Significance
Adult neurons are not able to make new connections as easily as developing neurons can; however, future therapies aimed at regeneration and repair of neural circuits in the adult nervous system depend critically on the formation of such connections. Here, we studied a recently discovered cell population that has the unusual ability to make new connections into adulthood, but under normal conditions does not grow new axons or dendrites, so that no new cells are contacted. We manipulated this cell population to induce axon and dendrite outgrowth using transgenic methods and determined that this results in stable, functional connections with new cells.
Keywords: plasticity, bipolar cell, avoidance, retina, dendritic arbor
Abstract
Mature mammalian neurons have a limited ability to extend neurites and make new synaptic connections, but the mechanisms that inhibit such plasticity remain poorly understood. Here, we report that OFF-type retinal bipolar cells in mice are an exception to this rule, as they form new anatomical connections within their tiled dendritic fields well after retinal maturity. The Down syndrome cell-adhesion molecule (Dscam) confines these anatomical rearrangements within the normal tiled fields, as conditional deletion of the gene permits extension of dendrite and axon arbors beyond these borders. Dscam deletion in the mature retina results in expanded dendritic fields and increased cone photoreceptor contacts, demonstrating that DSCAM actively inhibits circuit-level plasticity. Electrophysiological recordings from Dscam−/− OFF bipolar cells showed enlarged visual receptive fields, demonstrating that expanded dendritic territories comprise functional synapses. Our results identify cell-adhesion molecule-mediated inhibition as a regulator of circuit-level neuronal plasticity in the adult retina.
The visual system is an exemplary model for studies of nervous system development and plasticity. During development, retinal neurons in mammals readily extend axons and dendrites to make novel synaptic connections and refine them (1–3), but when development concludes, the ability to form new synaptic connections diminishes (4–7). Known factors limiting neuronal plasticity and regeneration in the adult central nervous system (CNS) include the postmitotic state of neurons, synaptic activity (8–10), immune system elements (11, 12), reduced availability of growth factors (13), neuron–glia interactions (14–16), and modifications to the extracellular milieu (16). Together, these factors help balance the need for adaptation to the natural stimulus environment with the need to maintain circuit-level stability required for vision (17).
Retinal bipolar cells (BCs) are excitatory neurons that receive dendritic input from the photoreceptors and send glutamatergic synaptic output to retinal ganglion cells (RGCs), which form the optic nerve and project to central brain nuclei. Because BCs are the unique link connecting the input and output layers of the retina, understanding BC plasticity is an important objective for approaches aimed at neuronal regeneration and repair of photoreceptors or RGCs into existing circuitry following retinal diseases, such as diabetic retinopathy and macular degeneration. However, the extent and mechanisms of BC plasticity at the cellular and circuit-level remain unclear. Here, we report that OFF-type BCs, those responsive to light decrements, in the intact adult retina retain the ability to make new synapses and continue to increase synapse number and dendritic arbor complexity out to at least 6 mo of age, well after the mouse retina is considered mature (18). This continued elaboration of synaptic architecture is different from that of ON-type BCs, which stabilizes during development (19).
The observed continued synaptogenesis in WT mice occurred within the tiled dendritic territory of each BC (20). Tiling is an organizational principal common to both vertebrate and invertebrate nervous systems in which the dendritic or axonal arbors of adjacent cells have minimal overlap (21). Axon tiling of some neuron types in Drosophila has been shown to require Dscam2, but other Drosophila genes or genes responsible for dendritic tiling or tiling in vertebrates have not been described (22). Guided by previous reports that BC tiling is regulated by homotypic interactions (23), we identified the Down syndrome cell adhesion molecule (Dscam) as required for OFF BC axon and dendrite tiling. Previous studies of Dscam function within the vertebrate retina focused on studies of neuron soma localization and neurite lamination in cell types whose axonal and dendritic arbors do not tile (24, 25). In chick, the DSCAM protein is localized to a narrow band of the retinal inner plexiform layer (IPL) and has been proposed to function in laminar targeting through adhesion (25). In contrast, in mouse, ground squirrel, and macaque, DSCAM is broadly distributed throughout the IPL and at the cone terminal in the outer plexiform layer (OPL) (26–29). Previous studies in mouse found that major DSCAM functions are to limit excessive adhesion between the dendrites of cells that normally overlap and in regulating developmental cell death, possibly in the context of a larger adhesion code with other factors providing cell-type–specific identity cues (24, 30). By labeling and tracing fields of single BCs, we found that Dscam preserves tiling and limits plasticity in retinal OFF BCs by inhibiting outgrowth of both dendritic and axonal arbors through interactions between homotypic cells. Electrophysiological whole-cell recordings from genetically identified OFF BCs lacking Dscam revealed that these cells have enlarged spatial visual receptive fields, demonstrating that the expanding arbors of Dscam-null OFF BCs establish functional synaptic connections with cones beyond the limits of their typical tiled receptive fields. To test whether these enlarged fields also formed after developmental maturity, we deleted Dscam in the mature retina. Delayed deletion, too, activated BC dendrite outgrowth and increased the number of cones contacted per BC. These results identify a function for DSCAM proteins in a mature neural circuit. They also establish blocking Dscam function as a paradigm for triggering synaptogenesis and circuit-level plasticity in the adult mouse retina.
Results
We used Htr2a transgenic mice (31, 32) and immunohistochemistry (IHC) against cell-type–specific markers (33) to study structural development and neuronal plasticity of OFF-type BCs during development [postnatal days (P) 15 and 21] and following developmental maturity (P35, 3 mo, and 6 mo). We quantified dendritic and axonal arbor morphology of more than 1,500 type 3b and type 4 BCs (BC3s, BC4s) through manual tracing in confocal fluorescence image stacks of retinal whole-mounts obtained at five time points (Fig. S1 and Movie S1), and assessed functional correlates of observed morphological changes with electrophysiological recordings of fluorescence-labeled BC4s in the whole-mount retina, in vitro.
BC4s Form Novel Dendritic Connections with a Fixed Set of Cones After Developmental Maturity.
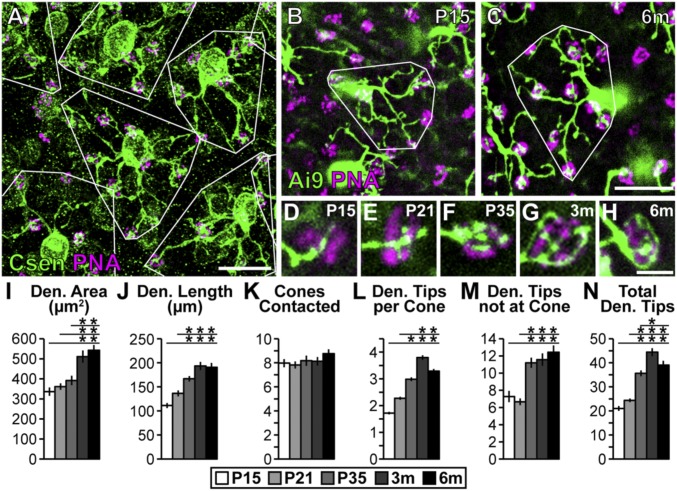
Confocal imaging of retinal whole mounts (Fig. 1A) showed that BC4 dendritic arbors in the adult retina tile the retinal outer plexiform layer with minimal overlap, consistent with previous reports (20, 33–35). Comparing BC4 dendritic arbor morphology from P15 through 6 mo (Fig. 1 B–H), we discovered that BC4s continue to elaborate their dendritic arbors after reaching developmental maturity at ∼1 mo of age (18). This anatomical plasticity was unexpected, and contrasts with ON-type BCs, which establish their adult complement of synaptic connections early in development, at ∼P14 (36).
Fig. 1.
BC4s elaborate their dendritic arbors into adulthood. (A) Whole-mount retina (3 mo) stained with anticalsenilin antibody and PNA to visualize BC4s and cone photoreceptors, respectively. Dendrite areas are outlined in white to show tiling. (Scale bar, 25 μm.) (B and C) Genetically labeled BC4s (HTR:Cre × Ai9) at P15 (B) and 6 mo (C). Dendritic arbors occupy more area and elaborate their arbors throughout development and into adulthood. (Scale bar, 25 μm.) (D–H) High-magnification images of BC4 connections with cones from P15 to 6 mo. BC4s elaborate their innervation of cones over time until they become almost paddle shaped. (Scale bar, 5 μm.) (I–N) Quantification of BC4 characteristics at P15, P21, P35, 3 mo, and 6 mo. Significant increases in all measurements except number of cones contacted were detected (ANOVA P values in I, J, L–N: P < 0.0001; in K: P = 0.48). Horizontal lines above columns in I–N represent comparisons between the column under the left end of the line and all columns to the right. An asterisk above a point on the line represents a post hoc significance test value, indicating a significant difference between the left-most column under the line and the column under the asterisk. Traced cells: n = 36, 39, 40, 40, and 30 at P15, P21, P35, 3 mo, and 6 mo, respectively. Error bars = SEM. WT data included also in Figs. 2 and 3 and Figs. S4 and S5. Csen, calsenilin; Den., dendrite; m, month; P, postnatal day.
To determine which aspects of the BC4 dendritic arbor change with age, we used quantitative measures to compare dendritic arbor morphology from P15 to 6 mo. We found a modest increase in dendritic field area and total length of BC4 dendrites starting at P15 and continuing to 6 mo (Fig. 1 I and J). Furthermore, we found that while the total number of cones contacted per BC4 remained constant (Fig. 1K), both the number of BC4 dendritic tips at each cone terminal, each of which contains multiple synaptic ribbons, and the number of dendritic tips not at cones increased over time (Fig. 1 L–N). This was surprising and indicates that mouse BC4s may continue to form new anatomical connections with cones well after retinal maturity (ages > 1 mo). Because the number of cones contacted did not increase in WT after P15 (Fig. 1K), these new connections were apparently confined to the set of cones within each cell’s tiled dendritic field established during development. Based on these results, we speculated that mature BC4s might form synaptic connections with an increasing number of cones when the constraints on dendritic outgrowth are lifted, for example, by removing factors that enforce dendritic field tiling.
DSCAM Limits BC4 Plasticity by Enforcing Tiling of Dendritic Arbors.
We hypothesized that the ability of adult BC4s to expand dendritic arbors and contact previously not-contacted cones in WT mice is constrained by homotypic inhibition from neighboring BC4s. An important candidate for blocking circuit-level plasticity by preventing dendritic outgrowth is Dscam. Dscam is expressed in all OFF-type BCs, except BC1s (27, 37). To test the role of Dscam in establishing and maintaining BC4 tiling, we utilized genetic knockout mice and a floxed allele of Dscam in combination with HTR:Cre mice. HTR:Cre selectively targets Dscam deletion to BC4s with minimal disruption to retinal organization and no detectable change in BC4 cell number, common complications of Dscam deletion in other cell types (24) (Figs. S2 and S3). Dscams have been shown to regulate neurite laminar targeting through adhesion in Drosophila (38), chick (25), and at least one retinal cell type in mouse (26). Since altered laminar targeting may impact cell physiology (39), we first tested if selective deletion of Dscam in BC4 cells affects their laminar targeting. We found that this was not the case: conditional deletion of Dscam altered neither laminar targeting of BC4 axons (Fig. S2 A–C and H) nor the distribution and density of upstream cone photoreceptors (Fig. S3 A–F).
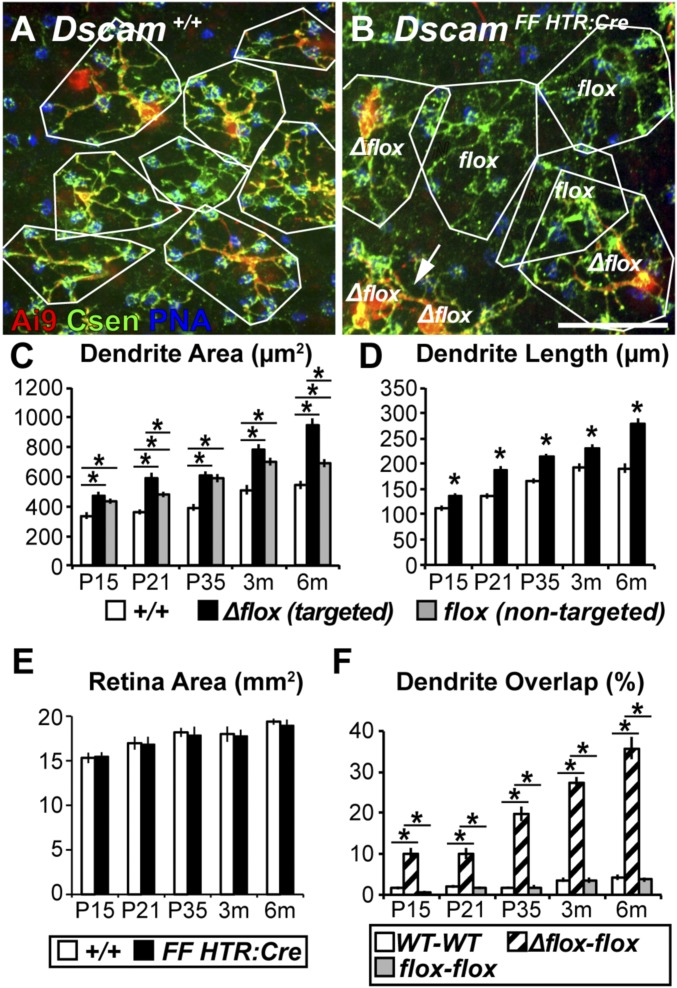
Next, we compared BC4 morphology in Dscam+/+ and DscamFF HTR:Cre retinas from P15 through 6 mo (Fig. 2 A and B). Fluorescence coexpression and IHC validation showed that HTR:Cre deleted Dscam in many BC4s (DscamΔflox BC4s) (Fig. 2 A and B, red + green), but some nontargeted cells remained (∼20%; Dscamflox BC4s) (Fig. 2 A and B, green). This chimeric expression pattern allowed structural comparison of mutant and WT BC4s within the same retina (Fig. S2D). Since DSCAM in vertebrates and invertebrates acts as a homotypic cell-adhesion molecule (25, 40), we should expect a structural phenotype also in Dscamflox BC4s directly adjacent to DscamΔflox BC4s. We found that the dendritic area of DscamΔflox BC4s was increased compared with Dscam+/+ BC4s at all time points (P15 to 6 mo), while Dscamflox BC4s showed an intermediate phenotype (Fig. 2C). Dendrite length was greater in DscamΔflox BC4s compared with Dscam+/+ BC4s (Fig. 2D) but the retinal area was not (Fig. 2E).
Fig. 2.
Dscam is necessary to establish and maintain BC4 dendritic tiling. (A and B) Whole-mount retinas where BC4s are labeled genetically (HTR:Cre × Ai9), cones are stained with PNA, and anticalsenilin antibody to label all BC4s. Dendrite areas outlined in white. (A) Dscam+/+ retina, BC4 dendrite arbors tile. (B) DscamFF HTR:Cre retina, BC4s invade each other’s territory when DscamΔflox BC4s neighbor each other (arrow) or when a DscamΔflox BC4 neighbors a Dscamflox BC4. When Dscamflox BC4s neighbor each other, tiling remained intact. Apparently substantial overlap between DscamΔflox–DscamΔflox was not quantified because all cells expressed the same complement of fluorescent proteins. (Scale bar, 25 μm.) (C) Quantification of BC4 dendrite area. Significant increases in dendrite area were detected in DscamΔflox and Dscamflox BC4s compared with Dscam+/+ BC4s (ANOVA P values at all ages: P < 0.0001). (D) Quantification of dendrite length. DscamΔflox BC4s have significantly more dendrites than Dscam+/+ BC4s (t test, P15, 3 mo P < 0.001; P21, P35, 6 mo P < 0.0001). (E) Quantification of total retina area. No significant differences were detected when comparing Dscam+/+ and DscamFF HTR:Cre retinas at any time-point (t test, P15 P = 0.85; P21 P = 0.90; P35 P = 0.77; 3 mo P = 0.85; 6 mo P = 0.64). (F) Quantification of dendrite overlap. Significant increases in dendrite overlap were detected in DscamΔflox–Dscamflox BC4 pairs compared with Dscam+/+ and Dscamflox pairs (ANOVA, P15, P21, P35, 3 mo, 6 mo, all P < 0.0001). Asterisks indicate t test P < 0.05 or post hoc significance test indicating a significant difference between the left-most column under the line and the column under the asterisk. Cell trace n = 36 (WT), 30 (Δflox), and 38 (flox) at P15; 39 (WT), 30 (Δflox), and 40 (flox) at P21; 40 (WT), 40 (Δflox), and 40 (flox) at P35; 40 (WT), 40 (Δflox), and 40 (flox) at 3 mo; and 30 (WT), 30 (Δflox), and 40 (flox) at 6 mo. Error bars = SEM. Dscam+/+ data are also presented in Fig. 1 and Fig. S4. Image in A is also used in Fig. S4. Csen, calsenilin.
Increased dendritic field area without a change in retinal area implies increased dendritic field overlap concomitant with loss of dendritic tiling (41). To test this, we mapped the dendritic territories of neighboring BC4s in the Dscam+/+ and DscamFF HTR:Cre retinas (Fig. 2F). While dendritic field overlap between Dscam+/+ BC4 pairs was negligible, as expected, overlap between neighboring DscamΔflox–Dscamflox and DscamΔflox–DscamΔflox BC4 pairs (Fig. 2B) was substantial. Furthermore, DscamFF HTR:Cre retinas showed the predicted chimeric recombination pattern comprising WT-like tiling between Dscamflox BC4 pairs and loss of tiling between DscamΔflox and Dscamflox BC4 pairs (Fig. 2B). Based on these data, we conclude that DSCAM acts between mouse BC4s in a cell-type–autonomous manner (i.e., between cells of same type). Using genetic knockout mice, we found a Dscam gene dosage-dependent change in BC4 tiling (WT < heterozygous < homozygous for the Dscam mutation), consistent with previous results (42, 43) (Fig. S4).
DSCAM Prevents BC4s from Contacting Cones Outside of Their Tiled Dendrite Territories.
Next, we asked if loss of tiling is accompanied by an increase in the number of cone → BC4 anatomical connections. To answer this question, we counted the number of cones contacted by individual Dscam+/+, DscamΔflox, and Dscamflox BC4s at ages between P15 and 6 mo (Fig. 3 A–C). We found a significant increase in the number of cones contacted as a function of age in DscamΔflox and Dscamflox BC4s, but not Dscam+/+ BC4s (Fig. 3D). We also found a significant increase in the total number of dendritic tips, number of dendritic tips per cone, and number of dendritic tips not at cones in DscamΔflox BC4s compared with Dscam+/+ BC4s (Fig. 3 E–G). To determine whether dendritic tips not ending at cones contacted rods, we stained retinas with dystroglycan, a marker specific to synaptic clefts and peanut agglutinin (PNA), which labels the cone axon terminal membrane (Fig. 3H). We found no difference in the number of rod contacts in Dscam+/+ vs. DscamΔflox BC4s. However, the number of dendritic tips ending at either photoreceptor type was increased in DscamΔflox BC4s, compared with Dscam+/+ BC4s (Fig. 3I). This phenotype, too, was dependent on Dscam gene dosage (WT < heterozygous < homozygous) (Fig. S5). Taken together, these data support a model in which Dscam-mediated recognition enforces BC4 dendritic tiling by preventing dendrite overlap.
Fig. 3.
BC4s lacking Dscam inhibition project more dendrites and connect to more photoreceptors. (A–C) Whole-mount retinas (3 mo) with genetically labeled BC4s (HTR:Cre × Ai9) and cones stained with PNA. (A) Dscam+/+ BC4. (B) DscamΔflox BC4 in DscamFF HTR:Cre retina. (C) Dscamflox BC4 in DscamFF HTR:Cre retina. Dscamflox BC4s are calsenilin+, Ai9− because they have not been targeted by HTR:Cre. (Scale bar, 25 μm.) (D) Quantification of cones contacted by BC4s. DscamΔflox and Dscamflox BC4s contact significantly more cones compared with Dscam+/+ BC4s and they continued to contact more cones over time (ANOVA: P15 P = 0.02; P21 P < 0.01; P35 P = 0.01; 3 mo and 6 mo P < 0.0001). (E–G) Quantification of BC4 dendrite tips. Compared with Dscam+/+ BC4s, DscamΔflox BC4s have statistically more dendrite tips (E, t test, all ages, P < 0.0001), tips per cone (F, t test: P15, P21, P35 P ≤ 0.0001; 3 mo P < 0.01; 6 mo P = 0.02), and tips not at cones (G, t test: P15 and P21 P < 0.0001; P35 P = 0.03; 3 mo P < 0.01; 6 mo P = 0.03). (H) Confocal images of P35 retinas stained with markers against rod (dystroglycan) and cones (PNA) (scale bar, 25 μm; Insets magnified 7.5×), showing instances of colocalization (Right Inset, arrows) or lack thereof (Left Inset, arrow). (I) Dscam+/+ and DscamΔflox BC4s contacted similar numbers of rods, while DscamΔflox BC4s had statistically more dendrite tips ending in neither photoreceptors (t test, rods, P = 0.11; other, P < 0.0001). Asterisks indicate t test P < 0.05 or post hoc significance test indicating a significant difference between the left-most column under the line and the column under the asterisk. Data collected from the number of cell traces = 36 (WT), 30 (Δflox), and 38 (flox) at P15; 39 (WT), 30 (Δflox), and 40 (flox) at P21; 40 (WT), 40 (Δflox), and 40 (flox) at P35; 40 (WT), 40 (Δflox), and 40 (flox) at 3 mo; and 30 (WT), 30 (Δflox), and 40 (flox) at 6 mo. Rod contact data collected from 30 reconstructured cells each WT and (Δflox). Error bars = SEM. Dscam+/+ data in D–G is also presented in Fig. 1 and Fig. S5. Image in A is also used in Fig. S5. Dystro, dystroglycan. Csen, calsenilin; Den., dendrite.
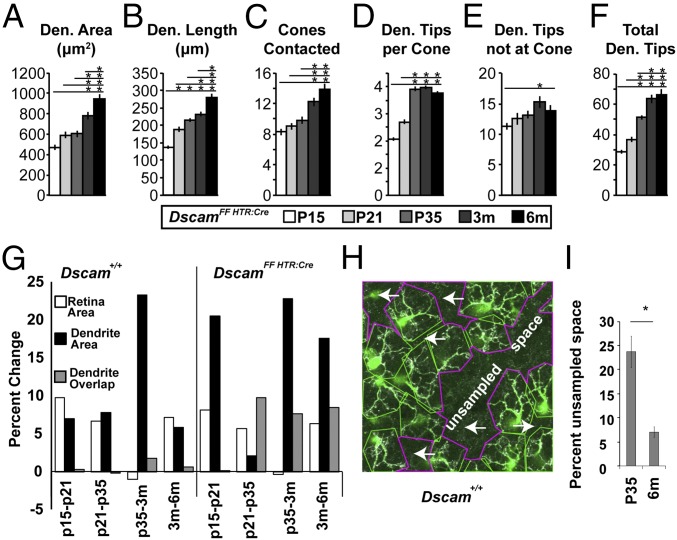
In support of this model, we found that DscamΔflox BC4 dendritic fields continue to grow, with significant increases in dendritic length and dendritic area between 3 and 6 mo of age (Fig. 4 A–F). This contrasts with WT BC4s, in which length and area do not increase between 3 and 6 mo (Fig. 1 I and J). Comparing increases in retinal area that occur as the retina ages to changes in BC4 dendritic area and overlap, we found that increases in dendritic area in DscamΔflox BC4s exceed retinal area growth and correlate with increases in BC4 overlap (Fig. 4G). Increases in WT BC4 dendritic area either match growth in retinal area or are not correlated with increases in dendrite overlap between adjacent BC4s (Fig. 4G). Increases in WT dendritic area between P35 and 3 mo occurred without similar changes in retina area or BC4 overlap. This suggested that BC4 tiling becomes tighter in the WT retina after P35. To test this, we measured unsampled space, defined as retinal area at the level of the OPL that was not sampled by the dendrites of any BC4 (Fig. 4H). We found a significant decrease in unsampled space in the WT retina between P35 and 6 mo of age (Fig. 4I).
Fig. 4.
Dendrite growth dynamics. (A–F) Quantification of DscamΔflox BC4 characteristics at P15, P21, P35, 3 mo, and 6 mo. Significant increases in all measurements were detected (ANOVA P values: A–D, and F: P < 1.3 × 10−12; E: P = 1.4 × 10−2). Horizontal lines above columns in A–F represent comparisons between the column under the left end of the line and all columns to the right. An asterisk above a point on the line represents a post hoc significance test value, indicating a significant difference between the left-most column under the line and the column under the asterisk. Traced cells: n = 30, 30, 40, 40, and 30 at P15, P21, P35, 3 mo, and 6 mo, respectively. Error bars = SEM. DscamΔflox data included also in Figs. 2 and 3. (G) Graphs showing the percent change between the means for successive time points for retina area and dendrite area and the difference between the means for successive time points for dendrite overlap. (H and I) Percent unsampled space (H, arrows) was reduced between P35 and 6 mo. (t test P = 4.9 × 10−4, H scale, 106 μm × 106 μm, n = 10 images per age.) Den., dendrite; m, month; P, postnatal day.
Isoneuronal Organization Is Disrupted at the Cone Synapse in Dscam Mutant BC4s.
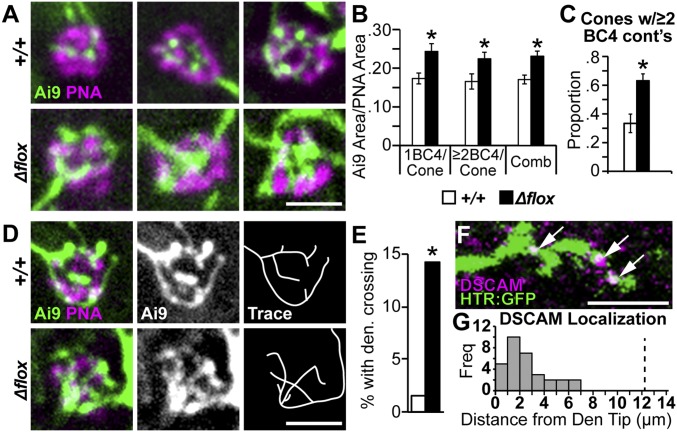
Mouse Dscam prevents excessive adhesion between cells of a given cell type, while Drosophila Dscam1 helps to provide isoneuronal cues—those intrinsic to individual neurons—that prevent excessive overlap of a given cell’s own dendrites (44–47). Isoneuronal recognition is provided in retinal starburst amacrine cells by γ-protocadherins, but how isoneuronal avoidance is mediated in most retinal cell types remains unknown (48). We next measured if isoneuronal BC4 dendrite organization was disrupted in the Dscam mutant retina. Compared with Dscam+/+ BC4s, Dscam-null BC4 innervation of cones was highly disorganized and the dendrites of a single BC4 appeared to clump together (Fig. 5A). Indeed, DscamΔflox BC4 dendrites covered significantly more of the cone (Fig. 5B) compared with Dscam+/+ BC4s, while the total number of multiply innervated cones in the DscamFF HTR:Cre retina was increased (Fig. 5C). Reconstructions of individual BC4 cone innervations showed a significant increase in the proportion of cells with crossing dendrites in DscamΔflox BC4s compared with Dscam+/+ BC4s (Fig. 5 D and E). Conceivably, this could be caused by clumping of multiple BC4 dendrite tips constraining dendrite avoidance, but we found increased overlap in BC4 dendrites even in cases where a cone was innervated by a single Dscam mutant BC4 (Fig. 5B). Consistent with the increase in dendrite self-crossing and DSCAM’s role in isoneuronal avoidance in Drosophila, we found that DSCAM protein was localized along distal BC4 dendrites, not just at the dendritic tips (Fig. 5 F and G).
Fig. 5.
Dscam is necessary to inhibit self crossing at the cone synapse in BC4s. (A) High-magnification images of single BC4s contacting cones at 3 mo of age in whole-mount retinas. Dscam+/+ BC4s connections with cones are highly organized and vary in their amount of contacts at a single cone. DscamΔflox BC4s connections with cones are disorganized and their dendrites clump. Images show representative examples; WT cones, n = 101; Δflox cones, n = 118. (Scale bar, 3 μm.) (B) Quantification of total density of BC4 dendrites localized within PNA area. Significant increases in coverage were observed in DscamΔflox BC4s compared with Dscam+/+ BC4s, regardless if the cones were contacted by 1 BC4 or ≥two BC4s (t test, 1 BC4 per cone, P < 0.01; ≥two BC4 per cone, P = 0.03; combined, P < 0.0001). (C) Quantification of cones contacted by two or more BC4s. A significant increase was observed in DscamFF HTR:Cre compared with Dscam+/+ (t test, P = 0.01). (D) High-magnification images of single BC4s making contact with cones at 3 mo of age. Dscam+/+ BC4s dendrites do not cross. DscamΔflox BC4s dendrites frequently cross. Images show representative examples; WT, n = 63; Δflox, n = 42. (Scale bar, 3 μm.) (E) Quantification of observed dendrite crossing at the cone. Statistically more dendrites cross in DscamΔflox BC4s compared with Dscam+/+ BC4s (hypothesis-test, P = 0.01). (F) High-magnification image of DSCAM colocalization with Dscam+/+ BC4 dendrites in cryo-sections. Arrows indicate colocalization. (Scale bar, 3 μm.) (G) Quantification of the distance between DSCAM puncta and the nearest BC4 dendritic tip. DSCAM was observed as far away as 7 μm from the dendrite tip. Vertical dotted line at 12 μm indicates the average dendrite length from dendrite terminal to cell body. *P < 0.05. Error bars = SEM. Retinas collected at 3 mo of age. comb, combined; cont’s, contacts; Den., dendrite freq, frequency.
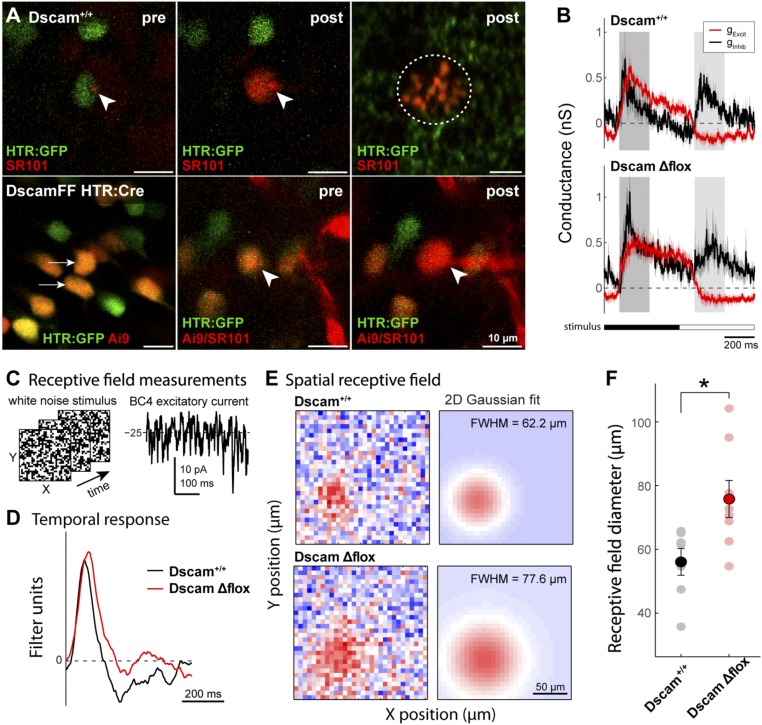
Loss of DSCAM Causes Enlarged BC4s Visual Receptive Fields.
Our anatomical data show that BC4s lacking Dscam have enlarged dendritic arbors. To test whether these cells make functional synaptic connections with cone photoreceptors throughout their extended dendritic field, we performed whole-cell recordings of fluorescence-labeled BC4s in the whole-mount retina in vitro (Fig. 6 A and B), and measured their spatial receptive fields using binary white noise stimulation (Fig. 6C). DscamΔflox BC4s, identified by HTR:GFP and Ai9 coexpression in DscamFF HTR:Cre mice, showed similar time course and strength of excitatory and inhibitory synaptic conductance compared with Dscam+/+ BC4s, identified by HTR:GFP expression in Dscam+/+ mice, including both ON and OFF inhibition and excitation (Fig. 6 A–C). These data indicate that presynaptic neurons and basic response properties of DscamΔflox BC4 cells were unchanged. However, DscamΔflox BC4s had significantly larger receptive fields compared with Dscam+/+ BC4s (Fig. 6 E and F), demonstrating that extended dendrites make functional synaptic connections with cone photoreceptors over a larger retinal area compared with WT BC4s.
Fig. 6.
BC4s lacking DSCAM have enlarged visual receptive fields. (A) Two-photon fluorescence images of genetically identified bipolar cells in the whole-mount retina in vitro. (Upper) HTR:GFP expressing bipolar cells (green) were targeted for electrophysiological whole-cell recording. Intracellular solution contained fluorescent dye, sulforhodamine 101 (SR101, red) to confirm cell type based on dye fill at the level of the soma (Center) and axonal arbor (Right, red circled). (Lower) HTR:GFP and Ai9 coexpressing cells in the DscamFF HTR:Cre retina (arrows) were targeted for recording as in Dscam+/+. Arrowheads indicate the pipette tip at the recorded cell. post, image after recording; pre, image before recording. Abundant Ai9 expression in DscamFF HTR:Cre mice precluded verification of axonal arbor from dye fills. (B) Plots of the light-evoked excitatory (red) and inhibitory (black) conductance in Dscam+/+ (Upper) and DscamΔflox BC4s (Lower) during stimulation with a contrast modulated spot on a gray background (100-µm diameter; time course shown at bottom). OFF and ON-evoked conductances were quantified by averaging over the time windows indicated by the shaded bars; see Results for details. (C) BC4 visual receptive fields were measured using white-noise checkerboard stimuli. (Left) Example stimulus frames; (Right) example response segment. (D) Temporal response of example Dscam+/+ and DscamΔflox BC4s, computed by cross-correlating the white-noise evoked response and the visual stimulus. No significant difference was detected comparing OFF or ON excitation or inhibition; OFF excitation: 0.41 ± 0.11 (WT) vs. 0.39 ± 0.05 (DscamΔflox) nS; ON excitation: −0.09 ±0.06 (WT) vs. −0.16 ± 0.05 (DscamΔflox) nS ; OFF inhibition: 0.48 ± 0.03 (WT) vs. 0.26 ± 0.11 (DscamΔflox) nS; ON inhibition: 0.37 ± 0.12 (wt) vs. 0.24 ± 0.03 (DscamΔflox) nS; n = 4 Dscam+/+, 5 DscamFF HTR:Cre; t test, P = 0.89, 0.40, 0.06, 0.39. (E) Spatial receptive field maps (Left) of example Dscam+/+ and DscamΔflox BC4s, computed by cross-correlating the white-noise evoked response and the visual stimulus. Receptive field size was quantified using 2D Gaussian fits to the spatial receptive field at the peak response (∼85 ms). Receptive field diameter is expressed as full width at half maximum of the Gaussian fits (FWHM = 2.36 s). A significant increase in the receptive field of DscamΔflox BC4s was observed compared with Dscam+/+ BC4s (35.1% increase; FWHM of Gaussian fit: 75.8 ± 5.81, n = 8 vs. 56.1 ± 4.18 µm, n = 7; mean ± SEM; t test, P = 0.019). (F) Receptive field diameters for the recorded BC4 population (Dscam+/+ n = 7; DscamΔflox n = 8; *P = 0.019).
DSCAM Enforces Tiling of BC4 Axonal Arbors.
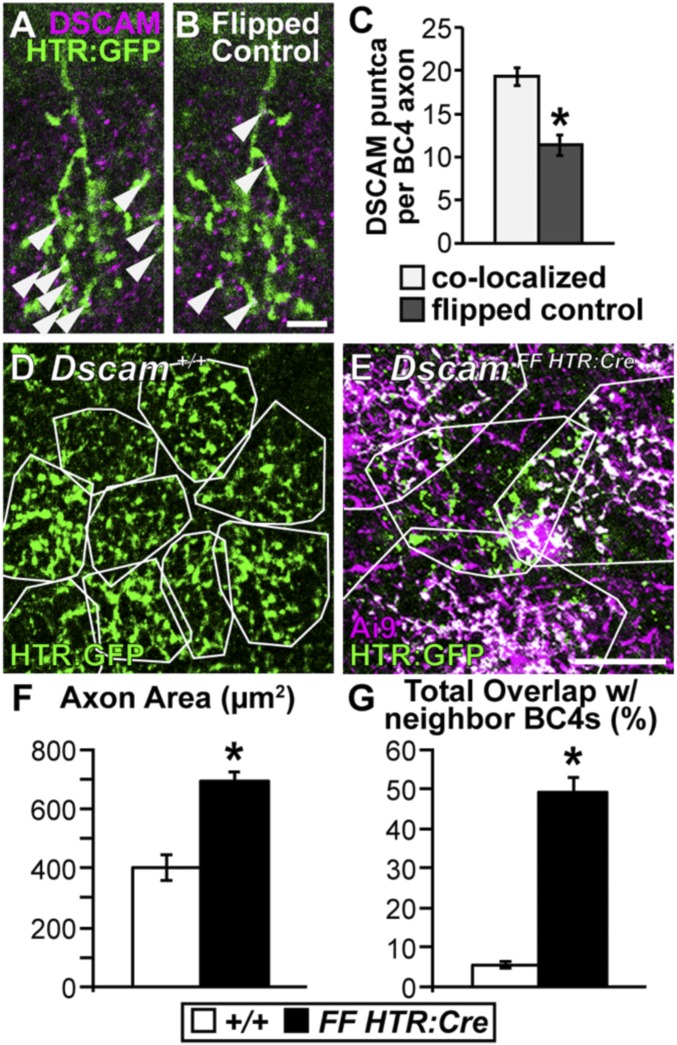
BCs are strongly polarized neurons characterized by axonal and dendritic arbors extending in opposite directions from the soma. DSCAM expression in BC4s is localized to the distal dendrites (27) (Fig. 5 F and G). In RGCs, the function of DSCAM differs depending on cellular compartment. DSCAM prevents clumping in dendrites (24) while promoting growth in axons (49). To determine whether DSCAM has similar compartment-dependent functions in BCs, we first confirmed that DSCAM protein was localized to BC4 axons (Fig. 7 A–C). Next, we compared axonal arbor territories in DscamFF HTR:Cre vs. Dscam+/+ retinas (Fig. 7 D and E). Dscamflox BC4 axonal arbors were larger and overlapped more with neighboring BC4 axons compared with Dscam+/+ BC4 axons (Fig. 7 F and G). These results show that DSCAM has a similar function in BC4 dendrites and axons (i.e., to maintain tiling).
Fig. 7.
Dscam is necessary to establish BC4 axon tiling. (A–C) Colocalization of DSCAM protein with BC4 axons. All analyses were performed in serial z-stack images; shown here is a single confocal plane from an analyzed stack. (A and B) Cryo-sections of retina (3 mo) stained with anti-DSCAM and HTR:GFP to determine colocalization. (Scale bar, 5 μm.) (A) Image showing DSCAM/BC4 axon colocalization. (B) Axon channel flipped about the horizontal axis as a control for incidental colocalization. Arrowheads point to colocalized DSCAM puncta on BC4 axons. (C) Quantification of DSCAM puncta colocalization per BC4 axon. A significant increase in DSCAM puncta was detected when comparing the original images to the flipped controls (t test, P < 0.001). (D and E) Whole-mount retina (3 mo), BC4s are labeled genetically (HTR:Cre × Ai9 and HTR:GFP or HTR:GFP only). (Scale bar, 20 μm.) (D) Dscam+/+, BC4 axons outlined in white to visualize tiling. (E) DscamFF HTR:Cre, axons outlined in white. Only the green axon is Dscamflox. The others outlined are DscamΔflox. Axons invade each other’s territory when one or both lack DSCAM. (F and G) Quantifications of axon area (F) and total overlap with neighboring BC4 axons (G). Dscamflox BC4 axons become significantly larger (t test < 0.0001) and overlap significantly more with neighboring DscamΔflox BC4 axons (t test, P = <0.0001) compared with Dscam+/+ BC4 axons. *P < 0.05. Sample size WT, n = 30; Δflox, n = 30. Error bars = SEM.
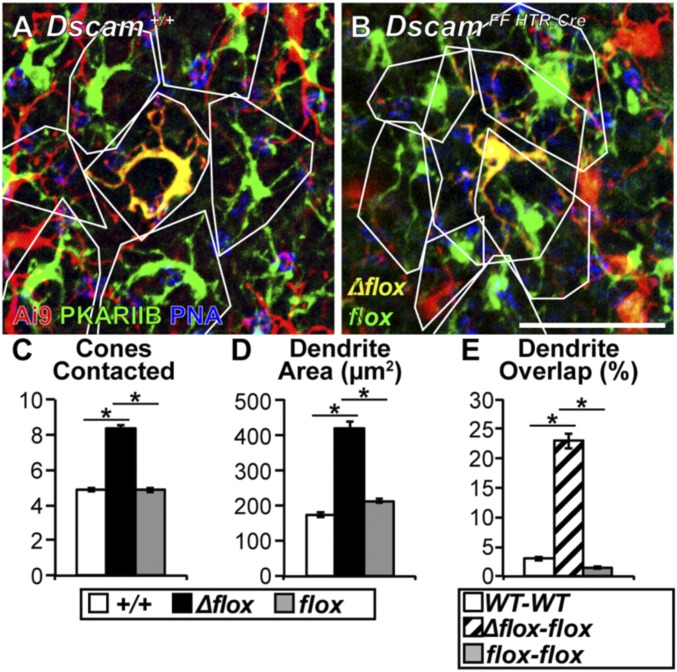
DSCAM Enforces Dendrite Tiling in BC3bs.
Dscam is expressed in multiple OFF BC types (27). To assess whether the results obtained from BC4s generalize also to other OFF BC types, we next measured how loss of DSCAM impacts dendrite outgrowth of another Dscam expressing OFF BC, BC3b. Analysis of BC3bs was possible because HTR:Cre targets ∼1% of BC3bs (31), allowing for the sparse labeling and targeting of Dscam in BC3bs (Fig. 8 A and B). BC3bs were readily distinguished from BC4s by counterstaining with the BC3b selective molecular marker anti-PKARIIβ (protein kinase A RII β) (50). We found significant increases in dendritic area and number of cones contacted in DscamΔflox BC3bs compared with Dscam+/+ and Dscamflox BC3bs (Fig. 8 C and D). Furthermore, neighboring DscamΔflox–Dscamflox BC3b pairs showed more dendritic field overlap compared with Dscam+/+ or Dscamflox BC3b pairs (Fig. 8E). Because of sparse recombination in BC3Bs, most Dscamflox BC3bs are adjacent to other Dscamflox BC3bs, and Dscamflox BC3b phenotypes were not significantly different from WT BC3bs (Fig. 8 C–E). Based on these data, we conclude that DSCAM plays a similar role in controlling dendritic field area in BC3b and BC4.
Fig. 8.
Dscam is necessary for establishing BC3b dendritic tiling. (A and B) Whole-mount retinas (1 mo) genetically labeled (HTR:Cre × Ai9) and counterstained with PKARIIB and PNA. Because HTR:Cre targets ∼1% of BC3bs, sparse BC3bs can be labeled genetically while concurrently deleting Dscam when the mouse is homozygous for the floxed Dscam allele. BC4s can be eliminated from the analysis because they are not PKARIIB+. (A) Dscam+/+, BC3bs outlined in white to visualize tiling. (B) DscamFF HTR:Cre, DscamΔflox BC3bs become significantly larger and lose tiling with neighboring Dscamflox BC3bs (ANOVA, P < 0.0001). (Scale bar, 20 μm.) (C–E) Quantification of cones contacted (C), dendrite area (D), and dendrite overlap (E). Significant increases in cones contacted and dendrite area were detected when comparing DscamΔflox BC3bs to Dscam+/+ or Dscamflox BC3bs (ANOVA, P < 0.0001). Significant increases in dendrite overlap were detected when comparing DscamΔflox–Dscamflox BC3b pairs to Dscam+/+ or Dscamflox pairs (ANOVA, P < 0.0001). An asterisk represents a post hoc test value indicating a significant difference. Cell traces n = 105 (WT), 27 (Δflox), and 80 (flox). Error bars = SEM. Retinas collected at 1 mo of age.
DSCAM Actively Inhibits BC4 Plasticity After Development.
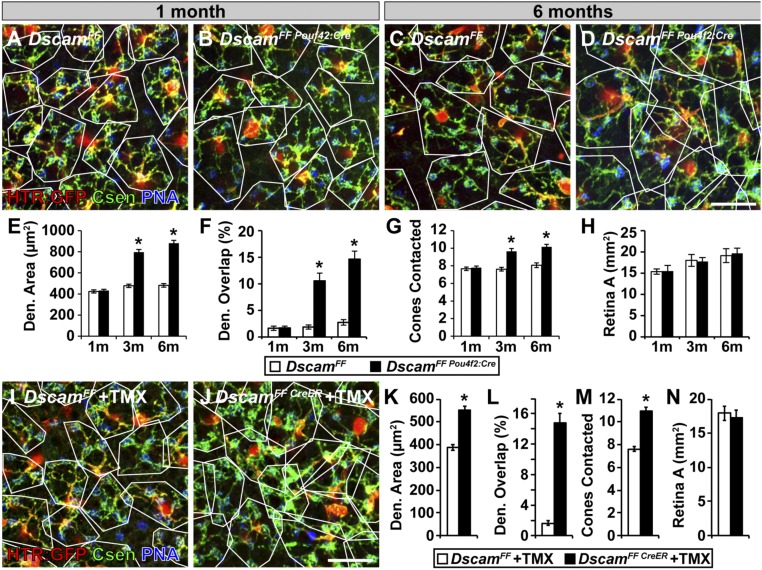
The results presented thus far show that constitutive loss of Dscam expression leads to expanded BC3b and BC4 dendritic fields. Conceivably, this phenotype is caused by the absence of DSCAM during development. Alternatively, it may reflect an active role for DSCAM in maintaining BC dendritic territory after development is complete (>1 mo). To distinguish between these two scenarios, we deleted Dscam in BCs in adult mice using two independent approaches. In the first approach, late deletion of Dscam was mediated by Pou4f2:Cre. Pou4f2:Cre targets the conditional allele of Dscam in BCs after 1 mo of age (51). Using this approach, we found no differences in dendrite area, dendrite overlap, or cones contacted in DscamFF vs. DscamFF Pou4f2:Cre BC4s before recombination (up to 1 mo of age) (Fig. 9 A, B, and E–G). However, at this time point a phenotype is clearly observed in the DscamFF Htr:Cre retina compared with WT (Fig. 2F). After recombination, at ages 3 and 6 mo, we found significant increases in each of these measurements (Fig. 9 C–G). We found no change in retinal area at any of the time points (Fig. 9H).
Fig. 9.
Dscam is necessary to maintain BC4 dendritic tiling in the adult retina. Deletion of the Dscam floxed allele after 1 mo of age was accomplished with Pou4f2:Cre (A–H), which effectively recombines Dscam by P45, and with the inducible CreER (I–N). DscamFF BC4s were compared with DscamFF Pou4f2:Cre BC4s before Pou4f2:Cre mediated recombination of Dscam occurs, 1 mo (A and B), and then compared at two more time-points after recombination occurs, 3 and 6 mo (C and D). (A–D) Whole-mount retinas with BC4s labeled with HTR:GFP and stained with PNA and calsenilin. Dendrite areas mapped in white. (Scale bar, 20 μm.) (E–H) Quantifications of dendrite area (E), dendrite overlap (F), and cones contacted (G). DscamFF BC4s and DscamFF Pou4f2:Cre BC4s are not statistically different for any of these measures at 1 mo of age. However, DscamFF Pou4f2:Cre BC4s become statistically greater for all of these measurements at 3 and 6 mo (t test P values: dendrite area: 1 mo P = 0.66, 3 mo P < 0.0001, 6 mo P < 0.0001; dendrite overlap: 1 mo P = 0.93, 3 mo P < 0.0001, 6 mo P < 0.0001; cones contacted: 1 mo P = 0.62, 3 mo P < 0.0001, 6 mo P < 0.0001; retina area: 1 mo P = 0.87, 3 mo P = 0.77, 6 mo P = 0.68). (H) Quantification of total retina area. No statistical differences were observed between DscamFF and DscamFF Pou4f2 retinas. (I and J) DscamFF and DscamFF CreER mice were injected with tamoxifen at 1 mo and retinas were collected at 3 mo. (Scale bar, 20 μm.) (K–N) Quantifications of dendrite area (K), dendrite overlap (L), cones contacted (M). DscamFF CreER BC4s are statically larger for all of these measurements (t test, dendrite area, P < 0.0001; dendrite overlap, P < 0.0001; cones contacted, P < 0.0001; retina area, P = 0.69). (N) Quantification of total retina area. No statistical differences observed between DscamFF and DscamFF CreER retinas. An asterisk above a point on the line represents a t test value of 0.05 or less between the left-most column and the column under the asterisk. Data collected from number of cell traces (Pou2f-Cre and controls) = 60 (control/flox) and 60 (Δflox) at 1 mo; 60 (control/flox) and 45 (Δflox) at 3 mo; 45 (control/flox) and 45 (Δflox) at 6 mo; (CreER and controls/flox) = 50 (controls/flox) and 50 (Δflox). Error bars = SEM. Den., dendrite.
In the second approach, we used the tamoxifen-dependent CreER system (52, 53), which allowed greater flexibility in the timing of Dscam deletion. DscamFF and DscamFF CreER mice were injected with tamoxifen at 1 mo and retinas harvested and analyzed at 3 mo. Comparing CreER-targeted vs. non-CreER–targeted BC4s (Fig. 9 I and J), we found significant increases in dendrite area, dendrite overlap, and number of cones contacted (Fig. 9 K–M). As before, we found no change in retina area (Fig. 9N). These data demonstrate that DSCAM actively enforces dendritic tiling throughout the lifespan. By blocking dendritic outgrowth, DSCAM prevents BCs from contacting additional cones over time, thus inhibiting circuit-level BC plasticity in the mature retina.
Discussion
We provide insight into the regulation of neuronal plasticity. We report that OFF-type retinal BC3s and BC4s retain the ability to extend and refine components of their dendritic arbors within a defined tiled territory long after the retina is considered developmentally mature. We identify Dscam as a gene that enforces BC axonal and dendritic tiling and inhibits BC plasticity through interactions between homotypic cells. We demonstrate that abolishing Dscam expression in BC3bs and BC4s promotes dendritic outgrowth, and show that BC4 dendritic extensions make functional synaptic connections with more cone photoreceptors than in WT controls, resulting in enlarged visual receptive fields. Finally, we show that deleting Dscam in the adult retina is sufficient for triggering BC dendrite outgrowth. This demonstrates that Dscam is required for maintaining dendritic territories throughout life. This is a demonstration of cell-adhesion molecule-mediated inhibition of axon and dendrite outgrowth as a regulator of circuit-level plasticity. Because adult plasticity is a key objective for clinical approaches to retinal regeneration and repair, interfering with Dscam or its downstream signaling pathways may serve as a potential strategy for promoting neurite outgrowth and synaptogenesis in the adult CNS.
Several factors limit neuronal plasticity in the mature CNS. In the spinal cord, myelin and extracellular matrix proteins provide inhibitory cues that prevent sprouting and outgrowth of neurons following damage (54–58). Within the retina, restriction of axon outgrowth in mature RGCs is mediated, in part, by the down-regulation of factors that promote cytoskeletal dynamics (59). Throughout the CNS, the bioavailability of growth factors critical for promoting plasticity during development and for learning and memory is reduced with age (17). Here, we expand our understanding of the factors contributing to governing circuit-level plasticity by identifying a role for the cell-adhesion protein DSCAM.
Intrinsic Plasticity of OFF BCs.
Our mechanistic understanding of BC dendrite and axon development is based primarily on studies in ON BCs (23, 36, 60, 61). Here, we demonstrate that OFF BC dendritic arbor and synaptic development is qualitatively different from ON BCs. Whereas ON BCs have the same number of anatomical connections in the adult retina as they do around the time of eye-opening (18, 60), we show that OFF BCs continue to make and refine anatomical connections within their dendritic territories until at least 6 mo of age. The difference in dendritic plasticity between ON and OFF BCs may be related to the different innervation patterns of ON and OFF BCs in the mammalian retina. While ON cells make invaginating contacts at synaptic ribbon-containing sites, OFF cells make flat contacts at the base of synaptic invaginations, possibly leaving their dendrite tips free to explore and expand (62, 63). An additional difference between ON cone BCs and mouse OFF BC3a, BC3b, and BC4s is that the latter make direct synaptic contacts with rods in addition to cones, analogous to the primate DB3 BCs (33, 50, 64). The dendritic field of these OFF BC types reportedly contains an excess of potential presynaptic rod contacts, with only about one in five rods directly contacted (64, 65). We did not observe an increase in the number of rods contacted, even as dendritic field size increased. Thus, synaptic input to BCs appears to be controlled independent of dendritic arbor size, with reported feedback from axonal contacts as a potential mechanism (66).
Loss of DSCAM-Dependent Axon and Dendrite Tiling Results in Formation of New Cone Contacts.
While most retinal neurons have axonal and dendritic fields that overlap extensively, ranging from about 3-fold in nonfoveal ganglion cells to about 30-fold for starburst amacrine cells (67–69), BCs have tiled dendritic and axonal arbors (20). In this study, we identify Dscam as the regulator of tiling in two Dscam-expressing OFF BC types (BC3b, BC4) (27). The tiled organization of dendritic and axonal arbors of these BC types suggest that a similar mechanism acts to prevent overlap in other BC types, but the genes that mediate this, as well as tiling in other vertebrate neuron populations, remain to be identified. Four types of OFF BCs express Dscam and all have dendrites that converge onto the cone synapse (27) or axonal arbors that overlap, and yet DSCAM protein appears to function by preventing dendrite and axonal overlap independently in these cell types. This is consistent with the extensive overlap of the dendritic arbors of different Dscam-expressing cell types in the IPL and a model wherein Dscam does not provide cell-type–specific recognition cues, but rather acts to prevent excessive adhesion or preserve tiling by acting in the context of a larger cell-type identity code (24). This contrasts with Dscam function in chick where DSCAM protein is localized to a narrow band of the IPL and plays a role in laminar targeting, a function that may be mediated in mouse by semaphorins and plexins (70, 71), contactins (72), and cadherins (39). The localization of DSCAM protein in the mouse IPL at the adherens junctions suggests that cadherins are good candidates for such an identity code (28). Drosophila Dscam1 has been shown to mediate repulsion (45) after homotypic binding and genetic and biochemical studies in Drosophila have identified an interaction between Dscam1 and tubulin-folding cofactor D that results in microtubule disruption (73). Conservation of such a system in mouse would be consistent with the findings of this study that Dscam maintains tiling and inhibits dendrite and axonal outgrowth by acting in conjunction with additional factors for cell-type specificity.
BC tiling facilitates efficient transmission of spatiotemporal information from photoreceptors to RGCs (35). Interestingly, we report that the physiological tiling of OFF BCs is less strict than the anatomical tiling, with the anatomical BC visual receptive field diameter roughly twofold larger than the dendritic field diameter. This is likely due to cone–cone gap junction coupling (74). By developing a model system in which we can selectively and inducibly increase the receptive field of identified BC types, we can now test directly how loss of tiling impacts information encoding at the level of the retinal ganglion cells. Since Dscam is widely expressed throughout the CNS (75–77), similar perturbations of receptive and projective fields in the brain should be of potential interest for exploring links between mutations in Dscam and autism in humans (78, 79).
DSCAM Deletion in Mature Neurons Triggers Dendrite Outgrowth and Synaptic Contact.
During development, Dscams promote axon growth and guidance (49, 80–84), laminar targeting (25, 38), and avoidance (22, 24, 44, 45, 85, 86). In this study, we report that DSCAM acts in mature neurons to actively suppress dendrite growth, a novel mechanism to inhibit plasticity. Widespread ablation of photoreceptors has been shown to result in rod BC dendrite sprouting and innervation of rods located outside of the ablated region, helping to restore visual function in the ablated region (87). Surviving cone BCs, on the other hand, failed to sprout and innervate cones outside of the lesion (87). Our study predicts that targeting Dscam in this context will activate BC dendrite outgrowth and facilitate restoration of cone-driven visual function in BCs proximal to the lesion site.
Other cell-adhesion molecules that regulate neuron self-organization through homotypic binding include the MEGF proteins and the γ-protocadherin complex (48, 88), while semaphorins and plexins restrict axon and dendrite arbors across the vertical plane of the retina (i.e., photoreceptors to RGCs) (70, 71, 89, 90). Whether deletion of factors like MEGF and semaphorin proteins in the adult retina is also sufficient to induce neurite outgrowth is not known but worth exploring.
The identification of signaling pathways downstream of Dscam and the availability of pharmacological agents to up- and down-regulate these pathways (91, 92) suggest that targeted intervention could be performed to promote or inhibit neurite outgrowth in cases where DSCAM function may be lacking or overexhuberant, including in autism (78, 79, 93), fragile-X syndrome, and Down syndrome (94, 95). Because of its sensitivity to graded changes in Dscam expression (e.g., homozygous vs. heterozygous for Dscam mutations), the model system presented here provides a powerful research platform for understanding how manipulations of neurite outgrowth impact neural circuit function.
Materials and Methods
Mouse Strains.
All animal procedures were performed in accordance with protocols approved by the Animal Use and Care Committees at the University of Idaho and the University of Louisville, and were in compliance with National Institutes of Health guidelines. We used two different transgenic mouse lines manipulating Dscam: (i) Dscamtm1Pfu and (ii) Dscamtm1.1Pfu; one Cre-dependent reporter mouse: Gt(ROSA)26Sortm9(CAG-tdTomato)Hze (referred to as Ai9; The Jackson Laboratory, stock no: 007909) (96); three Cre recombinase-expressing transgenic mouse lines: (i) Tg(Htr2a-cre)KM207Gsat (referred to as HTR:Cre; MMRRC stock no: 036750) (31), (ii) Pou4f2:Cre (courtesy of Vann Bennett, Duke University, Durham, NC); (iii) Gt(ROSA)26Sortm1(Cre/ERT2)Tyi (referred to as CreER; The Jackson Laboratory, stock no: J008463) (52); and one GFP transgenic mouse: Htr2a-EGFP (referred to as HTR:GFP; MMRRC, stock no: DQ118) (32). Mice were maintained on a mixed genetic background containing C57BL/6, C3H, 129, and FVB. Mutant alleles of Pde6b were crossed out of the colony. Mice were fed ad libitum and kept on a 12-h light/dark cycle. A minimum of three retinas from three mice were used in each measurement, except for Pou4f2-Cre at 6 mo of age, in which case four retinas from two mice were used.
Genotyping.
Mice were genotyped following standard procedures using previously reported primer sequences (97). Morphological analyses were performed blind to genotype. All mice taken for study were given a unique alphabetical code that was dissociated from the genotypes to those performing experiments until data collection was complete. Tail biopsies from each mouse were saved for post hoc verification of genotype, if needed.
Microscopy.
Micrographs were captured using either a Nikon Spinning Disk confocal microscope or an Olympus Scanning Laser confocal microscope (FV1000). Image processing was performed using FIJI (National Institutes of Health) or Adobe Photoshop (Adobe Systems) and was limited to adjustments of brightness or contrast applied uniformly across the image.
Immunohistochemistry.
IHC was performed as previously described (27). In all images containing HTR:GFP, the GFP was amplified using IHC; the tdTomato RFP from the Ai9 reporter was not amplified. See SI Materials and Methods for details about antibodies and stains.
Statistical Analysis.
Statistical analyses were performed in Microsoft Excel or Matlab. A summary of the statistical tests performed and P values for each measurement can be found in Table S1; resources can be found in Table S2. To test for statistical differences between two groups with numerical data, we used the Student’s t test; for three or more groups with numerical data we used a one-way ANOVA with a Tukey–Kramer post hoc test; for categorical data we used a hypothesis test comparing two independent proportions; for IPL lamination data we used a Kruskal–Wallis test with a Dunn’s post hoc test. A P value < 0.05 was considered statistically different and is denoted by an asterisk in the graphs in the figures. Horizontal lines above columns in figures represent comparisons between the column under the left end of the line and all columns to the right. An asterisk above a point on the line represents a significant difference between the left-most column and the column under the asterisk. Error bars show mean ± SEM.
Cell Counts, Cell Spacing, and Retinal Thickness.
For total cell counts, eight images (four central, four peripheral), equally sampled from the dorsal, ventral, nasal, and temporal regions, were captured from whole-mount retinas stained with cell type specific markers at 1-μm increments along the z axis. A 60× magnification (44,944-μm2 field size) was used to capture BC4s, BC3bs, and cones. A 20× magnification (160,000-μm2 field size) was used to capture dopaminergic amacrine cells (DACs). Image stacks were imported into FIJI and cell bodies were manually marked using the multipoint tool. The total number of cells per retina was calculated by extrapolating the number of cells in the eight sample areas to the total retinal area. Retinal area was determined by montage imaging of the entire whole-mount retina and using the polygon tool in FIJI. To determine cell spacing, x–y coordinates of each annotated cell soma were exported from FIJI and imported into WinDRP to compute the nearest-neighbor regularity index (NNRI) and the packing factor (98). Procedures for quantifying morphological characteristics of identified BC types are described in SI Materials and Methods.
DSCAM Localization.
All DSCAM localization was performed in cryo-sections of Dscam+/+ retinas by staining them with antibodies against HTR:GFP, calsenilin, and DSCAM. Confocal images were then taken using a scanning-laser confocal microscope at 1,200× magnification, sampling at 0.5-μm increments about the z axis. Image stacks were then taken into FIJI where they were analyzed by the following metrics.
DSCAM localization on BC4 dendrites.
Dendrite tips and cell bodies of BC4s and DSCAM colocalized to BC4 dendrites were identified and marked using the multipoint tool. This distance from DSCAM puncta to the nearest dendrite tip was measured by tracing from the puncta, along the dendrite, to the terminal. The length of each dendrite was measured in the same way, but from the dendrite tip back to the cell body.
DSCAM colocalization with BC4 axons.
Using images stacks, volumes surrounding isolated BC4 axons were identified and all of the DSCAM puncta within that area were marked with the BC4 axon channel hidden. Then the channel was made visible and the puncta colocalized with the axon were recorded. As a control, the BC4 axon channel was flipped about the horizontal axis and the measurement was performed again.
Electrophysiological Recordings.
Whole-cell electrophysiological recordings from fluorescence-labeled bipolar cells were performed as described previously (99). Each retina was mounted photoreceptor-side down on nitrocellulose filter paper, centered on a square pattern of four 1.3-mm-diameter apertures to permit visual stimulation from below and electrode access from above. A fifth aperture, offset ventrally, was used to focus the stimulus onto the photoreceptors. Recordings were performed on a custom-built two-photon fluorescence microscope (Olympus BX-51) controlled by Scanimage software (www.scanimage.org), with concentric and parfocal IR bright field and two-photon imaging capability. BC4 cells were selectively recorded by targeting HTR:GFP-expressing, or HTR:GFP and Ai9 coexpressing cells in inner nuclear layer of Dscam+/+ and DscamFF HTR:Cre mice, respectively (for details, see ref. 100). Typical access resistance was 25–40 MΩ; typical input resistance was 350–700 MΩ. Pipette internal solution contained: 120 mM Cs-methanesulfonate, 5 mM TEA-Cl, 10 mM Hepes, 10 mM BAPTA, 3 mM NaCl, 2 mM QX-314-Cl, 4 mM ATP-Mg, 0.4 mM GTP-Na2, and 10 mM phosphocreatine-Tris2 (pH 7.3, 280 mOsm), and a red fluorescent dye (Sulforhodamine 101; Thermo Fisher). Excitatory currents were recorded at a holding potential near the reversal potential for chloride, ECl (−67 mV), corrected for the liquid junction potential (−9 mV); inhibitory currents were recorded at the reversal potential for cations, Ecat (0 mV).
Visual Stimulation.
Visual stimuli were generated with an iMac computer using Matlab and Psychophysics Toolbox software (Mathworks; www.psychtoolbox.org). Stimuli were displayed using a DLP video projector (60 fps refresh rate; HP AX325AA; Hewlett-Packard), with the image projected onto the photoreceptor layer using a 20× objective (Olympus) in place of the microscope condenser. The stimulus was focused under visual guidance and focus accuracy was verified post hoc for each recorded cell. Pixel size at level of the photoreceptors was 3.25 × 3.25 µm. Stimuli comprised binary white noise checkerboard (30 × 30 checks, check size 6.5 × 6.5 µm; 100% Michelson contrast, six stimulus frames per second) to measure spatial receptive fields and contrast-modulated spots (100% Michelson contrast; 100-µm diameter; 1 Hz) to measure excitatory and inhibitory conductances.
Tamoxifen Dosing.
Tamoxifen (T5648; Sigma Aldrich) was dissolved at 20 mg/mL in sesame seed oil by incubation at 37 °C until dissolved (about 4 h). Next, 100 μL of tamoxifen solution was administered by intraperitoneal injection (1 mg/10 g body weight) daily for 5 d starting at postnatal day 30. Mice were maintained for an additional 3 mo before being taken for study.
Supplementary Material
Acknowledgments
We thank Aaron McGee and Deborah Stenkamp for useful comments on the manuscript. This research was supported by National Eye Institute Grants EY020857 (to P.G.F.) and EY028297 (to P.G.F. and B.G.B.) and a grant from the E. Matilda Ziegler Foundation (to B.G.B.). Imaging support was provided by NIH Grants P20 RR016454, P30 GM103324-01, and P20 GM103408.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.L.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1713548114/-/DCSupplemental.
References
- 1.Wong RO. Differential growth and remodelling of ganglion cell dendrites in the postnatal rabbit retina. J Comp Neurol. 1990;294:109–132. doi: 10.1002/cne.902940109. [DOI] [PubMed] [Google Scholar]
- 2.Sanes JR, Yamagata M. Many paths to synaptic specificity. Annu Rev Cell Dev Biol. 2009;25:161–195. doi: 10.1146/annurev.cellbio.24.110707.175402. [DOI] [PubMed] [Google Scholar]
- 3.Ford K, Feller M. Formation of early retinal circuits in the inner-plexiform layer. In: Kolb H, Fernandez E, Nelson R, editors. Webvision: The Organization of the Retina and Visual System. University of Utah Health Sciences Center; Salt Lake City, UT: 1995. [PubMed] [Google Scholar]
- 4.Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen DF, Jhaveri S, Schneider GE. Intrinsic changes in developing retinal neurons result in regenerative failure of their axons. Proc Natl Acad Sci USA. 1995;92:7287–7291. doi: 10.1073/pnas.92.16.7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duan X, et al. Subtype-specific regeneration of retinal ganglion cells following axotomy: Effects of osteopontin and mTOR signaling. Neuron. 2015;85:1244–1256. doi: 10.1016/j.neuron.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smirnakis SM, et al. Lack of long-term cortical reorganization after macaque retinal lesions. Nature. 2005;435:300–307. doi: 10.1038/nature03495. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg JL, et al. Retinal ganglion cells do not extend axons by default: Promotion by neurotrophic signaling and electrical activity. Neuron. 2002;33:689–702. doi: 10.1016/s0896-6273(02)00602-5. [DOI] [PubMed] [Google Scholar]
- 9.Condic ML. Neural development: Axon regeneration derailed by dendrites. Curr Biol. 2002;12:R455–R457. doi: 10.1016/s0960-9822(02)00944-2. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg JL, Klassen MP, Hua Y, Barres BA. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002;296:1860–1864. doi: 10.1126/science.1068428. [DOI] [PubMed] [Google Scholar]
- 11.Huh GS, et al. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu Y, et al. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc Natl Acad Sci USA. 2010;107:7975–7980. doi: 10.1073/pnas.0913449107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipton SA, Wagner JA, Madison RD, D’Amore PA. Acidic fibroblast growth factor enhances regeneration of processes by postnatal mammalian retinal ganglion cells in culture. Proc Natl Acad Sci USA. 1988;85:2388–2392. doi: 10.1073/pnas.85.7.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bei F, et al. Restoration of visual function by enhancing conduction in regenerated axons. Cell. 2016;164:219–232. doi: 10.1016/j.cell.2015.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg JL, et al. An oligodendrocyte lineage-specific semaphorin, Sema5A, inhibits axon growth by retinal ganglion cells. J Neurosci. 2004;24:4989–4999. doi: 10.1523/JNEUROSCI.4390-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hübener M, Bonhoeffer T. Neuronal plasticity: Beyond the critical period. Cell. 2014;159:727–737. doi: 10.1016/j.cell.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 18.Gibson R, et al. Functional and neurochemical development in the normal and degenerating mouse retina. J Comp Neurol. 2013;521:1251–1267. doi: 10.1002/cne.23284. [DOI] [PubMed] [Google Scholar]
- 19.Okawa H, Hoon M, Yoshimatsu T, Della Santina L, Wong ROL. Illuminating the multifaceted roles of neurotransmission in shaping neuronal circuitry. Neuron. 2014;83:1303–1318. doi: 10.1016/j.neuron.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wässle H, Puller C, Müller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci. 2009;29:106–117. doi: 10.1523/JNEUROSCI.4442-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jan YN, Jan LY. The control of dendrite development. Neuron. 2003;40:229–242. doi: 10.1016/s0896-6273(03)00631-7. [DOI] [PubMed] [Google Scholar]
- 22.Millard SS, Flanagan JJ, Pappu KS, Wu W, Zipursky SL. Dscam2 mediates axonal tiling in the Drosophila visual system. Nature. 2007;447:720–724. doi: 10.1038/nature05855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SC, et al. Homotypic regulation of neuronal morphology and connectivity in the mouse retina. J Neurosci. 2011;31:14126–14133. doi: 10.1523/JNEUROSCI.2844-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuerst PG, et al. DSCAM and DSCAML1 function in self-avoidance in multiple cell types in the developing mouse retina. Neuron. 2009;64:484–497. doi: 10.1016/j.neuron.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008;451:465–469. doi: 10.1038/nature06469. [DOI] [PubMed] [Google Scholar]
- 26.Li S, et al. DSCAM promotes refinement in the mouse retina through cell death and restriction of exploring dendrites. J Neurosci. 2015;35:5640–5654. doi: 10.1523/JNEUROSCI.2202-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Andrade GB, Long SS, Fleming H, Li W, Fuerst PG. DSCAM localization and function at the mouse cone synapse. J Comp Neurol. 2014;522:2609–2633. doi: 10.1002/cne.23552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Andrade GB, Kunzelman L, Merrill MM, Fuerst PG. Developmentally dynamic colocalization patterns of DSCAM with adhesion and synaptic proteins in the mouse retina. Mol Vis. 2014;20:1422–1433. [PMC free article] [PubMed] [Google Scholar]
- 29.Garrett AM, Tadenev AL, Hammond YT, Fuerst PG, Burgess RW. Replacing the PDZ-interacting C-termini of DSCAM and DSCAML1 with epitope tags causes different phenotypic severity in different cell populations. eLife. 2016;5:e16144. doi: 10.7554/eLife.16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuerst PG, Koizumi A, Masland RH, Burgess RW. Neurite arborization and mosaic spacing in the mouse retina require DSCAM. Nature. 2008;451:470–474. doi: 10.1038/nature06514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu Q, Ivanova E, Ganjawala TH, Pan ZH. Cre-mediated recombination efficiency and transgene expression patterns of three retinal bipolar cell-expressing Cre transgenic mouse lines. Mol Vis. 2013;19:1310–1320. [PMC free article] [PubMed] [Google Scholar]
- 32.Lu Q, Ivanova E, Pan ZH. Characterization of green fluorescent protein-expressing retinal cone bipolar cells in a 5-hydroxytryptamine receptor 2a transgenic mouse line. Neuroscience. 2009;163:662–668. doi: 10.1016/j.neuroscience.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haverkamp S, et al. Type 4 OFF cone bipolar cells of the mouse retina express calsenilin and contact cones as well as rods. J Comp Neurol. 2008;507:1087–1101. doi: 10.1002/cne.21612. [DOI] [PubMed] [Google Scholar]
- 34.Wässle H, Riemann HJ. The mosaic of nerve cells in the mammalian retina. Proc R Soc Lond B Biol Sci. 1978;200:441–461. doi: 10.1098/rspb.1978.0026. [DOI] [PubMed] [Google Scholar]
- 35.Sterling P, Laughlin S. Principles of Neural Design. The MIT Press; Cambridge, MA: 2015. [Google Scholar]
- 36.Dunn FA, Wong RO. Diverse strategies engaged in establishing stereotypic wiring patterns among neurons sharing a common input at the visual system’s first synapse. J Neurosci. 2012;32:10306–10317. doi: 10.1523/JNEUROSCI.1581-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Euler T, Haverkamp S, Schubert T, Baden T. Retinal bipolar cells: Elementary building blocks of vision. Nat Rev Neurosci. 2014;15:507–519. doi: 10.1038/nrn3783. [DOI] [PubMed] [Google Scholar]
- 38.Tadros W, et al. Dscam proteins direct dendritic targeting through adhesion. Neuron. 2016;89:480–493. doi: 10.1016/j.neuron.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duan X, Krishnaswamy A, De la Huerta I, Sanes JR. Type II cadherins guide assembly of a direction-selective retinal circuit. Cell. 2014;158:793–807. doi: 10.1016/j.cell.2014.06.047. [DOI] [PubMed] [Google Scholar]
- 40.Wojtowicz WM, et al. A vast repertoire of Dscam binding specificities arises from modular interactions of variable Ig domains. Cell. 2007;130:1134–1145. doi: 10.1016/j.cell.2007.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reese BE, Keeley PW. Design principles and developmental mechanisms underlying retinal mosaics. Biol Rev Camb Philos Soc. 2015;90:854–876. doi: 10.1111/brv.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keeley PW, et al. Neuronal clustering and fasciculation phenotype in Dscam- and Bax-deficient mouse retinas. J Comp Neurol. 2012;520:1349–1364. doi: 10.1002/cne.23033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blank M, et al. The Down syndrome critical region regulates retinogeniculate refinement. J Neurosci. 2011;31:5764–5776. doi: 10.1523/JNEUROSCI.6015-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matthews BJ, et al. Dendrite self-avoidance is controlled by Dscam. Cell. 2007;129:593–604. doi: 10.1016/j.cell.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 45.Hughes ME, et al. Homophilic Dscam interactions control complex dendrite morphogenesis. Neuron. 2007;54:417–427. doi: 10.1016/j.neuron.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen BE, et al. The molecular diversity of Dscam is functionally required for neuronal wiring specificity in Drosophila. Cell. 2006;125:607–620. doi: 10.1016/j.cell.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 47.Neves G, Chess A. Dscam-mediated self- versus non-self-recognition by individual neurons. Cold Spring Harb Symp Quant Biol. 2004;69:485–488. doi: 10.1101/sqb.2004.69.485. [DOI] [PubMed] [Google Scholar]
- 48.Lefebvre JL, Kostadinov D, Chen WV, Maniatis T, Sanes JR. Protocadherins mediate dendritic self-avoidance in the mammalian nervous system. Nature. 2012;488:517–521. doi: 10.1038/nature11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bruce FM, Brown S, Smith JN, Fuerst PG, Erskine L. DSCAM promotes axon fasciculation and growth in the developing optic pathway. Proc Natl Acad Sci USA. 2017;114:1702–1707. doi: 10.1073/pnas.1618606114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mataruga A, Kremmer E, Müller F. Type 3a and type 3b OFF cone bipolar cells provide for the alternative rod pathway in the mouse retina. J Comp Neurol. 2007;502:1123–1137. doi: 10.1002/cne.21367. [DOI] [PubMed] [Google Scholar]
- 51.Simmons AB, et al. Pou4f2 knock-in Cre mouse: A multifaceted genetic tool for vision researchers. Mol Vis. 2016;22:705–717. [PMC free article] [PubMed] [Google Scholar]
- 52.Ventura A, et al. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 53.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: A tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 54.Caroni P, Schwab ME. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J Cell Biol. 1988;106:1281–1288. doi: 10.1083/jcb.106.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwab ME, Caroni P. Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron. 2008;60:404–405. doi: 10.1016/j.neuron.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 56.Jones LL, Yamaguchi Y, Stallcup WB, Tuszynski MH. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci. 2002;22:2792–2803. doi: 10.1523/JNEUROSCI.22-07-02792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steinmetz MP, et al. Chronic enhancement of the intrinsic growth capacity of sensory neurons combined with the degradation of inhibitory proteoglycans allows functional regeneration of sensory axons through the dorsal root entry zone in the mammalian spinal cord. J Neurosci. 2005;25:8066–8076. doi: 10.1523/JNEUROSCI.2111-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cafferty WB, McGee AW, Strittmatter SM. Axonal growth therapeutics: Regeneration or sprouting or plasticity? Trends Neurosci. 2008;31:215–220. doi: 10.1016/j.tins.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steketee MB, et al. Regulation of intrinsic axon growth ability at retinal ganglion cell growth cones. Invest Ophthalmol Vis Sci. 2014;55:4369–4377. doi: 10.1167/iovs.14-13882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dunn FA, Della Santina L, Parker ED, Wong RO. Sensory experience shapes the development of the visual system’s first synapse. Neuron. 2013;80:1159–1166. doi: 10.1016/j.neuron.2013.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morgan JL, Dhingra A, Vardi N, Wong RO. Axons and dendrites originate from neuroepithelial-like processes of retinal bipolar cells. Nat Neurosci. 2006;9:85–92. doi: 10.1038/nn1615. [DOI] [PubMed] [Google Scholar]
- 62.Boycott BB, Hopkins JM. Cone bipolar cells and cone synapses in the primate retina. Vis Neurosci. 1991;7:49–60. doi: 10.1017/s0952523800010932. [DOI] [PubMed] [Google Scholar]
- 63.Hopkins JM, Boycott BB. Synaptic contacts of a two-cone flat bipolar cell in a primate retina. Vis Neurosci. 1992;8:379–384. doi: 10.1017/s0952523800005125. [DOI] [PubMed] [Google Scholar]
- 64.Tsukamoto Y, Omi N. Some OFF bipolar cell types make contact with both rods and cones in macaque and mouse retinas. Front Neuroanat. 2014;8:105. doi: 10.3389/fnana.2014.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Behrens C, Schubert T, Haverkamp S, Euler T, Berens P. Connectivity map of bipolar cells and photoreceptors in the mouse retina. eLife. 2016;5:e20041. doi: 10.7554/eLife.20041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnson RE, Kerschensteiner D. Retrograde plasticity and differential competition of bipolar cell dendrites and axons in the developing retina. Curr Biol. 2014;24:2301–2306. doi: 10.1016/j.cub.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Farajian R, Raven MA, Cusato K, Reese BE. Cellular positioning and dendritic field size of cholinergic amacrine cells are impervious to early ablation of neighboring cells in the mouse retina. Vis Neurosci. 2004;21:13–22. doi: 10.1017/s0952523804041021. [DOI] [PubMed] [Google Scholar]
- 68.Devries SH, Baylor DA. Mosaic arrangement of ganglion cell receptive fields in rabbit retina. J Neurophysiol. 1997;78:2048–2060. doi: 10.1152/jn.1997.78.4.2048. [DOI] [PubMed] [Google Scholar]
- 69.Borghuis BG, Ratliff CP, Smith RG, Sterling P, Balasubramanian V. Design of a neuronal array. J Neurosci. 2008;28:3178–3189. doi: 10.1523/JNEUROSCI.5259-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matsuoka RL, et al. Transmembrane semaphorin signalling controls laminar stratification in the mammalian retina. Nature. 2011;470:259–263. doi: 10.1038/nature09675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matsuoka RL, et al. Class 5 transmembrane semaphorins control selective mammalian retinal lamination and function. Neuron. 2011;71:460–473. doi: 10.1016/j.neuron.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peng YR, et al. Satb1 regulates contactin 5 to pattern dendrites of a mammalian retinal ganglion cell. Neuron. 2017;95:869–883.e6. doi: 10.1016/j.neuron.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Okumura M, Sakuma C, Miura M, Chihara T. Linking cell surface receptors to microtubules: Tubulin folding cofactor D mediates Dscam functions during neuronal morphogenesis. J Neurosci. 2015;35:1979–1990. doi: 10.1523/JNEUROSCI.0973-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Raviola E, Gilula NB. Gap junctions between photoreceptor cells in the vertebrate retina. Proc Natl Acad Sci USA. 1973;70:1677–1681. doi: 10.1073/pnas.70.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamakawa K, et al. DSCAM: A novel member of the immunoglobulin superfamily maps in a Down syndrome region and is involved in the development of the nervous system. Hum Mol Genet. 1998;7:227–237. doi: 10.1093/hmg/7.2.227. [DOI] [PubMed] [Google Scholar]
- 76.Agarwala KL, Nakamura S, Tsutsumi Y, Yamakawa K. Down syndrome cell adhesion molecule DSCAM mediates homophilic intercellular adhesion. Brain Res Mol Brain Res. 2000;79:118–126. doi: 10.1016/s0169-328x(00)00108-x. [DOI] [PubMed] [Google Scholar]
- 77.Barlow GM, Lyons GE, Richardson JA, Sarnat HB, Korenberg JR. DSCAM: An endogenous promoter drives expression in the developing CNS and neural crest. Biochem Biophys Res Commun. 2002;299:1–6. doi: 10.1016/s0006-291x(02)02548-2. [DOI] [PubMed] [Google Scholar]
- 78.Turner TN, et al. Genome sequencing of autism-affected families reveals disruption of putative noncoding regulatory DNA. Am J Hum Genet. 2016;98:58–74. doi: 10.1016/j.ajhg.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang T, et al. De novo genic mutations among a Chinese autism spectrum disorder cohort. Nat Commun. 2016;7:13316. doi: 10.1038/ncomms13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu G, et al. DSCAM functions as a netrin receptor in commissural axon pathfinding. Proc Natl Acad Sci USA. 2009;106:2951–2956. doi: 10.1073/pnas.0811083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Andrews GL, et al. Dscam guides embryonic axons by Netrin-dependent and -independent functions. Development. 2008;135:3839–3848. doi: 10.1242/dev.023739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shi L, Yu HH, Yang JS, Lee T. Specific Drosophila Dscam juxtamembrane variants control dendritic elaboration and axonal arborization. J Neurosci. 2007;27:6723–6728. doi: 10.1523/JNEUROSCI.1517-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schmucker D, et al. Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell. 2000;101:671–684. doi: 10.1016/s0092-8674(00)80878-8. [DOI] [PubMed] [Google Scholar]
- 84.Ly A, et al. DSCAM is a netrin receptor that collaborates with DCC in mediating turning responses to netrin-1. Cell. 2008;133:1241–1254. doi: 10.1016/j.cell.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Matthews BJ, Grueber WB. Dscam1-mediated self-avoidance counters netrin-dependent targeting of dendrites in Drosophila. Curr Biol. 2011;21:1480–1487. doi: 10.1016/j.cub.2011.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hattori D, Millard SS, Wojtowicz WM, Zipursky SL. Dscam-mediated cell recognition regulates neural circuit formation. Annu Rev Cell Dev Biol. 2008;24:597–620. doi: 10.1146/annurev.cellbio.24.110707.175250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Beier C, et al. Deafferented adult rod bipolar cells create new synapses with photoreceptors to restore vision. J Neurosci. 2017;37:4635–4644. doi: 10.1523/JNEUROSCI.2570-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kay JN, Chu MW, Sanes JR. MEGF10 and MEGF11 mediate homotypic interactions required for mosaic spacing of retinal neurons. Nature. 2012;483:465–469. doi: 10.1038/nature10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun LO, et al. On and off retinal circuit assembly by divergent molecular mechanisms. Science. 2013;342:1241974. doi: 10.1126/science.1241974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matsuoka RL, et al. Guidance-cue control of horizontal cell morphology, lamination, and synapse formation in the mammalian outer retina. J Neurosci. 2012;32:6859–6868. doi: 10.1523/JNEUROSCI.0267-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim JH, Wang X, Coolon R, Ye B. Dscam expression levels determine presynaptic arbor sizes in Drosophila sensory neurons. Neuron. 2013;78:827–838. doi: 10.1016/j.neuron.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Purohit AA, et al. Down syndrome cell adhesion molecule (DSCAM) associates with uncoordinated-5C (UNC5C) in netrin-1-mediated growth cone collapse. J Biol Chem. 2012;287:27126–27138. doi: 10.1074/jbc.M112.340174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Iossifov I, et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–221. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cvetkovska V, Hibbert AD, Emran F, Chen BE. Overexpression of Down syndrome cell adhesion molecule impairs precise synaptic targeting. Nat Neurosci. 2013;16:677–682. doi: 10.1038/nn.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grossman TR, et al. Over-expression of DSCAM and COL6A2 cooperatively generates congenital heart defects. PLoS Genet. 2011;7:e1002344. doi: 10.1371/journal.pgen.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Madisen L, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fuerst PG, Bruce F, Rounds RP, Erskine L, Burgess RW. Cell autonomy of DSCAM function in retinal development. Dev Biol. 2012;361:326–337. doi: 10.1016/j.ydbio.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rodieck RW. The density recovery profile: A method for the analysis of points in the plane applicable to retinal studies. Vis Neurosci. 1991;6:95–111. doi: 10.1017/s095252380001049x. [DOI] [PubMed] [Google Scholar]
- 99.Borghuis BG, Looger LL, Tomita S, Demb JB. Kainate receptors mediate signaling in both transient and sustained OFF bipolar cell pathways in mouse retina. J Neurosci. 2014;34:6128–6139. doi: 10.1523/JNEUROSCI.4941-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fransen JW, Borghuis BG. Temporally diverse excitation generates direction-selective responses in ON- and OFF-type retinal starburst amacrine cells. Cell Rep. 2017;18:1356–1365. doi: 10.1016/j.celrep.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 101.Longair MH, Baker DA, Armstrong JD. Simple Neurite Tracer: Open source software for reconstruction, visualization and analysis of neuronal processes. Bioinformatics. 2011;27:2453–2454. doi: 10.1093/bioinformatics/btr390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.