Abstract
Background
BRAF V600E mutation defines a specific colorectal cancer (CRC) subgroup with poor prognosis. Promising preclinical data showed synthetically lethal activity of mitotic spindle poisons on BRAF-mutated and BRAF-like CRC models. We designed a phase II trial to test the activity of vinorelbine in patients with BRAF V600E mutated metastatic CRC (mCRC).
Patients and methods
Patients progressed to or not deemed eligible for standard treatments received oral (60 mg/sqm) or intravenous (25 mg/sqm) vinorelbine, on days 1 and 8 every 21 days. Primary endpoint was objective response rate (ORR).
Results
Twenty patients were enrolled; 75% of them were highly pretreated. No responses were observed (0%); only one patient had a confirmed disease stabilisation (5%). Median progression-free survival was 1 month (95% CI 0.8 to 1.8), median overall survival was 2.1 months (95% CI 1.6 to 3.7). No serious adverse events were observed.
Conclusions
Despite encouraging preclinical data, our study did not show signs of clinical activity for vinorelbine in this patients’ population. Further investigations on molecular heterogeneity and dynamic evolution of BRAF V600E mutated mCRC are needed.
Keywords: Vinorelbine, BRAFV600E, metastatic colorectal cancer
Key question.
What is already known about this subject?
Patients with BRAF V600E metastatic colorectal cancer (mCRC) have an awfully poor prognosis and derive limited benefit from available treatments.
Combined targeted strategies against murine sarcoma viral oncogene homolog B (BRAF), Epidermal Growth Factor Receptor (EGFR) and Mitogen-activated protein kinase (MEK)/phosphatidylinositol-3-kinases (PI3K) are under evaluation.
Robust preclinical data highlight the potential efficacy of mitotic spindle poisons in this disease subtype.
What does this study adds?
Neither a minimal sign of activity was found with vinorelbine in a cohort of 20 pretreated patients with BRAF V600E mutated mCRC.
No Response Evaluation Criteria in Solid Tumour response were reported and only one disease stabilisation lasting more than 2 months was observed.
No feasibility or safety concerns were evident.
How might this impact on clinical practice?
Vinorelbine should not be recommended as a potential option for the treatment of patients with mCRC with BRAF V600E mutated tumours, either in advanced lines.
Further investigations on molecular heterogeneity and dynamic evolution of BRAF V600E mutated mCRC are needed.
Moving back to the bench is highly recommended before moving to more ambitious and large clinical projects.
Background
BRAF V600E mutation is found in about 8% of metastatic colorectal cancer (mCRC) and is an acknowledged marker of poor prognosis, defining a disease subgroup with specific clinical and pathological characteristics.1 Targeted dual/triple combinations achieved promising results in pretreated patients with BRAF-mutated mCRC,2–6 but the possibility to obtain a long-term disease control seems limited by the rapid emergence of acquired resistance.7 8
A specific pattern of BRAF-like gene signature has been discovered, thus, deepening the knowledge of disease biology and allowing to identify BRAF wild-type tumours with a similar aggressive clinical behaviour.9 A recent work looked for specific vulnerability genes whose suppression could interfere with cancer progression in BRAF-like models.10 RANBP2 gene was deemed responsible for the progression of the mitotic spindle, and its suppression caused death in BRAF-like, but not in non-BRAF-like cell lines. These findings led to test the hypothesis of a potential susceptibility to mitotic spindle poisons of CRC models, and vinorelbine (VNR) was found the most active drug in BRAF-like models, showing no activity in non-BRAF-like ones. In a retrospective analysis of a previous study investigating vinca alkaloids in patients with mCRC, a BRAF-like gene signature was found in the only patient achieving a prolonged complete response. Drawing from such background, we carried out a phase II study aimed at investigating the activity of VNR in patients with BRAF V600E mutated mCRC.
Patients and methods
Study population
Patients with histologically confirmed mCRC harbouring BRAF V600E mutation were eligible if they had progressed during or within 3 months from the last administration of all standard treatment options (ie, fluoropyrimidines, irinotecan, oxaliplatin, bevacizumab, aflibercept, regorafenib, cetuximab or panitumumab) or were deemed not eligible for such therapies at investigator’s judgement. Other inclusion criteria were age ≥18 years, Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) 0–1, at least one measurable lesion as defined by Response Evaluation Criteria in Solid Tumours (RECIST) V.1.1 and adequate hepatic, renal and bone marrow function. BRAF V600E mutation was detected either on primary tumours or metastatic lesions by Pyrosequencing or Sequenom Mass Array, as per local laboratory procedure. Mismatch Repair (MMR) status was evaluated by immunohistochemistry or multiplex PCR. The protocol was approved by local Ethics Committees at all participating institutions. All patients provided written informed consent before study entry.
Study design and statistical analysis
This was a multicentre, single-arm, phase 2 trial evaluating activity and safety of VNR in patients with BRAF V600E mutated mCRC. Enrolled patients received VNR orally at a dose of 60 mg/sqm or intravenously at a dose of 25 mg/sqm days 1 and 8 every 21 days. Treatment was continued until disease progression (PD), unacceptable toxicity, death or consent withdrawal.
The primary endpoint was objective response rate (ORR), defined as the proportion of patients that achieved a confirmed complete response or a partial response as best response according to RECIST V.1.1. Radiographic assessment of tumour response was carried out every 9 weeks. Secondary endpoints were progression-free survival (PFS), defined as the time from treatment start to the evidence of PD, or death due to any cause, whichever occurred first; overall survival (OS), defined as the time from treatment start to the date of death due to any cause and safety profile. The Kaplan-Meier method was used to estimate PFS and OS durations with 95% CI. Adverse events (AEs) were assessed according to the National Cancer Institute common toxicity criteria (V.4.0).
Considering the usual refractoriness of BRAF V600E mutated advanced mCRC to standard treatments, we assumed the hypothesis of achieving a 20% ORR with VNR monotherapy as acceptable for demonstrating a promising clinical activity.
Simon’s optimal two-stage design11 was adopted. The null hypothesis was set at 5% and tested against a one-sided alternative. According to the study design, in the first stage, n=21 patients would have been accrued. If two or more responses had been observed in the initial cohort, the study would have continued to the second step, and 20 additional patients would have been accrued for a total of 41. The null hypothesis would have been rejected in case of five or more responses. This design yielded a type I error rate of 0.05 (one sided) and a power of 90% for a true response rate of 20%.
Results
Study population
Between May 2016 and May 2017, a total of 20 patients were enrolled at four Italian centres. Baseline patients’ and disease characteristics are summarised in table 1. The majority of patients were male (70%), aged <70 years (85%), with ECOG PS 0 (55%). Primary tumours were more frequently located in right colon (65%). Mucinous histology was reported in 33% of cases and MMR deficiency was found in only 17% of cases. Ninety per cent of patients had more than one metastatic site at the time of enrolment and 75% had received at least two lines of systemic treatments. Previous regimens included fluoropyrimidines (90%), oxaliplatin (90%), irinotecan (80%), bevacizumab (75%), anti-Epidermal Growth Factor Receptor (EGFR)s (30%), regorafenib (30%) and TAS-102 (15%). Three patients had previously received BRAF targeted combinations, while one patient with a MMR-deficient tumour had been exposed to the immune checkpoint inhibitor nivolumab. Two patients had not received any treatment before VNR.
Table 1.
Patients’ and tumours’ characteristics
| Characteristics | n=20 N (%) |
| Sex | |
| Female | 6 (30) |
| Male | 14 (70) |
| Age | |
| Median (range) | 64 (28–80) |
| ≥70 | 3 (15) |
| <70 | 17 (85) |
| Eastern Cooperative Oncology Group performance status | |
| 0 | 11 (55) |
| 1 | 9 (45) |
| Primary tumour location | |
| Right | 13 (65) |
| Left | 5 (25) |
| Extraperitoneal rectum | 2 (10) |
| Primary tumour resected | |
| Yes | 18 (90) |
| No | 2 (10) |
| Grading | |
| G1–G2 | 13 (65) |
| G3–G4 | 5 (25) |
| Gx | 2 (10) |
| Mucinous histology | |
| Yes | 6 (33) |
| No | 12 (67) |
| NA | 2 |
| Microsatellite instability (MSI) status | |
| MSI-H | 3 (17) |
| Microsatellite stability (MSS)/MSI-L | 15 (73) |
| Not tested | 2 |
| Presentation of metastases | |
| Synchronous | 12 (60) |
| Metachronous | 8 (40) |
| Adjuvant chemotherapy | |
| Fluoropyrimidine+oxaliplatin | 5 (25) |
| Fluoropyrimidine | 1 (5) |
| No adjuvant | 14 (70) |
| Number of previous lines of therapy for metastatic disease | |
| 0 | 2 (10) |
| 1 | 3 (15) |
| 2 | 9 (45) |
| ≥3 | 6 (30) |
| Number of metastatic sites | |
| 1 | 2 (10) |
| ≥2 | 18 (90) |
| Baseline Carcino-Embryonic Antigen | |
| Normal | 5 (29) |
| > Upper Limit of Normal (5 ng/mL) | 12 (71) |
| Not available | 3 |
Activity and efficacy
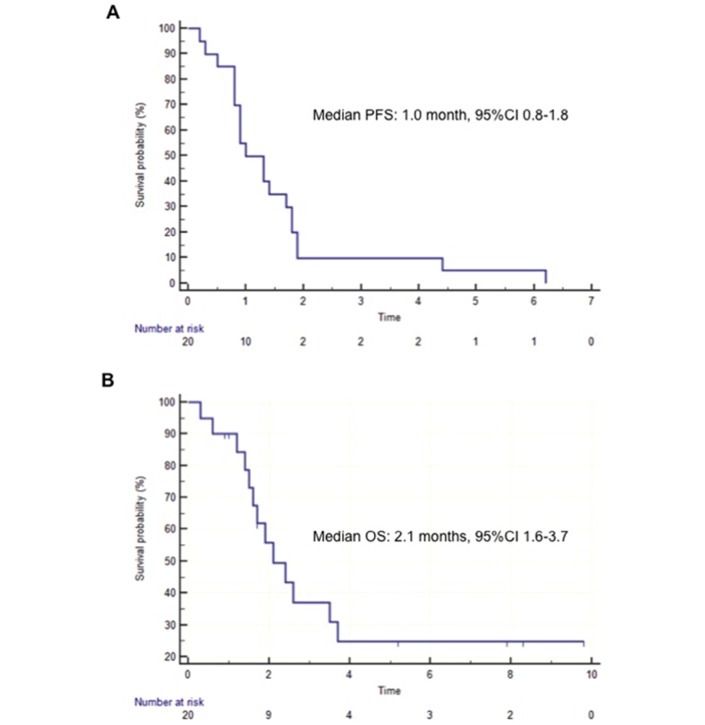
No response was observed (ORR 0%). Stable disease was reported at the first CT scan reassessment in two (10%) patients and confirmed at the second assessment only in one case. Thus, the primary endpoint of the study was not met and the trial was stopped at the first step, not reaching the prespecified number of responses to move forward to the second step. At a median follow-up of 7.4 months, all patients experienced PD and 12 (60%) patients died. The median PFS was 1.0 month (95% CI 0.8 to 1.8) and the median OS was 2.1 months (95% CI 1.6 to 3.7) (figure 1). Overall, six patients (30%) received poststudy therapy: regorafenib in four (20%) patients, chemotherapy rechallenge in one patient (5%), while one (5%) case with Methyl Guanine Methyl Transferase (MGMT) methylation responded to temozolomide.
Figure 1.
Kaplan-Meier curves of the probability of (A) progression-free survival (PFS) and (B) overall survival (OS).
Safety
All patients received at least one cycle of treatment and were assessed for safety. The median number of cycles administered per patient was 2 (range 1–9). Four (9%) out of 44 administered cycles were delayed because of toxicity. Only one patient required a dose reduction and another one early interruption due to toxicity. Treatment-related AEs are summarised in table 2. The most frequent grade ≥3 AE was neutropenia that occurred in three patients (15%) and became febrile in one case. No serious AEs or toxic deaths were reported.
Table 2.
Treatment-related adverse events according to NCI-CTCAE V.4.0
| Adverse events | G1 N (%) |
G2 N (%) |
G3 N (%) |
G4 N (%) |
| Nausea | 3 (15) | 0 | 1 (5) | 0 |
| Vomiting | 1 (5) | 0 | 1 (5) | 0 |
| Diarrhoea | 0 | 3 (15) | 1 (5) | 0 |
| Gastrointestinal pain | 0 | 2 (10) | 0 | 0 |
| Fatigue | 1 (5) | 3 (15) | 0 | 0 |
| Anaemia | 1 (5) | 2 (10) | 1 (5) | 0 |
| Thrombocytopenia | 0 | 0 | 0 | 0 |
| Neutropenia | 0 | 1 (5) | 3 (15) | 1 (5) |
| Febrile neutropenia | – | – | 1 (5) | 0 |
Discussion
The identification of efficacious treatment options for patients bearing BRAF-mutated tumours is one of the most challenging unmet needs in the landscape of mCRC. While outcome results with conventional therapies are extremely poor and FOLFOXIRI plus bevacizumab is regarded by international guidelines as a preferred first-line choice for these patients,12 targeted approaches recently provided encouraging but not outstanding results in pretreated patients. Considering the association of BRAF mutation with microsatellite instability, immunotherapy is emerging as a breakthrough option, but only in about one-third of patients.13
Based on the recent preclinical experience by Vecchione et al,10 a robust and sound biological rationale supported the potential efficacy of VNR and thus encouraged the immediate translation of these findings in the clinical setting.
Unfortunately, results of our phase II study definitely failed to reveal even a minimal signal of activity in pretreated BRAF-mutated patients. Different explanations of this discrepancy can be hypothesised.
First, preclinical data suggested a potential activity of VNR in cancer cells bearing the BRAF-like signature and not in BRAF-mutated cells. However, although the concordance between BRAF mutation and BRAF-like signature is not absolute, this gene expression profile is found in more than 90% of BRAF-mutated tumours.9 Thus, though acknowledging the limited size of our proof-of-concept trial, it seems rather unlike that none of our patients actually harboured the BRAF-like signature.
Second, no data are currently available with regard to the dynamic evolution of the BRAF-like signature across the time and under the pressure of multiple lines of treatment, so that the choice to mostly include pretreated patients might have affected our results.
Thirdly, BRAF-mutated tumours show a relevant molecular heterogeneity that might translate into clinical differences, both in terms of prognosis and sensitivity to different agents.14
In conclusion, based on our data, we believe that before moving to larger clinical trials investigating the potential role of VNR in BRAF-mutated or BRAF-like mCRC, a new step back to the bench should be taken in order to address these unanswered questions. For instance, the adoption of patient-derived xenografts as preclinical models might represent a good attempt to better mimic the human setting. Based on these findings, the BRAF-mutated population could be further dissected into different subgroups and a niche of benefit for VNR alone or in combination with other synthetically lethal agents could be found.
Footnotes
Contributors: CC, FP and FL contributed to study conception and design. CC, FP, VD, RM and FL contributed to manuscript writing. All authors collected and interpreted the data and read and approved the manuscript in its final version.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Ethics Committee Istituto Oncologico Veneto, Padua.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Tie J, Gibbs P, Lipton L, et al. Optimizing targeted therapeutic development: analysis of a colorectal cancer patient population with the BRAF(V600E) mutation. Int J Cancer 2011;128:2075–84. 10.1002/ijc.25555 [DOI] [PubMed] [Google Scholar]
- 2.Corcoran RB, André T, Yoshino T, et al. Efficacy and circulating tumor DNA (ctDNA) analysis of the BRAF inhibitor dabrafenib (D), MEK inhibitor trametinib (T), and anti-EGFR antibody panitumumab (P) in patients (pts) with BRAF V600E–mutated (BRAFm) metastatic colorectal cancer (mCRC). Ann Oncol 2016;27:455O 10.1093/annonc/mdw370.04 [DOI] [Google Scholar]
- 3.Hong DS, Morris VK, El Osta B, et al. Phase IB study of vemurafenib in combination with irinotecan and cetuximab in patients with metastatic colorectal cancer with BRAFV600E mutation. Cancer Discov 2016;6:1352–65. 10.1158/2159-8290.CD-16-0050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kopetz S, McDonough SL, Morris VK, Lenz HJ, et al. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG 1406). J Clin Oncol 2017;35:520 10.1200/JCO.2017.35.4_suppl.520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Geel R, Tabernero J, Elez E, et al. A phase Ib dose-escalation study of encorafenib and cetuximab with or without alpelisib in metastatic BRAF-mutant colorectal cancer. Cancer Discov 2017;7:610–9. 10.1158/2159-8290.CD-16-0795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yaeger R, Cercek A, O’Reilly EM, et al. Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutant metastatic colorectal cancer patients. Clin Cancer Res 2015;21:1313–20. 10.1158/1078-0432.CCR-14-2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahronian LG, Sennott EM, Van Allen EM, et al. Clinical acquired resistance to RAF inhibitor combinations in BRAF-mutant colorectal cancer through MAPK pathway alterations. Cancer Discov 2015;5:358–67. 10.1158/2159-8290.CD-14-1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pietrantonio F, Oddo D, Gloghini A, et al. MET-driven resistance to dual EGFR and BRAF blockade may be overcome by switching from EGFR to MET inhibition in BRAF-mutated colorectal cancer. Cancer Discov 2016;6:963–71. 10.1158/2159-8290.CD-16-0297 [DOI] [PubMed] [Google Scholar]
- 9.Popovici V, Budinska E, Tejpar S, et al. Identification of a poor-prognosis BRAF-mutant-like population of patients with colon cancer. J Clin Oncol 2012;30:1288–95. 10.1200/JCO.2011.39.5814 [DOI] [PubMed] [Google Scholar]
- 10.Vecchione L, Gambino V, Raaijmakers J, et al. A vulnerability of a subset of colon cancers with potential clinical utility. Cell 2016;165:317–30. 10.1016/j.cell.2016.02.059 [DOI] [PubMed] [Google Scholar]
- 11.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989;10:1–10. 10.1016/0197-2456(89)90015-9 [DOI] [PubMed] [Google Scholar]
- 12.Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386–422. 10.1093/annonc/mdw235 [DOI] [PubMed] [Google Scholar]
- 13.Andre T, Lonardi S, Wong KJM, et al. Combination of nivolumab (nivo)+ipilimumab (ipi) in the treatment of patients (pts) with deficient DNA mismatch repair (dMMR)/high microsatellite instability (MSI-H) metastatic colorectal cancer (mCRC): CheckMate 142 study. J Clin Oncol 2017;35. [Google Scholar]
- 14.Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med 2015;21:1350–6. 10.1038/nm.3967 [DOI] [PMC free article] [PubMed] [Google Scholar]