Abstract
Alcohol metabolism in the liver generates highly toxic acetaldehyde. Breakdown of acetaldehyde by aldehyde dehydrogenase 2 (ALDH2) in the mitochondria consumes NAD+ and generates reactive oxygen/nitrogen species, which represents a fundamental mechanism in the pathogenesis of alcoholic liver disease (ALD). A mitochondria-targeted lipophilic ubiquinone (MitoQ) has been shown to confer greater protection against oxidative damage in the mitochondria compared to untargeted antioxidants. The present study aimed to investigate if MitoQ could preserve mitochondrial ALDH2 activity and speed up acetaldehyde clearance, thereby protects against ALD. Male C57BL/6 J mice were exposed to alcohol for 8 weeks with MitoQ supplementation (5 mg/kg/d) for the last 4 weeks. MitoQ ameliorated alcohol-induced oxidative/nitrosative stress and glutathione deficiency. It also reversed alcohol-reduced hepatic ALDH activity and accelerated acetaldehyde clearance through modulating ALDH2 cysteine S-nitrosylation, tyrosine nitration and 4-hydroxynonenol adducts formation. MitoQ ameliorated nitric oxide (NO) donor-mediated ADLH2 S-nitrosylation and nitration in Hepa-1c1c7 cells under glutathion depletion condition. In addition, alcohol-increased circulating acetaldehyde levels were accompanied by reduced intestinal ALDH activity and impaired intestinal barrier. In accordance, MitoQ reversed alcohol-increased plasma endotoxin levels and hepatic toll-like receptor 4 (TLR4)-NF-κB signaling along with subsequent inhibition of inflammatory cell infiltration. MitoQ also reversed alcohol-induced hepatic lipid accumulation through enhancing fatty acid β-oxidation. Alcohol-induced ER stress and apoptotic cell death signaling were reversed by MitoQ. This study demonstrated that speeding up acetaldehyde clearance by preserving ALDH2 activity critically mediates the beneficial effect of MitoQ on alcohol-induced pathogenesis at the gut-liver axis.
Keywords: Aldehyde dehydrogenase 2, Posttranslational modification, Alcoholic liver disease, MitoQ
Highlights
-
•
PTMs of ALDH2 participated in the pathogenesis of alcoholic liver disease.
-
•
MitoQ treatment accelerated acetaldehyde detoxification.
-
•
MitoQ ameliorated acetaldehyde-related tight junction disruption.
-
•
MitoQ reversed TLR4-mediated inflammatory response in alcoholic liver disease.
-
•
MitoQ counteracts alcohol-induced ER stress and cell apoptosis.
1. Introduction
Long-term heavy alcohol drinking causes alcoholic liver disease (ALD), a leading cause of liver- related morbidity and mortality [1], [2]. The clinical spectrum of ALD includes steatosis, steatohepatitis, cirrhosis, and hepatocellular carcinoma. Although much effort on exploring the pathogenesis of ALD has been made, FDA-approved therapies are still not available for any stages of ALD. Increasing evidence from both clinic and animal studies suggest that mitochondria damage/dysfunction is one of the hallmarks of ALD [3], [4], [5]. Alcohol consumption causes mitochondrial structural abnormalities, such as giant mitochondria and loss of cristae. Alcohol-induced hepatic mitochondrial dysfunction has been demonstrated by reduced ATP levels, mitochondrial fatty acid β-oxidation proteins, and mitochondrial DNA [6], [7], [8]. Our recent study demonstrated that chronic alcohol exposure decreased the protein levels of respiratory chain complexes and caused overproduction of reactive oxygen species (ROS) and lipid peroxidation products [9]. Consequently, alcohol activated mitochondrial cell death as indicated by increased mitochondrial Bcl-2-associated X protein levels and cytochrome c release from mitochondria to the cytosol as well as increased caspase-3 activation and DNA fragmentation. These previous findings suggest that mitochondria are central players in the pathogenesis of ALD.
The liver is the primary organ for alcohol metabolism. Alcohol is first metabolized to acetaldehyde by different pathways, including alcohol dehydrogenase (ADH), cytochrome P450 2E1 (CYP2E1) and catalase. However, previous reports have shown that chronic alcohol consumption only induces CYP2E1 in the liver [10]. Alcohol breakdown by CYP2E1 is known to generate ROS. Both acetaldehyde and ROS are highly reactive and can damage proteins and DNAs. Aldehyde dehydrogenase 2 (ALDH2) is the major enzyme catalyzed the conversion of acetaldehyde into non-toxic acetate, which is localized in the mitochondrial matrix [11]. In addition, ALDH2 also detoxifies lipid aldehydes such as 4-horoxynonenal (4-HNE) [12]. Inactivation of ALDH2 by ROS or reactive nitrogen species (RNS) through post-translation modifications (PTMs) under oxidative conditions has been reported [13], [14], [15]. Hepatic ALDH2 has been shown to be S-nitrosylated in alcohol-exposed liver of rats, which led to reduction of ALDH activity [16]. Inactivation of ALDH2 by tyrosine nitration has been reported as one of the critical causes in acetaminophen-induced liver injury [17]. Furthermore, 4-HNE has been shown to inhibit ALDH2 activity by forming adducts [15]. These findings highlight an essential role of oxidative/nitrosative stress in regulating ALDH2 functions. Our previous study showed that activating ALDH2 by Alda-1 reversed chronic alcohol-induced steatosis and apoptosis, suggesting that mitochondrial ALDH2 is a molecular target for treating ALD [10].
Mitochondria-targeted ubiquinone (MitoQ) comprises coenzyme Q10 and lipophilic triphenylphosphonium (TPP), which make it accumulate easily within the mitochondria to prevent mitochondrial oxidative damage [18], [19]. MitoQ has been demonstrated to be protective against various oxidative stress-related diseases, including liver injury in hepatitis C patients [20], LPS-induced sepsis [21], renal ischemia-reperfusion injury [22], DSS-induced colitis [23], high fat diet induced metabolic syndrome [24], [25], tubular injury in diabetic kidney disease [26]. Chacko et al. have demonstrated that MitoQ alleviated alcoholic steatosis and ROS/RNS production associated downstream effects such as inhibiting protein nitration and protein aldehyde formation [27]. However, the mechanisms of MitoQ-mediated protection have not been fully defined. In this study, we determined if MitoQ may speed up acetaldehyde detoxification through regulating ALDH2 post-translation modifications, and thereby ameliorate alcohol-induced pathogenesis at the gut-liver axis in a mouse model of ALD.
2. Research design and methods
2.1. Animal experimental design
Male C57BL/6 J mice were obtained from the Jackson Laboratory (Bar Harbor, ME). All mice were treated according to the experimental procedures approved by Institutional Animal Care and Use Committee of North Carolina Research Campus. Ten-week-old mice were pair-fed a modified Lieber-DeCarli alcohol (alcohol-fed, AF) or isocaloric maltose dextrin control (pair-fed, PF) liquid diet for 8 weeks (n=8) with or without 5 mg/kg/d MitoQ supplementation (PF/MitoQ and AF/MitoQ, n=8) for the last 4 weeks. The ethanol content (%, w/v) in the diet was 3.6 for the first 2 weeks and increased by 0.3% every 2 weeks, reaching to 4.5% for the last 2 weeks. Food intakes of all the 4 groups were measured daily. The amount of food given to the pair-fed mice was the same as what the alcohol-fed mice consumed in the previous day. Four hours before tissue collection, the mice were gavaged with ethanol (3 g/kg) or isocaloric dextrin (5.2 g/kg). Mice were anesthetized with inhalational isoflurane, and tissues were collected.
2.2. Histopathology and Oil red O staining
Histopathology and Oil red O staining were performed as previously described [28]. Briefly, liver tissues were fixed in 10% formalin, and processed for paraffin embedding. Paraffin sections were cut in 5 µm and processed with hematoxylin and eosin (H&E) staining. For analysis of fat accumulation, liver samples were frozen in Tissue-Tek OCT (Optimum Cutting Temperature) Compound (VWR, Batavia, IL), and stained with Oil red O solution for neutral lipids.
2.3. Immunohistochemistry
Hepatic 4-hydroxynonenal (4-HNE) and 3-nitrotyrosine (3-NT) levels were detected by immunohistochemical staining. Liver tissue paraffin sections were incubated with 3% hydrogen peroxide for 10 min to inactivate endogenous peroxidases. The endogenous mouse IgG was blocked by incubation with a mouse-to-mouse blocking reagent (ScyTek Laboratories, Logan, UT). Tissue sections were then incubated with a monoclonal mouse anti-4-HNE antibody (Northwest Life Science Specialties, Vancouver, WA) or 3-NT antibody (Abcam, Cambridge, MA) at 4 °C overnight, followed by incubation with EnVision+ Labelled Polymer-HRP- conjugated anti-mouse IgG (DAKO, Carpinteria, CA) at room temperature for 30 min. Diaminobenzidine (DAB) was used as HRP substrate for visualization.
2.4. Western blot and Immunoprecipitation (IP)
Whole protein lysates of livers were extracted using 10% Nonidet P-40 lysis buffer supplemented with protease inhibitor and phosphatase inhibitor (Sigma-Aldrich). Aliquots containing 50 µg of proteins were loaded onto 8–12% SDS-PAGE, trans-blotted onto PVDF membrane, blocked with 5% nonfat milk in Tris-buffered saline solution with 0.1% Tween-20 for 1 h at room temperature, and incubated with NOX4, 3-NT, S-NO-cys, OXPHOS, MTCO1, ALDH2, CYP2E1 (Abcam, Cambridge, MA), P-AMPKα, IRE1α, PERK, P- PERK, ATF4, cleaved caspase-3, PARP, BCL2, NF-κB (Cell Signaling Technology, Denver, MA), SOD2 (Millipore, Billerica, MA), 4-HNE (Northwest Life Science Specialties, Vancouver, WA), ADH, ATF6, XBP-1, CHOP, LC3B (Novus Biologicals, Littleton, CO), CPT-1, ACOX-1 (Proteintech, Rosemont, IL), β-actin, HNF-4α (Santa Cruz Biotechnologies, Santa Cruz, CA), P-PPARα, PPARα and TLR4 (Thermo Scientific, Waltham, MA) respectively. Membranes were washed and incubated with horseradish peroxidase–conjugated secondary antibodies (Thermo Scientific, Rockford, IL, USA). Bound complexes were detected via enhanced chemiluminescence (GE Healthcare, Piscataway, NJ, USA). Bands were quantified, and the ratio to β-actin was calculated and given as fold changes, setting the values of pair-fed or control at 1.
For immunoprecipitation studies, the IgG faction of anti-ALDH2 antibody was covalently immobilized onto immobilized protein G agarose beads (Abcam, Cambridge, MA) following the manufacturer's instruction. Immunoprecipitation of ALDH2 proteins was performed following the procedure described previously [29]. The immune purified ALDH2 proteins were subjected to western blot analysis with each of the antibodies.
2.5. qPCR analysis
Total RNA was isolated from the liver or small intestine and reverse-transcribed with TaqMan Reverse Transcription Reagents (Applied Biosystems, Foster City, CA). The gene expression of related mRNA was measured in triplicate by the comparative cycle threshold method using a 7500 real-time PCR system (Applied Biosystems, Foster City, CA). The primer sequences (Integrated DNA Technologies, Skokie, IL) are shown in Supplemental Table 1. The data were normalized to 18 S rRNA mRNA levels and presented as fold changes, setting the value of the PF mice as 1.
2.6. Immunofluorescence
To detect macrophages and tight junction proteins, cryostat sections of liver and ileum were incubated with rabbit anti-CD11b antibody (BD Biosciences, San Jose, CA, and rat anti-zona occludens-1 (ZO-1) antibody (Millipore, Billerica, MA) overnight at 4 °C, respectively, followed by incubation with alexa fluor 594-conjugated anti-rat IgG or anti-rabbit IgG (Jackson ImmunoResearch Laboratories, USA) for 30 min at room temperature.
2.7. Measurement of ethanol and acetaldehyde
Plasma and hepatic ethanol and acetaldehyde levels were determined by Headspace Gas Chromatography Mass Spectrometry (GC-MS) analytical techniques using an Agilent 7890B GC system (Agilent Technologies, Santa Clara, CA) equipped with a Gerstel autosampler as described in our previous report [28].
2.8. Cell culture
Hepa-1c1c7 cells obtained from American Type Culture Collection (Rockville, MD, USA) were cultured in Alpha minimum essential medium without nucleosides (AMEM; Life technologies, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA), 100 U/mL penicillin and 100 μg/mL streptomycin (Invitrogen), at 37 °C in a humidified atmosphere of 5% CO2. Hepatocytes were pretreated with BSO (500 μmol/L) for 16 h. BSO was then replaced with either normal medium or S-Nitroso-N-Acetyl-DL-Penicillamine (SNAP) (1 mmol/L) for 8 h in the absence or presence of MitoQ (0.5 μmol/L). After treatments, cells were washed and collected for further study. Cell variability was measured with Annexin Red Assay commercial Kit (Millipore, Billerica, MA), and the resultant fluorescence was detected with flow cytometer (Millipore, Billerica, MA).
2.9. Statistics
Data are expressed as mean ± standard deviation (SD). Results were analyzed using the one sample t-test or one-way analysis of variance (ANOVA) followed by Least Significant Difference. In all tests, p values less than 0.05 were considered statistically significant.
3. Results
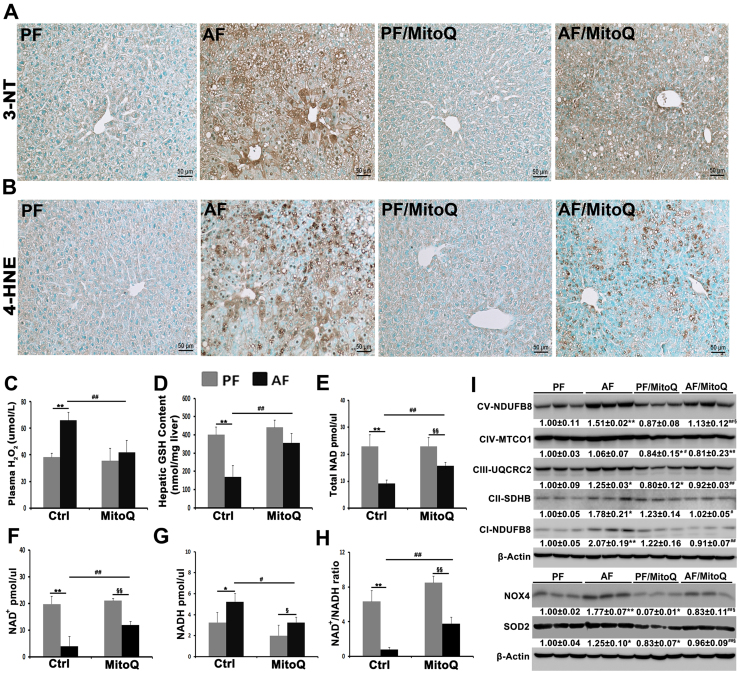
3.1. MitoQ suppressed alcohol-induced oxidative and nitrosative stress
Hepatic 3-nitrotyrosine levels (Fig. 1A) and 4-HNE adduct formation (Fig. 1B) were increased in the liver of alcohol-fed mice, and MitoQ treatment reduced not only the positive cell numbers but also the staining intensity. Chronic alcohol exposure significantly increased plasma H2O2 levels, and MitoQ reduced it to normal levels (Fig. 1C). Hepatic GSH concentrations were reduced by more than 50% in the alcohol-fed mice compared to pair-fed mice, which was normalized by MitoQ (Fig. 1D). MitoQ treatment also ameliorated alcohol-reduced GSH/GSSG ratio in the liver (Supplementary Fig. 1). Furthermore, chronic alcohol feeding dramatically reduced hepatic levels of total NAD (Fig. 1E), NAD+ (Fig. 1G) and NAD+/NADH ratio (Fig. 1H); all these were attenuated by MitoQ.
Fig. 1.
Effects of MitoQ on hepatic nitrosative and oxidative stress in alcohol-fed mice. (A) Immunohistiochemistry of hepatic 3-NT. (B) Immunohistiochemistry of hepatic 4-HNE. (C) Plasma hydrogen peroxide concentrations. (D) Hepatic GSH concentrations. (E) Hepatic total NAD concentrations. (F) Hepatic NAD+ concentrations. (G) Hepatic NADH concentrations. (H) Hepatic NAD+/NADH ratio. (I) Western blot of mtETC complex subunits, NOX4 and SOD2 in the liver. Scale bar: 50 µm. * P < 0.05 vs PF, ** P < 0.01 vs PF, # P<0.05 vs. AF, ## P<0.01 vs. AF, § P<0.05 vs. PF/MitoQ, §§ P<0.01 vs. PF/MitoQ.
To determine whether alcohol-induced oxidative and nitrosative stress are associated with mitochondrial dysfunction, the protein levels of hepatic mitochondrial electron transport chain (mtETC) complexes were examined. Chronic alcohol feeding increased the protein levels of complexes I (CI-NDUFB8), II (CII-SDHB), III (CIII-UQCRC2) and V (CV- NDUFB8), but not IV (MTCO1) (Fig. 1I). MitoQ treatment reversed alcohol-increased mtETC complexes to pair-fed levels. Induction of mitochondrial NOX4 has been shown to contribute alcohol-induced hepatic ROS generation. Chronic alcohol feeding significantly increased hepatic NOX4 protein levels, which was reversed by MitoQ treatment (Fig. 1I). Mitochondrial SOD2 is known to be responsible to cellular superoxide increase. Alcohol increased hepatic SOD2 protein levels, which was reversed by MitoQ (Fig. 1I).
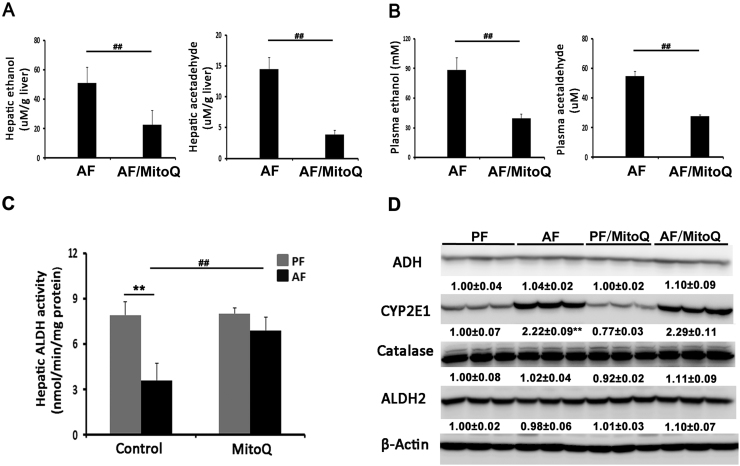
3.2. MitoQ treatment accelerated acetaldehyde clearance and restored alcohol-reduced aldehyde dehydrogenase activity
To determine the effects of MitoQ on ethanol clearance, the levels of ethanol and acetaldehyde in the plasma and liver were measured. Chronic alcohol feeding increased ethanol and acetaldehyde levels in both the liver (Fig. 2A) and plasma (Fig. 2B), and MitoQ treatment significantly reduced ethanol and acetaldehyde levels in both the liver and plasma compared to the AF mice. Chronic alcohol exposure dramatically decreased hepatic ALDH activity by more than 50%, and MitoQ treatment restored it to PF levels (Fig. 2C). Hepatic CYP2E1 protein levels were increased by alcohol feeding regardless of MitoQ treatment (Fig. 2D). The protein levels of ADH, catalase and ALDH2 were not affected by either alcohol or MitoQ.
Fig. 2.
MitoQ administration alleviated alcohol-impaired ALDH activity along with accelerated acetaldehyde clearance. (A) Hepatic ethanol and acetaldehyde concentrations. (B) Plasma ethanol and acetaldehyde concentrations. (C) Hepatic ALDH activity. (D) Expression of hepatic ethanol and aldehyde metabolizing enzymes. ** P < 0.01 vs PF, ## P<0.01 vs. AF.
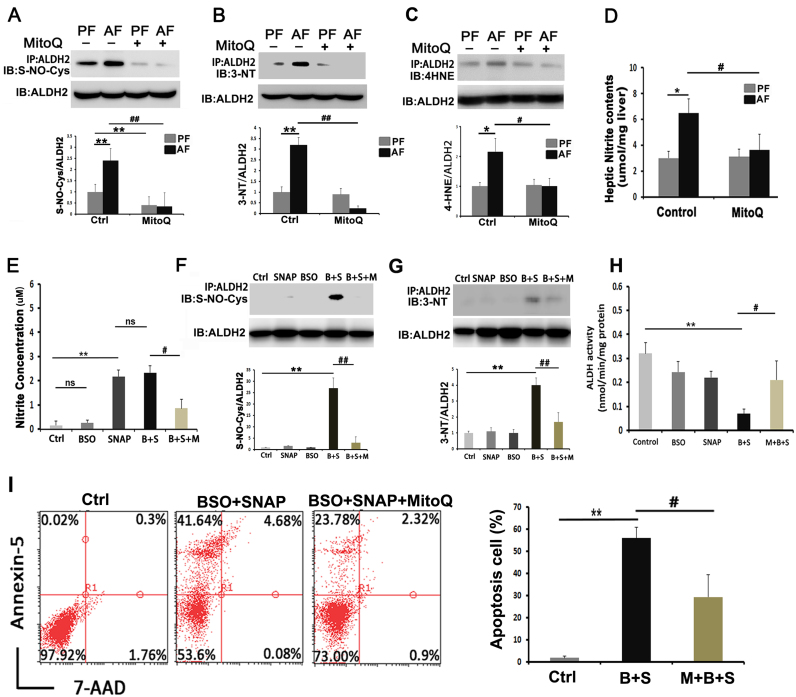
3.3. MitoQ reversed alcohol-induced ALDH2 S-nitrosylation, nitration and 4-HNE adduct formation
To understand the mechanism of how alcohol suppresses, and MitoQ restores, ALDH activity without affecting ALDH2 protein levels, posttranslational modification of ALHD2 was assessed. Immunoblot analysis with the anti-S-NO-Cys antibody or anti-3-NT antibody showed increased S-nitrosylated cysteine band (Fig. 3A) and nitrated tyrosine bands (Fig. 3B) in immunoprecipitated ALDH2 of the AF mice compared to the PF mice. The S-nitrosylated and nitrated ALDH2 bands were significantly diminished by MitoQ treatment. MitoQ also ameliorated alcohol-induced ALDH2-4HNE adduct formation (Fig. 3C). Because nitration and S-ntrosylation are the major forms of ALDH2 posttranslational modification, hepatic nitrite levels were measured. Chronic alcohol exposure elevated hepatic nitrite levels by 2-fold, which was reversed by MitoQ treatment (Fig. 3D).
Fig. 3.
MitoQ enhanced ALDH activity in alcohol-fed mice through modulating ALDH2 post- translation modifications. Hepatic ALDH2 proteins from 4 groups were purified and subjected to immunoblot analysis with the specific anti-ALDH2, anti-S-nitrosocysteine antibody, anti-3-NT antibody or 4- HNE antibody respectively (A-C). (D) Hepatic nitrite contents. * P < 0.05 vs PF, ** P < 0.01 vs PF, # P<0.05 vs. AF, ## P<0.01 vs. AF. For in vitro studies, hepatocytes were pretreated with BSO (500 μmol) for 16 h. BSO was then replaced with either medium or SNAP (1 mM) for 8 h in the absence or presence of 0.5 μM MitoQ. (E) Nitrite concentrations. (F-G) Immunoblot analysis for immunopurified ALDH2 proteins from Hepa1c1c7 cells with anti-ALDH2, anti-S- nitrosocysteine antibody or anti-3-nitrotyrosine antibody. (H) Hepatocyte ALDH activity. (I) Flow cytometry to measure cell death. (J) Apoptosis rate in Hepa1c1c7 cells. ** P < 0.01 vs control, # P<0.05 vs. BSO plus SNAP, ## P<0.01 vs. BSO plus SNAP.
To determine the link between nitrosative stress, cystein S-nitrosylation and nitration of ALDH2, and ALDH activity, a NO donor (SNAP) in GSH depletion condition was introduced to Hepa-1c1c7 cells to mimic nitrosative stress condition. GSH-depletion was achieved by treatment with buthionine sulfoximine (BSO) which blocks GSH biosynthesis. As shown in Fig. 3E, SNAP or SNAP plus BSO, but not BSO alone, increased the nitrite concentrations, and MitoQ significantly inhibited SNAP-increased nitrite levels. ALDH2 proteins were purified from the treated Hepa-1c1c7 cells for analysis of cystein S-nitrosylation and tyrosine nitration. Either BSO or SNAP did not induced cysteine S-nirosylation (Fig. 3F) and tyrosine nitration (Fig. 3G) of ALDH2. However, combination of BSO and SNAP significantly increased both the ALDH2 cysteine S-nitrosylation (Fig. 3F) and tyrosine nitration (Fig. 3G); these effects were significantly reversed by MitoQ administration. BSO and SNAP synergistically also reduced cellular ALDH activity, whereas either BSO or SNAP alone had minor inhibitory effect (Fig. 3H). MitoQ remarkably ameliorated the synergistic effect of BSO and SNAP on ALDH activity. Cytotoxicity of nitrosative stress was assessed by analysis of annexin-5 and 7-AAD fluorescence intensity. As shown in Figs. 3I and 3J, MitoQ inhibited cell death caused by SNAP under GSH-depleted conditions.
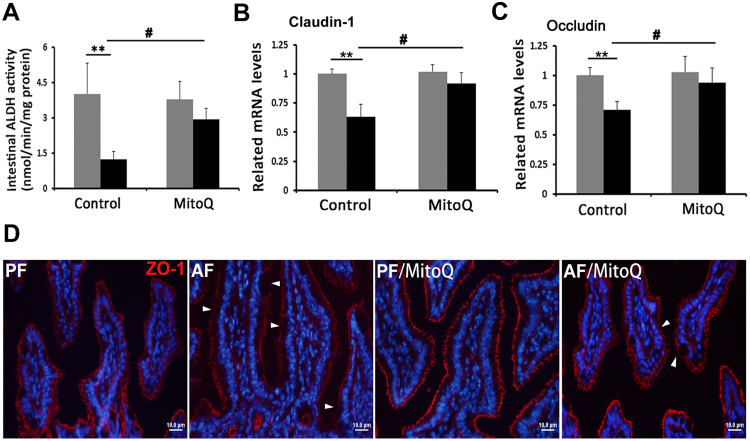
3.4. MitoQ ameliorated alcohol-induced intestinal ALDH inactivation and gut barrier disruption
The effect of MitoQ supplementation on ileal ALDH activity was measured. Chronic alcohol feeding dramatically reduced the ALDH activity of the ileum (Fig. 4A). MitoQ restored alcohol-reduced ileal ALDH activity. Chronic alcohol feeding repressed the ileal expression of tight junction proteins, claudin-1 (Fig. 4B) and occludin (Fig. 4C), which was normalized by MitoQ supplementation. Immunofluorescence staining of tight junction protein ZO-1 showed that alcohol exposure reduced the distribution and intensity of ZO-1 at the top of the ileal epithelium (Fig. 4D). This pathophysiological change was reversed by MitoQ supplementation.
Fig. 4.
MitoQ reversed alcohol-induced gut barrier disruption along with enhanced intestinal ALDH activity. (A) Intestinal ALDH activity (B) Bar graphs shown mRNA levels of Claudin 1 determined by RT-qPCR. (C) Bar graphs shown mRNA levels of Occludin determined by RT- qPCR. (D) Immunofluorescent staining of ZO-1 in the ileum. Scale bar: 10 µm. Red: ZO-1; blue: 4′,6-diamidino-2-phenylindole counterstaining of the nuclei; white trigangle: disassembled tight junction proteins. * P < 0.05 vs PF, ** P < 0.01 vs PF, # P<0.05 vs. AF.
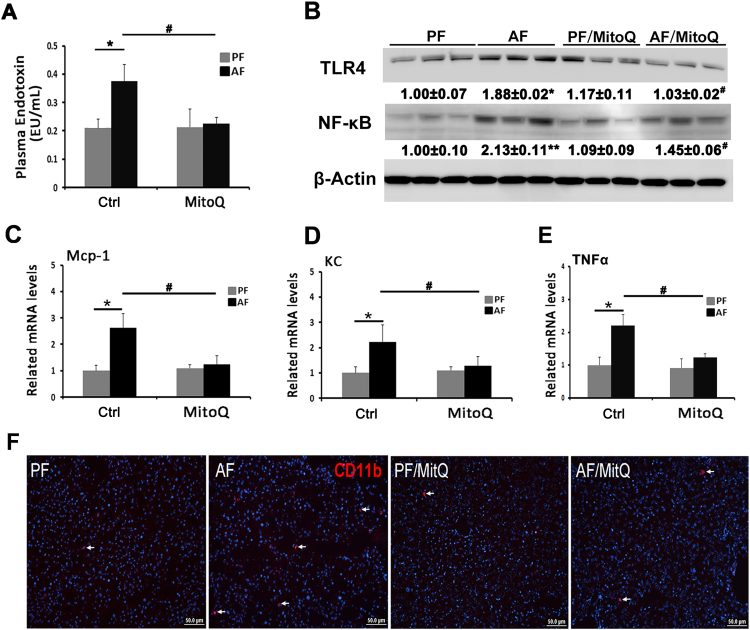
3.5. MitoQ ameliorated alcohol-induced endotoxemia and subsequent hepatic pro-inflammatory response
In accordance with change of gut barrier integrity, the plasma bacteria endotoxin (LPS) levels were increased by chronic alcohol exposure, and normalized by MitoQ supplementation (Fig. 5A). Consequently, hepatic TLR4, which mediates LPS signaling, was increased by alcohol exposure (Fig. 5B). MitoQ supplementation normalized alcohol-increased hepatic TLR4 protein levels and attenuated alcohol-induced NF-κB nuclear translocation.
Fig. 5.
Effects of MitoQ on endotoxemia and TLR4-NF-κB signaling pathway activation. (A) Plasma LPS levels. (B) Immunoblot bands of hepatic TLR4 and NF-κB. (C) Bar graphs shown mRNA levels of hepatic Mcp-1. (D) Bar graphs shown mRNA levels of hepatic KC. (E) Bar graphs shown mRNA levels of hepatic TNFα. (F) Immunofluorescent staining of CD11b+ in mouse liver. Scale bar: 50 µm. Arrows: CD11b+ cells; blue: 4′,6-diamidino-2-phenylindole counterstaining of the nuclei. * P < 0.05 vs PF, # P<0.05 vs. AF.
To investigate the contribution of TLR4-NF-κB pathway-mediated pro-inflammatory response, hepatic gene expression of cytokine and chemokines including tumor necrosis factor (TNFα), monocyte chemoattractant protein-1 (Mcp-1) and chemokine (C-X-C motif) ligand 1 (CXCL-).
(1/KC) was measured. Chronic ethanol exposure significantly up-regulated the expressions of hepatic TNFα, Mcp-1 and KC, which were effectively blocked by MitoQ supplementation (Fig. 5C-E). Immunofluorescence staining showed that alcohol feeding increased the number of inflammatory CD11b+ cells in the liver compared to the PF mice, which was attenuated by MitoQ supplementation (Fig. 5F).
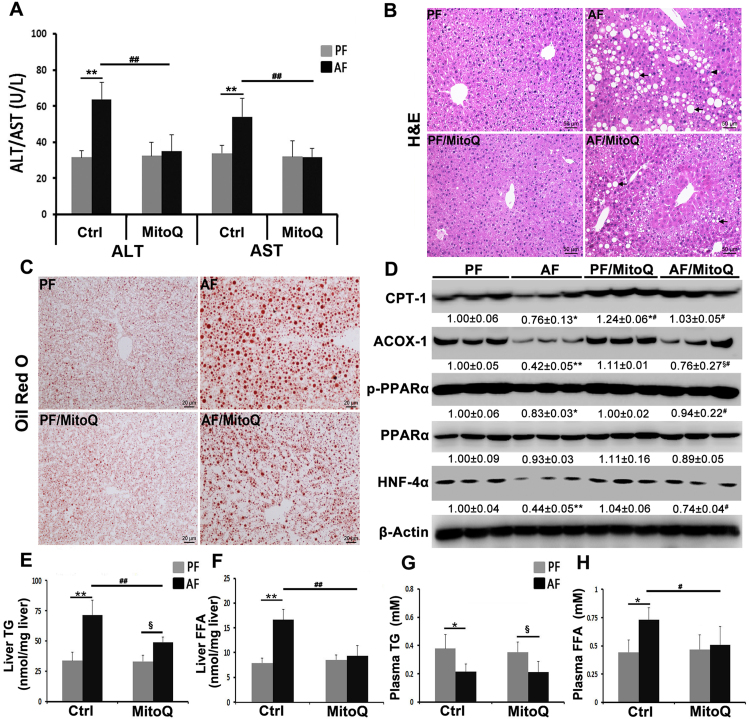
3.6. MitoQ treatment ameliorated alcohol-induced liver injury and alterations in lipid metabolism
Liver damage was assessed by measuring plasma ALT and AST levels and hepatic histopathological changes. Chronic alcohol feeding elevated plasma ALT and AST levels (Fig. 6A) and caused hepatic accumulation of macrovesicular lipid droplets and infiltration of inflammatory cells (Fig. 6B), which was attenuated by MitoQ administration. Lipid accumulation was further measured by Oil red O staining of neutral lipids and quantitative assay of TG and FFAs levels. Hepatic accumulation of neutral lipid droplets was found in alcohol-fed mice (Fig. 6C), and MitoQ supplementation reduced the size and number of neutral lipid droplets. In accordance, MitoQ diminished alcohol-increased hepatic TG and FFAs accumulation (Fig. 6D and E). MitoQ supplementation did not affect alcohol reduced plasma TG levels (Fig. 6F), but reversed alcohol-increased plasma FFAs levels (Fig. 6G).
Fig. 6.
MitoQ ameliorated alcohol-induced liver injury and lipid metabolism alterations. (A) Levels of plasma ALT and AST in each group. (B) Liver histopathological changes in each group shown by H&E staining (arrows: lipid droplets, arrowheads: inflammatory cells) Scale bar: 50 µm. (C) Oil red O staining of neutral lipids. Scale bar: 20 µm. (D) Levels of proteins involved in lipid metabolism. (E) Liver TG content. (F) Liver FFA concentration. (G) Plasma TG content. (H) Plasma FFA concentration. Proteins levels were quantitated by NIH image J. All values are denoted as means ± SD. * P < 0.05 vs PF, ** P < 0.01 vs PF, # P<0.05 vs. AF, ## P<0.01 vs. AF, §, P<0.05 vs. PF/MitoQ.
To further explore the potential mechanism of MitoQ in regulating lipid homeostasis, we measured the protein levels of hepatic lipid metabolism enzymes and lipid metabolism regulators. As shown in Fig. 6D, chronic alcohol feeding significantly reduced hepatic fatty acid β-oxidation-related proteins, mitochondrial CPT-1 and peroxisomal ACOX-1, which was reversed by MitoQ administration (Fig. 6D). MitoQ attenuated alcohol-reduced hepatic protein levels of p-PPARα and HNF4α (Fig. 6D). MitoQ treatment also normalized alcohol-decreased p-AMPKα protein levels (supplementary Fig. 2).
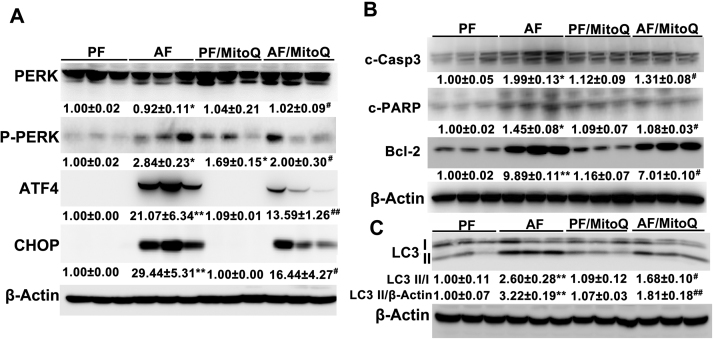
3.7. MitoQ supplementation inhibited alcohol-induced hepatic ER stress and apoptotic signaling
To examine whether MitoQ affects alcohol-induced endoplasmic reticulum (ER) stress activation, the protein levels of ER stress markers, including PERK, ATF4 and CHOP were measured. Chronic alcohol exposure significantly increased the protein levels of p-PERK, ATF4 and CHOP, and all these changes were reversed by MitoQ supplementation (Fig. 7A). Furthermore, MitoQ supplementation inhibited alcohol-induced hepatic PARP and caspase-3 cleavage (Fig. 7B). The protein levels of BCL-2 were increased by alcohol feeding, which were ameliorated by MitoQ supplementation (Fig. 7B). Because ER stress is a potent trigger for autophagy which, in turn, regulates apoptosis, autophagy was assessed by measuring LC3 activation. As shown in the Fig. 6C, chronic alcohol exposure-increased hepatic LC3II/LC3I ratio was reversed by MitoQ supplementation.
Fig. 7.
MitoQ reversed alcohol-induced hepatic ER stress along with attenuation of apoptosis. (A) Western blot of proteins involved in ER stress (B) Western blot of proteins involved in apoptosis. (C) Expression of hepatic LC3I/II. * P < 0.05 vs PF, ** P < 0.01 vs PF, # P<0.05 vs. AF, ## P<0.01 vs. AF, § P<0.05 vs. PF/MitoQ.
4. Discussion
There are growing evidence supporting a causative role of acetaldehyde toxicity in the initiation and progression of ALD. Acetaldehyde exerts hepatotoxicity through induction of oxidative stress, mitochondrial abnormalities, apoptosis, gut hyper-permeability, dysregulation of lipid metabolism and inflammatory response [30], [31], [32], [33], [34], [35], [36]. The present study demonstrated for the first time that reactivation of ALDH2 via posttranslational modifications represents a novel mechanism underlying the protective effect of MitoQ against ALD. Reactivation of ALDH2 accounts for MitoQ-enhanced acetaldehyde/lipid aldehydes detoxification and the consequent protective effects on alcohol-induced pathogenesis at the gut-liver axis, including ER stress, lipid dyshomeostasis, apoptosis, gut barrier dysfunction and hepatic inflammatory response. To the best of our knowledge, this study for the first time demonstrated that MitoQ provides protection against ALD by preserving ALDH2 function.
Mitochondria are the main source of oxidative stress and/or nitrosative stress [37], [38]. Under normal physiological conditions, ROS/RNS serve as 'redox messengers' in the regulation of intracellular signaling [39]. However, under elevated oxidative/nitrosative stress conditions, DNA, proteins and lipids undergo oxidative modifications, leading to disruption of their functions [40]. Many mitochondrial proteins, including ALDH2, can be oxidatively modified under elevated nitroxidative stress conditions through posttranslational modifications and generally inactivated [41]. On the basis of these findings, we hypothesized that mitochondria-targeted antioxidant could potentially prevent ALDH2 PTMs, which may enhance acetaldehyde detoxification in response to chronic alcohol exposure. MitoQ is a well-established mitochondria-targeted antioxidant with strongly antioxidant property as demonstrated by scavenging various radicals [42], [43]. MitoQ also has been shown to prevent peroxynitrite-mediated protein modification and 4-HNE formation [44]. Indeed, we found that MitoQ treatment significantly reversed alcohol-impaired ALDH activity along with normalization of hepatic ALDH2 cysteine residue(s) S-nitrosylation, tyrosine residue(s) nitration and 4-HNE adduct formation but not acetylation (data not shown). MitoQ supplementation significantly decreased alcohol-increased hepatic 4-HNE, 3-NT levels and nitrite contents. It has been reported that excessive NO in the mitochondria could impair mitochondrial respiration [66]. Inducible nitric oxide synthase (iNOS) plays an important role in the pathogenesis of ALD. Mice lacking iNOS are significantly prevented from alcohol-induced inflammation and steatosis [65]. However, Chacko et al. reported that MitoQ treatment significantly decreased RNS in the liver of alcohol-fed rats but did not have any effect on the induction of iNOS protein, which suggest that the mode of action of MitoQ is on the downstream [27]. Alcohol-decreased hepatic GSH contents were reversed by MitoQ supplementation. In this study, MitoQ ameliorated NO donor-induced hepatocyte ALDH2 cysteine residue(s) S-nitrosylation and tyrosine residue(s) nitration under GSH-depletion condition in vitro. Alcohol feeding significantly increased protein levels of mitochondrial complexes I, II, III, and V but not IV, which was ameliorated by MitoQ treatment. The protein levels of SOD2, which responds to elevated oxidative stress in mitochondria, were increased in alcohol-fed mice and strongly decreased after MitoQ treatment. Alcohol-increased mitochondrial NOX4 expression was remarkably reversed by MitoQ. Our previous data have shown that administration of an NOX4 inhibitor, GKT137831, ameliorated mitochondria superoxide and protected against alcohol-induced hepatic steatosis and apoptosis in mice [45]. Exogenous NAD+ supplementation blocked oxidative stress and cell death suggesting a stress resistant role of NAD+ [46]. As a co-factor, NAD+ is the limiting substrate for alcohol metabolism by ADH in the cytoplasm and acetaldehyde metabolism by ALDH2 in mitochondria [47]. Greater NAD availability and cycling may enhance ADH and ALDH2 activity in the liver. In this study, MitoQ restored alcohol-decreased NAD+ levels and NAD+/NADH ratio and enhanced ethanol metabolism. Above all, these data suggest that restored NAD+ levels and NAD+/NADH ratio, reversed mitochondrial respiratory alteration, enhanced GSH levels, and inhibition of NOX4 may contribute to the beneficial effects of MitoQ on ALDH2 post-translational modifications and related acetaldehyde detoxification in the alcohol-fed mice.
Acetaldehyde plays an important role in regulating alcohol-induced intestinal barrier dysfunction. In vitro studies have shown that tight junction proteins, such as ZO-1 and occludin, were disrupted and dissociated from actin cytoskeleton of Caco-2 cell monolayers by acetaldehyde [48]. Our previous data showed that nicotinic acid supplementation modulates intestinal ALDH gene expression and luminal acetaldehyde levels [49]. We also found that zinc supplementation improved tight junction genes expression in association with increased intestinal ALDH expression and activity [50], [51]. In this study, MitoQ treatment did not affects ileal ALDH2 protein levels in alcohol-fed mice (data not shown). However, enhanced ALDH activity in small intestine indicates a more effective response of the intestinal epithelium to counteract the acetaldehyde-related damage in alcohol-fed mice. Interestingly, this affect may indicate that MitoQ potentially regulates posttranslational modulation of intestinal ALDHs. One possibility could be the antioxidant capacity of MitoQ. Indeed, oxidative stress is a causal factor in cellular stress-related tight junction dysfunction in the intestinal epithelium [52]. It has been shown that DSS-induced barrier dysfunction and tight junction disruption can be reversed by antioxidant administration [53]. Therefore, MitoQ may alleviate the oxidative processes, and eventually protect the function of ALDHs as well as tight junction.
Endotoxemia has been well documented in alcoholic patients [54]. Both clinical and animal studies have shown that the plasma endotoxins and hepatic cytokine/chemokine were increased by alcohol [55], [56]. In this study, alcohol feeding-induced LPS translocation in association with impaired gut barrier function. LPS of Gram-negative bacterial cell wall are the major pathogen-associated molecular patterns (PAMPs) and a natural ligand of toll-like receptors (TLR). Studies have reported that the activation of TLR4 is involved in the development and progression of ALD [57]. In this study, MitoQ treatment ameliorated alcohol-induced LPS penetration to the circulation and TLR4-NF-κB signaling pathway activation. These findings were associated with a marked inhibition of alcohol-induced TNFα, Mcp-1 and Cxcl-1 gene expression.
Fatty liver is one of the earliest pathological changes in the progression of ALD, and acetaldehyde may participate in the pathogenesis through modulating hepatic lipid metabolism and lipid homeostasis [58]. An in vitro study using recombinant HepaG2 cells demonstrated that only cells producing acetaldehyde can induce early triglyceride and growth response-1 accumulation after alcohol exposure [59]. Chacko et al. have reported that MitoQ treatment significantly decreased ethanol-dependent micro and macro hepatosteatosis in rat, however only high dose MitoQ (25 mg/kg/d) administration has modestly effect on AMPK signaling [27]. In our alcohol-fed mouse model, MitoQ treatment at 5 mg/kg/d significantly reversed alcohol-decreased p-AMPKα protein levels. The differences on animal species and alcohol feeding method may contribute to this discrepancy. In the present study, alcohol-induced steatosis resulted from decreased fatty acid oxidation and de-regulated lipid metabolism regulators. Two alcohol- perturbed fatty acid β-oxidation enzymes (CPT-1 and ACOX-1) were significantly reversed by MitoQ treatment. MitoQ significantly ameliorated alcohol-decreased protein levels of HNF-4α and p-PPARα, two transcription factors that play critical roles in the regulation of hepatic lipid homeostasis. CPT-1 and ACOX-1 are both classical target genes regulated by PPARα. HNF-4α is a master regulator of hepatic gene expression, and liver-specific knockout of HNF-4α led to severe steatosis in mice. In our previous study, activation of HNF-4α by zinc attenuated alcohol- induced hepatic steatosis along with upregulated gene expressions including CPT-1 and ACOX-1. We also showed that acetaldehyde and 4-HNE could decrease hepatocyte P-PPARα and HNF-4α protein levels [60]. These data suggest that modulation of fatty acid uptake-related proteins and lipid metabolism regulators contribute to the beneficial effects of MitoQ on alcohol-induced hepatosteatosis.
Alcohol-fed mice exhibited steatohepatitis along with induction of ER stress [61]. It has been suggested that ALDH2 may have a beneficial role against ER stress-induced cardiac anomalies [62]. ER stress disturbs hepatic lipid metabolism by deregulation of lipogenic genes, promotion of apoptosis, and activation of several pro-inflammatory pathways [63], [64]. Alcohol-increased oxidative stress, enhanced inflammatory response and accumulated free fatty acids in the liver all could mediate unfolded protein response (UPR) activation, which contributes to ALD development. In this study, the unfolded protein response transducers IRE-1α and P-PERK were activated after alcohol exposure. As a consequence, the transcription factor CHOP, which is known to modulate cell death, along with cleaved caspase-3 and PARP protein levels were increased. Anti-apoptosis protein BCL-2 and autophagy-related proteins LC3B levels were increased in alcohol-fed mice, which may serve as an adaptive response to ethanol toxicity, and significantly prevented by MitoQ. MitoQ ameliorated alcohol-induced ER stress and apoptosis demonstrated a mechanistic link between mtROS and ER stress in alcohol-induced apoptotic cell death. The beneficial effect of MitoQ on ER stress and apoptosis could be exerted by a complex mechanism underlying its modulatory effect on aldehydes detoxification and also by counteracting oxidative stress, alcoholic lipotoxicity and pro-inflammatory cytokine-dependent inflammation as a result of its anti-oxidant and anti-inflammation activity, as previous suggested.
5. Conclusions
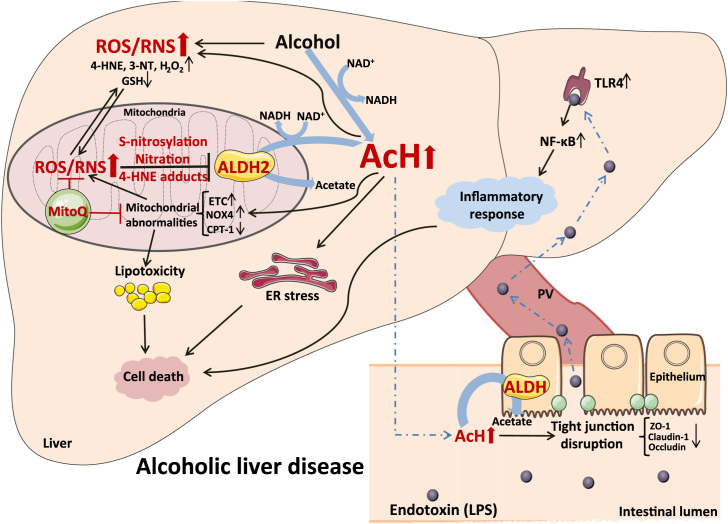
Acceleration of acetaldehyde and lipid aldehyde clearance accounts for the protective effect of dietary MitoQ supplementation on alcohol-induced hepatic lipid metabolic disorder, ER stress, apoptosis and inflammation. Restoration of ALDH activity by preventing oxidative/nitrosative posttranslational modification of mitochondrial ALDH2 represents a novel molecular mechanism underlying MitoQ-accelerated clearance of acetaldehyde and lipid aldehyde and the consequent protective effects against alcohol-induced pathogenesis at the gut-liver axis (Fig. 8).
Fig. 8.
Depicting the possible molecular mechansims by which MitoQ prevents alcoholic liver disease through modulating ALDH2 posttranslational modifications. After chronic alcohol abuse, oxidative and nitrosative stress were increased, which impaired mitochondrial ALDH2 activity via cysteine S-nitrosylation, tyrosine nitration and 4-HNE adducts formation. Acetaldehyde accumulated in the system result in ileal tight junction disruption and increased plasma endotoxin and subsequently inflammatory response in the liver. Excess acetaldehyde accumulation in the liver leads to enhanced lipotoxicity, ER stress and cell apoptosis pathway activation. MitoQ supplementation prevented alcohol-induced ALDH2 posttranslational modifications and accelerated acetaldehyde clearance, which against alcohol-induced pathogenesis at the gut-liver axis.
Funding
This research was supported by the National Institutes of Health grants (R01AA018844, R01AA020212).
Conflicts of interest
No potential conflicts of interest relevant to this article are reported.
Author contributions
S.Q., W.Z., W.L.Z., X. and X.S. conducted animal feeding experiment. H.L., S.Q, and X.S. performed data analysis. H.L. conducted cell culture experiment. H.L. drafted the manuscript, and W.Z., W.L.Z., and Z.Z. participated in the manuscript preparation. H.L. is the guarantor of this work and, as such, had full access to all the data for the study and takes responsibility for its integrity and for the accuracy of the data analysis.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2017.11.005.
Appendix A. Supplementary material
Supplementary material
References
- 1.Masarone M., Rosato V., Dallio M., Abenavoli L., Federico A., Loguercio C., Persico M. Epidemiology and Natural History of Alcoholic Liver Disease. Rev. Recent Clin. Trials. 2016;11(3):167–174. doi: 10.2174/1574887111666160810101202. [DOI] [PubMed] [Google Scholar]
- 2.Gao B., Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141(5):1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song B.J., Abdelmegeed M.A., Henderson L.E., Yoo S.H., Wan J., Purohit V., Hardwick J.P., Moon K.H. Increased nitroxidative stress promotes mitochondrial dysfunction in alcoholic and nonalcoholic fatty liver disease. Oxid. Med. Cell. Longev. 2013;2013:781050. doi: 10.1155/2013/781050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoek J.B., Cahill A., Pastorino J.G. Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology. 2002;122(7):2049–2063. doi: 10.1053/gast.2002.33613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantena S.K., King A.L., Andringa K.K., Eccleston H.B., Bailey S.M. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radic. Biol. Med. 2008;44(7):1259–1272. doi: 10.1016/j.freeradbiomed.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mansouri A., Gaou I., De Kerguenec C., Amsellem S., Haouzi D., Berson A., Moreau A., Feldmann G., Letteron P., Pessayre D., Fromenty B. An alcoholic binge causes massive degradation of hepatic mitochondrial DNA in mice. Gastroenterology. 1999;117(1):181–190. doi: 10.1016/s0016-5085(99)70566-4. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Checa J.C., Hirano T., Tsukamoto H., Kaplowitz N. Mitochondrial glutathione depletion in alcoholic liver disease. Alcohol. 1993;10(6):469–475. doi: 10.1016/0741-8329(93)90067-x. [DOI] [PubMed] [Google Scholar]
- 8.Clugston R.D., Jiang H., Lee M.X., Piantedosi R., Yuen J.J., Ramakrishnan R., Lewis M.J., Gottesman M.E., Huang L.S., Goldberg I.J., Berk P.D., Blaner W.S. Altered hepatic lipid metabolism in C57BL/6 mice fed alcohol: a targeted lipidomic and gene expression study. J. Lipid Res. 2011;52(11):2021–2031. doi: 10.1194/jlr.M017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Q., Zhong W., Zhang W., Zhou Z. Defect of mitochondrial respiratory chain is a mechanism of ROS overproduction in a rat model of alcoholic liver disease: role of zinc deficiency. Am. J. Physiol. Gastrointest. liver Physiol. 2016;310(3):G205–G214. doi: 10.1152/ajpgi.00270.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong W., Zhang W., Li Q., Xie G., Sun Q., Sun X., Tan X., Sun X., Jia W., Zhou Z. Pharmacological activation of aldehyde dehydrogenase 2 by Alda-1 reverses alcohol-induced hepatic steatosis and cell death in mice. J. Hepatol. 2015;62(6):1375–1381. doi: 10.1016/j.jhep.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res. Health.: J. Natl. Inst. Alcohol Abus. Alcohol. 2006;29(4):245–254. [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong H., Yin H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: focusing on mitochondria. Redox Biol. 2015;4:193–199. doi: 10.1016/j.redox.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song B.J., Abdelmegeed M.A., Yoo S.H., Kim B.J., Jo S.A., Jo I., Moon K.H. Post- translational modifications of mitochondrial aldehyde dehydrogenase and biomedical implications. J. Proteom. 2011;74(12):2691–2702. doi: 10.1016/j.jprot.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon K.H., Abdelmegeed M.A., Song B.J. Inactivation of cytosolic aldehyde dehydrogenase via S-nitrosylation in ethanol-exposed rat liver. FEBS Lett. 2007;581(21):3967–3972. doi: 10.1016/j.febslet.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doorn J.A., Hurley T.D., Petersen D.R. Inhibition of human mitochondrial aldehyde dehydrogenase by 4-hydroxynon-2-enal and 4-oxonon-2-enal. Chem. Res. Toxicol. 2006;19(1):102–110. doi: 10.1021/tx0501839. [DOI] [PubMed] [Google Scholar]
- 16.Moon K.H., Hood B.L., Kim B.J., Hardwick J.P., Conrads T.P., Veenstra T.D., Song B.J. Inactivation of oxidized and S-nitrosylated mitochondrial proteins in alcoholic fatty liver of rats. Hepatology. 2006;44(5):1218–1230. doi: 10.1002/hep.21372. [DOI] [PubMed] [Google Scholar]
- 17.Abdelmegeed M.A., Jang S., Banerjee A., Hardwick J.P., Song B.J. Robust protein nitration contributes to acetaminophen-induced mitochondrial dysfunction and acute liver injury. Free Radic. Biol. Med. 2013;60:211–222. doi: 10.1016/j.freeradbiomed.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apostolova N., Victor V.M. Molecular strategies for targeting antioxidants to mitochondria: therapeutic implications. Antioxid. Redox Signal. 2015;22(8):686–729. doi: 10.1089/ars.2014.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James A.M., Sharpley M.S., Manas A.R., Frerman F.E., Hirst J., Smith R.A., Murphy M.P. Interaction of the mitochondria-targeted antioxidant MitoQ with phospholipid bilayers and ubiquinone oxidoreductases. J. Biol. Chem. 2007;282(20):14708–14718. doi: 10.1074/jbc.M611463200. [DOI] [PubMed] [Google Scholar]
- 20.Gane E.J., Weilert F., Orr D.W., Keogh G.F., Gibson M., Lockhart M.M., Frampton C.M., Taylor K.M., Smith R.A., Murphy M.P. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int.: Off. J. Int. Assoc. Study Liver. 2010;30(7):1019–1026. doi: 10.1111/j.1478-3231.2010.02250.x. [DOI] [PubMed] [Google Scholar]
- 21.Lowes D.A., Thottakam B.M., Webster N.R., Murphy M.P., Galley H.F. The mitochondria- targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Free Radic. Biol. Med. 2008;45(11):1559–1565. doi: 10.1016/j.freeradbiomed.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Dare A.J., Bolton E.A., Pettigrew G.J., Bradley J.A., Saeb-Parsy K., Murphy M.P. Protection against renal ischemia-reperfusion injury in vivo by the mitochondria targeted antioxidant MitoQ. Redox Biol. 2015;5:163–168. doi: 10.1016/j.redox.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dashdorj A., Jyothi K.R., Lim S., Jo A., Nguyen M.N., Ha J., Yoon K.S., Kim H.J., Park J.H., Murphy M.P., Kim S.S. Mitochondria-targeted antioxidant MitoQ ameliorates experimental mouse colitis by suppressing NLRP3 inflammasome-mediated inflammatory cytokines. BMC Med. 2013;11:178. doi: 10.1186/1741-7015-11-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feillet-Coudray C., Fouret G., Ebabe Elle R., Rieusset J., Bonafos B., Chabi B., Crouzier D., Zarkovic K., Zarkovic N., Ramos J., Badia E., Murphy M.P., Cristol J.P., Coudray C. The mitochondrial-targeted antioxidant MitoQ ameliorates metabolic syndrome features in obesogenic diet-fed rats better than Apocynin or Allopurinol. Free Radic. Res. 2014;48(10):1232–1246. doi: 10.3109/10715762.2014.945079. [DOI] [PubMed] [Google Scholar]
- 25.Fouret G., Tolika E., Lecomte J., Bonafos B., Aoun M., Murphy M.P., Ferreri C., Chatgilialoglu C., Dubreucq E., Coudray C., Feillet-Coudray C. The mitochondrial-targeted antioxidant, MitoQ, increases liver mitochondrial cardiolipin content in obesogenic diet-fed rats. Biochim. Et. Biophys. Acta. 2015;1847(10):1025–1035. doi: 10.1016/j.bbabio.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 26.Xiao L., Xu X., Zhang F., Wang M., Xu Y., Tang D., Wang J., Qin Y., Liu Y., Tang C., He L., Greka A., Zhou Z., Liu F., Dong Z., Sun L. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 2017;11:297–311. doi: 10.1016/j.redox.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chacko B.K., Srivastava A., Johnson M.S., Benavides G.A., Chang M.J., Ye Y., Jhala N., Murphy M.P., Kalyanaraman B., Darley-Usmar V.M. Mitochondria-targeted ubiquinone (MitoQ) decreases ethanol-dependent micro and macro hepatosteatosis. Hepatology. 2011;54(1):153–163. doi: 10.1002/hep.24377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Q., Zhang W., Zhong W., Sun X., Zhou Z. Dietary fisetin supplementation protects against alcohol-induced liver injury in Mice. Alcohol., Clin. Exp. Res. 2016;40(10):2076–2084. doi: 10.1111/acer.13172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moon K.H., Kim B.J., Song B.J. Inhibition of mitochondrial aldehyde dehydrogenase by nitric oxide-mediated S-nitrosylation. FEBS Lett. 2005;579(27):6115–6120. doi: 10.1016/j.febslet.2005.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roman J., Colell A., Blasco C., Caballeria J., Pares A., Rodes J., Fernandez-Checa J.C. Differential role of ethanol and acetaldehyde in the induction of oxidative stress in HEP G2 cells: effect on transcription factors AP-1 and NF-kappaB. Hepatology. 1999;30(6):1473–1480. doi: 10.1002/hep.510300623. [DOI] [PubMed] [Google Scholar]
- 31.Farfan Labonne B.E., Gutierrez M., Gomez-Quiroz L.E., Konigsberg Fainstein M., Bucio L., Souza V., Flores O., Ortiz V., Hernandez E., Kershenobich D., Gutierrez-Ruiz M.C. Acetaldehyde-induced mitochondrial dysfunction sensitizes hepatocytes to oxidative damage. Cell Biol. Toxicol. 2009;25(6):599–609. doi: 10.1007/s10565-008-9115-5. [DOI] [PubMed] [Google Scholar]
- 32.Brandt M., Garlapati V., Oelze M., Sotiriou E., Knorr M., Kroller-Schon S., Kossmann S., Schonfelder T., Morawietz H., Schulz E., Schultheiss H.P., Daiber A., Munzel T., Wenzel P. NOX2 amplifies acetaldehyde-mediated cardiomyocyte mitochondrial dysfunction in alcoholic cardiomyopathy. Sci. Rep. 2016;6:32554. doi: 10.1038/srep32554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menegola E., Broccia M.L., Di Renzo F., Giavini E. Acetaldehyde in vitro exposure and apoptosis: a possible mechanism of teratogenesis. Alcohol. 2001;23(1):35–39. doi: 10.1016/s0741-8329(00)00132-4. [DOI] [PubMed] [Google Scholar]
- 34.Rao R.K. Acetaldehyde-induced barrier disruption and paracellular permeability in Caco-2 cell monolayer. Methods Mol. Biol. 2008;447:171–183. doi: 10.1007/978-1-59745-242-7_13. [DOI] [PubMed] [Google Scholar]
- 35.Lieber C.S. Metabolic effects of acetaldehyde. Biochem. Soc. Trans. 1988;16(3):241–247. doi: 10.1042/bst0160241. [DOI] [PubMed] [Google Scholar]
- 36.Dong D., Zhong W., Sun Q., Zhang W., Sun X., Zhou Z. Oxidative products from alcohol metabolism differentially modulate pro-inflammatory cytokine expression in Kupffer cells and hepatocytes. Cytokine. 2016;85:109–119. doi: 10.1016/j.cyto.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lazaro J.J., Jimenez A., Camejo D., Iglesias-Baena I., Marti Mdel C., Lazaro-Payo A., Barranco-Medina S., Sevilla F. Dissecting the integrative antioxidant and redox systems in plant mitochondria. Effect of stress and S-nitrosylation. Front. Plant Sci. 2013;4:460. doi: 10.3389/fpls.2013.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kramer P.A., Ravi S., Chacko B., Johnson M.S., Darley-Usmar V.M. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: implications for their use as bioenergetic biomarkers. Redox Biol. 2014;2:206–210. doi: 10.1016/j.redox.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trachootham D., Lu W., Ogasawara M.A., Nilsa R.D., Huang P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008;10(8):1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grimsrud P.A., Xie H., Griffin T.J., Bernlohr D.A. Oxidative stress and covalent modification of protein with bioactive aldehydes. J. Biol. Chem. 2008;283(32):21837–21841. doi: 10.1074/jbc.R700019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song B.J., Akbar M., Abdelmegeed M.A., Byun K., Lee B., Yoon S.K., Hardwick J.P. Mitochondrial dysfunction and tissue injury by alcohol, high fat, nonalcoholic substances and pathological conditions through post-translational protein modifications. Redox Biol. 2014;3:109–123. doi: 10.1016/j.redox.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith R.A., Murphy M.P. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann. New Y. Acad. Sci. 2010;1201:96–103. doi: 10.1111/j.1749-6632.2010.05627.x. [DOI] [PubMed] [Google Scholar]
- 43.Murphy M.P., Smith R.A. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu. Rev. Pharmacol. Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 44.Reily C., Mitchell T., Chacko B.K., Benavides G., Murphy M.P., Darley-Usmar V. Mitochondrially targeted compounds and their impact on cellular bioenergetics. Redox Biol. 2013;1(1):86–93. doi: 10.1016/j.redox.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun Q., Zhang W., Zhong W., Sun X., Zhou Z. Pharmacological inhibition of NOX4 ameliorates alcohol-induced liver injury in mice through improving oxidative stress and mitochondrial function. Biochim. Et. Biophys. Acta. 2017;1861(1 Pt A):2912–2921. doi: 10.1016/j.bbagen.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu Y., Zhao K.K., Tong Y., Zhou Y.L., Wang Y.X., Zhao P.Q., Wang Z.Y. Exogenous NAD(+) decreases oxidative stress and protects H2O2-treated RPE cells against necrotic death through the up-regulation of autophagy. Sci. Rep. 2016;6:26322. doi: 10.1038/srep26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cederbaum A.I. Alcohol metabolism. Clin. liver Dis. 2012;16(4):667–685. doi: 10.1016/j.cld.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunagan M., Chaudhry K., Samak G., Rao R.K. Acetaldehyde disrupts tight junctions in Caco-2 cell monolayers by a protein phosphatase 2A-dependent mechanism. Am. J. Physiol. Gastrointest. liver Physiol. 2012;303(12):G1356–G1364. doi: 10.1152/ajpgi.00526.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhong W., Li Q., Zhang W., Sun Q., Sun X., Zhou Z. Modulation of intestinal barrier and bacterial endotoxin production contributes to the beneficial effect of nicotinic acid on alcohol-induced endotoxemia and hepatic inflammation in Rats. Biomolecules. 2015;5(4):2643–2658. doi: 10.3390/biom5042643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhong W., McClain C.J., Cave M., Kang Y.J., Zhou Z. The role of zinc deficiency in alcohol-induced intestinal barrier dysfunction. Am. J. Physiol. Gastrointest. liver Physiol. 2010;298(5):G625–G633. doi: 10.1152/ajpgi.00350.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhong W., Zhao Y., McClain C.J., Kang Y.J., Zhou Z. Inactivation of hepatocyte nuclear factor-4 alpha mediates alcohol-induced downregulation of intestinal tight junction proteins. Am. J. Physiol. Gastrointest. liver Physiol. 2010;299(3):G643–G651. doi: 10.1152/ajpgi.00515.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meyer T.N., Schwesinger C., Ye J., Denker B.M., Nigam S.K. Reassembly of the tight junction after oxidative stress depends on tyrosine kinase activity. J. Biol. Chem. 2001;276(25):22048–22055. doi: 10.1074/jbc.M011477200. [DOI] [PubMed] [Google Scholar]
- 53.Wang A., Keita A.V., Phan V., McKay C.M., Schoultz I., Lee J., Murphy M.P., Fernando M., Ronaghan N., Balce D., Yates R., Dicay M., Beck P.L., MacNaughton W.K., Soderholm J.D., McKay D.M. Targeting mitochondria-derived reactive oxygen species to reduce epithelial barrier dysfunction and colitis. Am. J. Pathol. 2014;184(9):2516–2527. doi: 10.1016/j.ajpath.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fujimoto M., Uemura M., Nakatani Y., Tsujita S., Hoppo K., Tamagawa T., Kitano H., Kikukawa M., Ann T., Ishii Y., Kojima H., Sakurai S., Tanaka R., Namisaki T., Noguchi R., Higashino T., Kikuchi E., Nishimura K., Takaya A., Fukui H. Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: relation to severity of liver disturbance. Alcohol., Clin. Exp. Res. 2000;24(4 Suppl):48S–54S. [PubMed] [Google Scholar]
- 55.Nolan J.P. The role of intestinal endotoxin in liver injury: a long and evolving history. Hepatology. 2010;52(5):1829–1835. doi: 10.1002/hep.23917. [DOI] [PubMed] [Google Scholar]
- 56.Ferluga J., Kaplun A., Allison A.C. Protection of mice against endotoxin-induced liver damage by anti-inflammatory drugs. Agents Actions. 1979;9(5–6):566–574. doi: 10.1007/BF01968129. [DOI] [PubMed] [Google Scholar]
- 57.Petrasek J., Mandrekar P., Szabo G. Toll-like receptors in the pathogenesis of alcoholic liver disease. Gastroenterol. Res. Pract. 2010;2010 doi: 10.1155/2010/710381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ceni E., Crabb D.W., Foschi M., Mello T., Tarocchi M., Patussi V., Moraldi L., Moretti R., Milani S., Surrenti C., Galli A. Acetaldehyde inhibits PPARgamma via H2O2-mediated c-Abl activation in human hepatic stellate cells. Gastroenterology. 2006;131(4):1235–1252. doi: 10.1053/j.gastro.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 59.Thomes P.G., Osna N.A., Davis J.S., Donohue T.M., Jr. Cellular steatosis in ethanol oxidizing-HepG2 cells is partially controlled by the transcription factor, early growth response-1. Int. J. Biochem. Cell Biol. 2013;45(2):454–463. doi: 10.1016/j.biocel.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang X., Zhong W., Liu J., Song Z., McClain C.J., Kang Y.J., Zhou Z. Zinc supplementation reverses alcohol-induced steatosis in mice through reactivating hepatocyte nuclear factor-4alpha and peroxisome proliferator-activated receptor-alpha. Hepatology. 2009;50(4):1241–1250. doi: 10.1002/hep.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ji C. New insights into the pathogenesis of alcohol-induced ER stress and liver diseases. Int. J. Hepatol. 2014;2014:513787. doi: 10.1155/2014/513787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang B., Zhang Y., La Cour K.H., Richmond K.L., Wang X.M., Ren J. Mitochondrial aldehyde dehydrogenase obliterates endoplasmic reticulum stress-induced cardiac contractile dysfunction via correction of autophagy. Biochim. Et. Biophys. Acta. 2013;1832(4):574–584. doi: 10.1016/j.bbadis.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ji C. Dissection of endoplasmic reticulum stress signaling in alcoholic and non-alcoholic liver injury. J. Gastroenterol. Hepatol. 2008;23(Suppl 1):S16–S24. doi: 10.1111/j.1440-1746.2007.05276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tam A.B., Mercado E.L., Hoffmann A., Niwa M. ER stress activates NF-kappaB by integrating functions of basal IKK activity, IRE1 and PERK. PloS One. 2012;7(10):e45078. doi: 10.1371/journal.pone.0045078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Venkatraman A., Shiva S., Wigley A., Ulasova E., Chhieng D., Bailey S.M., Darley-Usmar V.M. The role of iNOS in alcohol-dependent hepatotoxicity and mitochondrial dysfunction in mice. Hepatology. 2004;40(3):565–573. doi: 10.1002/hep.20326. [DOI] [PubMed] [Google Scholar]
- 66.Shiva S., Brookes P.S., Patel R.P., Anderson P.G., Darley-Usmar V.M. Nitric oxide partitioning into mitochondrial membranes and the control of respiration at cytochrome c oxidase. Proc. Natl. Acad. Sci. USA. 2001;98(13):7212–7217. doi: 10.1073/pnas.131128898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material