Abstract
Objective
Epidemiological studies strongly suggest that lipid factors independent of LDL cholesterol contribute significantly to cardiovascular disease (CVD) risk. Because circulating lipoproteins comprise only a small fraction of total body cholesterol, the mobilization and excretion of cholesterol from plasma and tissue pools may be an important determinant of CVD risk. Our hypothesis is that fecal excretion of endogenous cholesterol is protective against atherosclerosis.
Approach and Results
Cholesterol metabolism and carotid intima-media thickness (CIMT) were quantitated in 86 non-diabetic adults. Plasma cholesterol was labeled by intravenous infusion of cholesterol-d7 solubilized in a lipid emulsion and dietary cholesterol by cholesterol-d5 and the nonabsorbable stool marker sitostanol-d4. Plasma and stool samples were collected while subjects consumed a cholesterol- and phytosterol-controlled metabolic kitchen diet and were analyzed by mass spectrometry. CIMT was negatively correlated with fecal excretion of endogenous cholesterol (r=−0.426, P<0.0001), total cholesterol (r=−0.472, P=<0.0001), and daily percent excretion of cholesterol from the rapidly-mixing cholesterol pool (r=−0.343, P=0.0012), and was positively correlated with percent cholesterol absorption (r=+0.279, P=0.0092). In a linear regression model controlling for age, sex, systolic blood pressure, hemoglobin A1c, LDL, HDL cholesterol, and statin drug use, fecal excretion of endogenous cholesterol remained significant (P=0.0008).
Conclusions
Excretion of endogenous cholesterol is strongly, independently, and negatively associated with CIMT. The reverse cholesterol transport pathway comprising the intestine and the rapidly-mixing plasma and tissue cholesterol pool could be an unrecognized determinant of CVD risk not reflected in circulating lipoproteins. Further work is needed to relate measures of reverse cholesterol transport to atherosclerotic disease.
Keywords: cholesterol absorption, excretion, controlled diet, stable isotopes, clinical trial, mass spectrometry
Subject Codes: Metabolism, lipids and cholesterol
Introduction
Guidelines for preventing and treating atherosclerotic disease are heavily focused on LDL cholesterol because of strong clinical trial evidence and readily-available plasma measurements.1 Atherosclerosis originates from cholesterol deposition in the artery from β-lipoproteins, including LDL. However, circulating LDL is a small contributor to total body cholesterol, comprising about 3 g in a person with circulating LDL cholesterol concentration of 100 mg/dL compared to about 72 g of total body cholesterol.2–6 Moreover, LDL cholesterol is a static measurement that contains no kinetic information about rates of cholesterol production or excretion.
The reverse transport of cholesterol is a multi-step pathway beginning with cholesterol extraction from peripheral tissues and macrophages, followed by transfer into a rapidly-mixing body cholesterol pool, secretion into the intestine, and excretion in the stool.7 Reverse cholesterol transport (RCT) may mitigate the effect of LDL cholesterol by removing cholesterol deposited in the arterial wall. This work focuses on the distal portion of RCT, emphasizing the rapidly-mixing body cholesterol pool and the intestine. Using multiple stable isotopic tracers to label both dietary and endogenous cholesterol and employing a metabolic kitchen diet to provide a constant baseline for measurement, it is possible to quantitate total fecal cholesterol excretion and to characterize it as originating from the endogenous pool, the diet, or unlabeled hepatobiliary components.8 The final common pathway for RCT is the fecal excretion of endogenous cholesterol (FEEC). That measurement, along with related cholesterol fluxes, forms the basis for this report. We define endogenous cholesterol as body cholesterol derived principally from biosynthesis and, to a lesser extent, from absorbed dietary cholesterol. The endogenous cholesterol was labeled by the intravenous administration of cholesterol-d7 tracer.
Although little evidence exists on the relationship between FEEC and CVD risk, epidemiological studies have examined plasma biomarkers for the related processes of cholesterol absorption and synthesis, but the results have been inconsistent.9, 10 Case-control studies also have been controversial, in part because the proposed biomarkers are not fully understood and have not been validated.11–16 Actual measurements of whole body cholesterol metabolism, rather than its biomarkers, are needed to establish a possible relationship between whole body cholesterol metabolism and CVD.
Increased CIMT has been associated with increased risk of cardiovascular events such as myocardial infarction and stroke.17–20 The Atherosclerosis Risk in Communities study showed a strong relationship between CIMT and cardiovascular events.20 More recently, a study of the Framingham Offspring Study cohort showed that both the maximum internal CIMT and mean common CIMT predict cardiovascular outcomes.21 Thus, CIMT is a well-accepted imaging biomarker of atherosclerosis and CVD risk. In the present observational study, we explored whether FEEC was related to CIMT in adults.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Subjects
Among the one hundred subjects screened, 94 were eligible and entered the study, and 86 completed the protocol. Subjects included 52 females and 34 males with mean age of 53.7 ± 13.0 years. Table 1 shows the subject characteristics upon entry into the study. They comprised a wide range of non-diabetic individuals. Subjects over age 30 were selected to increase the strength of the relation of CVD risk factors to CIMT. Statin drug use was allowed because this is a very common medication and does not appear to influence whole body cholesterol metabolism significantly.22
Table 1.
Subject Characteristics at Baseline
| Mean ± SD | |
|---|---|
| Sex: Women/Men (n) | 52/34 |
| Race: White/Black/Other (n) | 62/16/8 |
| Age (y) | 53.7 ± 13.0 |
| BMI (Kg/m2) | 28.1 ± 4.7 |
| CIMT (mm) | 0.68 ± 0.13 |
| Lipids (mg/dL) | |
| Total cholesterol | 181.6 ± 33.8 |
| LDL cholesterol | 101.2 ± 27.6 |
| HDL cholesterol | 59.6 ± 19.3 |
| Triglycerides | 101.1 ± 49.5 |
| HbA1C (%) | 5.51 ± 0.36 |
| Fasting glucose (mg/dL) | 95.2 ± 11.5 |
| Fasting insulin (µU/mL) | 12.4 ± 11.6 |
| HOMA-IR | 3.08 ± 3.36 |
| Blood pressure (mmHg) | |
| Systolic | 124 ± 17 |
| Diastolic | 74 ± 11 |
| Non-cholesterol sterols | |
| 5α-cholestanol (µg/mL) | 2.52 ± 0.64 |
| Lathosterol (µg/mL) | 1.87 ± 0.92 |
| 5α-cholestanol/Lathosterol | 1.76 ± 1.32 |
| Medications (n): | |
| Statins | 11 |
| Anti-hypertensive | |
| Calcium channel blockers | 6 |
| Angiotensin II receptor antagonists | 5 |
| Angiotensin converting enzyme | 8 |
| Diuretic | 1 |
Abbreviations: BMI, body mass index; HbA1C, hemoglobin A1c; HOMA-IR, homeostasis model of insulin resistance.
CIMT and traditional CVD risk factors
Pearson correlations between CIMT and several traditional CVD risk factors are shown in Table 2. Age, systolic blood pressure, HbA1c and fasting plasma glucose were associated with increased CIMT, as expected. However, there was no significant relation between circulating lipids or lipoproteins and CIMT in this sample.
Table 2.
Pearson Correlation between CIMT and Traditional Risk Factors
| CIMT | ||
|---|---|---|
| Correlation coefficient | P value | |
| Age | 0.513 | < 0.0001 |
| BMI | 0.200 | NS |
| Systolic blood pressure | 0.425 | < 0.0001 |
| Glucose homeostasis | ||
| HbA1c | 0.420 | < 0.0001 |
| Fasting glucose | 0.272 | 0.0114 |
| Fasting insulin | 0.152 | NS |
| HOMA-IR | 0.143 | NS |
| Plasma lipids and lipoproteins | ||
| Plasma total cholesterol | −0.042 | NS |
| Plasma LDL cholesterol | −0.061 | NS |
| Plasma HDL cholesterol | −0.053 | NS |
| Plasma triglycerides | 0.104 | NS |
Abbreviations: BMI, body mass index; HbA1c, hemoglobin A1c; HOMA-IR, homeostasis model of insulin resistance.
Cholesterol metabolism
Cholesterol metabolism results are shown in Table 3. Total daily fecal cholesterol excretion measured was 0.58 g/day. Of this amount, 68% was accounted for by recovery of the plasma tracer cholesterol-d7 and therefore had an endogenous origin, whereas 16% was due to unabsorbed dietary cholesterol measured with cholesterol-d5. An additional 16% of fecal cholesterol was not labeled and may represent newly-synthesized or other non-equilibrating hepatobiliary cholesterol. Percent intestinal cholesterol absorption was 57.4%. The rapidly-mixing cholesterol pool, which includes both circulating lipoproteins and a portion of tissue cholesterol, averaged 25.3 g. Since the calculated plasma pool of cholesterol was only about 5.4 g, the rapidly-mixing cholesterol pool was comprised of 79% tissue cholesterol that equilibrated rapidly with plasma. Percent cholesterol excretion, the percent of the rapidly-mixing plasma cholesterol pool excreted daily into the stool as cholesterol and bacterial cholesterol metabolites, was 1.64%. These measurements emphasize that endogenous tissue cholesterol dominates whole body cholesterol metabolism.
Table 3.
Pearson Correlation between CIMT and Cholesterol Metabolic Parameters
| Mean ± SD | CIMT | ||
|---|---|---|---|
|
| |||
| Correlation coefficient |
P value | ||
| Fecal excretion of total cholesterol (g/day) | 0.58 ± 0.16 | −0.472 | < 0.0001 |
| Endogenous origin (g/day) | 0.39 ± 0.13 | −0.426 | < 0.0001 |
| Dietary origin (g/day) | 0.09 ± 0.02 | −0.252 | 0.0193 |
| Unlabeled (g/day) | 0.09 ± 0.09 | −0.141 | 0.1956 |
| Percent cholesterol excretion (%/day) | 1.64 ± 0.67 | −0.343 | 0.0012 |
| Fecal total fecal bile acid excretion (g/day) | 0.52 ± 0.14 | −0.088 | 0.4220 |
| Cholesterol rapidly-mixing pool size (g) | 25.3 ± 5.6 | −0.075 | 0.4908 |
| Endogenous cholesterol production rate (g/day) | 0.89 ± 0.20 | −0.425 | < 0.0001 |
| Endogenous cholesterol secretion into intestine (g/day) | 0.95 ± 0.35 | −0.274 | 0.011 |
| Percent cholesterol absorption (%) | 57.4 ± 8.4 | 0.279 | 0.0092 |
| Plasma cholesterol-d7 enrichment change (%) | 71.1 ± 5.8 | 0.285 | 0.0083 |
Pearson correlations between factors related to fecal cholesterol excretion and carotid intima-media thickness (CIMT) in 86 subjects. Fecal excretion of total cholesterol includes bacterial degradation products and is also known as fecal neutral sterols. Percent cholesterol excretion is the percent of the rapidly-mixing cholesterol pool excreted in the stool per day. Endogenous cholesterol production rate is the sum of fecal cholesterol and bile acid excretion minus unabsorbed dietary cholesterol. Plasma cholesterol-d7 enrichment change is the decline in plasma cholesterol enrichment from day 2 to day 15 expressed as a positive percent of the day 2 value; it is related primarily to the rate of dilution of plasma cholesterol by unlabeled endogenous cholesterol.
CIMT and whole-body cholesterol metabolism
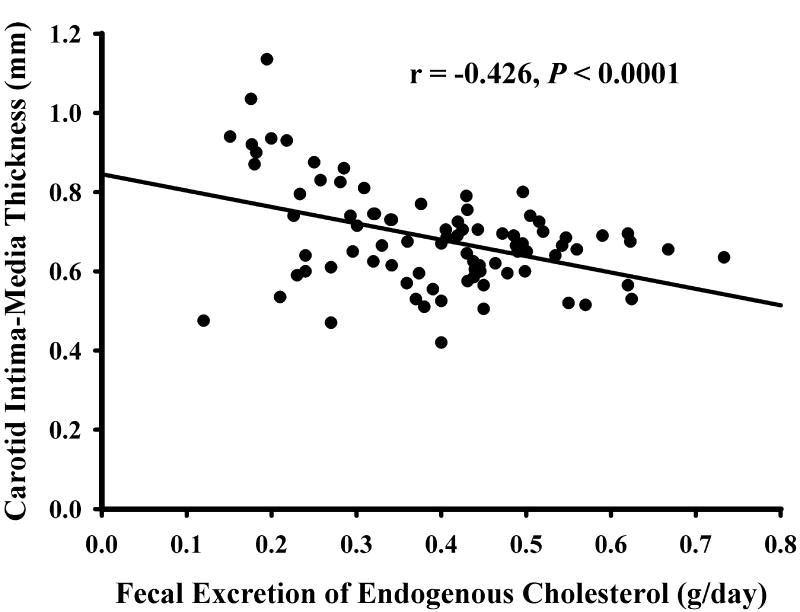
Table 3 shows that CIMT was strongly and negatively correlated with several measures of fecal cholesterol elimination, including total cholesterol excretion (P < 0.0001), endogenous cholesterol excretion (P < 0.0001), unabsorbed dietary cholesterol excretion (P = 0.019) and percent cholesterol excretion (P = 0.0012). Figure 1 shows a scatterplot of the relation between CIMT and FEEC. There was no relation of CIMT to the small amount of unlabeled stool cholesterol, suggesting that unrecognized cholesterol pools do not contribute significantly to CIMT. Percent cholesterol absorption, an important determinant of fecal cholesterol excretion, was positively correlated with CIMT (P = 0.0092). Secretion of endogenous cholesterol into the intestine, another determinant of fecal cholesterol excretion, was negatively correlated with CIMT (P = 0.011). Although turnover of plasma cholesterol was not measured in the study, it was estimated by the decline in plasma cholesterol enrichment over 13 days after infusion of intravenous tracer. This measurement was positively correlated with CIMT (P = 0.0083).
Figure 1. Relation between CIMT and FEEC.
Simple regression of carotid intima-media thickness (CIMT) (mm) with fecal excretion of endogenous cholesterol (FEEC) (g/day) is presented for 86 subjects.
Potential plasma biomarkers for FEEC
Non-cholesterol sterols were measured in plasma in order to assess their potential use as biomarkers for FEEC in larger studies. FEEC was positively correlated with the ratio of plasma lathosterol/cholesterol (r = 0.283, P = 0.0083) and marginally negatively correlated with the ratio of plasma 5α-cholestanol/cholesterol (r = −0.211, P = 0.051). The ratio of 5α-cholestanol/lathosterol, reflecting the balance of cholesterol absorption to cholesterol biosynthesis, was significantly correlated with FEEC (r = −0.373, P = 0.0004).
Regression Model
FEEC explained 18.1% of variation in CIMT (P < 0.0001) in simple linear regression, and 7.6% in a multiple regression model that controlled for age, sex, systolic blood pressure, HbA1c, LDL cholesterol, HDL cholesterol, and statin drug use (model R2 = 0.52, P = 0.0008 for FEEC).
Discussion
This work shows that FEEC, the principal pathway of cholesterol elimination in humans, is negatively correlated with CIMT. The data provide a link between whole body cholesterol metabolism and CIMT, a measure of early atherosclerosis. The association is quite strong and similar to that of traditional risk factors such as age, systolic blood pressure, and hemoglobin A1c in a study group consisting of 86 relatively healthy non-diabetic subjects between the ages of 30 and 80. Moreover, FEEC appears to be a stronger risk factor than HDL and LDL cholesterol, which were not related to CIMT in this small study.
The cholesterol production rate (1.0 g/day) in the present study was similar to previous reports of 1.1 g/day23 and 1.14 g/day.24 The rapidly mixing cholesterol pool of 25.3 g in the current study also was similar to the reported pool sizes of 23.423 and 25.9 g.24 The cholesterol flux from the rapidly mixing cholesterol pool out of the body (production rate over the size of the rapidly mixing pool) (4.0%) in the present study was comparable to previous reported values of 4.7%23 and 4.4%.24 Others have reported a higher production rate (2.1 g/day)25 and a lower rapid pool size (11.7 g),26 probably due to differences in tracer and/or methods used.
The primary objective in this work was to quantitate fecal cholesterol excretion and precisely determine its origins. The analytical methods also allowed us to correlate several secondary endpoints with CIMT. The complete results form a coherent model for how body cholesterol metabolism might influence CIMT and CVD risk. Figure 2 depicts a working model of cholesterol compartments (circles) and cholesterol fluxes (arrows). The rapidly-mixing body cholesterol pool includes all of the cholesterol in liver and blood as well as part of the cholesterol in other tissues. The rapid cholesterol pool is particularly important because it is the pool from which cholesterol is excreted from the body and into which cholesterol is absorbed. The size of the rapidly-mixing cholesterol pool was not directly related to CIMT, but cholesterol fluxes into and out of the pool were significantly correlated with CIMT, either negatively (solid arrows) or positively (dashed arrows). Cholesterol transport processes tending to increase the rapidly-mixing pool, such as inflow from unlabeled endogenous cholesterol and reabsorption of intestinal cholesterol, were positively correlated with CIMT, whereas processes tending to reduce the pool size, such as secretion of endogenous cholesterol into the intestine and elimination of cholesterol in the stool, were negatively correlated. Fecal bile acids were not correlated to CIMT and were not included in the model. The results suggest that cholesterol homeostasis is maintained by competing cholesterol transport processes and that individuals who have greater cholesterol excretion have more favorable CIMT values.
Figure 2. Partial model of body and intestinal endogenous cholesterol metabolism.
Solid arrows depict cholesterol pathways with statistically significant negative correlations to carotid intima-media thickness (CIMT) while dotted lines show pathways with significant positive correlations to CIMT. Dietary cholesterol was controlled at a low level during measurements and is not included in the model. Cholesterol pathways that tend to reduce the rapidly-mixing body cholesterol pool are associated with reduced CIMT. Primary data are found in Table 3. Endogenous cholesterol input into the rapidly-mixing pool is estimated as the percent fall in plasma enrichment between days 2 and 15.
LDL and HDL cholesterol have great utility in assessing CVD risk, but the information obtained is limited to a static measurement, obtained at a single point in time. HDL cholesterol concentration does not provide information about the quantity of cholesterol flowing into and out of the HDL particles. Previous investigators also found that whole body cholesterol turnover studied with an intravenous cholesterol tracer was not related to circulating HDL or LDL cholesterol,27 which may be due to the fact that RCT is the combined actions of various lipoproteins such as HDL and LDL. More attention has been given to functional assays for HDL. For example, cholesterol efflux capacity of HDL, a functional measure of cholesterol efflux from macrophages, has been shown to relate to atherosclerosis better than HDL cholesterol alone.28 What distinguishes many cholesterol metabolic parameters in the current study is that they are kinetic and represent cholesterol flows over time. It is possible that kinetic measurements may supplement lipoprotein concentration measurements to provide a stronger prediction of CVD risk.
Reverse cholesterol transport is an important emerging pathway in which cholesterol efflux from macrophages and peripheral tissues is traced into HDL, through the plasma, and into the intestine, where it is excreted as neutral sterols and bile acids.7, 29 Although attention has been focused on the arterial macrophage as a first step, the current work shows that cholesterol flowing from all tissues into and out of the rapidly-mixing cholesterol pool and intestine may contribute importantly to RCT. In particular, the intestine may have a central role in regulating body cholesterol metabolism and possibly in protecting against the development of atherosclerotic disease.30 The data presented in Figure 1 show a large inter-individual variability in FEEC and support previous work demonstrating similar variability in percent cholesterol absorption.31 This is likely due to inter-individual differences in metabolic regulation rather than analytical error because our previous work shows that repeated FEEC measurements in the same individual have a coefficient of variation of about 10%.8
Because of the difficulty of performing metabolic tracer studies such as this one, very few data exist that address the relation of whole body cholesterol metabolism to CVD. It would be highly desirable to have a plasma biomarker for cholesterol excretion. Since cholesterol absorption from the intestine is not complete (averaging 57% in this study), cholesterol homeostasis must be maintained by increased cholesterol synthesis, increased cholesterol absorption, or a combination of these processes to avoid cholesterol deficiency. We used a composite plasma biomarker, the ratio of 5α-cholestanol/lathosterol, to reflect the balance between cholesterol absorption, represented by 5α-cholestanol, and cholesterol biosynthesis, represented by lathosterol, within each individual. The ratio was negatively correlated with FEEC (r = −0.373, P = 0.0004) and positively correlated with CIMT (r = 0.415, P < 0.0001). Previous investigators have shown that similar plasma absorption/synthesis ratios are associated with CVD.16 It is possible that links to CVD in the absorption/synthesis biomarker literature might be explained, in part, by reduced FEEC as the final common pathway most closely linked to increased CIMT. Conversely, lower intestinal cholesterol absorption would result in cholesterol deficiency and increased compensatory biosynthesis, resulting in a lower absorption/synthesis ratio, higher fecal cholesterol excretion, and lower CVD risk. More work is needed to connect biomarkers with measurements of cholesterol metabolism, transport, CIMT, and/or CVD outcome.
Although CIMT is not a direct measure of clinical outcomes or events, it does reflect current information on the development of early atherosclerosis and there is abundant evidence that it is strongly related to well-validated CVD risk factors. The present study demonstrates that FEEC is negatively related to CIMT independently of established CVD risk factors, pointing to FEEC and whole-body cholesterol metabolism as a novel potential target for modulating CVD risk.
It has been demonstrated that bile acid sequestrants effectively reduce coronary artery disease outcomes, probably by reducing LDL cholesterol and increasing sterol excretion or RCT.32, 33 Failure of CETP inhibitors to reduce cardiovascular events has indicated that it is not sufficient to simply raise HDL cholesterol.34 The functional capacity of HDL in RCT might be more important mechanistically. Increased fecal cholesterol excretion is a prominent mechanism of action of phytosterols in foods and supplements,35 but there have been no interventional studies with CVD outcomes to firmly establish the treatment effectiveness. A recent clinical trial of the drug ezetimibe, an inhibitor of cholesterol absorption, resulted in fewer CVD events even in the face of optimum statin drug treatment.36 Although the result has been attributed to LDL cholesterol lowering, the actual mechanism is unknown and recent work shows that FEEC is increased about 70% by ezetimibe.8 Further work is needed to establish the utility of treatments directed toward whole body cholesterol metabolism.
Supplementary Material
Highlights.
The quantity and efficiency of cholesterol excretion were strongly and negatively correlated with carotid intima-media thickness (CIMT) in non-diabetic adults aged 30–80 years old.
The association of fecal cholesterol excretion with CIMT was independent of age, sex, systolic blood pressure, hemoglobin A1c, LDL cholesterol, and HDL cholesterol.
Acknowledgments
We thank the Alvin J. Siteman, Cancer Center at Washington University School of Medicine for use of the Biologic Therapy Core Facility. We are grateful for the skilled assistance from the Center for Clinical Studies, the Clinical Research Unit nursing staff and the metabolic kitchen staff at Washington University Institute of Clinical and Translational Sciences. We appreciate the dedication of the study participants.
Source of Funding
This work was supported by NIH grants R01-108160 to REO, Washington University Mass Spectrometry Resource RR-00954, Washington University Diabetes Research Center P30DK020579, Washington University Nutrition Obesity Research Center P30 DK056341, and Washington University Institute of Clinical and Translational Sciences UL1 TR000448.
Nonstandard Abbreviations and Acronyms
- RCT
Reverse cholesterol transport
- CIMT
Carotid intima-media thickness
- TICE
Trans-intestinal cholesterol excretion
- FEEC
Fecal excretion of endogenous cholesterol
- HOMA-IR
Homeostasis model of insulin resistance
Footnotes
Clinical Trial Registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT01603758
Disclosure
The authors have nothing to disclose.
References
- 1.Expert Panel on Detection. Executive summary of the third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Goodman DS, Smith FR, Seplowitz AH, Ramakrishnan R, Dell RB. Prediction of the parameters of whole body cholesterol metabolism in humans. Journal of lipid research. 1980;21:699–713. [PubMed] [Google Scholar]
- 3.Samuel P, Lieberman S. Improved estimation of body masses and turnover of cholesterol by computerized input--output analysis. Journal of lipid research. 1973;14:189–196. [PubMed] [Google Scholar]
- 4.Goodman DS, Noble RP, Dell RB. Three-pool model of the long-term turnover of plasma cholesterol in man. Journal of lipid research. 1973;14:178–188. [PubMed] [Google Scholar]
- 5.Ostlund RE., Jr A minimal model for human whole body cholesterol metabolism. Am J Physiol. 1993;265:E513–520. doi: 10.1152/ajpendo.1993.265.3.E513. [DOI] [PubMed] [Google Scholar]
- 6.Ostlund RE, Jr, Matthews DE. [13c]cholesterol as a tracer for studies of cholesterol metabolism in humans. J Lipid Res. 1993;34:1825–1831. [PubMed] [Google Scholar]
- 7.Rosenson RS, Brewer HB, Jr, Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ, Remaley AT, Rothblat GH, Tall AR, Yvan-Charvet L. Cholesterol efflux and atheroprotection: Advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–1919. doi: 10.1161/CIRCULATIONAHA.111.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin X, Racette SB, Ma L, Wallendorf M, Ostlund RE., Jr Ezetimibe increases endogenous cholesterol excretion in humans. Arterioscler Thromb Vasc Biol. 2017;37:990–996. doi: 10.1161/ATVBAHA.117.309119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel MD, Thompson PD. Phytosterols and vascular disease. Atherosclerosis. 2006;186:12–19. doi: 10.1016/j.atherosclerosis.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Weingartner O, Bohm M, Laufs U. Controversial role of plant sterol esters in the management of hypercholesterolaemia. Eur Heart J. 2009;30:404–409. doi: 10.1093/eurheartj/ehn580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fassbender K, Lutjohann D, Dik MG, Bremmer M, Konig J, Walter S, Liu Y, Letiembre M, von Bergmann K, Jonker C. Moderately elevated plant sterol levels are associated with reduced cardiovascular risk--the lasa study. Atherosclerosis. 2008;196:283–288. doi: 10.1016/j.atherosclerosis.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 12.Pinedo S, Vissers MN, von Bergmann K, Elharchaoui K, Lutjohann D, Luben R, Wareham NJ, Kastelein JJ, Khaw KT, Boekholdt SM. Plasma levels of plant sterols and the risk of coronary artery disease: The prospective epic-norfolk population study. Journal of lipid research. 2007;48:139–144. doi: 10.1194/jlr.M600371-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Matthan NR, Pencina M, LaRocque JM, Jacques PF, D'Agostino RB, Schaefer EJ, Lichtenstein AH. Alterations in cholesterol absorption/synthesis markers characterize framingham offspring study participants with chd. Journal of lipid research. 2009;50:1927–1935. doi: 10.1194/jlr.P900039-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silbernagel G, Fauler G, Renner W, Landl EM, Hoffmann MM, Winkelmann BR, Boehm BO, Marz W. The relationships of cholesterol metabolism and plasma plant sterols with the severity of coronary artery disease. Journal of lipid research. 2009;50:334–341. doi: 10.1194/jlr.P800013-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Weingartner O, Weingartner N, Scheller B, Lutjohann D, Graber S, Schafers HJ, Bohm M, Laufs U. Alterations in cholesterol homeostasis are associated with coronary heart disease in patients with aortic stenosis. Coron Artery Dis. 2009;20:376–382. doi: 10.1097/MCA.0b013e32832fa947. [DOI] [PubMed] [Google Scholar]
- 16.Weingartner O, Lutjohann D, Vanmierlo T, Muller S, Gunther L, Herrmann W, Bohm M, Laufs U, Herrmann M. Markers of enhanced cholesterol absorption are a strong predictor for cardiovascular diseases in patients without diabetes mellitus. Chem Phys Lipids. 2011;164:451–456. doi: 10.1016/j.chemphyslip.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: The rotterdam study. Circulation. 1997;96:1432–1437. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- 18.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular health study collaborative research group. The New England journal of medicine. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 19.van der Meer IM, Bots ML, Hofman A, del Sol AI, van der Kuip DA, Witteman JC. Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: The rotterdam study. Circulation. 2004;109:1089–1094. doi: 10.1161/01.CIR.0000120708.59903.1B. [DOI] [PubMed] [Google Scholar]
- 20.Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, Clegg LX. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: The atherosclerosis risk in communities (aric) study, 1987–1993. Am J Epidemiol. 1997;146:483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 21.Polak JF, Pencina MJ, Pencina KM, O'Donnell CJ, Wolf PA, D'Agostino RB., Sr Carotid-wall intima-media thickness and cardiovascular events. The New England journal of medicine. 2011;365:213–221. doi: 10.1056/NEJMoa1012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldberg IJ, Holleran S, Ramakrishnan R, Adams M, Palmer RH, Dell RB, Goodman DS. Lack of effect of lovastatin therapy on the parameters of whole-body cholesterol metabolism. The Journal of clinical investigation. 1990;86:801–808. doi: 10.1172/JCI114777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldberg IJ, Holleran S, Ramakrishnan R, Adams M, Palmer RH, Dell RB, Goodman DS. Lack of effect of lovastatin therapy on the parameters of whole-body cholesterol metabolism. J Clin Invest. 1990;86:801–808. doi: 10.1172/JCI114777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith FR, Dell RB, Noble RP, Goodman DS. Parameters of the three-pool model of the turnover of plasma cholesterol in normal and hyperlipidemic humans. J Clin Invest. 1976;57:137–148. doi: 10.1172/JCI108253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidson MH, Voogt J, Luchoomun J, Decaris J, Killion S, Boban D, Glass A, Mohammad H, Lu Y, Villegas D, Neese R, Hellerstein M, Neff D, Musliner T, Tomassini JE, Turner S. Inhibition of intestinal cholesterol absorption with ezetimibe increases components of reverse cholesterol transport in humans. Atherosclerosis. 2013;230:322–329. doi: 10.1016/j.atherosclerosis.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Turner S, Voogt J, Davidson M, Glass A, Killion S, Decaris J, Mohammed H, Minehira K, Boban D, Murphy E, Luchoomun J, Awada M, Neese R, Hellerstein M. Measurement of reverse cholesterol transport pathways in humans: In vivo rates of free cholesterol efflux, esterification, and excretion. J Am Heart Assoc. 2012;1:e001826. doi: 10.1161/JAHA.112.001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer RH, Nichols AV, Dell RB, Ramakrishnan R, Lindgren FT, Gong EL, Blum CB, Goodman DS. Lack of relationship in humans of the parameters of body cholesterol metabolism with plasma levels of subfractions of hdl or ldl, or with apoe isoform phenotype. Journal of lipid research. 1986;27:637–644. [PubMed] [Google Scholar]
- 28.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009;50(Suppl):S189–194. doi: 10.1194/jlr.R800088-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo Sasso G, Murzilli S, Salvatore L, D'Errico I, Petruzzelli M, Conca P, Jiang ZY, Calabresi L, Parini P, Moschetta A. Intestinal specific lxr activation stimulates reverse cholesterol transport and protects from atherosclerosis. Cell Metab. 2010;12:187–193. doi: 10.1016/j.cmet.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Miettinen TA, Tilvis RS, Kesaniemi YA. Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am J Epidemiol. 1990;131:20–31. doi: 10.1093/oxfordjournals.aje.a115479. [DOI] [PubMed] [Google Scholar]
- 32.Dorr AE, Gundersen K, Schneider JC, Jr, Spencer TW, Martin WB. Colestipol hydrochloride in hypercholesterolemic patients--effect on serum cholesterol and mortality. J Chronic Dis. 1978;31:5–14. doi: 10.1016/0021-9681(78)90076-0. [DOI] [PubMed] [Google Scholar]
- 33.Insull W., Jr Clinical utility of bile acid sequestrants in the treatment of dyslipidemia: A scientific review. South Med J. 2006;99:257–273. doi: 10.1097/01.smj.0000208120.73327.db. [DOI] [PubMed] [Google Scholar]
- 34.Kosmas CE, DeJesus E, Rosario D, Vittorio TJ. Cetp inhibition: Past failures and future hopes. Clin Med Insights Cardiol. 2016;10:37–42. doi: 10.4137/CMC.S32667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ostlund RE., Jr Phytosterols in human nutrition. Ann. Rev. Nutr. 2002;22:533–549. doi: 10.1146/annurev.nutr.22.020702.075220. [DOI] [PubMed] [Google Scholar]
- 36.Cannon CP, Blazing MA, Braunwald E. Ezetimibe plus a statin after acute coronary syndromes. The New England journal of medicine. 2015;373:1476–1477. doi: 10.1056/NEJMc1509363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.