Abstract
Introduction
Cognitive training improves cognitive performance and delays functional impairment, but its effects on dementia are not known. We examined whether three different types of cognitive training lowered the risk of dementia across 10 years of follow-up relative to control and if greater number of training sessions attended was associated with lower dementia risk.
Methods
The Advanced Cognitive Training in Vital Elderly (NCT00298558) study was a randomized controlled trial (N = 2802) among initially healthy older adults, which examined the efficacy of three cognitive training programs (memory, reasoning, or speed of processing) relative to a no-contact control condition. Up to 10 training sessions were delivered over 6 weeks with up to four sessions of booster training delivered at 11 months and a second set of up to four booster sessions at 35 months. Outcome assessments were taken immediately after intervention and at intervals over 10 years. Dementia was defined using a combination of interview- and performance-based methods.
Results
A total of 260 cases of dementia were identified during the follow-up. Speed training resulted in reduced risk of dementia (hazard ratio [HR] 0.71, 95% confidence interval [CI] 0.50–0.998, P = .049) compared to control, but memory and reasoning training did not (HR 0.79, 95% CI 0.57–1.11, P = .177 and HR 0.79, 95% CI 0.56–1.10, P = .163, respectively). Each additional speed training session was associated with a 10% lower hazard for dementia (unadjusted HR, 0.90; 95% CI, 0.85–0.95, P < .001).
Discussion
Initially, healthy older adults randomized to speed of processing cognitive training had a 29% reduction in their risk of dementia after 10 years of follow-up compared to the untreated control group.
Keywords: Cognitive training, Cognitive intervention, Dementia, Nonpharmacological intervention, Useful field of view training
Highlights
-
•
A randomized trial examined the efficacy of three cognitive training programs.
-
•
Speed of processing cognitive training significantly reduced dementia risk.
-
•
Each session of speed training completed was associated with reduced dementia risk.
1. Introduction
Dementia affects 14% of persons aged 71 years and older and 30% of those over the age 90 [1]. A 2010 study estimated that 34.4 million people have dementia worldwide with estimated formal and informal care costs of $422 billion [2]. Interventions that postpone dementia onset by even two years would cut projected dementia prevalence in 2047 by 22% [3].
The Advanced Training in Vital Elderly study (ACTIVE) [4] was a randomized trial on the efficacy of three different types of cognitive training to preserve cognitive and daily function in older adults. Participants were randomized to either strategy-based memory or reasoning training, speed of processing training, or no-contact control conditions [4]. Cognitive training produced longitudinal improvements on the targeted cognitive outcomes, and trained participants self-reported less difficulty completing instrumental activities of daily living (IADL) 10 years later [5], [6], [7]. As dementia by definition involves functional impairments, of interest is whether these interventions reduced dementia risk. Previous analysis of ACTIVE using a combination of self-report and performance-based definitions of dementia found no difference in rate of dementia by training arm at 5 years [8].
Importantly, ACTIVE subanalyses have shown that, as hypothesized [4], exposure to booster training was associated with larger improvements in cognitive performance and wider transfer to daily function, particularly for the reasoning and speed arms [5], [9], [10]. Participants randomized to greater doses of speed training demonstrated improved functional performance at 1, 2, and 5 years [5], [9]. Exposure to booster training was associated with additional improvement in targeted cognitive performance at 10 years for participants receiving reasoning and speed training [5], [6], [7], [9], [10]. Thus, consideration of training dose is necessary.
Given the additional follow-up in ACTIVE and the indications that booster training enhances outcomes, it was of interest to reexamine the relation between training and dementia across 10 years. We hypothesized that exposure to cognitive training would lower the risk of dementia and that the benefit would be greatest for those attending more training sessions (i.e., booster training).
2. Methods
2.1. Study design and participants
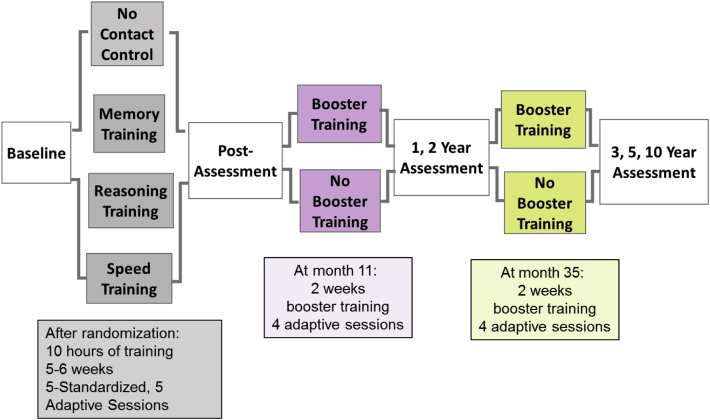
ACTIVE was a multi-site, single-blind, 4-arm, randomized trial (NCT00298558, see Fig. 1). Participants were community-dwelling adults aged 65 years and older. Participants were excluded if they had significant cognitive dysfunction (Mini-mental State Examination [MMSE] < 23), any functional impairment (self-reported difficulty indexed by the Minimum Data Set [MDS] home care), poor vision, self-reported diagnoses of Alzheimer's disease, stroke, certain cancers, or communication difficulties [4]. Written informed consent was obtained. The study was approved by site Institutional Review Boards.
Fig. 1.
The Advanced Cognitive Training in Vital Elderly study design. Participants were randomized to one of four training arms and assessed immediately after training or an equivalent delay. Assessments were completed at 1, 2, 3, 5, and 10 years. A subset of participants completed four additional booster training sessions at 11 months and again at 35 months.
2.2. Procedures
The study protocol is detailed elsewhere [4]. Briefly, eligible participants completed baseline assessments of cognitive (i.e., memory, reasoning, and speed of processing) and functional abilities (i.e., self-report and performance-based measures of functional abilities) and were randomized (Fig. 1). Memory training focused on instruction and practice in strategy use for verbal episodic memory. Reasoning training focused on instruction and practice in strategy use related to problem-solving and serial patterns. Speed training focused on computerized, visual-perceptual exercises designed to increase the amount and complexity of information quickly processed. Each training arm consisted of ten 60–75 minute sessions over 5 to 6 weeks, delivered to small groups of participants. A subset of participants completing at least 80% of the training sessions was randomly selected to receive booster training (four 75-minute sessions) at 11 and 35 months after completion of the initial training. Thus, the total “dose” of each type of training could range from 0–18 sessions. Outcome assessments occurred immediately after training and at 1, 2, 3, 5, and 10 years after training.
2.3. Measures
The measures are detailed elsewhere [4], with brief descriptions of those relevant to analyses provided here. The memory composite outcome included Hopkins Verbal Learning Test, Rey Auditory-Verbal Learning Test, and Rivermead Behavioral Memory Test (immediate recall). The reasoning composite included Letter Series, Letter Sets, and Word Series. The speed composite included the four subtests of the Useful Field of View, reverse-scaled so that higher scores indicated better performance. Participants' vocabulary scores were also considered. Test scores were normalized to the control group to form Z-scores. The average of the component Z-scores formed four domain-specific cognitive composites.
Baseline demographic and health variables were captured by self-report including age; sex; race; education; marital status; smoking; alcohol consumption; depressive symptoms (assessed by the Center for Epidemiological Scales for Depression); and the presence of diabetes, myocardial infarction, angina, congestive heart failure (CHF), stroke, hypertension, and high cholesterol.
2.4. Outcome
Adapting our earlier approach [8] and consistent with research-based diagnostic criteria [11], we defined dementia as the first occurrence of any of the following:
-
1.
Cognitive and functional impairment defined as follows: a) memory composite score at or below −1.5 SD of the baseline sample mean and reasoning composite, speed composite, or vocabulary score at or below −1.5 SD of the baseline mean (for assessment details see [4]), and b) MDS IADL total score at or below the 10th percentile of the baseline (self-reported).
-
2.
A score of <22 on the MMSE, with all subsequent MMSE assessments at <22 or missing [12].
-
3.
Self- or proxy-report of diagnosis of dementia or Alzheimer's disease during the follow-up.
Our earlier approach [8] included two additional criteria, institutionalization and deactivation due to family refusal. We did not include these two criteria in the primary analysis because neither designation is specific to dementia. Dementia is the cause of nursing home placement in only 48% of admissions [13], and families may restrict participant engagement for reasons apart from dementia. For comparability to earlier analyses, we included these two markers in sensitivity analyses (Section 2.5.1).
2.5. Statistical analysis
Statistical analyses were performed using SAS 9.4 software. Descriptive statistics are presented using means and standard deviations for continuous variables and frequencies and proportions for categorical variables. The effect of cognitive training on dementia risk was evaluated using Weibull regression analyses for interval-censored data, as markers of dementia were only known within discrete intervals of time. Accelerated failure time analysis using Weibull regression model was used to estimate training effects while controlling for confounding effects of potential risk factors [14]. We determined whether randomization to cognitive training lowered dementia risk by comparing each of the training groups to the control arm. Second, we examined whether there was a relationship between dementia and number of sessions attended for each training arm. Training sessions ranged from 0 to 18 and were treated as a time-varying covariate in the model. The approach proposed by Sparling et al. [15] was used to handle the time-varying covariate for interval-censored data.
Unadjusted hazard ratios (HRs) of risk factors were first estimated, and those significant at the .05 level were then included in a multivariable model via a backward elimination procedure. Adjusted HRs and their 95% confidence intervals (CIs) were estimated based on the final model to assess the effect of these factors on dementia risk.
2.5.1. Sensitivity analyses
Sensitivity analyses were performed to examine the effect of variations in dementia criteria. Training effects were estimated using different combinations of the criteria (Section 2.4) including #1, #2, and #3; #1 only, #2 only, and #3 only; and #1 and #2, #1 and #3, and #2 and #3. In addition, we examined the dementia criteria previously applied [8], which included institutionalization and deactivation from the study due to family refusal. These results were compared with the primary results to examine whether the effects were dependent on the dementia definition.
To further evaluate the effect of training sessions attended, three sets of sensitivity analyses were performed. The first set examined the effect of dementia criteria on the relation between number of sessions attended and dementia risk as detailed above.
The second set of sensitivity analyses for the effect of training sessions attended examined whether unmeasured participant characteristics associated with invitation to booster training may account for the relation between training sessions and dementia risk as there could be differences between participants who completed fewer/more training sessions. Restricting the analysis to two subgroups of more homogeneous participants, who initially completed at least eight sessions of training and were or were not randomized to booster training, we examined the adjusted effect of training sessions on dementia risk and compared results to the primary analysis. The goal was to determine whether the relation between training and dementia risk was evident in these two subgroups of participants.
The third set of sensitivity analyses examined the effect of different patterns of attrition on the relation between training sessions and dementia risk. Utilizing the ideas of pattern mixture models, we restricted the analysis to three subgroups of participants by dropout patterns: early dropouts (those who dropped out of the study before 5 years), late dropouts (those who dropped out of the study after 5 years), and completers (those who remained in the study at 10 years). The adjusted effect of training sessions on dementia risk was estimated and compared to the primary analysis to determine whether the relation between training sessions and dementia risk was of similar magnitude in these three subgroups of participants.
3. Results
3.1. Demographics
Demographics, health characteristics, and attrition were similar by training arm (ps > .05, see Table 1). At baseline, the overall sample had an average age of 73.6 years (SD 5.9), preserved cognitive status as indicated by MMSE (M 27.3, SD 2.0), and included individuals who were predominately white (73.3%) and female (76.2%). Each training arm had comparable rates of health conditions including diabetes, hypertension, myocardial infarction, stroke, and depressive symptoms. The total number of training sessions attended, including the initial and booster sessions, were not different across treatment arms. Of the 2785 participants in the analytic sample, 1220 completed the 10-year follow-up. Among participants who did not complete the 10-year follow-up, 627 were censored due to death, and the remaining 938 were censored prior to the 10-year follow-up due to attrition (30.6% attrition). The rate of nonparticipation due to death, withdrawal, and loss to follow-up was in expected ranges given the age of the sample at baseline, and, importantly, did not differ by training arm.
Table 1.
Participant characteristics by training arm (count and % unless otherwise noted)
| Variable | Memory (N = 702) | Reasoning (N = 690) | Speed (N = 698) | Control (N = 695) |
|---|---|---|---|---|
| Demographics | ||||
| Age, yrs, M (SD) | 73.5 (6.0) | 73.5 (5.7) | 73.4 (5.8) | 74.0 (6.0) |
| Female | 537 (76.5) | 536 (77.7) | 537 (76.9) | 513 (73.8) |
| White | 523 (74.5) | 497 (72) | 520 (74.5) | 501 (72.1) |
| Education, yrs, M (SD) | 13.6 (2.7) | 13.5 (2.7) | 13.6 (2.7) | 13.4 (2.7) |
| Married | 256 (36.5) | 241 (34.9) | 238 (34.2) | 257 (37.0) |
| Health | ||||
| Smoking | 57 (8.1) | 46 (6.7) | 50 (7.2) | 54 (7.8) |
| Alcohol consumption | ||||
| Nondrinker | 297 (42.4) | 296 (43.1) | 292 (42.0) | 350 (50.7) |
| Light drinker | 343 (49.0) | 344 (50.2) | 362 (52.0) | 312 (45.2) |
| Heavy drinker | 60 (8.6) | 46 (6.7) | 42 (6.0) | 29 (4.2) |
| MMSE, M (SD) | 27.3 (2.1) | 27.3 (1.9) | 27.4 (1.9) | 27.3 (2.0) |
| CES-D, M (SD) | 5.1 (5.3) | 5.5 (5.3) | 5.2 (4.9) | 5.07 (4.9) |
| Chronic conditions | ||||
| Diabetes | 95 (13.5) | 97 (14.1) | 87 (12.5) | 76 (11.0) |
| Myocardial infarction | 79 (11.3) | 78 (11.4) | 76 (11.0) | 74 (10.7) |
| Angina | 108 (15.5) | 115 (16.9) | 93 (13.5) | 102 (14.8) |
| CHF | 30 (4.3) | 44 (6.5) | 27 (3.9) | 37 (5.4) |
| Stroke | 46 (6.6) | 53 (7.8) | 50 (7.2) | 44 (6.4) |
| Hypertension | 372 (53.2) | 365 (53.3) | 350 (50.4) | 336 (48.8) |
| Participation status | ||||
| Participated at 10 years | 300 (42.7) | 316 (45.8) | 319 (45.7) | 285 (41.0) |
| Censored at death | 151 (21.5) | 145 (21.0) | 168 (24.1) | 163 (23.5) |
| Participant withdrew | 145 (20.7) | 135 (19.6) | 121 (17.3) | 148 (21.3) |
| Site's decision to withdraw | 80 (11.4) | 60 (8.7) | 66 (9.5) | 68 (9.8) |
| Loss to follow-up | 17 (2.4) | 22 (3.2) | 9 (1.3) | 13 (2.9) |
| Family refusal | 9 (1.3) | 12 (1.7) | 14 (2) | 15 (2.2) |
Abbreviations: MMSE, Mini-mental State Examination; CES-D, Center for Epidemiological Studies Depression Scale range 0–36; CHF, congestive heart failure.
3.2. Characteristics of participants with dementia
A total of 260 participants developed dementia during the 10-year follow-up (12% met the psychometric criteria for dementia only, 28% met the MMSE criterion for dementia only, 43% met the reported diagnosis of dementia criterion only, 15% met two of the definitions, and 2% met all three of the definitions). Participants who developed dementia during the follow-up were older, male, of nonwhite race, less educated, more likely nondrinkers, with more depressive symptoms, and more likely to have diabetes or CHF (Table 2).
Table 2.
Demographic and clinical characteristics by dementia status (count and % unless otherwise noted)
| Variable | No dementia (N = 2525) | Dementia (N = 260) | Hazard ratio (95%CI) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age, years, M (SD) | 73.4 (5.8) | 75.8 (6.0) | 1.10 (1.08–1.13) | <.001 |
| Female | 1885 (76.8) | 183 (70.4) | 0.65 (0.49–0.85) | .002 |
| White | 1871 (74.1) | 170 (65.4) | 0.59 (0.45–0.76) | <.001 |
| Education, years, M (SD) | 13.6 (2.7) | 13.1 (2.7) | 0.90 (0.86–0.95) | <.001 |
| Married | 898 (35.6) | 94 (36.3) | 0.91 (0.70–1.17) | .444 |
| Health | ||||
| Smoking | 191 (7.6) | 16 (6.2) | 1.14 (0.69–1.9) | .603 |
| Alcohol consumption | ||||
| None | 1104 (43.9) | 131 (50.6) | 1.00 (reference) | |
| Light | 1243 (49.4) | 118 (45.6) | 0.77 (0.60–0.99) | .042 |
| Heavy | 167 (6.6) | 10 (3.9) | 0.54 (0.28–1.04) | .065 |
| MMSE, M (SD) | 27.4 (1.9) | 26.2 (2.1) | 0.71 (0.67–0.76) | <.001 |
| CES-D, M (SD) | 5.1 (5.1) | 6.5 (5.4) | 1.06 (1.04–1.08) | <.001 |
| Memory, M (SD) | 0.1 (0.3) | 0.1 (0.3) | 0.99 (0.66–1.49) | .98 |
| Reasoning, M (SD) | 0.1 (0.3) | 0.1 (0.3) | 1.23 (0.83–1.83) | .31 |
| Speed, M (SD) | 0.1 (0.3) | 0.1 (0.3) | 1.08 (0.73–1.59) | .70 |
| Vocabulary, M (SD) | 0.7 (0.2) | 0.6 (0.2) | 0.18 (0.10–0.31) | <.001 |
| Chronic conditions | ||||
| Diabetes | 313 (12.4) | 42 (16.2) | 1.56 (1.12–2.17) | .009 |
| Myocardial infarction | 280 (11.2) | 27 (10.4) | 1.20 (0.80–1.79) | .374 |
| Angina | 380 (15.2) | 38 (14.8) | 1.10 (0.78–1.55) | .586 |
| CHF | 123 (4.9) | 15 (5.8) | 2.02 (1.20–3.40) | .009 |
| Stroke | 172 (6.9) | 21 (8.1) | 1.30 (0.83–2.03) | .252 |
| Hypertension | 1308 (52.1) | 115 (44.6) | 0.84 (0.65–1.07) | .156 |
Abbreviations: MMSE, Mini-mental State Examination; CES-D, Center for Epidemiological Studies Depression Scale; CHF, congestive heart failure.
3.3. Cognitive training and number of sessions attended
Speed training resulted in lower risk of dementia across 10 years as compared to control (see Table 3). The hazard of dementia was 29% lower for speed training than control (HR, 0.71; 95% CI, 0.50–0.998, P = .049). The risk of dementia for memory and reasoning training was not significantly different compared to control (see Table 3). A greater number of memory sessions was associated with reduced dementia risk (HR, 0.95; 95% CI, 0.90–1.00, P = .038) but was not significant after adjusting for risk factors. The lower risk of dementia for speed training was more prominent for those who completed a greater number of training sessions (Table 3). Each additional speed training session was associated with a 10% lower hazard for dementia (unadjusted HR, 0.90, 95% CI, 0.85–0.95, P < .001). The effect of number of speed training sessions remained significant after controlling for age, sex, race, depressive symptoms, diabetes, and congestive heart failure (adjusted HR, 0.90; 95% CI, 0.85–0.95, P < .001). Among participants who completed five or more booster training sessions, indicators of dementia were evident in 5.9% of participants from the speed arm and 9.7–10.1% among those completing the memory and reasoning booster training arms, respectively (See Supplemental Table 1).
Table 3.
Effect of training and number of training sessions attended on risk of dementia
| Variable | No dementia (N = 2525) | Dementia (N = 260) | Hazard ratio (95% CI) | P value |
|---|---|---|---|---|
| Training group, N (%) | ||||
| Control | 620 (24.6) | 75 (28.8) | 1.00 (reference) | |
| Memory | 639 (25.3) | 63 (24.2) | 0.79 (0.57–1.11) | .177 |
| Reasoning | 627 (24.8) | 63 (24.2) | 0.79 (0.56–1.10) | .163 |
| Speed | 639 (25.3) | 59 (22.7) | 0.71 (0.50–0.998) | .049 |
| Number of training sessions, M (SD)∗ | ||||
| Memory | 11.9 (5.2) | 11.6 (5.7) | 0.95 (0.90–1.00) | .038 |
| Reasoning | 12.0 (5.0) | 12.9 (4.1) | 0.96 (0.91–1.02) | .240 |
| Speed | 12.1 (4.9) | 10.8 (4.8) | 0.90 (0.85–0.95) | <.001 |
Abbreviation: CI, confidence interval.
Hazard ratios for number of training sessions indicate association with dementia per each training session attended.
3.4. Sensitivity analyses for effects of cognitive training
When dementia was defined using all three criteria (Section 2.4) in all combinations and more broadly also using the previously applied [8] criteria (i.e., institutionalization and deactivation due to family refusal), the hazard of dementia was consistently lower for participants in the speed training arm compared to controls. The estimated HR ranged from 0.64 to 0.87, magnitudes consistent with the results from the primary analyses (Supplemental Table 2).
3.5. Sensitivity analyses for effect of training sessions
3.5.1. Variations in dementia definition
For the effect of training sessions, the estimated HRs of dementia were again consistent with the primary analysis when dementia was defined using different combinations of the criteria. The estimated HRs after adjusting for age, sex, race, depressive symptoms, and diabetes ranged from 0.90 to 0.92 (Supplemental Table 3), indicating that a greater number of speed of processing training was associated with lower dementia risk.
3.5.2. Assignment to booster
Among 639 participants in the speed training arm who completed at least 8 initial training sessions (hence eligible for booster training), an additional training session was associated with an 11% lower risk of dementia (adjusted HR, 0.89; 95% CI, 0.82–0.98). Similarly, the adjusted HR was 0.83 (95% CI, 0.74–0.92) for an additional training session among 365 participants in the speed training arm who completed at least 8 initial training sessions and were randomized to booster training. These results are consistent with the primary analyses. That is, when two relatively homogeneous subgroups (8–10 initial sessions attended, 8–10 initial sessions attended and randomized to booster) were selected from the speed training arm, we still see the same trend for decreased risk of dementia with increased training session exposure.
3.5.3. Patterns of attrition
The three dropout patterns (prior to 5-year follow-up, after 5-year follow-up, and completers) had HRs of similar magnitude as found in the primary analysis. The HRs for each additional training session were 0.89 for early dropouts, 0.94 for late dropouts, and 0.89 for completers. Although the statistical significance was not consistent as in the primary analysis (due to limited power from small subsamples), results for dropout patterns yielded effect sizes similar in magnitude indicating lower risk of dementia associated with attending more speed training sessions.
4. Discussion
Initially healthy, well-functioning older adults randomized to speed of processing cognitive training had a 29% reduction in their risk of dementia after 10 years of follow-up compared to an untreated control group. This relationship seemed to be driven in part by number of training sessions attended (greater risk reduction with more training sessions attended). Cognitive training focused on memory or reasoning was not associated with decreased risk of dementia. To our knowledge, this is the first study to show that any intervention (behavioral or pharmacologic) can lower risk of dementia.
This relationship was not detectable in the ACTIVE sample after 5 years of follow-up [8]. At 5 years, there were 189 dementia cases compared to 260 cases at 10 years. The increased number of outcomes improved our power to detect a relationship. We also applied new analysis by examining the role of number of training sessions attended, and found that is an important driver of the effect.
Speed training is distinct from memory and reasoning training as a perceptual/cognitive technique aimed at enhancing basic information processing efficiency with implicit learning mechanisms. In contrast, the memory and reasoning training arms are strategy-based and operate through explicit memory systems. Older adults at higher risk for dementia due to older age, low education, or mild cognitive impairment are actually more likely to benefit from speed training [9], [16], [17]. Meta-analysis of speed training indicates effects are broad [18] including enhanced quality of life [19], [20], lower risk of depression [21], and improved physical function [22]. Importantly, multiple randomized trials indicate that speed training results in improved everyday functioning including both performance-based and self-report indices of IADL [17], [23], [24], [25], [26]. Given that functional decline is a hallmark of dementia [27], it is logical that speed training reduces dementia risk. A recent critique of cognitive training in general is that participants' beliefs and expectations may influence their performance [28]. However, results across randomized trials indicate that speed of processing training produces equivalent training gains as compared to either active control conditions or no-contact controls and that speed training effects cannot be attributed to beliefs or expectations [18], [29], [30].
To place our results in a broader context, the dementia risk reduction of 22.7% for speed training vs. 28.8% for control yields a relative risk of 78.8% across 10 years. The magnitude of this effect is greater than the relative risk reduction antihypertensive medications provide against major cardiovascular events like stroke, coronary heart disease, or heart failure, in which treatment is associated with a 20–40% relative risk reduction over 3 to 5 years [31].
The underlying mechanism for the dementia risk reduction is not yet clear but could relate to positive changes in brain reserve as a result of cognitive training [32]. The brain reserve concept arose, in part, as a way to understand the well-documented protective effect of education on the display of clinical brain diseases in epidemiological studies. Speed training may lower dementia risk by increasing brain reserve capacity through compensatory changes in function (e.g., enhanced capacity or efficiency of the brain) or via direct effects promoting viability of healthy tissue or decreasing the amount or effect of pathologic proteins and processes [8], [33]. Biomarker studies or changes in brain structure and function taken at intervals during training may help identify mechanisms of action underlying the protective effects of speed training.
This study includes strengths such as the experimental design, a large diverse sample, multi-center treatment delivery and outcome assessments, and longitudinal follow-up. Limitations are also noted including the absence of a clinical diagnosis, attrition during follow-up, and the method of booster training assignment. ACTIVE did not have dementia as a primary outcome, so results are from secondary analyses. We acknowledge that the association between number of training sessions and the risk for dementia could be due to reverse causality. As such, we have appropriately moderated the interpretation of the exposure to training results toward association with risk. Our dementia criteria were defined a priori [8]. There are of course limitations to these criteria, for example, self- and proxy-reports of dementia diagnosis are not infallible, MDS-IADL function was self-reported and thus biased, low MMSE is not a sensitive dementia marker, and overlap among the dementia criteria was low. A definitive study of the efficacy of cognitive training on dementia requires a clinical diagnosis as the primary outcome. That said, our approach to approximating a clinical diagnosis of dementia is reasonable and yielded a similar proportion of cases with dementia as prior research [1]. Our criteria were based on standard diagnostic criteria and published quantitative cut points. The psychometric criteria tie directly to the definition of dementia from the National Institute on Aging/Alzheimer's Association-loss of cognitive function associated with impairment in activities of daily living [34]. Furthermore, results confirmed that known risk factors for dementia (e.g., age, education, CHF, and diabetes) were similarly associated with our dementia criteria [35], [36]. Finally, sensitivity analyses systematically examined variations in the dementia definition and found effects of similar magnitude with every variant.
Attrition always presents a challenge when the sample comprises adults over age 65, and the follow-up interval is long. Typically, such studies see attrition rates of 2.5–9% per year [37], [38], [39]. The overall attrition rate in ACTIVE of 5.5% per year over a 10-year-period falls within this range. Importantly, in ACTIVE, there was no differential attrition by training arm, either quantitatively or by reason for participant loss. Finally, our sensitivity analyses comparing effects of early dropout, late dropouts, and completers consistently indicated similar magnitude of speed training effects on dementia risk reduction regardless of timing of dropout. Thus, the results are robust and are likely a valid indication of the influence of speed training on dementia.
A design limitation in ACTIVE was the method of assigning participants to booster training after the initial training was completed. Participants were randomized to booster, but invitation to complete booster was conditioned on initial training adherence. While this helps to assure delivery of the treatment, it also opens the range of interpretation of booster effects. One of the sensitivity analyses we conducted examined if participant factors related to completing 8+ initial sessions and hence being eligible for booster training could explain the dementia risk reduction. The relation of increased training exposure to lower risk of dementia was detected in each group to the same degree; therefore, differential participant characteristics linked to booster assignment is likely not responsible for our pattern of findings.
We have shown that a specific form of cognitive training, speed of processing, reduced the risk of dementia in initially well-functioning older adults followed up to 10 years. This is the first report of an intervention significantly reducing dementia risk. Future research should examine ways to increase the potency of this form of training intrinsically (e.g., increasing dose) and possibly by adding other putative protective interventions (e.g., exercise and diet). Replication of results using clinical diagnosis of dementia as a primary outcome is needed. Further examination to elucidate mechanisms of action is also warranted.
Research in Context.
-
1.
Systematic review: We conducted systematic literature reviews in Pubmed and PsychInfo to identify randomized clinical trials of cognitive training among healthy older adults. The Advanced Cognitive Trial in Vital Elderly is the only randomized clinical trial to examine the effects of cognitive training on dementia risk.
-
2.
Interpretation: Our results indicate that cognitive speed of processing training, a computerized technique aimed at improving useful field of view, significantly reduced dementia risk across 10 years. Multiple clinical trials indicate that speed training improves older adults' everyday function. As functional decline is a hallmark of dementia, it is consistent that speed training reduces dementia risk. We provide new evidence that certain nonpharmacological, cognitive interventions (i.e., speed of processing training) have potential to reduce dementia risk and improve public health.
-
3.
Future directions: Future work should clarify the mechanisms of effective cognitive training and determine the dose required to derive health benefits.
Acknowledgments
ACTIVE was supported by grants from the National Institute of Nursing Research (U01 NR04508, U01 NR04507), the National Institute on Aging (U01 AG14260, U01 AG 14282, U01 AG 14263, U01 AG14289, U01 AG 014276), the Indiana Alzheimer Disease Center (P30AG10133), and the Cognitive and Aerobic Resilience for the Brain Trial (R01 AG045157). This study is registered at Clinicaltrials.gov as NCT00298558.
We thank the ACTIVE study participants and acknowledge the ACTIVE study sites: Hebrew SeniorLife–Richard N. Jones, PhD, John N. Morris, PhD, and Adrienne L. Rosenberg MS; Indiana University School of Medicine–F.W.U., PhD, David Smith, MD, Daniel F. Rexroth PsyD., Fredric D. Wolinsky PhD, and Lyndsi Moser CCRP; Johns Hopkins University–George W. Rebok, PhD, Jason Brandt, PhD, Kay Cresci PhD, RN, Joseph Gallo MD, MPH, and Laura Talbot PhD, EdD, RN, CS; New England Research Institutes (Data Coordinating Center) – Sharon L. Tennstedt, PhD, Kathleen Cannon BS, Michael Doherty MS, Henry Feldman PhD, Patricia Forde BS, Nancy Gee MPH, Eric Hartung EdD, Linda Kasten MS, Ken Kleinman ScD, Herman Mitchell PhD, George Reed PhD, Anne Stoddard ScD, Yan Xu MS, and Elizabeth Wright PhD; Pennsylvania State University–Sherry L. Willis PhD, Pamela Davis MS, Scott Hofer PhD, K. and Warner Schaie PhD; University of Alabama at Birmingham–Karlene Ball, PhD, Cynthia Owsley PhD, Dan Roenker PhD, David Vance PhD, Virginia Wadley PhD, and Martha Graham; University of Florida/Wayne State University–Michael Marsiske, PhD, Jason Allaire, PhD, Manfred K. Diehl, PhD, Ann L. Horgas, RN, PhD, FAAN, and Peter A. Lichtenberg, PhD, ABPP. We also acknowledge the following NIH project officers: Jared Jobe, Daniel Berch, Jeffrey Elias, Sidney Stahl, and Jonathan King of the National Institute on Aging and Taylor Harden, Karin Helmers, Mary Leveck, Nell Armstrong, Kathy Koepke, and Susan Marden of the National Institute of Nursing Research. J.D.E. additionally thanks Michael Merzenich, Ph.D. and Jasmine Alicea.
Declaration of interests. J.D.E. worked between 1996 to 2005 as a consultant conducting related research studies for Visual Awareness, Inc., who owned the intellectual property surrounding the speed of processing training software. Posit Science now markets the newest version of the training program. Over an approximate three-month period in 2008, J.D.E. worked as a limited consultant to Posit Science, Inc. to analyze data and prepare a publication. J.D.E. currently serves on the data safety and monitoring board of NIH grants awarded to employees of Posit Science. J.D.E. worked as a consultant to Wilson, Sonsini, Goodrich & Rosati across an approximate three month period between May-August of 2015. L.T.G. is employed by Moderna Therapeutics. F.W.U. received research support from Posit Science, Inc., in the form of site licenses for cognitive training programs for investigator-initiated research projects (2009–2012). D.O.C., H.X., and L.A.R. have no conflicts of interest relevant to these analyses.
Contributions to the manuscript. J.D.E. was responsible for study design, data collection, literature searches, data analysis and interpretation, and writing of the manuscript. H.X. was responsible for statistical analyses, data analysis and interpretation, figures, and writing of the manuscript. D.O.C. was responsible for literature searches, data interpretation, and writing of the manuscript. L.T.G. was responsible for data analysis and interpretation. L.A.R. was responsible for data collection, literature searches, and writing of the manuscript. F.W.U. helped obtaining funding, study design, data collection, data analysis and interpretation, and writing of the manuscript.
Sponsors role: Representatives of the National Institute on Aging and the National Institute of Nursing Research were directly involved in the design of the ACTIVE study and monitored study conduct.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2017.09.002.
Supplementary data
References
- 1.Plassman B.L., Langa K.M., Fisher G.G., Herringa S.G., Ofstedal M.B., Burke J.R. Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wimo A., Winblad B., Jonsson L. The worldwide societal costs of dementia: Estimates for 2009. Alzheimers Dement. 2010;6:98–103. doi: 10.1016/j.jalz.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Brookmeyer R., Johnson E., Ziegler-Graham K., Arrighi H.M. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:181–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 4.Jobe J.B., Smith D.M., Ball K.K., Tennstedt S.L., Marsiske M., Willis S.L. ACTIVE: a cognitive intervention trial to promote independence in older adults. Control Clin Trials. 2001;22:453–479. doi: 10.1016/s0197-2456(01)00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ball K.K., Berch D.B., Helmers K.F., Jobe J.B., Leveck M.D., Marsiske M. Effects of cognitive training interventions with older adults: A randomized controlled trial. JAMA. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rebok G.W., Ball K., Guey L.T., Jones R.N., Kim H.Y., King J.W. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc. 2014;62:16–24. doi: 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willis S.L., Tennstedt S.L., Marsiske M., Ball K.K., Elias J., Koepke K.M. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296:2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unverzagt F.W., Guey L.T., Jones R.N., Marsiske M., King J.W., Wadley V.G. ACTIVE cognitive training and rates of incident dementia. J Int Neuropsychol Soc. 2012;18:669–677. doi: 10.1017/S1355617711001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ball K.K., Ross L.A., Roth D.L., Edwards J.D. Speed of processing training in the ACTIVE study: How much is needed and who benefits? J Aging Health. 2013;25:65S–84S. doi: 10.1177/0898264312470167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willis S.L., Caskie G.I. Reasoning training in the ACTIVE study: how much is needed and who benefits? J Aging Health. 2013;25:43S–64S. doi: 10.1177/0898264313503987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plassman B.L., Langa K.M., Fisher G.G., Heeringa S.G., Weir D.R., Ofstedal M.B. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148:427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDowell I., Kristjansson B., Hill G.B., Hébert R. Community screening for dementia: The Mini Mental State Exam (MMSE) and modified Mini-Mental State Exam (3MS) compared. J Clin Epidemiol. 1997;50:377–383. doi: 10.1016/s0895-4356(97)00060-7. [DOI] [PubMed] [Google Scholar]
- 13.Van Rensbergen G., Nawrot T. Medical conditions of nursing home admissions. BMC Geriatr. 2010;10:46. doi: 10.1186/1471-2318-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odell P.M., Anderson K.M., D'Agostino R.B. Maximum likelihood estimation for interval-censored data using a Weibull-based accelerated failure time model. Biometrics. 1992;48:951–959. [PubMed] [Google Scholar]
- 15.Sparling Y.H., Younes N., Lachin J.M. Parametric survival models for interval-censored data with time-dependent covariates. Biostatistics. 2006;7:599–614. doi: 10.1093/biostatistics/kxj028. [DOI] [PubMed] [Google Scholar]
- 16.Clark D.O., Xu H., Unverzagt F.W., Hendrie H. Does targeted cognitive training reduce educational disparities in cognitive function among cognitively normal older adults? Int J Geriatr Psychiatry. 2016;31:809–817. doi: 10.1002/gps.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin F., Heffner K.L., Ren P., Tivarus M.E., Brasch J., Chen D.G. Cognitive and neural effects of vision-based speed-of-processing training in older adults with amnestic mild cognitive impairment: A pilot study. J Am Geriatr Soc. 2016;64:1293–1298. doi: 10.1111/jgs.14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards JD, Fausto BA, Tetlow AM, Corona RT, Valdes EG. Systematic review and meta-analyses of useful field of view cognitive training. submitted. [DOI] [PubMed]
- 19.Wolinsky F.D., Vander Weg M.W., Martin R., Unverzagt F.W., Willis S.L., Marsiske M. Does cognitive training improve internal locus of control among older adults? J Gerontol B Psychol Sci Soc Sci. 2010;65:591–598. doi: 10.1093/geronb/gbp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolinsky F.D., Unverzagt F.W., Smith D.M., Jones R., Stoddard A., Tennstetdt S.L. The ACTIVE cognitive training trial and health-related quality of life: Protection that lasts for 5 years. J Gerontol A Biol Sci Med Sci. 2006;61:1324–1329. doi: 10.1093/gerona/61.12.1324. [DOI] [PubMed] [Google Scholar]
- 21.Wolinsky F.D., Mahncke H.W., Vander Weg M.W., Martin R., Unverzagt F.W., Jones R.N. The effect of speed-of-processing training on depressive symptoms in ACTIVE. J Gerontol A Biol Sci Med Sci. 2009;64A:468–472. doi: 10.1093/gerona/gln044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith-Ray R.L., Makowski-Woidan B., Hughes S.L. A randomized trial to measure the impact of a community-based cognitive training intervention on balance and gait in cognitively intact black older adults. Health Educ Behav. 2014;41:62S–69S. doi: 10.1177/1090198114537068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards J.D., Wadley V.G., Myers R., Roenker D.L., Cissell G.M., Ball K.K. Transfer of a speed of processing intervention to near and far cognitive functions. Gerontology. 2002;48:329–340. doi: 10.1159/000065259. [DOI] [PubMed] [Google Scholar]
- 24.Edwards J.D., Wadley V.G., Vance D.E., Roenker D.L., Ball K.K. The impact of speed of processing training on cognitive and everyday performance. Aging Ment Health. 2005;9:262–271. doi: 10.1080/13607860412331336788. [DOI] [PubMed] [Google Scholar]
- 25.Vance D.E., Fazeli P.L., Ross L.A., Wadley V.G., Ball K.K. Speed of processing training with middle-age and older adults with HIV: A pilot study. J Assoc Nurses AIDS Care. 2012;23:500–510. doi: 10.1016/j.jana.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolinsky F.D., Vander Weg M.W., Howren M.B., Jones M.P., Dotson M.M. The effect of cognitive speed of processing training on the development of additional IADL difficulties and the reduction of depressive symptoms: results from the IHAMS randomized controlled trial. J Aging Health. 2015;27:334–354. doi: 10.1177/0898264314550715. [DOI] [PubMed] [Google Scholar]
- 27.Ratner E., Atkinson D. Response to Dr. Amit Lampit et al. J Am Geriatr Soc. 2015;63:2614–2615. doi: 10.1111/jgs.2_13825. [DOI] [PubMed] [Google Scholar]
- 28.Simons D.J., Boot W.R., Charness N., Gathercole S.E., Chabris C.F., Hambrick D.Z. Do “brain training” programs work? Psychol Sci Public Interest. 2016;17:103–186. doi: 10.1177/1529100616661983. [DOI] [PubMed] [Google Scholar]
- 29.Kaur J., Dodson J.E., Steadman L., Vance D.E. Predictors of improvement following speed of processing training in middle-aged and older adults with HIV: A pilot study. J Neurosci Nurs. 2014;46:23–33. doi: 10.1097/JNN.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharpe C., Holup A.A., Hansen K.E., Edwards J.D. Does self-efficacy affect responsiveness to cognitive speed of processing training? J Aging Health. 2014;26:786–806. doi: 10.1177/0898264314531615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blood pressure lowering treatment trialists' collaboration Effects of different blood-pressure-lowering regimens on major cardiovascular events: Results of prospectively-designed overviews of randomized trials. Lancet. 2003;362:1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 32.Barulli D., Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends Cogn Sci. 2013;17:502–509. doi: 10.1016/j.tics.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satz P. Brain reserve capacity on symptom onset after brain injury: a formulation and review of evidence for threshold theory. Neuropsychology. 1993;7:273–295. [Google Scholar]
- 34.McKhann G.M., Knopman D.S., Chertkow H., Hyman B., Jack C.R., Jr., Kawas C. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:236–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norton S., Matthews F.E., Barnes D.E., Yaffe K., Brayne C. Potential for primary prevention of Alzheimer's disease: An analysis of population-based data. Lancet Neurol. 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 36.Xu W., Tan L., Wang H.F., Jiang T., Tan M.S., Tan L. Meta-analysis of modifiable risk factors for Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2015;86:1299–1306. doi: 10.1136/jnnp-2015-310548. [DOI] [PubMed] [Google Scholar]
- 37.Hendrie H.C., Ogunniyi A., Hall K.S., Baiyewu O., Unverzagt F.W., Gureje O. Incidence of dementia and Alzheimer disease in 2 communities: Yoruba residing in Ibadan, Nigeria, and African Americans residing in Indianapolis, Indiana. JAMA. 2001;285:739–747. doi: 10.1001/jama.285.6.739. [DOI] [PubMed] [Google Scholar]
- 38.Evans D.A., Bennett D.A., Wilson R.S., Bienias J.L., Morris M.C., Scherr P.A. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60:185–189. doi: 10.1001/archneur.60.2.185. [DOI] [PubMed] [Google Scholar]
- 39.Tang M.X., Cross P., Andrews H., Jacobs D.M., Small S., Bell K. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.