Abstract
The insecticidal Vip3 proteins from Bacillus thuringiensis (Bt), along with the classical Bt Cry proteins, are currently used in Bt-crops to control insect pests, since they do not share the same mode of action. Here we characterized the response of Spodoptera exigua larvae after Vip3 challenge. The expression profile of 47 genes was analyzed in larvae challenged with three concentrations of Vip3Ca. Results showed that the up-regulated genes were mainly involved in immune response, whereas the down-regulated genes were mainly involved in the digestion process. Other mechanisms of cellular response to the damage such as apoptosis were analyzed. For this analysis, sections from the midguts were examined by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining. The nuclei of the midgut epithelial cells were stained at the highest concentration of the Vip3Ca protein and at lower concentrations of Vip3Aa in agreement with the different potency of the two proteins. In addition, apoptosis was also examined by the analysis of the expression of five caspase genes. The present study shows that exposure of S. exigua larvae to sublethal concentrations of Vip3 proteins activates different insect response pathways which trigger the regulation of some genes, APN shedding, and apoptotic cell death.
Introduction
The concern over the excessive use of chemical insecticides has increased in recent years, due to the ecological impact, as well as to the selection for resistance in field insect populations1. Among the different biological alternatives for pest control, one of the most popular is the use of bioinsecticies based on Bacillus thuringiensis 2. This bacterium produces a wide range of insecticidal proteins which are active against a number of agricultural pest species3. Insecticidal crystal proteins (Cry proteins) are being used to control insect pests in formulated sprays since 19384 and, since 1996, they have been expressed in transgenic crops to protect them from insect attack5. More recently, a novel class of insecticidal proteins (Vip3 proteins) have also been introduced in transgenic crops to complement the toxic action of the Cry proteins, as well as to reduce the risk of insect resistance development in the field6 (ISAAA GM Approval Database. http://www.isaaa.org/gmapprovaldatabase).
Cry proteins are highly specific against their target insects and are generally recognized as pore-forming toxins (PFTs)7. The mode of action of these proteins has been extensively studied for more than 20 years, especially for Cry1A proteins8,9. In general, it is accepted that these toxins are solubilized in the insect gut and then activated by the action of digestive enzymes. The active forms bind to specific receptors in the brush border of epithelial midgut cells and induce pores in the membrane which eventually lead to septicemia and insect death9. Nowadays, different models have been proposed to explain how these proteins exert their cytotoxicity, however some aspects remain unclear8,10. Much less is known about the mode of action of Vip3 proteins. The Vip3Aa protein was the first member of the family of Vip3 proteins being described11 and most studies dealing with the mode of action of Vip3 proteins have been performed with this protein12. The available information supports that these proteins act by forming pores in the midgut epithelial cells13,14. As for Cry proteins, Vip3 proteins are synthesized as protoxins which are processed by proteases in the larva midgut rendering the active form, which then binds to specific receptors in the brush border of epithelial midgut cells15–18. After binding, the Vip3 protein induces disruption of the midgut epithelial cells18–21 by its pore forming activity13,14. One very interesting feature of the Vip3 proteins mode of action is that they do not share membrane binding sites with Cry proteins, a property which does not only complement the spectrum of activity of Cry proteins, but also decreases the chances of cross-resistance13,14,17,22,23.
The insect gut is not only an organ of digestion but also constitutes the first physical barrier that protects the host against penetration of both pathogenic and commensal microorganisms24,25. In mammals, some studies have identified the mechanisms that regulate gut mucosal immunity, revealing a central role of innate immunity in these processes26. Despite the fact only few studies have been conducted in insects, the available data suggest that midgut epithelial tissue of the insects challenged with either pathogenic or nonpathogenic bacteria27–29 is able to trigger an immune response to reduce the cellular and tissue damage. Therefore, the insecticidal activity of Cry and Vip proteins might be affected by the host defense response, since they exert their toxic action in the midgut of the target insects.
Transcriptomic and proteomic approaches are being helpful to elucidate which mechanisms are involved in the host responses to B. thuringiensis proteins in non-model insects of agricultural importance30–37. In general, these analyses point out that, after protein exposure, the insects usually increase their immune function in addition to reduce their digestive activity38,39. Some reports have shown that the expression of some components of the mitogen-activated protein kinase (MAPK) cascade were up-regulated in response to Cry proteins in Coleoptera, Diptera, and Lepidoptera40–43. Additionally, other gene families have been described to be transcriptionally regulated in response to Cry and Vip3 proteins in Spodoptera exigua larvae30,31,44–46, such as the response to pathogens (REPAT) genes, though their specific role in host response still remains unclear38,45.
Apoptosis has also been described as a mechanism of cellular response after the exposure of cultured cells with different PFTs47–50. In vitro experiments with midgut primary culture cells from Heliothis virescens showed that Cry toxins induced apoptosis in epithelial cells51. More recently, similar results were observed when CF1 or Sf9 cultured cells were exposed to Cry1A or Vip3Aa proteins, respectively52,53. In vivo assays also showed that apoptosis could be observed in insect midgut epithelial cells when Cry proteins were administered to both Culex pipiens and Bombyx mori larvae54,55.
The activation of different mechanisms of response in S. exigua larvae after the exposure to different B. thuringiensis proteins (Cry and Vip3 proteins) has been reported30,31,38,56. These mechanisms of response might contribute to reduce the damage produced by B. thuringiensis proteins to the insect. To date, the genes identified in the S. exigua response to B. thuringiensis proteins have been found to be involved in many different aspects of the insect biology such as: metabolism, immune-response, detoxification, and epithelial renewal, among others38. The present work extends previous studies carried out on S. exigua larvae with the analysis of the expression profile of 47 genes after Vip3Ca exposure. These selected genes were previously found differentially expressed after the exposure to other B. thuringiensis proteins and other pathogens31,56,57. Thus, the data obtained in the present study could help understand whether the S. exigua response to Vip3Ca is specific or, on the contrary, it is a conserved feature independent of the toxic agent to which the insects are exposed to. Furthermore, the damage produced by Vip3Ca and Vip3Aa proteins has been characterized by measuring the in vivo response of the midgut epithelial cells (APN shedding and apoptosis).
Results
Growth inhibition assays
Susceptibility of S. exigua 4th instar larvae against the Vip3Ca protein was determined in terms of the effect on larval growth inhibition. The results showed a dose-response relationship, with an EC50 of 38.4 ng/cm2 (Fig. 1). Therefore, despite the fact that Vip3Ca has negligible activity, in terms of mortality, against S. exigua 58, it has a clear effect on larval growth inhibition.
Figure 1.
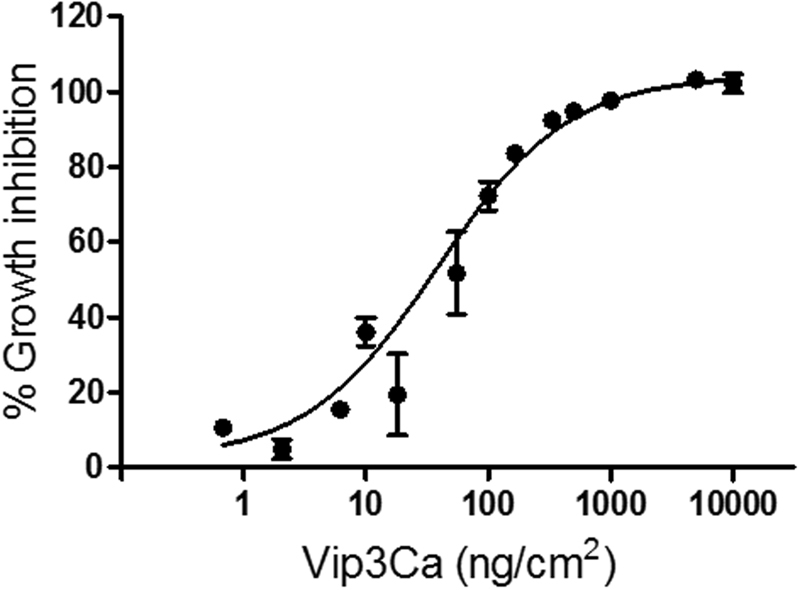
Growth inhibition dose-response curve of newly molted 4th instar S. exigua larvae after exposure to Vip3Ca for 24 h. Each value represents the mean of at least three independent experiments (±SEM).
Analysis of the gene expression upon Vip3Ca challenge
The expression profile of 47 genes was analyzed in S. exigua midguts by qRT-PCR, after 24 h exposure to Vip3Ca. To be able to compare gene expression results with those previously reported after Vip3Aa challenge31,56, the concentration of Vip3Ca to cause 99% growth inhibition on 4th instar S. exigua larvae (1000 ng/cm2) was used. In order to test whether a lower or higher concentration of Vip3Ca could alter the regulatory effect on these genes, their expression levels were also determined after a challenge with either 100 or 10000 ng/cm2 of Vip3Ca.
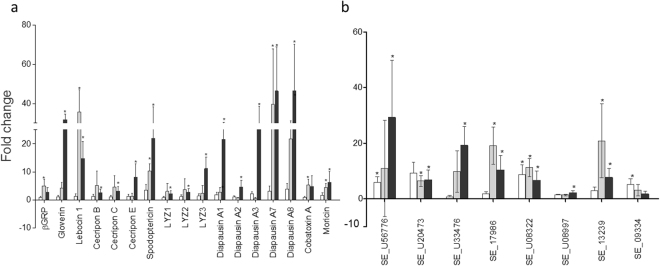
The results showed that the number of genes whose expression was altered was different depending on the exposure condition (concentration of Vip3Ca) (Fig. 2). At the lowest concentration of Vip3Ca (100 ng/cm2), only 5 genes (about 11%) were regulated (Fig. 2 and Supplementary Table S1), whereas at the other two concentrations tested, 1000 and 10000 ng/cm2, the number of regulated genes was 20 (about 43%) and 29 (around 62%), respectively. The distribution of up- and down-regulated genes, according to the concentration of Vip3Ca used in each treatment, is summarized in Supplementary Fig. S1. Almost all the regulated genes at 100 and 1000 ng/cm2 were also found regulated at 10000 ng/cm2 of Vip3Ca, suggesting that the response can be dose-dependent. The ratio of up-regulated and down-regulated genes at 100 and 1000 ng/cm2 was similar (3 up- and 2 down-regulated, and 12 up- and 8 down-regulated, respectively). However, at 10000 ng/cm2, the ratio of up-regulated genes and down-regulated genes was higher (22 vs. 7, respectively). The levels of transcriptional activation ranged from 2.8-fold to 46.5-fold, whereas the levels of transcriptional repression ranged from 2.5-fold to 653-fold (Figs 3, 4 and Supplementary Table S1).
Figure 2.
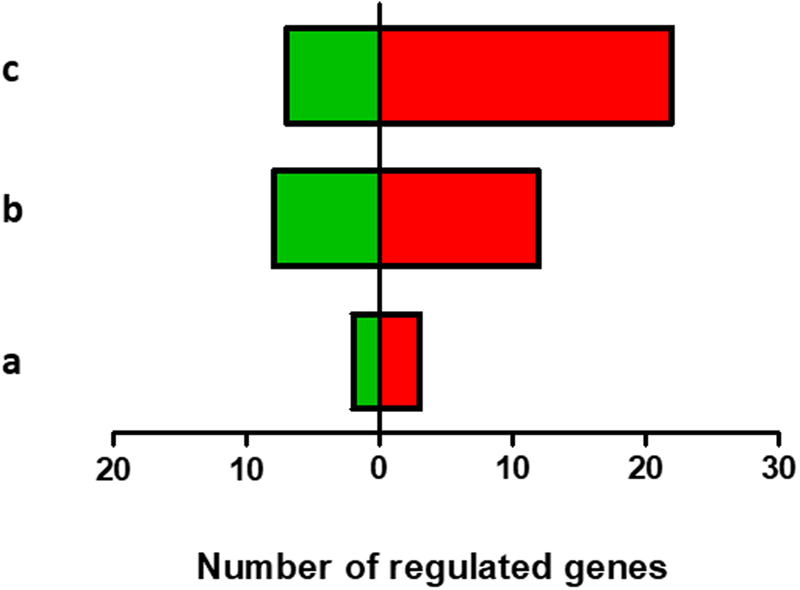
Overview of the regulated genes in S. exigua midgut after Vip3Ca challenged at 100 (a), 1000 (b), and 10000 (c) ng/cm2. A total of 47 genes were analyzed. The number of up- and down-regulated genes are indicated in red and green bars, respectively.
Figure 3.
Analysis of the genes whose expression is up-regulated after 24 h exposure at three concentrations of Vip3Ca. (a) Immuno-related genes (b) Non immuno-related genes. White, grey, and black bars represent the gene expression of each transcript after Vip3Ca challenged at 100, 1000, and 10000 ng/cm2, respectively. Abbreviations: βGRP: beta-1,3-glucan recognition protein. The expression of each gene in the gut of Vip3Ca exposed larvae was compared to its control in the gut of control larvae (exposed to WK6). Fold-changes were determined by using the REST MCS software. Each bar represents the mean of three independent experiments (±SD). Significant differences were indicated by an asterisk.
Figure 4.
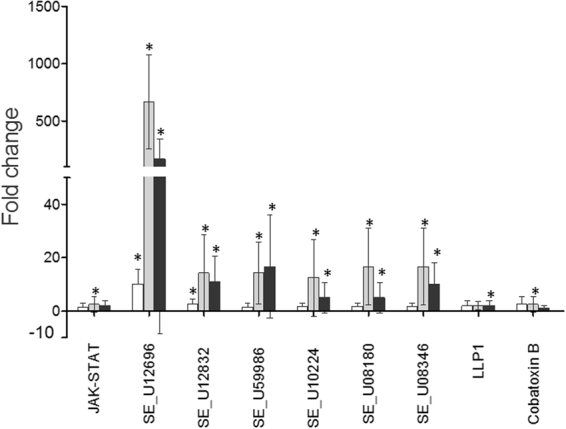
Analysis of the genes whose expression is down-regulated after exposure 24 h at three concentrations of Vip3Ca. White, grey, and black bars represent the gene expression of each transcript after Vip3Ca challenged at 100, 1000, and 10000 ng/cm2, respectively. The expression of each gene in the gut of Vip3Ca exposed larvae was compared to its control in the gut of control larvae (exposed to WK6). Fold-changes were determined by using the REST MCS software. Each bar represents the mean of three independent experiments (±SD). Significant differences were indicated by an asterisk.
At 100 ng/cm2, the up-regulated genes included a member of the REPAT family (REPAT2), a gene coding for a juvenile hormone binding protein (SE_U56776), and one for for a pancreatic lipase (SE_U08322). Of the two down-regulated genes, one had homology with a lipase gene, whereas the other two had no homology to known genes from public sequence databases (SE_U12696).
At the concentration of Vip3Ca to provoke 99% growth inhibition on 4th instar S. exigua larvae (1000 ng/cm2) most of the up-regulated genes (7 out of 12) encoded antimicrobial peptides (AMPs), being the most up-regulated ones Diapausin A6 and Lebocin 1. The gene coding for the beta-1,3-glucan recognition protein (β-GRP) was only slightly overexpressed. Additionally, the other up-regulated genes showed homology with genes that encoded juvenile hormone binding proteins (SE_U17986 and SE_U13239) and for pancreatic lipases (SE_U20473 and SE_U08322). Around 88% of the down-regulated genes showed homology to known genes from public sequence databases, including those coding for lipases, proteases and chitin deacetylases (Supplementary Table S1). The biggest repression (653-fold) was observed for one gene which no homology to known gene (SE_U12696). The Cobatoxin B gene, as well as the immune signaling pathway JAK-STAT gene, were also found slightly down-regulated.
At the highest concentration used (10000 ng/cm2) the scenario observed was similar to the one described for the larvae exposed to 1000 ng/cm2. Fifteen of the twenty-two up-regulated genes encoded AMPs (Fig. 3). These 15 AMPs belong to different groups such as the cysteine-rich peptides (Diapausin A1-A3, A6 and A7, and Spodoptericin), glycine- and proline-rich peptides (Gloverin), amphipatic peptides (Cecropin B, C, and E, and Moricin) and lysozymes (LYZ1, 2, and 3). Other up-regulated genes showed homology with genes that encoded juvenile hormone binding proteins (SE_56776, SE_U17986, and SE_U13239), for a Diapausin precursor (SE_U33476), for pancreatic lipases (SE_U20473 and SE_U08322), and for a gene with no homology to any known gene (SE_U08997). The down-regulated genes showed homology with those coding for lipases, proteases and chitin deacetylases (Supplementary Table S1). Again, the biggest repression (167-fold) was observed for the gene SE_U12696. The gene encoding for the AMP LLP1 was also found slightly down-regulated.
Determination of epithelial damage by APN shedding assays
Shedding of membrane-bound APN to the lumen, as a marker for epithelial damage, was measured after 24 h exposure to Vip3 proteins at the concentration of 100 ng/cm2 of Vip3Aa and at 3 different concentrations of Vip3Ca (100, 1000, and 10000 ng/cm2). The results showed that, in larvae exposed to Vip3Ca, the APN activity in the luminal fluid increased ca. 3-fold and 6-fold at 1000 or 10000 ng/cm2, respectively, though no significant change was observed at 100 ng/cm2 (Fig. 5). A correlation between growth inhibition and the amount of APN released into the luminal fluid produced by Vip3Ca protein was observed (Supplementary Fig. S2).
Figure 5.
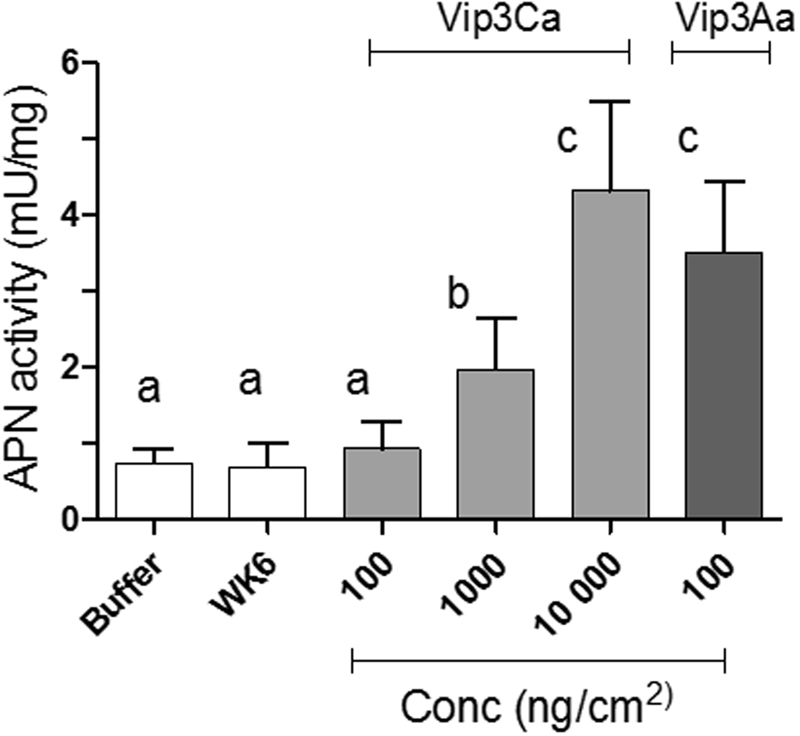
Activity of the APN protein in the midgut lumen of larvae exposed for 24 h to WK6 proteins (control larvae), Vip3Ca (three concentrations) or Vip3Aa (one concentration). Means were compared by one-way ANOVA, followed by Dunnett’s comparison test (P < 0.001). Significant differences between control larvae and treated larvae were indicated by different letters.
The luminal APN activity of those larvae exposed to 100 ng/cm2 of Vip3Aa increased ca. 5-fold. Interestingly, the APN activity in the lumen fluid of larvae exposed to a concentration that produces a 99% of growth inhibition for each respective Vip3 protein (100 ng/cm2 for Vip3Aa and 1000 ng/cm2 for Vip3Ca) was significantly higher for Vip3Aa.
Determination of epithelial cell damage by the TUNEL assay
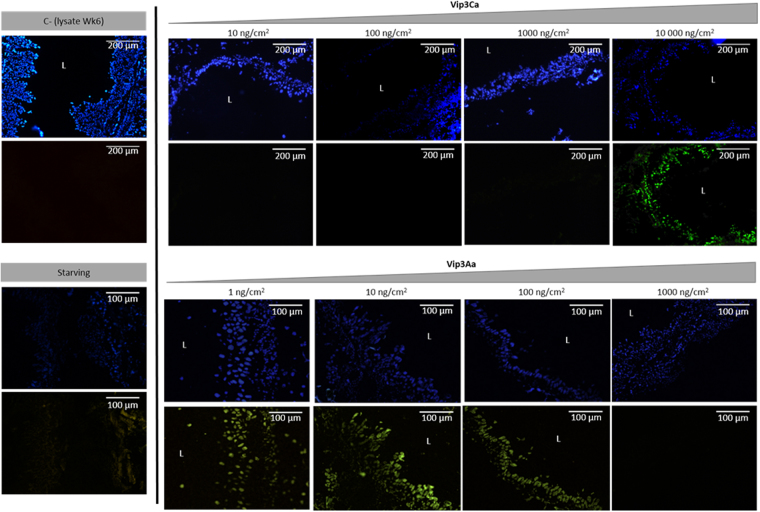
To test whether exposure to sublethal concentrations of Vip3Ca could trigger a signaling pathway leading to the death of the epithelial cells by apoptosis, midguts of larvae exposed for 24 h to Vip3Ca were sectioned and stained with the DeadEndTM Fluorimentric TUNEL system. Midguts of larvae exposed to Vip3Aa were used for comparative purposes. The results showed that in control larvae (exposed to WK6 proteins) and in starving larvae, no TUNEL-positive cells were observed. No TUNEL-positive cells were observed either after Vip3Ca treatment at the two lowest concentrations used (10 and 100 ng/cm2). However, a few TUNEL-positive cells were observed in the gut of larvae intoxicated with 1000 ng/cm2 of Vip3Ca and, at the highest concentration used (10000 ng/cm2), almost all the cells were TUNEL-positive (Fig. 6). In the case of Vip3Aa challenge, TUNEL-positive cells were observed in the gut of the larvae intoxicated with the three lowest concentrations (1, 10 and 100 ng/cm2) and no TUNEL-positive cells were found at the highest concentration (1000 ng/cm2) (Fig. 6).
Figure 6.
Effect of sublethal concentrations of Vip3Ca and Vip3Aa proteins to Spodoptera exigua midgut epithelial cells. L4 newly molted larvae were intoxicated with Vip3 proteins for 24 h and then midgut tissue sections were prepared and stained with TUNEL (green signal) and DAPI (blue signal). As controls, larvae fed with the empty vector (WK6) and 24 h starving larvae were used. Magnification was 100× for the Vip3Ca protein and 200× for the Vip3Aa protein. L, gut lumen.
Analysis of the expression levels of apoptosis-related genes in S. exigua larval midgut challenged with Vip3Ca
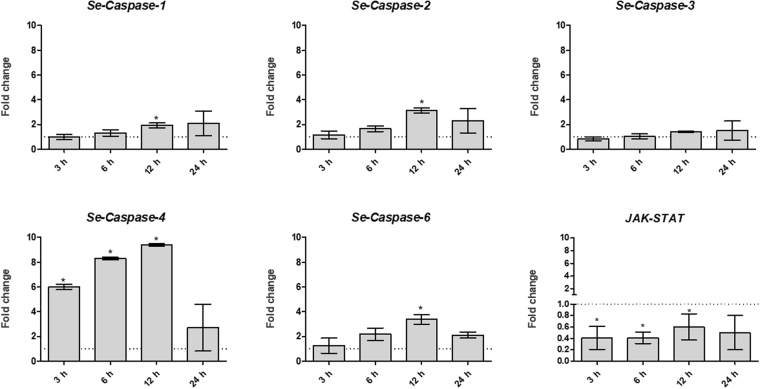
The induction of the apoptotic process was analyzed in the midgut epithelial cells, at the molecular level, by measuring the change in transcription levels of 5 caspase genes by qRT-PCR. The transcription levels of the gene encoding a component of the JAK-STAT pathway was also analyzed since this pathway has been related with the renewal of the midgut tissue59,60. To analyze the time course of apoptosis, the expression level of the six genes were monitored at 4 different time points: 3 h, 6 h, 12 h, and 24 h after Vip3Ca challenge at 10000 ng/cm2. After either 3 h or 6 h of exposure, only Se-Caspase-4 was found up-regulated, whereas after 12 h of exposure four of the 5 caspase genes studied were found up-regulated (Fig. 7). In contrast, none of the 5 caspase genes studied were found regulated after 24 h exposure. These data suggest that the main transcriptional induction of apoptotic machinery occurs after 12 h exposure. The gene coding a component of the JAK-STAT pathway was found down-regulated after 3 h, 6 h, and 12 h of Vip3Ca challenge (Fig. 7).
Figure 7.
Analysis of the transcriptional induction of apoptotic related genes at 10000 ng/cm2 of Vip3Ca for 3 h, 6 h, 12 h, and 24 h. The expression of each gene in the gut of larvae exposed to Vip3Ca was compared to its control in the gut of control larvae (exposed to WK6 empty vector). Fold-changes were determined by using the REST MCS software. Each bar represents the mean of three independent experiments (±SD). Significant differences were indicated by an asterisk.
Fitness cost analysis
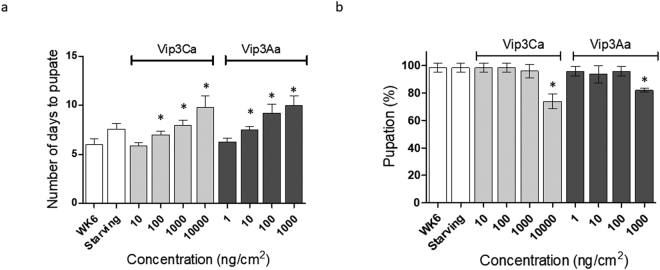
Since the exposure to Vip3 proteins affected the transcriptional pattern of S. exigua larvae and caused epithelial and cellular damage, we wanted to determine whether the exposure also had an associated fitness cost. The results showed significant differences in the time to pupation for those larvae exposed to higher concentrations of either Vip3Aa (10, 100, and 10000 ng/cm2) or Vip3Ca (100, 1000, and 10000 ng/cm2), as compared to control larvae and with larvae exposed to lower concentrations (Fig. 8a). Interestingly, the time to pupation was also found significantly longer in starving larvae. The percentage of pupation was found significantly lower in those larvae exposed to the highest concentration of either Vip3Aa or Vip3Ca (Fig. 8b).
Figure 8.
Analysis of the associated fitness cost on S. exigua 4th instar larvae after Vip3Ca or Vip3Aa exposure. Two parameters were measured: (a) number of days to pupation, and (b) % of pupation. Means were compared by one-way ANOVA, followed by Dunnett’s comparison test (P < 0.001). Significant differences between control larvae and treated larvae were indicated by an asterisk.
Discussion
A better knowledge of the different mechanisms of insect response after the exposure to different B. thuringiensis proteins will broaden our understanding of how larvae response might help to reduce the damage produced by these insecticidal proteins. In the present study, the expression profile of 47 selected genes was analyzed in S. exigua larvae challenged with three concentrations of Vip3Ca. These genes were previously found differentially expressed after the exposure to Vip3Aa, Cry1Ca, and other pathogens31,56,57. In order to compare our results with those obtained previously by Bel et al.31 and by Crava et al.56, a concentration of Vip3Ca which caused a 99% of growth inhibition was used as a starting point. Thus, the data obtained could help understand whether the S. exigua response to Vip3Ca is specific or, on the contrary, it is a conserved feature independent of the toxic agent to which they are exposed to.
The gene expression results showed that at the lowest concentration of Vip3Ca tested (100 ng/cm2) the number of regulated genes was lower (about 11%) than when larvae were exposed to 1000 or 10000 ng/cm2 of Vip3Ca (around 43% and 62%, respectively). Moreover, almost all the regulated genes at 100 and 1000 ng/cm2 were also found regulated at 10000 ng/cm2 of Vip3Ca, suggesting that the host response is dose-dependent. In general, our results agree with previous gene expression studies which showed the up-regulation of genes involved in immune system and hormone modulation (e.g. JH binding protein), and the down-regulation of genes involved in the digestion process (e.g. serine proteases) and peritrophic membrane permeability (e.g. chitin deacetylases), upon exposure to different B. thuringiensis proteins28,30,31,35,36,61–65. The highest value of down-regulation (653-fold) found in this study was obtained for one gene with unknown function when larvae were exposed to 1000 ng/cm2 of Vip3Ca. Similar results were observed by Bel et al.31 when S. exigua larvae were exposed to Vip3Aa. This gene encodes a putative protein called REVIP because it was detected in Response-to-Vip intoxication.
In our study we have included different S. exigua immune-related genes which were classified in three categories by Pascual et al.66: (a) pathogen recognition, (b) immune signaling pathways and melanization process, and (c) antimicrobial effectors (Supplementary Table S1). A general upregulation of the immune-related genes was observed after Vip3Ca exposure at 1000 and 10000 ng/cm2, though it is worth to note that the genes belonging to the antimicrobial effectors were more clearly regulated after Vip3Ca exposure than the other genes. These antimicrobial effectors are produced to act as barriers against the progress of bacterial infections67. Here we analyzed the transcriptional response of 24 genes coding for 21 AMPs and 3 lysozymes. These genes were described in a detailed study performed by Crava et al.56 where most of the 24 genes were found up-regulated after Vip3Aa exposure at a concentration to provoke 99% growth inhibition on 4th instar S. exigua larvae. Our results showed that none of these genes were found regulated in response to the lowest concentration of Vip3Ca used. Conversely, when S. exigua larvae were exposed at 1000 or 10000 ng/cm2 of Vip3Ca, the number of regulated AMPs genes was 8 and 15, respectively. Our data agree with previous data obtained from Crava et al.56 in that the response of the S. exigua larvae might be associated to the level of cell damage produced by the different B. thuringiensis proteins used and not to the mode of action of these proteins.
Genes from the three main immune signaling pathways (Toll, Imd, and JAK-STAT) were also represented in our study. The results showed that neither the Toll-like receptor gene nor the Imd gene were found to be regulated at any of the 3 concentrations tested. In contrast, the gene encoding a component of the JAK-STAT pathway was down-regulated after 24 h exposure to 1000 ng/cm2 of Vip3Ca. Additionally, the expression of this gene was also found down-regulated when larvae were exposed at 10000 ng/cm2 of Vip3Ca for lower times (3 h, 6 h, and 12 h). The JAK-STAT pathway has been shown to be involved in the activation of the midgut renewal by the proliferation and differentiation of the stem cells59,60,68. Thus, we speculate that the negative regulation of this pathway might be affecting the renewal of the midgut cells that have been damaged by the action of the Vip3Ca protein. This negative regulation might be a host defense response for gut healing processes after entomopathogen exposure39. The lack of regulation of the Toll and Imd immune signaling pathways was also described when S. exigua larvae were exposed to Vip3Aa protein31. It is worth to note that the activation of most of the immune signaling pathways relies mainly on post-translational modifications (e.g. phosphorylation) and to a lesser extent on transcriptional regulations40,69.
The effect of Vip3Aa and Vip3Ca proteins on the midgut epithelial cells was measured by determining the APN activity into the luminal fluid. The results showed that both Vip3 proteins cause APN shedding into the luminal fluid in S. exigua larvae. The ability of some B. thuringiensis proteins and other pore forming toxins (PFTs) to cause shedding of cell surface proteins has already been reported70–72. In agreement with our results, Valaitis et al.70 found that exposure to Vip3Aa induced shedding of APN in Lymantria dispar larvae. However, they hypothesized that the APN shedding would not be involved in host defense to B. thuringiensis proteins, since the inhibition of the APN shedding by cyclic AMP did not affect their toxicity70. Here we observed a clear dose-response relationship between the growth inhibition produced by increasing concentrations of Vip3Ca and the APN activity in the lumen of the larvae. This result supports that the depletion of the APN is stronger when the damage produced to the epithelial cells is larger. Moreover, when comparing the APN shedding observed after exposure using a concentration of either Vip3Aa or Vip3Ca proteins which produces 99% growth inhibition (100 and 1000 ng/cm2, respectively), the results showed that the luminal APN activity is higher in those larvae exposed to Vip3Aa than in those exposed to Vip3Ca. This result suggests that the cell damage produced by the Vip3Aa protein might be larger than the one produced by the Vip3Ca protein. Moreover, this result is in agreement with the results obtained in the gene expression analysis, since the exposure to Vip3Aa seems to regulate the expression of more genes that the exposure to Vip3Ca, at the same dose, in S. exigua larvae.
The analysis of the S. exigua midguts exposed to sublethal concentrations of Vip3Aa and Vip3Ca proteins by TUNEL assays showed the presence of TUNEL-positive cells at different concentrations of each protein (Fig. 6). TUNEL-positive cells were clearly observed at the highest concentration (10000 ng/cm2) of the Vip3Ca protein. In contrast, TUNEL-positive cells were detected at the lowest and intermediate concentrations of Vip3Aa (1, 10, and 100 ng/cm2). No TUNEL-positive cells were observed at the highest concentration of Vip3Aa (1000 ng/cm2), probably due to the fact that, at this concentration, epithelial cells might be responding by other mechanisms and/or by other signal-transduction pathways to respond to such an attack73,74 or it might happen that most of the cell membrane was disrupted. The histopathological effects produced in larvae from Mamestra brassicae after exposure to either Vip3Aa or Vip3Ca has been described by Gomis-Cebolla et al. (2016)18. The results showed that feeding with both Vip3 proteins produced disruption of the midgut epithelium, though the damage caused by Vip3Aa protein was detected earlier, in agreement with its higher toxicity. Consistent with our data, Tanaka et al.55 reported the presence of TUNEL-positive midgut cells in B. mori larvae when treated with sublethal concentrations of Cry1Aa and few TUNEL-positive cells when larvae were exposed to lethal concentrations. On the basis of these observations, we can speculate that disruption of midgut cell membranes, by the pore formation activity, is a main event that occurs when the insect species are exposed to a high dose of an active B. thuringiensis protein. Nevertheless, other events, such as apoptosis, may happen when larvae are exposed to sublethal doses. Thus, apoptotic events might involve a host defense response to the damage produced by the toxic agent, leading to renewal of the epithelial layer39,73.
Although the mode of action of Vip3 proteins is still not completely resolved, it is commonly accepted that, similarly to Cry proteins, they bind to specific receptors and form pores in the brush border of the epithelial cells13,15,17,18. Additionally, some studies have reported that Cry proteins can activate different intracellular cascade pathways, leading to apoptotic cell death10,51,52,54,55. The ability to produce pores in their target cells and also to activate different intracellular cascade pathways has also been described for other toxins (e.g. aerolysin and alpha-toxin) produced by other bacteria75,76. Apoptosis is a special and highly regulated type of programmed cell death that can be induced by different factors. Apoptosis has a fundamental role in biological process such as: development, tissue homeostasis, DNA damage response, and immune response77. In mammalian cells, PFTs kill cells by two different mechanisms: (1) apoptosis, characterized by the activation of initiator caspases that trigger effector- caspases to cleave cellular substrates, and (2) inflammatory responses by the activation of inflammatory caspases78. Caspases (cysteine-dependent aspartate-specific proteases) are a family of evolutionary conserved proteins that have been described for playing a key role in apoptosis79. In addition to the TUNEL assays, apoptosis was monitored by the analysis of the expression levels of five caspase genes. The results showed that expression level of the Se-Caspase-4 was highly up-regulated after 3 h, 6 h, and 12 h. This gene has special sequence features and its function has not been assessed yet79. Nevertheless, as this gene is regulated after 3 h of exposure we can hypothesized that it might play a role in the pro-apoptotic proteolytic cascade as an initiator caspase. The expression levels of the initiator Se-Caspase-6 and the effector Se-Caspases-1 and -2 genes were found up-regulated after 12 h of Vip3Ca exposure. The expression levels of the gene coding Se-Caspase-3 was not regulated at the different times tested, indicating that maybe it would not be involved in the host response to Vip3 exposure.
Here we show for the first time that Vip3Aa and Vip3Ca trigger apoptosis in S. exigua midgut epithelial cells in vivo. However, further research will be required to define the apoptotic signal transduction pathway induced by both Vip3 proteins in S. exigua larvae. It was recently reported that Vip3Aa can induce apoptosis in Sf9 cultured cells and that this is mediated by the mitochondrial and caspase dependent pathways53. Portugal et al.52 showed that Cry1A proteins induced apoptosis in CF1 cultured cells. Interestingly, the authors observed that the pore formation activity of the Cry1A proteins is necessary to induce apoptosis. It is possible that, with Vip3 proteins, pore formation is also a necessary step for cells to enter the apoptotic pathway.
In summary, the results from the present study show that exposure of S. exigua larvae to sublethal concentrations of Vip3Ca (a protein with low activity against this insect) activates different insect response pathways which trigger the regulation of some genes (such as the antimicrobial effectors, caspases), induces APN shedding, and triggers other signals that lead to apoptotic cell death. Understanding the host response process to the B. thuringiensis proteins currently used in insect control will help to shed light on insect defensive mechanisms to toxic agents.
Materials and Methods
Insects
Larvae from the S. exigua FRA colony were kindly supplied by M. López-Ferber, INRA (St Christol les Alés, France). The insects were reared on artificial diet at 25 °C with a relative humidity of 70% RH and a photoperiod of 16 h/8 h (light/dark). The FRA colony had been maintained for more than 10 years without exposure to pathogens80.
Expression and purification of Vip3Aa and Vip3Ca proteins
The Vip3Aa protein (NCBI accession No. AAW65132) was overexpressed in recombinant Escherichia coli BL21 carrying the vip3Aa16 gene. The Vip3Ca protein (NCBI accession No. AEE98106) was overexpressed in E. coli WK6 carrying the expression vector pMaab 10 (kindly supplied by Bayer CropScience N.V., Ghent, Belgium). Protein expression and lysis of Vip3Aa and Vip3Ca was carried out following the conditions described by Abdelkefi-Mesrati et al.81 and Gomis-Cebolla et al. (2016)18, respectively.
Vip3Aa and Vip3Ca proteins in the cell lysates were further purified by isoelectric point precipitation (Ipp) as described by Chakroun et al.82. The pH of the lysates was lowered with acetic acid to pH 5.5 for Vip3Aa, and to pH 5.9 for Vip3Ca. After centrifugation, the precipitated proteins were resuspended in solubilization buffer (20 mM Tris-HCl, 150 mM NaCl, pH 9) and dialyzed against the same buffer overnight. The amount of Vip3 proteins was quantified by densitometry after SDS-PAGE electrophoresis using the TotalLab 1D v13.01 software. As a control, the empty E. coli WK6 strain was cultured and processed in the same way as described above for the Vip3Ca producing strain.
Growth inhibition assays
Susceptibility of newly molted 4th instar S. exigua larvae to Vip3Ca was determined by growth inhibition assays using surface contamination method as previously described80. Sixteen larvae were individually exposed to each concentration for 24 h. As a control, 16 larvae were exposed to a protein preparation from the empty E. coli WK6 strain. For this, the pellet obtained after the Ipp of the empty E. coli WK6 was prepared in the same way as with the Vip3Ca protein. Five independent biological replicates were performed. The percentage of growth inhibition (% GI) was calculated as described Herrero et al.83, using the formula: % GI = [1 − (RGt/RGc)] × 100, where RGt and RGc represent the relative growth of larvae after exposure to either Vip3Ca or the empty E. coli WK6 strain preparation (control larvae), respectively. Relative growth was calculated as RG = [(W1 − W0)/W0], where W0 and W1 are the initial and final weight of the larva, respectively. The effective concentrations which produced a reduction in the larval growth of 50% and 99% were calculated using the GraphPad Prism v. 5.1 (GraphPad Inc., La Jolla, CA, US) using a non-linear logistic model (Hill equation).
Effects on larva development after Vip3 protein exposure
The effect of Vip3Ca and Vip3Aa proteins on two parameters of fitness was determined: time to pupation and percentage of pupation. Sixteen 4th instar larvae were individually exposed to 10, 100, 1000, and 10000 ng/cm2 of Vip3Ca or to 1, 10, 100, and 1000 ng/cm2 of Vip3Aa. As a control, 16 larvae were exposed to a protein preparation from the empty E. coli WK6 strain. After 24 h exposure, larvae were transferred to non-intoxicated diet to complete their development. Three independent biological replicates were performed. The developmental time from 4th instar larvae to pupa and the number of pupae was daily recorded.
Gene expression analysis
The expression profile of 47S. exigua genes, which were previously described as responding to a Vip3Aa challenge or other pathogens31,56,57 (Supplementary Table S1), was analyzed under two different conditions: WK6 exposed (used as a control) and Vip3Ca exposed. For this purpose, sixteen newly molted 4th instar larvae were individually exposed to 100, 1000, and 10000 ng/cm2 of Vip3Ca for 24 h. For the analysis of the expression levels of apoptosis-related genes, five S. exigua caspase genes were selected79 (Supplementary Table S2). Six newly molted 4th instar larvae were individually exposed to 10000 ng/cm2 of Vip3Ca for 3 h, 6 h, 12 h and 24 h. Then, larvae were dissected and midguts pooled and stored at −80 °C until used. Three independent biological replicates were performed.
RNA was purified from larval midguts using the RNAzol RT reagent (Sigma-Aldrich, St. Louis, MO, US) according to the manufacturer’s instructions. Purified RNA (0.5 μg) was then treated with DNase I (Invitrogen, Carlsbad, CA, US) and subsequently reverse-transcribed to cDNA using oligo-(dT) primers and the SuperScript II Reverse Transcriptase (Invitrogen), following the manufacturer’s protocol. Quantitative real-time PCR (qRT-PCR) was carried out using EvaGreen® (Biotis, Vilnius, Lithuania) following standard protocols and measured in an ABI Prism 7700 Sequence Detection System (Applied Biosystems). Each reaction was performed in a final volume of 20 μl, which contained 4 μl of cDNA (300 ng). Forward and reverse primers were added to a final concentration of 300 pM. Primers used in this study are described in Supplementary Tables S1 and S2. The 47 selected genes analyzed in this study, including the house-keeping gene, were previously designed and their efficiency tested by other authors31,56,57. Specific primers for the five S. exigua caspase genes used in this study were designed on the basis of their sequences from the NCBI database (HQ328953, HQ328958, HQ328966, HQ328975, and HQ328993) using Primer Express Software from Applied Biosystems (Carlsbad, CA, US) (Supplementary Table S2). Prior to quantifying differential expression among different treatments, the efficiency of each pair of primers was evaluated by performing 3-fold dilution series experiments. The specific amplification of transcripts was verified by dissociation curve analysis. The Rest MCS software (version 2) was used to obtain the expression ratios (-fold change)84.
Measurement of aminopeptidase activity in the midgut lumen
The APN activity in the midgut lumen from S. exigua larva treated with either Vip3Ca or Vip3Aa proteins was measured as a marker to evaluate the damage produced by the proteins after 24 h exposure. At least three independent replicates were performed for each condition. In each replicate, sixteen 4th instar newly molted larvae were exposed to four different concentrations of Vip3Ca (10 100, 1000, and 10000 ng/cm2) and to one single concentration of Vip3Aa (100 ng/cm2). The concentration of Vip3Aa used produced a 99% of growth inhibition according to a previous study31. As a control, WK6 proteins prepared as for the Vip3Ca sample were used. The contents from at least 10 midguts (for each condition) were obtained and transferred into 100 μl of 50 mM Tris-HCl, pH 7.5, 1 mM PMSF. Then, midgut contents were vortexed for 30 s, centrifuged at 21 000 x g for 5 min at 4 °C, and the supernatant was used for activity assays. Protein concentration was determined by Bradford85. APN activity was determined using 4 mM L-leucyl-p-nitroanilide in 50 mM Tris-HCl (pH 7.5) buffer as substrate. The released of p-nitroanilide was monitored at 405 nm for 2 min using a Tecan Infinite 200 plate reader (Switzerland). An extinction coefficient of 9.9 mM−1 cm−1 for p-nitroanilide was used.
Sectioning of insect tissues and TUNEL staining
Fragmentation of the DNA in the midgut epithelial cells from S. exigua larva exposed to either Vip3Ca or Vip3Aa proteins was measured as a marker of apoptosis. DNA fragmentation was measured using the principle of TUNEL (TdT-mediated dUTP Nick-End Labeling), which consists on the catalytical incorporation of a labelled dUTP (2′-deoxyuridine 5′-triphosphate) at the 3′- hydroxyl (-OH) group of the DNA end using a terminal deoxynucleotidyl transferase. For TUNEL staining assays, 4th instar newly molted larvae were treated for 24 h under four different conditions: WK6 exposed (used as a control), Vip3Ca exposed, Vip3Aa exposed, and starving (larvae kept without food for 24 h). In each assay, 16 larvae were exposed to four different concentrations of Vip3Ca (10, 100, 1000, and 10000 ng/cm2) or Vip3Aa (1, 10, 100, and 1000 ng/cm2). Three independent biological replicates for each treatment were performed. After 24 h exposure, only larvae actively eating (as determined by observing the food bites) were selected, flash frozen and kept at −80 °C until used. Sections of 10 μm were prepared by the microscopy facilities at the Universitat de València using the cryostat microtome Leica CM 1510 S. Slides with the tissue sections were stored at −20 °C until used. Tissue sections were treated with the DeadEndTM Fluorimentric TUNEL system (Promega) following the manufacturer’s instructions. Nuclear DNA was stained with DAPI (4′,6-diamidino-2-phenylindole) as described by Chazotte86. Coverslips were mounted using mounting medium from Sigma. Tissue sections were then examined using Leica DMI2500 microscope equipped with a digital color camera (Leica DFC300 FX). Tissue sections were stained with hematoxylin and eosin as was described elsewhere87 to check the quality of the midgut sections (Supplementary Fig. S3).
Electronic supplementary material
Acknowledgements
Research was supported by the Spanish Ministry of Science and Innovation (Ref. AGL2015-70584-C2-1-R), by grants of the Generalitat Valenciana (GVPROMETEOII-2015-001 and GVISIC2013-004) and by European FEDER funds. J.G.C was recipient of a PhD grant from the Spanish Ministry of Economy and Competitivity (grant ref. BES-2013-065848). We thank Rosa María González-Martínez and Óscar Marín for their help with insect rearing.
Author Contributions
P.H.M., J.F., and B.E., contributed to the design of the study. P.H.M., and J.G.C., performed the experiments. P.H.M., J.F., J.G.C., and B.E., analyzed the data. P.H.M., and J.F., wrote the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-16406-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chattopadhyay P, Banerjee G, Mukherjee S. Recent trends of modern bacterial insecticides for pest control practice in integrated crop management system. 3 Biotech. 2017;7:60. doi: 10.1007/s13205-017-0717-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lacey LA, et al. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015;132:1–41. doi: 10.1016/j.jip.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 3.van Frankenhuyzen K. Cross-order and cross-phylum activity of Bacillus thuringiensis pesticidal proteins. J. Invertebr. Pathol. 2013;114:76–85. doi: 10.1016/j.jip.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim MA, Griko N, Junker M, Bulla LA. Bacillus thuringiensis: a genomics and proteomics perspective. Bioeng. Bugs. 2010;1:31–50. doi: 10.4161/bbug.1.1.10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shelton AM, Zhao JZ, Roush RT. Economic, ecological, food safety, and social consequences of the deployment of Bt transgenic plants. Annu. Rev. Entomol. 2002;47:845–881. doi: 10.1146/annurev.ento.47.091201.145309. [DOI] [PubMed] [Google Scholar]
- 6.Burkness EC, Dively G, Patton T, Morey AC, Hutchison WD. Novel Vip3A Bacillus thuringiensis (Bt) maize approaches high-dose efficacy against Helicoverpa zea (Lepidoptera: Noctuidae) under field conditions: Implications for resistance management. GM Crops. 2010;1:337–343. doi: 10.4161/gmcr.1.5.14765. [DOI] [PubMed] [Google Scholar]
- 7.Schnepf E, et al. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 1998;62:775–806. doi: 10.1128/mmbr.62.3.775-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pardo-Lopez L, Soberón M, Bravo A. Bacillus thuringiensis insecticidal three-domain Cry toxins: mode of action, insect resistance and consequences for crop protection. FEMS Microbiol. Rev. 2013;37:3–22. doi: 10.1111/j.1574-6976.2012.00341.x. [DOI] [PubMed] [Google Scholar]
- 9.Adang MJ, Crickmore N, Jurat-Fuentes JL. Diversity of Bacillus thuringiensis crystal toxins and mechanism of action. Adv. in Insect Phys. 2014;47:39–87. doi: 10.1016/B978-0-12-800197-4.00002-6. [DOI] [Google Scholar]
- 10.Zhang X, Candas M, Griko NB, Taussig R, Bulla LA. A mechanism of cell death involving an adenylyl cyclase/PKA signaling pathway is induced by the Cry1Ab toxin of Bacillus thuringiensis. Proc. Nal. Acad. Sci.USA. 2006;103:9897–9902. doi: 10.1073/pnas.0604017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estruch JJ, et al. Vip3A, a novel Bacillus thuringiensis vegetative insecticidal protein with a wide spectrum of activities against lepidopteran insects. Proc. Natl. Acad. Sci. USA. 1996;93:5389–5394. doi: 10.1073/pnas.93.11.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakroun M, Banyuls N, Bel Y, Escriche B, Ferré J. Bacterial vegetative insecticidal proteins (Vip) from entomopathogenic bacteria. Microbiol. Mol. Biol. Rev. 2016;80:329–350. doi: 10.1128/MMBR.00060-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee MK, Walters FS, Hart H, Palekar N, Chen JS. The mode of action of the Bacillus thuringiensis vegetative insecticidal protein Vip3A differs from that of Cry1Ab delta-endotoxin. Appl. Environ. Microbiol. 2003;69:4648–4657. doi: 10.1128/AEM.69.8.4648-4657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu JG, Yang AZ, Shen XH, Hua BG, Shi GL. Specific binding of activated Vip3Aa10 to Helicoverpa armigera brush border membrane vesicles results in pore formation. J. Invertebr. Pathol. 2011;108:92–97. doi: 10.1016/j.jip.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Sena JAD, Hernández-Rodríguez CS, Ferré J. Interaction of Bacillus thuringiensis Cry1 and Vip3A proteins with Spodoptera frugiperda midgut binding sites. Appl. Environ. Microbiol. 2009;75:2236–2237. doi: 10.1128/AEM.02342-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caccia S, Chakroun M, Vinokurov K, Ferré J. Proteolytic processing of Bacillus thuringiensis Vip3A proteins by two Spodoptera species. J. Insect Physiol. 2014;67:76–84. doi: 10.1016/j.jinsphys.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Chakroun M, Ferré J. In vivo and in vitro binding of Vip3Aa to Spodoptera frugiperda midgut and characterization of binding sites by (125)I radiolabeling. Appl. Environ. Microbiol. 2014;80:6258–6265. doi: 10.1128/AEM.01521-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomis-Cebolla J, et al. Proteolytic processing and in vivo binding of the Bacillus thuringiensis Vip3Ca insecticidal protein. J. Invertebr. Pathol. 2017;142:60–67. doi: 10.1016/j.jip.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Abdelkefi-Mesrati L, et al. Study of the Bacillus thuringiensis Vip3Aa16 histopathological effects and determination of its putative binding proteins in the midgut of Spodoptera littoralis. J. Invertebr. Pathol. 2011;106:250–254. doi: 10.1016/j.jip.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Abdelkefi-Mesrati L, et al. Investigation of the steps involved in the difference of susceptibility of Ephestia kuehniella and Spodoptera littoralis to the Bacillus thuringiensis Vip3Aa16 toxin. J. Inverebr. Pathol. 2011;107:198–201. doi: 10.1016/j.jip.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Yu CG, Mullins MA, Warren GW, Koziel MG, Estruch JJ. The Bacillus thuringiensis vegetative insecticidal protein Vip3A lyses midgut epithelium cells of susceptible insects. Appl. Environl. Microbiol. 1997;63:532–536. doi: 10.1128/aem.63.2.532-536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pickett, B. R., Gulzar, A., Ferré, J. & Wright, D. J. Bacillus thuringiensis Vip3Aa toxin resistance in Heliothis virescens (Lepidoptera: Noctuidae). Appl. Environ. Microbiol. 83, 10.1128/aem.03506-16 (2017). [DOI] [PMC free article] [PubMed]
- 23.Kurtz RW. A review of Vip3A mode of action and effects on Bt Cry protein-resistant colonies of lepidopteran larvae. Southwest. Entomol. 2010;35:391–394. doi: 10.3958/059.035.0321. [DOI] [Google Scholar]
- 24.Sansonetti PJ. War and peace at mucosal surfaces. Nat. Rev. Immunol. 2004;4:953. doi: 10.1038/nri1499. [DOI] [PubMed] [Google Scholar]
- 25.Huang J-H, Jing X, Douglas AE. The multi-tasking gut epithelium of insects. Insect biochem. Mol. Biol. 2015;67:15–20. doi: 10.1016/j.ibmb.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahluwalia B, Magnusson MK, Öhman L. Mucosal immune system of the gastrointestinal tract: maintaining balance between the good and the bad. Scand. J. Gastroenterol. 2017;12:1–9. doi: 10.1080/00365521.2017.1349173. [DOI] [PubMed] [Google Scholar]
- 27.Freitak D, Wheat CW, Heckel DG, Vogel H. Immune system responses and fitness costs associated with consumption of bacteria in larvae of Trichoplusia ni. BMC Biol. 2007;5:56–56. doi: 10.1186/1741-7007-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell host Microbe. 2009;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Hernández‐Martínez P, et al. Increase in midgut microbiota load induces an apparent immune priming and increases tolerance to Bacillus thuringiensis. Environ. Microbiol. 2010;12:2730–2737. doi: 10.1111/j.1462-2920.2010.02241.x. [DOI] [PubMed] [Google Scholar]
- 30.Hernández-Martínez P, et al. Constitutive activation of the midgut response to Bacillus thuringiensis in Bt-resistant Spodoptera exigua. PLoS One. 2010;5:e12795. doi: 10.1371/journal.pone.0012795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bel Y, Jakubowska AK, Costa J, Herrero S, Escriche B. Comprehensive analysis of gene expression profiles of the beet armyworm Spodoptera exigua larvae challenged with Bacillus thuringiensis Vip3Aa toxin. PLoS One. 2013;8:e81927. doi: 10.1371/journal.pone.0081927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song F, et al. Transcriptional profiling analysis of Spodoptera litura larvae challenged with Vip3Aa toxin and possible involvement of trypsin in the toxin activation. Sci. Rep. 2016;6:23861. doi: 10.1038/srep23861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Contreras E, Rausell C, Real MD. Proteome response of Tribolium castaneum larvae to Bacillus thuringiensis toxin producing strains. PLoS One. 2013;8:e55330. doi: 10.1371/journal.pone.0055330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cancino-Rodezno A, et al. Comparative proteomic analysis of Aedes aegypti larval midgut after intoxication with Cry11Aa toxin from Bacillus thuringiensis. PLoS One. 2012;7:e37034. doi: 10.1371/journal.pone.0037034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oppert B, et al. Transcriptome profiling of the intoxication response of Tenebrio molitor larvae to Bacillus thuringiensis Cry3Aa protoxin. PLoS One. 2012;7:e34624. doi: 10.1371/journal.pone.0034624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sparks ME, Blackburn MB, Kuhar D, Gundersen-Rindal DE. Transcriptome of the Lymantria dispar (gypsy moth) larval midgut in response to infection by Bacillus thuringiensis. PloS one. 2013;8:e61190. doi: 10.1371/journal.pone.0061190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Canton PE, Cancino-Rodezno A, Gill SS, Soberón M, Bravo A. Transcriptional cellular responses in midgut tissue of Aedes aegypti larvae following intoxication with Cry11Aa toxin from Bacillus thuringiensis. BMC Genomics. 2015;16:1042. doi: 10.1186/s12864-015-2240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herrero S, Bel Y, Hernández-Martínez P, Ferré J. Susceptibility, mechanisms of response and resistance to Bacillus thuringiensis toxins in Spodoptera spp. Curr. Opin. Insect Sci. 2016;15:89–96. doi: 10.1016/j.cois.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Castagnola A, Jurat-Fuentes JL. Intestinal regeneration as an insect resistance mechanism to entomopathogenic bacteria. Curr. Opin. Insect Sci. 2016;15:104–110. doi: 10.1016/j.cois.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cancino-Rodezno A, et al. The mitogen-activated protein kinase p38 is involved in insect defense against Cry toxins from Bacillus thuringiensis. Insect Biochem. Mol. Biol. 2010;40:58–63. doi: 10.1016/j.ibmb.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huffman DL, et al. Mitogen-activated protein kinase pathways defend against bacterial pore-forming toxins. Proc. Nat. Acad. Sci. USA. 2004;101:10995–11000. doi: 10.1073/pnas.0404073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bishop AH, Robinson CV. Bacillus thuringiensis HD-1 Cry-: development of a safe, non-insecticidal simulant for Bacillus anthracis. J. Appl. Microbiol. 2014;117:654–662. doi: 10.1111/jam.12560. [DOI] [PubMed] [Google Scholar]
- 43.Qiu, L. et al. Knockdown of the MAPK p38 pathway increases the susceptibility of Chilo suppressalis larvae to Bacillus thuringiensis Cry1Ca toxin. Sci. Rep. 7 (2017). [DOI] [PMC free article] [PubMed]
- 44.Herrero S, et al. REPAT, a new family of proteins induced by bacterial toxins and baculovirus infection in Spodoptera exigua. Insect Biochem.Mol. Biol. 2007;37:1109–1118. doi: 10.1016/j.ibmb.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Navarro‐Cerrillo G, Ferré J, de Maagd R, Herrero S. Functional interactions between members of the REPAT family of insect pathogen‐induced proteins. Insect Mol. Biol. 2012;21:335–342. doi: 10.1111/j.1365-2583.2012.01139.x. [DOI] [PubMed] [Google Scholar]
- 46.Navarro-Cerrillo G, Hernández-Martínez P, Vogel H, Ferré J, Herrero S. A new gene superfamily of pathogen-response (repat) genes in Lepidoptera: Classification and expression analysis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2013;164:10–17. doi: 10.1016/j.cbpb.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Galindo CL, et al. Aeromonas hydrophila cytotoxic enterotoxin activates mitogen-activated protein kinases and induces apoptosis in murine macrophages and human intestinal epithelial cells. J. Biol. Chem. 2004;279:37597–37612. doi: 10.1074/jbc.M404641200. [DOI] [PubMed] [Google Scholar]
- 48.Chen Y, Zychlinsky A. Apoptosis induced by bacterial pathogens. Microb. Pathog. 1994;17:203–212. doi: 10.1006/mpat.1994.1066. [DOI] [PubMed] [Google Scholar]
- 49.Timmer AM, et al. Streptolysin O promotes group A Streptococcus immune evasion by accelerated macrophage apoptosis. J. Biol. Chem. 2009;284:862–871. doi: 10.1074/jbc.M804632200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Genestier A-L, et al. Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J. Clin. Invest. 2005;115:3117. doi: 10.1172/JCI22684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loeb MJ, et al. Apoptosis in cultured midgut cells from Heliothis virescens larvae exposed to various conditions. Arch. Insect Biochem Physiol. 2000;45:12–23. doi: 10.1002/1520-6327(200009)45:1<12::AID-ARCH2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 52.Portugal L, Muñóz-Garay C, de Castro DLM, Soberón M, Bravo A. Toxicity of Cry1A toxins from Bacillus thuringiensis to CF1 cells does not involve activation of adenylate cyclase/PKA signaling pathway. Insect Biochem. Mol Biol. 2017;80:21–31. doi: 10.1016/j.ibmb.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Jiang K, et al. Vip3Aa induces apoptosis in cultured Spodoptera frugiperda (Sf9) cells. Toxicon. 2016;120:49–56. doi: 10.1016/j.toxicon.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 54.Smouse, D. & Nishiura, J. A Bacillus thuringiensis δ-endotoxin induces programmed cell death in mosquito larvae. Cell Death Differ. 4 (1997). [DOI] [PubMed]
- 55.Tanaka S, Yoshizawa Y, Sato R. Response of midgut epithelial cells to Cry1Aa is toxin‐dependent and depends on the interplay between toxic action and the host apoptotic response. FEBS J. 2012;279:1071–1079. doi: 10.1111/j.1742-4658.2012.08499.x. [DOI] [PubMed] [Google Scholar]
- 56.Crava CM, Jakubowska AK, Escriche B, Herrero S, Bel Y. Dissimilar regulation of antimicrobial proteins in the midgut of Spodoptera exigua larvae challenged with Bacillus thuringiensis toxins or baculovirus. PLoS One. 2015;10:e0125991. doi: 10.1371/journal.pone.0125991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jakubowska AK, Vogel H, Herrero S. Increase in gut microbiota after immune suppression in baculovirus-infected larvae. PLoS Pathog. 2013;9:e1003379. doi: 10.1371/journal.ppat.1003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palma L, et al. Vip3C, a novel class of vegetative insecticidal proteins from Bacillus thuringiensis. App. Environ. Microbiol. 2012;78:7163–7165. doi: 10.1128/AEM.01360-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Callus BA, Mathey-Prevot B. SOCS36E, a novel Drosophila SOCS protein, suppresses JAK/STAT and EGF-R signalling in the imaginal wing disc. Oncogene. 2002;21:4812. doi: 10.1038/sj.onc.1205618. [DOI] [PubMed] [Google Scholar]
- 60.Jiang H, et al. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Munster M, et al. Altered gene expression in Choristoneura fumiferana and Manduca sexta in response to sublethal intoxication by Bacillus thuringiensis Cry1Ab toxin. Insect Mol. Biol. 2007;16:25–35. doi: 10.1111/j.1365-2583.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 62.Meunier L, Prefontaine G, Van Munster M, Brousseau R, Masson L. Transcriptional response of Choristoneura fumiferana to sublethal exposure of Cry1Ab protoxin from Bacillus thuringiensis. Insect Mol.Biol. 2006;15:475–483. doi: 10.1111/j.1365-2583.2006.00659.x. [DOI] [PubMed] [Google Scholar]
- 63.Lei Y, et al. Midgut transcriptome response to a Cry toxin in the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) Gene. 2014;533:180–187. doi: 10.1016/j.gene.2013.09.091. [DOI] [PubMed] [Google Scholar]
- 64.Yao J, Buschman LL, Lu N, Khajuria C, Zhu KY. Changes in gene expression in the larval gut of Ostrinia nubilalis in response to Bacillus thuringiensis Cry1Ab protoxin ingestion. Toxins (Basel) 2014;6:1274–1294. doi: 10.3390/toxins6041274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodríguez-Cabrera L, Trujillo-Bacallao D, Borrás-Hidalgo O, Wright DJ, Ayra-Pardo C. Molecular characterization of Spodoptera frugiperda–Bacillus thuringiensis Cry1Ca toxin interaction. Toxicon. 2008;51:681–692. doi: 10.1016/j.toxicon.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 66.Pascual L, et al. The transcriptome of Spodoptera exigua larvae exposed to different types of microbes. Insect Biochem.Mol. Biol. 2012;42:557–570. doi: 10.1016/j.ibmb.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 67.Yi H-Y, Chowdhury M, Huang Y-D, Yu X-Q. Insect antimicrobial peptides and their applications. App. Microbiol. Biotechnol. 2014;98:5807–5822. doi: 10.1007/s00253-014-5792-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karsten P, Häder S, Zeidler MP. Cloning and expression of Drosophila SOCS36E and its potential regulation by the JAK/STAT pathway. Mech. Dev. 2002;117:343–346. doi: 10.1016/S0925-4773(02)00216-2. [DOI] [PubMed] [Google Scholar]
- 69.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 70.Valaitis AP. Bacillus thuringiensis pore-forming toxins trigger massive shedding of GPI-anchored aminopeptidase N from gypsy moth midgut epithelial cells. Insect Biochem. Mol. Biol. 2008;38:611–618. doi: 10.1016/j.ibmb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 71.Walev I, Vollmer P, Palmer M, Bhakdi S, Rose-John S. Pore-forming toxins trigger shedding of receptors for interleukin 6 and lipopolysaccharide. Proc. Natl. Acad. Sci. USA. 1996;93:7882–7887. doi: 10.1073/pnas.93.15.7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Popova TG, et al. Acceleration of epithelial cell syndecan-1 shedding by anthrax hemolytic virulence factors. BMC Microbiol. 2006;6:8. doi: 10.1186/1471-2180-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aroian R, van der Goot FG. Pore-forming toxins and cellular non-immune defenses (CNIDs) Curr Opin Microbiol. 2007;10:57–61. doi: 10.1016/j.mib.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 74.Gonzalez MR, et al. Pore-forming toxins induce multiple cellular responses promoting survival. Cell Microbiol. 2011;13:1026–1043. doi: 10.1111/j.1462-5822.2011.01600.x. [DOI] [PubMed] [Google Scholar]
- 75.Nelson KL, Brodsky RA, Buckley JT. Channels formed by subnanomolar concentrations of the toxin aerolysin trigger apoptosis of T lymphomas. Cell. Microbiol. 1999;1:69–74. doi: 10.1046/j.1462-5822.1999.00009.x. [DOI] [PubMed] [Google Scholar]
- 76.Menzies BE, Kourteva I. Staphylococcus aureus α-toxin induces apoptosis in endothelial cells. FEMS Immunol.Med. Microbiol. 2000;29:39–45. doi: 10.1111/j.1574-695X.2000.tb01503.x. [DOI] [PubMed] [Google Scholar]
- 77.Elmore SA. A review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cancino-Rodezno A, Porta H, Soberón M, Bravo A. Defense and death responses to pore forming toxins. Biotechnol Genet Eng Rev. 2010;26:65–82. doi: 10.5661/bger-26-65. [DOI] [PubMed] [Google Scholar]
- 79.Courtiade J, Pauchet Y, Vogel H, Heckel DG. A comprehensive characterization of the caspase gene family in insects from the order Lepidoptera. BMC Genomics. 2011;12:357. doi: 10.1186/1471-2164-12-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hernández-Martínez P, Ferré J, Escriche B. Susceptibility of Spodoptera exigua to 9 toxins from Bacillus thuringiensis. J.Invert.Pathol. 2008;97:245–250. doi: 10.1016/j.jip.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 81.Abdelkefi-Mesrati L, Rouis S, Sellami S, Jaoua S. Prays oleae midgut putative receptor of Bacillus thuringiensis vegetative insecticidal protein Vip3LB differs from that of Cry1Ac toxin. Mol. Biotechnol. 2009;43:15–19. doi: 10.1007/s12033-009-9178-4. [DOI] [PubMed] [Google Scholar]
- 82.Chakroun M, et al. Susceptibility of Spodoptera frugiperda and S. exigua to Bacillus thuringiensis Vip3Aa insecticidal protein. J. Invertebr. Pathol. 2012;110:334–339. doi: 10.1016/j.jip.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 83.Herrero S, Borja M, Ferré J. Extent of variation of the Bacillus thuringiensis toxin reservoir: the case of the geranium bronze, Cacyreus marshalli Butler (Lepidoptera: Lycaenidae) Appl. Environ. Microbiol. 2002;68:4090–4094. doi: 10.1128/AEM.68.8.4090-4094.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36–e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 86.Chazotte, B. Labeling Nuclear DNA Using DAPI. Cold Spring Harb. Protoc. pdb.prot5556; 10.1101/pdb.prot5556 (2011). [DOI] [PubMed]
- 87.Lillie, R. D. Histopathologic technic and practical histochemistry. (Blakiston; New York, 1947).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.