Abstract
Background:
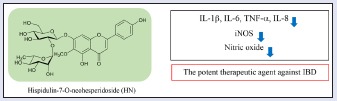
Inflammatory bowel disease (IBD) is characterized by chronic inflammation of the gastrointestinal tract and involves secretion of inflammatory mediators. The flavone diglycoside hispidulin-7-O-neohesperidoside (HN) isolated from the methanolic extract of aerial parts of Cirsium japonicum var. ussuriense, but its pharmacologic activities, with the exception of alleviation of alcohol toxicity, have not been investigated to date.
Objective:
The aim of the present study was to investigate the anti-inflammatory activities of HN for the treatment of chronic inflammatory illnesses, including IBD.
Materials and Methods:
In lipopolysaccharide (LPS)-induced RAW264.7 cells and HT-29 cells, the effects of HN on cell viability and nitric oxide (NO) production were examined via MTT assay and the Griess reaction, respectively. The expression levels of interleukin (IL)-1α, IL-8, and tumor necrosis factor (TNF)-α and inducible nitric oxide synthase (iNOS) protein levels were measured by enzyme-linked immunosorbent assay and Western blotting, respectively.
Results:
HN concentration-dependently inhibited NO production in LPS-induced RAW 264.7 cells. Treatment with HN considerably downregulated the levels of the pro-inflammatory cytokines, IL-1β and TNF-α and the iNOS protein level in LPS-induced RAW 264.7 cells. Furthermore, HN inhibited the production of the chemotactic cytokine, IL-8, in LPS-induced HT-29 cells.
Conclusion:
HN has potential as an anti-inflammatory agent to prevent and/or treat IBD.
SUMMARY
Hispidulin-7-O-neohesperidoside (HN) is flavone diglycoside isolated from the methanolic extract of aerial parts of Cirsium japonicum var. ussuriense.
HN concentration-dependently inhibited NO production and considerably downregulated the levels of the proinflammatory cytokines, IL-1β and TNF-α, and the iNOS protein level in LPS-induced RAW 264.7 cells.
HN inhibited the production of the chemotactic cytokine, IL-8, in LPS-induced HT-29 cells.
HN has potential as an anti-inflammatory agent to prevent and/or treat IBD.
Abbreviations used: IBD: Inflammatory bowel disease, HN: hispidulin-7-O-neohesperidoside, LPS: lipopolysaccharide, NO: nitric oxide, IL: interleukin, TNF: tumor necrosis factor, iNOS: inducible nitric oxide synthase, CD: Crohn's disease, UC: ulcerative colitis, RT: room temperature, DMEM: Dulbecco's modified Eagle's medium, FBS: fetal bovine serum, PBS: phosphate buffered saline, SDS: sodium dodecyl sulfate, PVDF: polyvinylidene difluoride, SD: standard deviation
Keywords: Hispidulin-7-O-neohesperidoside, Cirsium japonicum var. ussuriense, inflammation, inflammatory bowel disease
INTRODUCTION
Inflammation is a biologic response to stimuli such as pathogen infection and presents a major obstacle in maintaining a high quality of life.[1] Chronic or recurrent inflammatory reactions within the colon possibly due to viruses or bacteria may initiate or promote colon cancer development or progression.[2] Inflammatory bowel disease (IBD) is characterized by chronic inflammation of the gastrointestinal tract and is categorized as Crohn's disease (CD) and ulcerative colitis (UC).[3] For development of new therapeutic strategies against these diseases, a better understanding of the processes that initiate, modulate, and perpetuate intestinal mucosal inflammation is required.[4] Also, the chronic immune response in IBD may be regulated by increased secretion of pro-inflammatory cytokines due to an inappropriate response to initial stimulating events and/or impaired downregulation of cytokine secretion.[4]
Nitric oxide (NO), an important mediator of inflammation, is synthesized by nitric oxide synthase (NOS), which exists as three isoforms, endothelial, neuronal, and inducible NOS. Among them, inducible nitric oxide synthase (iNOS) plays a pivotal role in regulation of inflammation as well as ultimate repair of injury and carcinogenesis.[5] Lipopolysaccharides (LPSs), the major outer membrane constituent of Gram-negative bacteria, stimulate production of pro-inflammatory cytokines such as iNOS, interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor-alpha (TNF-α), as well as immune mediators such as NO, in various cell types, including RAW264.7 macrophages and HT-29 cells.[6,7,8]
Cirsium japonicum DC var. ussuriense (Rege) Kitam (Compositae) is a perennial herb indigenous to Korea. The aerial parts of this plant are used in oriental medicine as a diuretic, tonic, neuralgia stomachic, antiphlogistic, and detoxicant.[9] C. japonicum var. ussuriense is also known as “Korean thistle.” Pharmacologic studies on extract of C. japonicum var. ussuriense have reported hepatoprotective, anti-inflammatory, antitumor, antimutagenic, elimination, and antioxidant effects.[9,10,11,12] Polyacetylene and flavonoid have been reported as major constituents of C. japonicum var. ussuriense.[9,13] Hispidulin-7-O-neohesperidoside (HN) is a flavone diglycoside from C. japonicum var. ussuriense that alleviates alcohol toxicity by enhancing ethanol oxidation and inhibiting lipid peroxidation.[9,14] However, the pharmacologic activities of HN have not been investigated to date.
In this study, to assess the anti-inflammatory activities of HN, we used LPS-stimulated RAW 264.7 macrophages and HT-29 colonic epithelial cells. HN isolated from C. japonicum var. ussuriense inhibited the production of NO and pro-inflammatory mediators. Our findings indicate that HN can modulate inflammation, suggesting it to have potential for treatment of IBD.
MATERIALS AND METHODS
Plant and phytochemical materials
The aerial parts of C. japonicum var. ussuriense were collected from Sanchung, Kyungnam, Korea, on July 20, 1997. A voucher specimen (NM018) was deposited at the herbarium of Sunchon National University, Suncheon, Korea. Dried and pulverized C. japonicum var. ussuriense aerial parts were extracted with methanol using an ultrasonic apparatus at room temperature (RT). Methanolic extract of C. japonicum var. ussuriense aerial parts was concentrated in vacuo to give a crude extract, which was suspended in H2O and partitioned successively in CHCl3, n-butanol, and H2O. HN was isolated from the n-butanol fraction of C. japonicum var. ussuriense aerial parts.[14]
Cell culture
HT-29 human colonic epithelial cells and RAW264.7 macrophages were obtained from the Korean Cell Line Bank (Seoul, Korea). These cell lines were separately maintained as monolayers in Dulbecco's modified Eagle's medium (DMEM; Hyclone, Logan, UT, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Hyclone, Logan, UT, USA), containing 100 IU/mL penicillin and 100 μg/mL streptomycin (Hyclone, Logan, UT, USA) at 37°C in a humidified atmosphere of 95% air–5% CO2. Experiments were performed with RAW264.7 cells or HT-29 cells treated with HN at final concentrations of 25, 50, and 100 μM for 1 h and then 1 μg/mL lipopolysaccharide (LPS; Sigma-Aldrich, St. Louis, MO, USA) was applied for 24 h to induce inflammation.
Cell viability
Cells were seeded in 96-well plates at a density of 1 × 105 cells/well and incubated for 24 h. HT-29 and RAW264.7 cells were treated with vehicle or HN for 24 h. Cell viability was assessed by MTT assay, in which MTT (final concentration 0.5 mg/mL) was directly added to cultures, followed by incubation at 37°C for 2 h. Subsequently, the supernatant was aspirated and 100 μL of DMSO was added to dissolve the formazan. Following dissolution of the insoluble crystals, absorbance at 570 nm was measured using a microplate reader. Data are expressed as percentages of viable cells relative to that of the control cultures.
Estimation of NO production
RAW264.7 cells were treated with HN for 1 h and then exposed to 1 μg/mL LPS. After incubation for 24 h, the nitrite level in culture medium was measured to evaluate NO production using Griess reagent. The supernatant was harvested and then 100 μL aliquots were mixed with an equal volume of Griess reagent [equal volumes of 1% (w/v) sulfanilamide in 5% (v/v) phosphoric acid and 0.1% (w/v) naphtylethylene] in a 96-well plate and incubated at RT for 10 min. The absorbance at 550 nm was measured using a microplate reader (BioTek Instruments, Inc., Highland Park, Winooski, USA). Serum-free culture medium was used as the blank in all experiments. Nitrite was quantified by generating a standard curve using serial dilutions of NaNO2. Relative NP (%) was calculated as (NP of sample treated – NP of control)/(NP of LPS-treated-NP of control) × 100 (%).[15]
Pro-inflammatory cytokine expression
RAW264.7 and HT-29 cells were plated overnight in 96-well plates at a density of 1 × 105 and 2 × 104 cells/well, respectively. The cells were treated with samples for 1 h before exposure to 1 μg/mL LPS. After incubation for 24 h, the supernatants were collected and stored at –70°C until cytokine assay. IL-1β, TNF-α, and IL-6 levels in RAW264.7 cells were determined using mouse ELISA kits (Cusabio, Wuhan, China) and IL-8 level in HT-29 human colonic epithelial cells was determined using ELISA kits (BD OptEIATM, CA, USA).
Protein extraction and Western blot analysis
RAW264.7 cells were plated overnight in six-well plates at 1 × 105 cells/well. The medium was exchanged for fresh medium and cells were treated with samples for 1 h before exposure to 1 μg/mL LPS. After incubation for 24 h, cells were washed twice with phosphate-buffered saline (PBS). Cell lysates were prepared using ice-cold lysis buffer (50 mM Tris, pH 7.4, 1 mM EDTA, 0.1% Triton X-100, 1 mM PMSF, 25 μg/mL leupeptin, and 20 μg/mL pepstatin). Protein content was determined using Bio-Rad protein assay reagent according to the manufacturer's instructions. Equal amounts of protein (30 μg) were resolved in 10% sodium dodecyl sulfate (SDS)-polyacrylamide gels. Proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA), which were blocked with TBST (10 mM Tris [pH 7.4], 100 mM NaCl, and 0.5% Tween 20 containing 3% nonfat milk) for 1 h at RT. For immunodetection, membranes were incubated overnight with primary antibodies, including anti-iNOS (1:1000 dilution, Cell Signaling, BA, USA) in TBST containing 1% skim milk powder. After washing three times with TBST, immunoreactive bands were visualized using immunopure peroxidase-conjugated goat anti-mouse-IgG (1:1000, Santa Cruz, CA, USA). Finally, after rinsing in wash buffer, the membranes were visualized by enhanced chemiluminescence (ECL-kit, Thermo-Fisher Scientific, USA). The membranes were exposed to ECL detection reagents and quantified using a Bio imaging system (Micro Chemi 4.2 Chemilumineszenz-System, Israel).
Statistical analysis
Data are expressed as mean ± standard deviation (SD) of at least three independent experiments. One-way ANOVA was used for comparisons of multiple group means followed by t-test and statistical significance was considered at P less than 0.05.
RESULT AND DISCUSSION
IBD, such as UC and CD, is a chronic and relapsing inflammatory condition of the gastrointestinal tract. Although the etiology of IBD is unknown, heredity, infection, environmental factors, and immunologic disorders have been suggested to be involved, and several models of experimental colitis have been developed to study the cellular and molecular mechanisms of inflammation and immunologic abnormality.[16] To identify natural products with anti-inflammatory effects against IBD, we used LPS-stimulated RAW 264.7 cells and HT-29 colonic epithelial cells.
Cirsium japonicum DC var. ussuriense (Rege) Kitam (Compositae), also known as “Korean thistle,” is a perennial herb indigenous to Korea. The aerial parts of this plant are used in oriental medicine as a diuretic, tonic, neuralgia stomachic, antiphlogistic, and detoxicant and have demonstrated hepatoprotective, anti-inflammatory, antitumor, antimutagenic, cytotoxic, and antioxidant effects in pharmacologic studies.[9,10,12] Flavonoids are reported to be major constituents of C. japonicum var. ussuriense.[9] These are plant-derived secondary metabolites distributed throughout the plant kingdom. In many studies, flavonoids such as quercetin and rutin have shown anti-inflammatory activities in cellular and rodent models.[17] HN isolated from the methanolic extract of the aerial parts of C. japonicum var. ussuriense is a flavone diglycoside [Figure 1].[14] Various pharmacologic activities of flavone glycosides have been reported in cellular and rodent models. HN has a neohesperidosyl [α-l-rhammnopyranosy-(1→2)-β-d-glucopyranosyl] moiety at the seventh position of hispidulin. Hispidulin is a natural bioactive flavone with various pharmacologic effects, for example, antioxidant, anticancer, antiepileptic, antihypnotic, anti-osteoclastogenesis, anti-inflammatory, anti-influenza, antidiabetic, antitrypanosomal, and hepatoprotective activities.[18,19] However, the pharmacologic activities, including the anti-inflammatory effect, of HN have not been investigated to date, with the exception of alleviation of alcohol toxicity. In this study, we evaluated the effects on inflammatory mediators of HN treatment in LPS-treated RAW264.7 and HT-29 cells.
Figure 1.
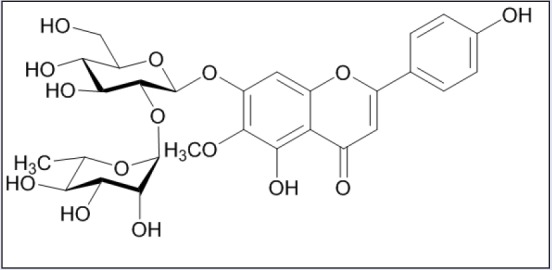
The chemical structure of hispidulin-7-O-neohesperidoside (HN)
In LPS-induced RAW264.7 cells, the cytotoxicity of HN (25-100 μM) was measured by MTT assay. HN did not exert a cytotoxic effect at up to 100 μM compared with untreated control cells [Figure 2]. Thus, subsequent experiments used 25-100 μM HN. To elucidate its effects on inflammation, the inhibitory effect of HN on NO production was examined in LPS-induced RAW264.7 cells via the Griess reaction. HN showed potent inhibitory effects on NO production in LPS-induced RAW264.7 cells [Figure 3]. NO, which is involved in the development of intestinal inflammation, is generated by iNOS and contributes to inflammation. Therefore, we assessed the effect of HN on the iNOS protein level in LPS-induced RAW264.7 cells by Western blot analysis. The results confirmed that HN decreased the iNOS protein level in a concentration-dependent manner [Figure 4].
Figure 2.
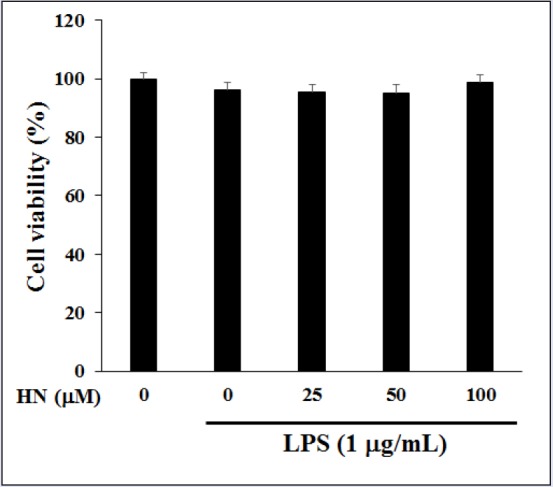
Effect of HN on the viability in LPS-induced RAW264.7 cells. Cytotoxicity on RAW264.7 cells treated with various concentrations of HN for 24 h. HN cytotoxicity was assessed by MTT assay. Data are mean ± SD (n = 3) of three independent experiments
Figure 3.
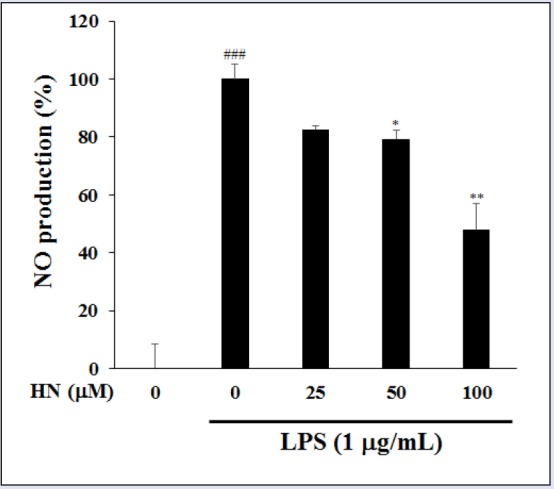
Inhibitory effect of HN on nitrite production in LPS-induced RAW264.7 cells. Nitrite in culture medium was quantified by Griess assay. Data are mean ± SD (n = 3) of three independent experiments. ###P < 0.001, compared with the untreated control; *P < 0.05 and **P < 0.01, compared with the LPS-treated control
Figure 4.
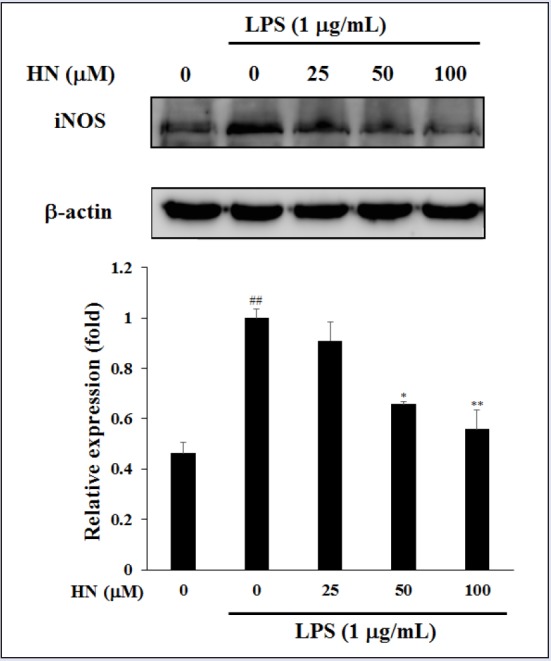
Inhibitory effect of HN on iNOS protein expression in LPS-induced RAW264.7 cells. iNOS and β-actin protein levels were determined by Western blotting. Data are mean ± SD (n = 3) of three independent experiments. ##P < 0.01, compared with the untreated control; *P < 0.05 and **P < 0.01, compared with the LPS-treated control
Moreover, HN at more than 25 μM suppressed the production of LPS-induced pro-inflammatory cytokines, IL-1β and TNF-α. In IBD patients, macrophages and intestinal immune cells secrete large amounts of IL-1β and TNF-α [Figure 5].[17] IL-1β is produced by both inflammatory cells and mucosal epithelial cells during colonic inflammation. TNF-α is a potent cytokine with multiple immunologic and inflammatory effects related to IBD.[19] Both IL-1β and TNF-α may regulate, amplify, and perpetuate mucosal inflammation by various mechanisms and increase the release of potent chemotactic cytokines such as IL-8, which is found in increased quantities in inflamed mucosa.[4] The increased IL-8 production within the intestine of IBD patients contribute to neutrophil activation by interacting with IL-1β and TNF-α, and thus, may initiate or maintain IBD.[20] Thus, inhibitors of IL-8 may be used to treat immune-associated diseases such as CD and UC. IL-8 is produced by various cell types, such as neutrophils, epithelial cells, and endothelial cells; in particular, LPS stimulates IL-8 secretion in HT-29 cells.[21] To investigate the inhibitory effect of HN on IL-8 production, we evaluated its effect on IL-8 levels in LPS-induced HT-29 cells by ELISA. HT-29 cells were pretreated with HN or vehicle overnight, followed by a 1-h exposure to LPS. MTT assay indicated that HN at 50-200 μM was not toxic to LPS-induced HT-29 cells compared with the control group not treated with LPS [Figure 6]. HN treatment decreased IL-8 levels in a concentration-dependent manner [Figure 7].
Figure 5.
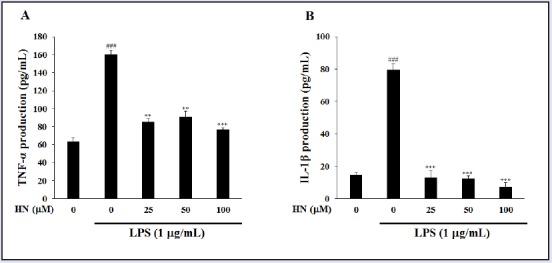
Inhibitory effect of HN on pro-inflammatory cytokine production in LPS-induced RAW264.7 cells. TNF-α was quantified by ELISA. (A) Cells were incubated with various concentrations of HN for 24 h. (B) Protein levels were determined by ELISA. Data are mean ± SD (n = 3) of three independent experiments. ###P < 0.001, compared with the untreated control; **P < 0.01 and ***P < 0.001, compared with the LPS-treated control
Figure 6.
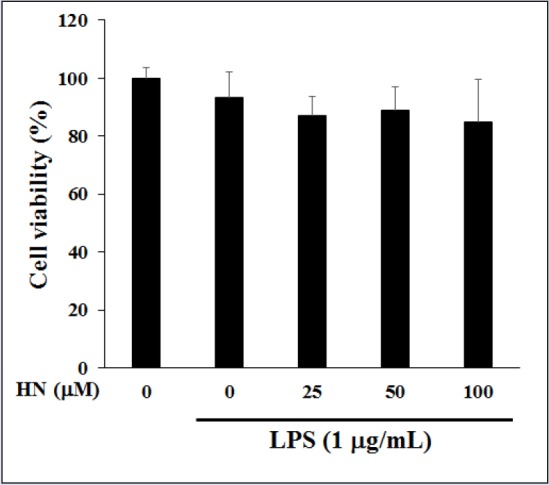
Effect of HN on the viability of LPS-induced HT-29 cells. Cells were pretreated with HN at various concentrations for 24 h, and cytotoxicity was analyzed by MTT assay. Data are mean ± SD (n = 3) of three independent experiments
Figure 7.
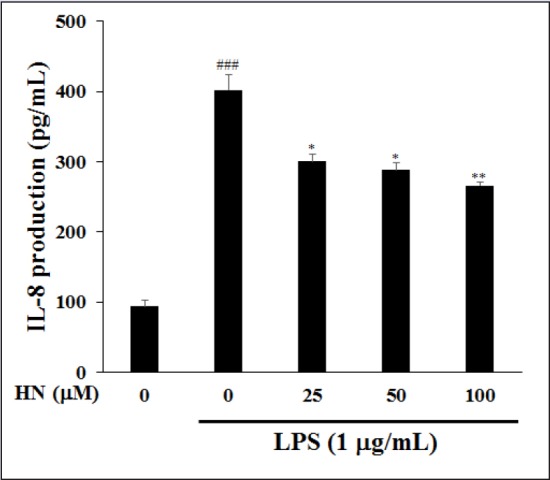
Inhibitory effect of HN on IL-8 expression in LPS-induced HT-29 colonic epithelial cells. IL-8 was quantified by ELISA. Values are means ± SD (n = 3) of three independent experiments. ###P < 0.001, compared with the untreated control; *P < 0.05 and **P < 0.01, compared with the LPS-treated control
HN, an active flavone diglycoside, was isolated from the methanolic extract of the aerial parts of C. japonicum var. ussuriense and significantly suppressed NO production in LPS-induced macrophages. HN downregulated the levels of the pro-inflammatory cytokines, IL-1β and TNF-α, as well as the iNOS protein level, in LPS-induced RAW 264.7 cells. In addition, HN inhibited the production of IL-8 in LPS-induced HT-29 colonic epithelial cells. Cytokines play a key role in the regulation of the intestinal immune system.[22,23] Improvement in research of the immunology of IBD and in bioengineering have led to new therapeutic concepts that target aspect of the inflammatory process.[24] Especially, the blockade of TNF is the currently most efficacious therapeutic target for IBD. Therefore, these results indicated that HN inhibits the production of inflammatory mediators and could be a candidate therapeutic against IBD.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. NRF-2014R1A1A2056624). This study was supported by Sunchon National University Research Fund in 2014 and Suncheon Research Center for Natural Medicines.
REFERENCES
- 1.Sung MJ, Davaatseren M, Kim SH, Kim MJ, Hwang JT. Boehmeria nivea attenuates LPS-induced inflammatory markers by inhibiting p38 and JNK phosphorylations in RAW264.7 macrophages. Pharm Biol. 2013;51:1131–6. doi: 10.3109/13880209.2013.781196. [DOI] [PubMed] [Google Scholar]
- 2.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–59. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 3.Verma N, Ahuja V, Paul J. Profiling of ABC transporters during active ulcerative colitis and in vitro effect of inflammatory modulators. Dig Dis Sci. 2013;58:2282–92. doi: 10.1007/s10620-013-2636-7. [DOI] [PubMed] [Google Scholar]
- 4.Reinecker HC, Steffen M, Witthoeft T, Pflueger I, Schreiber S. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993;94:174–81. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang KH, Kong CS, Seo Y, Kim MM, Kim SK. Anti-inflammatory effect of coumarins isolated from Corydalis heterocarpa in HT-29 human colon carcinoma cells. Food Chem Toxicol. 2009;47:2129–34. doi: 10.1016/j.fct.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 6.Arenzana-Seisdedos F, Virelizier JL. Interferons as macrophage-activating factors. II. Enhanced secretion of interleukin 1 by lipopolysaccharide-stimulated human monocytes. Eur J Immunol. 1983;13:437–40. doi: 10.1002/eji.1830130602. [DOI] [PubMed] [Google Scholar]
- 7.Bischoff SC. Quercetin: potentials in the prevention and therapy of disease. Curr Opin Clin Nutr Metab Care. 2008;11:733–40. doi: 10.1097/MCO.0b013e32831394b8. [DOI] [PubMed] [Google Scholar]
- 8.Shon M, Choi S, Kahng G, Nam S, Sung N. Antimutagenic, antioxidant and free radical scavenging activity of ethyl acetate extracts from white, yellow and red onions. Food Chem Toxicol. 2004;42:659–66. doi: 10.1016/j.fct.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Park JC, Hur JM, Park JG, Kim SC, Park JR, Choi SH. Effects of methanol extract of Cirsium japonicum var. ussuriense and its principle, hispidulin-7-O-neohesperidoside on hepatic alcohol-metabolizing enzymes and lipid peroxidation in ethanol-treated rats. Phytother Res. 2004;18:19–24. doi: 10.1002/ptr.1299. [DOI] [PubMed] [Google Scholar]
- 10.Lee D, Kim K, Li B, Choi H, Keo S, Jun K. Anti-inflammatory effect of the Cirsium japonicum var. ussuriense 70% ethanolic extract in RAW264. 7 cells by heme oxygenase-1 expression. Kor J Pharmacogn. 2012 [Google Scholar]
- 11.Lee H, Kim J, Kim N, Park S, Kim M, Yu C. Antioxidant, antimutagenicity and anticancer activities of extracts from Cirsium japonicum var. ussuriense KITAMURA. Korean J Med Crop Sci. 2003;11:53–61. [Google Scholar]
- 12.Yoo OK, Choi BY, Park JO, Lee JW, Park BK, Joo CG. Ethanol extract of Cirsium japonicum var. ussuriense kitamura exhibits the activation of nuclear factor erythroid 2-related factor 2-dependent antioxidant response element and protects human keratinocyte HaCaT cells against oxidative DNA damage. J Cancer Prev. 2016;21:66–72. doi: 10.15430/JCP.2016.21.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thao NT, Cuong TD, Hung TM, Lee JH, Na M, Son JK. Simultaneous determination of bioactive flavonoids in some selected Korean thistles by high-performance liquid chromatography. Arch Pharm Res. 2011;34:455–61. doi: 10.1007/s12272-011-0314-x. [DOI] [PubMed] [Google Scholar]
- 14.Park JC, Lee JH, Choi JS. A flavone diglycoside from Cirsium japonicum var. ussuriense. Phytochemistry. 1995;39:261–2. [Google Scholar]
- 15.Lee MA, Lee HK, Kim SH, Kim YC, Sung SH. Chemical Constituents of Alnus firma and their inhibitory activity on lipopolysaccharide-induced nitric oxide production in BV2 microglia. Planta Med. 2010;76:1007–10. doi: 10.1055/s-0029-1240911. [DOI] [PubMed] [Google Scholar]
- 16.Kwon KH, Murakami A, Tanaka T, Ohigashi H. Dietary rutin, but not its aglycone quercetin, ameliorates dextran sulfate sodium-induced experimental colitis in mice: attenuation of pro-inflammatory gene expression. Biochem Pharmacol. 2005;69:395–406. doi: 10.1016/j.bcp.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Subramanian S, Rhodes JM, Hart CA, Tam B, Roberts CL, Smith SL. Characterization of epithelial IL-8 response to inflammatory bowel disease mucosal E. coli and its inhibition by mesalamine Inflamm. Bowel Dis. 2008;14:162–75. doi: 10.1002/ibd.20296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atif M, Ali I, Hussain A, Hyder SV, Niaz B, Khan FA. Pharmacological assessment of hispidulin—a natural bioactive flavone. Acta Pol Pharm. 2015;72:829–42. [PubMed] [Google Scholar]
- 19.Grecco Sdos S, Felix MJ, Lago JH, Pinto EG, Tempone AG, Romoff P. Anti-trypanosomal phenolic derivatives from Baccharis uncinella. Nat Prod Commun. 2014;9:171–3. [PubMed] [Google Scholar]
- 20.Laveti D, Kumar M, Hemalatha R, Sistla R, GM Naidu V, Talla V. Anti-inflammatory treatments for chronic diseases: a review. Inflamm Allergy Drug Targets. 2013;12:349–61. doi: 10.2174/18715281113129990053. [DOI] [PubMed] [Google Scholar]
- 21.Mitsuyama K, Toyonaga A, Sasaki E, Watanabe K, Tateishi H, Nishiyama T. IL-8 as an important chemoattractant for neutrophils in ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1994;96:432–6. doi: 10.1111/j.1365-2249.1994.tb06047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Wang Y, Zhang F, Wang K, Liu G, Yang M. Allyl methyl disulfide inhibits IL-8 and IP-10 secretion in intestinal epithelial cells via the NF-κB signaling pathway. Int Immunopharmacol. 2015;27:156–63. doi: 10.1016/j.intimp.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Rogler G, Andus T. Cytokines in inflammatory bowel disease. World J Surg. 1998;22:382–9. doi: 10.1007/s002689900401. [DOI] [PubMed] [Google Scholar]
- 24.Baumgart D.C, Sandborn W.J. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–57. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]


