Abstract
This study aimed at determining whether audiovisual training without linguistic material has a remediating effect on reading skills and central auditory processing in dyslexic children. It was found that this training resulted in plastic changes in the auditory cortex, indexed by enhanced electrophysiological mismatch negativity and faster reaction times to sound changes. Importantly, these changes were accompanied by improvement in reading skills. The results indicate that reading difficulties can be ameliorated by special training programs and, further, that the training effects can be observed in brain activity. Moreover, the fact that the present training effects were obtained by using a program including no linguistic material indicates that dyslexia is at least partly based on a general auditory perceptual deficit.
Neural dysfunctions underlying dyslexia are still largely unknown despite decades of research. Dyslexia has been identified as a problem of phonological processing (1–5), although other difficulties like those in visual processing have also been reported (6–8). Dyslexic individuals might actually suffer from a more general auditory-perception problem, which may underlie their difficulties in phonological perception (9–18). For example, some authors suggest that these individuals have problems in processing temporal aspects of the speech signal, such as rapid acoustic transitions or tone-order reversals (9, 14–17). However, even some other aspects of sounds, such as rhythm or pitch, are problematic for individuals with dyslexia (13, 18). The evidence suggesting that these individuals have dysfunctions also in their nonlinguistic auditory and visual perception (for a review, see ref. 17) supports the view that a general sensory-processing disorder is involved. However, the role of these dysfunctions in the etiology of dyslexia is unclear. It is possible that deficits in nonspeech sound discrimination in dyslexia might just co-occur with it, having no causal role in the reading problems.
The high incidence of dyslexia and its associated role in learning impairments (19) accentuate the importance of finding effective methods for remediating dyslexic children. Because of the central nervous system's higher plasticity in early than later developmental stages (20, 21), remediation programs should be started as early as possible. Therefore, in the present study, we tested the effects of a training program for dyslexic children in the first grade of school. A training program containing no linguistic material was used to determine whether perceptual training that does not involve the phonological system could have a beneficial effect on reading impairment. We used a training “game” in which dyslexic individuals have been found to perform significantly worse than nondyslexic control subjects (22). The effects of the training program were evaluated by measuring subjects' brain activity, behavioral stimulus discrimination, and reading skills.
Brain activity was measured by recording the mismatch negativity (MMN), which accurately reflects auditory discrimination without such biasing factors as attention, decision making, or motor response (23). The MMN can be elicited by any discriminable change in a sequence of auditory stimuli, its amplitude and latency indicating how well sound changes are discriminated; the easier the discrimination, the larger the amplitude and shorter the latency (24). Although the MMN amplitude can be modulated by attention under some conditions (25), it is even elicited with no behavioral task, which enables one to determine sound-discrimination ability even in individuals with communication problems such as aphasic or comatose patients and infants (26–28). Furthermore, previous studies have shown that the auditory dysfunctions in language-impaired and dyslexic individuals can be evaluated with the MMN (T.K., S.B., M.T., and R.N., unpublished observations; refs. 3, 13, 18, 29). For instance, it was found that the MMN amplitude for a consonant change occurring within a syllable (3), for pitch changes in tones (18), for rhythm changes in sound patterns (13), and for tone-order reversals (T.K., S.B., M.T., and R.N., unpublished observations) is diminished in dyslexia. Moreover, the MMN reduction was paralleled by a decrement in behavioral performance in dyslexic individuals (13, 18).
In addition, several studies (30–33) have shown that the MMN can be used as a measure of cortical plastic changes induced by successful discrimination training. In these studies, no MMN was initially elicited by slight sound changes that subjects could not discriminate. However, after discrimination training, the MMN emerged in those subjects who learned behaviorally to discriminate the stimulus changes.
Materials and Methods
Reading-Skill Tests.
Four basic reading-skill measures (34) were included in the reading-skill evaluations of the present study. These measures are subtests of a Finnish battery devised to diagnose the reading skills in preschool and first-grade children. These measures included spelling (counting the syllables of words), deleting the first phoneme of words, and reading short words (correctness and speed). The normative data for this test battery for the correctness and speed in reading short words had been obtained from first-grade children in their spring semester (age 7 years; 227 children) and those for spelling (176 children) and deleting the first phoneme (193 children) from preschool children (age 6 years).
Subjects.
Forty-eight 7-year-old children, who were initially screened as reading impaired by the school, participated in the present study. To ensure that these children indeed were reading impaired, their reading skills were compared with those of six age-matched peers who were typically developing in reading skills. It was found that the dyslexic children performed significantly worse in each subtest than did children who were making normal progress in reading skills [F(1,52) = 6.30–14.48, P < 0.02–0.001; one-way ANOVA]. The average test scores in each subtest were for nondyslexic and dyslexic children, respectively: spelling, 9.8 and 7.1 of the maximum of 10 points (SEM 0.2 and 0.4); deleting the first phoneme, 9.8 and 6.3 (10-point scale, SEM 0.2 and 0.36); correctly read words, 29.8 and 23.6 (30-point scale, SEM 0.2 and 1.0); reading speed, 0.9 and 5.7 (seconds/word, SEM 0.2 and 0.5).
The dyslexic children were divided into two groups, training group (9 girls, 15 boys), participating in the audiovisual training, and control group (11 girls, 13 boys), in a pseudorandom manner in the beginning of their first spring semester. The primary criterion in forming two comparable groups was the children's scores in the reading test (34). In addition, the children's scores in the test version of the audiovisual training program (to be explained later) and their gender were taken into account in forming these groups. Statistical analyses confirmed that before the training period, there were no significant differences in the scores of the reading-skill test or the test version of the computer game between the two groups (see Table 1 and Fig. 2). The reading skills and the performance in the training program of these children were measured again after the training period.
Table 1.
Performance data
| Training group (SEM)
|
Control group (SEM)
|
|||
|---|---|---|---|---|
| 1st measurement | 2nd measurement | 1st measurement | 2nd measurement | |
| IQ | 98.00 (3.50) | 93.00 (4.00) | ||
| Words/correct* | 23.79 (0.97) | 29.38 (0.56) | 23.34 (1.22) | 26.78 (0.96) |
| Speed; s/word | 5.85 (0.77) | 2.34 (0.29) | 5.60 (0.53) | 3.37 (0.45) |
| Spelling | 6.71 (0.54) | 8.96 (0.35) | 7.54 (0.52) | 9.33 (0.22) |
| Rem. 1st phon. | 6.42 (0.52) | 8.87 (0.26) | 6.21 (0.51) | 8.08 (0.48) |
| Comp. test | 21.71 (1.10) | 27.75 (0.52) | 21.04 (1.02) | 24.96 (0.70) |
| Hit % | 89.33 (4.17) | 92.50 (2.50) | 80.83 (8.83) | 88.33 (5.57) |
| FA % | 23.67 (12.17) | 11.17 (7.33) | 23.00 (6.50) | 20.00 (11.00) |
| RT (ms) | 690.00 (59.00) | 583.00 (59.00) | 801.00 (64.00) | 779.00 (45.00) |
Significant differences between the groups are marked with bold (IQ, Hit%, FA% from subgroups of 11 children).
Words/correct, the number of correctly read words (maximum 30); speed; s/word, reading speed, seconds/word; Spelling, counting the number of syllables in words; Rem 1st phon., removing the 1st phoneme of words; Comp. test, the score in the computer test (maximum 30); Hit %, hit rate; and FA%, false-alarm rate.
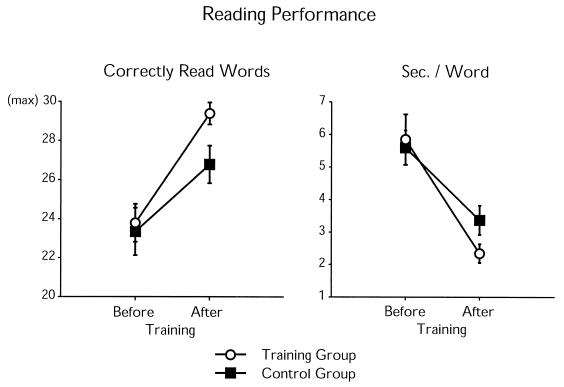
Figure 2.
Reading performance before and after the training period. (Left) The average number (with standard errors of the mean) of correctly read words. (Right) The average reading speed (seconds per word). No significant group differences were found before the training period, whereas after it, the training group read correctly significantly more words than the control group (24 children in each group) and was nearly significantly faster in reading.
Subgroups of the dyslexic children including 11 children in each group (five girls and six boys in the training group and six girls and five boys in the control group) participated in additional measurements, which involved event-related potentials (ERPs) (to obtain the MMN) as well as behavioral target detection (administered after the ERP recording session) before and after the training period. In addition, the performance IQ of these children was evaluated before the training period with WISC III (subtests: picture completion, coding, picture arrangement, block design, and object assembly), which indicated no significant IQ differences between the groups (Table 1).
Training Procedure.
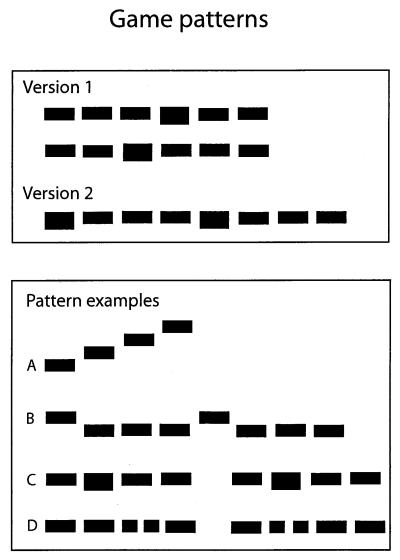
A computer game consisting of abstract, nonverbal tasks that require audiovisual matching (22) was used for training. In this game, various sound patterns with 3–15 elements were graphically presented on a computer screen as horizontal sequences of rectangles (Fig. 1). The sound elements varied in pitch, duration, and intensity, which were visually represented by the vertical position, length, and thickness of the rectangles on the screen, respectively. Both easy and difficult patterns were randomly presented throughout the training period. Each training session began with a stimulus block with a 1,000-ms stimulus (element)-onset asynchrony (SOA) and a 550-ms sound duration. During the sessions, subjects could change the SOA within a window of 200-1800 ms and the sound duration within a window of 30–80% of the SOA (60–1440 ms).
Figure 1.
(Upper) Task examples of the computer game version 1 and 2 are presented. In version 1, the child had to choose which of the two visual patterns corresponds to the sound pattern that is played. In version 2, only one pattern is displayed on the screen, after which a sound pattern is played. The child has to press the space bar when the sound corresponding to the last element of the visual pattern is being played. (Lower) Examples of the patterns used in the game. An elevation of a visual element corresponds to a higher pitch sound, whereas sound intensity is visually coded with element thickness and sound duration with element length. (A) A simple good Gestalt formed by the upward scale. Although the pattern itself is simple, the lack of repetition complicates the perception of the structure. (B) Finding subgroups according to good Gestalts. Although there is no pause between the subgroups, the accents clearly mark their beginnings, which helps one to follow the pattern. (C) The subgroups clearly marked by the pause. The accents are perceived the easiest as the beginnings of the subgroups; the positions of the accents here thus require some structuring against good Gestalts. (D) Patterns having the same elements but different structures. Because the most probable expectation on the second group of elements is the repetition of the first, this task requires the change of expectations. Perceiving differences within the subgroups is also needed, for the beginnings and ends of the groups are similar.
Two versions of the game were used in the present study. In version 1, two patterns were drawn on the screen. After a couple of seconds, a sound sequence was played that corresponded to one of the patterns. The player's task was to indicate which one of the patterns was played. In version 2, one pattern was first drawn on the screen and then a corresponding sound sequence was played. The player's task was to follow the pattern (from left to right) as it was being played and to press the space bar at the moment when the last element of the pattern was being played. After a correct response, the subject was rewarded by a smiling face and the color change of the screen, whereas after an incorrect response, the same pattern repeated showing the correct timing so that the color of the rectangle changed at the moment when the sound corresponding to it was played.
There were 14 training sessions with the computer game during a period of 7 weeks. Each training session lasted for about 10 min and occurred twice a week. Both versions of the game were used, version 1 during the first four training sessions and version 2 during the remaining sessions. The training was started with game version 1, for it involved no time pressure in responding and was therefore easier than version 2 for the child to get familiarized with the training program.
Electrophysiological Recordings.
The MMN was recorded to infrequent order reversals of tone pairs (35). Subjects were presented with sequences of tone pairs with a constant pair-onset asynchrony of 600 ms. Each tone was 40 ms in duration and 70 dB (sound pressure level) in intensity. In the standard pair, which occurred on 90% of the trials, the first tone was 500 Hz and the second one 750 Hz, separated by a 10-ms silent gap. In the deviant pair, occurring randomly on 10% of the trials, the tone order was reversed.
ERPs were obtained from the nose-referenced electroencephalogram (0.1–100 Hz, sampling rate 250 Hz) by off-line averaging electroencephalogram epochs separately for the standard and deviant stimulus pairs. The analysis epoch began 100 ms before and ended 600 ms after the stimulus-pair onset. Voltage variation caused by horizontal eye movements was monitored with an electrode attached to the outer canthus of the right eye and that caused by vertical eye movements with an electrode on the forehead. Epochs contaminated by eye movements or other extracerebral artifacts producing voltage variation exceeding ±60 μV at any electrode were omitted.
ERPs were separately averaged for standard and deviant pairs and digitally filtered by using a bandpass of 1–30 Hz. Before data analysis, the responses were re-referenced to the average of the left and right mastoids to maximize the MMN signal. The MMN was delineated in the difference waveforms, obtained by subtracting the ERP elicited by the standard pair from that elicited by the deviant pair. In this way, an estimate of the neural activity associated with the discrimination of deviant stimuli from standard stimuli could be obtained (23). The MMN amplitude was quantified by measuring the MMN in the individual difference waveforms using consecutive 25-ms measurement windows between 200 and 325 ms from tone-pair onset. The amplitude comparisons were performed with one-way ANOVA at the Fz electrode, where the signal-to-noise ratio for the MMN is largest (23).
Behavioral Recordings.
The behavioral performance of the children was evaluated in two ways. First, we investigated how the children behaviorally discriminated the tone-order reversals in a target-discrimination task administered after the MMN sessions. A brief training session was conducted first in which the child was presented with single tones (500- and 750-Hz tones as the standard and deviant stimuli, respectively) to familiarize him/her with the task. The behavioral session consisted of 48 trains of four stimulus pairs (divided to four separate stimulus blocks), with 600-ms SOA of the pairs within the trains. Each sound-pair train was separated from the next one with a silent pause of 3 s. In one-half of the sound-pair trains, the fourth stimulus pair was a deviant pair, whereas in the rest it was a standard pair. The order of the sound-pair trains was randomized. The child's task was to press a response key with the thumb of the preferred hand when he/she heard a deviant sound pair. Button presses occurring within 200–3,000 ms after the onset of a deviant sound pair were regarded as hits and those occurring at any other time as false alarms.
Second, the performance in the test version of the computer game was measured. This test version (a variant of game version 2) includes a set of 30 audiovisual matching tasks. The stimulus elements were presented with a 1,000-ms SOA and sounds with a duration of 550 ms throughout the test. The number of hits was registered by space-bar presses occurring during the time window when the last sound of the pattern is played.
Results
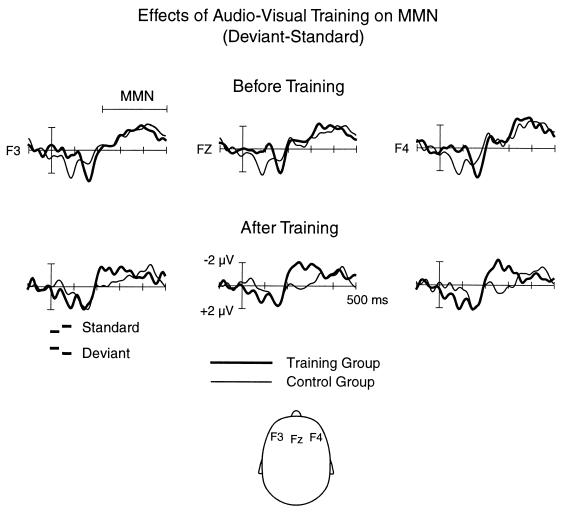
Group differences following the audiovisual training period were found in the reading-skill test as well as in the electrophysiological and behavioral measures. The group comparison of the reading-skill measures¶ revealed that whereas there were no group differences before the training, the children in the training group read significantly more words correctly after the training period than did the control-group children [F(1,46) = 5.54, P < 0.03; Fig. 2 and Table 1]. In addition, after the training, the reading speed was marginally significantly higher in the training group than in the control group [F(1,46) = 3.67, P < 0.07]. Training effects were also found in the electrophysiological and behavioral measures (obtained from the subgroups of the children), whereas the groups did not significantly differ from each other in the performance IQ (see Table 1). In the two groups, the MMNs elicited by the tone-order reversals were very similar in amplitude and morphology before the training (see Fig. 3), there being no significant amplitude differences. In contrast, after the training period, the MMN amplitude was considerably increased in the training group but not in the control group, with a significant difference between the groups [F(1,20) = 5.01–7.20, P < 0.03; 225–275 ms from pair onset]. Moreover, the MMN amplitude of the training group was significantly larger after the training compared with that before the training [F(1,10) = 10.15, P < 0.03; 225–250 ms from pair onset], whereas no such amplitude change was found for the control group.
Figure 3.
Electrophysiological responses of dyslexic children in the training and control groups (11 children in each group) at the frontal scalp to tone-order reversals (the response elicited by the standard pair subtracted from that elicited by the deviant pair). The responses of the training group are presented with the thick line and those of the control group (with no audiovisual training) with the thin line. Responses before (Upper) and after (Lower) the audiovisual training are shown. No significant group differences were obtained for the responses measured before the training. In contrast, after the training, the earlier parts of the MMN response were enhanced in the training group as compared with those of the control group.
The results were further analyzed by testing the correlation of the change in the MMN amplitude from the first to the second recording session with the change in the reading-skill scores. The change of the reading-skill scores from the first to the second reading test for each child was calculated as the difference between the unweighted sums of the scores of the four reading-skill measures. A significant correlation was found between the MMN amplitude change and the change in reading performance (r = 0.42, P < 0.03, one-tailed Spearman test).
The analyses of the behavioral data indicated no significant differences between the groups or between the first and second recording sessions in hit or false-alarm rates. However, the reaction time (RT) was shorter in the training group than in the control group in the second recording session [F(1,17) = 7.13, P < 0,02; two children from the training group and one child from the control group were missing from the analyses because of their refusal to participate], whereas no group differences were found in the first recording session. In addition, the training group performed significantly better than the control group in the second recording session [F(1,46) = 10.36, P < 0.002] in the test version of the computer game, whereas there were no significant group differences in the first recording session (all 48 children included).
Discussion
Our results suggest that perceptual training with nonlinguistic audiovisual stimuli causes plastic changes in the neural substrate of sound discrimination and an improvement in reading skills. This reading-skill improvement was associated with changes at the early automatic neural level of sound discrimination, as reflected by the MMN, and at the behavioral level in the RTs and performance in the test version of the computer game. The fact that training even altered the early preattentive stage of sound discrimination while also improving reading performance gives support to the view that reading difficulties in dyslexic individuals, at least in part, stem from bottom-up processing constraints (see also ref. 36).
Several studies have shown a correlation between nonlinguistic processing and reading skills (37) as well as between acoustic temporal-processing problems and language-related impairments, including dyslexia (12–17, 19). Our results support this view that difficulties in dyslexia are based, at least to some extent, on the dysfunction of general sensory discrimination rather than on deficit specific to phonological processing. However, no previous study has shown that training to discriminate nonspeech stimuli improves language-related abilities. For example, Tallal et al. (36) and Merzenich et al. (38) used nonspeech as well as speech stimuli in training language learning-impaired children, which does not permit one to specify the role of nonspeech stimuli in causing the improvement in children's ability to discriminate speech contrasts. The current results indicating that training with nonlinguistic stimuli does improve language functions, such as those involved in reading, suggests that the language-processing system is at least partly hierarchically built on acoustic nonspeech representations. To determine to what extent this is so, a further study should be conducted to compare the effects of training using phonetic and nonphonetic stimuli with each other.
According to the hypothesis suggesting a more general auditory dysfunction than a deficit specific to the phonological system in dyslexic individuals, the perceptual difficulties in dyslexia especially relate to the discrimination of rapid acoustic changes (9, 14–17). While supporting the general auditory dysfunction view of dyslexia, our results suggest that this dysfunction is an even more fundamental problem of auditory perception than one involving the discrimination of rapid acoustic transitions. This is because the present training effects were obtained with stimuli containing no rapid transitions and with stimulus sequences that were not rapidly presented (the fastest pace being 200-ms element onset-to-onset time). Nevertheless, this training improved the discrimination of the order reversals of brief (40-ms) sounds, as reflected by the enhanced MMNs and decreased RTs in the training group after the training period. It might be that learning to structure sensory input also affects the processing of faster stimulus elements than those originally used in the training.
Our finding of reading improvement as a result of audiovisual training was paralleled by functional changes in the brain of the trained children as reflected in the amplification of the early parts of the MMN and in the decreased RT to targets. These MMN and behavioral findings in the trained children reflect an improved sound-discrimination accuracy. The training-induced enhancement of the MMN amplitude reflects, presumably, an increased accuracy of cortical auditory representations (30–33, 39). Changes of the sensory representations in the brain as a result of training were demonstrated by several animal and human studies (30–33, 40–42). Moreover, in the present study, there was a significant correlation between the change in the MMN amplitude and in the reading-skills scores, indicating a close relation between the cortical discrimination of auditory nonspeech sound elements and the reading ability.
The effectiveness of the present training program during the relatively short training period might be attributed to two factors. First, we trained the children as early as possible, for plastic changes take place more easily and are more profound in immature than mature brain structures (20, 21). Problems in acquiring readings skills were observed during the first fall semester in these children, and our study was started soon thereafter (when the children were 7 years old). Second, the present training program closely imitates reading performance with the exception that it is less complicated. Excluding semantic processing in the training program enables the child to concentrate on the perceptual features of the stimuli. Simplicity in the early steps of training is important; if training is started with difficult tasks that are impossible for the child to carry out successfully, then the learning process might not be initiated at all. Motivational problems might also arise, owing to too slow progression or to experiences of failure. As previous studies (21, 30, 40, 42) have shown, attention and motivation are important factors in causing plastic neural changes in the brain.
The present results are encouraging with respect to both understanding and remediating dyslexia. However, to develop an optimal training method, the factors underlying the beneficial effects of the present computer program should be determined in further studies. For example, the possible independent roles of the auditory and visual systems in training as compared with training involving the interaction of these systems should be determined. In addition, it is important to exclude the possible nonspecific (placebo) effects that might relate to task performance itself by using a group that has a control task. Moreover, because there are different types of dyslexia (e.g., dysphonetic and dyseidetic dyslexia; refs. 17 and 19), the remediation efficacy of the training program should be separately determined for each of these groups.
Acknowledgments
We are very grateful to Dr. Elyse Sussman for her comments on the previous version of this manuscript. This study was supported by Academy of Finland Grant 73038.
Abbreviations
- MMN
mismatch negativity
- ERP
event-related potential
- SOA
stimulus (element)-onset asynchrony
- RT
reaction time
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
In the first test session, administered in the beginning of the spring semester (before the training was started), the children in the training and control groups were in the correct reading of words in percentile 5 of the normative data, and in the reading speed, the respective percentile was 10. In counting syllables, the groups were in percentile 57; in deleting the first phoneme of words, the groups were in percentile 97.
References
- 1.Mody M, Studdert-Kennedy M, Brady S. J Exp Child Psychol. 1998;64:199–231. doi: 10.1006/jecp.1996.2343. [DOI] [PubMed] [Google Scholar]
- 2.Rayner K, Pollatsek A, Bilsky A B. Psychon Bull Rev. 1995;2:501–507. doi: 10.3758/BF03210985. [DOI] [PubMed] [Google Scholar]
- 3.Schulte-Körne G, Deimel W, Bartling J, Remschmidt H. NeuroReport. 1998;9:337–340. doi: 10.1097/00001756-199801260-00029. [DOI] [PubMed] [Google Scholar]
- 4.Studdert-Kennedy M, Mody M. Psychon Bull Rev. 1995;2:508–514. doi: 10.3758/BF03210986. [DOI] [PubMed] [Google Scholar]
- 5.Godfrey J J, Syrdal-Lasky A K, Millay K K, Knox C M. J Exp Child Psychol. 1981;32:401–424. doi: 10.1016/0022-0965(81)90105-3. [DOI] [PubMed] [Google Scholar]
- 6.Demp J B, Boynton G M, Heeger D J. J Neurosci. 1998;18:6939–6951. doi: 10.1523/JNEUROSCI.18-17-06939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salmelin R, Service E, Kiesilä P, Uutela K, Salonen O. Ann Neurol. 1996;40:157–162. doi: 10.1002/ana.410400206. [DOI] [PubMed] [Google Scholar]
- 8.Livingstone M S, Rosen G D, Drislane F W, Galaburda A M. Proc Natl Acad Sci USA. 1991;88:7943–7947. doi: 10.1073/pnas.88.18.7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tallal P. Brain Lang. 1980;9:182–198. doi: 10.1016/0093-934x(80)90139-x. [DOI] [PubMed] [Google Scholar]
- 10.Wright B A, Lombardino L J, King W M, Puranik C S, Leonard C M, Merzenich M M. Nature (London) 1997;387:176–178. doi: 10.1038/387176a0. [DOI] [PubMed] [Google Scholar]
- 11.Helenius P, Uutela K, Hari K. Brain. 1999;122:907–913. doi: 10.1093/brain/122.5.907. [DOI] [PubMed] [Google Scholar]
- 12.Hari R, Kiesilä P. Neurosci Lett. 1996;205:138–140. doi: 10.1016/0304-3940(96)12393-4. [DOI] [PubMed] [Google Scholar]
- 13.Kujala T, Myllyviita K, Tervaniemi M, Alho K, Kallio J, Näätänen R. Psychophysiology. 2000;37:262–266. [PubMed] [Google Scholar]
- 14.Nagarajan S, Mahncke H, Salz T, Tallal P, Roberts T, Merzenich M M. Proc Natl Acad Sci USA. 1999;96:6483–6488. doi: 10.1073/pnas.96.11.6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Temple E, Poldrack R A, Protopapas A, Nagarajan S, Salz T, Tallal P, Merzenich M M, Gabrieli J D. Proc Natl Acad Sci USA. 2000;97:13907–13912. doi: 10.1073/pnas.240461697. . (First Published November 28, 2000; 10.1073/pnas.240461697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed M A. J Exp Child Psychol. 1989;48:270–292. doi: 10.1016/0022-0965(89)90006-4. [DOI] [PubMed] [Google Scholar]
- 17.Farmer M E, Klein R M. Psychon Bull Rev. 1995;2:460–493. doi: 10.3758/BF03210983. [DOI] [PubMed] [Google Scholar]
- 18.Baldeweg T, Richardson A, Watkins S, Foale C, Gruzelier J. Ann Neurol. 1999;45:495–503. doi: 10.1002/1531-8249(199904)45:4<495::aid-ana11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 19.Snowling M J. Dyslexia. Oxford: Blackwell; 2000. [Google Scholar]
- 20.Pantev C, Oostenveld R, Engelien A, Ross B, Roberts L E, Hoke M. Nature (London) 1998;392:811–814. doi: 10.1038/33918. [DOI] [PubMed] [Google Scholar]
- 21.Singer W. Science. 1995;270:758–763. doi: 10.1126/science.270.5237.758. [DOI] [PubMed] [Google Scholar]
- 22.Karma K. In: Auditory Structuring in Explaining Dyslexia, Proceedings of the Eighth International Workshop on the Cognitive Science of Natural Language Processing (CSNLP-8), August 9–11 1999. Mc Kevitt P, Mulvihill C, Nualláin S, O'Riordan C, editors. National University of Ireland, Galway, Ireland: Information Technology Centre; 1999. [Google Scholar]
- 23.Näätänen R. Attention and Brain Function. Hillsdale, NJ: Lawrence Erlbaum; 1992. [Google Scholar]
- 24.Tiitinen H, May P, Reinikainen K, Näätänen R. Nature (London) 1994;327:90–92. doi: 10.1038/372090a0. [DOI] [PubMed] [Google Scholar]
- 25.Szymanski M D, Yund E W, Woods D L. J Acoust Soc Am. 1999;106:3492–3505. doi: 10.1121/1.428202. [DOI] [PubMed] [Google Scholar]
- 26.Aaltonen O, Tuomainen J, Laine M, Niemi P. Brain Lang. 1993;44:139–152. doi: 10.1006/brln.1993.1009. [DOI] [PubMed] [Google Scholar]
- 27.Kane N M, Curry S H, Butler S R, Gummins B H. Lancet. 1993;341:688. doi: 10.1016/0140-6736(93)90453-n. [DOI] [PubMed] [Google Scholar]
- 28.Cheour M, Ceponiene R, Lehtokoski A, Luuk A, Allik J, Alho K, Näätänen R. Nat Neurosci. 1998;1:351–353. doi: 10.1038/1561. [DOI] [PubMed] [Google Scholar]
- 29.Kraus N, McGee T J, Carrell T D, Zecker S G, Nicol T G, Koch D B. Science. 1996;273:971–973. doi: 10.1126/science.273.5277.971. [DOI] [PubMed] [Google Scholar]
- 30.Näätänen R, Schröger E, Karakas S, Tervaniemi M, Paavilainen P. NeuroReport. 1993;4:503–506. doi: 10.1097/00001756-199305000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Tremblay K, Kraus N, McGee T. NeuroReport. 1998;9:3557–3560. doi: 10.1097/00001756-199811160-00003. [DOI] [PubMed] [Google Scholar]
- 32.Kraus N, McGee T J, Carrell T D, King C, Tremblay K. J Cognit Neurosci. 1995;7:27–34. doi: 10.1162/jocn.1995.7.1.25. [DOI] [PubMed] [Google Scholar]
- 33.Winkler I, Kujala T, Tiitinen H, Sivonen P, Alku P, Lehtokoski A, Czigler I, Csepe V, Ilmoniemi R, Näätänen R. Psychophysiology. 1999;36:638–642. [PubMed] [Google Scholar]
- 34.Poskiparta E, Niemi P, Lepola J. Diagnostic Tests: I. Reading and Writing. University of Turku, Finland: The Center of Learning Research; 1994. [Google Scholar]
- 35.Tervaniemi M, Radil T, Radil J, Kujala T, Näätänen R. Audiol Neurootol. 1999;4:303–310. doi: 10.1159/000013854. [DOI] [PubMed] [Google Scholar]
- 36.Tallal P, Miller S L, Bedi G, Byma G, Wang X, Nagarajan S S, Schreiner C, Jenkins W M, Merzenich M M. Science. 1996;271:81–84. doi: 10.1126/science.271.5245.81. [DOI] [PubMed] [Google Scholar]
- 37.Talcott J B, Witton C, McLean M F, Hansen P C, Rees A, Green G G R, Stein J. Proc Natl Acad Sci USA. 2000;97:2952–2957. doi: 10.1073/pnas.040546597. . (First Published February 25, 2000; 10.1073/pnas.040546597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merzenich M M, Jenkins W M, Johnston P, Schreiner C, Miller S L, Tallal P. Science. 1996;271:77–81. doi: 10.1126/science.271.5245.77. [DOI] [PubMed] [Google Scholar]
- 39.Näätänen R, Lehtokoski A, Lennes M, Cheour M, Huotilainen M, Iivonen A, Vainio M, Alku P, Ilmoniemi R J, Luuk A, et al. Nature (London) 1997;385:432–434. doi: 10.1038/385432a0. [DOI] [PubMed] [Google Scholar]
- 40.Recanzone G H, Schreiner C E, Merzenich M M. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E. Science. 1995;270:305–307. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- 42.Ahissar E, Vaadia E, Ahissar M, Bergman H, Arieli A, Abeles M. Science. 1992;257:1412–1415. doi: 10.1126/science.1529342. [DOI] [PubMed] [Google Scholar]