Abstract
Chronic ethanol exposure causes white matter (WM) atrophy and degeneration with major impairments in the structural integrity of myelin. Since myelin is composed of oligodendrocyte lipid-rich membranes, understanding the consequences and reversibility of alcohol-related oligodendrocyte dysfunction in relation to myelin structure could provide new insights into the pathogenesis of WM degeneration and potential strategies for treatment. Adult male Long Evans rats were pair-fed with isocaloric liquid diets containing 0% or 26% ethanol (caloric) for 3 or 8 weeks. During the last 2 weeks of feeding, the ethanol groups were binged with 2 g/kg of ethanol by intraperitoneal (i.p.) injection on Mondays, Wednesdays, and Fridays; controls were treated with i.p. saline. For recovery effects, at the 6-week time point, ethanol exposures were tapered over 2 days, and then discontinued, rendering the rats ethanol-free for 12 days. Anterior corpus callosum WM lipid ion profiles were analyzed using matrix-assisted laser desorption ionization-imaging mass spectrometry (MALDI-IMS) and correlated with histopathology. Ethanol exposures caused progressive atrophy and reductions in myelin staining intensity within the corpus callosum, whereas short-term recovery partially reversed those effects. MALDI-IMS demonstrated striking ethanol-associated alterations in WM lipid profiles characterized by reduced levels of phosphatidylinositols, phosphatidylserines, phosphatidylethanolamines, and sulfatides, and partial “normalization” of lipid expression with recovery. Ethanol exposure duration and recovery responses were further distinguished by heatmap hierarchical dendrogram and PCA plots. In conclusion, chronic+binge ethanol exposures caused progressive, partially reversible WM atrophy with myelin loss associated with reduced expression of WM phospholipids and sulfatides. The extent of WM lipid abnormalities suggests that ethanol broadly impairs molecular and biochemical functions regulating myelin synthesis, degradation, and maintenance in oligodendrocytes.
Keywords: Chronic+binge alcohol, Alcohol recovery, MALDI, Imaging mass spectrometry
Introduction
Chronic heavy alcohol abuse leads to disproportionate atrophy of white matter (WM) (S. M. de la Monte, 1988; C. G. Harper, Kril, & Holloway, 1985) with cognitive impairment characterized mainly by loss of executive functions (Chanraud et al., 2007), which can progress to dementia and disability (Li, 2008; Schmidt et al., 2005). Neuroimaging and postmortem studies have shown that severities of WM atrophy and degeneration correlate with maximum daily and lifetime alcohol exposures (S. M. de la Monte & Kril, 2014; C. Harper, Dixon, Sheedy, & Garrick, 2003). Despite its diffuse nature, alcohol-related brain degeneration (ARBD) most prominently targets the corpus callosum, and prefrontal, temporal, and cerebellar WM (S. M. de la Monte & Kril, 2014; Phillips, Harper, & Kril,1987), indicating that the distribution of injury is non-random. Studies have focused on corpus callosumatrophy in ARBD (Chanraud et al., 2007; Estruch et al., 1997; Pfefferbaum, Lim, Desmond, & Sullivan, 1996; Pfefferbaum, Rosenbloom, Adalsteinsson, & Sullivan, 2007); damage to the corpus callosum disrupts inter-hemispheric communication, compromising exchange of sensory, motor, and cognitive information. Diffusion Tensor Imaging revealed that corpus callosum atrophy correlates with altered WM micro-structural integrity (Pfefferbaum, Adalsteinsson, & Sullivan, 2006; Schulte, Sullivan, Müller-Oehring, Adalsteinsson, & Pfefferbaum, 2005). These observations are supported by recent in vivo studies showing that ARBD-associated WM atrophy is mediated by combined effects of demyelination, dysmyelination, and axonal degeneration (Papp-Peka, Tong, Kril, de la Monte, & Sutherland, 2016). However, clinical studies provide some evidence that abstinence can partially reverse WM pathology in humans (Bartsch et al., 2007; Estilaei et al., 2001; Gazdzinski, Durazzo, Mon, Yeh, & Meyerhoff, 2010; Monnig, Tonigan, Yeo, Thoma, & McCrady, 2013). Therefore, improved understanding of its pathogenesis could lead to novel therapeutic interventions that reduce the burden of ARBD-related cognitive impairment, dementia, and disability.
Despite the wealth of information about ethanol's adverse effects on WM, the underlying basis of its degeneration in ARBD is not well understood, in part because the tools needed to study WM myelin have not been widely accessible until recently. WM myelin is a major component of the oligodendrocyte membrane that functions to insulate nerve fibers and thereby support neurotransmission. Myelin is largely composed of lipids (70–85% of its dry weight), including cholesterol, sphingolipids, and phospholipids (Schmitt, Castelvetri, & Simons, 2015). Sphingolipids (sphingomyelins, cerebrosides, and sulfatides) are located in the extracellular membrane leaflet and have a major role in myelin formation and maintenance and neuronal plasticity (Honke, 2013; Schmitt et al., 2015; Takahashi & Suzuki, 2012). Sphingolipids, together with cholesterol, form membrane microdomains (lipid rafts) that regulate membrane fluidity, protein trafficking, and signal transduction (Korade & Kenworthy, 2008). Phospholipids (phosphatidylserines, phosphatidylethanolamines, and phosphatidylinositols) are localized along the inner cytosolic leaflet exposed to the cell surface, and function by regulating intracellular signaling and membrane trafficking (Di Paolo & De Camilli, 2006; Fernandis & Wenk, 2007; Van Meer, Voelker, & Feigenson, 2008). Given these varied and critical functions of myelin lipids, specialized analytical approaches are needed to gain a better understanding of how alcohol compromises WM integrity. Fortunately, recent advances in lipidomics now enable efficient in situ characterization of myelin lipid profiles in relation to disease, including experimental ARBD (Yalcin, Nunez, Tong, & de la Monte, 2015), without the need to generate tissue extracts (Roux et al., 2016, 2015; Wang, Jackson, Post, & Woods, 2008). We hypothesize that progressive ethanol-mediated WM atrophy and degeneration are associated with major alterations in myelin lipid composition, reflecting oligodendrocyte dysfunction, and that abstinence-induced recovery and restoration of WM integrity will at least in part normalize myelin lipid profiles. This study utilized matrix-assisted laser desorption ionization-imaging mass spectrometry (MALDI-IMS) to address this hypothesis in an experimental chronic+binge ethanol exposure model with a subgroup evaluated after short-term recovery.
Methods
Experimental model
The use of experimental animals in this research was approved by the Institutional Animal Care and Use Committee (IACUC) at the Lifespan/Rhode Island Hospital. The protocols followed guidelines established by the National Institutes of Health. Rats were housed under standardized conditions with 12-h light/dark cycles and controlled temperature (70–74 °F). The chronic+binge ethanol exposure model was used because it is an accepted approach for producing alcohol-related diseases that more closely mimic human pathology (Bertola, Mathews, Ki, Wang, & Gao, 2013; Bertola, Park, & Gao, 2013), including WM degeneration and cognitive impairment (Tong, Yu, Deochand, & de la Monte, 2015; Tong et al., 2015). Male 6-week-old Long Evans rats were pair-fed isocaloric liquid diets containing 0% or 26% ethanol by caloric content (0 or 6% v/v) for 3 or 8 weeks (n = 6/group). During the last 2 weeks of ethanol feeding, the ethanol groups were binged with 2 g/kg of ethanol by intraperitoneal (i.p.) injection on Mondays, Wednesdays, and Fridays; controls were treated with i.p. saline. To study the effects of abstinence/recovery, an additional six rats were subjected to 6 weeks of chronic+binge ethanol exposures, followed by tapering of ethanol to 0% over a 2-day period, after which the rats were rendered ethanol-free for 12 days.
On the morning of sacrifice, 30 min after binge exposure, peak blood alcohol concentrations were measured using an Analox GM7 analyzer (Analox Instruments, MA, USA). Rats were sacrificed by isoflurane inhalation and their brains were harvested immediately. A standardized 3-mm thick coronal slice that flanked the infundibulum was frozen and stored at −80°C for later MALDI-IMS studies, and the adjacent posterior 3-mm slice was fixed in 10% neutral buffered formalin for paraffin embedding. Histological sections (8-µm thick) were stained with Luxol fast blue hematoxylin and eosin (LHE).
MALDI-IMS
Detailed methods have been described in previous reports (Yalcin & de la Monte, 2015). In brief, cryo-sections (8-µm thick) were thaw-mounted onto indium tin oxide (ITO)-coated slides (Delta Technologies, Loveland, CO), rinsed with 50-mM ammonium formate buffer (pH 6.4), and sublimed with 5-dehydroxybenzoic acid (DHB; Sigma-Aldrich Co, St. Louis, MO). Adjacent sections stained with LHE were used to determine the region of interest (ROI) for imaging data acquisition. MALDI-IMS was performed with MALDI-time-of-flight (TOF/TOF) Ultraflextreme mass spectrometer (Bruker Daltonics, Billerica, MA) in the negative ion mode by focusing a Smartbeam II Nd:YAG laser onto ~100-µm2 areas in corpus callosum WM. After determining that the within-group variability was low, four brains per group were used for IMS data acquisitions. The spectra from three datasets were combined for data reduction and further analysis in ClinProTools. External mass calibration was performed using 1 µL of a mixture of standard peptides (Peptide Calibration Standard II, Bruker Daltonics) and matrix (α-Cyano-4-hydroxycinnamic acid [HCCA]; Bruker Daltonics) deposited onto the slide after sublimation. This mixture provides seven calibration points in the mass range between 377 and 2463 Da and allows mass accuracy for phospholipids and sphingolipids.
Data analysis
MALDI-IMS data normalization to total ion count and visualization was performed with FlexImaging v4.0 software. Imaging spectra were processed by ClinProTools v3.0 (Bruker Daltonics). Each spectrum was baseline corrected, recalibrated, and a peak picking procedure was applied for further statistical analysis. Lipid identification was achieved by comparing mass-to-charge (m/z) values of precursor and product ions obtained by tandem mass spectrometry (MS/MS) analysis with those cataloged in the LIP-IDMAPS database. When MS/MS was inconclusive for lipid structure assignments, identification was made based on previously published reports. The average intensity of lipid ions per ROI was used in R-generated heatmaps and data barplots (version 3.2) to compare time course and recovery responses of ethanol relative to control samples. Barplots were generated in R using the ggplot2 module to visualize the mean percent changes in lipid ion expression. Heatmap hierarchical clustering analysis and data barplots were generated as reported (Nunez et al., 2016). Principal component analysis (PCA) generated by ClinProTools was used to compare lipid ion expression patterns among groups.
Results
Effects of chronic+binge ethanol exposures and recovery on brain weight and WM histopathology
Both control and ethanol-exposed rats tolerated their diets and continued to gain weight over the course of study. The slightly lower mean body weights in the ethanol groups were not statistically significant (Table 1). Blood alcohol concentrations were elevated at baseline and 30 min after binge exposure as previously reported (Zabala et al., 2015). There were no significant age-matched inter-group differences in mean brain weight after 3 or 8 weeks of liquid diet feeding. However, LHE-stained histological sections of brain revealed progressive ethanol exposure-associated reductions in myelin staining intensity and thickness of the corpus callosum relative to controls, and partial reversal of these ethanol effects after 12 days of recovery (Fig. 1).
Table 1.
Characteristics of time course of ethanol exposure and recovery model.
| 3 weeks exposure
|
8 weeks exposure
|
||||
|---|---|---|---|---|---|
| Control | Ethanol | Control | Ethanol | Recovery | |
| Body weight (g) | 342.9 ± 25.2 | 336.3 ± 26.0 | 455.3 ± 27.7 | 439.7 ± 34.8 | 474.8 ± 62.4 |
| Brain weight (g) | 1.9 ± 0.1 | 1.8 ± 0.1 | 2.0 ± 0.1 | 1.9 ± 0.1 | 1.9 ± 0.1 |
| Brain/body weight ratio | 0.0056 ± 0.0003 | 0.0055 ± 0.0004 | 0.0045 ± 0.0003 | 0.0044 ± 0.0003 | 0.0040 ± 0.0005 |
| Blood alcohol (mg/dL) | 20.8 ± 13.3 | 251.3 ± 30.7 | 17.3 ± 4.1 | 141.2 ± 54.1 | 18.0 ± 3.1 |
Rats were weighed weekly and weight gains in each group were similar. Body weight, brain weight, and calculated body/brain weight ratios (mean ± S.D.) at sacrifice are shown. Age-dependent inter-group comparisons at the 3-week and 8-week time points demonstrated no significant differences by unpaired two-tailed T tests. Blood alcohol concentrations measured with the Analox GM7 Analyzer (Analox Instruments, Lunenburg, MA) 30min after binge administration of 2g/kg ethanol were significantly higher in the ethanol-exposed group relative to the control and recovery groups (all p < 0.0001).
Fig. 1.
White matter histological responses to short-term (3 weeks) or long-term (8 weeks) chronic+binge ethanol exposures, and short-term recovery. Formalin-fixed, paraffin-embedded sections (8 µm thick) at the infundibulum level including dorsal hippocampus (hip) (A–E) and corpus callosum (F–J and asterisks in A–E) were stained with Luxol fast blue, hematoxylin and eosin. Representative results from the 3-week control (A, F); 3-week ethanol (B, G); 8-week control (C, H); 8-week ethanol (D, I); and 8-week ethanol/recovery groups (E, J) are shown. Luxol fast blue stains myelin blue; staining intensity corresponds with degree of myelination. The bars in Panels F–J have the same length; the degrees to which the bars overlap with cortex (ctx) reflect atrophy of the corpus callosum. Original magnifications prior to scaling: A–E, 80×; F–J, 320× (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
WM lipid profiles
The Peak Statistic report of the combined datasets identified 183 ions with mass-to-charge (m/z) ratios between 600 and 1000 Da. Structural assignments made by tandem mass spectrometry and previously published reports revealed that 151 ions were distinct lipids detected in their deprotonated forms ([M−H]−), and 32 were C13 isotopes (Supplementary Figure SFig. 1). Among the 183 lipids, 45 were characterized as sphingolipids (25%), 97 as phospholipids (53%), and 41 were unknown (22%). Sphingolipids were composed of 40 sulfatides (ST) (89%), 3 lactosylceramides (7%), and 2 sphingomyelins (4%). Phospholipids were characterized as phosphatidylserine (PS) (29; 30%), phosphatidylethanolamine (PE) (16; 16%), phosphatidylinositol (PI) (31; 32%), phosphatidylglycerol (PG) (5; 5%), or not explicitly identifiable (16%). Sphingolipids followed closely by PIs were the most abundantly expressed lipids in the corpus callosum. PSs were intermediate in abundance whereas PEs and PGs were expressed at very low levels.
Principal component analysis (PCA)
The 3-dimensional PCA plots distinguished ethanol exposure, duration, and recovery effects on WM sphingolipid and phospholipid profiles from controls. The ethanol-exposure clusters were distinctly separated from controls (Fig. 2A and B). In contrast, the recovery cluster, although distinct, partly overlapped with either the ethanol or control group (Fig. 2C), consistent with partial reversal of ethanol's effects on WM lipid ion profiles in the corpus callosum.
Fig. 2.
Principal Component Analysis (PCA) of white matter lipid profiles. MALDI-IMS (negative ion mode) lipid ion data (600–1000 Da mass range) from white matter were analyzed using PCA plots generated with ClinProTools. PCA plots compare results between control and ethanol-exposed rats at the 3-week (A1–A3) or 8-week (B1–B3) time points, and the 8-week control, 8-week ethanol, and 8-week ethanol + recovery groups (C1–C3). In the recovery group, rats were exposed to ethanol for 6 weeks, then ethanol was tapered over 72 h, and thereafter discontinued. Note the separation of control (blue) and ethanol (red) clusters in A and B and partial overlap of the recovery clusters (green) with those of the control and ethanol-exposed groups. Each PCA plot depicts data from separate age-matched groups (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Time course and recovery effects on sphingolipid and phospholipid expression
R-generated heatmaps with hierarchical clustering demonstrated differential ethanol exposure duration and recovery effects on sphingolipid and phospholipid expression (Fig. 3).
Fig. 3.
Heatmaps illustrating ethanol exposure duration and recovery effects on the expression levels of sphingolipids (A), phosphatidylserines (B), phosphatidylinositols (C), phosphatidylethanolamines (D), and phosphatidylglycerols (E) in white matter. Ion intensities are displayed using a 6-color palette corresponding to z-scores scaled to have a mean of 0 and standard deviation of 1.5. Hierarchical clustering dendrograms are shown for each lipid class. C13 isotopes were indicated (**). See Supplementary Figure SFig.1 for lipid ion characteristics. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Sphingolipids
The sphingolipid heatmap included 40 STs, 3 lactosylceramides, 2 sphingomyelins, and 2 dominant hierarchical clusters (Fig. 3A). In the upper section (Cluster a), high levels of sphingolipid expression were present in control WM, and lower levels were expressed in ethanol-exposed samples. The a1 and a2 sub-clusters were further distinguished by progressive declines in lipid abundance with longer durations of ethanol exposure, and partial reversal of these trends with respect to ST(40:0), ST(42:3), and C13 isotopes of ST(40:3[OH])** and LacCer(38:2)** following recovery. In contrast, in sub-cluster a3, ethanol-associated reductions in lipid expression were not duration-dependent and responses to recovery were mixed. The lower Cluster b, mainly composed of hydroxylated STs, was associated with very low lipid ion levels in control WM, moderately higher levels after 3 weeks of ethanol exposure, further increases after 8 weeks of ethanol exposure, and partial normalization following recovery.
Phosphatidylserines
Both major clusters in the hierarchical heatmap revealed PSs to be most abundantly expressed in control WM (Fig. 3B). Ethanol exposures reduced PS expression, but the effects varied with duration such that responses were detectable after 3 or 8 weeks. When they occurred after 3 weeks, the levels were either static or progressive with longer durations of ethanol exposure. Cluster a and Cluster b differed mainly with respect to the effects of short-term abstinence. In Cluster a, recovery did not reverse the inhibitory effects of ethanol, but in Cluster b, short-term abstinence broadly increased PS expression, partially abrogating the suppressive effects of ethanol. Further subgroup analysis revealed that recovery abetted expression of the 36- and 40-carbon PS ions, but not the 38-carbon PS ions.
Phosphatidylinositols
The PI heatmap, containing 29 lipids, had 2 dominant hierarchical clusters (a, b) (Fig. 3C). Sub-clusters a1, a2, and a3 were distinguished by high (a1), intermediate (a2), or low (a3) PI expression in control samples, but were alike in their common sharp reductions in PI expression after 3 weeks of ethanol exposure, and increased expression after longer duration of ethanol exposure, with or without a short period of recovery. Cluster b was characterized by high levels of PI expression in control WM, and progressive reductions in PI expression with longer durations of ethanol exposure. Short-term abstinence increased the levels of some species, including PI(34:1), PI(36:0), PI(36:4), PI(38:3), PI(38:4), PI(38:4)-O, and PI(38:5), but had no abrogating effects on ethanol's suppression of the 5 phosphorylated PI species detected, i.e., PIP(36:5), PI(P-38:3), PI(P-36:0), PIP(38:3), and PIP(38:4).
Phosphatidylethanolamines
Control WM had higher expression levels of all PEs detected compared with those measured in the ethanol-exposure groups, and for 11 of the 16 PEs, longer durations of ethanol exposure further reduced the levels of lipid ion expression (Fig. 3D). For 3 lipids, the expression levels were unchanged and for the remaining 2, the levels were increased after 8 weeks compared with 3 weeks of ethanol exposure. Short-term recovery partly reversed the inhibitory effects of ethanol on 5 lipids, i.e., PE(36:1), PE(36:2), PE(38:3), and C13 isotopes of PE(38:4)** and PE(38:5)**, and normalized expression of PE(38:5). In contrast, the short period of abstinence had either no effect or further reduced expression of 10 lipids, including mainly plasmalogens (PlsEtn).
Phosphatidylglycerols
Although PGs were very low in abundance and they comprised the smallest subgroup of lipid ions detected, their relative levels of expression were uniformly higher in controls than in all ethanol-exposed groups, with or without recovery (Fig. 3E). The time course effects of ethanol were variable, but recovery was consistently associated with the lowest or nearly the lowest levels of PG expression.
Data bar depiction of ethanol exposure and recovery effects on relative lipid ion expression
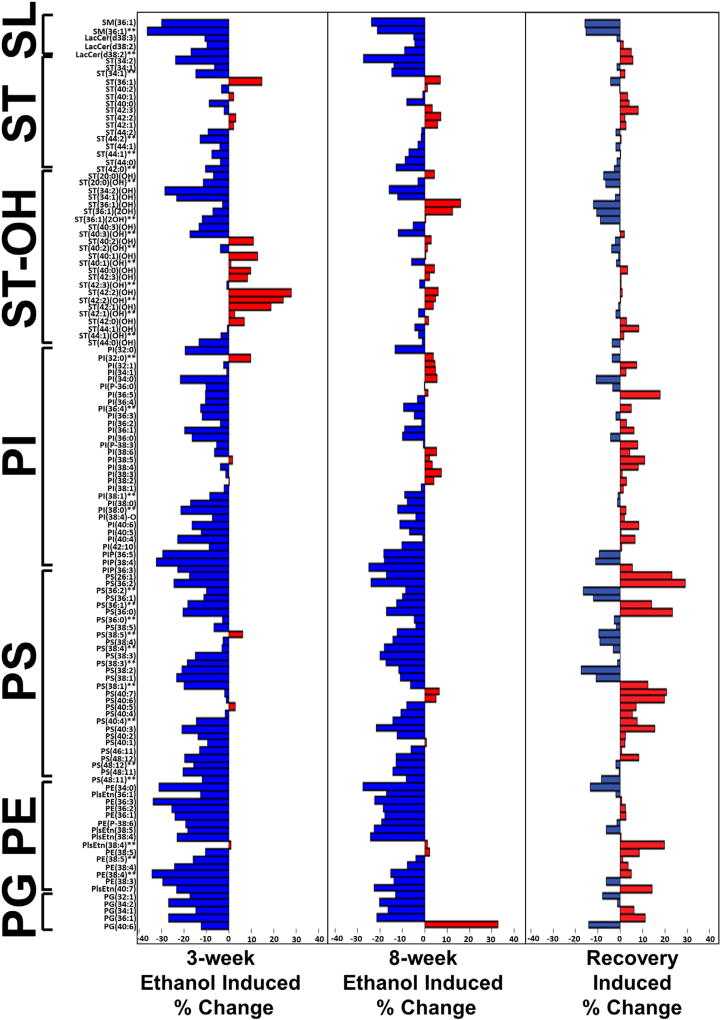
Data barplots subdivided by lipid class and ordered by m/z within each lipid class were used to make side-by-side comparisons of the 3-week and 8-week effects of ethanol exposure, relative to corresponding controls (Fig. 4). The results depicted in the data barplots reflect the calculated mean percentage changes in peak intensity (lipid ion abundance). Blue bars to the left depict reductions in lipid abundance, and red bars to the right reflect increased lipid ion expression in ethanol relative to control samples. Trends regarding the effects of ethanol were analyzed using Chi-square tests. Another advantage of the data barplots is that they depict proportional responses across the different classes of lipids irrespective of their relative abundance in WM.
Fig. 4.
Paired analysis of ethanol's effects on relative lipid ion abundance (peak intensity) after 3 weeks (A), 8 weeks (B), and short-term recovery (C). Data barplots were generated with the Ggplot2 module in R to depict the mean percentage changes in lipid ion abundance calculated for the 126 ions (n = 3 per group) identified as sphingolipids (SL), sulfatides (ST), hydroxylated sulfatides (ST-OH), phosphatidylinositols (PI), phosphatidylserines (PS), phosphatidylethanolamines (PE), or phosphatidylglycerols (PG). Lipids are listed in the ascending order based on the total number of carbon atoms in the structure. C13 isotopes were labeled with two asterisks (**) (Also see SFig. 1). Ethanol-associated reductions in lipid expression are represented by the blue bars to the left, and increases by the red bars to the right. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The data barplots show remarkably similar overall effects of ethanol on each lipid subtype after 3- or 8-week exposures. The major differences were that the inhibitory or stimulatory effects of ethanol were generally greater at the 3-week compared with the 8-week time point, suggesting that adaptive metabolic or age-related responses partially muted some of the early effects of ethanol exposure. Chi-square analysis of the overall time course-dependent (3 weeks versus 8 weeks) ethanol-mediated changes (increased, unchanged, decreased) was statistically significant (X2 = 6.25, 2 df; p = 0.044) (Fig. 5). Including the effects of recovery in the analysis demonstrated highly statistically significant inter-group differences in the relative shifts in lipid ion expression (X2 = 51.33, 4 df; p < 0.0001). Regarding sulfatides, Chi-square analysis of the time-dependent effects of ethanol revealed no significant difference in the relative responses of sulfatides. However, with recovery responses included in the analysis, there was a significant effect on the total pool of sulfatides (X2 = 11.22, 4 df; p = 0.024), a statistical trend effect on hydroxylated sulfatide expression (X2 = 9.07, 4 df; p = 0.059), but no significant effect on non-hydroxylated sulfatide expression.
Fig. 5.
Summary effects of ethanol exposure duration and recovery on lipid ion expression. Ethanol's aggregate effects on the expression levels of different lipid sub-classes relative to controls were evaluated statistically using Chi-square tests. The percentages of sulfatides (A), all phospholipids (B), phosphatidylethanolamines (C), phosphatidylglycerols (D), phosphatidylinositols (E), or phosphatidylserines (F) that were increased, unchanged, or decreased after 3 or 8 weeks of ethanol exposure, or 6 weeks exposure followed by 12 days recovery are depicted in the graphs. Chi-square test results are reported under Results. Significant p values are shown in the panels.
Ethanol exposure overwhelmingly reduced expression of all classes of phospholipids, including PS (93%), PE (94%), PI (90%), and PG (100%). Chi-square analysis revealed significant overall (including all subtypes) time course-dependent effects of ethanol on phospholipid ion expression (X2 = 126.4, 2 df; p < 0.0001); however, regarding phospholipid subtypes, significant ethanol time-course effects were only detected for PI (X2 = 8.51, 2 df; p = 0.014). Analysis of data from all groups revealed significant effects of ethanol and short-term recovery on PS (X2 = 27.61, 4 df; p < 0.0001), PE (X2 = 16.57, 4 df; p = 0.0023), PI (X2 = 21.58, 4 df; p = 0.0002), and all phospholipids combined (X2 = 201.5, 4 df; p < 0.0001), but not PG. The significant effects of ethanol were mainly due to suppression of lipid ion expression, whereas the effects of abstinence were largely due to differential reversal of ethanol's inhibitory effects on phospholipid expression.
Discussion
This study utilized three different time courses of chronic+binge ethanol exposures in Long Evans rats: short-term (3 weeks), long-term (8 weeks), and long-term (6 weeks) followed by recovery (12 days). The goal was to investigate the effects of these exposure conditions on WM lipid profiles in the corpus callosum. This chronic+binge ethanol exposure model was informative because it produced progressive time course-dependent WM atrophy with histopathological evidence of myelin loss within the corpus callosum, corresponding with abnormalities observed in human brains with ARBD. Furthermore, the partial recovery of WM myelin thickness and staining intensity after a short period of recovery is reminiscent of the abstinence effects in human alcoholics (Bartsch et al., 2007; Gazdzinski et al., 2010; Monnig et al., 2013; Pfefferbaum et al., 2006). The partial reversibility of histopathological changes suggests that the biochemical, metabolic, and molecular mediators of some aspects of ARBD-mediated WM atrophy are amenable to treatment. The rapidity of the responses suggests that the “correctable” defects pertain to mechanisms of myelin homeostatic maintenance. On the other hand, the incompleteness of recovery, and therefore possibly irreversible nature of injury/ degeneration, is likely due to other pathogenic factors, including axonal attrition and degeneration, as previously demonstrated by electron microscopy (Papp-Peka et al., 2016; Pfefferbaum et al., 2006). In addition to white matter degeneration, long-term chronic + binge ethanol exposure causes neurodegeneration with impairments in spatial learning and memory, and aberrant expression of mature neuronal-specific proteins in the temporal lobe (Tong et al., 2015; Tong et al., 2015).
The proportions of sphingolipids (25%) and phospholipids (53%) and the spectra of sphingolipids (sulfatides, sphingomyelins, and lactosylceramides) and phospholipids (phosphatidylserines, phosphatidylinositols, phosphatidylethanolamines, plasmalogens, and phosphatidylglycerols) identified in WM are consistent with previous reports in which MALDI-TOF IMS was performed in the negative ionization mode (Nunez et al., 2016; O'Brien & Sampson, 1965; Yalcin et al., 2015). In contrast, other abundant lipids in myelin, including cholesterol, phosphatidylcholine, and sphingomyelin, which form protonated ([M+H]+), sodiated ([M+Na]+), or potassiated ([M+K]+) adducts, were not detected because they are preferentially accessible via positive ionization mode mass spectrometry (Berry et al., 2011; Löhmann, Schachmann, Dandekar, Villmann, & Becker, 2010). These studies were focused on negative ionization mode MALDI-IMS to better characterize time-course effects of ethanol and abstinence on sulfatide expression, because previous reports suggested that sphingolipids were disproportionately targeted by alcohol exposure as well as other forms of WM degeneration (Roux et al., 2015; Yalcin et al., 2015).
Sulfatides, synthesized from galactose, ceramide, and sulfate via sequential reactions catalyzed by ceramide galactosyltransferase and cerebroside sulfotransferase, are important structural and functional constituents of myelin, including its formation (Honke, 2013; Takahashi & Suzuki, 2012) and stability (Coetzee et al., 1996). Therefore, the striking reductions in sulfatide caused by ethanol exposures may have contributed to the reductions in myelin (decreased Luxol fast blue staining) and atrophy of WM by impairing myelin formation and stability. In the context of normal myelin turnover, chronic+binge ethanol exposure could compromise both de novo biosynthesis and structural stability, leading to hypomyelination and enhanced myelin degradation. Increased degradation of sulfatides leads to increased ceramide levels. Ceramides play key roles in cell signaling pathways that mediate differentiation, senescence, proliferation, inflammation, and apoptosis (Adam, Heinrich, Kabelitz, & Schütze, 2002; Kolesnick & Krönke, 1998; Summers, 2006; Venable, Lee, Smyth, Bielawska, & Obeid, 1995). Moreover, their neurotoxic effects cause insulin resistance, which itself promotes oxidative injury, metabolic dysfunction, impairments in neuronal plasticity, myelin maintenance, and neuronal and glial cell survival in the brain (S. M. de la Monte et al., 2010). Correspondingly, increased ceramide levels have been reported in association with neurodegeneration (S. de la Monte, Derdak, & Wands, 2012; S. M. de la Monte, 2012; S. M. de la Monte, Re, Longato, & Tong, 2012; Yu et al., 2016), including ARBD (S. de la Monte et al., 2012; S. M. de la Monte, 2013; S. M. de la Monte, Longato, Tong, DeNucci, & Wands, 2009).
In contrast to its dominant inhibitory effects on sulfatides, ethanol exposure increased expression (abundance) of hydroxylated sulfatides. The very low abundance of hydroxylated sulfatides in control brains is consistent with an earlier MALDI-IMS study demonstrating that hydroxylated sulfatide expression is restricted to gray matter (Yuki et al., 2011). Hydroxylation at the 2-carbon position, as detected in half the WM sulfatides in ethanol-exposed brains, is catalyzed by fatty acid 2-hydroxylases (FA2H) (Alderson, Maldonado, Kern, Bhat, & Hama, 2006). The significance of this finding is unclear, because earlier studies suggested that hydroxylation increases intermolecular interactions that lead to reduced structural stability (Boggs, Koshy, & Rangaraj, 1984; Shah, Atienza, Rawlings, & Shipley, 1995), whereas later genetic studies suggest that hydroxylated sphingolipids positively influence myelin stability in aged brains (Edvardson et al., 2008; Zöller et al., 2008).
Phospholipids have important roles in regulating signal transduction at the plasma membrane level. PSs activate Akt networks that mediate cell survival, growth, proliferation, and metabolism via insulin-like growth factor, type 1 (IGF-1) signaling (Huang, Akbar, Kevala, & Kim, 2011) and enhance CNS WM myelination (Flores et al., 2008). The finding that ethanol inhibited expression of 93% of PSs suggests that the previously reported impairments in insulin and IGF-1 signaling through Akt pathways in various ethanol-exposure models (Andreani, Tong, Gundogan, Silbermann, & de la Monte, 2016; S.; de la Monte et al., 2012; S. M.; de la Monte & Wands, 2002; Deochand, Tong, Agarwal, Cadenas, & de la Monte, 2016; Ewenczyk, Ziplow, Tong, Le, & de la Monte, 2012; Steen et al., 2005; Xu et al., 2003), as well as in human ARBD (S. M. de la Monte, 2013; S. M. de la Monte et al., 2008), was mediated in part by reductions in myelin PS content. Another effect of long-term chronic+binge ethanol exposure was to reduce expression of PS(40:6), whose metabolite docosahexaenoic acid (DHA) is an omega-3 fatty acid that is essential for brain development and function (Kim, Akbar, & Kim, 2010). The recovery-associated partial normalization of PS expression, including PS(40:6), suggests that therapeutic measures designed to support or stabilize PS in myelin membranes could have broad effects in ameliorating structural and functional CNS WM pathology in ARBD.
PIs represent a diverse class of phospholipids that serve as substrates for lipid kinases that phosphorylate hydroxyl groups, and phospholipases, generating second messenger molecules for regulating cellular signaling, lipid signaling, and membrane fluidity, permeability, and trafficking (Cockcroft & Carvou, 2007; Crews et al., 1986; Gurtovenko & Anwar, 2009; Patra et al., 2006). Therefore, ethanol-mediated reductions in WM PI content could substantially impair a broad range of biological functions, including PI3 kinase activation of Akt pathways. An additional potential effect of ethanol is increased activation of phospholipases that hydrolyze phospholipid fatty acyl ester bonds. Ethanol stimulation of phospholipase C alters signaling by increasing formation of inositol-1,4,5-trisphosphate, Ca2+ release from intracellular stores sites leading to the formation of diacylglycerol, and stimulation of protein kinase C (Hoek & Rubin, 1990). Ethanol-mediated increases in PKC activity could lead to inhibition of myelin and chondroitin sulfate proteoglycans on axonal regeneration (Sivasankaran et al., 2004), and thereby contribute to WM atrophy and degeneration.
In conclusion, this study demonstrates temporal effects of chronic + binge ethanol exposures and short-term recovery on sphingolipid and phospholipid profiles in relation to WM atrophy. The results show that WM degeneration in ARBD is associated with broadly inhibited expression of lipids that have diverse roles in maintaining the structural and functional integrity of myelin, and to a lesser degree, axons. One potential mediator of these effects is that ethanol may impair expression and function of upstream enzymes that regulate oligodendrocyte myelin membrane lipid homeostasis.
Supplementary Material
Acknowledgments
Supported by F32 AA024018-01 (EBY), T32MH020068 (TMcL), R25GM083270 (TMcL), and AA-11431 (SmdlM) from the National Institutes of Health.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.alcohol.2017.05.008.
References
- Adam D, Heinrich M, Kabelitz D, Schütze S. Ceramide: Does it matter for T cells? Trends in Immunology. 2002;23:1–4. doi: 10.1016/s1471-4906(01)02091-9. [DOI] [PubMed] [Google Scholar]
- Alderson NL, Maldonado EN, Kern MJ, Bhat NR, Hama H. FA2H-dependent fatty acid 2-hydroxylation in postnatal mouse brain. Journal of Lipid Research. 2006;47:2772–2780. doi: 10.1194/jlr.M600362-JLR200. http://dx.doi.org/10.1194/jlr.M600362-JLR200. [DOI] [PubMed] [Google Scholar]
- Andreani T, Tong M, Gundogan F, Silbermann E, de la Monte SM. Differential effects of 3rd Trimester-Equivalent binge ethanol and tobacco-specific nitrosamine ketone exposures on brain insulin signaling in adolescence. Journal of Diabetes and Related Disorders. 2016;1:105–114. [PMC free article] [PubMed] [Google Scholar]
- Bartsch AJ, Homola G, Biller A, Smith SM, Weijers HG, Wiesbeck GA, et al. Manifestations of early brain recovery associated with abstinence from alcoholism. Brain. 2007;130:36–47. doi: 10.1093/brain/awl303. http://dx.doi.org/10.1093/brain/awl303. [DOI] [PubMed] [Google Scholar]
- Berry KA, Hankin JA, Barkley RM, Spraggins JM, Caprioli RM, Murphy RC. MALDI imaging of lipid biochemistry in tissues by mass spectrometry. Chemical Reviews. 2011;111:6491–6512. doi: 10.1021/cr200280p. http://dx.doi.org/10.1021/cr200280p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nature Protocols. 2013;8:627–637. doi: 10.1038/nprot.2013.032. http://dx.doi.org/10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertola A, Park O, Gao B. Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury in mice: A critical role for e-selection. Hepatology. 2013;58:1814–1823. doi: 10.1002/hep.26419. http://dx.doi.org/10.1002/hep.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs J, Koshy KM, Rangaraj G. Effect of fatty acid chain length, fatty acid hydroxylation, and various cations on phase behavior of synthetic cerebroside sulfate. Chemistry and Physics of Lipids. 1984;36:65–89. https://doi.org/10.1016/0009-3084(84)90090-2. [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, et al. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. http://dx.doi.org/10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Cockcroft S, Carvou N. Biochemical and biological functions of class I phosphatidylinositol transfer proteins. Biochimica et Biophysica Acta. 2007;1771:677–691. doi: 10.1016/j.bbalip.2007.03.009. http://dx.doi.org/10.1016/j.bbalip.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Coetzee T, Fujita N, Dupree J, Shi R, Blight A, Suzuki K, et al. Myelination in the absence of galactocerebroside and sulfatide: Normal structure with abnormal function and regional instability. Cell. 1996;86:209–219. doi: 10.1016/s0092-8674(00)80093-8. [DOI] [PubMed] [Google Scholar]
- Crews FT, Gonzales RA, Palovcik R, Phillips MI, Theiss C, Raizada M. Changes in receptor stimulated phosphoinositide hydrolysis in brain during ethanol administration, aging, and other pathological conditions. Psychopharmacology Bulletin. 1986;22:775–780. [PubMed] [Google Scholar]
- Deochand C, Tong M, Agarwal AR, Cadenas E, de la Monte SM. Tobacco smoke exposure impairs brain insulin/IGF signaling: Potential co-factor role in neurodegeneration. Journal of Alzheimer's Disease. 2016;50:373–386. doi: 10.3233/JAD-150664. http://dx.doi.org/10.3233/JAD-150664. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. http://dx.doi.org/10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- Edvardson S, Hama H, Shaag A, Gomori JM, Berger I, Soffer D, et al. Mutations in the fatty acid 2-hydroxylase gene are associated with leukodystrophy with spastic paraparesis and dystonia. American Journal of Human Genetics. 2008;83:643–648. doi: 10.1016/j.ajhg.2008.10.010. http://dx.doi.org/10.1016/j.ajhg.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estilaei MR, Matson GB, Payne GS, Leach MO, Fein G, Meyerhoff DJ. Effects of abstinence from alcohol on the broad phospholipid signal in human brain: An in vivo 31P magnetic resonance spectroscopy study. Alcoholism: Clinical and Experimental Research. 2001;25:1213–1220. [PubMed] [Google Scholar]
- Estruch R, Nicolas JM, Salamero M, Aragon C, Sacanella E, Fernandez-Sola J, et al. Atrophy of the corpus callosum in chronic alcoholism. Journal of the Neurological Sciences. 1997;146:145–151. doi: 10.1016/s0022-510x(96)00298-5. [DOI] [PubMed] [Google Scholar]
- Ewenczyk A, Ziplow J, Tong M, Le T, de la Monte SM. Sustained impairments in brain insulin/IGF signaling in adolescent rats subjected to binge alcohol exposures during development. Journal of Clinical and Experimental Pathology. 2012;2:106. doi: 10.4172/2161-0681.1000106. http://dx.doi.org/10.4172/2161-0681.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandis AZ, Wenk MR. Membrane lipids as signaling molecules. Current Opinion in Lipidology. 2007;18:121–128. doi: 10.1097/MOL.0b013e328082e4d5. http://dx.doi.org/10.1097/MOL.0b013e328082e4d5. [DOI] [PubMed] [Google Scholar]
- Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G, et al. Constitutively active Akt induces enhanced myelination in the CNS. The Journal of Neuroscience. 2008;28:7174–7183. doi: 10.1523/JNEUROSCI.0150-08.2008. http://dx.doi.org/10.1523/JNEUROSCI.0150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Mon A, Yeh PH, Meyerhoff DJ. Cerebral white matter recovery in abstinent alcoholics–a multimodality magnetic resonance study. Brain. 2010;133:1043–1053. doi: 10.1093/brain/awp343. http://dx.doi.org/10.1093/brain/awp343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtovenko AA, Anwar J. Interaction of ethanol with biological membranes: the formation of non-bilayer structures within the membrane interior and their significance. The Journal of Physical Chemistry B. 2009;113:1983–1992. doi: 10.1021/jp808041z. http://dx.doi.org/10.1021/jp808041z. [DOI] [PubMed] [Google Scholar]
- Harper C, Dixon G, Sheedy D, Garrick T. Neuropathological alterations in alcoholic brains. Studies arising from the new south Wales tissue resource centre. Progress in Neuropsychopharmacology and Biological Psychiatry. 2003;27:951–961. doi: 10.1016/S0278-5846(03)00155-6. http://dx.doi.org/10.1016/S0278-5846(03)00155-6. [DOI] [PubMed] [Google Scholar]
- Harper CG, Kril JJ, Holloway RL. Brain shrinkage in chronic alcoholics: A pathological study. British Medical Journal. 1985;290:501–504. doi: 10.1136/bmj.290.6467.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek JB, Rubin E. Alcohol and membrane-associated signal transduction. Alcohol and Alcoholism. 1990;25:143–156. doi: 10.1093/oxfordjournals.alcalc.a044989. [DOI] [PubMed] [Google Scholar]
- Honke K. Biosynthesis and biological function of sulfoglycolipids. Proceedings of the Japan Academy Series B, Physical and Biological Sciences. 2013;89:129–138. doi: 10.2183/pjab.89.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang BX, Akbar M, Kevala K, Kim HY. Phosphatidylserine is a critical modulator for Akt activation. The Journal of Cell Biology. 2011;192:979–992. doi: 10.1083/jcb.201005100. http://dx.doi.org/10.1083/jcb.201005100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Akbar M, Kim YS. Phosphatidylserine-dependent neuroprotective signaling promoted by docosahexaenoic acid. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2010;82:165–172. doi: 10.1016/j.plefa.2010.02.025. http://dx.doi.org/10.1016/j.plefa.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnick RN, Kronke M. Regulation of ceramide production and apoptosis. Annual Review of Physiology. 1998;60:643–665. doi: 10.1146/annurev.physiol.60.1.643. http://dx.doi.org/10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- Korade Z, Kenworthy AK. Lipid rafts, cholesterol, and the brain. Neuropharmacology. 2008;55:1265–1273. doi: 10.1016/j.neuropharm.2008.02.019. http://dx.doi.org/10.1016/j.neuropharm.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TK. Quantifying the risk for alcohol-use and alcohol-attributable health disorders: Present findings and future research needs. Journal of Gastroenterology and Hepatology. 2008;23(Suppl 1):S2–S8. doi: 10.1111/j.1440-1746.2007.05298.x. http://dx.doi.org/10.1111/j.1440-1746.2007.05298.x. [DOI] [PubMed] [Google Scholar]
- Löhmann C, Schachmann E, Dandekar T, Villmann C, Becker CM. Developmental profiling by mass spectrometry of phosphocholine containing phospholipids in the rat nervous system reveals temporospatial gradients. Journal of Neurochemistry. 2010;114:1119–1134. doi: 10.1111/j.1471-4159.2010.06836.x. http://dx.doi.org/10.1111/j.1471-4159.2010.06836.x. [DOI] [PubMed] [Google Scholar]
- Van Meer G, Voelker DR, Feigenson GW. Membrane lipids: Where they are and how they behave. Nature Reviews. Molecular Cell Biology. 2008;9:112–124. doi: 10.1038/nrm2330. http://dx.doi.org/10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnig MA, Tonigan JS, Yeo RA, Thoma RJ, McCrady BS. White matter volume in alcohol use disorders: A meta-analysis. Addiction Biology. 2013;18:581–592. doi: 10.1111/j.1369-1600.2012.00441.x. http://dx.doi.org/10.1111/j.1369-1600.2012.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM. Disproportionate atrophy of cerebral white matter in chronic alcoholics. Archives of Neurology. 1988;45:990–992. doi: 10.1001/archneur.1988.00520330076013. [DOI] [PubMed] [Google Scholar]
- de la Monte SM. Metabolic derangements mediate cognitive impairment and Alzheimer's disease: Role of peripheral insulin-resistance diseases. Pan-minerva Medica. 2012;54:171–178. [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM. Alcohol-induced liver and brain degeneration: Roles of insulin resistance, toxic ceramides, and endoplasmic reticulum stress. In: Watson RR, Preedy VR, Zibadi S, editors. Alcohol, nutrition, and Health consequences. Humana Press; 2013. pp. 507–522. [Google Scholar]
- de la Monte S, Derdak Z, Wands JR. Alcohol, insulin resistance and the liver-brain axis. Journal of Gastroenterology and Hepatology. 2012;27(Suppl 2):33–41. doi: 10.1111/j.1440-1746.2011.07023.x. http://dx.doi.org/10.1111/j.1440-1746.2011.07023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Kril JJ. Human alcohol-related neuropathology. Acta Neuropathologica. 2014;127:71–90. doi: 10.1007/s00401-013-1233-3. http://dx.doi.org/10.1007/s00401-013-1233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Longato L, Tong M, DeNucci S, Wands JR. The liver-brain axis of alcohol-mediated neurodegeneration: Role of toxic lipids. International Journal of Environmental Research and Public Health. 2009;6:2055–2075. doi: 10.3390/ijerph6072055. http://dx.doi.org/10.3390/ijerph6072055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Re E, Longato L, Tong M. Dysfunctional pro-ceramide, ER stress, and insulin/IGF signaling networks with progression of Alzheimer's disease. Journal of Alzheimer's Disease. 2012;30(Suppl 2):S217–S229. doi: 10.3233/JAD-2012-111728. http://dx.doi.org/10.3233/JAD-2012-111728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Tong M, Cohen AC, Sheedy D, Harper C, Wands JR. Insulin and insulin-like growth factor resistance in alcoholic neuro-degeneration. Alcoholism: Clinical and Experimental Research. 2008;32:1630–1644. doi: 10.1111/j.1530-0277.2008.00731.x. http://dx.doi.org/10.1111/j.1530-0277.2008.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Tong M, Nguyen V, Setshedi M, Longato L, Wands JR. Ceramide-mediated insulin resistance and impairment of cognitive-motor functions. Journal of Alzheimer's Disease. 2010;21:967–984. doi: 10.3233/JAD-2010-091726. http://dx.doi.org/10.3233/JAD-2010-091726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Wands JR. Chronic gestational exposure to ethanol impairs insulin-stimulated survival and mitochondrial function in cerebellar neurons. Cellular and Molecular Life Sciences. 2002;59:882–893. doi: 10.1007/s00018-002-8475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez K, Kay J, Krotow A, Tong M, Agarwal AR, Cadenas E, et al. Cigarette smoke-induced alterations in frontal white matter lipid profiles demonstrated by MALDI-imaging mass Spectrometry: Relevance to Alzheimer's disease. Journal of Alzheimer's Disease. 2016;51:151–163. doi: 10.3233/JAD-150916. http://dx.doi.org/10.3233/JAD-150916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JS, Sampson EL. Lipid composition of the normal human brain: Gray matter, white matter, and myelin. Journal of Lipid Research. 1965;6:537–544. [PubMed] [Google Scholar]
- Papp-Peka A, Tong M, Kril JJ, de la Monte SM, Sutherland GT. The differential effects of alcohol and nicotine-specific nitrosamine ketone on white matter Ultrastructure. Alcohol and Alcoholism. 2016;52:165–171. doi: 10.1093/alcalc/agw067. http://dx.doi.org/10.1093/alcalc/agw067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra M, Salonen E, Terama E, Vattulainen I, Faller R, Lee BW, et al. Under the influence of alcohol: The effect of ethanol and methanol on lipid bilayers. Biophysical Journal. 2006;90:1121–1135. doi: 10.1529/biophysj.105.062364. http://dx.doi.org/10.1529/biophysj.105.062364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiology of Aging. 2006;27:994–1009. doi: 10.1016/j.neurobiolaging.2005.05.007. http://dx.doi.org/10.1016/j.neurobiolaging.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Desmond JE, Sullivan EV. Thinning of the corpus callosum in older alcoholic men: A magnetic resonance imaging study. Alcoholism: Clinical and Experimental Research. 1996;20:752–757. doi: 10.1111/j.1530-0277.1996.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Adalsteinsson E, Sullivan EV. Diffusion tensor imaging with quantitative fibre tracking in HIV infection and alcoholism comorbidity: Synergistic white matter damage. Brain. 2007;130(Pt 1):48–64. doi: 10.1093/brain/awl242. http://dx.doi.org/10.1093/brain/awl242. [DOI] [PubMed] [Google Scholar]
- Phillips SC, Harper CG, Kril J. A quantitative histological study of the cerebellar vermis in alcoholic patients. Brain. 1987;110(Pt 2):301–314. doi: 10.1093/brain/110.2.301. [DOI] [PubMed] [Google Scholar]
- Roux A, Muller L, Jackson SN, Baldwin K, Womack V, Pagiazitis JG, et al. Chronic ethanol consumption profoundly alters regional brain ceramide and sphingomyelin content in rodents. ACS Chemical Neuroscience. 2015;6:247–259. doi: 10.1021/cn500174c. http://dx.doi.org/10.1021/cn500174c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux A, Muller L, Jackson SN, Post J, Baldwin K, Hoffer B, et al. Mass spectrometry imaging of rat brain lipid profile changes over time following traumatic brain injury. Journal of Neuroscience Methods. 2016;272:19–32. doi: 10.1016/j.jneumeth.2016.02.004. http://dx.doi.org/10.1016/j.jneumeth.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KS, Gallo JL, Ferri C, Giovannetti T, Sestito N, Libon DJ, et al. The neuropsychological profile of alcohol-related dementia suggests cortical and subcortical pathology. Dementia and Geriatric Cognitive Disorders. 2005;20:286–291. doi: 10.1159/000088306. http://dx.doi.org/10.1159/000088306. [DOI] [PubMed] [Google Scholar]
- Schmitt S, Castelvetri LC, Simons M. Metabolism and functions of lipids in myelin. Biochimica et Biophysica Acta. 2015;1851:999–1005. doi: 10.1016/j.bbalip.2014.12.016. http://dx.doi.org/10.1016/j.bbalip.2014.12.016. [DOI] [PubMed] [Google Scholar]
- Schulte T, Sullivan EV, Müller-Oehring EM, Adalsteinsson E, Pfefferbaum A. Corpus callosal microstructural integrity influences interhemispheric processing: A diffusion tensor imaging study. Cerebral Cortex. 2005;15:1384–1392. doi: 10.1093/cercor/bhi020. http://dx.doi.org/10.1093/cercor/bhi020. [DOI] [PubMed] [Google Scholar]
- Shah J, Atienza JM, Rawlings AV, Shipley GG. Physical properties of ceramides: Effect of fatty acid hydroxylation. Journal of Lipid Research. 1995;36:1945–1955. [PubMed] [Google Scholar]
- Sivasankaran R, Pei J, Wang KC, Zhang YP, Shields CB, Xu XM, et al. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nature Neuroscience. 2004;7:261–268. doi: 10.1038/nn1193. http://dx.doi.org/10.1038/nn1193. [DOI] [PubMed] [Google Scholar]
- Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease-is this type 3 diabetes? Journal of Alzheimer's Disease. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- Summers SA. Ceramides in insulin resistance and lipotoxicity. Progress in Lipid Research. 2006;45:42–72. doi: 10.1016/j.plipres.2005.11.002. http://dx.doi.org/10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Suzuki T. Role of sulfatide in normal and pathological cells and tissues. Journal of Lipid Research. 2012;53:1437–1450. doi: 10.1194/jlr.R026682. http://dx.doi.org/10.1194/jlr.R026682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong M, Yu R, Deochand C, de la Monte SM. Differential contributions of alcohol and the nicotine-derived nitrosamine ketone (NNK) to insulin and insulin-like growth factor resistance in the adolescent rat brain. Alcohol and Alcoholism. 2015;50:670–679. doi: 10.1093/alcalc/agv101. http://dx.doi.org/10.1093/alcalc/agv101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong M, Yu R, Silbermann E, Zabala V, Deochand C, de la Monte SM. Differential contributions of alcohol and nicotine-derived nitrosamine ketone (NNK) to white matter pathology in the adolescent rat brain. Alcohol and Alcoholism. 2015;50:680–689. doi: 10.1093/alcalc/agv102. http://dx.doi.org/10.1093/alcalc/agv102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venable ME, Lee JY, Smyth MJ, Bielawska A, Obeid LM. Role of ceramide in cellular senescence. The Journal of Biological Chemistry. 1995;270:30701–30708. doi: 10.1074/jbc.270.51.30701. [DOI] [PubMed] [Google Scholar]
- Wang HY, Jackson SN, Post J, Woods AS. A minimalist approach to MALDI imaging of Glycerophospholipids and sphingolipids in rat brain sections. International Journal of Mass Spectrometry. 2008;278:143–149. doi: 10.1016/j.ijms.2008.04.005. http://dx.doi.org/10.1016/j.ijms.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Yeon JE, Chang H, Tison G, Chen GJ, Wands J, et al. Ethanol impairs insulin-stimulated neuronal survival in the developing brain: Role of PTEN phosphatase. The Journal of Biological Chemistry. 2003;278:26929–26937. doi: 10.1074/jbc.M300401200. http://dx.doi.org/10.1074/jbc.M300401200. [DOI] [PubMed] [Google Scholar]
- Yalcin EB, de la Monte SM. Review of matrix-assisted laser desorption ionization-imaging mass spectrometry for lipid biochemical histopathology. The Journal of Histochemistry and Cytochemistry. 2015;63:762–771. doi: 10.1369/0022155415596202. http://dx.doi.org/10.1369/0022155415596202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin EB, Nunez K, Tong M, de la Monte SM. Differential sphin-golipid and phospholipid profiles in alcohol and nicotine-derived nitrosamine ketone-associated white matter degeneration. Alcoholism: Clinical and Experimental Research. 2015;39:2324–2333. doi: 10.1111/acer.12909. http://dx.doi.org/10.1111/acer.12909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Deochand C, Krotow A, Leão R, Tong M, Agarwal AR, et al. Tobacco smoke-induced brain white matter myelin Dysfunction: Potential Co-Factor role of smoking in neurodegeneration. Journal of Alzheimer's Disease. 2016;50:133–148. doi: 10.3233/JAD-150751. http://dx.doi.org/10.3233/JAD-150751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki D, Sugiura Y, Zaima N, Akatsu H, Hashizume Y, Yamamoto T, et al. Hydroxylated and non-hydroxylated sulfatide are distinctly distributed in the human cerebral cortex. Neuroscience. 2011;193:44–53. doi: 10.1016/j.neuroscience.2011.07.045. http://dx.doi.org/10.1016/j.neuroscience.2011.07.045. [DOI] [PubMed] [Google Scholar]
- Zabala V, Tong M, Yu R, Ramirez T, Yalcin EB, Balbo S, et al. Potential contributions of the tobacco nicotine-derived nitrosamine ketone (NNK) in the pathogenesis of steatohepatitis in a chronic plus binge rat model of alcoholic liver disease. Alcohol and Alcoholism. 2015;50:118–131. doi: 10.1093/alcalc/agu083. http://dx.doi.org/10.1093/alcalc/agu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zöller I, Meixner M, Hartmann D, Büssow H, Meyer R, Gieselmann V, et al. Absence of 2-hydroxylated sphingolipids is compatible with normal neural development but causes late-onset axon and myelin sheath degeneration. The Journal of Neuroscience. 2008;28:9741–9754. doi: 10.1523/JNEUROSCI.0458-08.2008. http://dx.doi.org/10.1523/JNEUROSCI.0458-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.