Abstract
What effects might arise from early life exposures to high iron? This review considers the specific effects of high iron on the brain, stem cells, and the process of erythropoiesis and identifies gaps in our knowledge of what molecular damage may be incurred by oxidative stress that is imparted by high iron status in early life. Specific areas to enhance research on this topic include the following: longitudinal behavioral studies of children to test associations between iron exposures and mood, emotion, cognition, and memory; animal studies to determine epigenetic changes that reprogram brain development and metabolic changes in early life that could be followed through the life course; and the establishment of human epigenetic markers of iron exposures and oxidative stress that could be monitored for early origins of adult chronic diseases. In addition, efforts to understand how iron exposure influences stem cell biology could be enhanced by establishing platforms to collect biological specimens, including umbilical cord blood and amniotic fluid, to be made available to the research community. At the molecular level, there is a need to better understand stress erythropoiesis and changes in iron metabolism during pregnancy and development, especially with respect to regulatory control under high iron conditions that might promote ineffective erythropoiesis and iron-loading anemia. These investigations should focus not only on factors such as hepcidin and erythroferrone but should also include newly identified interactions between transferrin receptor-2 and the erythropoietin receptor. Finally, despite our understanding that several key micronutrients (e.g., vitamin A, copper, manganese, and zinc) support iron’s function in erythropoiesis, how these nutrients interact remains, to our knowledge, unknown. It is necessary to consider many factors when formulating recommendations on iron supplementation.
Keywords: brain, early growth, erythropoiesis, excess iron, iron metabolism, iron toxicity, pregnancy, stem cells
INTRODUCTION
The coordinated relation between iron and oxygen underlies the basis for life but also gives rise to the metal’s toxicity and its ability to potentially interfere with the body’s normal development and function. This review was conducted to support discussions that took place during a workshop that was focused on iron screening and supplementation in pregnant women and young children. The focus of this review is the continuum that may exist in high, excessive, and toxic exposures to iron relative to molecular changes as well the nature of the research gaps that need to be addressed to answer questions about the consequences of high iron exposure. Pregnant women and young children may be particularly vulnerable to the adverse effects of excessive iron exposure, but at the same time, these development stages are often associated with recommendations to ensure iron intake.
Two-thirds of the body’s iron content is in hemoglobin, which transports oxygen from the lungs to the peripheral tissues. Myoglobin provides a local supply of oxygen to muscles, whereas neuroglobin, which is expressed in the central nervous system, is thought to protect against ischemic injury. Iron also plays an essential role in energy metabolism as a constituent of many mitochondrial enzymes of the electron transport chain, thereby participating in ATP production and oxygen consumption. However, excess iron promotes Fenton chemistry (Figure 1) to produce hydroxyl radicals and other reactive oxygen species (ROS), which damage lipids, DNA, and other cellular constituents. Because of the intimate relation between iron and oxygen metabolism, tissues and organs that have high oxidative metabolism are very susceptible to ROS damage and even more sensitive to adverse consequences of iron loading and its related toxicities.
FIGURE 1.
Targets of iron toxicity. Iron promotes Fenton chemistry, which promotes ROS. Organs that have high oxidative metabolism are the most susceptible to ROS damage and include the liver, heart, and pancreas. In early life, the brain may also be a sensitive target. Epigenetic changes that are induced by early life exposures may present in adult chronic diseases. ROS, reactive oxygen species.
Disorders that are associated with excessive iron help to elucidate some of the key topics of interest for excessive iron exposure. Excess iron can accumulate in both genetic and acquired overload disorders (1). Hereditary hemochromatosis or primary hemochromatosis originates from mutations in genes encoding HFE, hepcidin, hemojuvelin, transferrin receptor-2 (TfR2), and ferroportin. Of these conditions, HFE-associated hemochromatosis is the most common in Western populations and is usually detected in the fourth to fifth decades of life, whereas other forms are less frequent and can present clinical symptoms earlier in life (2). Excess iron that is accumulated because of such conditions can be readily measured by high serum ferritin, the iron-saturation state of transferrin, and the presence of nontransferrin-bound iron in the circulation. High circulating iron concentrations are also a complication of diseases such as thalassemia as a result of repeated blood transfusions. Iron loading from these and other disorders is considered to be acquired or secondary forms of hemochromatosis (3). Hemochromatosis patients load excess iron from the circulation into tissues and organs, particularly the liver, where iron is normally stored. Both primary and secondary iron overloads are associated with liver fibrosis and cirrhosis, but cardiac problems and pancreatic dysfunction are also observed. The effects of high iron are not necessarily reversible, but they can be managed by lowering amounts of iron through phlebotomy in cases of hereditary hemochromatosis (4). When erythroid function is defective, iron chelators may also be required.
In addition to these iron-loading disorders where circulating iron concentrations are high, a number of chronic diseases such as cancer, diabetes, cardiovascular disease, and many neurodegenerative conditions are also associated with excess tissue iron stores. In these pathologies, iron does not originate from excess circulating concentrations but may reflect intracellular iron accumulation that is due to chronic stress or inflammation (5–7). Although many studies are underway to evaluate whether iron depletion may help to improve or prevent chronic diseases, to our knowledge, it is not known whether the high amounts of tissue iron reflect a cause or consequence of the disease state.
Targets of particular interest relative to excessive iron exposure include the brain, stem cells, and the metabolic process of erythropoiesis. These targets are also particularly relevant to the growth and development that are associated with pregnancy and young childhood.
BRAIN
Certain aspects of neurodegenerative disease bolster the idea that the brain is a particularly sensitive target for adverse consequences of excessive iron (Figure 1). Brain iron increases with age, and higher amounts of iron have been linked to Alzheimer disease, Parkinson disease, and other degenerative conditions (8). Increased brain iron alters the behavior and mood (9). Animal studies have provided us with some information about these effects. After iron loading by intraperitoneal injection, rats displayed greater anxiety responses when tested by elevated plus maze (10). Adult rats that were fed a high–carbonyl-iron diet (20,000 parts per million) also displayed impaired behaviors (11). In genetic models of hemochromatosis associated with HFE 67D, mice showed increased repetitive behavior, thereby suggesting that behavioral dysfunction may be associated with iron-overload diseases as well (12). Nonetheless, relatively few studies have followed the influence of iron loading on emotional behavior in humans, and for the purposes of this workshop, note that iron supplementation of anemic women has been reported to improve depression and stress scales along with cognitive function (13).
Iron influx into the brain is more rapid during the postnatal period for rodents (14–16). Therefore, this time of life confers a susceptibility to iron overload, and many studies have documented effects on learning and memory during the postnatal development of mice (17–20). In contrast, relatively little is known about brain iron uptake or its consequences during early human development. On the basis of the data from animal studies, it is probable that exposure to excess iron during the postnatal period could negatively influence emotional, motor, and cognitive behaviors. There are ≥2 human studies that support this idea. Tamura et al. (21) measured cord blood ferritin concentrations and found that the high concentrations (>188 μg/L) were associated with low full-scale intelligence-quotient scores. In a second study, Lozoff et al. (22) assessed developmental outcomes in 10-y-old children who were given an iron-fortified formula (∼13 mg/L) or a low-iron formula (∼2.3 mg/L) and reported adverse effects with higher iron concentrations. Another article in these proceedings (23) addresses anthropomorphic differences that have been observed for infants who were fed higher amounts of iron, thus supporting a general conclusion that more iron is not necessarily better for early childhood development, although sufficient iron is definitely essential.
What are the molecular mechanisms through which excess iron might alter early development, particularly in the human brain? It is generally thought that conditions that underlie oxidative stress contribute to the developmental origins of many forms of adult chronic disease (24). Thus, ROS that are generated by excess tissue iron loading may provoke changes in redox metabolism that alter the pattern of DNA methylation. The chemical oxidation of 5-methylcytosine to 5-hydroxymethylcytosine in cytosine and guanine bases that are separated by one phosphate disrupt interactions with methyl-binding proteins. Altered methylation patterns confer epigenetic regulation and metabolic reprogramming (25). What tissue-specific epigenetic changes are induced by oxidative stress in response to high iron exposures could be better defined through animal research, and subsequent longitudinal human studies could include screens for specific epigenetic marks to confirm associations with early life iron exposure and adult disease. Ultimately, metabolic indicators that reflect iron deficiency (ID) and iron-overload status must be established.
STEM CELLS
Embryonic or adult stem cells differentiate into specialized cells of the body. Embryonic stem cells are pluripotent and can divide and multiply into any cell type, but very little is known about the effects of excess iron during early stages of development or how differentiation programs might be altered to induce the fetal origins of adult disease. Much of the focus of scientific research in this area has been on ID, where the most important concerns about human development have been established (26). One reason why the influence of iron excess has not been well studied is the notion that fetal iron concentrations are buffered from maternal iron by the placenta. Although an adequate supply of iron is provided at the expense of maternal iron stores, it is thought that excess iron would not be transferred (27). Thus, many questions remain unanswered.
One window of research opportunity to better understand these interactions may be provided through the collection of umbilical cord blood. Cord blood contains fetal and adult hematopoietic progenitor stem cells that could be isolated for further study (28). These progenitor cells give rise to differentiated blood cells and, therefore, have a unique functional relation with iron metabolism. Several studies have shown that maternal iron does not correlate with umbilical cord blood iron (27, 29, 30), but it has been reported that a high maternal serum ferritin concentration is associated with reduced numbers of hematopoietic stem cells (HSCs) in cord blood (30). This effect could possibly reflect fetal-iron metabolism in HSC development (see Erythropoiesis), but further research is necessary to define whether HSCs are lower because of impaired maternal iron delivery or the presence of iron excess during fetal development. Multipotent stem cells are also present in amniotic fluid and can be induced to differentiate into a variety of cell types (e.g., adipose, bone, muscle, and liver). The availability of these cells represents a second biological resource for future research efforts to understand how iron status might influence early embryonic and fetal stem cell development (31).
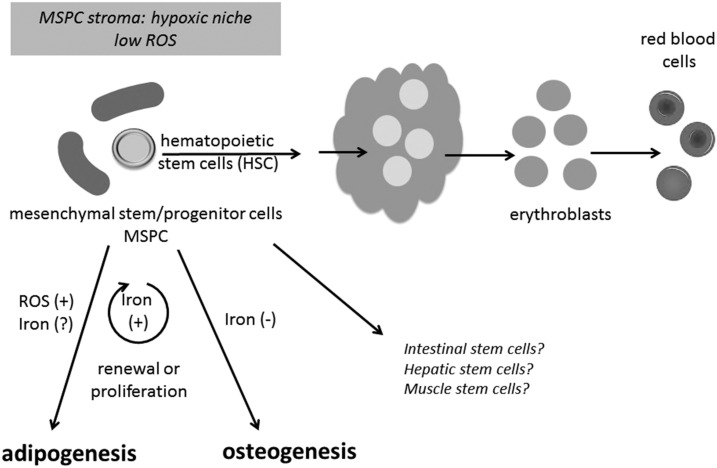
Unlike embryonic stem cells, adult stem cells have a more limited capacity to develop into different cell types, but they are able to regenerate through cell division and can be induced experimentally to become tissue-specific cells with a highly specialized function. Adult stem cells are present in bone marrow, adipose tissue, the endothelium, and other tissues. Their differentiation program to specialized functions is typically reflected in the stem cell’s microenvironment or niche. Depending on the locale, different cues are present that induce adult stem cells to differentiate into specific cell types. Figure 2 depicts stem cells in the bone marrow. Here, mesenchymal stem/progenitor cells (MSPCs) provide a stromal niche for HSCs that can regenerate and differentiate into erythroid cells. This microenvironment is believed to be hypoxic to keep ROS concentrations low. The low oxygen environment would protect against DNA damage for cell renewal and hematopoiesis. This niche can also promote events that lead to an early metabolic switch from glycolytic to oxidative metabolism during proliferation and lineage commitment (i.e., metabolic memory). High iron conditions would interfere with the microenvironment and metabolic switches that are necessary to support HSCs and hematopoiesis. In addition, MSPCs themselves can differentiate to a number of cell types including osteoblasts, myocytes, and adipocytes (Figure 2). ROSs are known to have a strong influence on MSPC proliferation and differentiation (24). It has been shown that the iron loading of MSPCs in vitro increases cell proliferation but blocks their differentiation to osteogenic stem cells (32, 33). In contrast, ROSs appear to increase adipogenesis (34). How iron status contributes to MSPC growth and proliferation has yet to be fully explored, but note that adult patients with a genetic iron-overload disease have defects in bone mineral density (35) and that excess iron has been associated with diabetes and metabolic syndrome (36). These observations raise further questions about whether oxidative stress that is promulgated by the use of iron-fortified infant formula, e.g., could promote the early origins of adult disease.
FIGURE 2.
Bone marrow stem cell niche is hypoxic and sensitive to oxidative stress. Bone marrow MSPCs provide a stromal niche for HSCs that can differentiate into erythroid cells. This hypoxic microenvironment keeps ROS concentrations low, protecting against DNA damage for cell renewal and hematopoiesis and promoting events that lead to an early metabolic switch from glycolytic to oxidative metabolism during proliferation and lineage commitment (i.e., metabolic memory). MSPCs also differentiate to adipocytes and osteoblasts, and lineage development is sensitive to iron and ROS. HSC, hematopoietic stem cell; MSPC, mesenchymal stem/progenitor cell; ROS, reactive oxygen species.
Although the influence of iron on MSPC differentiation programs remains to be more fully determined, the differentiation program of HSCs to produce myeloid, lymphoid, or erythroid cells has been much-better studied. With respect to iron metabolism, the differentiation of HSCs to erythropoietic progenitors is significant because of the functional need for hemoglobin synthesis. Iron uptake from transferrin is critical at a very early stage because heme production must precede globin synthesis. In mice lacking a heme exporter, the accumulation of excess intracellular heme, which can increase oxidative stress, leads to ineffective erythropoiesis (37). These findings suggest that exposures to high iron during hematopoiesis in early life might induce anemia with profound developmental effects.
ERYTHROPOIESIS
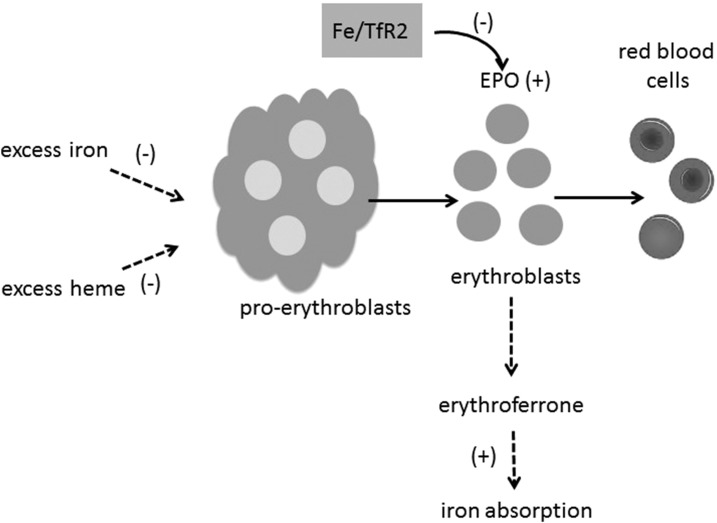
As previously mentioned, erythropoiesis and iron metabolism are connected by the need for iron to fulfill heme synthesis during red blood cell (RBC) production. In pregnancy and early development, this demand is high because of RBC expansion. Iron intake is controlled by the actions of erythroferrone, a hormone produced by erythroblasts (early erythroid progenitor cells) as a negative regulator of the liver-derived hormone hepcidin; therefore, erythroferrone is a positive regulator of intestinal iron absorption (38). Erythropoietin is produced by the kidneys and is another positive regulator of iron absorption. Erythropoietin induces the proliferation of proerythroblasts, which, in turn, differentiate into erythroblasts (39). During murine fetal development and pregnancy, high erythropoietin concentrations are known to induce “stress erythropoiesis” (40), thereby resulting in conditional ID. This conditional deficiency drives erythropoietin synthesis to increase erythroblasts and, thus, erythroferrone concentrations and, thereby, reduce hepcidin concentrations to enhance iron uptake (Figure 3).
FIGURE 3.
Erythropoiesis and iron metabolism are connected by red blood cell production. Iron intake is controlled by the actions of erythroferrone, a hormone produced by erythroblasts as a positive regulator of intestinal iron absorption. EPO also positively regulates iron absorption, thereby inducing the proliferation of proerythroblasts, which, in turn, differentiate into erythroblasts. This process is negatively regulated by excess iron or heme. The iron saturation of transferrin modulates TfR2, which interacts with the EPO receptor and reduces EPO sensitivity. EPO, erythropoietin; TfR2, transferrin receptor-2.
To our knowledge, how excess iron or ROS might directly alter these regulatory pathways is unknown except for the well-established effects on hepatic hepcidin synthesis (41). Evidence supporting the notion that erythropoiesis is a target for iron toxicity has been indirect. Iron-chelating agents improve hemoglobin concentrations and reduce the need for blood transfusions in iron-loaded patients with myelodysplastic syndrome and myelofibrosis, suggesting that high iron may exert negative effects (42). There has been limited evidence from in vitro studies that high iron blocks early progenitor development and erythroblast differentiation that are due to ROS (42), but no definitive conclusions can be made about the influence of iron status in healthy individuals. Recent studies in knockout mice have suggested that high transferrin saturation (reflecting high iron status) could influence erythropoietin function through TfR2. Humans and mice with defects in TfR2 develop iron overload, and this receptor is considered to be an iron sensor for the liver. TfR2 also forms a complex with the erythropoietin receptor in erythroblasts, and the presence of high iron may alter erythropoietin sensitivity to limit erythropoiesis by modulating the number of functional erythropoietin receptors (43). The multiple inputs reflecting high iron status during erythropoiesis are highlighted in Figure 3.
Other micronutrient deficiencies strongly influence erythropoiesis and may modify iron metabolism (44). For example, vitamin A deficiency is associated with anemia, and its effects might involve functions in erythropoiesis, the mobilization of iron stores, or the immune response (45). Copper is required for ferroxidase function to transport and mobilize iron stores, and hepatic iron overload is observed in copper-deficient humans. Zinc-dependent transcription factors help to maintain adequate erythropoiesis, thus pointing to the need for zinc sufficiency to support iron’s function. Finally, mitochondrial manganese superoxide dismutase function is necessary for erythrocyte production in mice (46), and connections between human iron and manganese metabolism are well known. Iron supplementation in the presence of other micronutrient deficiencies is ineffective, and thus it is worth considering whether the lack or excess of interacting micronutrients influences iron’s adverse effects on various functions that are related to early development. Because single micronutrients are usually a research focus for investigation, nutrient-nutrient interactions remain very poorly understood. This is another area that might be incorporated into initiatives to learn more about the effects of iron exposures during pregnancy and early childhood.
RESEARCH GAPS
Considering the paucity of information about the effects of excess iron during pregnancy and early childhood, we need to rely on evidence from animal studies to project what areas of research need to be better addressed in human studies. Rodent studies have indicated that postnatal exposure to high iron, at a time of greater influx to the brain, promotes neuronal damage that affects cognition and memory. There are additional lines of evidence that argue that iron status can affect behavior and mood. The possible links between iron exposure and brain development in children should be explored in longitudinal behavioral studies. Moreover, increasing evidence has revealed that oxidative stress, which may be imparted by tissue iron accumulation, may induce early epigenetic changes that could lead to chronic disease later in life. The age-dependent influence of iron on epigenetics could be documented through better animal-research studies. Results of such analyses might pave the way for a more detailed investigations into the human life course to better understand how early life exposures influence the progression of adult disease. The development of tools to monitor epigenetic markers that are associated with iron exposures is just one critical aspect that will be necessary to explore these interactions.
Effects of iron on stem cell proliferation and differentiation also deserve investigation with a focus on the understanding of disease implications later in life (e.g., osteoporosis and metabolic syndrome). In particular, the role of iron in HSC ROS regulation could be better defined. In this regard, efforts to establish biological resources such as umbilical cord blood and amniotic fluid samples could help explore early stem cell effects. For example, specimen-collection banks could be established for materials to be made available for researchers to explore these fundamental questions.
In conclusion, whether high iron affects erythropoiesis and, in particular, stress erythropoiesis is not clear. The influence of the iron sensor TfR2 on erythropoietin sensitivity may be relevant in iron-loading anemia. Ineffective erythropoiesis will evolve an anemic state that has been repeatedly shown to be detrimental to early development. Molecular studies to determine what steps in erythropoiesis are sensitive to a high iron condition could provide insight into potential interventions. Similarly, despite our knowledge that several key micronutrients (e.g., vitamin A, copper, manganese, and zinc) support iron’s function in erythropoiesis, how these nutrients interact remains unknown to our knowledge. It is necessary to consider many factors when formulating recommendations for iron supplementation because these nutrient-nutrient interactions could possibly contribute to iron-induced toxicity. Research on mixtures of micronutrients can be carried out in both human and animal studies to establish a more comprehensive and holistic view of nutritional needs during pregnancy and early childhood.
Acknowledgments
The author’s responsibilities were as follows—the sole author prepared the manuscript and read and approved the final manuscript. The author reported no conflicts of interest related to the study.
Footnotes
Abbreviations used: HSC, hematopoietic stem cell; ID, iron deficiency; MSPC, mesenchymal stem/progenitor cell; RBC, red blood cell; ROS, reactive oxygen species; TfR2, transferrin receptor-2.
REFERENCES
- 1.Silva B, Faustino P. An overview of molecular basis of iron metabolism regulation and the associated pathologies. Biochim Biophys Acta 2015;1852:1347–59. [DOI] [PubMed] [Google Scholar]
- 2.Vujić M. Molecular basis of HFE-hemochromatosis. Front Pharmacol 2014;5:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siddique A, Kowdley KV. Review article: the iron overload syndromes. Aliment Pharmacol Ther 2012;35:876–93. [DOI] [PubMed] [Google Scholar]
- 4.Rombout-Sestrienkova E, van Kraaij MG, Koek GH. How we manage patients with hereditary haemochromatosis. Br J Haematol 2016;175:759–70. [DOI] [PubMed] [Google Scholar]
- 5.Torti SV, Torti FM. Iron and cancer: more ore to be mined. Nat Rev Cancer 2013;13:342–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vinchi F, Muckenthaler MU, Da Silva MC, Balla G, Balla J, Jeney V. Atherogenesis and iron: from epidemiology to cellular level. Front Pharmacol 2014;5:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 2014;13:1045–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivera-Mancía S, Pérez-Neri I, Ríos C, Tristán-López L, Rivera-Espinosa L, Montes S. The transition metals copper and iron in neurodegenerative diseases. Chem Biol Interact 2010;186:184–99. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, Wessling-Resnick M. Iron and mechanisms of emotional behavior. J Nutr Biochem 2014;25:1101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sobotka TJ, Whittaker P, Sobotka JM, Brodie RE, Quander DY, Robl M, Bryant M, Barton CN. Neurobehavioral dysfunctions associated with dietary iron overload. Physiol Behav 1996;59:213–9. [DOI] [PubMed] [Google Scholar]
- 11.Maaroufi K, Had-Aissouni L, Melon C, Sakly M, Abdelmelek H, Poucet B, Save E. Effects of prolonged iron overload and low frequency electromagnetic exposure on spatial learning and memory in the young rat. Neurobiol Learn Mem 2009;92:345–55. [DOI] [PubMed] [Google Scholar]
- 12.Chang J, Kueon C, Kim J. Influence of lead on repetitive behavior and dopamine metabolism in a mouse model of iron overload. Toxicol Res 2014;30:267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beard JL, Hendricks MK, Perez EM, Murray-Kolb LE, Berg A, Vernon-Feagans L, Irlam J, Isaacs W, Sive A, Tomlinson M. Maternal iron deficiency anemia affects postpartum emotions and cognition. J Nutr 2005;135:267–72. [DOI] [PubMed] [Google Scholar]
- 14.Taylor EM, Morgan EH. Developmental changes in transferrin and iron uptake by the brain in the rat. Brain Res Dev Brain Res 1990;55:35–42. [DOI] [PubMed] [Google Scholar]
- 15.Roskams AJ, Connor JR. Iron, transferrin, and ferritin in the rat brain during development and aging. J Neurochem 1994;63:709–16. [DOI] [PubMed] [Google Scholar]
- 16.Focht SJ, Snyder BS, Beard JL, Van Gelder W, Williams LR, Connor JR. Regional distribution of iron, transferrin, ferritin, and oxidatively-modified proteins in young and aged Fischer 344 rat brains. Neuroscience 1997;79:255–61. [DOI] [PubMed] [Google Scholar]
- 17.Fredriksson A, Schroder N, Eriksson P, Izquierdo I, Archer T. Maze learning and motor activity deficits in adult mice induced by iron exposure during a critical postnatal period. Brain Res Dev Brain Res 2000;119:65–74. [DOI] [PubMed] [Google Scholar]
- 18.Schröder N, Fredriksson A, Vianna MR, Roesler R, Izquierdo I, Archer T. Memory deficits in adult rats following postnatal iron administration. Behav Brain Res 2001;124:77–85. [DOI] [PubMed] [Google Scholar]
- 19.Fredriksson A, Schroder N, Eriksson P, Izquierdo I, Archer T. Neonatal iron exposure induces neurobehavioural dysfunctions in adult mice. Toxicol Appl Pharmacol 1999;159:25–30. [DOI] [PubMed] [Google Scholar]
- 20.de Lima MN, Polydoro M, Laranja DC, Bonatto F, Bromberg E, Moreira JC, Dal-Pizzol F, Schroder N. Recognition memory impairment and brain oxidative stress induced by postnatal iron administration. Eur J Neurosci 2005;21:2521–8. [DOI] [PubMed] [Google Scholar]
- 21.Tamura T, Goldenberg RL, Hou J, Johnston KE, Cliver SP, Ramey SL, Nelson KG. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J Pediatr 2002;140:165–70. [DOI] [PubMed] [Google Scholar]
- 22.Lozoff B, Castillo M, Clark KM, Smith JB. Iron-fortified vs low-iron infant formula: developmental outcome at 10 years. Arch Pediatr Adolesc Med 2012;166:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lönnerdal B. Excess iron intake as a factor in growth, infections, and development of infants and young children. Am J Clin Nutr 2017;106(Suppl):1681S–7S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanhees K, Vonhogen IG, van Schooten FJ, Godschalk RW. You are what you eat, and so are your children: the impact of micronutrients on the epigenetic programming of offspring. Cell Mol Life Sci 2014;71:271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hitchler MJ, Domann FE. Redox regulation of the epigenetic landscape in cancer: a role for metabolic reprogramming in remodeling the epigenome. Free Radic Biol Med 2012;53:2178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen LH. Anemia and iron deficiency: effects on pregnancy outcome. Am J Clin Nutr 2000;71:1280S–4S. [DOI] [PubMed] [Google Scholar]
- 27.Hay G, Refsum H, Whitelaw A, Melbye EL, Haug E, Borch-Iohnsen B. Predictors of serum ferritin and serum soluble transferrin receptor in newborns and their associations with iron status during the first 2 y of life. Am J Clin Nutr 2007;86:64–73. [DOI] [PubMed] [Google Scholar]
- 28.Broxmeyer HE, Srour E, Orschell C, Ingram DA, Cooper S, Plett PA, Mead LE, Yoder MC. Cord blood stem and progenitor cells. Methods Enzymol 2006;419:439–73. [DOI] [PubMed] [Google Scholar]
- 29.Siddappa AM, Rao R, Long JD, Widness JA, Georgieff MK. The assessment of newborn iron stores at birth: a review of the literature and standards for ferritin concentrations. Neonatology 2007;92:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pope B, Hokin B, Grant R. Effect of maternal iron status on the number of CD34+ stem cells harvested from umbilical cord blood. Transfusion 2014;54:1876–80. [DOI] [PubMed] [Google Scholar]
- 31.De Coppi P, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 2007;25:100–6. [DOI] [PubMed] [Google Scholar]
- 32.Balogh E, Tolnai E, Nagy B Jr, Nagy B, Balla G, Balla J, Jeney V.. Iron overload inhibits osteogenic commitment and differentiation of mesenchymal stem cells via the induction of ferritin. Biochim Biophys Acta 2016;1862:1640–9. [DOI] [PubMed] [Google Scholar]
- 33.Borriello A, Caldarelli I, Speranza MC, Scianguetta S, Tramontano A, Bencivenga D, Stampone E, Negri A, Nobili B, Locatelli F, et al. Iron overload enhances human mesenchymal stromal cell growth and hampers matrix calcification. Biochim Biophys Acta 2016;1860:1211–23. [DOI] [PubMed] [Google Scholar]
- 34.Atashi F, Modarressi A, Pepper MS. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: a review. Stem Cells Dev 2015;24:1150–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guggenbuhl P, Deugnier Y, Boisdet JF, Rolland Y, Perdriger A, Pawlotsky Y, Chales G. Bone mineral density in men with genetic hemochromatosis and HFE gene mutation. Osteoporos Int 2005;16:1809–14. [DOI] [PubMed] [Google Scholar]
- 36.Simcox JA, McClain DA. Iron and diabetes risk. Cell Metab 2013;17:329–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doty RT, Phelps SR, Shadle C, Sanchez-Bonilla M, Keel SB, Abkowitz JL. Coordinate expression of heme and globin is essential for effective erythropoiesis. J Clin Invest 2015;125:4681–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kautz L, Nemeth E. Molecular liaisons between erythropoiesis and iron metabolism. Blood 2014;124:479–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oburoglu L, Romano M, Taylor N, Kinet S. Metabolic regulation of hematopoietic stem cell commitment and erythroid differentiation. Curr Opin Hematol 2016;23:198–205. [DOI] [PubMed] [Google Scholar]
- 40.Paulson RF, Shi L, Wu DC. Stress erythropoiesis: new signals and new stress progenitor cells. Curr Opin Hematol 2011;18:139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ganz T. Systemic iron homeostasis. Physiol Rev 2013;93:1721–41. [DOI] [PubMed] [Google Scholar]
- 42.Lu W, Zhao M, Rajbhandary S, Xie F, Chai X, Mu J, Meng J, Liu Y, Jiang Y, Xu X, et al. Free iron catalyzes oxidative damage to hematopoietic cells/mesenchymal stem cells in vitro and suppresses hematopoiesis in iron overload patients. Eur J Haematol 2013;91:249–61. [DOI] [PubMed] [Google Scholar]
- 43.Nai A, Lidonnici MR, Rausa M, Mandelli G, Pagani A, Silvestri L, Ferrari G, Camaschella C. The second transferrin receptor regulates red blood cell production in mice. Blood 2015;125:1170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan LN, Mike LA. The science and practice of micronutrient supplementations in nutritional anemia: an evidence-based review. JPEN J Parenter Enteral Nutr 2014;38:656–72. [DOI] [PubMed] [Google Scholar]
- 45.Semba RD, Bloem MW. The anemia of vitamin A deficiency: epidemiology and pathogenesis. Eur J Clin Nutr 2002;56:271–81. [DOI] [PubMed] [Google Scholar]
- 46.Case AJ, Madsen JM, Motto DG, Meyerholz DK, Domann FE. Manganese superoxide dismutase depletion in murine hematopoietic stem cells perturbs iron homeostasis, globin switching, and epigenetic control in erythrocyte precursor cells. Free Radic Biol Med 2013;56:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]