Abstract
Introduction
Later-life brain alterations in former tackle football players are poorly understood, particularly regarding their relationship with repetitive head impacts (RHIs) and clinical function. We examined white matter signal abnormalities (WMSAs) and their association with RHIs and clinical function in former National Football League (NFL) players.
Methods
Eighty-six clinically symptomatic former NFL players and 23 same-age reportedly asymptomatic controls without head trauma exposure underwent magnetic resonance imaging and neuropsychological testing. FreeSurfer calculated WMSAs. A cumulative head impact index quantified RHIs.
Results
In former NFL players, increased volume of WMSAs was associated with higher cumulative head impact index scores (P = .043) and worse psychomotor speed and executive function (P = .015). Although former NFL players had greater WMSA volume than controls (P = .046), these findings are inconclusive due to recruitment of controls based on lack of clinical symptoms and head trauma exposure.
Discussion
In former NFL players, WMSAs may reflect long-term microvascular and nonmicrovascular pathologies from RHIs that negatively impact cognition.
Keywords: White matter signal abnormalities, White matter hyperintensities, Repetitive head impacts, Chronic traumatic encephalopathy, Alzheimer's disease, Cognitive function, Concussion, Subconcussive
Highlights
-
•
Repetitive head impact exposure was positively associated with WMSAs in former NFL players.
-
•
In former NFL players, greater WMSAs was associated with worse psychomotor speed and executive function.
-
•
The pathologies of WMSAs may contribute to the clinical presentation of chronic traumatic encephalopathy.
1. Introduction
Chronic traumatic encephalopathy (CTE) is a neurodegenerative disease associated with recurrent concussive and subconcussive injuries (i.e., repetitive head impacts [RHIs]) [1], [2] and is commonly observed in former boxers and professional American football players [1], [2], [3], [4]. CTE can currently only be diagnosed through neuropathological examination [5], in part, because biomarkers that can accurately detect CTE in vivo do not yet exist. Research has begun to use neuroimaging to study the long-term effects of RHIs on the brain and to identify methods to detect neurological sequelae from RHIs (including CTE), focusing on former professional football players due to their substantial exposure to RHIs. Structural [6], [7], functional [7], [8], and neurochemical [6], [9] brain changes have been found in this cohort, believed to be a result of RHIs, and may affect cognitive and neuropsychiatric function [10], [11]. Later-life brain alterations in former football players are not fully characterized, particularly in terms of their relationship with RHIs and clinical function. Former NFL players are presumably at high risk for CTE [4] and can serve as a population to provide insight into the potential in vivo structural brain changes associated with CTE (e.g., Hart et al. [7], Koerte et al. [12]).
White matter signal abnormalities (WMSAs) may be common magnetic resonance imaging (MRI) findings that correlate with clinical function in former football players. WMSAs refer to regions in the white matter that appear hyperintense on T2 fluid-attenuated inversion recovery (FLAIR) but hypointense on T1-weighted images, such as magnetization-prepared rapid gradient echo (MPRAGE) scans. The etiology underlying WMSAs is nonspecific and may be multifaceted. WMSAs have been documented to accompany aging and cardiovascular disease (CVD) and usually are interpreted to reflect small-vessel cerebrovascular disease from microvascular hypoperfusion [13], [14], [15], [16], [17].
Reduced cerebral perfusion has been observed in active and former National Football League (NFL) players [7], [8], and previous research reports greater total and deep volume WMSAs in 10 former NFL players with cognitive deficits, compared with age-matched controls [7]. Subcortical or periventricular WMSAs were also observed in 7/9 former NFL players from a clinical case series [18]. Former professional football players may be at risk for the pathologies of WMSAs due to their high rates of CVD [19], [20]. WMSAs may also reflect long-term microvascular and nonmicrovascular pathologies from RHIs. In the setting of acute mild traumatic brain injury (mTBI), WMSAs can be common, perhaps from decreases in cerebral perfusion [21], [22], [23], and they predict post-mTBI cognitive outcomes [21], [24]. RHIs are also associated with impaired cerebral hemodynamics in active boxers [25], and WMSAs in active collegiate ice hockey players have been shown to occur closer to the gray and white matter interface compared with controls [26]. This sulcal depth location of WMSAs was suggestive of head trauma as the precipitant cause, particularly given that the diagnostic lesion of CTE is the perivascular deposition of hyperphosphorylated tau (p-tau) at the base of the sulci [5]. There may indeed be a vascular component, including blood-brain barrier leakage, involved in the RHI and neurofibrillary tangle formation relationship [27], [28], [29], [30]. In autopsy cases of “dementia pugilistica,” decreased microvascular density and fragmented vessels in the frontal cortex were observed, and tau and microvascular pathology correlated [29]. Along with microvascular changes, RHIs and CTE are also associated with neurometabolic disturbances (e.g., gliosis, neuroinflammation) [2], [9], [31], [32], especially diffuse axonal injury [2], [7], [33], [34], that could result in WMSAs [35], [36], [37].
WMSAs have long been studied in Alzheimer's disease (AD). WMSAs predict increased risk for AD dementia [38]. In fact, WMSAs have even been postulated to be a core feature of AD, given WMSAs were increased in autosomal-dominant AD patients before symptom onset and predicted cerebrospinal fluid amyloid β levels in carriers only [39]. Although the clinical presentation of CTE is heterogeneous and includes a constellation of various cognitive (including nonmemory impairments), behavior, and mood symptoms [3], [4], [40], the pathologies of WMSAs may contribute to the clinical presentation of CTE as they do in AD.
This study examined the association between RHIs (using the cumulative head impact index [CHII]; [10]) and volume of WMSAs from T1-weighted MPRAGE MRI in symptomatic former NFL players. The association between WMSAs and cognitive and neuropsychiatric function was also investigated. It was hypothesized that increased volume of WMSAs would be associated with greater exposure to RHIs and worse clinical function.
2. Methods
2.1. Study design(s)
The current sample included 86 former NFL players from the National Institutes of Health–funded “Diagnosing and Evaluating Traumatic Encephalopathy using Clinical Tests” (DETECT) study. The purpose of DETECT is to identify in vivo biomarkers for CTE. Eligibility criteria for the former NFL players included the following: male, age of 40–69 years, a minimum of two NFL seasons and 12 years of organized tackle football, and self-reported cognitive, behavioral, and/or mood symptoms. Also recruited was a same-age comparison group (n = 23), who had no history of contact sport participation, service in the military, or self-reported TBI, and denied symptoms (at telephone screen); because of inherent recruitment bias, they served as “controls” for secondary analyses. Exclusion criteria for the DETECT study included MRI or lumbar puncture contraindications, presence of another central nervous system disease, and/or primary language other than English. The sample size was reduced from 96 former NFL players and 25 same age controls following exclusion of participants who either did not complete MRI or whose structural MRI data acquisition was of inadequate quality. Participants completed a 2- to 3-day visit involving demographic, medical, and athletic history interview(s), neurological evaluation, neuropsychological testing, structured psychiatric interview, self-report behavior/mood measures, and MRI. Study protocols were approved by the local Institutional Review Boards, and participants provided written informed consent.
2.2. Measures
2.2.1. MRI
All participants underwent MRI on a 3T Siemens Verio MRI scanner with a 32-channel head coil. Three-dimensional T1-weighted sequences (MPRAGE [1 × 1 × 1 mm3, TR = 1800 ms, TE = 3.36 ms]) were acquired. The T1-weighted images were visually inspected for artifacts and intrascan misalignments, and those with significant artifacts were excluded. Postprocessing of all images was conducted by the Psychiatry Neuroimaging Laboratory, Brigham and Women's Hospital. Brain masks were automatically generated based on T2-weighted images and were manually corrected. T2-weighted data optimized contrasts between the brain and the surrounding exterior cerebrospinal fluid (CSF). The T1-weighted images and the brain masks were imported into FreeSurfer 5.3 software. The FreeSurfer automated segmentation process of brain tissue has been described elsewhere [41], [42], but it subdivides the brain into regions of gray matter, white matter, and hypointense regions within the white matter using a combination of segmentation and a set of priors. Total volume of white matter hypointensities is an averaged composite of the left and right hemispheres. Visual quality assessment was performed following the FreeSurfer segmentation to ensure FreeSurfer included the entire brain and for overall completeness of the FreeSurfer segmentation. Quality control also included checking for proper detection of white and gray matter borders. If FreeSurfer did not detect white and gray matter borders correctly (leading to distorted segmentations), fiducials were set in neighboring white matter regions allowing the software to correctly define the tissue. Total volume of WMSAs (i.e., white matter hypointensities) and supratentorial volume (SV) were extracted from FreeSurfer output. SV served as a covariate in analyses.
2.2.2. Cumulative head impact index
The CHII was developed in a sample of former amateur American football players to estimate cumulative head impact exposure from football [10]. The CHII is based on self-reported number of seasons of football played and position(s) at each level played, and estimated head impact exposure frequencies from published helmet accelerometer studies in former amateur football players. Published helmet accelerometer studies at the professional level do not exist. For the present sample of former NFL players, college-level estimates of head impact frequencies were applied for their professional football exposure, in addition to the self-reported seasons and positions played in the NFL. Previous work in former NFL players from the DETECT study has linked CHII scores with higher total concentrations of plasma total tau [43]. Higher CHII reflects greater exposure to RHIs.
2.2.3. Neuropsychological and neuropsychiatric measures
Participants were administered a neuropsychological battery to evaluate attention, executive function, verbal and visual episodic memory, language, and visuospatial function. Participants completed semi-structured interviews and self-report measures of neuropsychiatric function (e.g., depression, aggression). The tests administered have been previously reported [44]. Neuropsychological test raw scores were transformed to standard scores that account for age, gender, and/or education. As described elsewhere [44], principal component analysis generated four composite factors measuring the following domains: behavioral/mood, psychomotor speed/executive function, and verbal and visual memory.
2.2.4. Modified Framingham Stroke Risk Profile
The Framingham Stroke Risk Profile (FSRP) is a widely used metric of CVD [45] and was included to account for CVD as a possible etiology of the WMSAs. The FSRP is calculated using an algorithm based on age, systolic blood pressure, hypertensive medication, diabetes, cigarette smoking, prior CVD, atrial fibrillation, and left ventricular hypertrophy. History of atrial fibrillation was deduced from medication status, whereas history of diabetes, cigarette smoking, and prior CVD (myocardial infarction, heart disease) were self-reported. Thirty-eight former NFL players were ≤54 years and were assigned a score of 0 for age. The FSRP raw risk score was calculated without left ventricular hypertrophy because this was not part of data collection. This has been done previously [46].
2.3. Statistical analyses
For analyses that examined WMSAs as the outcome, the WMSA variable was log-transformed due to a positively skewed distribution. In the 86 former NFL players, multivariable linear regression tested the association between the CHII and log-WMSAs. Linear regressions were used to examine the correlations between volume of WMSAs and the clinical domains (behavior/mood, psychomotor speed/executive function, and verbal and visual episodic memory). A separate model was performed for each domain. For analyses examining clinical function, the sample size was reduced to 76 due to missing data on tests that make up the domain factor scores (only participants with complete data on all of the factor scores were included in the analyses), and two were excluded due to objective test evidence of intentional symptom exaggeration. All analyses controlled for age, SV, and the mFSRP. Analyses were also repeated with race as a covariate, given 41.9% of the former NFL players were African American. Body mass index (BMI) was not included as a covariate, given the confound of BMI would largely stem from its association with CVD (as accounted for by the mFSRP). BMI is highly associated with the CHII (r = 0.34, P = .002) and is perhaps serving as a proxy for RHI exposure, as it reflects the person's playing position, that is, offensive linemen tend to have the highest level of RHI exposure [47] and the highest BMI [48]. In this sample, linemen had a higher BMI, compared with nonlinemen (mean difference = 2.78, P = .009).
For the secondary analyses, analysis of covariance compared log-WMSAs between the 86 symptomatic former NFL players and 23 same-age asymptomatic controls. Covariates included age and SV. The groups had nearly identical mFSRP scores (Table 1); therefore, the mFSRP was not included as a covariate. Race and BMI were not included as covariates in the between-group comparison. There is not a large enough sample of African American controls (n = 1) to accurately estimate the differential effects of race on WMSAs between the former NFL players and controls. The mFSRP and SV are examined and accounted for and thus attenuate concerns for confound from race. BMI was not included as a covariate, given the groups were similar in vascular status.
Table 1.
Sample characteristics of 86 former NFL players and 23 controls
| Demographic/athletic/health variables | NFL (n = 86) | Control (n = 23) | P value∗ |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD) years | 54.86 (7.91) | 57.22 (6.89) | .20 |
| Education, mean (SD) years | 16.43 (0.97) | 17.30 (2.14) | .07 |
| African American, n (%) | 36 (41.9) | 1 (4.3) | <.001 |
| Athletics | |||
| Duration of football play, mean (SD) years | 18.41 (3.44) | – | – |
| Cumulative head impact index, mean (SD) | 20,489.97 (7178.64) | – | – |
| Primary position group, n (%) | |||
| Offensive line | 25 (29.1) | – | – |
| Running back | 8 (9.3) | – | – |
| Tight end | 4 (4.7) | – | – |
| Offensive skill | 1 (1.2) | – | – |
| Defensive line | 13 (15.1) | – | – |
| Linebacker | 20 (23.3) | – | – |
| Defensive back | 15 (17.4) | – | – |
| Cardiovascular disease status | |||
| Body mass index, mean (SD) | 32.96 (4.99) | 28.02 (3.90) | <.001 |
| Systolic blood pressure, mean (SD) | 129.70 (15.78) | 135.09 (12.94) | .14 |
| Treated for hypertension, n (% yes) | 57 (66.3) | 17 (73.9) | .49 |
| History of diabetes, n (% yes) | 7 (8.1) | 1 (4.3) | 1.00 |
| History of cigarette smoking, n (% yes) | 3 (3.5) | 0 | 1.00 |
| History of cardiovascular disease, n (% yes) | 10 (11.6) | 0 | .12 |
| History of atrial fibrillation, n (% yes) | 4 (4.7) | 0 | .58 |
| †Modified Framingham Stroke Risk Profile, mean (SD) | 6.42 (3.34) | 6.39 (2.50) | .97 |
Abbreviations: NFL, National Football League; SD, standard deviation.
NOTE. Eligibility criteria for the control group required lack of reported symptoms and no history of head trauma or participation in contact sports.
Independent sample t tests were performed for continuous outcomes, and Fisher's exact test was used to compare group differences in race, history of diabetes, history of cigarette smoking, history of cardiovascular disease, and history of atrial fibrillation; χ2 test was used to examine differences on those treated for hypertension.
The Framingham Stroke Risk Profile is a modified version and did not include left ventricular hypertrophy.
3. Results
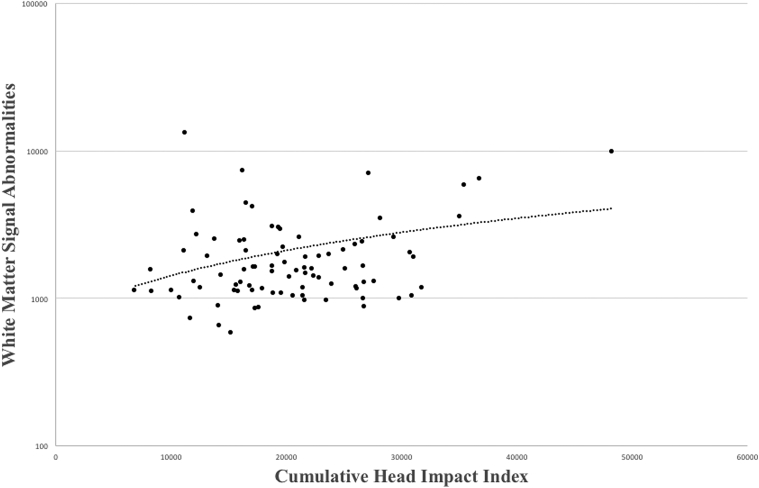
In the former NFL players, a higher CHII score was associated with increased volume of WMSAs (P = .043; Fig. 1), and increased volume of WMSAs predicted worse psychomotor speed/executive function (P = .015). See Table 2 for clinical test performance and Table 3 for a summary of the linear regression analyses. There were no significant effects for the other cognitive domains (P > .10). In the former NFL players, analysis of covariance controlling for age and SV showed that African Americans had greater volume of WMSAs (marginal mean difference = −0.17, 95% confidence interval [CI] = −0.28, −0.05, P = .008) and worse psychomotor speed and executive function (only controlling for age; marginal mean difference = −0.78, 95% CI = −1.13, −0.44, P < .001), compared to Caucasians. When race was added as a covariate to the analyses in the former NFL players, the effect of the CHII on WMSAs remained statistically significant (P = .021) and the association between WMSAs and psychomotor speed and executive function was diminished to a statistical trend (P = .061). Additional secondary analyses showed that the former NFL players had greater volume of WMSAs compared with the asymptomatic controls without a history of head trauma (marginal mean difference = −0.11, 95% CI = −0.22, −0.002, F = 4.07, P = .046).
Fig. 1.
Greater exposure to repetitive head impacts is associated with increased volume of white matter signal abnormalities. Scatter plot shows the relationship between the cumulative head impact index (x-axis) and volume of white matter signal abnormalities (y-axis, log-scale). This relationship was statistically significant after controlling for age, supratentorial volume, and the modified Framingham Stroke Risk Profile score (P = .043).
Table 2.
Clinical test performance in the former NFL players
| Measures | Mean (standard deviation) |
|---|---|
| Verbal episodic memory (T-scores) | |
| NAB List Learning Short Delay | 46.06 (12.75) |
| NAB List Learning Long Delay | 42.77 (13.94) |
| NAB Story Learning Immediate Recall | 39.73 (7.99) |
| NAB Story Learning Delayed Recall | 42.63 (7.53) |
| Visual episodic memory (T-scores) | |
| ROCFT Immediate Copy, Presence, and Accuracy | 48.96 (9.72) |
| ROCFT Delayed Presence and Accuracy | 49.57 (10.68) |
| Psychomotor speed and executive function (T-scores) | |
| Trail Making Test Part A | 49.69 (11.13) |
| Trail Making Test Part B | 44.89 (15.69) |
| WAIS-R Digit-Symbol Test (scaled score) | 10.25 (2.02) |
| DKEFS Color-Word Interference Test (scaled score) | 10.89 (2.81) |
| Controlled Oral Word Association Test | 49.93 (11.34) |
| Behavior and mood (raw scores) | |
| Beck Depression Inventory-II | 16.13 (11.44) |
| Center for Epidemiologic Studies Depression Scale | 22.14 (13.22) |
| Hamilton Depression Rating Scale | 9.54 (7.93) |
| Beck Hopelessness Scale | 4.75 (5.66) |
| Barratt Impulsivity Scale | 65.07 (14.77) |
| Apathy Evaluation Scale | 35.42 (9.70) |
| Brown-Goodwin Lifetime History of Aggression | 18.75 (5.05) |
| BRIEF-A Behavioral Regulation Index (T-score) | 63.04 (12.79) |
Abbreviations: BRIEF-A, Behavior Rating Inventory of Executive Function–Adult (self-report form); DKEFS, Delis-Kaplan Executive Function System; NAB, Neuropsychological Assessment Battery; NFL, National Football League; ROCFT, Rey-Osterrieth complex figure test; SD, standard deviation; WAIS-R, Wechsler Adult Intelligence Scale–Revised.
NOTE. All cognitive test results are presented as demographically corrected T-scores (mean = 50, SD = 10) except where noted. The tests presented include those that make up that four PCA-generated factor composite scores examined in this study, i.e., behavior/mood, psychomotor speed and executive function, and verbal and visual episodic memory. Sample size varies (N = 81 to N = 84) across tests due to missing data and two individuals are excluded from clinical analyses due to objective test evidence of intentional symptom exaggeration.
Table 3.
Linear regression models showing relationships between white matter signal abnormalities, repetitive head impact exposure, and psychomotor/speed executive function in the former NFL players (N = 86)
| Model variables | b (SE) | 95% CI | P-value |
|---|---|---|---|
| Log-white matter signal abnormalities | |||
| Age | 0.01 (0.004) | 0.002, 0.02 | .021 |
| Supratentorial volume | 0.0004 (0.0003) | −0.0002, 0.001 | .17 |
| Modified FSRP | 0.01 (0.01) | −0.01, 0.03 | .30 |
| CHII | 0.01 (0.004) | 0.0002, 0.02 | .043 |
| Psychomotor speed/executive function | |||
| Age | 0.02 (0.01) | −0.01, 0.05 | .12 |
| Supratentorial volume | 0.003 (0.001) | 0.001, 0.005 | .001 |
| Modified FSRP | −0.06 (0.03) | −0.12, 0.01 | .08 |
| White matter signal abnormalities | −0.11 (0.04) | −0.19, −0.02 | .015 |
Abbreviations: CHII, cumulative head impact index; CI, confidence interval; FSRP, Framingham Stroke Risk Profile.
NOTE. The FSRP is a modified version and did not include left ventricular hypertrophy. Age, supratentorial volume, and the modified FSRP were entered in block 1 of the regression model, and the CHII and white matter signal abnormalities were in block 2 (of separate models). Supratentorial volume, CHII, and white matter signal abnormalities were divided by 1000 to facilitate model fit. Bolded text indicates statistical significance.
4. Discussion
In this study, greater exposure to RHIs predicted increased volume of WMSAs in the former NFL players, after controlling for age, CVD, and SV (and race). In the former NFL players, increased volume of WMSAs corresponded to worse performance on tests of psychomotor speed and executive function, but not with measures of episodic memory or neuropsychiatric function. These findings provide evidence that exposure to RHIs may result in long-term pathological changes that manifest, at least in part, as WMSAs on MRI and are associated with cognition.
Secondary analyses further showed that the symptomatic former NFL players exhibited greater volume of WMSAs compared with asymptomatic male controls without a history of head trauma or contact sport participation. It is important to note that the former NFL players were required to be symptomatic (at telephone screen), whereas the controls must have been asymptomatic (at telephone screen) and have no history of head trauma. Therefore, the higher levels of WMSAs observed in the former NFL players compared with controls may not only be a result of exposure to RHIs but may also be attributed to differences in symptom status. Overall, future work that uses a more appropriate control group (i.e., clinically symptomatic controls without head trauma history) is needed to better understand the etiology and differences of WMSAs in former NFL players.
Although WMSAs are nonspecific neuroimaging findings, two etiologies that may underpin the association between exposure to RHIs and later-life WMSAs in former NFL players are included in the following sections.
4.1. Microvascular pathologies from RHIs and CTE
WMSAs typically accompany aging and CVD and reflect small-vessel ischemic disease due to microvascular cerebral hypoperfusion [13], [14], [15], [16], [17]. Microvascular cerebral hypoperfusion and resulting ischemia likely contribute to the association between RHIs and WMSAs. A single mTBI can cause acute decreases in cerebral perfusion, and such changes to perfusion levels may explain findings that link mTBI with WMSAs on MRI [22], [23]. RHIs may lead to persisting alterations in cerebral perfusion. For example, chronically impaired cerebral hemodynamic was observed in 12 professional boxers and was associated with cumulative rounds of and intensity of sparring [25]. Previous research also reports reductions in cerebral perfusion in former NFL players [7], [8]. Furthermore, CTE has been well-documented in former NFL players [2], and microvascular injury from RHIs has been theorized to contribute to the pathogenesis of the perivascular nests of p-tau deposits at the base of the sulci in CTE [27], [28], [29]. This is based on neuropathological evidence linking microvascular and tau pathology in boxers [29] and a case study correlating blood-brain barrier leakage (which can underlie small-vessel disease [49]) with CTE pathology [30]. These RHI-related microvascular changes can be captured on MRI as WMSAs, and MRI WMSAs have been found at the gray and white matter junction in active collegiate ice hockey players [26]. Importantly, the presence of CTE in the current sample is unknown. It is plausible that underlying CTE neuropathology is present in this sample of former NFL players because of their symptomatic status and high level of exposure to RHIs. Indeed, a recent autopsy case series found neuropathological evidence of CTE in 110/111 former NFL players who donated their brain for research [4]. Clinicopathological correlation studies will increase our understanding on the relationships among RHIs, WMSAs, CVD, and CTE.
4.2. Nonmicrovascular pathologies from RHIs and CTE
Gliosis and neuroinflammation are long-term correlates of RHIs [2], [9], [31] and CTE [2], [5] that can lead to WMSAs [35], [36], [37]. Diffuse axonal and white matter injuries are a characteristic consequence of concussion and subconcussive head trauma [50] that can persist into later life in individuals exposed to RHIs [33], [34]. White matter injury and degeneration is also found in most cases of CTE [2], [5]. Based on diffusion tensor imaging (DTI) studies in acute mTBI [51], WMSAs may reflect diffuse axonal injury associated with RHIs. Evaluation of WMSAs is clinically routine and thus the focus of this study. However, DTI has increased sensitivity to microstructural alterations (compared to WMSAs) and would be optimal for the early detection of white matter injury associated with exposure to RHIs and/or CTE. Previous work in former NFL players supports the utility of DTI in this setting [7]. Multimodal neuroimaging studies that correlate WMSAs with RHI- and CTE-related pathologies (e.g., p-tau from positron emission tomography imaging and axonal injury from DTI) will help to clarify the etiology of WMSAs in former NFL players.
4.3. WMSAs and clinical function
The pathologies of WMSAs contribute to the clinical presentation of AD [14], [39], [52], [53], [54], and similar effects may be present in CTE. CTE is associated with cognitive, behavioral, and mood deficits, with heterogeneity in severity and course potentially due to modifiers like WMSAs [3], [44]. In this study, greater volume of WMSAs predicted worse psychomotor speed and executive function—domains impacted by microvascular disease and CTE. There was no relationship between WMSAs and behavioral/mood and episodic memory, perhaps, because brain regions that modulate these clinical functions were not affected by WMSAs. The clinical effects of the pathologies of WMSAs may be dependent on their specific anatomical location [38], [53].
4.4. Limitations
There are several limitations associated with this study. To calculate the CHII in this sample of former NFL players, head impact frequencies from published helmet accelerometer studies in college football players were used to estimate professional exposure, and therefore, the CHII actually underestimated the extent of RHI exposure in this sample. Accurate retrospective quantification of recurrent concussion and subconcussive head injuries in former NFL players is challenging due to the remote and ambiguous nature of these events. Development and validation of RHI metrics should continue to be a target of future research. The etiologies of WMSAs are multifaceted, but often related to CVD. The mFSRP is not an exhaustive index of CVD and several of the components that make up the FSRP rely on self-report rather than direct assessment (e.g., fasting glucose levels). Overall, CVD is a prevalent comorbidity in former NFL players [19], [20], and it cannot be ruled out as a potential contributor to WMSAs in this sample of former NFL players.
It is unlikely that there is a competing neurodegenerative disease (e.g., AD) in the present sample due to the young age of the former NFL players (mean age = 55) [4]. The presence and severity of CTE in the former NFL players are unknown, and on average, the neuropsychological status of the sample was largely within normal limits. Only a subset of individuals who may be driving the current findings may have meaningful pathology. This is exemplified by a previous study showing significant variability in plasma total tau concentrations in the DETECT sample [43]. There is variability in both RHI exposure and WMSA burden in this sample (Fig. 1). For example, the one participant with the highest CHII score also had significant WMSA burden (relative to the other former NFL players) and clinical impairment (i.e., 1 SD below the mean on psychomotor speed and executive function). It is participants like this that possibly represent the target population, that is, those with likely CTE, and are driving the current findings. The present study needs to be repeated once CTE can be diagnosed during life. Indeed, not all individuals who play football or are exposed to RHIs develop long-term neurological consequences, and other risk factors, for example, apolipoprotein E (APOE), may interact with RHIs to increase one's susceptibility to the long-term effects of RHIs on the brain.
We did not examine the spatial distribution of WMSAs. FreeSurfer provides an automated approach to quantify total volume of T1 white matter hypointensities. Although this approach is frequently used in clinical research investigations on WMSAs, FreeSurfer's labeling of WMSAs tends to be more restricted to certain regions of the brain tissue (e.g., periventricular) [55], [56], [57]. The spatial distribution of WMSAs may be distinct across different neurodegenerative diseases [52] and can also provide insight into the cognitive domains they may influence. FLAIR also has increased sensitivity to WMSAs and is routine in the clinical evaluation of WMSAs because they appear as bright spots. Future work that uses FLAIR MRI is needed to clarify the spatial patterns of WMSAs in former professional football players compared with older adults, as well as other neurodegenerative disease comparison groups (e.g., AD).
Finally, there was one African American in the control group, whereas 41.9% of the former NFL players were African American. A similar proportion (39%) of African Americans was found in 3439 former NFL players [19]. Because only one control was African American, race was not included as covariate in the between-group analyses as it would not allow for accurate estimation of the differential effects of race on WMSAs across the two study groups. In the former NFL players, the relationship between the CHII and WMSAs remained statistically significant after race was added as a covariate; the association between WMSAs and psychomotor speed and executive function was diminished to a statistical trend. Compared to Caucasians, African Americans had greater volume of WMSAs and worse psychomotor speed and executive function. This is consistent with research that shows African Americans can exhibit more severe WMSAs [58], which may contribute to the association between African American race and poor neurological outcomes, including increased risk for AD dementia [59]. This relationship, however, appears to be dependent on genetic, clinical, and environmental factors [59]. To better understand race and WMSAs in former NFL players, the present study needs to be repeated with a larger and demographically matched control group.
5. Conclusions
WMSAs in former professional American football players may reflect long-term microvascular and nonmicrovascular pathologies from RHIs that are associated with cognition. Future studies that longitudinally examine WMSAs and clinical function in individuals at high risk for CTE and investigate the associations among WMSAs and RHI- and CTE-specific pathology will increase our understanding of the clinical implications of WMSAs in CTE.
Research in Context.
-
1.
Systematic review: We reviewed the literature using PubMed and references of research articles. Neuroimaging has been used to study the long-term neurological effects of repetitive head impacts (RHIs) and identify methods to detect chronic traumatic encephalopathy (CTE) during life. No study, however, has examined the presence and clinical correlates of white matter signal abnormalities (WMSAs) in former National Football League (NFL) players. This is despite WMSAs having been examined in the setting of acute mild traumatic brain injury and Alzheimer's disease; these studies are appropriately cited.
-
2.
Interpretation: In former NFL players, WMSAs may reflect long-term microvascular and nonmicrovascular pathologies from RHIs that are associated with cognition.
-
3.
Future directions: Multimodal neuroimaging studies that examine WMSAs and RHI- and CTE-related pathologies will likely clarify the etiology of WMSAs in NFL players. Once CTE can be diagnosed in life, longitudinal studies should examine whether WMSAs predict risk for and clinical progression of CTE.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH; P30 AG13846; R01NS078337; R56 9500304025; U01NS093334; 1U01NS086659-01). This publication was also supported by the National Center for Advancing Translational Sciences, NIH, through BU-CTSI Grant Number 1UL1TR001430. M.L.A. and the research reported in this publication are supported by the NIH under grant number 1F32NS096803-01 and a Pilot Grant from the Boston University Alzheimer's Disease Center (AG013846). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. There is no sponsor.
Footnotes
R.A.S. is a member of the Mackey-White Committee of the NFL Players Association. He is a paid consultant to Eli Lilly (Indianapolis, IN, USA), Avanir Pharmaceuticals (Aliso Viejo, CA, USA), and Biogen (Cambridge, MA, USA). He is a member of the Board of Directors of King-Devick Technologies, Inc. (Chicago, IL, USA), and he receives royalties for published neuropsychological tests from Psychological Assessment Resources, Inc. (Lutz, FL, USA). R.C.C. is a paid consultant to the NFL Head Neck and Spine Committee, NOCSAE, Concussion Legacy Foundation, royalties from book publications, and compensation from expert legal opinion. A.C.M. has received funding from the NFL and WWE and is a member of the Mackey-White Committee of the NFL Players Association. A.P.L. is a paid consultant for Agios pharmaceuticals and Moncton MRI. He is also a co-founder of BrainSpec Inc. The remaining authors have no conflicts of interest to disclose.
References
- 1.Bieniek K.F., Ross O.A., Cormier K.A., Walton R.L., Soto-Ortolaza A., Johnston A.E. Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol. 2015;130:877–889. doi: 10.1007/s00401-015-1502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKee A.C., Stern R.A., Nowinski C.J., Stein T.D., Alvarez V.E., Daneshvar D.H. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stern R.A., Daneshvar D.H., Baugh C.M., Seichepine D.R., Montenigro P.H., Riley D.O. Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81:1122–1129. doi: 10.1212/WNL.0b013e3182a55f7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mez J., Daneshvar D.H., Kiernan P.T., Abdolmohammadi B., Alvarez V.E., Huber B.R. Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA. 2017;318:360–370. doi: 10.1001/jama.2017.8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKee A.C., Cairns N.J., Dickson D.W., Folkerth R.D., Keene C.D., Litvan I. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016;131:75–86. doi: 10.1007/s00401-015-1515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coughlin J.M., Wang Y., Munro C.A., Ma S., Yue C., Chen S. Neuroinflammation and brain atrophy in former NFL players: An in vivo multimodal imaging pilot study. Neurobiol Dis. 2015;74:58–65. doi: 10.1016/j.nbd.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hart J., Jr., Kraut M.A., Womack K.B., Strain J., Didehbani N., Bartz E. Neuroimaging of cognitive dysfunction and depression in aging retired National Football League players: a cross-sectional study. JAMA Neurol. 2013;70:326–335. doi: 10.1001/2013.jamaneurol.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amen D.G., Willeumier K., Omalu B., Newberg A., Raghavendra C., Raji C.A. Perfusion Neuroimaging Abnormalities Alone Distinguish National Football League Players from a Healthy Population. J Alzheimers Dis. 2016;53:237–241. doi: 10.3233/JAD-160207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coughlin J.M., Wang Y., Minn I., Bienko N., Ambinder E.B., Xu X. Imaging of Glial Cell Activation and White Matter Integrity in Brains of Active and Recently Retired National Football League Players. JAMA Neurol. 2017;74:67–74. doi: 10.1001/jamaneurol.2016.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montenigro P.H., Alosco M.L., Martin B.M., Daneshvar D.H., Mez J., Chaisson C.E. Cumulative head impact exposure predicts later-life depression, apathy, executive dysfunction, and cognitive impairment in former high school and college football players. J Neurotrauma. 2017;34:328–340. doi: 10.1089/neu.2016.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright M.J., Woo E., Birath J.B., Siders C.A., Kelly D.F., Wang C. An index predictive of cognitive outcome in retired professional American Football players with a history of sports concussion. J Clin Exp Neuropsychol. 2016;38:561–571. doi: 10.1080/13803395.2016.1139057. [DOI] [PubMed] [Google Scholar]
- 12.Koerte I.K., Hufschmidt J., Muehlmann M., Tripodis Y., Stamm J.M., Pasternak O. Cavum septi pellucidi in symptomatic former professional football players. J Neurotrauma. 2016;33:346–353. doi: 10.1089/neu.2015.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garde E., Mortensen E.L., Krabbe K., Rostrup E., Larsson H.B. Relation between age-related decline in intelligence and cerebral white-matter hyperintensities in healthy octogenarians: a longitudinal study. Lancet. 2000;356:628–634. doi: 10.1016/S0140-6736(00)02604-0. [DOI] [PubMed] [Google Scholar]
- 14.Brickman A.M., Guzman V.A., Gonzalez-Castellon M., Razlighi Q., Gu Y., Narkhede A. Cerebral autoregulation, beta amyloid, and white matter hyperintensities are interrelated. Neurosci Lett. 2015;592:54–58. doi: 10.1016/j.neulet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolters F.J., Zonneveld H.I., Hofman A., van der Lugt A., Koudstaal P.J., Vernooij M.W. Cerebral perfusion and the risk of dementia: a population-based study. Circulation. 2017;136:719–728. doi: 10.1161/CIRCULATIONAHA.117.027448. [DOI] [PubMed] [Google Scholar]
- 16.O'Sullivan M., Lythgoe D.J., Pereira A.C., Summers P.E., Jarosz J.M., Williams S.C. Patterns of cerebral blood flow reduction in patients with ischemic leukoaraiosis. Neurology. 2002;59:321–326. doi: 10.1212/wnl.59.3.321. [DOI] [PubMed] [Google Scholar]
- 17.Holland C.M., Smith E.E., Csapo I., Gurol M.E., Brylka D.A., Killiany R.J. Spatial distribution of white-matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging. Stroke. 2008;39:1127–1133. doi: 10.1161/STROKEAHA.107.497438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardner R.C., Possin K.L., Hess C.P., Huang E.J., Grinberg L.T., Nolan A.L. Evaluating and treating neurobehavioral symptoms in professional American football players: Lessons from a case series. Neurol Clin Pract. 2015;5:285–295. doi: 10.1212/CPJ.0000000000000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehman E.J., Hein M.J., Baron S.L., Gersic C.M. Neurodegenerative causes of death among retired National Football League players. Neurology. 2012;79:1970–1974. doi: 10.1212/WNL.0b013e31826daf50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tucker A.M., Vogel R.A., Lincoln A.E., Dunn R.E., Ahrensfield D.C., Allen T.W. Prevalence of cardiovascular disease risk factors among National Football League players. JAMA. 2009;301:2111–2119. doi: 10.1001/jama.2009.716. [DOI] [PubMed] [Google Scholar]
- 21.Tate D.F., Gusman M., Kini J., Reid M., Velez C.S., Drennon A.M. Susceptibility weighted imaging and white matter abnormality findings in service members with persistent cognitive symptoms following mild traumatic brain injury. Mil Med. 2017;182:e1651–e1658. doi: 10.7205/MILMED-D-16-00132. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y., Nelson L.D., LaRoche A.A., Pfaller A.Y., Nencka A.S., Koch K.M. Cerebral blood flow alterations in acute sport-related concussion. J Neurotrauma. 2016;33:1227–1236. doi: 10.1089/neu.2015.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bigler E.D., Abildskov T.J., Goodrich-Hunsaker N.J., Black G., Christensen Z.P., Huff T. Structural neuroimaging findings in mild traumatic brain injury. Sports Med Arthrosc. 2016;24:e42–e52. doi: 10.1097/JSA.0000000000000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark A.L., Sorg S.F., Schiehser D.M., Luc N., Bondi M.W., Sanderson M. Deep white matter hyperintensities affect verbal memory independent of PTSD symptoms in veterans with mild traumatic brain injury. Brain Inj. 2016;30:864–871. doi: 10.3109/02699052.2016.1144894. [DOI] [PubMed] [Google Scholar]
- 25.Bailey D.M., Jones D.W., Sinnott A., Brugniaux J.V., New K.J., Hodson D. Impaired cerebral haemodynamic function associated with chronic traumatic brain injury in professional boxers. Clin Sci (lond) 2013;124:177–189. doi: 10.1042/CS20120259. [DOI] [PubMed] [Google Scholar]
- 26.Jarrett M., Tam R., Hernandez-Torres E., Martin N., Perera W., Zhao Y. A prospective pilot investigation of brain volume, white matter hyperintensities, and hemorrhagic lesions after mild traumatic brain injury. Front Neurol. 2016;7:11. doi: 10.3389/fneur.2016.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geddes J.F., Vowles G.H., Nicoll J.A., Revesz T. Neuronal cytoskeletal changes are an early consequence of repetitive head injury. Acta Neuropathol. 1999;98:171–178. doi: 10.1007/s004010051066. [DOI] [PubMed] [Google Scholar]
- 28.McKee A.C., Cantu R.C., Nowinski C.J., Hedley-Whyte E.T., Gavett B.E., Budson A.E. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buee L., Hof P.R., Bouras C., Delacourte A., Perl D.P., Morrison J.H. Pathological alterations of the cerebral microvasculature in Alzheimer's disease and related dementing disorders. Acta Neuropathol. 1994;87:469–480. doi: 10.1007/BF00294173. [DOI] [PubMed] [Google Scholar]
- 30.Doherty C.P., O'Keefe E., Wallace E., Loftus T., Keaney J., Kealy J. Blood-Brain Barrier dysfunction as a hallmark pathology in chronic traumatic encephalopathy. J Neuropathol Exp Neurol. 2016;75:656–662. doi: 10.1093/jnen/nlw036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin A.P., Ramadan S., Stern R.A., Box H.C., Nowinski C.J., Ross B.D. Changes in the neurochemistry of athletes with repetitive brain trauma: preliminary results using localized correlated spectroscopy. Alzheimers Res Ther. 2015;7:13. doi: 10.1186/s13195-015-0094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cherry J.D., Tripodis Y., Alvarez V.E., Huber B., Kiernan P.T., Daneshvar D.H. Microglial neuroinflammation contributes to tau accumulation in chronic traumatic encephalopathy. Acta Neuropathol Commun. 2016;4:112. doi: 10.1186/s40478-016-0382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stamm J.M., Koerte I.K., Muehlmann M., Pasternak O., Bourlas A.P., Baugh C.M. Age at first exposure to football is associated with altered corpus callosum white matter microstructure in former professional football players. J Neurotrauma. 2015;32:1768–1776. doi: 10.1089/neu.2014.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koerte I.K., Lin A.P., Muehlmann M., Merugumala S., Liao H., Starr T. Altered Neurochemistry in former professional soccer players without a history of concussion. J Neurotrauma. 2015;32:1287–1293. doi: 10.1089/neu.2014.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fazekas F., Kleinert R., Offenbacher H., Schmidt R., Kleinert G., Payer F. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 36.Udaka F., Sawada H., Kameyama M. White matter lesions and dementia: MRI-pathological correlation. Ann N Y Acad Sci. 2002;977:411–415. doi: 10.1111/j.1749-6632.2002.tb04845.x. [DOI] [PubMed] [Google Scholar]
- 37.Wardlaw J.M., Valdes Hernandez M.C., Munoz-Maniega S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J Am Heart Assoc. 2015;4:001140. doi: 10.1161/JAHA.114.001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brickman A.M., Zahodne L.B., Guzman V.A., Narkhede A., Meier I.B., Griffith E.Y. Reconsidering harbingers of dementia: progression of parietal lobe white matter hyperintensities predicts Alzheimer's disease incidence. Neurobiol Aging. 2015;36:27–32. doi: 10.1016/j.neurobiolaging.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee S., Viqar F., Zimmerman M.E., Narkhede A., Tosto G., Benzinger T.L. White matter hyperintensities are a core feature of Alzheimer's disease: Evidence from the dominantly inherited Alzheimer network. Ann Neurol. 2016;79:929–939. doi: 10.1002/ana.24647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alosco M.L., Mez J., Kowall N.W., Stein T.D., Goldstein L.E., Cantu R.C. Cognitive Reserve as a Modifier of Clinical Expression in Chronic Traumatic Encephalopathy: A Preliminary Examination. J Neuropsychiatry Clin Neurosci. 2017;29:6–12. doi: 10.1176/appi.neuropsych.16030043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 43.Alosco M.L., Tripodis Y., Jarnagin J., Baugh C.M., Martin B., Chaisson C.E. Repetitive head impact exposure and later-life plasma total tau in former National Football League players. Alzheimers Dement (Amst) 2017;7:33–40. doi: 10.1016/j.dadm.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alosco M.L., Jarnagin J., Tripodis Y., Platt M., Martin B., Chaisson C.E. Olfactory Function and Associated Clinical Correlates in Former National Football League Players. J Neurotrauma. 2017;34:772–780. doi: 10.1089/neu.2016.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D'Agostino R.B., Wolf P.A., Belanger A.J., Kannel W.B. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994;25:40–43. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- 46.Llewellyn D.J., Lang I.A., Xie J., Huppert F.A., Melzer D., Langa K.M. Framingham Stroke Risk Profile and poor cognitive function: a population-based study. BMC Neurol. 2008;8:12. doi: 10.1186/1471-2377-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baugh C.M., Kiernan P.T., Kroshus E., Daneshvar D.H., Montenigro P.H., McKee A.C. Frequency of head-impact-related outcomes by position in NCAA division I collegiate football players. J Neurotrauma. 2015;32:314–326. doi: 10.1089/neu.2014.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Basra S.S., Pokharel Y., Hira R.S., Bandeali S.J., Nambi V., Deswal A. Relation between playing position and coronary artery calcium scores in retired National Football League players. Am J Cardiol. 2014;114:1836–1840. doi: 10.1016/j.amjcard.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 49.Wardlaw J.M., Makin S.J., Vales Hernandez M.C., Armitage P.A., Heye A.K., Chappell F.M. Blood-brain barrier failure as a core mechanism in cerebral small vessel disease and dementia: evidence from a cohort study. Alzheimers Dement. 2017;13:634–643. [Google Scholar]
- 50.Giza C.C., Hovda D.A. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75:S24–S33. doi: 10.1227/NEU.0000000000000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohammadian M., Roine T., Hirvonen J., Kurki T., Ala-Seppala H., Frantzen J. High angular resolution diffusion-weighted imaging in mild traumatic brain injury. Neuroimage Clin. 2017;13:174–180. doi: 10.1016/j.nicl.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lindemer E.R., Greve D.N., Fischl B., Augustinack J.C., Salat D.H., Alzheimer's Disease Neuroimaging Initiative Differential regional distribution of juxtacortical white matter signal abnormalities in aging and Alzheimer's disease. J Alzheimers Dis. 2017;57:293–303. doi: 10.3233/JAD-161057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lindemer E.R., Greve D.N., Fischl B.R., Augustinack J.C., Salat D.H. Regional staging of white matter signal abnormalities in aging and Alzheimer's disease. Neuroimage Clin. 2017;14:156–165. doi: 10.1016/j.nicl.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kandel B.M., Avants B.B., Gee J.C., McMillan C.T., Erus G., Doshi J. White matter hyperintensities are more highly associated with preclinical Alzheimer's disease than imaging and cognitive markers of neurodegeneration. Alzheimers Dement (Amst) 2016;4:18–27. doi: 10.1016/j.dadm.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jacobs H.I., Leritz E.C., Williams V.J., Van Boxtel M.P., van der Elst W., Jolles J. Association between white matter microstructure, executive functions, and processing speed in older adults: the impact of vascular health. Hum Brain Mapp. 2013;34:77–95. doi: 10.1002/hbm.21412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leritz E.C., Shepel J., Williams V.J., Lipsitz L.A., McGlinchey R.E., Milberg W.P. Associations between T1 white matter lesion volume and regional white matter microstructure in aging. Hum Brain Mapp. 2014;35:1085–1100. doi: 10.1002/hbm.22236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salat D.H., Tuch D.S., van der Kouwe A.J., Greve D.N., Pappu V., Lee S.Y. White matter pathology isolates the hippocampal formation in Alzheimer's disease. Neurobiol Aging. 2010;31:244–256. doi: 10.1016/j.neurobiolaging.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brickman A.M., Schupf N., Manly J.J., Luchsinger J.A., Andrews H., Tang M.X. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Arch Neurol. 2008;65:1053–1061. doi: 10.1001/archneur.65.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barnes L.L., Bennett D.A. Alzheimer's disease in African Americans: risk factors and challenges for the future. Health Aff (Millwood) 2014;33:580–586. doi: 10.1377/hlthaff.2013.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]