Abstract
Background
In September 2016, three acutely jaundiced (AJS) pregnant women were admitted to Am Timan Hospital, eastern Chad. We described the outbreak and conducted a case test-negative study to identify risk factors for this genotype of HEV in an acute outbreak setting.
Methods
Active case finding using a community based surveillance network identified suspected AJS cases. Pregnant or visibly ill AJS cases presenting at hospital were tested with Assure® IgM HEV rapid diagnostic tests (RDTs) and some with Polymerase Chain Reaction (PCR) in Amsterdam; confirmed cases were RDT-positive and controls were RDT-negative. All answered questions around: demographics, household makeup, area of residence, handwashing practices, water collection behaviour and clinical presentation. We calculated unadjusted odds ratios (ORs) and 95% confidence intervals (95% CI).
Results
Between September and April 2017, 1443 AJS cases (1293 confirmed) were detected in the town(attack rate: 2%; estimated 65,000 population). PCR testing confirmed HEV genotype 1e.
HEV RDTs were used for 250 AJS cases; 100 (40%) were confirmed. Risk factors for HEV infection, included: having at least two children under the age of 5 years (OR 2.1, 95%CI 1.1–4.3), having another household member with jaundice (OR 2.4, 95%CI 0.90–6.3) and, with borderline significance, living in the neighbourhoods of Riad (OR 3.8, 95%CI 1.0–1.8) or Ridina (OR 3.3, 95%CI 1.0–12.6). Cases were more likely to present with vomiting (OR 3.2, 9%CI 1.4–7.9) than controls; possibly due to selection bias. Cases were non-significantly less likely to report always washing hands before meals compared with controls (OR 0.33, 95%CI 0.1–1.1).
Discussion
Our study suggests household factors and area of residence (possibly linked to access to water and sanitation) play a role in HEV transmission; which could inform future outbreak responses. Ongoing sero-prevalence studies will elucidate more aspects of transmission dynamics of this virus with genotype 1e.
Background
Studies between 1990–2013 estimate the global burden of Hepatitis E virus (HEV) infection around 3.4 million cases of symptomatic illness, 70,000 deaths and 3000 stillbirth on an annual basis [1]. The disease is usually self-limiting but in severe cases can develop into fulminant hepatitis. Five genotypes are known to infect humans. HEV genotype 3 and 4 are zoonotic and infect a large range of hosts with pigs probably serving as the major reservoir. Genotype 1 and 2 infect only humans and are spread by faecal contamination of water supplies, but household transmission has been shown to play a role [2]. Most recently, a case of post-transplantation HEV infection with genotype 7 was reported, which had only been known to be associated with camels prior to this case [3]. The overall case fatality rate is estimated between 4–30%, but has been documented to reach up to 40% in pregnant women, particularly during the third trimester [4–6].
Outbreaks of HEV infection have been reported in sub-Saharan Africa, with the majority of documented experiences in outbreaks centralised in camps of refugees or internally displaced persons in Kenya, Uganda, Sudan, South Sudan, Ethiopia and Chad [6–10]. HEV outbreaks in Chad were reported in 1983–1984 in French soldiers and refugees in Goz Amer in 2004 [6,11]. The infection appears to be common in the country with 22% of hospitalised patients for non-hepatic causes displaying a history of infection [12].
In epidemiological week 33, 2016 (September), three females patients were admitted to the hospital in Am Timan (managed collaboratively between the Ministry of Health (MoH) of Chad and Médecins Sans Frontières [MSF]) with acute jaundice syndrome (AJS). Of these, two died (both pregnant) from fulminant hepatitis and one tested positive for HEV infection using the Assure® IgM HEV Rapid Diagnostic test (RDT) [13]. Am Timan is a town of approximately 65,000 persons in the eastern Salamat region of Chad. Following the identification of this cluster of positive HEV patients, MSF in collaboration with the MoH quickly established a community based surveillance network and initiated water and hygiene interventions including controlled bucket chlorination, hygiene promotion and hygiene kit distribution.
We report on the epidemiological and laboratory findings of an outbreak of HEV in the town of Am Timan, following its detection in September 2016 to April 2017. In addition, we conducted a case-test-negative study [14] to identify possible risk factors associated with being a confirmed case of HEV to inform future outbreak responses.
Methods
Case definitions
We defined suspected cases as those presenting with AJS at the hospital or in the community, in the region of Salamat from epidemiological week 33, 2016, onwards. AJS was defined as a person presenting with “yellow eyes”. This sensitive case definition has shown to be useful in previous outbreaks of HEV as it can be easily explained and non-medical persons can be trained on it easily. Confirmed cases were defined as suspected cases testing RDT positive for HEV. Discarded cases were suspected cases testing RDT negative for HEV.
Case finding and data collection
Active case finding was organised through a community based surveillance network, which used 160 community health workers to conduct house-to-house visits (on a bi-weekly basis) to screen for AJS and refer persons to hospital with AJS at risk for clinical complications (pregnant women, children <1 year of age and persons with AJS who were vomiting and/or had altered mental status). Community health workers filled in a structured paper-based surveillance questionnaire for each AJS case identified which collected information on demographics, household characteristics, water sources used, pregnancy status and symptoms.
All hospital referrals (and self-referred individuals) underwent a medical assessment to determine severity of illness at the hospital. AJS cases presenting at the hospital that were pregnant or severely ill were tested for HEV infection using Assure® IgM HEV RDTs. Health workers at the hospital also filled in a structured medical questionnaire for each AJS case.
As the surveillance and medical questionnaires were used for active surveillance in the outbreak, they were piloted and improved at the start of surveillance activities and immediately implemented for routine use. The collected data in the questionnaires was entered into an Excel datasheet by a data-entry clerk using unique identifiers for each case.
Laboratory investigation
In October 2016, blood specimens were sent to Sanquin Laboratories in Amsterdam, the Netherlands, and were tested for anti-HEV antibodies using the Wantai enzyme-linked immunosorbent assay (EIA) and for HEV RNA using polymerase chain reaction (PCR). Genotyping was also performed on HEV PCR positive samples. Differential diagnoses for malaria, hepatitis A, hepatitis B, hepatitis C, yellow fever, zika, dengue, west nile, rift valley and chikungunya were performed using EIAs.
Descriptive epidemiology
We calculated proportions for epidemiological characteristics by case definition category. We compared the differences in proportions between categorical variables using chi-squared tests.
Case test negative study
Using surveillance data, we compared epidemiological characteristics of cases (confirmed cases) with controls (discarded cases) by calculating unadjusted odds ratios (ORs) and their respective 95% confidence intervals (95%CI). Risk factors were considered to be associated with being a confirmed case of hepatitis E if the p-value of the association was <0.05. We conducted stratified analyses to exclude relevant confounding and effect modification. Multivariable logistic regression analysis was also conducted but has not been presented due to the small sample size of our data and a lack of plausible reasoning for the variables of interest to influence association.
Ethics
The Operational Centre Amsterdam Medical Director exempted this study from full review in accordance with the MSF Ethical Review Board guidelines, as it represented routine program monitoring and evaluation related work in the context of outbreak response where respondents were not exposed to risks. All data was anonymised for analysis and reported accordingly.
Results
Descriptive epidemiology
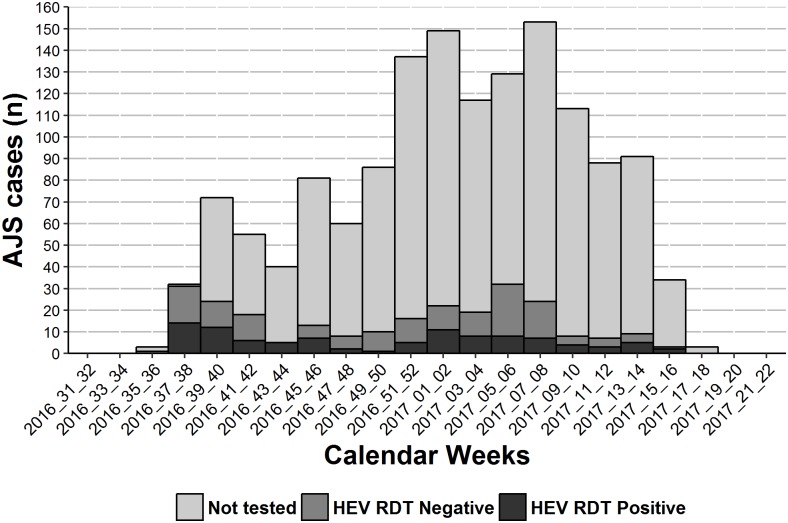
Between week 33/2016 and week 18/2017, 1443 suspected cases were identified. Cases peaked between week 51/2016 and week 8/2017, reaching 153 in weeks 7 and 8/2017. Of these 1443 suspected cases, 1193 (83%) were not tested; 250 (17%) were tested with a HEV RDT of which 100 (40%) were positive (confirmed) and 150 (60%) were negative (discarded) (Fig 1). The cumulative attack rate was 2% (1293 confirmed and suspected AJS cases in estimated 65,000 population).
Fig 1. Epidemic curve of suspected cases, confirmed HEV cases and discarded cases on bi-weekly epidemiological week periods.
Am Timan, Chad, 2016–2017 (suspected cases = 1193; discarded cases = 150, confirmed cases = 100).
The majority, 59% (n = 59) of confirmed cases were female compared to 47% (n = 562) of the suspected cases were females (p = 0.029) (Table 1). Confirmed cases were older; 62% (n = 61) of confirmed cases versus 42% (n = 503; p<0.001) of suspected cases belonged to the 15–44 year old age group.
Table 1. Characteristics of cases of acute jaundice syndrome and those testing positive and negative for HEV using RDTs RDTs during an acute outbreak of HEV in Am Timan, Chad, 2016–2017 (suspected cases = 1193; confirmed cases = 100; discarded cases = 150).
| Characteristic | Suspected cases | Confirmed cases | Discarded cases | P-valueⱡ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | N | % | N | N | % | n | N | % | ||
| Sex | ||||||||||
| Male | 631 | 1193 | 53 | 41 | 100 | 41 | 55 | 150 | 37 | 0.029 |
| Age group (years) | ||||||||||
| 0 to 4 | 153 | 1187 | 13 | 11 | 99 | 11 | 11 | 149 | 7 | 0.724 |
| 5 to 14 | 478 | 1187 | 40 | 23 | 99 | 23 | 32 | 149 | 21 | 0.001 |
| 15 to 44 | 503 | 1187 | 42 | 61 | 99 | 62 | 99 | 149 | 66 | <0.001 |
| ≥44 | 53 | 1187 | 4 | 4 | 99 | 4 | 7 | 149 | 5 | 1.000 |
| Household make up | ||||||||||
| ≥5 persons in household | 859 | 1020 | 84 | 47 | 53 | 89 | 84 | 108 | 78 | 0.497 |
| ≥ 2 children in household | 628 | 1008 | 62 | 37 | 53 | 70 | 55 | 105 | 52 | 0.339 |
| ≥ 5 children in household | 47 | 1008 | 5 | 6 | 53 | 11 | 1 | 105 | 1 | 0.065 |
| Other household member with jaundice | 131 | 1014 | 13 | 10 | 58 | 17 | 9 | 111 | 8 | 0.455 |
| Pregnant or post-partum | ||||||||||
| Pregnant | 16 | 42 | 38 | 15 | 29 | 52 | 33 | 59 | 56 | 0.371 |
| Post-partum | 1 | 8 | 12 | 2 | 16 | 12 | 1 | 23 | 4 | 1.000 |
| Clinical status | ||||||||||
| Hospitalised | 4 | 544 | 1 | 44 | 96 | 46 | 38 | 143 | 27 | <0.001 |
| Fever | 525 | 575 | 91 | 64 | 92 | 70 | 88 | 135 | 65 | <0.001 |
| Nausea | 308 | 572 | 54 | 53 | 93 | 57 | 71 | 137 | 52 | 0.651 |
| Vomiting | 590 | 703 | 84 | 57 | 65 | 88 | 72 | 104 | 69 | 0.536 |
| Epigastric pain | 348 | 571 | 61 | 54 | 91 | 59 | 74 | 137 | 54 | 0.861 |
| Itching | 405 | 569 | 71 | 51 | 91 | 56 | 62 | 138 | 45 | 0.005 |
| Diarrhoea | 85 | 563 | 15 | 12 | 93 | 13 | 17 | 138 | 12 | 0.693 |
| Abnormal mental state | 2 | 524 | 0 | 11 | 94 | 12 | 8 | 136 | 6 | <0.001 |
| Neighbourhood of residence | ||||||||||
| Anfandock | 38 | 1181 | 3 | 5 | 96 | 5 | 7 | 149 | 5 | 0.456 |
| Ganatir | 208 | 1193 | 17 | 7 | 100 | 7 | 27 | 150 | 18 | 0.011 |
| Ridina | 135 | 1181 | 11 | 8 | 96 | 8 | 4 | 149 | 3 | 0.449 |
| Riad | 76 | 1181 | 6 | 7 | 96 | 7 | 3 | 149 | 2 | 0.911 |
| Taradona (all) | 315 | 1193 | 26 | 19 | 100 | 19 | 33 | 150 | 22 | 0.132 |
ⱡ P-values derived from Pearson’s chi-squared test between suspected and confirmed cases
Amongst the 716 female cases reported, 562 were suspected cases (47%) and 59 were confirmed (8.2%). Eighty six women out of the 621 suspected and confirmed cases (13.8%) self-reported as being pregnant. For 31 pregnant women, a medical assessment was completed and out of these for four women we were able to document the outcome of their pregnancy during their hospitalisation. Two women experienced a spontaneous abortion (both in their second trimester and both confirmed HEV cases) and two women delivered a surviving baby (both in their third trimester, one confirmed HEV case).
For 1020 (71%) suspected cases and 53 (53%) confirmed cases, we had information on household makeup. Household makeup did not differ between suspected cases and confirmed cases with 89% (n = 47) of households among confirmed cases having > = 5 persons living in the household. Ten of 58 (17%) confirmed cases had another household member with jaundice, similar to suspected cases.
The proportion of pregnant and post-partum women were similar among suspected and confirmed cases; with 52% (n = 15) and 12% (n = 2) of female confirmed cases being pregnant and post-partum, respectively.
The most common clinical symptoms reported amongst confirmed cases with available information were vomiting (n = 57, 88%), fever (n = 64, 70%), epigastric pain (n = 54, 59%). Fewer confirmed cases reported fever compared to suspected cases (n = 64, 70% versus n = 525, 91%; p<0.001). Also more confirmed cases reported an abnormal mental state (n = 11, 12% versus n = 2, 0%, p<0.001) than suspected cases.
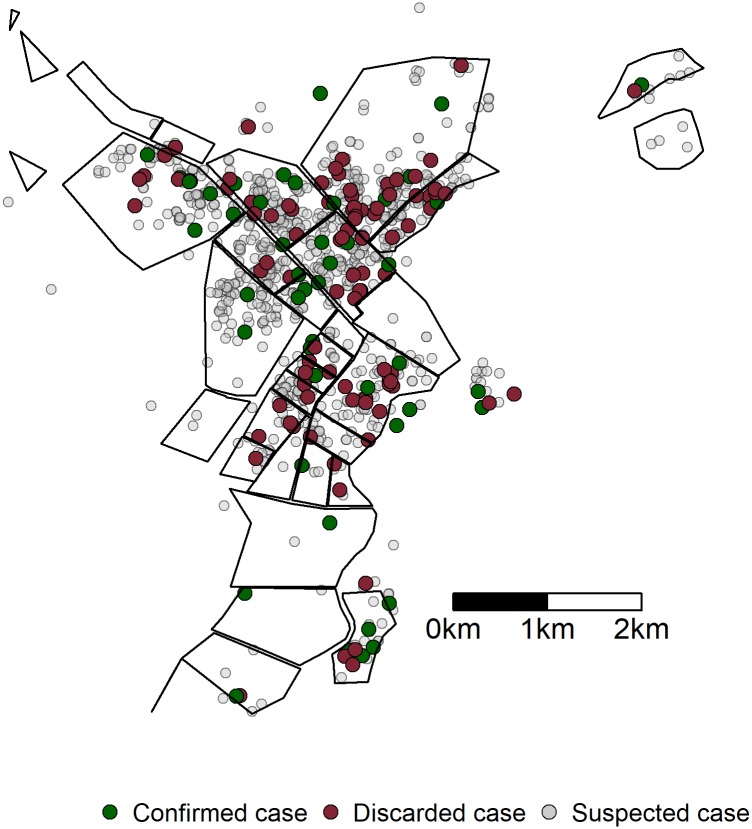
The highest proportions of suspected and confirmed cases were reported from the districts of Taradona, with 26% (n = 315) and 19% (n = 19), respectively. The proportion of confirmed cases (n = 7, 7%) reported from Ganatir was significantly less than suspected cases (n = 208, 17%; p = 0.011) (Fig 2).
Fig 2. Geospatial distribution of suspected cases, confirmed HEV cases and discarded cases during an outbreak in Am Timan, Chad, 2016–2017 (suspected cases = 1193; discarded cases = 150, confirmed cases = 100).
Forty-eight (8%), suspected and confirmed cases with information available were hospitalised; of these 44 (92%) were also confirmed cases. For 39 of the hospitalised patients (81%) a clinical outcome was recorded; 9 patients died (case fatality ratio hospitalised patients = 23%), 28 were discharged and two were lost to follow up. Amongst the hospitalised patients, there were 15 pregnant women, of whom three died (case fatality ratio = 20%).
Blood specimens of 37 confirmed and suspected cases were tested for anti-HEV antibodies in Sanquin Laboratories in Amsterdam. Of the specimens tested, 35 (95%) were positive for anti-HEV IgM of which 24 (68%) were also anti-HEV IgG positive. Eleven IgM positive specimens were tested for HEV RNA using polymerase chain reaction (PCR); all were positive. The genotype of the virus identified was Genotype 1e and was homologous to the HEV isolates from outbreaks in Sudan [15].
There was no difference in the proportion of confirmed and discarded cases that tested positive for malaria (31/96; 41.9% vs. 29/142; 34.9%; p = 0.4). More discarded cases tested positive for hepatitis B infection (17/142, 12%) compared to confirmed cases (3/96, 3.1%)(p = 0.02). Two discarded cases also tested positive for hepatitis C, two tested positive for west Nile virus infection and one for rift valley fever infection. This left 108 (72%) discarded cases with no alternative aetiology for AJS. No confirmed cases tested positive for other infectious disease aetiologies.
Case test negative study
We included 100 confirmed cases and 150 discarded cases (controls) in the analysis. We found no significant differences between cases and controls based on age or sex (Table 2). However, cases were more likely to live in households with more than two (OR 2.1, 95%CI 1.1–4.3) or more than five (OR 13, 95%CI 2.2–254) children under the age of 5 years compared with controls. Cases were also more likely, though not significantly, to have another household member with jaundice (OR 2.4, 95%CI 0.90–6.3) compared to controls.
Table 2. Risk factors for confirmed HEV infection with RDTs during an acute outbreak of HEV in Am Timan, Chad, 2016–2017.
| Characteristic | Confirmed cases | Discarded cases | OR | 95%CI | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | N | % | n | N | % | ||||
| Sex | |||||||||
| Male | 41 | 100 | 41 | 55 | 150 | 37 | 1.2 | 0.71–2.02 | 0.490 |
| Age group (years) | |||||||||
| 0 to 4 | 11 | 99 | 11 | 11 | 149 | 7 | 1.6 | 0.65–3.8 | 0.315 |
| 5 to 14 | 23 | 99 | 23 | 32 | 149 | 21 | 1.1 | 0.60–2.0 | 0.745 |
| 15 to 44 | 61 | 99 | 62 | 99 | 149 | 66 | 0.81 | 0.48–1.4 | 0.437 |
| ≥44 | 4 | 99 | 4 | 7 | 149 | 5 | 0.85 | 0.22–2.9 | 0.806 |
| Household make up | |||||||||
| ≥5 persons in household | 47 | 53 | 89 | 84 | 108 | 78 | 2.2 | 0.9–6.4 | 0.101 |
| ≥ 2 children in household | 37 | 53 | 70 | 55 | 105 | 52 | 2.1 | 1.1–4.3 | 0.038 |
| ≥ 5 children in household | 6 | 53 | 11 | 1 | 105 | 1 | 13 | 2.2–254 | 0.018 |
| Other household member with jaundice | 10 | 58 | 17 | 9 | 111 | 8 | 2.4 | 0.90–6.3 | 0.081 |
| Pregnant or post-partum | |||||||||
| Pregnant | 15 | 29 | 52 | 33 | 59 | 56 | 0.84 | 0.35–2.1 | 0.710 |
| Post-partum | 2 | 16 | 13 | 1 | 23 | 4 | 3.1 | 0.28–71 | 0.368 |
| Clinical status | |||||||||
| Hospitalised | 44 | 96 | 46 | 38 | 143 | 27 | 2.3 | 1.4–4.1 | 0.002 |
| Fever | 64 | 92 | 70 | 88 | 135 | 65 | 1.2 | 0.69–2.2 | 0.491 |
| Nausea | 53 | 93 | 57 | 71 | 137 | 52 | 1.2 | 0.73–2.1 | 0.441 |
| Vomiting | 57 | 65 | 88 | 72 | 104 | 69 | 3.2 | 1.4–7.9 | 0.008 |
| Epigastric pain | 54 | 91 | 59 | 74 | 137 | 54 | 1.2 | 0.73–2.1 | 0.428 |
| Itching | 51 | 91 | 56 | 62 | 138 | 45 | 1.6 | 0.92–2.7 | 0.100 |
| Diarrhoea | 12 | 93 | 13 | 17 | 138 | 12 | 1.1 | 0.47–2.3 | 0.895 |
| Abnormal mental state | 11 | 94 | 12 | 8 | 136 | 6 | 2.1 | 0.82–5.7 | 0.122 |
| Water point use | |||||||||
| City water | 11 | 43 | 26 | 17 | 85 | 20 | 1.4 | 0.57–3.25 | 0.472 |
| Borehole | 11 | 39 | 28 | 17 | 84 | 20 | 1.4 | 0.57–3.25 | 0.472 |
| Communal tap | 1 | 38 | 3 | 9 | 81 | 11 | 0.22 | 0.01–1.23 | 0.157 |
| River | 2 | 43 | 5 | 6 | 85 | 7 | 0.69 | 0.1–3.19 | 0.665 |
| Handwashing practices | |||||||||
| Always washing hands before eating (compared to sometimes washing hands) | 29 | 36 | 81 | 75 | 81 | 93 | 0.33 | 0.1–1.1 | 0.065 |
| Soap available for handwashing | 14 | 35 | 40 | 33 | 81 | 41 | 0.97 | 0.43–2.2 | 0.941 |
| Neighbourhood of residence | |||||||||
| Riad | 7 | 96 | 7 | 3 | 149 | 2 | 3.8 | 1.0–18 | 0.056 |
| Ridina | 8 | 96 | 8 | 4 | 149 | 3 | 3.3 | 1.0–12.6 | 0.057 |
| Ganatir | 7 | 100 | 7 | 27 | 150 | 18 | 0.34 | 0.13–0.78 | 0.016 |
Cases were more likely to present with vomiting (OR 3.2, 9%CI 1.4–7.9) and be hospitalised (OR 2.3, 95%CI 1.4–4.1) than controls. Stratified analyses suggested vomiting could be a negative confounder for the association between hospitalisation and being a case (hospitalisation adjusted OR: 4.7, 95%CI: 2.3–10, p < 0.001).
Water sources used by cases and controls and the availability of soap at the household level were similar for cases and controls. Cases were less likely to report always washing hands before meals compared with controls (OR 0.33, 95%CI 0.1–1.1), but this association was not statistically significant.
Compared with controls, cases were more likely to live in the neighbourhoods of Riad (OR 3.8, 95%CI 1.0–1.8) or Ridina (OR 3.3, 95%CI 1.0–12.6), in north-western Am Timan (though not statistically significant), but were significantly less likely to live in the neighbourhood of Ganatir (OR 0.34, 95%CI 0.13–0.78).
Discussion
This HEV outbreak of genotype 1e in Chad was the largest HEV outbreak in sub-Saharan Africa that has been detected in a setting that was not a refugee camp. The magnitude of the outbreak was smaller than those reported from Uganda 2007 and South Sudan in 2006 [2,16]. However, the clinical characteristics and case fatality of hospitalised and confirmed cases remained similar to those reported from other outbreaks. Even though the overall impact of this outbreak in an urban setting was limited and few deaths were reported, the observed clinical manifestations of HEV were similar to those seen in refugee and IDP camps.
The epidemic curve suggested a point source followed by continuous person-to-person transmission. However, despite the six week cycles, the successive waves of AJS likely resulted from the temporary stop on active case finding during week 44, 2016 due to a mass distribution of hygiene kits to all households in the town and periodic interruption of active case finding to undertake a mortality survey. The epidemic curve does nevertheless mirror the shape described in previous outbreaks [17–19].
The differences between suspected and confirmed cases suggest there were some biases to being tested for hepatitis E. The higher age of confirmed cases compared to that of suspected cases is also not unexpected as this has been determined in previous outbreaks [19,20]. The higher proportion of female confirmed cases compared to suspected cases is likely due to the targeted testing criteria for HEV in Am Timan during this outbreak for pregnant women with AJS. The observed difference between suspected and confirmed cases in presenting with fever and altered mental status may be due to the differences in self-reporting in the community versus being seen by medical professionals at the hospital; all confirmed cases went to the hospital. There is no clear explanation for why the proportion of confirmed cases coming from the neighbourhood in Ganatir would be lower than the suspected cases, but could suggest a different level of HEV transmission in this neighbourhood compared to the other neighbourhoods. Anecdotally, Riad and Ridina are considered to be the poorer neighbourhoods of Am Timan with limited sanitation infrastructure, which could explain a more efficient transmission of the virus.
Over half of tested cases were negative for HEV during this outbreak, with no other infectious aetiology found. In contrast, other types of hepatitis and malaria infection accounted for AJS cases detected during a large-scale outbreak in Uganda[21]. In Am Timan, the additionally detected AJS cases might be due to other reasons (anemia, nutritional deficiencies or other) of which we were unable to elucidate the cause [22]. Another possible reason for the high proportion of negatives could be linked to RDT performance, where PCR is the gold standard unfeasible for the setting [23,24].
We were unable to identify a single source of this outbreak. However, the epidemiological evidence suggests that, also in this HEV outbreak, transmission at household level played a role in its propagation as having another person with jaundice in the household and having more than five people in the household were predictive of being a confirmed case [10].
The higher risk of hospitalisation and vomiting among cases could be attributed to the clinical evolution of the disease, in that HEV infected persons HEV may present with more severe illness compared to those with jaundice from other causes. Alternately, it would suggest that confirmed cases were more encouraged to be hospitalised, but we do not have any information that supports that hypothesis. Nevertheless the confounding of hospitalisation by vomiting does support the notion that there was selective admission to hospital during this outbreak. This also explains why vomiting and hospitalisation as independently associated with being a case.
The possible role that small children play in the transmission of the virus is suggested in our findings and was already suggested in previous sero-prevalence studies for HEV during outbreaks in Uganda [25]. The role that behaviour around hygienic practices play in HEV transmission is also alluded to in our results in that people with poor self-reported handwashing practices were more likely to be confirmed cases. Thus, the continued need for targeted water, sanitation (latrine construction) and hygiene (health promotion and education around hygiene practices) interventions at household level during outbreaks with this virus is reaffirmed. The need for these interventions to also reach children is highlighted by our study, given their potential to act as spreaders of infection. These household characteristics (including children) have been described as risk factors in previous HEV outbreaks[17,25] as well as the storage of drinking water in large, open-mouthed containers and the sharing of basins for handwashing [2]. The sharing of handwashing basins as a risk factor is an interesting contrast to our findings and may be suggestive of differences in the baseline level of hygiene practices; furthermore in the setting of Am Timan, few people had basins and washed hands using a kettle for pouring water.
Limitations to active case finding, including periodic interruptions and the mobility of parts of the town population (semi-nomadic herders), suggests that detected AJS cases may be an underestimation of the true number. Three further limitations to the case test-negative study must be highlighted. First, several characteristics were self-reported and could thus have suffered from recall and social desirability bias. Second, due to small numbers, no multivariable logistic regression models could be constructed to take confounding or effect modification into consideration for the risk factor analysis. Finally, only a small proportion of AJS cases were eligible for testing in this outbreak response according to very strict criteria. Thus confirmed cases may not be representative of the AJS cases and controls might not be representative of the affected population. In addition, not all tested individuals had information on exposures available.
Despite the potential under-ascertainment of cases, the cumulative attack rate of 2% suggests the limited extent of this outbreak in Am Timan when compared to previous outbreaks with attack rates of 25% [18], 7.7 [10] and 6.3% [26]. Genotype 1e has been identified in larger and more severe outbreaks of HEV (in terms of case numbers and deaths)[27]. Therefore, the impact and geospatial extension of this HEV outbreak in Am Timan cannot be attributed to a less pathogenic virus. Thus, the only remaining hypothesis is that a proportion of the population in Am Timan was already immune to HEV when the current outbreak started. For this reason, a cross-sectional study is currently ongoing in Am Timan to estimate the seroprevalence of HEV and to better understand the role that children might play in the transmission of this virus at a community level.
Conclusions
Our study suggests that HEV outbreaks in an open setting, while presenting similarly in clinical characteristics and case fatality, may be of lesser magnitude than in Sub-Saharan African refugee camp settings. Further, our study suggests household factors and area of residence (possibly linked to access to water and sanitation) play a role in HEV transmission, underscoring the need for future HEV outbreak responses to incorporate timely water and hygiene interventions and targeted hygiene promotion activities in programming.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
We would like to acknowledge Djibrine Mahamatahmat for his eye for detail and constant support to the surveillance system throughout this outbreak. Thanks to Amian Boni, MSF Project coordinator (Chad Emergency Unit) who has managed to keep all activities going throughout this outbreak. We would like to acknowledge all community health workers in Am Timan who contributed to the establishment and functioning of this surveillance system. Finally we would like to thank Kostas Danis for his critical revision of this article.
All outbreak response activities described in this article were funded by MSF-OCA. Thus the surveillance data that we used for this study was from this humanitarian outbreak response activity. We designed the study within the operational context of this outbreak of hepatitis E. MSF-OCA had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript. The authors received no specific funding for this work.
Data Availability
MSF has a managed access system for data sharing. Data are available on request in accordance with MSF’s data sharing policy. Requests for access to data should be made to data.sharing@msf.org. For more information please see: 1. MSF’s Data Sharing Policy: http://fieldresearch.msf.org/msf/handle/10144/306501 2. MSF’s Data Sharing Policy PLOS Medicine article: http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1001562
Funding Statement
The authors received no specific funding for this work.
References
- 1.Murray CJL, Ortblad KF, Guinovart C, Lim SS, Wolock TM, Roberts DA, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384: 1005–70. doi: 10.1016/S0140-6736(14)60844-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howard CM, Handzel T, Hill VR, Grytdal SP, Blanton C, Kamili S, et al. Novel risk factors associated with hepatitis E virus infection in a large outbreak in northern Uganda: results from a case-control study and environmental analysis. Am J Trop Med Hyg. 2010;83: 1170–3. doi: 10.4269/ajtmh.2010.10-0384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee G-H, Tan B-H, Chi-Yuan Teo E, Lim S-G, Dan Y-Y, Wee A, et al. Chronic Infection With Camelid Hepatitis E Virus in a Liver Transplant Recipient Who Regularly Consumes Camel Meat and Milk. Gastroenterology. 2016;150: 355–357. e3 doi: 10.1053/j.gastro.2015.10.048 [DOI] [PubMed] [Google Scholar]
- 4.Haffar S, Bazerbachi F, Lake JR. Making the case for the development of a vaccination against hepatitis E virus. Liver Int. 2015;35: 311–6. doi: 10.1111/liv.12590 [DOI] [PubMed] [Google Scholar]
- 5.Labrique AB, Sikder SS, Krain LJ, West KP, Christian P, Rashid M, et al. Hepatitis E, a vaccine-preventable cause of maternal deaths. Emerg Infect Dis. 2012;18: 1401–4. doi: 10.3201/eid1809.120241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hepatitis E, Chad. Wkly Epidemiol Rec. 2004;79: 313 Available: http://www.ncbi.nlm.nih.gov/pubmed/15382402 [PubMed] [Google Scholar]
- 7.Ahmed JA, Moturi E, Spiegel P, Schilperoord M, Burton W, Kassim NH, et al. Hepatitis E outbreak, Dadaab refugee camp, Kenya, 2012. Emerg Infect Dis. 2013;19: 1010–2. doi: 10.3201/eid1906.130275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Browne LB, Menkir Z, Kahi V, Maina G, Asnakew S, Tubman M, et al. Notes from the field: hepatitis E outbreak among refugees from South Sudan—Gambella, Ethiopia, April 2014-January 2015. MMWR Morb Mortal Wkly Rep. 2015;64: 537 Available: http://www.ncbi.nlm.nih.gov/pubmed/25996097 [PMC free article] [PubMed] [Google Scholar]
- 9.Cummings MJ, Wamala JF, Komakech I, Lukwago L, Malimbo M, Omeke ME, et al. Hepatitis E in Karamoja, Uganda, 2009–2012: epidemiology and challenges to control in a setting of semi-nomadic pastoralism. Trans R Soc Trop Med Hyg. 2014;108: 648–55. doi: 10.1093/trstmh/tru123 [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC). Investigation of hepatitis E outbreak among refugees—Upper Nile, South Sudan, 2012–2013. MMWR Morb Mortal Wkly Rep. 2013;62: 581–6. Available: http://www.ncbi.nlm.nih.gov/pubmed/23884344 [PMC free article] [PubMed] [Google Scholar]
- 11.Coursaget P, Krawczynski K, Buisson Y, Nizou C, Molinié C. Hepatitis E and hepatitis C virus infections among French soldiers with non-A, non-B hepatitis. J Med Virol. 1993;39: 163–6. Available: http://www.ncbi.nlm.nih.gov/pubmed/8387572 [DOI] [PubMed] [Google Scholar]
- 12.Coursaget P, Buisson Y, N’Gawara MN, Van Cuyck-Gandre H, Roue R. Role of hepatitis E virus in sporadic cases of acute and fulminant hepatitis in an endemic area (Chad). Am J Trop Med Hyg. 1998;58: 330–4. Available: http://www.ncbi.nlm.nih.gov/pubmed/9546413 [DOI] [PubMed] [Google Scholar]
- 13.Assure HEV IgM Rapid Test for Hepatitis E [Internet].
- 14.De Serres G, Skowronski DM, Wu XW, Ambrose CS. The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of randomised placebo-controlled clinical trials. Euro Surveill. 2013;18 Available: http://www.ncbi.nlm.nih.gov/pubmed/24079398 [DOI] [PubMed] [Google Scholar]
- 15.Van Cuyck H, Juge F, Roques P. Phylogenetic analysis of the first complete hepatitis E virus (HEV) genome from Africa. FEMS Immunol Med Microbiol. 2003;39: 133–9. Available: http://www.ncbi.nlm.nih.gov/pubmed/14625096 [DOI] [PubMed] [Google Scholar]
- 16.Boccia D, Guthmann J-P, Klovstad H, Hamid N, Tatay M, Ciglenecki I, et al. High mortality associated with an outbreak of hepatitis E among displaced persons in Darfur, Sudan. Clin Infect Dis. 2006;42: 1679–84. doi: 10.1086/504322 [DOI] [PubMed] [Google Scholar]
- 17.Teshale EH, Grytdal SP, Howard C, Barry V, Kamili S, Drobeniuc J, et al. Evidence of person-to-person transmission of hepatitis E virus during a large outbreak in Northern Uganda. Clin Infect Dis An Off Publ Infect Dis Soc Am. 2010;50: 1006–1010. doi: 10.1086/651077 [DOI] [PubMed] [Google Scholar]
- 18.Teshale EH, Howard CM, Grytdal SP, Handzel TR, Barry V, Kamili S, et al. Hepatitis E epidemic, Uganda. Emerg Infect Dis. 2010;16: 126–9. doi: 10.3201/eid1601.090764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guthmann J-P, Klovstad H, Boccia D, Hamid N, Pinoges L, Nizou J-Y, et al. A large outbreak of hepatitis E among a displaced population in Darfur, Sudan, 2004: the role of water treatment methods. Clin Infect Dis An Off Publ Infect Dis Soc Am. 2006;42: 1685–1691. doi: 10.1086/504321 [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Subhadra S, Singh B, Panda BK. Hepatitis E virus: the current scenario. Int J Infect Dis. 2013;17: e228–e233. doi: 10.1016/j.ijid.2012.11.026 [DOI] [PubMed] [Google Scholar]
- 21.Gerbi GB, Williams R, Bakamutumaho B, Liu S, Downing R, Drobeniuc J, et al. Hepatitis E as a cause of acute jaundice syndrome in northern Uganda, 2010–2012. Am J Trop Med Hyg. The American Society of Tropical Medicine and Hygiene; 2015;92: 411–4. doi: 10.4269/ajtmh.14-0196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bechir M, Schelling E, Hamit MA, Tanner M, Zinsstag J. Parasitic Infections, Anemia and Malnutrition Among Rural Settled and Mobile Pastoralist Mothers and Their Children in Chad. Ecohealth. 2012;9: 122–131. doi: 10.1007/s10393-011-0727-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fogeda M, de Ory F, Avellón A, Echevarría JM. Differential diagnosis of hepatitis E virus, cytomegalovirus and Epstein-Barr virus infection in patients with suspected hepatitis E. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2009;45: 259–261. doi: 10.1016/j.jcv.2009.05.022 [DOI] [PubMed] [Google Scholar]
- 24.Pischke S, Schulze-Zur-Wiesch J, Lütgehetmann M, Kreuels B, Lueth S, Kapaun P, et al. High clinical manifestation rate in an imported outbreak of hepatitis E genotype 1 infection in a German group of travellers returning from India. Ann Hepatol. 2017;16: 57–62. doi: 10.5604/16652681.1226815 [DOI] [PubMed] [Google Scholar]
- 25.Patel RC, Kamili S, Teshale E. Hepatitis E virus infections in children age 0–15, Uganda outbreak, 2007. J Clin Virol. 2015;73: 112–4. doi: 10.1016/j.jcv.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 26.Mast EE, Polish LB, Favorov MO, Khudyakova NS, Collins C, Tukei PM, et al. Hepatitis E Among Refugees in Kenya: Minimal Apparent Person-to-person Transmission, Evidence for Age-dependent Disease Expression, and New Serologic Assays Viral Hepatitis and Liver Disease. Springer, Tokyo; 1994. pp. 375–378. [Google Scholar]
- 27.Hughes JM, Wilson ME, Teshale EH, Hu DJ, Holmberg SD. The Two Faces of Hepatitis E Virus. Clin Infect Dis. 2010;51: 328–334. doi: 10.1086/653943 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
MSF has a managed access system for data sharing. Data are available on request in accordance with MSF’s data sharing policy. Requests for access to data should be made to data.sharing@msf.org. For more information please see: 1. MSF’s Data Sharing Policy: http://fieldresearch.msf.org/msf/handle/10144/306501 2. MSF’s Data Sharing Policy PLOS Medicine article: http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1001562