Abstract
The NIH Office of Dietary Supplements convened a public workshop on iron screening and supplementation in iron-replete pregnant women and young children in 2016 in Bethesda, Maryland. The starting point for the workshop was the recent reports from the US Preventive Services Task Force concluding that there was insufficient evidence to evaluate the benefits and harms associated with iron screening and routine supplementation among asymptomatic pregnant women and young children (6–24 mo old) in the United States. The goal of the workshop was to explore and refine understanding about the existing knowledge gaps and research needs associated with these preventive services for these groups. Given the focus on the United States, planning for the workshop took into account the higher iron status in the United States compared with developing countries and, in turn, included a focus on iron-replete individuals consistent with the U-shaped risk curve for nutrient-health relations. Topic areas included adaptations in iron homeostasis associated with pregnancy and young childhood, the impact of inflammation, measurement of iron status, current estimates of iron status for pregnant women and young children in the United States and in Europe, and emerging evidence suggesting adverse effects associated with iron supplementation of iron-replete individuals. A crosscutting dialogue conducted at the close of the workshop formed the basis for a workshop summary that specified evidence gaps and research needs in a range of areas centered on the relation of these adaptations of iron homeostasis with the response to and risk from iron supplementation as well as the need for indicators informative of the full continuum of iron status and based on health outcomes, not just erythropoiesis.
Keywords: iron, pregnancy, infancy, young childhood, iron screening, iron supplementation
IMPETUS FOR THE WORKSHOP
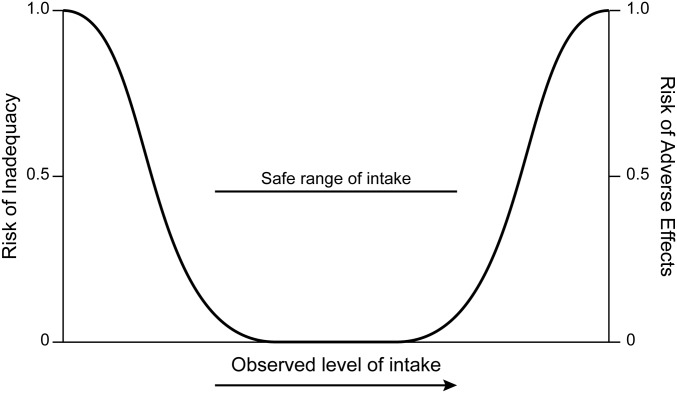
For decades health practitioners and the public have known dietary iron to be an essential dietary component, a foundation for development and health, a widely administered supplement for pregnant women, and a key ingredient in infant formula. Although iron may seem familiar, it is nonetheless characterized by substantive challenges and unknowns, ranging from measurement of iron status indicators in blood and their appropriate cutoffs to elucidation of alterations of its homeostatic mechanisms in pregnancy and infancy. A parallel consideration is that a U-shaped curve for risk across a range of intakes from low to high has been established for nutrient-health relations (Figure 1). Yet for iron the question of risk has largely focused on low or deficient intakes, that is, the left side of the curve. There has been less focus on risk associated with higher intakes beyond apparent iron needs, as reflected by the risk curve’s right side. This is understandable because iron deficiency (ID) and ID anemia (IDA) have been a major public health focus believed to affect many worldwide, especially among pregnant women and young children in developing countries. However, the right side of the risk curve is worthy of consideration, especially in developed countries with a lower prevalence of ID and IDA. Furthermore, in the United States, where preventing ID is a top public health goal, there is notable iron exposure due to widespread food fortification and routine supplementation that affects not only deficient individuals but also iron-replete individuals. This underscores the importance of exploring both ends of the U-shaped risk curve for iron.
FIGURE 1.
Generic depiction of the U-shaped curve for risk associated with nutrient intake and diet-health relations.
In 2015, questions about iron screening and supplementation in pregnant women and young children (6–24 mo old) were raised by reports from the US Preventive Services Task Force (USPSTF) (1, 2). Overall, reports issued by the USPSTF are derived from objective and systematic reviews of the totality of available data and have as their goal the evaluation of the nature of the evidence base underpinning preventive services in the United States, such as screening and nutrient supplementation. The questions for USPSTF reports center on the balance of benefits and harms. The reports are not structured to provide medical judgment or guidance for best practices. The 2015 USPSTF reports on iron indicated that, in the case of asymptomatic pregnant women in the United States, there was insufficient evidence for benefit relative to morbidity, mortality, and birth outcomes associated with screening for IDA or routinely supplementing with iron. In the case of US children 6–24 mo old, there was also insufficient evidence to indicate that screening for IDA improved health outcomes such as growth and cognition. These conclusions do not mean that screening and supplementation should not be done, but rather that the health benefits of these activities cannot be ascertained by using available evidence. Such a conclusion may be puzzling to some who understand the identification and elimination of IDA to be a long-established and desirable goal. Within the USPSTF analytic framework (1, 2), IDA and related hematological changes are intermediate outcomes and, although likely on the causal pathway to certain health outcomes, changes in measurements of IDA in asymptomatic individuals in the United States cannot be linked confidently nor consistently with improvements in health. These issues are of interest nevertheless in the important public health goals associated with reducing ID and IDA.
Taken together, the evidence gaps identified by the USPSTF and the interest in better elucidating alterations in the homeostatic mechanisms associated with iron in pregnancy and young childhood as well as the drivers for decisions to supplement pregnant women and young children in developed countries warranted an in-depth discussion to articulate the knowledge gaps and research needs for both ends of the risk curve. Examining the challenges surrounding the available screening tools was also essential because the hematological measurements used to indicate iron status are still evolving. They are often confounded, may not accurately estimate the prevalence of IDA and ID, may not clearly reflect the full range of status, and lack consistent cutoffs based on health outcomes. In September 2016 the NIH Office of Dietary Supplements convened an open, public workshop on iron screening and supplementation in iron-replete pregnant women and young children in Bethesda, Maryland (3). Other federal agencies acknowledged the importance of the issues and served as cosponsors. Importantly, the iron-replete considerations included in the workshop were complemented by the evolving scientific understandings associated with ID and iron status that served as a foundation for discussion. Participants with a widely varying expertise were tasked with offering reviews of the state of the evidence, taking part in a dialogue, and discussing research needs. The workshop was organized into major topic areas and concluded with a participant-wide discussion of crosscutting knowledge gaps. In the following sections we provide the background information that informed the organization of the workshop, and we introduce the articles that arose from the workshop and are published in this supplement issue.
As with all endeavors for which a range of scientific experts discuss a topic in common, clarification of terminology is a helpful starting point. Several key terms were used during the workshop (Table 1) and are reflected in the articles in this supplement.
TABLE 1.
Workshop terminology1
| Health outcomes | Measurements associated with biological and physiologic factors, symptoms, functioning, general health perceptions, and overall quality of life (4). Health outcomes may be clinical measurements, general health-related quality-of-life measurements, or disease-specific scales. |
| Indicators | Measurements associated with iron status and iron-related biological processes including physiologic and hematologic measurements and other chemical analyses of body tissues. For some, the term “biomarkers” reflects this concept; however, because IOM recommends that biomarkers be “objectively measured and evaluated” (5), the term is not used here to avoid its application to measurements that lack this level of scrutiny. Workshop participants at times also used the term “bioindicators” in lieu of “indicators” to emphasize the biological focus. |
| ID | A reduction or depletion in iron stores, which during its early stages is without consequences for erythropoiesis but transitions to iron deficiency erythropoiesis at the point of depletion. |
| IDA | Depletion of iron stores to the point that normal red blood cell production cannot be maintained, and hypochromic and microcytic cells are produced. Hemoglobin concentrations are decreased. |
| Iron replete | Iron stores sufficient to meet functional needs and at a level above that defined as iron deficient and below that defined as iron excess, iron overload, and iron toxicity. Such sufficiency, however, is not well defined and lacks established cutoffs. |
| Young children | Children 6–24 mo old, a definition used to be consistent with the USPSTF report. It also reflects nutritional interests because by 6 mo of age the infant has likely depleted its iron stores and would be entering a more vulnerable stage relative to iron needs. |
ID, iron deficiency; IDA, iron deficiency anemia; IOM, Institute of Medicine; USPSTF, United States Preventive Services Task Force.
BACKGROUND FOR THE WORKSHOP
An over-arching focus for the workshop planning was the context that surrounds iron screening and supplementation in developed countries, as well as foundational issues related to newer understandings of iron metabolism and homeostasis especially as it relates to pregnancy and young childhood. The context for iron status in developed countries, particularly the United States, is in contrast to that in developing countries. There is a lower prevalence of ID in the United States, there are notable amounts of iron in the food supply as well as ready access to iron-containing supplements, and iron supplementation during pregnancy and young childhood is common. However, other developed countries, such as those in Europe, approach iron screening and supplementation for pregnant women and young children somewhat differently. In turn, considerations of iron status and supplementation recommendations in European countries were informative. Current estimates of iron requirements and iron intake as well as recommendations for iron supplementation during pregnancy and young childhood laid the foundation for the workshop, as did emerging evidence on adverse effects associated with iron supplementation of iron-replete populations, particularly pregnant women and young children. Challenges related to the hematological measurements used to estimate iron status were also considered during planning.
The iron context in developed countries
Pregnant women
The Institute of Medicine (IOM) (now National Academy of Medicine) issued nutrient reference values, known as Dietary Reference Intakes, for iron in 2001 (6). The Dietary Reference Intakes that reflect the Estimated Average Requirement (50th percentile iron requirement) and the Recommended Dietary Allowance (97.5th percentile iron requirement) for pregnant women are shown in Table 2. They apply to both the US and Canadian populations. These reference values, or recommended intakes, differ notably from those for Europe, at least as specified by the European Food Safety Authority (11). The IOM values rest on the conclusion that iron intake should increase with pregnancy. The European Food Safety Authority has concluded that, unless there is a reason the individual may be at risk for deficiency, there is generally no need for increased iron intake during pregnancy, leaving an iron requirement equivalent to that for nonpregnant women. Some countries in Europe have issued their own intake requirements for iron, but they are not consistent. In 2010 the Scientific Advisory Committee on Nutrition in the United Kingdom commented that the substantial proportion of their population with apparent iron intakes below dietary recommendations is at odds with the low prevalence of poor iron status and could be attributable to uncertainties in Dietary Reference Values for iron intake which may be too high, particularly for girls and women of reproductive age (13).
TABLE 2.
Iron intake recommendations and estimated intake: pregnant women in the United States and Europe1
Recommendations from US health authorities for iron supplementation during pregnancy vary (Table 2). The frequently used software resource for clinicians UpToDate (7) notes as general advice the need to increase iron intake for pregnant women by 15–30 mg/d over the iron intake for nonpregnant women. The existing CDC guidelines were issued in 1998 (9) and recommend universal supplementation of pregnant women. The American College of Obstetricians and Gynecologists (8) recommends screening pregnant women as a first step and then supplementing with iron if IDA is detected. There are numerous guidelines issued by different European counties; overall European guidelines do not recommend universal supplementation (11).
Information on iron intake for nonpregnant women is often used to suggest a “baseline” intake for pregnant women. What We Eat in America (14) suggests that US women ≥20 y old consume ∼17 mg Fe/d, although this is based on only 1 d of intake data. A study that used NHANES 2003–2006 estimated usual iron intakes for women ≥19 y old at 25 and 14 mg Fe/d depending on whether they were users or nonusers of dietary supplements, respectively (15).
Nationally representative data reflecting iron intakes among pregnant women in the United States are limited, and available information should be interpreted cautiously. A study based on the 1988–1994 cycle of NHANES is often cited and, although reflective of older data, provides some insight (12). The study (n = 182) estimated that pregnant women consumed an average of 75 mg Fe/d (food and supplements), although the data were skewed with a median of 58 mg Fe/d (Table 2). During the second and third trimesters of pregnancy, ∼80% of these women were using iron-containing supplements. Pregnant women who consumed iron-containing supplements were taking in larger doses than the Tolerable Upper Intake Level (45 mg/d) for iron. Fewer than 15% who used supplements were being treated or had been treated for anemia in the past 3 mo. The researchers noted that supplement use among this group of pregnant women was not driven entirely by need. Sales data for prenatal vitamins help enhance the picture. Available data, which are limited and from different time periods, indicate that the more prevalent supplements, both prescription and over-the-counter, provide ∼30 mg Fe/d in a daily dose (K Andrews, USDA, personal communication, 2016). The annual sales of prenatal vitamins in the United States are in the hundreds of millions of dollars (J Johnson, New Hope Network, personal communication, 2016).
Data reviewed during the workshop planning indicated that ∼2–3% of pregnant women in the United States may be experiencing IDA, with the prevalence of ID estimated at 16% (7). These estimates are now confirmed and more clearly specified in an article in these proceedings (16). Although the absence of agreed-on measurements for iron repletion is acknowledged, available data can be interpreted to indicate that many pregnant women in the United States are likely to be iron replete. This interpretation does not negate concerns about IDA and ID, especially among at-risk subgroups. Some of these individuals may also be experiencing high levels of iron exposure, but the absence of agreed-on measurements for high iron stores limits interpretation of the data.
Young children
The IOM reference values for young children (6) are similar to those formulated in Europe (Table 3), but the iron content of infant formula products differ. Infant formula marketed in the United States generally provides 1.8 mg Fe/100 kcal (Table 3) (17). This amount of iron stems from considerations about iron bioavailability (17). Formulas in Europe contain less iron and may be differentiated on the basis of younger and older infants with those for older infants (so-called follow-on formula) providing less iron on the assumption that other iron-containing foods are entering the child’s diet (17).
TABLE 3.
Iron intake requirements, iron content of formula and estimated intake: young children (6–24 mo old) in the United States and Europe
Iron intake estimates of 17 and 10 mg Fe/d for children 6–12 mo old and 12–24 mo old, respectively, were reported in NHANES 2011–2012 (Table 3), albeit only for low-income groups (18). An informal calculation of the hypothetical iron intake of an 8-mo-old infant in the United States based on guidance from a professional association is shown in Table 4 and is differentiated by formula-fed and breastfed infants. According to this calculation, iron intakes may reach 18 mg Fe/d for formula-fed children, amounts greater than the iron Recommended Dietary Allowances of 11 mg Fe/d for 6–12-mo-old infants and 7 mg Fe/d for 12–24-mo-old infants. Not surprisingly, given the lower iron content of infant formula and the limited amount of iron fortification of foods compared with the United States, young children in Europe appear to have lower iron intakes (Table 3) (19).
TABLE 4.
Hypothetical daily iron intake of an 8-mo-old US infant based on sample menu1
| Iron intake, mg/d |
||
| Food | Formula-fed | Breastfed |
| Human milk2 (21) | — | 0.2 |
| Infant formula3 (21) | 8.5 | — |
| Iron-fortified cereal4 (21) | 4.8 | 4.8 |
| Meat5 (21, 22) | 2.4 | 2.4 |
| Vegetables6 (21, 22) | 1.8 | 1.8 |
| Other (fruit, dairy)7 (22) | 1 | 1 |
| Total | 18.5 | 10.2 |
From the American Academy of Pediatrics, sample menu for an 8–12-mo-old infant (20). All values are approximate based on average iron content of human milk, infant formula, and complementary foods. Includes dietary iron intakes only; does not include intake from supplements. Sources for iron content are from Baker and Greer (21) and the USDA (22).
Assumes daily intake of 720 mL human milk containing 0.35 mg Fe/L.
Assumes daily intake of 720 mL infant formula fortified with 12 mg Fe/L.
Assumes daily intake of 60 g dry fortified rice cereal mixed with fruit juice.
Assumes daily intake of 56 g pureed or crumbled ground beef and 70 g chicken from baby food jar.
Assumes daily intake of 112 g mashed baked sweet potato, 112 g creamed spinach from baby food jar, and 112 g strained green beans from a baby food jar.
Assumes daily intake of 3–4 servings of fruit, cheese, and/or other dairy products at ∼0.3 mg Fe/serving; no servings of legumes.
Nationally representative data on iron status for infants <12 mo old are not collected in NHANES. In the case of children 12–24 mo old, data available during the workshop planning (23) suggested that children 12–24 mo old had an estimated prevalence of <3% and <16% for IDA and ID, respectively. These estimates are now confirmed in an article in these proceedings (16). Again, as with pregnant women and with the recognition of the importance of addressing ID and IDA, a notable number of young children in the United States are likely to be iron replete.
Considerations for right side of U-shaped risk curve
A fundamental question is at what amount(s) of intake does risk attributable to iron intake arise. Clearly, low intakes increase risk of ID and IDA. However, emerging data suggest that concerns may exist for higher levels of iron intake, and these need to be considered especially if universal and/or routine iron supplementation are factors during pregnancy and infancy.
Although the evidence linking iron supplementation of iron-replete populations to adverse effects is at best preliminary and somewhat inconsistent, the general nature of the emerging data is shown in Table 5. Iron is pro-oxidative and in this capacity can affect biological systems even at less-than-toxic amounts. In the case of infants, examples of available data include reports of decreased growth attributable to iron supplementation among infants who were iron replete (Table 5). Associations have also been observed between greater iron stores and disturbances in glucose metabolism, including increased risk of gestational and type 2 diabetes among pregnant and postpartum women, respectively (Table 5). Furthermore, there are reports of changes in gut microbiota and increases in the amounts of gastrointestinal pathogenic bacteria with iron supplementation in young children (Table 5). The nature and possible causes of such effects were important discussion points for the workshop so as to examine both sides of the U-shaped risk curve.
TABLE 5.
Identified adverse outcomes associated with high iron exposure in pregnant women and young children1
| Outcome | Associated indicator of high iron exposure | Nature of evidence |
| Health outcome | ||
| GDM/T2D-PP (24–33) | Supplementation | Inconsistent |
| SF | Consistent | |
| Intake | Inconsistent | |
| Preterm birth (34–37) | Hemoglobin | Inconsistent |
| SF | Consistent | |
| Impaired fetal growth (35, 38) | Supplementation | Consistent |
| Hemoglobin | Inconsistent | |
| SF | Inconsistent | |
| Impaired infant/child growth (39–44) | Supplementation to iron replete | Consistent |
| Long-term impaired cognitive development (45) | Supplementation to iron replete | Limited |
| Diarrhea (46) | Supplementation or fortification | Inconsistent |
| Intermediate outcome | ||
| Microbiome change (47–50) | Supplementation | Inconsistent |
GDM, gestational diabetes mellitus; SF, serum ferritin; T2D-PP, type 2 diabetes postpartum.
Measurement of iron status as an underlying concern
Examples exist to demonstrate the key role that standardizing analytic and interpretative activities serve related to nutrient status measurements and, in turn, to establishing and clarifying links between nutrients and health outcomes. One such example is the Vitamin D Standardization Program, which has focused on ensuring accurate, harmonized, and reproducible measurements of serum 25-hydroxyvitamin D through standard reference methods, standard reference materials, and commutability (51). If nutrient status indicators can be harmonized and reported consistently, research can be more reliably compared and public health policies can rest on better foundations. These efforts are driven by collaborative partnerships with commitment to proficiency testing and other evaluative follow-on activities to ensure continued appropriate performance of the assays and procedures (52).
The uncertainties associated with the estimation of ID and IDA can perhaps be best typified by reports such as that from Petry et al. (53). One of the most common assertions in the iron literature is that half of the cases of anemia worldwide can be attributed to the onset of ID, making IDA the most common form of anemia. Yet factors such as inflammation and geographical region or even perhaps socioeconomic status suggest that the proportion of anemia attributable to iron may be different from typically estimated. In the case of the study by Petry et al. (53), IDA prevalence was estimated at 25% of the anemia cases rather than 50%. Moreover, there is an emerging interest with the potential for considerable impact as shown in the reports that ethnic differences have been documented in iron status. For instance, African Americans appear to be at increased risk of ID and have higher serum ferritin (SF) concentrations compared with the general population (54). These newer understandings further complicate the current approach to measuring iron status.
The biochemical measurement of iron status focuses almost exclusively on hematological indicators. Despite long-term use and considerable familiarity, most of these indicators have challenges relative to laboratory measurement and interpretation as well as adequate representation of the full spectrum of iron status. Table 6 lists examples of the known challenges. At times some of the challenges have been addressed by combining indicators into a “panel,” which as a group may be used to indicate status. However, problems or confounders associated with any one indicator will persist even if the indicator is situated within a panel.
TABLE 6.
Comparison of indicators of iron status1
| Indicator | Measurement | Pros | Cons |
| Hemoglobin | Anemia | Commonly available | Low specificity and sensitivity |
| Low complexity | Certain factors (e.g., elevation, age, ethnicity) may complicate interpretation | ||
| Ferritin | Size of iron stores | Commonly available | Confounded by inflammation |
| Transferrin saturation | Iron-deficient erythropoiesis | Commonly available | Diurnal and prandial variation |
| Erythrocyte protoporphyrin | Iron-deficient erythropoiesis | — | Reliability of field instrumentation |
| sTfR | Iron-deficient erythropoiesis | Less affected by inflammation | Limited availability |
| Assay differences | |||
| Ratio of sTfR-to-ferritin (derived by using various calculations) | Reflection of range of status | Less affected by inflammation | Requires 2 measurements |
| Hepcidin | Determinant of iron needs and utilization | Relatively sensitive | Experimental and under development |
| Possibly less affected by inflammation |
Christine Pfeiffer, CDC, personal communication as contribution to the compilation of this table, 2017. sTfR, soluble transferrin receptor.
Perhaps the most classic indicator for iron status is hemoglobin, which is used to estimate IDA although its specificity for IDA compared with anemia due to other causes is low. In the case of IDA estimations, hemoglobin concentrations are now usually interpreted in conjunction with measurements of SF, an established measure of iron stores. SF concentrations can signal a concern for iron status before the onset of changes in red blood cells. As such, SF concentrations are widely used to estimate ID, which has the advantage of identifying low iron status before the onset of IDA. Unfortunately, conditions of inflammation, which are receiving increased attention and may be more prevalent than previously understood, have the ability to elevate SF concentrations spuriously and thus mask ID, in turn offering a major challenge currently for iron status determinations based on SF concentrations.
Measurements of iron status have received considerable attention from WHO, which has issued guidance concerning indicators based on hemoglobin concentration (55) and has organized a guideline-development group focused on SF concentrations (56). Other activities include the collaborative research group Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia formed by the CDC, NIH’s National Institute for Child Health and Human Development, and the Global Alliance for Improved Nutrition (57). Attention is now turning to other indicators of iron status that may prove to be highly useful and relevant. These include soluble transferrin receptor concentration, the ratio of soluble transferrin receptor to SF concentrations, and hepcidin.
WORKSHOP SESSIONS
The workshop was organized into several topic sessions focused on different but interconnected issues surrounding iron. Considerable dialogue took place during each session, and a variety of panels critiqued and enhanced the presentations (3). A final summary session began with a workshop-wide conversation to integrate themes that emerged and was followed by discussions related to next steps.
Newer understandings about iron homeostasis drove the objectives for the presentations in Session 1. A particular focus was how iron metabolism may be altered during pregnancy and what characterizes homeostasis during the periods of rapid growth associated with infancy, as well as how such changes may affect determinations of status and iron requirements. Additional themes of interest included evidence that different organs of the body have different iron needs, the iron supply is prioritized to different organs, and different cell types within tissues may handle iron differently. Another focus was whether the requirement for iron should more appropriately reflect the variance of iron metabolism during pregnancy from trimester to trimester as opposed to the current basis which rests on third-trimester needs. Additionally, there was interest in the novel consideration that evolutionary factors may interface with the apparent need among breastfed infants at weaning for additional sources of iron, needs perhaps met by premastication of animal foods by the mother. Finally, questions of ethnic and racial differences in iron metabolism as well as emerging understandings from the study of chronic and acute inflammation were identified as topics for this session. Seven articles in this supplement address these issues.
Given the wide array of iron status indicators in use, objectives for Session 2 were to overview these markers and specify the nature of their challenges. The approach to harmonizing and standardizing the measurements was an intended consideration with a focus on the common approaches to establishing clinical indicators across a range of substances and endpoints. The implications of plasma volume expansion during pregnancy raised questions early in the workshop planning for confounders related to analytic methodologies, and the topic was included in Session 2. Furthermore, the basis for establishing cutoffs for the array of indicators was of interest. In addition, a key objective was to address the confounding of inflammation especially in the case of SF concentrations and to overview research needs to adjust for it or to modify relevant measurements according to the confounding. Five articles in this supplement address these issues.
As a prelude to discussions focused on emerging evidence suggesting adverse effects associated with iron supplementation of iron-replete individuals, Session 3 was arranged to provide an overview of current estimates of iron status among pregnant women and young children in both the United States and Europe. Presenters were asked to consider the spectrum of status including prevalence of deficiency, adequacy, and overload. Four articles in this supplement address this topic.
The objective of Session 4 was to array the nature of the evidence suggestive of concerns about supplementing iron-replete pregnant women and young children so that they could be considered as a whole as well as individually. The topics of interest were gestational diabetes; pregnancy outcomes; infant development, growth, and infection; and the gut microbiome. Discussions were to focus on the nature of the U-shaped risk curve for iron. Four articles in this supplement present these considerations.
A final session was organized to allow a workshop-wide discussion on how the information presented in the workshop could be integrated and, on this basis, what are the evidence and knowledge gaps as well as the key focus areas for the future. This discussion is reflected in the final article in this supplement, which serves as a workshop summary (58).
Acknowledgments
We thank Carol Haggans, who logged innumerable hours editing and formatting the manuscripts in this supplement before submission for peer review. Her work was invaluable. We thank Joyce Merkel, who managed the references and related formatting for the manuscripts and who worked wonders with manuscript graphics and tables. Her contributions are much appreciated. In addition, we thank Paul Coates, Director of the NIH Office of Dietary Supplements, who supported and encouraged this project from its inception.
The authors’ responsibilities were as follows—CLT: developed the manuscript; PMB: critiqued the manuscript and provided input; and both authors: read and approved the final manuscript. Neither of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: ID, iron deficiency; IDA, iron deficiency anemia; IOM, Institute of Medicine; SF, serum ferritin; USPSTF, US Preventive Services Task Force.
REFERENCES
- 1.USPSTF. Iron deficiency anemia in pregnant women: screening and supplementation [Internet]. 2015. [cited 2016 Oct 4]. Available from: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/iron-deficiency-anemia-in-pregnant-women-screening-and-supplementation.
- 2.USPSTF. Iron deficiency anemia in young children: screening [Internet]. 2015. [cited 2016 Oct 4]. Available from: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/iron-deficiency-anemia-in-young-children-screening?ds=1&s=iron%20deficiency.
- 3.NIH Office of Dietary Supplements. Iron screening and supplementation in iron-replete pregnant women and young children [Internet]. c2016. [cited 2017 Feb 13]. Available from: https://ods.od.nih.gov/pubs/NIH_Iron_Workshop_Agenda.pdf.
- 4.Velentgas P, Dreyer NA, Wu AW. Outcome definition and measurement Velentgas P, Dreyer NA, Wu AW, editors. Developing a protocol for observational comparative effectiveness research: a user’s guide. Rockville (MD): Agency for Healthcare Research and Quality; 2013. p. 71–92. [PubMed] [Google Scholar]
- 5.Institute of Medicine Committee on Qualifications of Biomarkers and Surrogate Endpoints in Chronic Disease. Evaluation of biomarkers and surrogate endpoints in chronic disease. Washington (DC): The National Academies Press; 2010. [PubMed] [Google Scholar]
- 6.Institute of Medicine Food Nutrition Board. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. Washington (DC): The National Academics Press; 2001. [PubMed] [Google Scholar]
- 7.Garner CD. Nutrition in pregnancy In: Post TW, editor. UpToDate. Waltham (MA): Wolters Kluwer; 2017. [Google Scholar]
- 8.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 95: anemia in pregnancy. Obstet Gynecol 2008;112:201–7. [DOI] [PubMed] [Google Scholar]
- 9.Recommendations to prevent and control iron deficiency in the United States. Centers for Disease Control and Prevention. MMWR Recomm Rep 1998;47:1–29. [PubMed] [Google Scholar]
- 10.Cogswell ME, Kettel-Khan L, Ramakrishnan U. Iron supplement use among women in the United States: science, policy and practice. J Nutr 2003;133:1974S–7S. [DOI] [PubMed] [Google Scholar]
- 11.EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific opinion on dietary reference values for iron. EFSA J 2015;13:4254. [Google Scholar]
- 12.Milman N. Iron prophylaxis in pregnancy–general or individual and in which dose? Ann Hematol 2006;85:821–8. [DOI] [PubMed] [Google Scholar]
- 13.Scientific Advisory Committee on Nutrition. Iron and Health. London: TSO; 2010. [Google Scholar]
- 14.USDA Agricultural Research Group Food Surveys Research Group. What we eat in America, NHANES 2011-2012, day 1 food and supplement intake data [Internet]. 2015. [cited 2017 Feb 7]. Available from: https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/food-surveys-research-group/.
- 15.Bailey RL, Fulgoni VL III, Keast DR, Dwyer JT. Dietary supplement use is associated with higher intakes of minerals from food sources. Am J Clin Nutr 2011;94:1376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta PM, Hamner HC, Suchdev PS, Flores-Ayala R, Mei Z. Iron status of toddlers, nonpregnant females, and pregnant females in the United States. Am J Clin Nutr 2017;106(Suppl):1640S–6S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Domellöf M, Braegger C, Campoy C, Colomb V, Decsi T, Fewtrell M, Hojsak I, Mihatsch W, Molgaard C, Shamir R, et al. . Iron requirements of infants and toddlers. J Pediatr Gastroenterol Nutr 2014;58:119–29. [DOI] [PubMed] [Google Scholar]
- 18.Food and Nutrition Board Committee to Review WIC Food Package. Review of WIC food packages: improving balance and choice: final report. Washington (DC): The National Academies Press; 2017. [PubMed] [Google Scholar]
- 19.Eussen S, Alles M, Uijterschout L, Brus F, van der Horst-Graat J. Iron intake and status of children aged 6-36 months in Europe: a systematic review. Ann Nutr Metab 2015;66:80–92. [DOI] [PubMed] [Google Scholar]
- 20.American Academy of Pediatrics. Sample menu for an 8 to 12 month old [Internet]. 2015. [cited 2016 Sep 13]. Available from: https://www.healthychildren.org/English/ages-stages/baby/feeding-nutrition/Pages/Sample-One-Day-Menu-for-an-8-to-12-Month-Old.aspx.
- 21.Baker RD, Greer FR. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0-3 years of age). Pediatrics 2010;126:1040–50. [DOI] [PubMed] [Google Scholar]
- 22.US DA Agricultural Research Service Nutrient Data Laboratory. USDA national nutrient database for standard reference, release 28 [Internet]. 2015. [cited 2016 Sep 13]. Available from: https://ndb.nal.usda.gov/ndb/.
- 23.Mahoney DH. Iron deficiency in infants and young children: screening, prevention, clinical manifestations, and diagnosis In: Post TW, editor. UpToDate. Waltham (MA); Wolters Kluwer; 2016. [Google Scholar]
- 24.Fu S, Li F, Zhou J, Liu Z. The relationship between body iron status, iron intake and gestational diabetes: a systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darling AM, Mitchell AA, Werler MM. Preconceptional iron intake and gestational diabetes mellitus. Int J Environ Res Public Health 2016;13E525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bao W, Chavarro JE, Tobias DK, Bowers K, Li S, Hu FB, Zhang C. Long-term risk of type 2 diabetes in relation to habitual iron intake in women with a history of gestational diabetes: a prospective cohort study. Am J Clin Nutr 2016;103:375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bo S, Menato G, Villois P, Gambino R, Cassader M, Cotrino I, Cavallo-Perin P. Iron supplementation and gestational diabetes in midpregnancy. Am J Obstet Gynecol 2009;201:158.e1–6. [DOI] [PubMed] [Google Scholar]
- 28.Helin A, Kinnunen TI, Raitanen J, Ahonen S, Virtanen SM, Luoto R. Iron intake, haemoglobin and risk of gestational diabetes: a prospective cohort study. BMJ Open 2012;2:e001730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowers K, Yeung E, Williams MA, Qi L, Tobias DK, Hu FB, Zhang C. A prospective study of prepregnancy dietary iron intake and risk for gestational diabetes mellitus. Diabetes Care 2011;34:1557–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowers KA, Olsen SF, Bao W, Halldorsson TI, Strom M, Zhang C. Plasma concentrations of ferritin in early pregnancy are associated with risk of gestational diabetes mellitus in women in the Danish National Birth Cohort. J Nutr 2016;146:1756–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X, Scholl TO, Stein TP. Association of elevated serum ferritin levels and the risk of gestational diabetes mellitus in pregnant women: The Camden study. Diabetes Care 2006;29:1077–82. [DOI] [PubMed] [Google Scholar]
- 32.Chan KK, Chan BC, Lam KF, Tam S, Lao TT. Iron supplement in pregnancy and development of gestational diabetes–a randomised placebo-controlled trial. BJOG 2009;116:789–97, discussion 97–8. [DOI] [PubMed] [Google Scholar]
- 33.Kinnunen TI, Luoto R, Helin A, Hemminki E. Supplemental iron intake and the risk of glucose intolerance in pregnancy: re-analysis of a randomised controlled trial in Finland. Matern Child Nutr 2016;12:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamura T, Goldenberg RL, Johnston KE, Cliver SP, Hickey CA. Serum ferritin: a predictor of early spontaneous preterm delivery. Obstet Gynecol 1996;87:360–5. [DOI] [PubMed] [Google Scholar]
- 35.Goldenberg RL, Tamura T, DuBard M, Johnston KE, Copper RL, Neggers Y. Plasma ferritin and pregnancy outcome. Am J Obstet Gynecol 1996;175:1356–9. [DOI] [PubMed] [Google Scholar]
- 36.Scholl TO. High third-trimester ferritin concentration: associations with very preterm delivery, infection, and maternal nutritional status. Obstet Gynecol 1998;92:161–6. [DOI] [PubMed] [Google Scholar]
- 37.Lao TT, Tam KF, Chan LY. Third trimester iron status and pregnancy outcome in non-anaemic women; pregnancy unfavourably affected by maternal iron excess. Hum Reprod 2000;15:1843–8. [DOI] [PubMed] [Google Scholar]
- 38.Hwang JY, Lee JY, Kim KN, Kim H, Ha EH, Park H, Ha M, Kim Y, Hong YC, Chang N. Maternal iron intake at mid-pregnancy is associated with reduced fetal growth: results from Mothers and Children’s Environmental Health (MOCEH) study. Nutr J 2013;12:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dewey KG, Domellof M, Cohen RJ, Landa Rivera L, Hernell O, Lonnerdal B. Iron supplementation affects growth and morbidity of breast-fed infants: results of a randomized trial in Sweden and Honduras. J Nutr 2002;132:3249–55. [DOI] [PubMed] [Google Scholar]
- 40.Domellöf M, Cohen RJ, Dewey KG, Hernell O, Rivera LL, Lönnerdal B. Iron supplementation of breast-fed Honduran and Swedish infants from 4 to 9 months of age. J Pediatr 2001;138:679–87. [DOI] [PubMed] [Google Scholar]
- 41.Majumdar I, Paul P, Talib VH, Ranga S. The effect of iron therapy on the growth of iron-replete and iron-deplete children. J Trop Pediatr 2003;49:84–8. [DOI] [PubMed] [Google Scholar]
- 42.Lind T, Seswandhana R, Persson LA, Lönnerdal B. Iron supplementation of iron-replete Indonesian infants is associated with reduced weight-for-age. Acta Paediatr 2008;97:770–5. [DOI] [PubMed] [Google Scholar]
- 43.Idjradinata P, Watkins WE, Pollitt E. Adverse effect of iron supplementation on weight gain of iron-replete young children. Lancet 1994;343:1252–4. [DOI] [PubMed] [Google Scholar]
- 44.Pasricha SR, Hayes E, Kalumba K, Biggs BA. Effect of daily iron supplementation on health in children aged 4-23 months: a systematic review and meta-analysis of randomised controlled trials. Lancet Glob Health 2013;1:e77–86. [DOI] [PubMed] [Google Scholar]
- 45.Lozoff B, Castillo M, Clark KM, Smith JB. Iron-fortified vs low-iron infant formula: developmental outcome at 10 years. Arch Pediatr Adolesc Med 2012;166:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paganini D, Uyoga MA, Zimmermann MB. Iron fortification of foods for infants and children in low-income countries: effects on the gut microbiome, gut inflammation, and diarrhea. Nutrients 2016;8:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dostal A, Lacroix C, Bircher L, Pham VT, Follador R, Zimmermann MB, Chassard C. Iron modulates butyrate production by a child gut microbiota in vitro. MBio 2015;6:e01453–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaeggi T, Kortman GA, Moretti D, Chassard C, Holding P, Dostal A, Boekhorst J, Timmerman HM, Swinkels DW, Tjalsma H, et al. . Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 2015;64:731–42. [DOI] [PubMed] [Google Scholar]
- 49.Dostal A, Baumgartner J, Riesen N, Chassard C, Smuts CM, Zimmermann MB, Lacroix C. Effects of iron supplementation on dominant bacterial groups in the gut, faecal SCFA and gut inflammation: a randomised, placebo-controlled intervention trial in South African children. Br J Nutr 2014;112:547–56. [DOI] [PubMed] [Google Scholar]
- 50.Zimmermann MB, Chassard C, Rohner F, N’Goran EK, Nindjin C, Dostal A, Utzinger J, Ghattas H, Lacroix C, Hurrell RF. The effects of iron fortification on the gut microbiota in African children: a randomized controlled trial in Cote d’Ivoire. Am J Clin Nutr 2010;92:1406–15. [DOI] [PubMed] [Google Scholar]
- 51.NIH Office of Dietary Supplements. Vitamin D standardization program [Internet]. 2010. [cited 2017 Feb 16]. Available from: https://ods.od.nih.gov/Research/vdsp.aspx.
- 52.Westgard JO, Darcy T. The truth about quality: medical usefulness and analytical reliability of laboratory tests. Clin Chim Acta 2004;346:3–11. [DOI] [PubMed] [Google Scholar]
- 53.Petry N, Olofin I, Hurrell RF, Boy E, Wirth JP, Moursi M, Donahue Angel M, Rohner F. The proportion of anemia associated with iron deficiency in low, medium, and high human development index countries: a systematic analysis of national surveys. Nutrients 2016;8E693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pan Y, Jackson RT. Insights into the ethnic differences in serum ferritin between black and white US adult men. Am J Hum Biol 2008;20:406–16. [DOI] [PubMed] [Google Scholar]
- 55.WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. VMNIS Vitamin and Mineral Nutrition Information System. Geneva (Switzerland): WHO; 2011. [Google Scholar]
- 56.WHO. WHO guideline development group – the use of ferritin concentrations to assess iron status in populations [Internet]. 2016. [cited 2017 Feb 15]. Available from: http://www.who.int/nutrition/events/2016_meeting_guidelinedevelopmentgroup_ferritin_2to4march/en/.
- 57.Suchdev PS, Namaste S, Aaron G, Raiten D, Brown KH, Flores-Ayala R. Overview of the biomarkers reflecting inflammation and determinants of anemia project. Adv Nutr 2016;7:349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brannon PM, Stover PJ, Taylor CL. Integrating themes, evidence gaps, and research needs identified by workshop on iron screening and supplementation in iron-replete pregnant women and young children. Am J Clin Nutr 2017;106(Suppl):1703S–12S. [DOI] [PMC free article] [PubMed] [Google Scholar]