349 was found to carry a frameshift deletion in CD33 predicted to remove over 40% of the protein. There is evidence that higher expression of CD33 in brains is associated with cognitive decline [58]. However, it may be that dysregulation of the protein, either through over or under expression, could contribute to AD risk. In addition, a rare alteration in SORL1, Thr588Ile, was identified within the vacuolar protein sorting 10 (VPS10) domain and may influence the processing of amyloid beta fragments, as has been shown for other AD associated variants in this gene [59]. Moreover, a potentially pathogenic alteration was identified in PSEN1 in a single individual, Glu318Gly; this variant was previously reported to result in higher tau and phosphorylated tau levels in cerebrospinal fluids [60]. Three affected individuals from family 1893 were found to share the Arg336His alteration in the NME8 gene, a change that fell within the first NDK domain of the protein. This gene has been associated with clinical features of AD including atrophy of the hippocampus and occipital gyrus [6,61]. These alterations, while not segregating within all affected individuals in the families, may play a contributing role in AD risk.
Conclusion
This study demonstrates how using large, extended families to evaluate exome data identifies segregating risk variants in potentially novel AD candidate genes. In contrast to GWAS studies that have grown from hundreds to thousands and tens of thousands of participants, this study design requires far fewer participants. Indeed, a single extended family may be sufficient to identify a novel AD candidate gene [18]. Moreover, WES has the sensitivity to directly detect both common and rare variants that may confer a risk to AD, while GWAS findings are limited to pinpointing a region of interest, but not necessarily the causative alterations. In the study presented here, rare changes potentially contributing to AD risk were found in genes implicated in the immune response, CD163L1 and CLECL1, and neuronal function, CTNNA1, GALR3, MIEF1, PLEKHG5 and THBS2. Variants were also identified in genes previously connected to both early and late-onset AD including AKAP9, INPP5D, SORL1 and UNC5C. Further investigation will be required to fully assess the cellular and molecular consequences of the alterations identified here as well as determine whether the novel genes found are involved in AD risk across larger datasets.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (R01 AG027944, R01 AG028786 to MAPV, R01 AG019085 to JLH, P20 MD000546); a joint grant from the Alzheimer’s Association (SG-14-312644) and the Fidelity Biosciences Research Initiative to MAPV; the BrightFocus Foundation (A2011048 to MAPV). NIA-LOAD Family-Based Study supported the collection of samples used in this study through NIH grants U24 AG026395 and R01 AG041797 and the MIRAGE cohort was supported through the NIH grants R01 AG025259 and R01 AG048927. We thank contributors, including the Alzheimer’s disease Centers who collected samples used in this study, as well as patients and their families, whose help and participation made this work possible. Study design: HNC, BWK, JLH, MAPV; Sample collection: MLC, JMV, RMC, LAF, JLH, MAPV; Whole exome sequencing and Sanger sequencing: SR, PLW; Sequencing data analysis: HNC, BWK, KLHN, SR, MAK, JRG, ERM, GWB, MAPV; Statistical analysis: BWK, KLHN, JMJ, MAPV; Preparation of manuscript: HNC, BWK. The authors jointly discussed the experimental results throughout the duration of the study. All authors read and approved the final manuscript.
References
- 1.Qiu C, Kivipelto M, Von Strauss E. Epidemiology of Alzheimer's disease: Occurrence, determinants and strategies toward intervention. Dialogues Clin Neurosci. 2009;11:111–128. doi: 10.31887/DCNS.2009.11.2/cqiu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gatz M, Reynolds CA, Fratiglioni L, Johansson B, Mortimer JA, et al. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry. 2006;63:168–174. doi: 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- 3.Van Cauwenberghe C, Van Broeckhoven C, Sleegers K. The genetic landscape of Alzheimer disease: Clinical implications and perspectives. Genet Med. 2015;18:421–430. doi: 10.1038/gim.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 5.Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, et al. ApolipoproteinE: High-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet. 2013;45:1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pottier C, Hannequin D, Coutant S, Rovelet-Lecrux A, Wallon D, et al. High frequency of potentially pathogenic SORL1 mutations in autosomal dominant early-onset Alzheimer disease. Mol Psychiatry. 2012;17:875–879. doi: 10.1038/mp.2012.15. [DOI] [PubMed] [Google Scholar]
- 8.Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, et al. TREM2 variants in Alzheimer's disease. N Engl J Med. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guerreiro RJ, Lohmann E, Kinsella E, Bras JM, Luu N, et al. Exome sequencing reveals an unexpected genetic cause of disease: NOTCH3 mutation in a Turkish family with Alzheimer's disease. Neurobiol Aging. 2012;33:1008.e17–1008.e23. doi: 10.1016/j.neurobiolaging.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, et al. Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med. 2013;368:107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benitez BA, Jin SC, Guerreiro R, Graham R, Lord J, et al. Missense variant in TREML2 protects against Alzheimer's disease. Neurobiol Aging. 2014;35:1510.e19–1510.e26. doi: 10.1016/j.neurobiolaging.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carney RM, Kohli MA, Kunkle BW, Naj AC, Gilbert JR, et al. Parkinsonism and distinct dementia patterns in a family with the MAPT R406W mutation. Alzheimers Dement. 2014;10:360–365. doi: 10.1016/j.jalz.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cruchaga C, Karch CM, Jin SC, Benitez BA, Cai Y, et al. Rare coding variants in the phospholipase D3 gene confer risk for Alzheimer's disease. Nature. 2014;505:550–554. doi: 10.1038/nature12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dolzhanskaya N, Gonzalez MA, Speriani F, Stefl S, Messing J, et al. A novel p.Leu(381)Phe mutation in presenilin 1 is associated with very early onset and unusually fast progressing dementia as well as lysosomal inclusions typically seen in Kufs disease. J Alzheimers Dis. 2014;39:23–27. doi: 10.3233/JAD-131340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logue MW, Schu M, Vardarajan BN, Farrell J, Bennett DA, et al. Two rare AKAP9 variants are associated with Alzheimer's disease in African Americans. Alzheimers Dement. 2014;10:609–618. e11. doi: 10.1016/j.jalz.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sassi C, Guerreiro R, Gibbs R, Ding J, Lupton MK, et al. Investigating the role of rare coding variability in Mendelian dementia genes (APP, PSEN1, PSEN2, GRN, MAPT and PRNP) in late-onset Alzheimer's disease. Neurobiol Aging. 2014;35:2881.e1–2881.e6. doi: 10.1016/j.neurobiolaging.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wetzel-Smith MK, Hunkapiller J, Bhangale TR, Srinivasan K, Maloney JA, et al. A rare mutation in UNC5C predisposes to late-onset Alzheimer's disease and increases neuronal cell death. Nat Med. 2014;20:1452–1457. doi: 10.1038/nm.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohli MA, Cukier HN, Hamilton-Nelson KL, Rolati S, Kunkle BW, et al. Segregation of a rare TTC3 variant in an extended family with late-onset Alzheimer disease. Neurol Genet. 2016;2:e41. doi: 10.1212/NXG.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Velez JI, Lopera F, Sepulveda-FAlla D, Patel HR, Jphar AS, et al. APOE*E2 allele delays age of onset in PSEN1 E280A Alzheimer’s disease. Mol Psychiatry. 2016;21:916–924. doi: 10.1038/mp.2015.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehling R, Nosková L, Stránecký V, Hartmannová H, Přistoupilová A, et al. Cerebellar dysfunction in a family harboring the PSEN1 mutation co-segregating with a cathepsin D variant p.A58V. J Neurol Sci. 2013;326:75–82. doi: 10.1016/j.jns.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Louwersheimer E, Cohn-Hokke PE, Pijnenburg YA, Weiss MM, et al. Rare genetic variant in SORL1 may increase penetrance of Alzheimer's disease in a family with several generations of APOE-ε4 Homozygosity. J Alzheimers Dis. 2017;56:63–74. doi: 10.3233/JAD-160091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunkle BW, Jaworski J, Barral S, Vardarajan B, Beecham GW, et al. Genome-wide linkage analyses of non-Hispanic white families identify novel loci for familial late-onset Alzheimer's disease. Alzheimers Dement. 2016;12:2–10. doi: 10.1016/j.jalz.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKhann G, Drachman D, Folstein M, Katzman R, Price D, et al. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 24.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institutes on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Durbin R. Fast and accurate short read alignment with Burrows- Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, et al. The genome analysis toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, et al. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raney BJ, Dreszer TR, Barber GP, Clawson H, Fujita PA, et al. Track data hubs enable visualization of user-defined genome-wide annotations on the UCSC genome browser. Bioinformatics. 2014;30:1003–1005. doi: 10.1093/bioinformatics/btt637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye K, Schulz MH, Long Q, Apweiler R, Ning Z. Pindel: A pattern growth approach to detect break points of large deletions and medium sized insertions from paired-end short reads. Bioinformatics. 2009;25:2865–2871. doi: 10.1093/bioinformatics/btp394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin--rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet. 2002;30:97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- 35.Wang K, Li M, Hakonarson H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li MX, Gui HS, Kwan JS, Bao SY, Sham PC. A comprehensive framework for prioritizing variants in exome sequencing studies of Mendelian diseases. Nucleic Acids Res. 2012;40:e53. doi: 10.1093/nar/gkr1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan A, Abecasis GR, Kang HM. Unified representation of genetic variants. Bioinformatics. 2015;31:2202–2204. doi: 10.1093/bioinformatics/btv112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kircher M, Witten DM, Jain P, O'Roak BJ, Cooper GM, et al. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46:310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glusman G, Caballero J, Mauldin DE, Hood L, Roach JC. Kaviar: An accessible system for testing SNV novelty. Bioinformatics. 2011;27:3216–3217. doi: 10.1093/bioinformatics/btr540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shearer AE, Eppsteiner RW, Booth KT, Ephraim SS, Gurrola J, et al. Utilizing ethnic-specific differences in minor allele frequency to recategorize reported pathogenic deafness variants. Am J Hum Genet. 2014;95:445–453. doi: 10.1016/j.ajhg.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richards S, Aziz N, Bale S, Bick D, Das S, et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Green RC, Cupples LA, Go R, Benke KS, Edeki T, et al. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002;287:329–336. doi: 10.1001/jama.287.3.329. [DOI] [PubMed] [Google Scholar]
- 43.Lee JH, Cheng R, Graff-Radford N, Foroud T, Mayeux R, et al. Analysis of the National Institute on Aging Late-Onset Alzheimer’s Disease Family Study: Implication of additional loci. Arch Neurol. 2008;65:1518–1526. doi: 10.1001/archneur.65.11.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu JZ, McRae AF, Nyholt DR, Medland SE, Wray NR, et al. A versatile gene-based test for genome-wide association studies. Am J Hum Genet. 2010;87:139–145. doi: 10.1016/j.ajhg.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryan EJ, Marshall AJ, Magaletti D, Floyd H, Draves KE, et al. Dendritic cell-associated lectin-1: A novel dendritic cell-associated, C-type lectin-like molecule enhances T cell secretion of IL-4. J Immunol. 2002;169:5638–5648. doi: 10.4049/jimmunol.169.10.5638. [DOI] [PubMed] [Google Scholar]
- 47.Gonzalez-Dominguez E, Samaniego R, Flores-Sevilla JL, Campos-Campos SF, Gomez-Campos G, et al. CD163L1 and CLEC5A discriminate subsets of human resident and inflammatory macrophages in vivo. J Leukoc Biol. 2015;98:453–466. doi: 10.1189/jlb.3HI1114-531R. [DOI] [PubMed] [Google Scholar]
- 48.Moeller JB, Nielson MJ, Reichhardt MP, Schlosser A, Sorensen GL, et al. CD163-L1 is an endocytic macrophage protein strongly regulated by mediators in the inflammatory response. J Immunol. 2012;188:2399–2409. doi: 10.4049/jimmunol.1103150. [DOI] [PubMed] [Google Scholar]
- 49.Minter MR, Taylor JM, Crack PJ. The contribution of neuroflammation to amyloid toxicity in Alzheimer'd disease. J Neurochem. 2016;136:457–474. doi: 10.1111/jnc.13411. [DOI] [PubMed] [Google Scholar]
- 50.Kask M, Pruunsild P, Timmusk T. Bidirectional transcription from human LRRTM2/CTNNA1 and LRRTm1/CTNNA2 gene loci leads to expression of N-terminally truncated CTNNA1 and CTNNA2 isoforms. Biochem Biophys Res Commun. 2011;411:56–61. doi: 10.1016/j.bbrc.2011.06.085. [DOI] [PubMed] [Google Scholar]
- 51.Kolakowski LF, O'Neill GP, Howard AD, Broussard SR, Sullivan KA, et al. Molecular characterization and expression of cloned human galanin receptors GALR2 and GALR3. J Neurochem. 1998;71:2239–2251. doi: 10.1046/j.1471-4159.1998.71062239.x. [DOI] [PubMed] [Google Scholar]
- 52.Steiner RA, Hohmann JG, Holmes A, Wrenn CC, Cadd G, et al. Galanin transgenic mice display cognitive and neurochemical deficits characteristic of Alzheimer's disease. Proc Natl Acad Sci U S A. 2001;98:4184–4189. doi: 10.1073/pnas.061445598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu F, Yao PJ. Clathrin-mediated endocytosis and Alzheimer's disease: An update. Ageing Res Rev. 2009;8:147–149. doi: 10.1016/j.arr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Garcia-Escudero V, Martin-Maestro P, Perry G, Avila J. Deconstructing mitochondrial dysfunction in Alzheimer disease. Oxid Med Cell Longev. 2013;2013:162152. doi: 10.1155/2013/162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maystadt I, Rezsohazy R, Barkats M, Duque S, Vannuffel P, et al. The nuclear factor kappaB-activator gene PLEKHG5 is mutated in a form of autosomal recessive lower motor neuron disease with childhood onset. Am J Hum Genet. 2007;81:67–76. doi: 10.1086/518900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Azzedine H, Zavadakova P, Plante-Bordeneuve V, Vaz Pato M, Pinto N, et al. PLEKHG5 deficiency leads to an intermediate form of autosomal-recessive Charcot-Marie-Tooth disease. Hum Mol Genet. 2013;22:4224–4232. doi: 10.1093/hmg/ddt274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 58.Karch CM, Jeng AT, Nowotny P, Cady J, Cruchaga C, et al. Expression of novel Alzheimer's disease risk genes in control and Alzheimer's disease brains. PLoS ONE. 2012;7:e50976. doi: 10.1371/journal.pone.0050976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vardarajan BN, Zhang Y, Lee JH, Cheng R, Bohm C, et al. Coding mutations in SORL1 and Alzheimer disease. Ann Neurol. 2015;77:215–227. doi: 10.1002/ana.24305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benitez BA, Karch CM, Cai Y, Jin SC, Cooper B, et al. The PSEN1, p.E318G variant increases the risk of Alzheimer's disease in APOE-epsilon4 carriers. PLoS Genet. 2013;9:e1003685. doi: 10.1371/journal.pgen.1003685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y, Yu JT, Wang HF, Hao XK, Yang YF, et al. Association between NME8 locus polymorphism and cognitive decline, cerebrospinal fluid and neuroimaging biomarkers in Alzheimer's disease. PLoS ONE. 2014;9:e114777. doi: 10.1371/journal.pone.0114777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.