Abstract
Objective
Depression among pregnant women is a prevalent public health problem associated with poor maternal and offspring development. Behavioral Activation (BA) is a scalable intervention aligned with pregnant women’s preference for non-pharmacological depression care. This is the first test of the effectiveness of BA for depression among pregnant women, which aimed to evaluate the effectiveness of BA as compared with treatment as usual (TAU).
Method
Pregnant women (mean age = 28.75; SD = 5.67) with depression symptoms were randomly assigned to BA (n = 86) or TAU (n = 77). Exclusion criteria included known bipolar or psychotic disorder or immediate self-harm risk. Follow-up assessment occurred 5 and 10 weeks post-randomization and 3 months postpartum using self-report measures of primary and secondary outcomes and putative targets.
Results
Compared with TAU, BA was associated with significantly lower depressive symptoms (d = 0.34, p = .04) and higher remission (56.3% vs. 30.3%, p = .003). BA also demonstrated significant advantage on anxiety and perceived stress. Participants attended most BA sessions and reported high satisfaction. Participants in BA reported significantly higher levels of behavioral activation (d = 0.69, p <.0002) and environmental reward (d = 0.54, p < .003) than those who received TAU, and early change in both of these putative targets significantly mediated subsequent depression outcomes.
Conclusions
BA is effective for pregnant women, offering significant depression, anxiety, and stress related benefits, with mediation analyses supporting the importance of putative targets of activation and environmental reward.
Keywords: depression, behavioral activation, pregnancy, postpartum, randomized controlled trial
Depression affects up to 10% of women during pregnancy and 20% of women in the first three months postpartum (Evans, Heron, Francomb, Oke, & Golding, 2001; Gavin et al., 2005); moreover, anxiety and stress are often comorbid with depression among most pregnant and postpartum women (Babb, Deligiannidis, Murgatroyd, & Nephew, 2015; Goodman, Chenausky, & Freeman, 2014). The occurrence of depression and its comorbidities among women at this life cycle stage is associated with increased risk of multiple adverse correlates and consequences for both women and their offspring (Stein et al., 2014). As a result, the identification of scalable, effective evidence-based psychotherapies for this population is an important public health goal, particularly given data suggesting low rates of screening and treatment of depression among perinatal women (Ko, Farr, Dietz, & Robbins, 2012). Behavioral Activation (BA) is a promising psychotherapy for this population for empirical, practical, and theoretical reasons. We conducted the first pragmatic effectiveness trial of BA for antenatal depression and examined the extent to which BA engaged core putative targets and to which early change in these targets mediated subsequent depression outcomes.
The contemporary use of BA is grounded in theory and applied clinical research conducted by behaviorists in the 1970s (Dimidjian, Barrera, Martell, Munoz, & Lewinsohn, 2011). Interest in this early work was revitalized after a series of studies conducted by Jacobson and colleagues (1996) and Dimidjian and colleagues (2006); in parallel to this work, Lejuez and Hopko and colleagues also examined the use of BA among depressed populations (Lejuez, Hopko, LePage, Hopko, & McNeil, 2001). Since then a strong evidence base has accumulated supporting the efficacy of BA in general populations, as summarized in systematic reviews and meta-analyses (Cuijpers, Andersson, Donker, & van Straten, 2011; Cuijpers, van Straten, Andersson, & van Oppen, 2008; Cuijpers, van Straten, & Warmerdam, 2007; Ekers, Richards, & Gilbody, 2008; Ekers et al., 2014; Mazzucchelli, Kane, & Rees, 2009; Mazzucchelli, Kane, & Rees, 2010).
Among depression treatments provided to pregnant women, pharmacotherapy is the most widely used, with estimates that at least 75% of pregnant women identified as depressed are referred for pharmacotherapy (Dietz et al., 2007). In fact, the rise in antidepressant use among pregnant women has been described as “dramatic,” from under 1% of pregnant women prior to 1988 to more than 7% in 2008 (Mitchell et al., 2011). This is noteworthy in light of women’s documented preferences for nonpharmacological depression care (Battle, Salisbury, Schofield, & Ortiz-Hernandez, 2013) and high rates of relapse among pregnant women who discontinue antidepressant use (L. S. Cohen et al., 2006; Roca et al., 2013). The pharmacological management of depression during pregnancy requires ongoing consideration of risks and benefits for maternal mental health and fetal development (Wisner et al., 2000; Yonkers et al., 2009). Moreover, some evidence suggests that antidepressant medication efficacy among general populations is moderated by patient severity such that specific pharmacological benefit is observed only among patients with severe baseline symptoms (Fournier et al., 2010; Kirsch et al., 2008); such findings raise the question of whether some pregnant women are exposed to potential adverse effects without specific pharmacological benefit. Because there have been no randomized controlled trials of antidepressants with pregnant women (nor are there likely to be), it is necessary to rely on placebo controlled pharmacotherapy and psychotherapy comparisons within the general population to guide intervention selection. The evidence base for BA speaks directly to this point. Relative to antidepressant medication in a sample of adults with major depression, BA was comparable in efficacy and superior with respect to retention (Dimidjian et al., 2006) and enduring effects over a two-year follow-up (Dobson et al., 2008).
Moreover, relative to other evidence-based psychotherapies, BA may offer scalability advantages that are relevant to pregnant and postpartum women. Multiple studies provide strong evidence suggesting that nonspecialists can deliver BA effectively among general populations in routine care medical settings (Chowdhary et al., 2016; Ekers, Richards, McMillan, Bland, & Gilbody, 2011). These advantages may be particularly salient for depressed pregnant women given documented low rates of treatment seeking and preference for care in non-specialty settings (Flynn, Henshaw, O’Mahen, & Forman, 2010; O’Mahen & Flynn, 2008). Moreover, clinical guidelines require obstetric and primary care providers to screen for depression and deliver care or refer women who screen positively for depression (ACOG, 2015; Siu et al., 2016); as a result, many obstetric settings are likely to face increased rates of detection without corresponding availability of mental health services, underscoring the importance of psychotherapies that can be broadly and effectively delivered.
Finally, another rationale for the investigation of BA for antenatal depression is that the model that guides BA emphasizes the importance of understanding and treating depression in context. Given the contextual shifts inherent to the perinatal transition for women, the conceptual underpinnings of BA are a logical match for the unique treatment needs and preferences of pregnant women. In BA, an emphasis is placed on the ways in which depression is related to shifts in life context and on the maladaptive links that can develop between mood and reduced activity, and increased withdrawal, avoidance, and routine disruption. Interventions target negative cycles of depressed mood and reduced activity with guided activation in an effort to help individuals become more active, increase contact with sources of reward, and solve problems. Despite the clear behavioral model that guides BA, few intervention studies have tested the extent to which BA engages the putative targets of activation and experience of reward, relative to comparison conditions, or the extent to which engaging these targets mediates improvement in depression (for a recent exception, see, for example, Ryba, Lejuez, & Hopko, 2014).
We conducted a pragmatic effectiveness randomized controlled trial to optimize relevance for clinical practice (Glasgow, Magid, Beck, Ritzwoller, & Estabrooks, 2005; Tunis, Stryer, & Clancy, 2003). The overall aim of the study was to examine the effectiveness of BA across four sites in different regions of the United States within the National Institute of Mental Health funded Mental Health Research Network (MHRN). We randomly assigned pregnant women to BA or treatment as usual (TAU) and assessed changes in outcomes from pregnancy through the third month postpartum. Specifically, we addressed the primary hypothesis that BA would demonstrate superior effectiveness, relative to TAU, on change in depressive symptoms and rates of remission as measured by the Patient Health Questionnaire (PHQ-9; Kroenke, Spitzer, & Williams, 2001). We also addressed the secondary hypotheses that BA would demonstrate superior outcomes, relative to TAU, on measures of anxiety and stress, given their common co-occurrence with depression during pregnancy, and maternal self-efficacy, as an index of early postpartum functional outcome. We also examined reports of treatment satisfaction as an index of responsiveness of the BA intervention to pregnant women’s developmental needs and preferences. Finally, we addressed the hypotheses that BA would engage putative targets of behavioral activation and environmental reward significantly more than TAU and that early change in these variables would mediate subsequent depression symptom improvement.
METHODS
Participants: Description and Informed Consent
A four-site randomized controlled clinical trial was conducted to evaluate the effectiveness of Behavioral Activation (BA). Women were recruited from four sites of the MHRN in the United States, including Seattle, Washington (Group Health Cooperative), Minneapolis, Minnesota (HealthPartners), Denver, Colorado (Kaiser Permanente Colorado), and Atlanta, Georgia (Kaiser Permanente Georgia). These large integrated healthcare systems provide general medical and mental health care to defined populations, including members enrolled via commercial insurance, Medicaid, and other low-income programs. Enrollment occurred between April 2012 and June 2013 and assessment concluded in October 2014. Outcomes were measured at baseline, 5 and 10 weeks after randomization, and 3 months postpartum; participants completed measures online using the DatStat Illume software platform (www.datstat.com), which supports 64-bit security and authentication. Participants received $25 (USD) for completing each study assessment; participants were not paid for intervention session attendance. The study was approved by the institutional review boards and by institutional authorization agreement at each of the implementation sites and the University of Colorado Boulder and Emory University. After complete description of the study to the participants, written informed consent was obtained.
Recruitment, Eligibility, Consent and Randomization
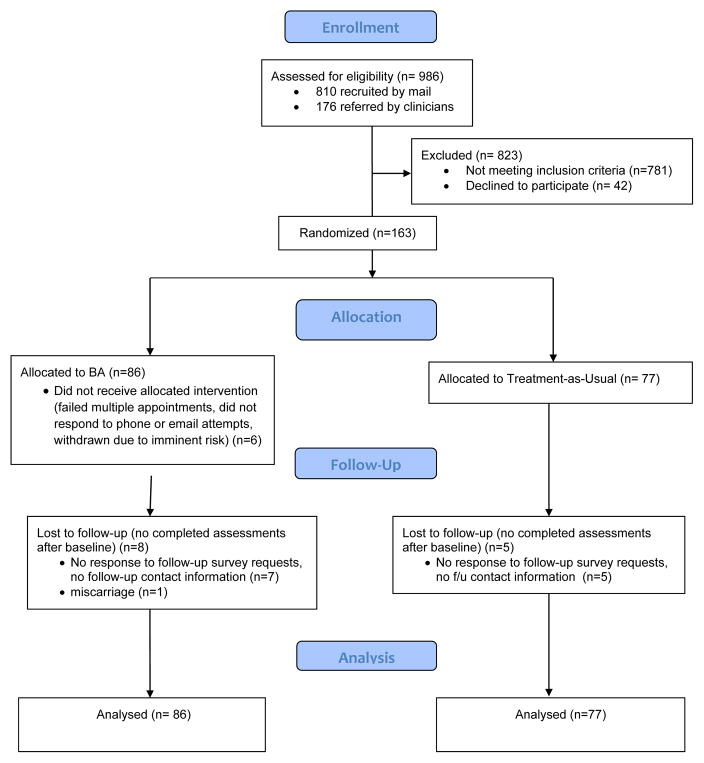
Inclusion and exclusion criteria were minimal: (a) pregnant; (b) receiving care at one of the four MHRN sites; (c) aged 18 or older; and (d) baseline score of ≥ 10 on the PHQ-9 (Kroenke et al., 2001); (e) English speaker; and (f) no known diagnosis of bipolar or psychotic disorder, active substance dependence, or immediate risk of self-harm or need for hospitalization. Consistent with a pragmatic trial design, prior or current use of medication or psychotherapy was not restricted. In addition, we expanded the eligibility criterion to a baseline score of ≥ 10 on the PHQ-9 (from the initial plan to require ≥ 15 given clinical guidelines in the delivery settings that recommended additional screening and intervention for such patients). Recruitment strategies included referral from obstetric care providers, self-referral in response to written information provided during or after obstetric or behavioral health visits, outreach calls to women with elevated scores at obstetric visits, or mailed screening with the PHQ-9. A study coordinator at each site telephoned referred patients, provided information about study procedures, assessed eligibility, and scheduled in-person appointments to complete consent and baseline assessment measures. Participants were randomly assigned to BA or TAU using a random number table with equal probability of assignment to groups using separate assignment tables for each site. To maintain allocation concealment, randomization was executed automatically on entry of eligibility information into the study database; assignment was completely concealed from study personnel during recruitment and assessment of eligibility. The CONSORT diagram illustrates participant flow (see Figure 1).
Figure 1.
Consolidated Standards for Reporting of Trials Flow Diagram
Interventions
BA
BA was delivered in a manner tailored for depressed pregnant women. In addition, all participants assigned to BA had full access to usual care. We allowed flexible delivery of BA with respect to location and timing of sessions to accommodate women’s preferences. BA sessions were delivered in the obstetric clinic, by telephone, or in women’s homes. We used a 10-session protocol for the intervention, but clinicians were encouraged to be flexible with the spacing and number of sessions to accommodate pregnant women’s scheduling demands. Ten providers were trained initially, and eight completed training and provided the study intervention; four had a nursing degree (nurse midwife, nurse practitioner), three had a master’s degree in behavioral health, and one was a registered occupational therapist. All were naïve to BA at the outset of the study.
BA training included 2 days of in-person workshops and self-paced reading followed by ongoing weekly group telephonic supervision (90 min) and individual supervision as needed (30 min). BA training and supervision was provided by two of the authors, SD and SSW. The BA providers followed procedures outlined in the BA treatment manual (Martell, Dimidjian, & Herman-Dunn, 2010), with customizations for delivery during pregnancy. Although initial studies of BA included up to 24 sessions (e.g., Dimidjian et al., 2006), the 10-session duration of treatment was used in this study given concerns about feasibility of delivery in the obstetric setting; the core strategies used in the initial BA study were implemented over this shorter duration of care. Treatment began with the provider and the participant collaboratively developing a case conceptualization and treatment plan based on the behavioral model of depression. Core treatment strategies included self-monitoring, structuring and scheduling activities, problem solving, and increasing social support. Interpersonal communication skills were emphasized as clinically indicated (Linehan, 1993). Sessions were structured and between-session homework was emphasized. Clinical examples and assignments were tailored to the circumstances and challenges of pregnancy and the early postpartum.
The Quality of Behavioral Activation Scale (QBAS; Dimidjian, Hubley, Martell, and Herman, unpublished measure) was used to assess the treatment fidelity of BA providers. Fourteen items were rated using a 7-point Likert-type scale with higher scores indicating higher quality. A score of 3 on each item corresponds to satisfactory skill in implementing the BA component delineated by that item. Although the QBAS is unpublished, it is the primary measure of quality of BA in use. QBAS ratings were made based on clinician participants’ performance in standardized role-plays with a hypothetical patient portrayed by trained research assistants. Role-plays, conducted over the telephone, were audio-recorded for later rating. Prior to each role-play, clinicians read a one-page vignette that detailed background information, depressive symptoms, depression history, and prior treatment of the client. Clinicians were assessed at baseline, posttraining and 9-month follow-up (one clinician was unable to complete the role-play at baseline because of logistical difficulties). Two BA experts, blind to time, completed all ratings. Reliability between the two experts for 12 overlapping ratings was acceptable with an ICC of .84, and scores at post-training (M = 3.56, SD = 1.05) and 9-month follow-up (M = 3.82, SD = 1.16) both exceeded the “satisfactory” threshold.
TAU
TAU consisted of receiving routine care and completing study assessments. If study assessment indicated an elevation in depression symptom severity, then the participant and the obstetric provider were notified, and referral to a behavioral health provider at the treatment site was provided as appropriate.
Measures
Baseline clinical characteristics were assessed with the self-reported Psychiatric Diagnostic Screening Questionnaire (PDSQ; Zimmerman & Mattia, 2001) subscales to screen for Diagnostic and Statistical Manual of Mental Disorders, (fourth edition; DSM-IV) diagnoses of posttraumatic stress disorder (PTSD), obsessive-compulsive disorder (OCD), panic disorder, and social anxiety; the recommended cutoffs are scores of 5, 1, 4, and 4 on these subscales, respectively, indicating greater likelihood of qualifying for the given diagnosis. Alcohol use was assessed with the Alcohol Use Disorders Identification Test – Consumption (AUDIT-C; Bush, Kivlahan, McDonell, Fihn, & Bradley, 1998). Additional questions assessed current and past depression treatment and demographic characteristics.
The primary outcome of depressive symptom severity was assessed at baseline and all follow-up assessments with the PHQ-9 (Kroenke et al., 2001), which has been validated against a structured diagnostic interview in pregnant women with a cutoff score of 10 indicating a diagnosis of depression with sensitivity (85%) and specificity (84%) (Lowe, Unutzer, Callahan, Perkins, & Kroenke, 2004). Consistent with a pragmatic trial design, the use of the self-report measure of depression severity parallels routine practice in clinical settings and has benefits over semistructured diagnostic interviews of being shorter and less costly.
Secondary outcomes measurement occurred at baseline and at all follow-ups, with the exception of maternal self-efficacy, which was only measured at 3 months postpartum. The Generalized Anxiety Disorder 7-item Questionnaire (GAD-7; Spitzer, Kroenke, Williams, & Lowe, 2006) assessed the presence and severity of GAD, and the Perceived Stress Scale (PSS-10; S. Cohen & Williamson, 1988) assessed stress; on both scales, higher scores indicate higher severity. Higher scores on the Maternal Self-Efficacy Questionnaire (Teti & Gelfand, 1991) indicate higher perceived maternal self-efficacy. Satisfaction with treatment was assessed among participants receiving BA at 10 weeks with the Client Satisfaction Questionnaire (CSQ; Attkisson & Zwick, 1982), with higher scores indicating greater satisfaction.
Two questionnaires assessed change on BA putative targets and were administered at baseline and all follow-ups. The Behavioral Activation for Depression Scale – Short Form (BADS-SF; Manos, Kanter, & Luo, 2011) measures extent of behavioral activation over the past week. The Environmental Reward Observation Scale (EROS; Armento & Hopko, 2007) measures the experience of environmental reward over the past several weeks. Higher scores indicate more activation and reward, respectively.
To characterize the nature and extent of other service use in both conditions, utilization of psychotropic medication dispensing and nonstudy behavioral health services was obtained from extraction of mental-health-related pharmacy, diagnosis, and procedure codes from a virtual data warehouse that is populated with electronic medical records data.
Sample Size and Power
A priori power estimates were based on a planned sample size of 85 per group (assuming a 15% attrition rate), a two-tailed alpha level of .05, a correlation between outcome assessments of 0.5, and an effect size defined as the time-averaged mean difference in PHQ-9 scores between BA and TAU groups divided by the within-group standard deviation. Power for three different hypothetical effect sizes of .3, .4, and .5 was 60%, 84%, and 96%, respectively, indicated adequate power to detect at least a medium clinical effect between BA and TAU.
Statistical Analyses
Baseline differences between the BA and TAU groups were examined using χ2 test for binary variables, Cochran Mantel Haenszel tests for categorical/ordinal variables and t tests for continuous variables. Depression, anxiety, and perceived stress outcomes were examined using general mixed-model analysis of variance (MMANOVA) implemented in PROC MIXED in SAS, version 9.3 (SAS Institute, Inc, 2011). The MMANOVA is a repeated-measures model that uses all available data, similar to hierarchical linear models (HLM; Raudenbush and Bryk, 2002). Unlike HLM, the MMANOVA does not require a mathematical relationship such as linear change between time and outcome; assessment point is treated as a fixed effect with baseline included as the first outcome measure. This resulted in an intent-to-treat analysis, including all randomized participants. Covariates included an assessment completion indicator, defined as failing to provide the final 3-month postpartum assessment (14% for TAU vs. 26% for BA, χ2 (1) = 3.21, p = .07), the assessment completion indicator by site interaction (21% within Colorado, 50% with Georgia, 9% within Minnesota, and 17% within Washington, χ2 (3) = 22.55, p < .001], baseline anxiety score (t(161) = 2.14, p = .034), and psychotherapy at the time of enrollment (χ2 (1) = 3.47, p = .062). Covariates included an assessment completion indicator, defined as failing to provide the final 3-month postpartum assessment (14% for TAU vs. 26% for BA, χ2 (1) = 3.21, p = .07), the assessment completion indicator by site interaction (21% within Colorado, 50% with Georgia, 9% within Minnesota, and 17% within Washington, χ2 (3) = 22.55, p < .001], baseline anxiety score (t(161) = 2.14, p = .034), and psychotherapy at the time of enrollment (χ2 (1) = 3.47, p = .062). MMANOVA included treatment-by-time interaction which allows the magnitude of the treatment differences to vary over the assessment, with no treatment difference at baseline due to randomization. MMANOVA allowed us to estimate the average change from baseline over the assessment period for each treatment, and the overall treatment effect is the contrast of the average change from baseline between the two treatments.
As a sensitivity analysis for missing data, we implemented a Markov Chain Monte Carlo (MCMC) imputation method through PROC MI of SAS 9.3 to create a complete data set using all observed clinical and demographic data (Yuan, 2011). MCMC constructs a Markov chain long enough for the distribution of the elements to stabilize to a common, stationary distribution. Data augmentation is applied to Bayesian inference with missing data by repeating a series of imputation and posterior steps. These two steps are iterated long enough for the results to be reliable for the imputed data set (Schafer, 1997). The goal is to have the iterates converge to their stationary distribution and then to simulate an approximately independent draw of the missing values. We subsequently ran 10 sets of MCMC imputations and ran MMANOVA on the average of the imputed data (Rubin, 1987).
To assess the clinical significance of differences between BA and TAU on depressive outcomes at the final 3-month postpartum assessment point, we used two approaches. We compared differences by group in rates of remission defined as achieving a final score < 5 on the PHQ-9, using the χ2 test for observed data. We also used the method for evaluating clinical significance proposed by Jacobson and Truax (1991) in which all dropouts and other missing data were scored as unimproved and included in the denominator of the calculations, providing a full intent-to-treat analysis.
For the maternal efficacy outcome, an Analysis of Covariance (ANCOVA) was used controlling for baseline PHQ-9 and site. Treatment satisfaction was acquired solely on the BA participants at the 10-week assessment point and was descriptively examined.
Assessment of mediation was performed with path analysis using multilevel models that examined the extent to which inclusion of the proposed mediator in the original analysis reduced the level of significance for the treatment effect. We used early change in the proposed mediators (baseline through week 10) and subsequent average PHQ-9 scores (week 10 through the 3-month postpartum), controlling for all specified covariates per the outcome model. To quantify the mediation effect, we utilized the Sobel z with the standard error, derived by using the second order Taylor Series approach (Krull & MacKinnon, 2001). We ensured the sample size across the sequential models was the same for all path models. Consistent with Shrout and Bolger (2002), we followed the use of bootstrap to test mediation, and with our repeated data structure we used the case resampling bootstrap approach. Significance levels for all analyses were evaluated at p < .05.
RESULTS
Sample Characteristics
Table 1 summarizes baseline demographic and clinical characteristics. Overall, participants reported moderately severe depressive symptoms on the PHQ-9 and symptoms of PTSD, OCD, and social anxiety that approximated or exceeded established clinical cutoff scores. Baseline measures also indicated moderate to severe generalized anxiety disorder (GAD) symptoms, high levels of perceived stress, and low levels of past-year alcohol use.
Table 1.
Demographic and Clinical Characteristics of the Sample
| Characteristic | BA (n = 86) | TAU (n = 77) | Total (N = 163) |
|---|---|---|---|
| Age, years, mean (SD) | 28.49(5.73) | 29.04(5.63) | 28.75(5.67) |
| Race, No. (%) | |||
| White | 51 (59.30) | 44 (57.14) | 95 (58.28) |
| Black | 25 (29.07) | 20 (25.97) | 45 (27.61) |
| Asian | 4 (4.65) | 3 (3.90) | 7 (4.29) |
| Other | 6 (6.98) | 10 (12.99) | 16 (9.82) |
| Hispanic ethnicity, No. (%) | 11 (12.79) | 14 (18.18) | 25 (15.34) |
| Marital status, No. (%) | |||
| Single, never married | 22 (25.58) | 21 (27.27) | 43 (26.38) |
| Married/cohabiting | 62 (72.09) | 52 (67.53) | 114 (69.94) |
| Divorced, separated | 2 (2.33) | 4 (5.19) | 6 (3.68) |
| Primiparous, No. (%) | 36 (41.86) | 28 (36.36) | 64 (39.26) |
| Education level, No. (%) | |||
| < High school | 4 (4.65) | 8 (10.39) | 12 (7.36) |
| High school | 19 (22.09) | 20 (26.97) | 39 (23.93) |
| Some college | 36 (41.86) | 23 (29.87) | 59 (36.20) |
| College | 19 (22.09) | 12 (15.58) | 31 (19.02) |
| Graduate school | 8 (9.30) | 14 (18.18) | 22 (13.50) |
| Yearly household income, No. (%) | |||
| <25k | 29 (33.72) | 31 (40.26) | 60 (36.81) |
| 25–50k | 25 (29.07) | 20 (25.97) | 45 (27.61) |
| 50–75k | 17 (19.77) | 11 (14.29) | 28 (17.18) |
| 75–100k | 9 (10.47) | 5 (6.49) | 14 (8.59) |
| >100k | 6 (6.98) | 9 (11.69) | 15 (9.20) |
| Chose not to answer | 0 (0) | 1 (1.30) | 1 (0.61) |
| Employed full- or part-time, No. (%) | 61 (70.93) | 54 (70.13) | 115 (70.55) |
| Past depression treatment, No. (%) | |||
| Psychotherapy | 8 (9.30) | 6 (7.79) | 14 (8.59) |
| Antidepressant | 16 (18.60) | 14 (18.18) | 30 (18.40) |
| Combination | 27 (31.40) | 31 (40.26) | 58 (35.58) |
| Treatment for depression at enrollment, No. (%) | |||
| Psychotherapy | 8 (9.30) | 15 (19.48) | 23 (14.11) |
| Antidepressant | 8 (9.30) | 7 (9.09) | 15 (9.20) |
| AUDIT-C, mean (SD) | 2.19 (1.77) | 2.52 (1.99) | 2.34 (2.14) |
| PTSD symptoms, mean (SD) | 4.87 (4.57) | 4.21 (4.99) | 4.56 (4.77) |
| OCD symptoms, mean (SD) | 1.55 (1.73) | 1.58 (1.67) | 1.56 (1.70) |
| Panic disorder symptoms, mean (SD) | 2.55 (2.21) | 2.92 (2.43) | 2.72 (2.32) |
| Social anxiety symptoms, mean (SD) | 5.27 (4.26) | 6.68 (4.20) | 5.94 (4.28) |
Note. BA = Behavioral Activation; TAU = Treatment as Usual; AUDIT-C = Alcohol Use Disorders Identification Test – Consumption; PTSD = Post-Traumatic Stress Disorder; OCD = Obsessive Compulsive Disorder; PTSD, OCD, panic, and social anxiety symptoms were measured with the self-reported Psychiatric Diagnostic Screening Questionnaire.
Treatment Attendance, Satisfaction, and Other Service Use
Two-thirds of participants assigned to BA completed five or more sessions (M = 6.43; SD = 3.64). Participants assigned to BA also reported a high degree of satisfaction on the CSQ-8 at the 10-week assessment, M = 27.76, SD = 3.83. Among participants with utilization data (n = 142), rates of nonstudy psychotherapy did not differ between groups (8.22% [n = 6] and 14.49% [n = 10] for BA and TAU, respectively, χ2(1) = 1.40, p = .24). Similarly, there were no differences between groups in rates of psychotropic medication dispensing (23.29% [n = 17] and 18.84% [n = 13] for BA and TAU, respectively, χ2(1) = .42, p = .52).
Depressive Symptom Severity, Response, and Remission
Consistent with our primary hypothesis, depressive symptom scores (PHQ-9) averaged across all follow-up time points were significantly lower in BA than TAU, F(1,152) = 4.39; p = .04, d = 0.34. Estimated average reduction from baseline across the full follow-up period were 5.57 (SD = 5.03) for BA and 3.98 (SD = 5.00) for TAU; descriptive statistics by group and time are presented in Table 2. An evaluation of whether assessment completion biased this intervention effect was conducted using the pattern-mixture model approach (Hedeker & Gibbons, 1997), which examines whether the intervention contrast varies substantially as a function of completion. Results indicated that the assessment completion status did not have an informative effect on the intervention contrast, F(1,151) = 0.57, p = .56. An additional sensitivity analysis for missing data that was based on imputation for missing data and observed data was conducted and yielded consistent findings with our observed data, F(1,152) = 5.18, p = .024, d = 0.37, with estimated average reduction from baseline across the full follow-up period of 5.06 (SD = 4.18) for BA and 3.51 (SD = 4.81) for TAU. We additionally assessed if the intervention effect was different across the four sites by adding a site-by-treatment interaction term to the model, which was non-significant, F(3,149) = 1.17, p = .32.
Table 2.
Observed depression, anxiety, and stress symptom severity and putative targets
| Baseline | 5 week follow-up | 10 week follow-up | 3 month postpartum follow-up | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| BA | TAU | BA | TAU | BA | TAU | BA | TAU | |
| PHQ-9 mean (SD), n | 14.83 (3.47) n = 86 |
14.60 (3.20) n =77 |
11.56 (4.77) N=67 |
12.00 (4.56) N=64 |
8.76 (5.39) N=70 |
10.46 (4.71) N=68 |
6.52 (6.67) N=64 |
8.10 (6.11) N=66 |
| GAD-7, mean (SD), n | 13.23 (4.30) n =86 |
13.50 (4.01) n =77 |
10.43 (4.80) N=67 |
11.50 (5.01) N=64 |
8.07 (5.50) N=70 |
10.87 (4.84) N=68 |
5.94 (5.77) N=64 |
8.80 (5.78) N=66 |
| PSS mean (SD), n | 26.40 (5.38) n =86 |
25.73 (4.76) n =77 |
23.49 (6.16) N=67 |
24.68 (6.70) N=64 |
19.08 (7.11) N=70) |
22.79 (5.90) N=68 |
15.96 (8.51) N=64 |
20.50 (7.10) N=66 |
| BADS mean (SD), n | 21.70 (7.45) n =86 |
22.21 (6.15) n =77 |
25.85 (7.88) N=67 |
22.51 (7.55) N=63 |
29.28 (10.49) N=70 |
24.40 (8.16) N=68 |
33.42 (9.41) N=64 |
28.49 (8.88) N=65 |
| EROS mean (SD), n | 22.48 (4.75) n =86 |
22.55 (3.76) n =77 |
23.97 (4.35) N=67 |
22.54 (4.21) N=63 |
26.79 (6.45) N=70 |
23.59 (4.71) N=68 |
29.35 (6.33) N=64 |
26.25 (5.62) N=65 |
Note. BA = Behavioral Activation; TAU = Treatment as Usual; PHQ-9 = Patient Health Questionnaire; GAD-7 = Generalized Anxiety Disorder 7-item Questionnaire; PSS = Perceived Stress Scale; BADS = Behavioral Activation for Depression Scale -- Short Form; EROS = Environmental Reward Observation Scale.
With respect to clinical significance, we found advantage for BA relative to TAU in rates of remission for women with final assessment data at 3-months postpartum. Among participants in BA, 56.3% (36 of 64) met criteria for remission as compared to 30.3% (20 of 66) of those assigned to TAU, χ2 (1) = 8.92, p = .003. Accounting for attrition, BA was significantly better in generating clinically significant improvement at 3-month postpartum, with 41.9% of participants in BA (36 of 86) compared to 26.0% in TAU (20 of 77) meeting the clinical significance threshold, χ2 (1) = 4.55, p < .04, odds ration [OR] = 2.05 (1.06–3.99).
Anxiety and Stress Symptom Severity
With respect to change in anxiety symptom severity (GAD-7), there was a significant benefit for BA compared to TAU, F(1,152) = 6.24; p = .014, d = 0.41. Estimated mean change from baseline across the full study period were 4.68 (SD = 4.33) for BA and 2.95 (SD = 4.96) for TAU. For perceived stress (PSS-10), there was a significant condition-by-time effect, F(1,152) = 3.89; p =.02, d = 0.33. There was no evidence of a significant difference between conditions from baseline to 5 weeks, F(1,152) = 3.22; p = .07, d = 0.30. However, participants in BA reported significantly lower perceived stress than TAU participants during the later assessment periods; specifically, between 5 and 10 weeks postrandomization, estimated mean scores were 18.98 (SD = 11.22) for BA and 23.50 (SD = 10.18) for TAU, F(1,152) = 17.93; p < .0001, d = 0.73; between 10 weeks postrandomization and 3 months postpartum, estimated mean scores were 16.35 (SD = 12.59) for BA and 21.32 (SD = 11.56) for TAU, F(1,152) = 15.13; p = 0.0001, d = 0.67. Descriptive statistics by group and time are presented in Table 2 for anxiety and stress symptoms.
Maternal Efficacy
The comparison between conditions of perceived maternal self-efficacy did not attain statistical significance, F(1,120) = 3.54; p = .062, d = 0.34; estimated means adjusting for baseline depressive severity and site were 34.93 (SD = 5.22) for BA and 36.04 (SD = 5.49) for TAU.
Engagement of Putative Targets
To test early engagement of the putative targets of activation and environmental reward (baseline through the 10-week assessment), we examined change over time and by treatment group; see Table 2. Using the mixed model framework, with linear time, there was a significant effect of time indicating that both conditions evidenced improvement, F(1,127) = 13.69; p = .0003; and consistent with our hypothesis, a significant effect was evident for treatment, F(1,128) = 15.10; p = < .0002, d = 0.69, with higher ratings of activation among participants in BA than TAU, with estimated means on the BADS-SF of 26.66 (SE = 1.12) for BA and 22.31 (SE = 1.02) for TAU. Focusing on early change in environmental reward (baseline through the 10-week assessment), there was a significant effect of time, indicating that both conditions evidenced improvement in environmental reward after baseline, F(1,127) = 27.24; p < .0001; and consistent with our hypothesis, a significant effect was evident for treatment, F(1,128) = 9.29; p=< .003, d = 0.54, with higher rating of environmental rewards among participants in BA than TAU, estimated means on the EROS of 25.11 (SE = 0.63) for BA and 23.22 (SE = 0.58) for TAU.
Mediation of Depressive Symptom Improvement by Putative Targets
To test the meditation of depression improvement by early engagement of activation as rated on the BADS-SF, we fit a sequential path model to examine the extent to which early change in BADS-SF (from baseline to 10 weeks) mediated subsequent depression symptom severity improvement (from 10 weeks to 3 months postpartum). In this model, consistent with our MMANOVA analyses, we found a significant intervention effect in early change in BADS-SF, t(137) = 3.08, p = .003, d = 0.53), being driven by more change within BA (7.22 ± 1.11) compared with TAU (2.48 ± 1.105). We also found a significant intervention effect on the average difference in depression severity from week 10 through 3 months postpartum, t(127) = 2.34, p = .02), being driven by lower average severity for BA compared with TAU, 1.745 (SE = 0.745), d = 0.42. Early change in BADS was a significant predictor of depressive severity from week 10 through 3 months postpartum, t(126) = 6.41, p < .0001), corresponding to those with higher change in the BADS-SF having on-average lower later depression severity with an on-average reduction in depression severity of 0.211 (SE = 0.033) units of per unit change in BADS-SF. Controlling for the BADS-SF early change, the intervention effect was no longer significant, t(126) = 0.95, p = .34, reducing to a 0.652 ± 0.686 difference with lower scores for BA compared with TAU. For the BADS-SF early change, we found a Sobel effect of 1.006 (SE = 0.36) corresponding to a significant Sobel z = 2.77, p = .009. Bootstrap estimates yielded a mean Sobel effect of 0.983 (SE = 0.39) with a 95% Confidence Interval of (+0.359 to +1.832), corresponding to a significant mediation effect.
To test the meditation of depression improvement by early environmental reward as rated on the EROS, we fit a sequential path model to examine the extent to which early change in EROS (from baseline to 10 weeks) mediated subsequent depression symptom severity improvement (from 10 weeks to 3 months postpartum). In this model, consistent with our MMANOVA analyses, we find a significant effect indicating more early change between baseline and 10 weeks within BA (4.202 ± 0.617) compared to TAU (1.115 ± 0.635), t(137) = 3.48, p = .0007, d = 0.59. We also found a significant intervention effect on the average difference in depression severity from week 10 through 3 months postpartum, t(127) = 2.34, p = .02, being driven by lower average severity for BA compared to TAU, 1.745 (SE = 0.745), d = 0.42. Early change in EROS was a significant predictor of depressive severity from week 10 through 3 months postpartum, t(126) = 8.19, p < .001, d = 1.49, corresponding to those with higher change in EROS having on-average lower later depression severity, with an on-average reduction in depression severity of 0.426 (SE = 0.052) units of per unit change in EROS. Controlling for the EROS early change, the intervention effect was no longer significant, t(126) = 0.52, p = .60, d = 0.09, reducing to a 0.331 ± 0.637 difference with lower scores for BA compared with TAU. For the EROS early change, we found a Sobel effect of 1.31 (SE = 0.41) corresponding to a significant Sobel z = 3.21, p = .002. Bootstrap estimates yielded a mean Sobel effect of 1.248 (SE = 0.391) with a 95% Confidence Interval of (+0.541 to +2.071), corresponding to significant mediation effect.
DISCUSSION
Our findings indicate that BA for pregnant women, relative to treatment as usual, was an effective therapy for depression. Compared with those in TAU, pregnant women receiving BA reported significantly lower depressive symptoms and higher rates of clinically significant change. In addition, BA demonstrated significant benefit with respect to anxiety and stress symptoms, which commonly co-occur with depressive symptoms during pregnancy (Babb et al., 2015; Goodman et al., 2014). Moreover, pregnant women demonstrated high compliance and satisfaction with BA. Finally, there is evidence that BA engaged putative targets of activation and environmental reward significantly more than usual care and that early change in these targets meditated subsequent depression symptom improvement.
This study reports the first application of BA with women during pregnancy. This is an important population to address given the unique needs of pregnant women, their children, and the healthcare providers who support them. Although untreated depression during pregnancy is associated with adverse correlates and consequences among both women and their offspring (Stein et al., 2014), there exists a shortage of viable treatment options. Antidepressant medication can effectively treat depression among pregnant women (Yonkers, Blackwell, Glover, & Forray, 2014); however, it is possible that most pregnant women taking antidepressants do not derive specific pharmacological benefit from antidepressants given results from placebo-controlled trials in general populations (Fournier et al., 2010). Moreover, few women continue antidepressant medication during pregnancy, and most experience high rates of relapse following medication discontinuation (L. S. Cohen et al., 2006). These data suggest that suggest pregnant women and their offspring may be exposed to the adverse effects of depression during pregnancy if interventions are not provided that are well aligned with women’s preferences. Although pregnant women express preference for psychotherapy (Battle et al., 2013; O’Mahen & Flynn, 2008), the paucity of scalable psychotherapies limits access for many pregnant women. To close the gap between treatment need and receipt among depressed pregnant women, it is imperative to provide access to effective, nonpharmacological intervention. The effects of BA on depression were clinically significant among pregnant women, with evidence of medium effect sizes also on anxiety and stress symptoms.
Our study builds on prior work indicating the scalability of BA. Although the efficacy of BA is well established, the effectiveness of BA in routine care settings has not been widely examined, which is a critical step in maximizing public health relevance according to the National Institute of Health Stage Model (Onken, Carroll, Shoham, Cuthbert, & Riddle, 2014). Our trial focused on effectiveness in the context of population-based recruitment and used a flexibly delivered protocol that was implemented by a diverse range of practitioners in real-world obstetric settings. Our study also builds on an emerging body of work indicating that BA can be delivered by nonspecialists in medical settings (Chowdhary et al., 2016; Ekers, Godfrey, et al., 2011; Ekers et al., 2008; Ekers, Richards, et al., 2011). In our study, we utilized both mental health and nonspecialty clinicians (including nurses and master’s level providers), suggesting that it is feasible to train a range of health providers who treat pregnant women to deliver BA. Although our study was not designed to test the extent to which specific mental health training is necessary to effectively deliver BA, this study underscores the importance of directly testing this question. In addition, emerging work suggests that BA may be delivered using Internet-based methods; among depressed postpartum women, Internet-based BA plus telephone support significantly outperformed usual care on depression, anxiety, and functional impairment outcomes (O’Mahen et al., 2014).
Our study also contributes to an emerging literature examining the process by which psychological interventions achieve clinical benefit. Data on mediators of change are essential to determining the extent to which interventions work in theory-specified manner. Although our study was not powered specifically to test mediation, our findings highlight the potential importance of early change in activation and environmental reward, consistent with behavioral models of depression that guide delivery of BA. Although we relied exclusively on self-reported change in putative outcomes, which may be confounded with depression symptoms, the focus on early change in putative targets predicting later change in symptoms was a strength of our approach. Nevertheless, we cannot infer a causal relationship between the meditators and outcome, and it is possible in study designs such as ours that a third variable influenced both mediator and outcome. It will be important for future studies to incorporate multimethod assessments of putative targets, including additional self-report measures of reward (e.g., Carvalho et al., 2011), behavioral tasks (e.g., Treadway, Buckholtz, Schwartzman, Lambert, & Zald, 2009), and experience sampling methods. In addition, it will be of value to test competing models of mediation that investigate processes specified as central to other psychological treatment protocols for depression and to power such studies specifically to test mediation.
The study had several limitations. We had a limited follow-up period of three months postpartum, which precludes us from examining potential enduring effects of BA. Given the relapsing nature of depression for most individuals, it is important to study a longer follow-up period among women treated with BA. That said, the effects on depression were comparable to studies of BA with general adult population samples, among whom BA has been demonstrated to be comparable to antidepressant medication in efficacy and superior in both acute treatment retention as well as to have enduring effects over a two-year follow-up (Dimidjian et al., 2006; Dobson et al., 2008). We also did not assess participants for major depressive disorder criteria and relied on a self-report measure as our primary outcome of depressive symptoms. However, pregnant women with elevated depression symptoms (and their offspring) have been found to be at risk for adverse outcomes; in a study comparing pregnant and postpartum women with elevated symptoms to those who met DSM-IV criteria for major depression, no differences were evident in the severity of depressive symptoms, rates of comorbid anxiety disorders, severity of anxiety symptoms, perceived stress, or social support (Goodman & Tully, 2009). Moreover, the use of self-report measures parallels routine practice and screening recommendations in most clinical settings. Finally, we did not measure treatment satisfaction in our control condition, and our measurement of maternal functional outcomes was limited to one self-report measure of perceived maternal self-efficacy, which had restricted range in this sample.
Clinical Implications
Effectively treating depression among pregnant women is an important public health goal given that up to one in seven women experience depression during this life transition (Gavin et al., 2005). The correlates and consequences of depression on women and their children are negative and potentially enduring (Stein et al., 2014). Moreover, available treatments often are not aligned with women’s preferences for care, thereby creating challenges for women and their healthcare providers. To the extent that recent guidelines will promote screening of depression during pregnancy (ACOG, 2015; Siu et al., 2016), it is essential that such screening practices are coupled with informing pregnant women about and providing options for effective nonpharmacological depression care. This study suggests that depression among pregnant women is effectively treated with BA, which can be flexibly delivered by trained behavioral and allied health providers. Future studies are needed to examine strategies to maximize the reach of this intervention, to test its effects on maternal functioning and offspring developmental outcomes, and to assess at multiple levels of analysis potential enduring effects and putative targets.
Public Health Significance Statement.
This study suggests that behavioral activation (BA) is an effective treatment for pregnant women with elevated depressive symptoms. Compared with usual care, women receiving BA reported clinically significant improvements in depressive, anxiety, and stress symptoms.
Acknowledgments
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number U19MH092201. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The trial was registered at clinicaltrials.gov (NCT01401231). Dr. Dimidjian receives royalties from Guilford Press for work related to behavioral activation. Drs. Goodman, Simon, Sherwood, Ludman, Gallop, Welch, Hubley, and Beck, Ms. Boggs, Ms. Metcalf, and Mr. Powers report no conflicts to disclose related to this study. The authors express their gratitude to Belinda Operskalski, Julia Anderson, June Bluespruce, and Deborah King at Group Health Collaborative, to Yolanda Gray-Rozier, Fonda Mitchell, Ashli Owen-Smith, and Robbin Ryan at Kaiser Permanente Georgia, to Allen Mallory and Erica Ahlich at Emory University, to Marcia G. Hayes, Dani Bredeson, Elisabeth M. Seburg, Ann Tucker, and Kristine Tromiczak at HealthPartners Institute, and to all of their dedicated BA study clinicians for assistance with this study.
Contributor Information
Sona Dimidjian, University of Colorado Boulder.
Sherryl H. Goodman, Emory University
Nancy E. Sherwood, HealthPartners Institute for Education and Research, Bloomington, MN
Greg E Simon, Group Health Research Institute, Group Health Cooperative, Seattle, WA.
Evette Ludman, Group Health Research Institute, Group Health Cooperative, Seattle, WA.
Robert Gallop, West Chester University, West Chester, PA.
Stacy Shaw Welch, Evidence-based Treatment Centers of Seattle, Seattle, WA.
Jennifer M. Boggs, Kaiser Permanente Colorado Institute for Health Research, Denver, CO
Christina A. Metcalf, University of Colorado Boulder
Sam Hubley, University of Colorado School of Medicine.
J. David Powers, Kaiser Permanente Colorado Institute for Health Research, Denver, CO.
Arne Beck, Kaiser Permanente Colorado Institute for Health Research, Denver, CO.
References
- ACOG. Screening for perinatal depression: Committee Opinion No 630. Obstetrics and Gynecology. 2015;125:1268–1271. doi: 10.1097/01.AOG.0000465192.34779.dc. [DOI] [PubMed] [Google Scholar]
- Armento ME, Hopko DR. The Environmental Reward Observation Scale (EROS): development, validity, and reliability. Behavior Therapy. 2007;38(2):107–119. doi: 10.1016/j.beth.2006.05.003. S0005-7894(06)00083-9 [pii] [DOI] [PubMed] [Google Scholar]
- Attkisson CC, Zwick R. The client satisfaction questionnaire: Psychometric properties and correlations with service utilization and psychotherapy outcome. Evaluation and Program Planning. 1982;5(3):233–237. doi: 10.1016/0149-7189(82)90074-x. [DOI] [PubMed] [Google Scholar]
- Babb JA, Deligiannidis KM, Murgatroyd CA, Nephew BC. Peripartum depression and anxiety as an integrative cross domain target for psychiatric preventative measures. Behavioural Brain Research. 2015;276:32–44. doi: 10.1016/j.bbr.2014.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battle CL, Salisbury AL, Schofield CA, Ortiz-Hernandez S. Perinatal antidepressant use: Understanding women’s preferences and concerns. Journal of Psychiatric Practice. 2013;19(6):443–453. doi: 10.1097/01.pra.0000438183.74359.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking. Archives of Internal Medicine. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- Carvalho JP, Gawrysiak MJ, Hellmuth JC, McNulty JK, Magidson JF, Lejuez CW, Hopko DR. The reward probability index: Design and validation of a scale measuring access to environmental reward. Behavior Therapy. 2011;42(2):249–262. doi: 10.1016/j.beth.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Chowdhary N, Anand A, Dimidjian S, Shinde S, Weobong B, Balaji M, … Patel V. The Healthy Activity Program lay counsellor delivered treatment for severe depression in India: Systematic development and randomised evaluation. British Journal of Psychiatry. 2016;208(4):381–388. doi: 10.1192/bjp.bp.114.161075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera AC, … Stowe ZN. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499–507. doi: 10.1001/jama.295.5.499. 295/5/499 [pii] [DOI] [PubMed] [Google Scholar]
- Cohen S, Williamson GM. Perceived Stress in a Probability Sample of the United States. Newbury Park: Sage Publications; 1988. [Google Scholar]
- Cuijpers P, Andersson G, Donker T, van Straten A. Psychological treatment of depression: Results of a series of meta-analyses. Nordic Journal of Psychiatry. 2011;65(6):354–364. doi: 10.3109/08039488.2011.596570. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, van Straten A, Andersson G, van Oppen P. Psychotherapy for depression in adults: A meta-analysis of comparative outcome studies. Journal of Consulting and Clinical Psychology. 2008;76(6):909–922. doi: 10.1037/a0013075. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, van Straten A, Warmerdam L. Behavioral activation treatments of depression: A meta-analysis. Clinical Psychology Review. 2007;27(3):318–326. doi: 10.1016/j.cpr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Dietz PM, Williams SB, Callaghan WM, Bachman DJ, Whitlock EP, Hornbrook MC. Clinically identified maternal depression before, during, and after pregnancies ending in live births. American Journal of Psychiatry. 2007;164(10):1515–1520. doi: 10.1176/appi.ajp.2007.06111893. [DOI] [PubMed] [Google Scholar]
- Dimidjian S, Barrera M, Jr, Martell C, Munoz RF, Lewinsohn PM. The origins and current status of behavioral activation treatments for depression. Annual Review of Clinical Psychology. 2011;7:1–38. doi: 10.1146/annurev-clinpsy-032210-104535. [DOI] [PubMed] [Google Scholar]
- Dimidjian S, Hollon SD, Dobson KS, Schmaling KB, Kohlenberg RJ, Addis ME, … Jacobson NS. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. Journal of Consulting and Clinical Psychology. 2006;74(4):658–670. doi: 10.1037/0022-006X.74.4.658. [DOI] [PubMed] [Google Scholar]
- Dobson KS, Hollon SD, Dimidjian S, Schmaling KB, Kohlenberg RJ, Gallop RJ, … Jacobson NS. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the prevention of relapse and recurrence in major depression. J Consult Clinical Psychol. 2008;76(3):468–477. doi: 10.1037/0022-006X.76.3.468. 2008-06469-011 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekers D, Godfrey C, Gilbody S, Parrott S, Richards DA, Hammond D, Hayes A. Cost utility of behavioural activation delivered by the non-specialist. British Journal of Psychiatry. 2011;199(6):510–511. doi: 10.1192/bjp.bp.110.090266. [DOI] [PubMed] [Google Scholar]
- Ekers D, Richards D, Gilbody S. A meta-analysis of randomized trials of behavioural treatment of depression. Psychological Medicine. 2008;38(5):611–623. doi: 10.1017/s0033291707001614. [DOI] [PubMed] [Google Scholar]
- Ekers D, Richards D, McMillan D, Bland JM, Gilbody S. Behavioural activation delivered by the non-specialist: phase II randomised controlled trial. British Journal of Psychiatry. 2011;198(1):66–72. doi: 10.1192/bjp.bp.110.079111. [DOI] [PubMed] [Google Scholar]
- Ekers D, Webster L, Van Straten A, Cuijpers P, Richards D, Gilbody S. Behavioural activation for depression; an update of meta-analysis of effectiveness and sub group analysis. PloS One. 2014;9(6):e100100. doi: 10.1371/journal.pone.0100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J, Heron J, Francomb H, Oke S, Golding J. Cohort study of depressed mood during pregnancy and after childbirth. BMJ. 2001;323(7307):257–260. doi: 10.1136/bmj.323.7307.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn HA, Henshaw E, O’Mahen H, Forman J. Patient perspectives on improving the depression referral processes in obstetrics settings: a qualitative study. General Hospital Psychiatry. 2010;32(1):9–16. doi: 10.1016/j.genhosppsych.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, Fawcett J. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47–53. doi: 10.1001/jama.2009.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression - A systematic review of prevalence and incidence. Obstetrics and Gynecology. 2005;106(5):1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- Glasgow RE, Magid DJ, Beck A, Ritzwoller D, Estabrooks PA. Practical clinical trials for translating research to practice: Design and measurement recommendations. Medical Care. 2005;43(6):551–557. doi: 10.1097/01.mlr.0000163645.41407.09. doi: http://www.jstor.org/stable/3768172. [DOI] [PubMed] [Google Scholar]
- Goodman JH, Chenausky KL, Freeman MP. Anxiety disorders during pregnancy: A systematic review. Journal of Clinical Psychiatry. 2014;75(10):e1153–1184. doi: 10.4088/JCP.14r09035. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Tully EC. Recurrence of depression during pregnancy: Psychosocial and personal functioning correlates. Depression and Anxiety. 2009;26(6):557–567. doi: 10.1002/da.20421. [DOI] [PubMed] [Google Scholar]
- Hedeker D, Gibbons RD. Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychological Methods. 1997;2(1):64–78. [Google Scholar]
- Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology. 1991;59(1):12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- Jacobson NS, Dobson KS, Truax PA, Addis ME, Koerner K, et al. A component analysis of cognitive-behavioral treatment for depression. Journal of Consulting and Clinical Psychology. 1996;64:295–304. doi: 10.1037//0022-006x.64.2.295. [DOI] [PubMed] [Google Scholar]
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: A meta-analysis of data submitted to the Food and Drug Administration. PLoS Medicine. 2008;5(2):e45. doi: 10.1371/journal.pmed.0050045.g001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JY, Farr SL, Dietz PM, Robbins CL. Depression and treatment among U.S. pregnant and nonpregnant women of reproductive age, 2005–2009. Journal of Women’s Health. 2012;21(8):830–836. doi: 10.1089/jwh.2011.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krull JL, MacKinnon DP. Mulitlevel modeling of individuals: Group level mediated effects. Multivariate Behavioral Research. 2001;36:249–277. doi: 10.1207/S15327906MBR3602_06. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Hopko DR, LePage JP, Hopko SD, McNeil DW. A brief behavioral activation treatment for depression. Cognitive and Behavioral Practice. 2001;8(2):164–175. [Google Scholar]
- Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. New York, NY, US: Guilford Press; 1993. [Google Scholar]
- Lowe B, Unutzer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the patient health questionnaire-9. Medical Care. 2004;42:1194–1201. doi: 10.1097/00005650-200412000-00006. [DOI] [PubMed] [Google Scholar]
- Manos RC, Kanter JW, Luo W. The Behavioral Activation for Depression Scale–Short Form: Development and Validation. Behavior Therapy. 2011;42(4):726–739. doi: 10.1016/j.beth.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Martell CR, Dimidjian S, Herman-Dunn R. Behavioral activation for depression: A clinician’s guide. New York: Guilford Press; 2010. [Google Scholar]
- Mazzucchelli T, Kane R, Rees C. Behavioral activation treatment of depression in adults: A meta-analysis and review. Clinical Psychology: Science and Practice. 2009;16:383–411. [Google Scholar]
- Mazzucchelli T, Kane RT, Rees CS. Behavioral activation interventions for well-being: A meta-analysis. Journal of Positive Psychology. 2010;5(2):105–121. doi: 10.1080/17439760903569154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernández-Díaz S. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. American Journal of Obstetrics and Gynecology. 2011;205(1):51.e51–51.e58. doi: 10.1016/j.ajog.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mahen HA, Flynn HA. Preferences and perceived barriers to treatment for depression during the perinatal period. J Womens Health (Larchmt) 2008;17(8):1301–1309. doi: 10.1089/jwh.2007.0631. [DOI] [PubMed] [Google Scholar]
- O’Mahen HA, Richards DA, Woodford J, Wilkinson E, McGinley J, Taylor RS, Warren FC. Netmums: a phase II randomized controlled trial of a guided Internet behavioural activation treatment for postpartum depression. Psychological Medicine. 2014;44:1675–1689. doi: 10.1017/S0033291713002092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onken LS, Carroll KM, Shoham V, Cuthbert BN, Riddle M. Reenvisioning clinical science: Unifying the discipline to improve the public health. Clinical Psychological Science. 2014;2(1):22–34. doi: 10.1177/2167702613497932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2. London: Sage Publications, Inc; 2002. [Google Scholar]
- Roca A, Imaz ML, Torres A, Plaza A, Subira S, Valdes M, … Garcia-Esteve L. Unplanned pregnancy and discontinuation of SSRIs in pregnant women with previously treated affective disorder. Journal of Affective Disorders. 2013;150(3):807–813. doi: 10.1016/j.jad.2013.02.040. [DOI] [PubMed] [Google Scholar]
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley and Sons; 1987. [Google Scholar]
- Ryba MM, Lejuez CW, Hopko DR. Behavioral activation for depressed breast cancer patients: the impact of therapeutic compliance and quantity of activities completed on symptom reduction. Journal of Consulting and Clinical Psychology. 2014;82(2):325–335. doi: 10.1037/a0035363. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS/STAT 9.3 user’s guide. Cary, NC: SAS Institute Inc; 2011. [Google Scholar]
- Schafer JL. Analysis of Incomplete Multivariate Data. New York, NY: Chapman and Hall; 1997. [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychological Methods. 2002:422–445. [PubMed] [Google Scholar]
- Siu AL, Bibbins-Domingo K, Grossman DC, Baumann LC, Davidson KW, Ebell M, … Pignone MP. Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(4):380–387. doi: 10.1001/jama.2015.18392. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JBW, Lowe B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Archives of Internal Medicine. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, … Pariante CM. Effects of perinatal mental disorders on the fetus and child. Lancet. 2014;384(9956):1800–1819. doi: 10.1016/s0140-6736(14)61277-0. [DOI] [PubMed] [Google Scholar]
- Teti DM, Gelfand DM. Behavioral competence among mothers of infants in the first year: The mediational role of maternal self-efficacy. Child Development. 1991;62(5):918–929. doi: 10.1111/j.1467-8624.1991.tb01580.x. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH. Worth the ‘EEfRT’? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PloS One. 2009;4(8):e6598. doi: 10.1371/journal.pone.0006598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: Increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290(12):1624–1632. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Zarin DA, Holmboe ES, Appelbaum PS, Gelenberg AJ, Leonard HL, Frank E. Risk-benefit decision making for treatment of depression during pregnancy. American Journal of Psychiatry. 2000;157(12):1933–1940. doi: 10.1176/appi.ajp.157.12.1933. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Blackwell KA, Glover J, Forray A. Antidepressant use in pregnant and postpartum women. Annual Review of Clinical Psychology. 2014;10:369–392. doi: 10.1146/annurev-clinpsy-032813-153626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers KA, Wisner KL, Stewart DE, Oberlander TF, Dell DL, Stotland N, … Lockwood C. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. General Hospital Psychiatry. 2009;31:403–413. doi: 10.1016/j.genhosppsych.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y. Multiple Imputation Using SAS Software. Journal of Statistical Software. 2011;45(6):1–25. doi: 10.18637/jss.v045.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M, Mattia J. The Psychiatric Diagnostic Screening Questionnaire: Development, reliability and validity. Comprehensive Psychiatry. 2001;42(3):175–189. doi: 10.1053/comp.2001.23126. [DOI] [PubMed] [Google Scholar]