Abstract
Peripheral artery disease (PAD) is a morbid condition whereby ischemic peripheral muscle causes pain and tissue breakdown. Interestingly, PAD risk factors, e.g. diabetes mellitus, cause endothelial dysfunction secondary to decreased nitric oxide (NO) levels, which could explain treatment failures. Previously, we demonstrated 670nm light (R/NIR) increased NO from nitrosyl-heme stores, therefore we hypothesized R/NIR can stimulate vasodilation in healthy and diabetic blood vessels. Vasodilation was tested by ex vivo pressure myography in wild type C57Bl/6, endothelial nitric oxide synthase (eNOS) knockout, and db/db mice (10 mW/cm2 for 5min with 10 min dark period). NOS inhibition with N-Nitroarginine methyl ester (L-NAME) or the NO scavenger Carboxy-PTIO (c-PTIO) tested the specificity of NO production. 4,5-Diaminofluorescein diacetate (DAF-2) measured NO in human dermal microvascular endothelial cells (HMVEC-d). R/NIR significantly increased vasodilation in wild type and NOS inhibited groups, however R/NIR dilation was totally abolished with c-PTIO and blood vessel denudation. Interestingly, the bath solution from intact R/NIR stimulated vessels could dilate light naïve vessels in a NO dependent manner. Characterization of the bath identified a NO generating substance suggestive of S-nitrosothiols or non heme iron nitrosyl complexes. Consistent with the finding of an endothelial source of NO, intracellular NO increased with R/NIR in HMVEC-d treated with and without L-NAME (1mM), yet c-PTIO (100μm) reduced NO production. R/NIR significantly dilated db/db blood vessels. In conclusion, R/NIR stimulates vasodilation by release of NO bound substances from the endothelium. In a diabetes model of endothelial dysfunction, R/NIR restores vasodilation, which lends the potential for new treatments for diabetic vascular disease.
Keywords: Microcirculation, Endothelial dysfunction, Diabetes Mellitus, Electromagnetic energy, Photobiomodulation, Nitric Oxide
Graphical abstract
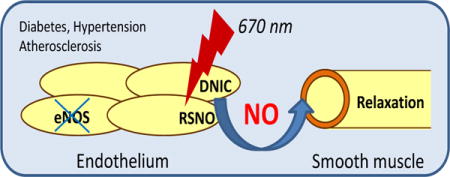
Introduction
Red/Near Infrared (R/NIR) light, has been used clinically for many years in the context of wound healing, plastic surgery, chronic joint pain and in the field of dermatology [1–3]. Many of the effects of R/NIR are related to observed effects on blood flow and angiogenesis, which are driven at least in part through nitric oxide dependent processes [4, 5]. While initially thought to be specific to laser light, similar effects were observed for other light sources, e.g. light emitting diodes. The biological chromophore(s) that absorb the light energy have been the focus of significant discussion with the copper site of cytochrome c oxidase a major candidate [4, 6–8]. However, many of the effects of R/NIR appear to be related to increased bioactivity of nitric oxide, and it is not clear how the effects of light energy could be accomplished via cytochrome c oxidase, other than perhaps the liberation of NO bound to this enzyme.
We have previously shown that nitrosyl heme groups, in hemoglobin and myoglobin, can surprisingly release NO, with very low efficiency, upon exposure to 670 nm radiation, and we proposed this as a possible mechanism for R/NIR cardioprotection [9]. Of interest was the observation that R/NIR acted synergistically with inorganic nitrite in this system, which increases cardiac MbNO levels. In addition, we have shown that R/NIR irradiation can stimulate collateral vessel growth after occlusion [10]. While R/NIR light has consistently been shown to stimulate the dilation of blood vessels in various vascular beds and preparations, and largely through NO-dependent processes, the fundamental mechanism of action of light remains unknown [11, 12]. The consequences of these observations are relevant to vascular pathologies where NO bioavailability is impaired and particularly in diabetic vasculopathy where current treatments often involve surgery and amputation.
In this study, we demonstrate that that 670nm light, (R/NIR) irradiation can directly stimulate blood vessel dilation in the absence of nitric oxide synthase through an NO dependent process. In addition, irradiation of isolated vessels produces a quasi-stable, transferable, vasodilator in the organ bath with the characteristics of an S-Nitrosothiol or a dinitrosyl iron complex (DNIC). Importantly, this mechanism of dilation in still available in both the eNOS knockout and diabetic mouse models, where conventional endothelial-derived dilation is impaired.
Materials and Methods
All experimental procedures and protocols used in this investigation were reviewed and approved by the Animal Care and Use Committee of the Medical College of Wisconsin. Furthermore, all conformed to the Guiding Principles in the Care and Use of Animals of the American Physiologic Society and were in accordance with the Guide for the Care and Use of Laboratory Animals.
R/NIR source
A 670 nm LED light source with power output up to 100 mW/cm2 was obtained from Quantum Devices (Barneveld, WI). Power output was measured with a light meter (×97 Irradiance meter, GigaHertz-Optik). A separate 670 nm light emitting diode source was utilized for all pressure myography experiments (NIR Technologies, Waukesha, WI). The light sources were placed 2.5cm from their target in all pressure myography experiments because this was the closest distance feasible between the light source and the chamber. In vitro, culture dishes and slides were placed directly on the light source. The irradiance intensities listed below reflect the values obtained at the chamber for ex vivo studies, or the bottom of the culture dish for in vitro studies.
DAF-2DA HPLC Method
Human Dermal Microvascular Endothelial Cells (HMVEC-d) (Lonza, Basel, Switzerland) were cultured under standard conditions. All experiments were performed using passages P6–P8. HMVEC were incubated with 5 μM DAF-2DA (Calbiochem, Billerica, MA) in Opti-MEM media (Thermo Fisher Scientific, Waltham, MA) for 30 minutes at 37 °C. The cells were exposed to 670 nm light at 25, 50, or 100 mW/cm2 for 0, 30, 60, or 120 seconds to 0, 0.75, 1.5, 3, 6, and 12 Joules/cm2 and then allowed to incubate another 15 minutes at 37 °C. Cells were then trypsinized, pelleted, and resuspended in Lysis Buffer (0.1% Triton X-100, 10mM NaPBS, 100uM DTPA). The cell lysate was passed through a 28G needle 10 times and centrifuged using a Y-10 Amicon Ultra filter (Millipore, Billerica, MA) at 12,000g for 90 minutes. The flow-through was injected onto an HPLC with a Kromasil C18 column (25 cm × 4.6 cm ID × 5 μm particle size). The mobile phase consisted of 10mM NaPBS (pH 7.5) and 5% Acetonitrile. A standard curve of DAF-2T (Genaxxon, Ulm, Germany) was used to determine concentration of DAF-2 in the samples. Positive controls for NO production include the calcium ionophore, A23187 (5μM, Cayman Chemical, Ann Arbor MI) and vascular endothelial growth factor (50 ng, EMD Millipore, Bellerica, MA). NO measurement by HPLC was represented as % increase over control to normalize the results since absolute DAF-2T levels can be affected by the intracellular concentration of anti-oxidants (e.g. ascorbic acid) [13, 14].
Ozone-based chemiluminescence measurements
S-Nitrosothiol formation were detected using a reducing media consisted of KI/I2, glacial acetic acid and dd H2O [15]. Sulfanilamide was added to the samples (10 % vol/vol of 100 mM in 2 N HCl) to remove nitrite. Mercuric chloride (10 % vol/vol of 50 mM HgCl2 dissolved in ddH2O) was added to the bath solution for inhibition of S-nitrosothiol or di-nitrosyl iron complex (DNIC).
Pressure Myography
Segments of facialis arteries (160–260 μm ID) from C57Bl6/J mice were transferred to a water-jacketed perfusion chamber and cannulated with two glass micropipettes (tip diameter ≈30 μm) at their in-situ length. The arteries were bathed in the PSS-equilibrated solution maintained at pH 7.4 and 37 °C. The micropipettes were connected to a reservoir filled with physiological saline solution and the arteries were pressurized to 60 mmHg. The internal diameter of the arteries was measured with a video system composed of a stereomicroscope (Olympus CK 40), a charge-coupled device camera (Panasonic GP-MF 602), a video monitor (Panasonic WV-BM 1410), and a video measuring apparatus (Boeckeler VIA-100).
After a 1hour equilibration period, the arteries were pre-constricted by ∼50% of their resting diameter with a thromboxane A2 analog, U-46619. To test the stability of U46619 to achieve stable blood vessel diameters through the end of a 30min period, we measured diameters in a separate group up to 45 min after steady state. Diameters did not change during this test period (data not shown). The blood vessels were placed in the dark and once steady-state contraction was obtained, they were treated with 10 mW/cm2 of 670 nm Light (NIR Technologies, Waukesha, WI) for 5 minutes. The blood vessels were then allowed to recover in the dark for 10 minutes and then the R/NIR light was reapplied for another 5 minutes with a subsequent5 minute recovery period in the dark. For the L-NAME and c-PTIO blood vessel dilation experiments the drugs were added into the bath solution before being pre-constricted with U46619 (Cayman Chemical, Ann Arbor, Michigan). For the denuded blood vessels, air was pushed through the vessel to remove the endothelium and then was hung on micropipettes. Vasodilator responses to papaverine (10−4 M) were determined at the end of experiments and were comparable in all tested groups. Vascular responses are expressed as percent maximal relaxation relative to U-46619 pre-constriction, with 100 % representing the passive baseline diameter with papaverine (Cayman Chemical, Ann Arbor, Michigan).
Statistics
All values are expressed as mean Standard Deviation (SD). All comparisons were made using a one-way ANOVA with post-hoc Tukey or Dunn’s t- test. Values for p<0.05 were considered significant.
Results
670 nm light dilates murine arteries in an endothelium dependent manner
To assess the efficacy of 670 nm light to dilate arteries, facial arteries from C57Bl/6 mice were pressurized and pre-constricted with U46619 in the dark. There was a time dependent increase in blood vessel diameter when exposed to 670 nm light, reaching 16.6 % (SD 8.9, p<0.001) increase over baseline after 5 min (Figure 1A). Upon withdrawal of the light source, there was a temporary decrease in diameter which reached a nadir after 4 min. By 10 min post light exposure, there was spontaneous dilation within the dark period. Since previous studies suggested the mediator of vasodilation required a replenishment period, the blood vessel was re-exposed to light energy at the same intensity [11]. This second exposure produced a 16.2 % (SD 10.8) increase in diameter over 5 min over the second baseline. Overall, both exposures within 20 min produced an absolute dilation of 36.1 % (SD 20.7). Withdrawal of light after the second exposure did not result in significant contraction.
Figure 1.
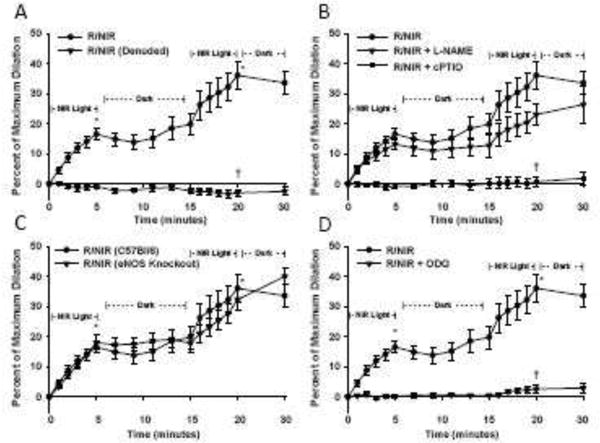
Facial arteries from C57Bl/6 mice were isolated and pressurized to 60 mmHg before pre-constriction with U46619. A) Energy exposure (R/NIR) produced a time dependent increase in vessel diameter (* p<0.001 vs baseline, n=21). Denudation of the vessel significantly attenuated dilation († p<0.001 vs. R/NIR, n=11). B) Pre-incubation with NOS inhibitor L-NAME (1 mM) did not significantly attenuate R/NIR dilation, however c-PTIO (100 μM) abolished the R/NIR effect (†=p<0.001 vs. R/NIR, n=10). C) Energy exposure (R/NIR) to vessels from eNOS knockout mice produced a similar time dependent increase in vessel diameter when compared to wild type control (*=p<0.001 vs baseline, n=10). D) Pre-incubation with ODQ (10μM) abolished the R/NIR effect (†=p<0.001 vs. R/NIR, n=8). R/NIR dilation curves in panels B–D are duplicated from Panel A.
To test whether an intact endothelium is necessary for 670 nm dilation, blood vessels were denuded and exposed to light under the same conditions. The previously observed dilation to 670 nm was abolished by removal of the endothelium at all measured time points (−0.8% SD 2.5 at 5 minutes, −0.6% SD 2.5 second exposure, and −2.9% SD 4.0 absolute dilation).
670 nm light stimulates vasodilation through nitric oxide-mediated mechanism
To determine if 670 nm light-dependent dilation is NO-dependent, the vessels were pre-incubated with the NO scavenger molecule c-PTIO. Light-dependent dilation was completely abolished at all measured time points by this treatment (Figure 1B, −0.6% SD 2.4 at 5 minutes, 0.6 % SD 1.8 second exposure, and 0.8 % SD 5.4 absolute dilation, p<0.001) indicating absolute NO-dependence of R/NIR-mediated vasodilatation.
Previously reported studies using polychromatic light suggested inhibition of NOS resulted in enhanced vasodilation of coronary arteries [11]. To examine the role of eNOS, we incubated facial arteries with the pan-NOS inhibitor, L-NAME prior to light exposure (Figure 1B). In the presence of L-NAME, vessel dilation in response to light exposure was similar to the 670nm light control group. The first period of exposure generated an absolute increase of 13.2 % (SD 6.2) with L-NAME, and the second exposure stimulated 10.2 % (SD 5.5). Interestingly, during the 10min dark period, the vessel diameter remained unchanged which suggests eNOS-generated NO exerts a secondary effect on light induced vasodilation. When compared to the 670nm control group, total dilation in the presence of with L-NAME was not significantly different (23.1 % (SD 11.6) vs. 36.1 % (20.7). The repeated dark period did not significantly stimulate vessel contraction or relaxation. These data suggest that eNOS activity may play some role in 670nm light-dependent dilation, but is not an absolute requirement.
To confirm the observation using L-NAME, vessels from eNOS knockout mice were stimulated with light under identical conditions (Figure 1C). The pattern of dilation was similar to the C57Bl/6 control. After 5 min of light the diameter increased to 18.0 % (SD 7.7). During the post- exposure dark period, the vessel diameter did not change, but once re-exposed to light, there was a modest increase in dilation of 14.5 % (SD 4.0). Similar to the L-NAME group, the second exposure achieved an absolute dilation of 32.3 % (SD 11.0)
670 nm light stimulates dilation through activation of soluble guanylate cyclase
To confirm the nitric oxide pathway is mediating light stimulated dilation, vessels were pretreated with ODQ (10μM), an irreversible inhibitor of the soluble guanylate cyclase. ODQ competes with NO binding to the heme moiety of the enzyme and prevents production of cGMP. Light dependent dilation was completely abolished (Figure 1D, 0.17% SD 1.5 at 5 min and absolute diameter increase 2.6% SD 3.2, p< 0.001). These data further support NO as the vasodilator produced by 670 nm light.
670 nm light stimulates dilation through production of an endothelium dependent substance
Although 670 nm light could produce significant dilation through a NO-mediated pathway, the observation that vessel diameter did not return to baseline suggested two possibilities. First, the blood vessel was permanently altered by 670 nm light and could not return to the baseline pre-constricted diameter; and second, that 670 nm light stimulated the production of a more long-lasting vasodilator substance. 670 nm light treatment did not interfere with U46619 activity, since blood vessel constriction was equally effective when pretreated U46619 (5 min of 670 nm light) or untreated U46619 was added to the bath (data not shown). Interestingly, after a vessel was exposed to 5 min of light, additional U46619 treatment did not result in constriction of the blood vessel (Supplemental Figure 1). However, if the bath fluid was replaced after light exposure, u46619 treatment restored blood vessel diameter to baseline. This raised the possibility that light exposure was producing a vasodilator substance.
To test whether 670nm energy produced a persistent vasodilator substance, blood vessels were treated with light for 10 minutes, which produced a 43.1 % (SD 7.5, n=8 vessels) increase in diameter (Figure 2A). After the energy treatment, the bath fluid was collected and added to a vessel which was not previously treated with light. This vessel significantly dilated 40.6% (SD 11.9) over 20 minutes. The bath fluid collected from a control, unexposed blood vessel caused little to no dilation ruling out mechanical stimuli as an etiology for the dilation (Figure 2B). In this latter experiment, the internal diameter remained unchanged from baseline (5%, SD 3.8, n=8 vessels) additionally reflecting the stability of the pressurized blood vessels over time. Importantly, the ability of the nascent vasodilatory substance to dilate a naive vessel was abolished by the NO scavenger cPTIO (Figure 2C) (3.9%, SD 1.2%, n=8 vessels). In addition, no vasodilatory substance was present in bath fluid after exposure of a denuded blood vessel to 670 nm energy (Figure 2D) (4.5%, SD 3.9%, n=8 vessels). This is in agreement with a requirement of an intact endothelium for light-mediated dilatation. To further characterize the downstream action of the vasodilatory substance, a denuded vessel was exposed to the bath fluid from an intact vessel exposed to 670 nm energy. The denuded vessel significantly dilated (38.5% SD 4.6, n=8 vessels), thus supporting the direct actions of the vasodilatory substance on smooth muscle (Figure 2E).
Figure 2.
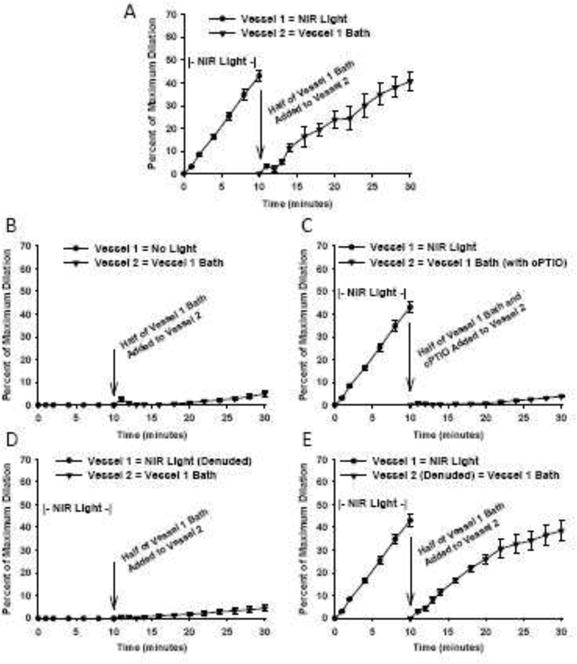
Facial arteries from C57Bl/6 mice were isolated and pressurized to 60 mmHg before pre-constriction with U46619 (n=8). A) After 10 min of light exposure, the bath solution from vessel 1 was added to vessel 2 which produces significant vasodilation. B) Control vessel unexposed to light shows no change in vessel 2 diameter (5.0%, SD 3.8). C) Vasodilation by the light produced substance is abolished in the presence of NO scavenger c-PTIO (3.9%, SD 1.2). D) Bath solution collected from light exposure on a denuded vessel cannot produce vasodilation (4.5%, SD 3.9). E) A denuded vessel will still dilate from the light produced substance of an intact vessel (38.5% SD 4.6).
To further investigate the nature of the vasodilatory species we examined the bath solution using ozone-based chemiluminescence. NO was not directly detectable in the bath fluid (data not shown), however, if bath fluid was injected into tri-iodide/acetic acid, after treatment with acid/sulfanilamide to remove nitrite, a chemiluminescence signal was detected that was depended on vessel irradiation (Figure 3). Substances that are known to give a chemiluminescence signal under these conditions include S-nitrosothiols and di-nitrosyl iron complex (DNIC). The chemiluminescence signal was attenuated by addition of mercuric chloride, suggesting one of the latter two species. Chemiluminescence data are quantified in Figure 3, using CysNO as a standard, and show that under these conditions, the level of the compound reached 40 nM CysNO equivalents.
Figure 3.
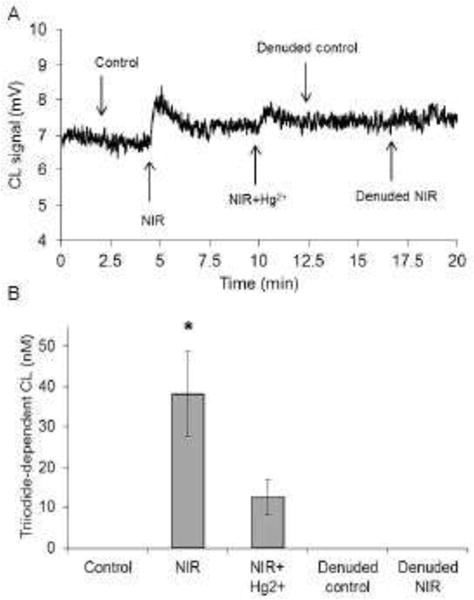
A) Representative tracing of ozone chemiluminescent signal. Bath solution from unexposed control, NIR exposure, and denuded vessel were analyzed with triiodide-dependent ozone-based chemiluminescence. Sulfanilamide solution (10 %) was added to bath samples to scavenge nitrite. Only NIR exposed bath could generate a triiodide dependent signal. Addition of mercuric chloride attenuated the signal. B) Quantification of the ozone chemiluminescent data (n=4, *p<0.001 vs control).
To further characterize the magnitude of light stimulated vasodilatation, facial arteries were stimulated with 40 nM GSNO and 40nM DNIC (Figure 4). Vessel diameter after 5 minutes of stimulation of GSNO (12.8% SD 4.4) and 40 nM DNIC (12.8% SD 4.9) was similar to 5 minutes of light treatment (16.6%, SD). These data indicate the quantity of light stimulated vasodilatory species measured in Figure 3 produces an increase in vessel diameter comparable to a similar quantity of purified S-nitrosothiol or DNIC.
Figure 4.
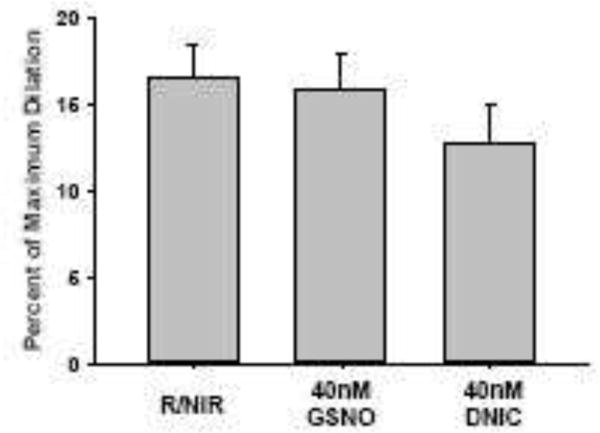
Facial arteries from C57Bl/6 mice were isolated and pressurized to 60 mmHg before pre-constriction with U46619. Percentage change in vessel diameter was compared after 5 min of R/NIR to a bath containing 40 nM GSNO and 40nM DNIC. N=5 vessels for each group.
670 nm light increases Nitric Oxide in Human Dermal Microvascular Endothelial Cells
We examined the ability of 670 nm light to increase NO production in a cellular model using DAF-2 to detect NO. This molecule generates a fluorescent triazole (DAF-2T) through the oxidative chemistry of NO, that is thought to be specific to NO. Since DAF-2 can generate nonspecific fluorescence products, we chose to measure the DAF-2 triazole directly by HPLC [16]. There was a significant time dependent increase in DAF-2T content when compared to the unexposed control (Figure 5A), 19.5% SD 4.9 at 3 J/cm2, p<0.001 n=5 independent experiments). DAF-2T content at 6 J/cm2 was significantly increased when compared to the unexposed control (13.1% SD 7.2, p<0.001), however the increase was similar to the cells exposed to 3J. To verify the DAF-2T method is sensitive to detect NO production in HMVEC-d, the calcium ionophore A23187 was used as a positive control. A23187 (5 μM) addition produced an 27 % increase (Figure 5B, SD 20, n=3). VEGF stimulates NOS in a calcium independent manner and was found to increase DAF-2T by 12 % (SD 12.8, n=3). To verify the specificity of the measured DAF-2T product, the NO scavenger, c-PTIO, was added prior to light exposure, and significantly attenuated the DAF-2T peak (Figure 5A, 12.1.0% (SD 11.4) at 3 J/cm2, p=0.001. However, inhibition of NOS with L-NAME modestly, but not significantly decreased DAF-2T, indicating light-dependent increases in NO were not entirely dependent on the presence of functional NOS.
Figure 5.
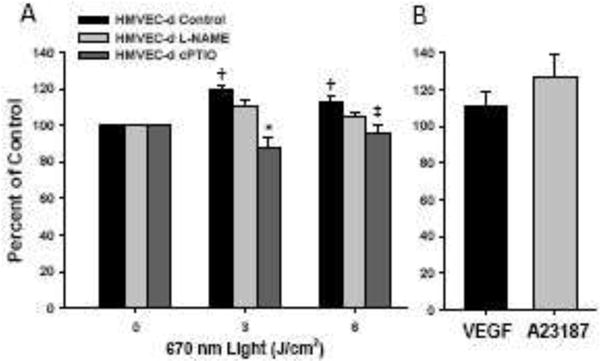
A) HMVEC-d were exposed to 670 nm energy (50 mW/cm2). Increases in DAF-2T signal were dose dependent (†=p<0.001 vs 0 Joules). HMVEC-d were treated with NO scavenger c-PTIO and eNOS inhibitor L-NAME, then exposed to 670 nm energy (50 mW/cm2). The observed increases in DAF-2T signal upon R/NIR exposure are inhibited with c-PTIO (* p=0.001, ‡ p=0.006 vs respective controls). N=5 individual experiments. B) Positive controls for NOS stimulation (50 ng VEGF and 5μM A23187 for comparison
670nm light can stimulate vasodilation in a model of NO depletion
Since our results indicate light-induced increases in NO levels and vasodilation do not require functional NOS, we hypothesized whether light could improve dilation in vessels with known impairment of NO dependent dilation. The db/db mouse has a genetic deletion of the leptin receptor, and is a well described animal model of type II diabetes mellitus and endothelial dysfunction [17]. When 670 nm light was applied to db/db vessels, there was a significant time dependent increase in vasodilation 11.9% (SD 7.0, p<0.001) (Figure 6). During the initial dark period dilation continued to occur, such that by the end of the 10 min the absolute change in vessel diameter was 27.2 % (SD 21.5). The magnitude of dilation to light after a second exposure was similar to the first exposure (13.5 % (SD 4.9) vs. 11.9% (SD 7.0)), confirming there is a consistency in the observed effect of 670 nm light on vessel diameter. Since the response of db/db vessels during the dark period was unexpected, there was concern the observed effects were non-specific. Therefore, c-PTIO was added to scavenge NO and energy mediated vasodilation was significantly abolished during all measured time-points (−0.2 % SD 4.1 at 5 minutes, 1.8 % SD 4.9 second exposure, and 5.7 % SD 6.0 absolute dilation, p<0.05).
Figure 6.
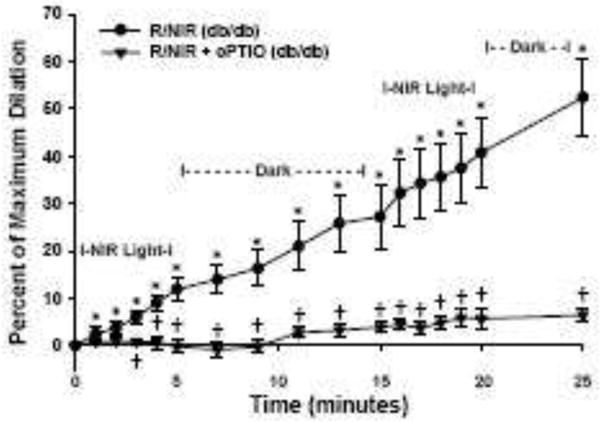
Facial arteries from db/db mice were isolated and pressurized to 60 mmHg before pre-constriction with U46619. Energy exposure (R/NIR) produced a time dependent increase in vessel diameter (*=p<0.001 vs baseline, n=10). Pre-incubation with NO scavenger, c-PTIO (100 μM), significantly attenuated dilation (†=p<0.001 vs. R/NIR, n=9).
Discussion
We show in this study that exposure mouse facial arteries to 670 nm light produces a sustained vasodilation that is not greatly affected by the NOS inhibitor L-NAME but is abolished by the NO scavenger c-PTIO and by endothelial denudation. This strongly points to the conclusion that light exposure results in vasodilation from an endothelial NO ‘store’ that is not immediately affected by the inhibition of NOS activity. Remarkably, light exposure results in the generation of a tri-iodide/ozone-based chemiluminescent-detectable compound in the bath surrounding the vessel that is able to stimulate vasodilation in un-irradiated vessels. This compound is only generated if the vessel has an intact endothelium. These data suggest a mechanism where 670 nm irradiation is able to stimulate the vascular endothelium to liberate a NO-dependent vasodilator substance that has the characteristics of an S-nitrosothiol or perhaps a DNIC. As this released compound can stimulate vasodilation even in the absence of light, it suggests the major-role of light is to stimulate the release of this mediator, rather than to liberate NO per se from the store.
The identity of this compound is still unknown however, we tentatively identify it as an S-nitrosothiol or a dinitrosyl iron complex. Both classes of molecule give ozone-based chemiluminescence signals when injected into tri-iodide/acetic acid, that are inhibitable by mercuric chloride. The mechanism by which long-wavelength light can stimulate the release of such compounds from the endothelium and the nature of the light receptor are so-far unknown. Previous studies measured dilation of porcine coronary vessels to polychromatic light in a force tension wire organ bath [11]. Light produced a maximal dilatory response after 1 min, however the potency of dilation decreased with successive exposure and could be regenerated under dark conditions. These results suggest the presence of a preformed and finite store within the vessel which could produce vasodilation. Porcine coronary artery rings exposed to 10 J/cm2 of 670 nm light energy [12] produced results similarly to Batenberg et al [11]. However, in our study, we found murine vessels incubated at a physiological pressure will dilate in response to light in a time dependent manner, and upon discontinuation of light, vessel diameter decreased or plateaued. These findings are contradictory to the above referenced citations, and the inability of the vessel to return to baseline during this dark period led us to investigate the possibility that light induced dilation must involve release of a stable vasodilator substance.
A functional eNOS enzyme is not required for light to stimulate vasodilatation, however NO dependence is confirmed since direct NO scavenging by c-PTIO could abolish dilation. This suggest that the endothelial stores do not require the presence of eNOS, but may be generated from the action of other NOS isoforms or inorganic nitrogen oxides, such as nitrite and nitrate. Much future work needs to be done regarding the formation, stability and magnitude of the endothelial source of the vasodilatory compound. Exposure of light energy in vitro results in modest increases in the intracellular levels of NO within cultured dermal endothelial cells, despite NOS inhibition, further supporting the actions of light to act on intracellular NO stores rather than stimulating NOS activity. These findings are consistent with our previous data where inhibition of NOS by L-NAME did not attenuate the effect of R/NIR on infarct size or collateral development, but the effect was abolished with direct NO scavenging by c-PTIO [9, 18]. Surprisingly, NOS may have a downstream role, as the gradual increasing vessel diameter seen during the dark period was attenuated with L-NAME. Recognizing that energy increases an endothelium dependent vasodilator, this dark period observation may imply other NOS isoforms could be activated by this substance and indirectly contribute to the observed vasodilation. To further characterize NOS dependent and NOS independent mechanisms for energy induced dilation, we repeated light induced dilations on eNOS knock out mice and observed a similar response to chemical NOS inhibition with L-NAME, but with a notable difference in that the dark period attenuation by L-NAME did not occur. Although our data indicate eNOS is not required for direct light dependent vasodilation, we remain open to the possibility that 670nm light energy could modulate NOS activity, since these enzymes contain a regulatory heme prosthetic group which can bind NO, and our previously published work suggests this bond has the potential to be disrupted by R/NIR. An additional hypothesis is that light energy could reverse the inhibitory S-nitrosation of the NOS enzyme by de-nitrosation, or NO mediated inhibition of NOS activity through direct binding of NO to the enzyme’s prosthetic heme group [19, 20]. Both possible mechanisms for light to enhance NOS activity are plausible when given our current understanding that 670 nm light energy releases NO from iron-nitrosyl moieties in blood and cardiac muscle, and could potentially degrade S-nitrosothiols. However, shorter wavelengths of light are likely much more facile at such photolysis.
The endothelial dependence of light-induced vasodilation differs from previous reports, all performed under static force tension, where the dilatory effect of polychromatic light on porcine arteries was enhanced when the endothelium was denuded and NOS was inhibited. The authors suggested a counter-regulatory mechanism by which loss of the endothelium decreases endothelial activated intermediate and small voltage gated potassium channels which can inhibit S-nitrosothiol formation [11, 21]. With the endothelium removed, S-nitrosothiol formation can occur, and modulate small and intermediate potassium channels in smooth muscle [11]. In our study an intact endothelium was required and is the source for the transferrable vasodilator substance identified in these studies. When adoptive transfer studies were performed using bath solution from blood vessels exposed to 670 nm light energy, light naïve vessels were stimulated to dilate by a vasodilator substance produced by the endothelium that was NO-dependent as c-PTIO inhibited vasodilation. As mentioned above, the mechanism for release of these substances into the bath is unknown, however exocytosis of S-nitrosothiol containing vesicles should be considered as there is evidence to suggest Flavin adenine dinucleotides stimulate release of nitrosyl factors in conscious rats [22].
Red-light mediated vasodilation was still apparent in vessels from an animal model with known endothelial dysfunction. Diabetes mellitus leads to a decrease of NO and impaired dilation through reductions in NOS protein and uncoupling of its NO production to generate superoxide [23]. Coronary vessels from db/db mice exhibit increased oxidative stress through increased production of superoxide within the vascular wall and impaired NO dependent vasodilation [24]. There is clear evidence that supplementation with NO can restore attenuated vasodilator responses observed in the db/db model. Cheang et al. treated db/db mice treated with a NOS stimulator and could restore vasodilation to flow and acetylcholine, which both lead to release of NO [25]. Similarly, Sindler et al. could show improved endothelial dependent vasodilation in db/db mice when administered sodium nitrite to produce NO [17]. Given the well-established chemical relationship between ROS and NO, future experiments will be necessary to characterize the impact of hydrogen peroxide and peroxynitrite on energy dependent vasodilation. Regardless, these experiments give credence to the concept that red-light could be beneficial to vascular function even in a setting of chronic endothelial pathology.
The characteristics of light within the red spectrum (670 nm) are ideal for clinical use where site specific increases in blood flow are required. Melanin does not absorb 670 nm energy, and is therefore this wavelength believed to have a greater depth of penetration into tissues. Also, 670 nm light does not produce heating effects which can lead to cellular damage [1, 26]. It is important to note light energy at shorter wavelengths can stimulate NO dependent vasodilation. Furchgott reported sunlight, and specifically UV range (310–355 nm) could stimulate vasodilation in an endothelial independent manner [27]. Sodium nitrite potentiated vasodilation through direct photolysis of nitrite to NO [28]. In addition, blue light (420–450 nm) can release NO from the skin [29]. However, the practical use of light in these wavelengths is limited due to the minimal depth of penetration (0.1–0.2 mm) and the deleterious health effects of UV exposure to the skin [30, 31].
In conclusion, 670nm light energy produces acute increases in vessel diameter at physiological pressures through the release of a vasodilator from the endothelium which we tentatively identify as a DNIC or an S-nitrosothiol. In vitro experiments identify and support the direct actions of light energy on cultured endothelial cells to produce NO independently from NOS enzyme activity. We anticipate under pathological conditions of NO depletion, light energy should be considered a viable means for increasing NO, as evidenced by the significant stimulation of dilation in diabetic vessels. Thus, the acute increases in dilation observed by light energy suggest it can provide an effective noninvasive source for NO delivery.
Supplementary Material
Supplemental Figure 1: Facial arteries from C57Bl/6 mice were isolated and pressurized to 60 mmHg before pre-constriction with U46619. Energy exposure (R/NIR) produced a time dependent increase in vessel diameter. U46619 did not significantly constrict the vessels after the energy exposure. Once the vessel bath was removed and replaced, U46619 constricted the vessels again.
Highlights.
670 nm light dilates blood vessels in an endothelial dependent mechanism.
Vasodilation requires nitric oxide but is independent of nitric oxide synthase.
Light acts on the endothelium to release a vasodilator substance.
S-nitrosothiol or dinitrosyl iron complexes are candidates for this vasodilator.
Light is an effective vasodilator in endothelial dysfunction, e.g. diabetes mellitus.
Acknowledgments
This study was funded by the Veteran Health Administration, 1IK2BX002426 (NL) and UL1TR001436 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources and the National Center for Advancing Translational Sciences (Award 5520327 to NL).
List of Abbreviations
- cGMP
cyclic Guanine Monophosphate
- CPTIO
2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium
- DAF-2
DA 4,5-diaminofluorescein diacetate
- DB/DB
Diabetic mouse strain with deletion of leptin receptor
- DNIC
Dinitrosyl iron complexes
- GSNO
S-nitrosoglutathione
- HUVEC
Human umbilical vein endothelial cells
- HMVEC-d
Human dermal microvascular endothelial cell
- HPLC
High-performance liquid chromatography
- L-NAME
L-NG-Nitroarginine Methyl Ester
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- PAD
Peripheral artery disease
- PBS
Phosphate-Buffered Saline
- ROS
Reactive oxygen species
- RSNO
S-nitrosothiol
- SD
Standard deviation
- λ670
Far Red Light
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Desmet KD, Paz DA, Corry JJ, Eells JT, Wong-Riley MT, Henry MM, Buchmann EV, Connelly MP, Dovi JV, Liang HL, Henshel DS, Yeager RL, Millsap DS, Lim J, Gould LJ, Das R, Jett M, Hodgson BD, Margolis D, Whelan HT. Clinical and experimental applications of NIR-LED photobiomodulation. Photomed Laser Surg. 2006;24:121–128. doi: 10.1089/pho.2006.24.121. [DOI] [PubMed] [Google Scholar]
- 2.Eells JT, Wong-Riley MT, VerHoeve J, Henry M, Buchman EV, Kane MP, Gould LJ, Das R, Jett M, Hodgson BD, Margolis D, Whelan HT. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion. 2004;4:559–567. doi: 10.1016/j.mito.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 3.Huang YY, Chen AC, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose Response. 2009;7:358–383. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karu TI, Pyatibrat LV, Afanasyeva NI. Cellular effects of low power laser therapy can be mediated by nitric oxide. Lasers Surg Med. 2005;36:307–314. doi: 10.1002/lsm.20148. [DOI] [PubMed] [Google Scholar]
- 5.Bibikova A, Belkin V, Oron U. Enhancement of angiogenesis in regenerating gastrocnemius muscle of the toad (bufo viridis) by low-energy laser irradiation. Anat Embryol. 1994;190:597–602. doi: 10.1007/BF00190110. [DOI] [PubMed] [Google Scholar]
- 6.Karu TI, Pyatibrat LV, Kolyakov SF, Afanasyeva NI. Absorption measurements of a cell monolayer relevant to phototherapy: Reduction of cytochrome c oxidase under near IR radiation. J Photochem Photobiol B. 2005;81:98–106. doi: 10.1016/j.jphotobiol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Ball KA, Castello PR, Poyton RO. Low intensity light stimulates nitrite-dependent nitric oxide synthesis but not oxygen consumption by cytochrome c oxidase: Implications for phototherapy. Journal of Photochemistry and Photobiology B: Biology. 2011;102:182–191. doi: 10.1016/j.jphotobiol.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Wong-Riley MT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, Kane M, Whelan HT. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: Role of cytochrome c oxidase. J Biol Chem. 2005;280:4761–4771. doi: 10.1074/jbc.M409650200. [DOI] [PubMed] [Google Scholar]
- 9.Lohr NL, Keszler A, Pratt P, Bienengraber M, Warltier DC, Hogg N. Enhancement of nitric oxide release from nitrosyl hemoglobin and nitrosyl myoglobin by red/near infrared radiation: Potential role in cardioprotection. J Mol Cell Cardiol. 2009;47:256–263. doi: 10.1016/j.yjmcc.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lohr NL, Ninomiya JT, Warltier DC, Weihrauch D. Far red/near infrared light treatment promotes femoral artery collateralization in the ischemic hindlimb. J Mol Cell Cardiol. 2013;62:36–42. doi: 10.1016/j.yjmcc.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batenburg WW, Kappers MH, Eikmann MJ, Ramzan SN, de Vries R, Danser AH. Light-induced vs. bradykinin-induced relaxation of coronary arteries: Do S-nitrosothiols act as endothelium-derived hyperpolarizing factors? J Hypertens. 2009;27:1631–1640. doi: 10.1097/HJH.0b013e32832bff54. [DOI] [PubMed] [Google Scholar]
- 12.Plass CA, Loew HG, Podesser BK, Prusa AM. Light-induced vasodilation of coronary arteries and its possible clinical implication. Ann Thorac Surg. 2012;93:1181–1186. doi: 10.1016/j.athoracsur.2011.12.062. [DOI] [PubMed] [Google Scholar]
- 13.Jourd’heuil D. Increased nitric oxide-dependent nitrosylation of 4, 5-diaminofluorescein by oxidants: Implications for the measurement of intracellular nitric oxide. Free Radical Biology and Medicine. 2002;33:676–684. doi: 10.1016/s0891-5849(02)00955-3. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Kim WS, Hatcher N, Potgieter K, Moroz LL, Gillette R, Sweedler JV. Interfering with nitric oxide measurements. 4,5-diaminofluorescein reacts with dehydroascorbic acid and ascorbic acid. J Biol Chem. 2002;277:48472–48478. doi: 10.1074/jbc.M209130200. [DOI] [PubMed] [Google Scholar]
- 15.Samouilov A, Zweier JL. Development of chemiluminescence-based methods for specific quantitation of nitrosylated thiols. Anal Biochem. 1998;258:322–330. doi: 10.1006/abio.1998.2609. [DOI] [PubMed] [Google Scholar]
- 16.Broillet M, Randin O, Chatton J. Photoactivation and calcium sensitivity of the fluorescent NO indicator 4, 5-diaminofluorescein (DAF-2): Implications for cellular NO imaging. FEBS Lett. 2001;491:227–232. doi: 10.1016/s0014-5793(01)02206-2. [DOI] [PubMed] [Google Scholar]
- 17.Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell. 2011;10:429–437. doi: 10.1111/j.1474-9726.2011.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lohr NL, Ninomiya JT, Warltier DC, Weihrauch D. Far red/near infrared light treatment promotes femoral artery collateralization in the ischemic hindlimb. J Mol Cell Cardiol. 2013 doi: 10.1016/j.yjmcc.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heiss EH, Dirsch VM. Regulation of eNOS enzyme activity by posttranslational modification. Curr Pharm Des. 2014;20:3503–3513. doi: 10.2174/13816128113196660745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas DD, Miranda KM, Colton CA, Citrin D, Espey MG, Wink DA. Heme proteins and nitric oxide (NO): The neglected, eloquent chemistry in NO redox signaling and regulation. Antioxidants and Redox Signaling. 2003;5:307–317. doi: 10.1089/152308603322110887. [DOI] [PubMed] [Google Scholar]
- 21.Batenburg WW, de Vries R, Saxena PR, Danser AH. L-S-nitrosothiols: Endothelium-derived hyperpolarizing factors in porcine coronary arteries? J Hypertens. 2004;22:1927–1936. doi: 10.1097/00004872-200410000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Hashmi-Hill MP, Sandock K, Bates JN, Robertson TP, Lewis SJ. Flavin adenine dinucleotide may release preformed stores of nitrosyl factors from the vascular endothelium of conscious rats. J Cardiovasc Pharmacol. 2007;50:142–154. doi: 10.1097/FJC.0b013e31805c1646. [DOI] [PubMed] [Google Scholar]
- 23.Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, Channon KM. Mechanisms of increased vascular superoxide production in human diabetes mellitus: Role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 24.Bagi Z, Koller A, Kaley G. Superoxide-NO interaction decreases flow- and agonist-induced dilations of coronary arterioles in type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol. 2003;285:H1404–10. doi: 10.1152/ajpheart.00235.2003. [DOI] [PubMed] [Google Scholar]
- 25.Cheang WS, Wong WT, Tian XY, Yang Q, Lee HK, He GW, Yao X, Huang Y. Endothelial nitric oxide synthase enhancer reduces oxidative stress and restores endothelial function in db/db mice. Cardiovasc Res. 2011;92:267–275. doi: 10.1093/cvr/cvr233. [DOI] [PubMed] [Google Scholar]
- 26.Bozkurt A, Onaral B. Safety assessment of near infrared light emitting diodes for diffuse optical measurements. Biomed Eng Online. 2004;3:9. doi: 10.1186/1475-925X-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.FURCHGOTT RF, EHRREICH SJ, GREENBLATT E. The photoactivated relaxation of smooth muscle of rabbit aorta. J Gen Physiol. 1961;44:499–519. doi: 10.1085/jgp.44.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsunaga K, Furchgott RF. Interactions of light and sodium nitrite in producing relaxation of rabbit aorta. J Pharmacol Exp Ther. 1989;248:687–695. [PubMed] [Google Scholar]
- 29.Opländer C, Deck A, Volkmar CM, Kirsch M, Liebmann J, Born M, Van Abeelen F, Van Faassen EE, Kröncke K, Windolf J. Mechanism and biological relevance of blue-light (420–453nm)-induced nonenzymatic nitric oxide generation from photolabile nitric oxide derivates in human skin in vitro and in vivo. Free Radical Biology and Medicine. 2013;65:1363–1377. doi: 10.1016/j.freeradbiomed.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 30.Meinhardt M, Krebs R, Anders A, Heinrich U, Tronnier H. Wavelength-dependent penetration depths of ultraviolet radiation in human skin. J Biomed Opt. 2008;13:044030–044030-5. doi: 10.1117/1.2957970. [DOI] [PubMed] [Google Scholar]
- 31.Gandini S, Montella M, Ayala F, Benedetto L, Rossi CR, Vecchiato A, Corradin MT, De Giorgi V, Queirolo P, Zannetti G. Sun exposure and melanoma prognostic factors. Oncology Letters. 2016;11:2706–2714. doi: 10.3892/ol.2016.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Facial arteries from C57Bl/6 mice were isolated and pressurized to 60 mmHg before pre-constriction with U46619. Energy exposure (R/NIR) produced a time dependent increase in vessel diameter. U46619 did not significantly constrict the vessels after the energy exposure. Once the vessel bath was removed and replaced, U46619 constricted the vessels again.


