Abstract
Purpose
Macular Telangiectasia Type 2 (MacTel) is a bilateral, progressive, potentially blinding retinal disease characterized by both vascular and neurodegenerative signs. Both the area of the break in the Ellipsoid Zone (EZ) seen in “en face” optical coherence tomographic (OCT) images and microperimetric focal retinal sensitivity loss have been proposed as potential measures of progression in MacTel. We aimed to assess the characteristics and interrelationship of these structural and functional disease markers from the data collected in a phase one clinical trial of ciliary neurotrophic factor (CNTF) in MacTel.
Methods
Orthogonal topographic (“en face”) maps of the EZ were generated from Heidelberg Spectralis OCT volume scans (15º×10º area, 30-μm B-scan intervals), or Cirrus HD-OCT4000 512×128 cube scans. Mesopic microperimetry was performed on CenterVue MAIA perimeters, using a Goldmann III stimulus in a custom test grid. Structural and functional data were analyzed by two methods: by calculating aggregate loss and by simple thresholding. The alignment quality of structural and functional data was also evaluated.
Results
Overall, the break area showed a good correlation with aggregate sensitivity loss (ρ =0.834, P<0.0001, 95%CI 0.716 to 0.906) but also with the number of test points below a threshold value (e.g. <20dB:ρ=0.843, p<0.0001 95%CI 0.755 to 0.902). Significant misalignment of the MAIA test grid was apparent in 13/48 visits of 7/14 eyes.
Conclusion
We found a good correlation between EZ break area and function loss. ‘En face’ OCT mapping of the EZ appears to demonstrate structural change before mesopic microperimetry can detect a focal loss of retinal sensitivity. Thresholding offers a quick alternative to calculating aggregate sensitivity loss.
Keywords: Retina, degeneration, telangiectasia, MacTel, imaging, Muller cells, OCT, microperimetry
BACKGROUND
Macular Telangiectasia Type 2 (MacTel) is a bilateral, slowly progressive, potentially blinding retinal disease in the juxtafoveal region of unknown cause.1 Its clinical phenotype is characterized by both vascular and neurodegenerative changes. Although many therapies have been tested in MacTel, none have been shown to be effective to-date.1, 2
One characteristic neurodegenerative sign apparent in optical coherence tomographic (OCT) images is a disruption (or ‘break’) in the line attributed to the junctions between photoreceptor inner and outer segments, IS/OS junction line; more recently, this signal was attributed to the inner segment ellipsoids, ‘ellipsoid zone’ (EZ), This sign was found to be associated with a loss of retinal sensitivity.3–9
Both EZ break area and focal retinal sensitivity loss, as reflected by mesopic microperimetry have been proposed as potential outcome measures in MacTel. In our previous studies we analyzed OCT volume scans acquired using Topcon 3DOCT-1000 devices and retinal sensitivity measurements using Nidek MP1 microperimeters.3, 4 The axial and lateral resolutions of the Topcon 3DOCT-1000 are limited and only a single raster scan pattern of 128 B-scans in an area of 20°×20° is available. The Nidek MP1 has a limited dynamic range (20dB) and uses flash photography for acquiring a reference color fundus image. Misalignments between test data and reference image are not infrequent. The CenterVue Maia microperimeter has a wider dynamic range (32dB) and uses an infrared scanning laser ophthalmoscope for capturing the reference fundus image. In our previous studies, retinal sensitivity changes were assessed by calculating aggregate sensitivity loss.3, 4 These calculations are relatively labor-intensive and require an accurate alignment of OCT ‘en face’ and microperimetric data.
In the current analyses our aim was to investigate further the characteristics and interrelationship of these structural and functional disease markers both by calculating aggregate loss as described previously and by a new, simple and fast assessment method, from data collected using Carl Zeiss Meditec HD-OCT4000 or Heidelberg Spectralis OCT devices and CenterVue Maia microperimeters in a phase one clinical trial of ciliary neurotrophic factor (CNTF) in MacTel.10
PARTICIPANTS AND METHODS
Study design
Data used in these analyses were collected in an open-label, non-randomized phase one clinical trial conducted at two centers: the Retina Associates of Cleveland, Inc and the Jules Stein Eye Institute, University of California Los Angeles. One eye of each patient (the more severely affected eye) received an encapsulated cell implant delivering ciliary neurotrophic factor (CNTF) to the retina.10 The study was registered with ClinicalTrials.gov (NCT01327911) and was conducted according to the guidelines of the Declaration of Helsinki. The protocol was approved by respective Institutional Review Boards, each participant provided signed informed consent. A Data and Safety Monitoring Committee was established to monitor participant safety. The study parameters and main findings have been published previously.10
Study Participants
Participants aged≥21 years with a diagnosis of bilateral MacTel, no previous history of intraocular surgery, best corrected visual acuity of ≥20/50 and the presence of a break in the EZ layer on OCT in at least one eye were eligible for the study.
Imaging
OCT scans were acquired at UCLA using Heidelberg Spectralis OCT (Heidelberg Engineering GmbH, Heidelberg, Germany), and at Cleveland using Cirrus HD-OCT 4000 (Carl Zeiss Meditec, Inc, Dublin, California, USA) devices. On the Spectralis, volume scans 15º×10º in size were recorded, with 30-μm B-scan intervals (97 B-scans/volume). On the Cirrus, a standard 512×128 cube scan covering a retinal area 20º×20º in size was acquired. Orthogonal topographic (“en face”) maps of the EZ were generated as described earlier.3, 4
Psychophysical testing
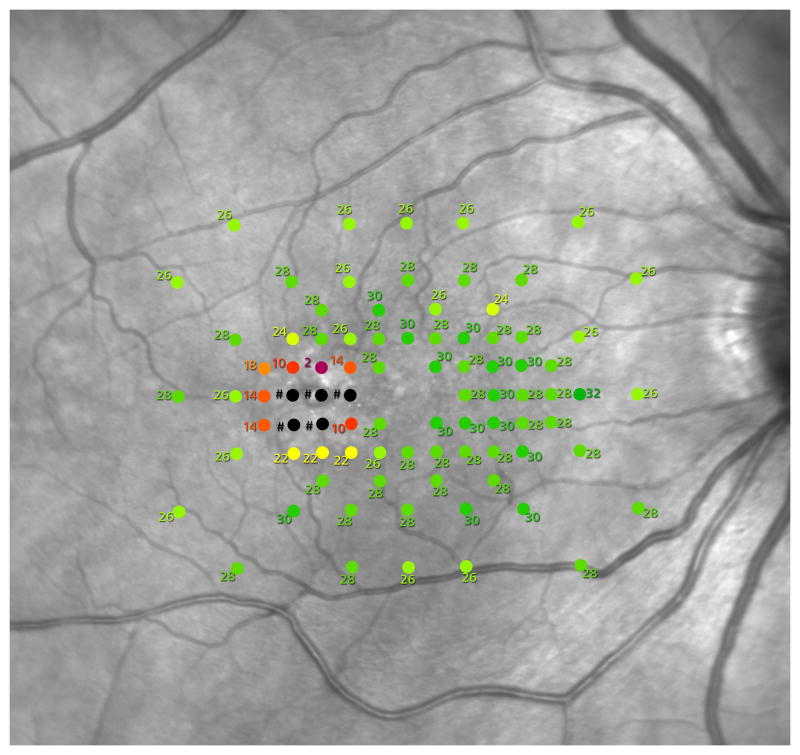
Fundus-correlated automated mesopic microperimetry was performed following pupil dilation with 1.0% tropicamide and 2.5% phenylephrine hydrochloride and 5 minutes of dark adaptation, on MAIA perimeters (CenterVueS.p.A., Padova, Italy), using a white Goldmann size III stimulus in a test grid adapted to the needs of this study (see Figure 1). This grid has a progressive spacing of test points. Close to the foveal centre (but excluding the immediate centre where the fixation target may interfere with the test stimuli), the spacing is 1° between test points. The test point spacing increases to 2° towards the periphery - as a compromise solution aiming to maximize the test area covered while minimising the test duration. Results were reported in decibels (dB).
Figure 1.
MAIA microperimeter test grid used in this study.
A sample retinal sensitivity report, the spacing of the grid centrally is one degree between test points. The stimulus used was white, size Goldmann III.
Image and psychophysical data analysis
OCT volume scans from both machines were imported into a dedicated 3D image analysis software (Visage Imaging Amira version 5.3, FEI Visualization Sciences Group, Hillsboro, Oregon, USA) and segmentation of the EZ layer was performed manually, as described earlier.11 Briefly, a slab from the mid-line of the EZ, one pixel in thickness was selected, orthogonal topographic maps (‘en face’ images) of this slab was created in Amira and exported into Adobe Photoshop CS6 (Adobe Systems Incorporated, San Jose, CA, USA). EZ break boundary delineation and area measurements were performed in Adobe Photoshop. Lesion delineation was performed through an initial thresholding followed by a manual correction of lesion edges as necessary, based on a close inspection of corresponding OCT volume data in B-scans and reconstituted scans perpendicular to B-scans (along the Y axis), in FEI Amira. OCT volume data was used in its original form with no enhancements or normalisation of individual B-scans with lower signal to noise ratio, instead information from these was evaluated in conjunction with information from adjacent higher quality B-scans. Since technical factors (including directionality of the incident light) may affect the intensity of the signal, an attenuated but discernible EZ signal was interpreted in this context as still present, an EZ break was defined as an area where the EZ stratification was no longer discernible.
‘En face’ SD-OCT images and retinal sensitivity data were superimposed over infrared images of the fundus and adjusted to attain exact correspondence based mainly on vascular landmarks. Aggregate sensitivity loss was calculated as described earlier,3 briefly: the mean of retinal sensitivity values within the grid at test points outside the area of the EZ break was calculated and considered the background sensitivity. Aggregate loss was defined as the sum of deviations from the background sensitivity of values measured at test points within the area of the EZ break. The repeatability of the method has been assessed previously. 3
We also analyzed the correlation of the break area size with the number of test points below threshold values chosen arbitrarily, as presented in Table 1.
Table 1.
Correlation of break area and the number of test points below threshold.
| threshold | Spearman’s ρ | p | 95% CI | n | |
|---|---|---|---|---|---|
| <24dB | 0.807 | <0.0001 | 0.701 | 0.878 | 65 |
| <20dB | 0.843 | <0.0001 | 0.755 | 0.902 | 65 |
| <16dB | 0.825 | <0.0001 | 0.728 | 0.890 | 65 |
| <12dB | 0.725 | <0.0001 | 0.584 | 0.823 | 65 |
| <8dB | 0.718 | <0.0001 | 0.574 | 0.818 | 65 |
| <4dB | 0.671 | <0.0001 | 0.510 | 0.786 | 65 |
Misalignment of the test grid relative to the background image was assessed at one location each in four quadrants centered on the fovea, based on vascular landmarks, considering the baseline data the reference. For each characteristic, one measurement was performed.
Statistical Methods
Spearman’s rank correlation coefficient was calculated to assess dependence between EZ break size and function loss. A p-value of <0.05 was accepted as statistically significant. All analyses were conducted using commercially available statistical software (MedCalc for Windows, version 12.5, MedCalc Software Ltd, Ostend, Belgium).
RESULTS
Fourteen eyes of 7 participants (age range 48–67 years) were examined at baseline and at 4 subsequent annual follow-up visits (a total of 70 observations, i.e. eyes × visits). At baseline, mean±SD age was 55.4±6.7 years (range 48–67 years), 2 were males, 5 females, 5 were Caucasian, one Asian, and one identified as ‘other race’, mean±SD BCVA was 76.9±9.4 letters (range 59–89 letters).
Fixation stability at baseline, expressed as the mean base contour ellipse area12 was BCEA@63%=0.61 deg2, (SD=0.49, Min=0.1, Max=1.60 deg2), mean BCEA@95%=5.54 deg2, (SD=4.44, Min=0.90, Max=14.40 deg2). Fixation stability measured as suggested by Fujii et al.13 was: mean P1 (1° radius)=91.4% (SD=8.5%, Min=74%, Max=100%), mean P2 (2° radius)=98.5% (SD=2.2% Min=93% Max=100%). The preferred retinal locus (PRL) was in all eyes within the fovea. At the last available visit (as detailed below) mean BCEA@63%=2.52 deg2, (SD=3.99, Min=0.1, Max=15.2 deg2), mean BCEA@95%=12.11 deg2, (SD=13.31, Min=1.20, Max=45.70 deg2). Fixation stability measured as suggested by Fujii et al.13 was: mean P1 (1° radius)=79.6% (SD=23.2%, Min=19%, Max=100%), mean P2 (2° radius)=94.4% (SD=11.0% Min=58% Max=100%). The PRL was within the fovea in 11 eyes (in 3 of these eyes on the edge of the fovea) and extrafoveal in 3 eyes.
One participant missed their 48 month visit, for one participant the MAIA test was not performed in the right eye at the 48 months’ visit; in these cases the data point from the visit preceding the missed visit was used instead (36 and 42 months respectively). An EZ break was apparent in 12 eyes at baseline and in all eyes at 48 months. One MAIA test report demonstrated diffusely low sensitivity values all over the grid, which was not seen in either the preceding or the subsequent MAIA test of the same eye; this was considered attributable to causes other than MacTel and not included in the analysis. Thus the total number of observations analyzed was 65.
Thresholding was performed using all MAIA data accepted as correct. In 5 eyes of 3 participants, the EZ break was present but consistently too small to be detected by functional testing. Aggregate loss calculation requires an overlap of break area and at least one MAIA test point. In some cases the EZ break was so small that it was located entirely within the space between MAIA test points. In cases where aggregate loss was not possible to calculate, we performed a simple surrogate analysis instead, calculating the grid mean and the difference between this mean and the lowest test point value within the grid. In observations with no EZ break, this ‘range’ was on average 5.5dB. In eyes with a present but too small EZ break - after elimination of outliers - it was 6.0dB. These values are close to the point-wise repeatability of the method reported in adults (5.7dB).14 Outliers included two individual test points directly over large blood vessels and two further test points near the disc margin with no visible retinal lesion but zero sensitivity data measured at one and normal sensitivity data at all other preceding and following visits.
The correlation of the break area size with the number of test points below threshold values chosen arbitrarily are presented in Table 1. The correlation was best for points with greater than 16dB loss with R2 greater than 0.80.
Overall, the break area size showed a good correlation also with aggregate sensitivity loss (ρ=0.834, P<0.0001, 95%CI 0.716 to 0.906, n=45). This correlation was stronger if the obvious misalignments of the MAIA grid were corrected (Spearman’s ρ=0.860, P<0.0001, 95%CI 0.758 to 0.921, n=45).
Misalignment of the test grid relative to baseline was >0.42° (the retinal image size of a Goldmann III stimulus) in at least one quadrant and along at least one axis (x/y) in a total of 13/48 visits of 7/14 eyes. The misalignment maximum was 2.1°, the median 0.07°, the mode 0.03°. In both eyes of one participant, this was clearly due to a misplacement of the full test grid; in other cases it was due to inconsistent geometry of the SLO reference image. The follow-up function was used in all tests reported.
DISCUSSION
Overall, we found an excellent correlation between EZ break area size and retinal function loss in MacTel, confirming the conclusion from a previous study.3, 4 Manual correction of obvious misalignments of the MAIA test grid relative to the reference fundus image improved the correlation only moderately. Considering the labor-intensity and the potential introduction of grader bias, this is not ideal for calculating aggregate loss. However, follow-up of individual test points can be considered valid only where it is confirmed that the specific test points were aligned with identical retinal locations at all visits over time. In case of geometric aberrations of the reference fundus image at follow-up visits, the manufacturer recommends use of the fundus image acquired at baseline for reference (personal communication).
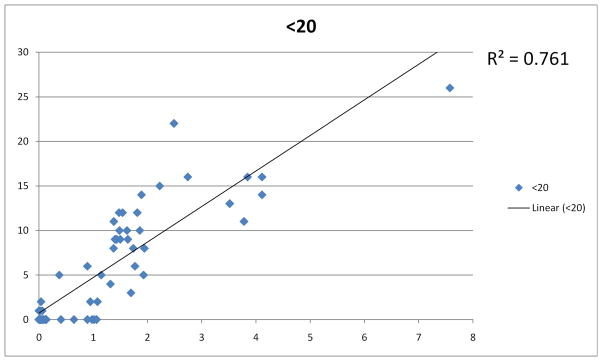
There was also a good correlation between the EZ break area and the size of the scotoma as reflected by the number of test points with specific lower sensitivity values, even when the topographic distribution of values was ignored (peak at <20dB, see Figure 2). This is attributable to the rather unique characteristics of the disease, in that the retinal sensitivity loss is highly localized and profound, limited to the area of outer retinal atrophy. Simple thresholding offers a quick alternative to calculating aggregate sensitivity loss, with mostly preserved specificity. It may also eliminate errors emanating from distortions or misalignment of the reference fundus image relative to the test grid data. One limitation of the method is that progression in retinal sensitivity is only detected in full increments of the threshold value. The threshold value should thus be selected in view of this as well as the repeatability of mesopic microperimetry14, 15 and the level of correlation between structural and functional measures (as reported in Table 1). 16dB seems a reasonable compromise.
Figure 2.
Correlation of the number of subthreshold test points with EZ break area.
Correlation of the the number of test points (y axis) with values below the selected threshold (in this graph 20dB) with EZ (IS/OS) break area size (x-axis). For easier reference, area sizes are presented here in approximate mm2 rather than pixels. It needs to be noted however, that since the refractive properties of the eyes tested were not recorded, metric conversions from measured pixels values are subject to variability in axial length and refractive power of respective eye.
In our sample OCT mapping of the EZ appears to demonstrate structural change even before mesopic microperimetry can detect a focal loss of retinal sensitivity typical of MacTel. This is partly due to the inherent limitation of the microperimetry technique used (mesopic range, stimulus size) as well as the limitations of the specific test grid. A denser grid would improve the detection of smaller lesions, however the potential increase in the number of test points is limited by the increase in overall test duration and ultimately patient fatigue.
In future therapeutic studies, if mesopic function is to be used as a measure of progression as described in our study, cases with early disease and small defects in the outer retina might be excluded. No such limitation exists if OCT were used to determine outcome. Although future psychophysical testing methods may provide improved sensitivity, it is evident from our data that OCT measurement of structural change gives an accurate assessment of visual loss as shown in two studies, and is currently more sensitive in measurement of defects in early cases, which appears ideal for assessment of benefit in therapeutic trials.
Summary statement.
We found a good correlation between Ellipsoid Zone (EZ) break area and function loss. ‘En face’ OCT mapping of the EZ may demonstrate structural change before mesopic microperimetry can detect a focal loss of retinal sensitivity. Thresholding offers a quick alternative to calculating aggregate sensitivity loss.
Acknowledgments
Financial support: The authors wish to thank the Lowy Medical Research Institute (LMRI) for providing support for funding this study. The LMRI also participated in the approval of the manuscript. The sponsor had no role in the study design, collection, analyses and interpretation of the data, or the writing of the manuscript. Tunde Peto is also funded by the NIHR BMRC at Moorfields Eye Hospital and UCL Institute of Ophthalmology.
This study is part of a non-randomized, uncontrolled phase one clinical trial, registered with ClinicalTrials.gov (NCT01327911).
Participating Centers and Investigators in the MacTel CNTF Safety Study:
Jules Stein Eye Institute, UCLA, Los Angeles, CA (USA): Steven Schwartz (PI), Jean-Pierre Hubschman, Allan Kreiger, Tara McCannel, Gad Heilweil, Joshua Udonetok, David Cupp, Hamid Hosseini, Ryan Wong, Sijit Itty, Logan Hitchcock, Rosaleen Ostrick, Nina Zelcer, Jennie Kageyama, Melissa Chun, Bita Shokouh, Nilo Davila, Robert Almanzor, Lauren Fash, Rachelle Bruce, Steven Nusinowitz, Jackie Sanguinet.
Retina Associates of Cleveland, Cleveland, OH (USA): Lawrence Singerman (PI), Michael Novak, Hernando Zegarra, Z. Nicholas Zakov, Scott Pendergast, David Miller, Joseph Coney, Jerome Schartman, George Michael Carson, Jennifer Peck, Michelle James, Susan Rath, Diane Weiss, Dianne Himmelman, Larraine Stone, Trina Nitzche, Kimberly DuBois, Stephanie Pelton, Vivian Tanner.
Reading Center (National Institute of Health Research Biomedical Research Centre for Ophthalmology at Moorfields Eye Hospital, National Health Service Foundation Trust and University College London, Institute of Ophthalmology, London, United Kingdom): Graham Holder, Tunde Peto, Ferenc B. Sallo, Irene Leung.
Coordinating Center (The EMMES Corporation), Rockville, MD (USA): Traci Clemons, Maria Figueroa, Dan Rosenberg.
Data Safety and Monitoring Committee (DSMC): David Musch (Chair), Mark Blumenkranz, Harry Flynn.
Joint Steering Committee: Alan Bird, Emily Chew, Martin Friedlander, Quentin Oswald, Rhett Schiffman, Richard Small.
Lowy Medical Research Institute: Jennifer Trombley.
Participating Principal Investigators and Centers in the MacTel Study
Jose-Alain Sahel, MD, PhD, Center Hopitalier National D’Optalmologie des Quinze-Vingts, Paris, France;
Robyn Guymer, MD, Center for Eye Research, East Melbourne, Australia;
Gisele Soubrane, MD, PhD, FEBO, Clinique Ophtalmologie de Creteil, Creteil, France;
Alain Gaudric, MD, Hopital Lariboisiere, Paris, France;
Jean-Pierre Hubschman, MD, Steven Schwartz, MD, Jules Stein Eye Institute, UCLA, Los Angeles, CA (USA);
Ian Constable, MD, Lions Eye Institute, Nedlands, Australia;
Michael Cooney, MD, MBA, Manhattan Eye, Ear, & Throat Hospital, New York, NY (USA);
Catherine Egan, MD, Moorfields Eye Hospital, London, England (UK);
Lawrence Singerman, MD, Retina Associates of Cleveland, Cleveland, OH (USA);
Mark C Gillies, MD, PhD, Save Sight Institute, Sydney, Australia;
Martin Friedlander, MD, PhD, Scripps Research Institute, La Jolla, CA (USA);
Daniel Pauleikhoff, Prof. Dr., St. Franziskus Hospital, Muenster, Germany;
Joseph Moisseiev, MD, Goldschleger Eye Institute, Tel Hashomer, Israel;
Richard Rosen, MD, New York Eye and Ear Infirmary, New York, NY (USA);
Robert Murphy, MD, Retina Group of Washington, Fairfax, VA (USA);
Frank Holz, MD, University of Bonn, Bonn Germany;
Grant Comer, MD, University of Michigan, Kellogg Eye Center, Ann Arbor, MI (USA);
Barbara Blodi, MD, University of Wisconsin, Madison, WI (USA);
Diana Do, MD, Wilmer Eye Institute, Baltimore, MD (USA);
Alexander Brucker, MD, Scheie Eye Institute, Philadelphia, PA (USA);
Raja Narayanan, MD, LV Prasad Eye Institute, Hyderabad, India;
Sebastian Wolf, MD, PhD, University of Bern, Bern, Switzerland;
Philip Rosenfeld, MD, PhD, Bascom Palmer, Miami, FL (USA).
Paul S Bernstein, MD, PhD, Moran Eye Center, University of Utah, UT (USA)
Joan W Miller, MD, Massachusetts Eye and Ear Infirmary, Harvard Medical School, Boston, MA (USA)
Lawrence Yannuzzi, MD, Vitreous, Retina, Macula Consultants of New York, NY, (USA)
Jacque Duncan, MD, University of California, San Francisco, CA, (USA)
Mina Chung, MD, University of Rochester Medical Center, Rochester, NY, (USA)
Jiong Yan, MD, Emory University, Atlanta, GA, (USA)
David Weinberg, MD, Medical College of Wisconsin, Milwaukee, WI, (USA)
Clasien Oomen, MD, Radboud University Medical Center, Nijmegen, Holland
Footnotes
Conflict of interest: FB Sallo: none, I Leung: none, TE Clemons: none, T Peto: none, EY Chew: none, D Pauleikhoff: none, AC Bird: none.
References
- 1.Charbel Issa P, Gillies MC, Chew EY, et al. Macular telangiectasia type 2. Prog Retin Eye Res. 2013;34:49–77. doi: 10.1016/j.preteyeres.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charbel Issa P, Kupitz EH, Heeren TF, Holz FG. Treatment for Macular Telangiectasia Type 2. Developments in ophthalmology. 2016;55:189–195. doi: 10.1159/000431263. [DOI] [PubMed] [Google Scholar]
- 3.Sallo FB, Peto T, Egan C, et al. The IS/OS junction layer in the natural history of type 2 idiopathic macular telangiectasia. Invest Ophthalmol Vis Sci. 2012;53:7889–7895. doi: 10.1167/iovs.12-10765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sallo FB, Peto T, Egan C, et al. “En face” OCT imaging of the IS/OS junction line in type 2 idiopathic macular telangiectasia. Invest Ophthalmol Vis Sci. 2012;53:6145–6152. doi: 10.1167/iovs.12-10580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maheshwary AS, Oster SF, Yuson RM, Cheng L, Mojana F, Freeman WR. The association between percent disruption of the photoreceptor inner segment-outer segment junction and visual acuity in diabetic macular edema. Am J Ophthalmol. 2010;150:63–67. e61. doi: 10.1016/j.ajo.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rangaswamy NV, Patel HM, Locke KG, Hood DC, Birch DG. A comparison of visual field sensitivity to photoreceptor thickness in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2010;51:4213–4219. doi: 10.1167/iovs.09-4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landa G, Su E, Garcia PM, Seiple WH, Rosen RB. Inner segment-outer segment junctional layer integrity and corresponding retinal sensitivity in dry and wet forms of age-related macular degeneration. Retina. 2011;31:364–370. doi: 10.1097/IAE.0b013e3181e91132. [DOI] [PubMed] [Google Scholar]
- 8.Eandi CM, Chung JE, Cardillo-Piccolino F, Spaide RF. Optical coherence tomography in unilateral resolved central serous chorioretinopathy. Retina. 2005;25:417–421. doi: 10.1097/00006982-200506000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Spaide RF, Koizumi H, Freund KB. Photoreceptor outer segment abnormalities as a cause of blind spot enlargement in acute zonal occult outer retinopathy-complex diseases. Am J Ophthalmol. 2008;146:111–120. doi: 10.1016/j.ajo.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 10.Chew EY, Clemons TE, Peto T, et al. Ciliary neurotrophic factor for macular telangiectasia type 2: results from a phase 1 safety trial. Am J Ophthalmol. 2015;159:659–666. e651. doi: 10.1016/j.ajo.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sallo FB, Peto T, Egan C, et al. “En face” OCT Imaging of the IS/OS Junction Line in Type 2 Idiopathic Macular Telangiectasia. Invest Ophthalmol Vis Sci. 2012;53:6145–6152. doi: 10.1167/iovs.12-10580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crossland MD, Dunbar HM, Rubin GS. Fixation stability measurement using the MP1 microperimeter. Retina. 2009;29:651–656. doi: 10.1097/IAE.0b013e318196bd65. [DOI] [PubMed] [Google Scholar]
- 13.Fujii GY, de Juan E, Jr, Sunness J, Humayun MS, Pieramici DJ, Chang TS. Patient selection for macular translocation surgery using the scanning laser ophthalmoscope. Ophthalmology. 2002;109:1737–1744. doi: 10.1016/s0161-6420(02)01120-x. [DOI] [PubMed] [Google Scholar]
- 14.Jones PR, Yasoubi N, Nardini M, Rubin GS. Feasibility of Macular Integrity Assessment (MAIA) Microperimetry in Children: Sensitivity, Reliability, and Fixation Stability in Healthy Observers. Invest Ophthalmol Vis Sci. 2016;57:6349–6359. doi: 10.1167/iovs.16-20037. [DOI] [PubMed] [Google Scholar]
- 15.Chen FK, Patel PJ, Xing W, et al. Test-retest variability of microperimetry using the Nidek MP1 in patients with macular disease. Invest Ophthalmol Vis Sci. 2009;50:3464–3472. doi: 10.1167/iovs.08-2926. [DOI] [PubMed] [Google Scholar]