Abstract
With the emergence of technologies to culture intestinal epithelial cells in vitro as various forms of intestinal organoids, there is growing interest in using such cultured intestinal cells as a source for tissue engineering and regenerative medicine. One such approach would be to combine the organoid technology with methodologies to engineer the culture environment, particularly the three-dimensional scaffold materials, to generate intestines that exquisitely recapitulate their original structures and functions. Another approach to use organoids for regenerative medicine would be to urge them to mature into functional intestines by implanting them into hosts. This process includes the tissue-engineered small intestine that uses synthetic scaffolds for tissue regeneration and direct organoid transplantation that takes advantage of submucosal tissues in the native intestines as a scaffold. Further study in these subfields could lead to the development of therapeutic options to use different types of organoids with various cell types in regenerative medicine for intestinal diseases in humans.
Keywords: Intestinal Organoid, Regenerative Medicine, Intestinal Stem Cells, Tissue Engineering, Transplantation
Abbreviations used in this paper: 2D, two-dimensional; 3D, three-dimensional; ECM, extracellular matrix; HIO, human intestinal organoid; IBD, inflammatory bowel disease; IEC, intestinal epithelial cell; ISC, intestinal stem cell; SBS, short bowel syndrome; TESI, tissue-engineered small intestine
Summary.
Based on the progress in organoid technology, researchers have explored the use of intestinal organoids for regenerative medicine. This article showcases recent progress in different strategies of organoid-based regenerative medicine, such as the generation of highly functional organoids in vitro, or direct transplantation of various forms of currently available organoids.
The intestinal epithelium constitutes a single-layered lining of cells that permits nutrient absorption in the body. It also acts as a protective barrier to restrict microbes and noxious substances in the intestinal lumen. Thus it is clear that the impairment of the intestinal epithelium leads to decreased nutrient absorption and increased ability of microorganisms and toxins to access the body, which may cause a variety of clinical manifestations in humans. Indeed, patients with severe forms of short bowel syndrome (SBS), which occurs as a result of extensive resection of the intestine,1, 2, 3 or those with congenital disorders4 suffer from malnutrition because of reduced or dysregulated absorptive function of the intestine. Also, disruption of the intestinal epithelial barrier function has been increasingly recognized as an important mechanism in the development and progression of human inflammatory bowel disease (IBD).5, 6 Many efforts have been made to restore the intestinal epithelial functions in severe forms of those diseases, such as an attempt to maximize the adaptive epithelial response to intestinal resections in SBS patients7 or to regain the intact barrier function of the intestinal epithelium in IBD patients.8 However, no standardized approach has been established.
In the last decade, there have been breakthroughs in culture technologies to maintain intestinal epithelial cells (IECs) in vitro. Studies have shown that when protein factors and extracellular matrix (ECM) scaffolds are adequately supplied, epithelial cells of the small intestine,9, 10, 11, 12, 13, 14 colon,10, 13, 15, 16, 17 and fetal intestine18, 19 of mice, humans, and many other species are organized into unique three-dimensional (3D) structures with efficient expansion of their stem cell populations. Also, there have been advances in maintaining IECs with mesenchymal cells20, 21 or inducing directed differentiation of the intestine from pluripotent cells,22, 23 both of which allow for mixed 3D culture of intestinal epithelial/non-epithelial cells in vitro. Although there have been various nomenclatures for those diverse 3D structures,24 the term organoid is now broadly accepted to describe a structure consisting of organ-specific cell types that self-organizes tissue-like structures in vitro.25, 26, 27, 28 We thus use organoid in this article to describe intestinal tissue–derived structures, irrespective of whether they contain mesenchyme or not, and also pluripotent stem cell–derived intestinal structures. On the basis of these methodologies to culture various types of intestinal organoids, there is a growing interest in using those cultured intestinal cells as a source for regenerative medicine. One such approach would be to place organoid cells in a highly engineered environment with various types of 3D scaffolds to assemble fully functional tissues that precisely recapitulate their original structures. Although this biofabrication approach is primarily aimed at generating biological systems to use for testing a variety of tissue functions, it can also be applied to the use of organoid cells in replacement therapy. Another approach would be to place intestinal organoids back into the body and urge them to undergo functional maturation for their use in regenerative medicine. In this article, we review recent studies that use organoid-based approaches for intestinal tissue engineering and regenerative medicine.
Intestinal Stem Cells and Their Niche Factors In Vivo
The intestinal epithelium of both mouse and human is in an immature state at birth and undergoes further maturation during postnatal periods.29, 30, 31 The mature tissue architecture in adult intestines (ie, crypt-villus structures in the small intestine and crypt structures in the colon, respectively) represents the functional unit of continuous self-renewal of the epithelial tissues.32, 33, 34, 35 Early studies revealed significant extracellular cues to regulate homeostasis of the IECs in vivo. Wnt signaling was demonstrated to be indispensable for their proliferation.36, 37 Moreover, animal studies showed that several other pathways such as tyrosine kinase receptor signaling38, 39 and Notch signaling40, 41 promote proliferation of IECs, whereas bone morphogenetic protein signaling has a negative effect on their growth.42, 43 Additional environmental cues that are provided by the ECM have also been suggested to be critical for survival of IECs in vivo, because the loss of attachment to the ECM induces a type of programmed cell death termed anoikis in IECs.44, 45
On the basis of these previous findings, Barker et al46 identified a gene encoding Lgr5, a member of the G-protein coupled receptor family of proteins, as the molecular marker of intestinal stem cells (ISCs). They demonstrated that Lgr5 is one of the Wnt target genes in IECs and labels the cells at the crypt base of both small intestine and colon in adults, which were found to be ISCs by genetic lineage tracing. Several other genes have been subsequently identified as markers of ISCs by using genetic lineage tracing or in vitro clonogenic assay of sorted single cells.47, 48, 49 Although a detailed description of the cells expressing these different markers is beyond the scope of this article, it is of note that identification of such ISC markers has stimulated the characterization of diverse ISC populations and their hierarchical and regulatory relationships. In addition, identification of ISC markers, as typified by Lgr5, has stimulated the cell-labeling technology that allows for the functional characterization of ISCs in various settings.50, 51, 52, 53, 54, 55
Intestinal Organoids
Early studies attempted to culture normal IECs in vitro.56, 57 For example, Evans et al56 developed a method to grow rat small intestinal cells seeded two-dimensionally in culture dishes and tested the effects of several growth factors and substrata in culture. However, such a protocol only allowed IECs to grow for around 10–14 days in the presence of contaminated subepithelial fibroblasts, indicating technical difficulties in maintaining long-term survival and proliferation of IECs in vitro. In the last decade, however, several technological breakthroughs have opened up new ways to grow normal, untransformed IECs containing ISC populations that unambiguously express stem cell markers with self-renewal and multi-differentiation capabilities. It was demonstrated that when crypt cells of the mouse small intestine are embedded in Matrigel, the pure epithelial cells grow for a long-term period with epidermal growth factor, noggin, and R-spondin in the culture medium.9 Under these well-defined conditions that mimic the physiological ISC niche environment, cells organize into 3D tissue structures composed of a central spherical domain and numerous budding structures that protrude outward. The outer parts recapitulate the crypt structure of the small intestine, with Lgr5+ ISCs and Paneth cells residing at their apex, whereas the central sphere is composed mostly of differentiated cells.9 Following this study, this type of organotypic epithelial culture was developed not only for small intestinal cells9, 10, 11, 12, 13, 14 but also for colonic cells10, 13, 15, 16, 17 and fetal intestinal progenitor cells18, 19 of mouse and other species including humans. Interestingly, several region-specific and species-specific differences in epithelial culture methodologies were noted. For example, Wnt ligands are required for culturing murine and human colonic cells.10, 15, 16, 17 Small intestinal cells of human origin also require Wnt factors in culture.14, 15 Such phenomenon may be due to low production levels of Wnt ligands in those epithelial organoids, which clearly contrasts with the mouse small intestinal epithelial organoids that contain Wnt3-producing Paneth cells.53 Intriguingly, when Wnt ligands are supplemented in mouse small intestinal epithelial organoid culture, the asymmetric architecture of organoids collapses due to the loss of local gradient of Wnt factors.53 It is of note that 1 prominent feature of the epithelial organoid system is its capability to expand cells nearly infinitely, which allows us to initiate culture from a small number of cells or tiny pieces of tissue and then obtain a large-scale epithelial culture that can be used in a wide variety of applications.
Another breakthrough was the development of a composite culture of epithelial and non-epithelial elements of the intestine. Ootani et al20 developed an air-liquid interface model to culture mouse intestinal spheres containing both epithelial and mesenchymal cell types in type I collagen gel. This study suggested that subepithelial myofibroblasts that lined the basal side of IECs might serve a role in supporting long-term culture and multi-lineage differentiation of ISCs in their system. Afterwards, Spence et al22 reported an elegant method to generate intestinal tissues by inducing step-wise differentiation of human pluripotent stem cells (embryonic stem cells and induced pluripotent stem cells) into definitive endoderm, mid/hindgut spheroids, and then intestinal spheroids. Obtained structures, termed human intestinal organoids (HIOs), are composed of both epithelial and mesenchymal cell types, with the epithelial lining allocated to the luminal surface and the stratified mesenchyme to the outer parts. These multi-layered organoids consisting of cells derived from different germ layers have been shown to recapitulate the complex cellular diversity in original tissues and will become useful tools to study epithelium-mesenchyme interactions or developmental/morphogenetic processes in the intestine. For details of technical aspects and applications of these different types of organoid systems, readers should refer to several recent reviews.25, 58, 59, 60, 61
Biofabrication Approach to Produce Intestines In Vitro
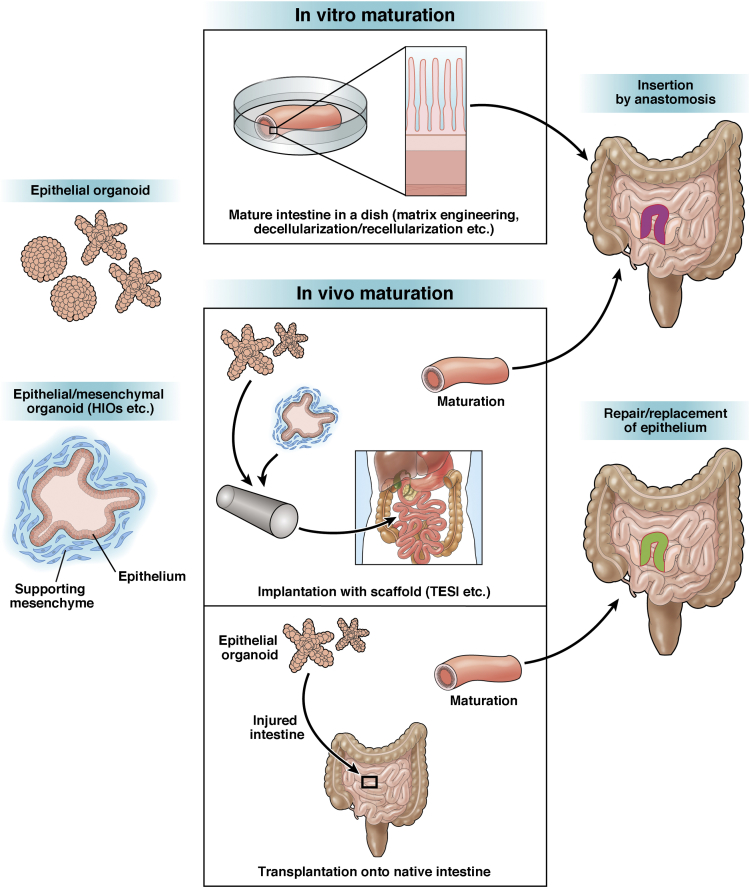
Despite the tremendous impact that organoid systems may have on multiple disciplines in intestinal research, it is true that the epithelial component in any organoid is not an exact copy of that in the intestine in vivo in terms of the size, structure, or function. For example, it remains a challenge to render the geometric patterning of the epithelial lining in organoids entirely equivalent to that in physiological intestines. Fully differentiated epithelial cells of the small intestine constitute the central sphere in the epithelial organoids but do not form finger-like projections that completely mimic villus structures.9 This is also the case with epithelia in organoids composed of mixed epithelial/non-epithelial populations. Epithelial cells in HIOs appear to form crypt-villus units, but they are thought to be functionally and architecturally similar to immature epithelia in a fetal stage.22, 62 On the basis of this background, recent studies have begun to take a newer approach to produce functional intestines that exquisitely recapitulate their original fine structures. Such an approach is called biofabrication, in which the cell culture technology is combined with material sciences and engineering technology to manufacture biological systems by using both biological and non-biological materials as their building blocks.63, 64 Several studies have shown that epithelial cells can be assembled into the structures that accurately mimic intestinal villi and crypts when seeded onto a synthetic scaffold fabricated into specifically designed 3D geometries.65, 66, 67, 68, 69, 70 Another study by Chen et al71 showed that by using a geometrically engineered 3D scaffold system, epithelial cells could form a hollow lumen with surrounding myofibroblasts, which were shown to represent proper epithelial functions such as mucus secretion into the lumen. Although many of these studies still use laboratory-adapted cell lines as a source of epithelia, several reports have shown that primary IECs obtained from mice could be incorporated in their systems.66, 68, 69, 70, 72 Studies in this rapidly growing research area to control spatial patterning of organoids will facilitate studies to produce in vivo–like intestinal architecture in vitro, which could potentially be used for clinical purposes in the future (Figure 1). Although the field is still in its early stages of development, this may also include an attempt to decellularize intestinal tissues through chemical and enzymatic digestion and use the resultant scaffolds for generating intestinal tissues by recellularization with organoid cells.70, 73
Figure 1.
Schematic illustration of intestinal tissue engineering and regenerative medicine based on organoid technology. Combining organoid technology with methodologies to engineer 3D scaffold materials may allow for generation of mature, transplantable intestines that finely recapitulate their original structures and functions. To urge intestinal organoids to mature into functional intestines by implanting them into hosts would be another strategy for regenerative medicine. This process includes TESI that utilizes synthetic scaffolds for tissue regeneration and direct organoid transplantation that takes advantage of submucosal tissues in the native intestines as a scaffold. Future research progress will offer a variety of options to utilize different types of organoids with various cell types for regenerative medicine for intestinal diseases in humans.
In addition to the structure, compositional and mechanical properties of the 3D ECM scaffold have been increasingly recognized as important factors in organoid technology (Figure 1). The original intestinal epithelial organoid culture was developed by using Matrigel as the 3D scaffold.9 It is now known that type I collagen serves as an alternative scaffold,17, 74 but it has been unclear how these 2 types of ECM act differently on epithelial organoids. In a recent study, Sachs et al75 showed that epithelial organoids of the small intestine, when embedded in type I collagen gel, can align and fuse, changing their structure from an enclosed cystic form to one that is hollow and tube-like. This phenomenon was observed only in the floating, contractible state of collagen gel but not in the adherent, incontractible state. Furthermore, organoid fusion does not occur when Matrigel is used as the ECM. Thus this study indicates that intestinal epithelial organoids intrinsically possess the potential to self-organize into tube-like structures in vitro, and this is achieved only when the composition and the mechanical factors of the ECM are appropriately provided. Another study by Gjorevski et al76 has also shown an intriguing relationship between the in vitro behavior of IECs and ECM. They demonstrated that a polyethylene glycol–based hydrogel, supplied with an Arg-Gly-Asp peptide, serves as a minimal, chemically defined ECM that supports ISC growth. By further designing the ECM polymers as a partially degradable system, they created a completely defined matrix system that is initially optimal for ISC expansion and then permissive to their morphogenetic differentiation through dynamic changes in the mechanical properties of polymers over time.76 This study strongly suggests that precisely controlling ECM composition in a sequential manner with completely defined factors will become an innovative approach to generate more complex, mature, and functional intestinal tissues in vitro.
Long-term maintenance and efficient expansion of epithelial cell populations in intestinal organoids have largely relied on their 3D culture conditions. However, the structural feature of organoids that invariably allocates the apical surface of the epithelium to inside of enclosed structure has limited the experimental manipulation of the inner space to study the effect of substances that gain access to the intestinal epithelium from the luminal side. To overcome this, several recent studies have adapted conventional 3D culture of intestinal epithelia to two-dimensional (2D) monolayer culture, which allowed investigation on the process of immunoglobulin A transcytosis across the epithelium77 or host-microbial interactions.78, 79 On the basis of the recent finding that the intestinal cells grown in 2D culture are capable of transitioning to 3D organoids and vice versa,80 these 2D culture methodologies will facilitate the study of intestinal cells, which compensates the limitation or shortcomings of conventional 3D organoid systems.
Tissue-Engineered Small Intestine
In vitro construction of functional intestine is one important step of tissue engineering in regenerative medicine for intestinal diseases. However, it may also be a reasonable strategy to implant intestinal cells, even though they are still in an immature state, and urge them to generate mature, complex tissues in host animals. In an early study before the development of current organoid technology, Choi et al81 seeded small intestinal cells, which were cultured as described by Evans et al,56 onto a synthetic tubular scaffold and then implanted them into the omentum of rats. Although the method did not allow the cells to expand extensively during culture, the implants developed into cystic structures in vivo with the epithelium facing the lumen, resembling mature small intestinal epithelia. This observation suggests the presence of some unknown in vivo factors that promote the maturation of implanted tissues. They further showed that development of such neointestinal cysts, named as tissue-engineered small intestine (TESI), involves angiogenesis,82 lymphangiogenesis,83 and recruitment of mucosal immune system84 in host animals. Moreover, it was shown that TESI could be anastomosed to the native small bowel after implantation, which allows its further maturation for 9 months,85, 86 with functionality to rescue the impaired intestinal function after massive small bowel resection.87 Grikscheit’s group has steadily made progress in this field and shown that TESI indeed includes the ISC population expressing Lgr5 by using transgenic mice.88 They also found that the human version of TESI, which is implanted into immunocompromised mice as a construct of human postnatal small intestinal tissues and biodegradable scaffolds, develops a tissue containing all 4 differentiated cell types of the human small intestinal epithelium and mesenchymal cell types. This finding suggests the availability of TESI technology to human cells.89, 90
The emergence of current organoid technology has further stimulated this area of research. It has been demonstrated that HIOs, produced in vitro by directed differentiation of human pluripotent stem cells, can engraft under the kidney capsule of immunocompromised mice.62 Importantly, transplantation of HIOs induced their maturation from displaying fetal-like to adult-type features, again suggesting some in vivo factors regulating tissue maturation. In the graft, all types of differentiated cells were located within appropriate regions of the crypt-villus axis as seen in adult tissues, and goblet cells and enteroendocrine cells showed more mature morphology than in those in culture. They also showed increases in mRNA/protein expression of markers of differentiated enterocytes and secretory cell types, further confirming the tissue maturation in grafts.62 Spence’s group has further extended the study and demonstrated that HIOs also develop into adult human intestine-like tissues when they are used as a source of TESI and implanted into the mouse abdomen.91 This finding suggests that new organoid technology, which allows for expansion of the stem cell pool in culture, could be coupled with the old TESI system that allows for surgical joining to native intestines. Interestingly, in a more recent study, Workman et al92 presented a method for incorporating the components of the enteric nervous system into HIOs by combining human pluripotent stem cell–derived neural crest cells in vitro and in vivo. Thus, sequential protocols to expand the intestinal epithelium with other intestinal elements, including enteric nervous system components, and implant them into a living body as TESI could become an attractive strategy to obtain the neointestine that has fully mature functions. In particular, combining this strategy with the development of methods to enlarge surface areas of TESI and connect them to native intestines in continuity would greatly enhance the development of treatment for severe SBS patients (Figure 1).
Direct Organoid Transplantation to Native Intestines
As seen in IBD patients, prolonged intestinal barrier dysfunction is associated not only with continuous fluid loss or malnutrition but also exposure to noxious antigens that further drive chronic inflammation.5, 6, 8 Thus, restoration of the epithelial barrier in damaged intestines by epithelial organoid transplantation might constitute a novel therapeutic approach for patients with those diseases. Patients with other diseases might also benefit from transplantation of intestinal epithelial organoids onto native intestines. For example, a loss-of-function mutation of genes encoding transporters93, 94 or enzymes95 that have nonredundant roles in intestinal functions causes clinical manifestations despite a normal intestinal surface area.4 Although these are usually rare disorders, the replacement of even a small area of the affected intestinal epithelium with gene-corrected epithelia might ameliorate the course of disease.
Even before the emergence of organoid technology, researchers made attempts to graft IECs onto the surface of native intestines. Tait et al96 developed a rat recipient model in which a freely isolated loop of the ascending colon was used as a scaffold of transplantation by applying surgical mucosectomy. Two weeks after transplanting rat small intestinal cells prepared according to the protocol of Evans et al,56 they found that the transplants ectopically regenerated the tissue containing epithelial lineages of the small intestine.96 Stelzner and his group also transplanted intestinal cells onto the native intestine.97 They isolated a part of the jejunum with its blood supply unaffected and stripped its epithelium by using ethylenediamine tetraacetic acid treatment in mice. They then showed that transplantation of small intestinal cells onto the jejunal portion resulted in the reconstitution of tissues that showed typical small bowel morphology indistinguishable from the adjacent native mucosa.97 They further showed that the cells obtained from the ileum could engraft the jejunum, keeping the expression of the Slc10A2 gene that encodes an ileal bile acid transporter.98 The neointestine that was generated by replacing the jejunal epithelium with the ileal one was capable of absorbing bile acids and corrected a clinical manifestation of malabsorption caused by intestinal resection.99 These studies were done before the development of current organoid technology that allows for expansion of stem cell populations before transplantation. However, those early studies demonstrated that the denuded surface of the intestine, which is generated by certain types of experimental procedures, could serve as a scaffold for cell transplantation.
The result of successful orthotopic transplantation of epithelial organoids, expanded by current organoid technology, was first described with colon epithelial organoids.17 Acute colitis was induced in recipient mice to generate denuded regions in the colon, and the cultured organoids were transplanted in recipient mice by means of enema. Shortly after transplantation, the transplanted cells were shown to cover the submucosa of injured recipient tissues. Importantly, mice that had successful engraftment regained their body weight faster than those of sham-transplanted mice. At 4 weeks after transplantation, transplanted cells contained proliferating cells and all terminally differentiated cell types, which indicated the presence of functional stem cells in the graft epithelium. Furthermore, extensively expanded colonic epithelial organoids, whose culture was initiated from a fluorescence-activated cell sorter–sorted single Lgr5+ colonic stem cell, were shown to engraft in multiple recipients, generating large areas of donor-derived colonic epithelium composed of normal colonic crypts.17 This study provides proof of principle that isolation, expansion, and transplantation of stem cell–containing epithelial organoids become a stem-cell therapy to repair damaged epithelia in certain types of diseases.
By using the same transplantation protocol, mouse fetal small intestinal epithelial organoids18 and in vitro–engineered endoderm progenitor cells, the latter of which were generated by transcription factor–driven conversion from fibroblasts,100 were shown to engraft to the colon. Interestingly, although the transplanted cells in these studies significantly differed from adult colonic cells, the graft tissues showed colonic epithelial phenotypes. This finding suggested that intestinal epithelial organoids composed of immature cells that preserve plasticity regarding differentiation can adapt to the colonic environment through phenotypic changes. By contrast, a subsequent study by Fukuda et al101 demonstrated that small intestinal epithelial organoids of adult mouse origin behave differently when transplanted into the colon. With a new recipient mouse model where ethylenediamine tetraacetic acid treatment and mechanical epithelial abrasion of the colon were used as a preparation for transplantation, the study showed that adult small intestinal organoids regenerate new epithelia that morphologically and functionally preserve mature small intestinal phenotypes even in the colonic environment. These collective data indicate that the mesenchymal component of the adult colon can provide some instructive signals as a scaffold to direct the fate of immature cells toward the colonic phenotype, but it is not enough to drive the differentiation status of adult small intestine cells into colonic ones. From the viewpoint of using organoids in clinical practice, this may indicate that there are options to use different types of epithelial organoids as a source of transplantation, depending on the availability of the donor cell source or the site of the diseased region in affected patients.
Transplanting epithelial organoids to the intestinal surface of living animals is becoming a unique methodology for many types of intestinal research. In a recent study, O’Rourke et al102 used the colitis recipient model and showed that patient-derived human colorectal cancer organoids engrafted to the colon in immunocompromised mice. In another report, Roper et al103 developed a novel approach to orthotopic organoid transplantation of colorectal cancer cells into mice by using colonoscopy-guided intramucosal injection. Although these studies mostly focused on the progression and metastatic processes of colorectal cancer in vivo, their data showed that orthotopic transplantation models in mice could become a useful tool even for the study of human cells. Indeed, we have recently established an orthotopic transplantation protocol that allows engraftment of normal human colonic stem cells in mouse colons to study their spatiotemporal dynamics in vivo.104 These studies, including ours, will provide evidence that human intestinal epithelial organoid cells will become a source of cell-based therapy. It remains unanswered whether the transplantation method can be adapted to any part of the intestine, or how this can be achieved. However, development of the method to repair injured epithelia directly or to partially replace the aberrant intestinal epithelia with normal ones would be a promising new therapeutic approach for several types of human diseases (Figure 1).
Conclusion
We have reviewed recent studies that represent important strategies in intestinal tissue engineering and regenerative medicine that are based on organoid culture technology. Fueled by the identification of ISCs and the development of groundbreaking organoid culture methodologies, this research area is growing rapidly. There have been many different challenges, ranging from the generation of highly organotypic structures in vitro to the repair or replacement of diseased intestinal epithelia by using healthy epithelial organoids. These various approaches are not exclusive to any one goal of intestinal regenerative medicine. Rather, concurrent advances in multiple fields will offer a variety of options to utilize different types of organoids with various cell types for regenerative medicine for intestinal diseases in humans.
Footnotes
Author contributions T.N. and T.S. conceived and wrote the manuscript.
Conflicts of interest The authors disclose no conflicts.
Funding This study was Supported by Japan Society for the Promotion of Science (JSPS KAKENHI grant number 17K19674).
Contributor Information
Tetsuya Nakamura, Email: nakamura.gast@tmd.ac.jp.
Toshiro Sato, Email: t.sato@keio.jp.
References
- 1.Jeppesen P.B. Spectrum of short bowel syndrome in adults: intestinal insufficiency to intestinal failure. JPEN J Parenter Enteral Nutr. 2014;38:8S–13S. doi: 10.1177/0148607114520994. [DOI] [PubMed] [Google Scholar]
- 2.Nightingale J., Woodward J.M., Small Bowel and Nutrition Committee of the British Society of Gastroenterology Guidelines for management of patients with a short bowel. Gut. 2006;55(Suppl 4):iv1–iv12. doi: 10.1136/gut.2006.091108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchman A.L., Scolapio J., Fryer J. AGA technical review on short bowel syndrome and intestinal transplantation. Gastroenterology. 2003;124:1111–1134. doi: 10.1016/s0016-5085(03)70064-x. [DOI] [PubMed] [Google Scholar]
- 4.Hong S.N., Dunn J.C., Stelzner M., Martín M.G. Concise review: the potential use of intestinal stem cells to treat patients with intestinal failure. Stem Cells Transl Med. 2017;6:666–676. doi: 10.5966/sctm.2016-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salim S.Y., Söderholm J.D. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:362–381. doi: 10.1002/ibd.21403. [DOI] [PubMed] [Google Scholar]
- 6.Martini E., Krug S.M., Siegmund B., Neurath M.F., Becker C. Mend your fences: the epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell Mol Gastroenterol Hepatol. 2017;4:33–46. doi: 10.1016/j.jcmgh.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeppesen P.B. Pharmacologic options for intestinal rehabilitation in patients with short bowel syndrome. JPEN J Parenter Enteral Nutr. 2014;38:45S–52S. doi: 10.1177/0148607114526241. [DOI] [PubMed] [Google Scholar]
- 8.Neurath M.F. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 2014;7:6–19. doi: 10.1038/mi.2013.73. [DOI] [PubMed] [Google Scholar]
- 9.Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J., Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 10.Mahe M.M., Aihara E., Schumacher M.A., Zavros Y., Montrose M.H., Helmrath M.A., Sato T., Shroyer N.F. Establishment of gastrointestinal epithelial organoids. Curr Protoc Mouse Biol. 2013;3:217–240. doi: 10.1002/9780470942390.mo130179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez L.M., Williamson I., Piedrahita J.A., Blikslager A.T., Magness S.T. Cell lineage identification and stem cell culture in a porcine model for the study of intestinal epithelial regeneration. PLoS One. 2013;8:e66465. doi: 10.1371/journal.pone.0066465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powell R.H., Behnke M.S. WRN conditioned media is sufficient for in vitro propagation of intestinal organoids from large farm and small companion animals. Biol Open. 2017;6:698–705. doi: 10.1242/bio.021717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dekkers J.F., Wiegerinck C.L., de Jonge H.R., Bronsveld I., Janssens H.M., de Winter-de Groot K.M., Brandsma A.M., de Jong N.W., Bijvelds M.J., Scholte B.J., Nieuwenhuis E.E., van den Brink S., Clevers H., van der Ent C.K., Middendorp S., Beekman J.M. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med. 2013;19:939–945. doi: 10.1038/nm.3201. [DOI] [PubMed] [Google Scholar]
- 14.Tüysüz N., van Bloois L., van den Brink S., Begthel H., Verstegen M.M., Cruz L.J., Hui L., van der Laan L.J., de Jonge J., Vries R., Braakman E., Mastrobattista E., Cornelissen J.J., Clevers H., Ten Berge D. Lipid-mediated Wnt protein stabilization enables serum-free culture of human organ stem cells. Nat Commun. 2017;8:14578. doi: 10.1038/ncomms14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato T., Stange D.E., Ferrante M., Vries R.G., Van Es J.H., Van den Brink S., Van Houdt W.J., Pronk A., Van Gorp J., Siersema P.D., Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 16.Jung P., Sato T., Merlos-Suárez A., Barriga F.M., Iglesias M., Rossell D., Auer H., Gallardo M., Blasco M.A., Sancho E., Clevers H., Batlle E. Isolation and in vitro expansion of human colonic stem cells. Nat Med. 2011;17:1225–1227. doi: 10.1038/nm.2470. [DOI] [PubMed] [Google Scholar]
- 17.Yui S., Nakamura T., Sato T., Nemoto Y., Mizutani T., Zheng X., Ichinose S., Nagaishi T., Okamoto R., Tsuchiya K., Clevers H., Watanabe M. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5⁺ stem cell. Nat Med. 2012;18:618–623. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- 18.Fordham R.P., Yui S., Hannan N.R., Soendergaard C., Madgwick A., Schweiger P.J., Nielsen O.H., Vallier L., Pedersen R.A., Nakamura T., Watanabe M., Jensen K.B. Transplantation of expanded fetal intestinal progenitors contributes to colon regeneration after injury. Cell Stem Cell. 2013;13:734–744. doi: 10.1016/j.stem.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mustata R.C., Vasile G., Fernandez-Vallone V., Strollo S., Lefort A., Libert F., Monteyne D., Pérez-Morga D., Vassart G., Garcia M.I. Identification of Lgr5-independent spheroid-generating progenitors of the mouse fetal intestinal epithelium. Cell Rep. 2013;5:421–432. doi: 10.1016/j.celrep.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Ootani A., Li X., Sangiorgi E., Ho Q.T., Ueno H., Toda S., Sugihara H., Fujimoto K., Weissman I.L., Capecchi M.R., Kuo C.J. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med. 2009;15:701–706. doi: 10.1038/nm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X., Nadauld L., Ootani A., Corney D.C., Pai R.K., Gevaert O., Cantrell M.A., Rack P.G., Neal J.T., Chan C.W., Yeung T., Gong X., Yuan J., Wilhelmy J., Robine S., Attardi L.D., Plevritis S.K., Hung K.E., Chen C.Z., Ji H.P., Kuo C.J. Oncogenic transformation of diverse gastrointestinal tissues in primary organoid culture. Nat Med. 2014;20:769–777. doi: 10.1038/nm.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spence J.R., Mayhew C.N., Rankin S.A., Kuhar M.F., Vallance J.E., Tolle K., Hoskins E.E., Kalinichenko V.V., Wells S.I., Zorn A.M., Shroyer N.F., Wells J.M. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCracken K.W., Howell J.C., Wells J.M., Spence J.R. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat Protoc. 2011;6:1920–1928. doi: 10.1038/nprot.2011.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stelzner M., Helmrath M., Dunn J.C., Henning S.J., Houchen C.W., Kuo C., Lynch J., Li L., Magness S.T., Martin M.G., Wong M.H., Yu J. NIH Intestinal Stem Cell Consortium. A nomenclature for intestinal in vitro cultures. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1359–G1363. doi: 10.1152/ajpgi.00493.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill D.R., Spence J.R. Gastrointestinal organoids: understanding the molecular basis of the host-microbe interface. Cell Mol Gastroenterol Hepatol. 2017;3:138–149. doi: 10.1016/j.jcmgh.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dedhia P.H., Bertaux-Skeirik N., Zavros Y., Spence J.R. Organoid models of human gastrointestinal development and disease. Gastroenterology. 2016;150:1098–1112. doi: 10.1053/j.gastro.2015.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clevers H. Modeling development and disease with organoids. Cell. 2016;165:1586–1597. doi: 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 28.Simian M., Bissell M.J. Organoids: a historical perspective of thinking in three dimensions. J Cell Biol. 2017;216:31–40. doi: 10.1083/jcb.201610056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henning S.J. Postnatal development: coordination of feeding, digestion, and metabolism. Am J Physiol. 1981;241:G199–G214. doi: 10.1152/ajpgi.1981.241.3.G199. [DOI] [PubMed] [Google Scholar]
- 30.Dehmer J.J., Garrison A.P., Speck K.E., Dekaney C.M., Van Landeghem L., Sun X., Henning S.J., Helmrath M.A. Expansion of intestinal epithelial stem cells during murine development. PLoS One. 2011;6:e27070. doi: 10.1371/journal.pone.0027070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chin A.M., Hill D.R., Aurora M., Spence J.R. Morphogenesis and maturation of the embryonic and postnatal intestine. Semin Cell Dev Biol. 2017;66:81–93. doi: 10.1016/j.semcdb.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bjerknes M., Cheng H. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology. 1999;116:7–14. doi: 10.1016/s0016-5085(99)70222-2. [DOI] [PubMed] [Google Scholar]
- 33.Wong M.H., Saam J.R., Stappenbeck T.S., Rexer C.H., Gordon J.I. Genetic mosaic analysis based on Cre recombinase and navigated laser capture microdissection. Proc Natl Acad Sci U S A. 2000;97:12601–12606. doi: 10.1073/pnas.230237997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshman E., Booth C., Potten C.S. The intestinal epithelial stem cell. Bioessays. 2002;24:91–98. doi: 10.1002/bies.10028. [DOI] [PubMed] [Google Scholar]
- 35.Beumer J., Clevers H. Regulation and plasticity of intestinal stem cells during homeostasis and regeneration. Development. 2016;143:3639–3649. doi: 10.1242/dev.133132. [DOI] [PubMed] [Google Scholar]
- 36.Korinek V., Barker N., Moerer P., van Donselaar E., Huls G., Peters P.J., Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 37.Pinto D., Gregorieff A., Begthel H., Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marchbank T., Goodlad R.A., Lee C.Y., Playford R.J. Luminal epidermal growth factor is trophic to the small intestine of parenterally fed rats. Clin Sci (Lond) 1995;89:117–120. doi: 10.1042/cs0890117. [DOI] [PubMed] [Google Scholar]
- 39.Wong V.W., Stange D.E., Page M.E., Buczacki S., Wabik A., Itami S., van de Wetering M., Poulsom R., Wright N.A., Trotter M.W., Watt F.M., Winton D.J., Clevers H., Jensen K.B. Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nat Cell Biol. 2012;14:401–408. doi: 10.1038/ncb2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Es J.H., van Gijn M.E., Riccio O., van den Born M., Vooijs M., Begthel H., Cozijnsen M., Robine S., Winton D.J., Radtke F., Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 41.Fre S., Huyghe M., Mourikis P., Robine S., Louvard D., Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- 42.Haramis A.P., Begthel H., van den Born M., van Es J., Jonkheer S., Offerhaus G.J., Clevers H. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303:1684–1686. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- 43.He X.C., Zhang J., Tong W.G., Tawfik O., Ross J., Scoville D.H., Tian Q., Zeng X., He X., Wiedemann L.M., Mishina Y., Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 44.Sträter J., Wedding U., Barth T.F., Koretz K., Elsing C., Möller P. Rapid onset of apoptosis in vitro follows disruption of beta 1-integrin/matrix interactions in human colonic crypt cells. Gastroenterology. 1996;110:1776–1784. doi: 10.1053/gast.1996.v110.pm8964403. [DOI] [PubMed] [Google Scholar]
- 45.Loza-Coll M.A., Perera S., Shi W., Filmus J. A transient increase in the activity of Src-family kinases induced by cell detachment delays anoikis of intestinal epithelial cells. Oncogene. 2005;24:1727–1737. doi: 10.1038/sj.onc.1208379. [DOI] [PubMed] [Google Scholar]
- 46.Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J., Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 47.Gracz A.D., Magness S.T. Defining hierarchies of stemness in the intestine: evidence from biomarkers and regulatory pathways. Am J Physiol Gastrointest Liver Physiol. 2014;307:G260–G273. doi: 10.1152/ajpgi.00066.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith N.R., Gallagher A.C., Wong M.H. Defining a stem cell hierarchy in the intestine: markers, caveats and controversies. J Physiol. 2016;594:4781–4790. doi: 10.1113/JP271651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barker N., van Oudenaarden A., Clevers H. Identifying the stem cell of the intestinal crypt: strategies and pitfalls. Cell Stem Cell. 2012;11:452–460. doi: 10.1016/j.stem.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 50.Snippert H.J., van der Flier L.G., Sato T., van Es J.H., van den Born M., Kroon-Veenboer C., Barker N., Klein A.M., van Rheenen J., Simons B.D., Clevers H. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 51.Ritsma L., Ellenbroek S.I., Zomer A., Snippert H.J., de Sauvage F.J., Simons B.D., Clevers H., van Rheenen J. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature. 2014;507:362–365. doi: 10.1038/nature12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruens L., Ellenbroek S.I.J., van Rheenen J., Snippert H.J. In vivo imaging reveals existence of crypt fission and fusion in adult mouse intestine. Gastroenterology. 2017;153:674–677.e3. doi: 10.1053/j.gastro.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sato T., van Es J.H., Snippert H.J., Stange D.E., Vries R.G., van den Born M., Barker N., Shroyer N.F., van de Wetering M., Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Snippert H.J., Clevers H. Tracking adult stem cells. EMBO Rep. 2011;12:113–122. doi: 10.1038/embor.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farin H.F., Jordens I., Mosa M.H., Basak O., Korving J., Tauriello D.V., de Punder K., Angers S., Peters P.J., Maurice M.M., Clevers H. Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature. 2016;530:340–343. doi: 10.1038/nature16937. [DOI] [PubMed] [Google Scholar]
- 56.Evans G.S., Flint N., Somers A.S., Eyden B., Potten C.S. The development of a method for the preparation of rat intestinal epithelial cell primary cultures. J Cell Sci. 1992;101(Pt 1):219–231. doi: 10.1242/jcs.101.1.219. [DOI] [PubMed] [Google Scholar]
- 57.Fukamachi H. Proliferation and differentiation of fetal rat intestinal epithelial cells in primary serum-free culture. J Cell Sci. 1992;103(Pt 2):511–519. doi: 10.1242/jcs.103.2.511. [DOI] [PubMed] [Google Scholar]
- 58.Sato T., Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 2013;340:1190–1194. doi: 10.1126/science.1234852. [DOI] [PubMed] [Google Scholar]
- 59.Leushacke M., Barker N. Ex vivo culture of the intestinal epithelium: strategies and applications. Gut. 2014;63:1345–1354. doi: 10.1136/gutjnl-2014-307204. [DOI] [PubMed] [Google Scholar]
- 60.Date S., Sato T. Mini-gut organoids: reconstitution of the stem cell niche. Annu Rev Cell Dev Biol. 2015;31:269–289. doi: 10.1146/annurev-cellbio-100814-125218. [DOI] [PubMed] [Google Scholar]
- 61.Merker S.R., Weitz J., Stange D.E. Gastrointestinal organoids: how they gut it out. Dev Biol. 2016;420:239–250. doi: 10.1016/j.ydbio.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 62.Watson C.L., Mahe M.M., Múnera J., Howell J.C., Sundaram N., Poling H.M., Schweitzer J.I., Vallance J.E., Mayhew C.N., Sun Y., Grabowski G., Finkbeiner S.R., Spence J.R., Shroyer N.F., Wells J.M., Helmrath M.A. An in vivo model of human small intestine using pluripotent stem cells. Nat Med. 2014;20:1310–1314. doi: 10.1038/nm.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schneeberger K., Spee B., Costa P., Sachs N., Clevers H., Malda J. Converging biofabrication and organoid technologies: the next frontier in hepatic and intestinal tissue engineering? Biofabrication. 2017;9:013001. doi: 10.1088/1758-5090/aa6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bajaj P., Schweller R.M., Khademhosseini A., West J.L., Bashir R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu Rev Biomed Eng. 2014;16:247–276. doi: 10.1146/annurev-bioeng-071813-105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sung J.H., Yu J., Luo D., Shuler M.L., March J.C. Microscale 3-D hydrogel scaffold for biomimetic gastrointestinal (GI) tract model. Lab Chip. 2011;11:389–392. doi: 10.1039/c0lc00273a. [DOI] [PubMed] [Google Scholar]
- 66.Costello C.M., Hongpeng J., Shaffiey S., Yu J., Jain N.K., Hackam D., March J.C. Synthetic small intestinal scaffolds for improved studies of intestinal differentiation. Biotechnol Bioeng. 2014;111:1222–1232. doi: 10.1002/bit.25180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Costello C.M., Sorna R.M., Goh Y.L., Cengic I., Jain N.K., March J.C. 3-D intestinal scaffolds for evaluating the therapeutic potential of probiotics. Mol Pharm. 2014;11:2030–2039. doi: 10.1021/mp5001422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y., Ahmad A.A., Sims C.E., Magness S.T., Allbritton N.L. In vitro generation of colonic epithelium from primary cells guided by microstructures. Lab Chip. 2014;14:1622–1631. doi: 10.1039/c3lc51353j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Y., Gunasekara D.B., Reed M.I., DiSalvo M., Bultman S.J., Sims C.E., Magness S.T., Allbritton N.L. A microengineered collagen scaffold for generating a polarized crypt-villus architecture of human small intestinal epithelium. Biomaterials. 2017;128:44–55. doi: 10.1016/j.biomaterials.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koppes A.N., Kamath M., Pfluger C.A., Burkey D.D., Dokmeci M., Wang L., Carrier R.L. Complex, multi-scale small intestinal topography replicated in cellular growth substrates fabricated via chemical vapor deposition of Parylene C. Biofabrication. 2016;8:035011. doi: 10.1088/1758-5090/8/3/035011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen Y., Lin Y., Davis K.M., Wang Q., Rnjak-Kovacina J., Li C., Isberg R.R., Kumamoto C.A., Mecsas J., Kaplan D.L. Robust bioengineered 3D functional human intestinal epithelium. Sci Rep. 2015;5:13708. doi: 10.1038/srep13708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shaffiey S.A., Jia H., Keane T., Costello C., Wasserman D., Quidgley M., Dziki J., Badylak S., Sodhi C.P., March J.C., Hackam D.J. Intestinal stem cell growth and differentiation on a tubular scaffold with evaluation in small and large animals. Regen Med. 2016;11:45–61. doi: 10.2217/rme.15.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maghsoudlou P., Totonelli G., Loukogeorgakis S.P., Eaton S., De Coppi P. A decellularization methodology for the production of a natural acellular intestinal matrix. J Vis Exp. 2013 Oct 7;(80) doi: 10.3791/50658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jabaji Z., Brinkley G.J., Khalil H.A., Sears C.M., Lei N.Y., Lewis M., Stelzner M., Martín M.G., Dunn J.C. Type I collagen as an extracellular matrix for the in vitro growth of human small intestinal epithelium. PLoS One. 2014;9:e107814. doi: 10.1371/journal.pone.0107814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sachs N., Tsukamoto Y., Kujala P., Peters P.J., Clevers H. Intestinal epithelial organoids fuse to form self-organizing tubes in floating collagen gels. Development. 2017;144:1107–1112. doi: 10.1242/dev.143933. [DOI] [PubMed] [Google Scholar]
- 76.Gjorevski N., Sachs N., Manfrin A., Giger S., Bragina M.E., Ordóñez-Morán P., Clevers H., Lutolf M.P. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539:560–564. doi: 10.1038/nature20168. [DOI] [PubMed] [Google Scholar]
- 77.Moon C., VanDussen K.L., Miyoshi H., Stappenbeck T.S. Development of a primary mouse intestinal epithelial cell monolayer culture system to evaluate factors that modulate IgA transcytosis. Mucosal Immunol. 2014;7:818–828. doi: 10.1038/mi.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.VanDussen K.L., Marinshaw J.M., Shaikh N., Miyoshi H., Moon C., Tarr P.I., Ciorba M.A., Stappenbeck T.S. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut. 2015;64:911–920. doi: 10.1136/gutjnl-2013-306651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.In J., Foulke-Abel J., Zachos N.C., Hansen A.M., Kaper J.B., Bernstein H.D., Halushka M., Blutt S., Estes M.K., Donowitz M., Kovbasnjuk O. Enterohemorrhagic Escherichia coli reduce mucus and intermicrovillar bridges in human stem cell-derived colonoids. Cell Mol Gastroenterol Hepatol. 2016;2:48–62.e3. doi: 10.1016/j.jcmgh.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y., DiSalvo M., Gunasekara D.B., Dutton J., Proctor A., Lebhar M.S., Williamson I., Speer J., Howard R.L., Smiddy N.M., Bultman S.J., Sims C.E., Magness S.T., Allbritton N.L. Self-renewing monolayer of primary colonic or rectal epithelial cells. Cell Mol Gastroenterol Hepatol. 2017;4:165–182. doi: 10.1016/j.jcmgh.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Choi R.S., Riegler M., Pothoulakis C., Kim B.S., Mooney D., Vacanti M., Vacanti J.P. Studies of brush border enzymes, basement membrane components, and electrophysiology of tissue-engineered neointestine. J Pediatr Surg. 1998;33:991–997. doi: 10.1016/s0022-3468(98)90520-6. [DOI] [PubMed] [Google Scholar]
- 82.Gardner-Thorpe J., Grikscheit T.C., Ito H., Perez A., Ashley S.W., Vacanti J.P., Whang E.E. Angiogenesis in tissue-engineered small intestine. Tissue Eng. 2003;9:1255–1261. doi: 10.1089/10763270360728161. [DOI] [PubMed] [Google Scholar]
- 83.Duxbury M.S., Grikscheit T.C., Gardner-Thorpe J., Rocha F.G., Ito H., Perez A., Ashley S.W., Vacanti J.P., Whang E.E. Lymphangiogenesis in tissue-engineered small intestine. Transplantation. 2004;77:1162–1166. doi: 10.1097/01.tp.0000121506.34924.3c. [DOI] [PubMed] [Google Scholar]
- 84.Tavakkolizadeh A., Berger U.V., Stephen A.E., Kim B.S., Mooney D., Hediger M.A., Ashley S.W., Vacanti J.P., Whang E.E. Tissue-engineered neomucosa: morphology, enterocyte dynamics, and SGLT1 expression topography. Transplantation. 2003;75:181–185. doi: 10.1097/01.TP.0000044101.03656.9F. [DOI] [PubMed] [Google Scholar]
- 85.Kaihara S., Kim S.S., Kim B.S., Mooney D., Tanaka K., Vacanti J.P. Long-term follow-up of tissue-engineered intestine after anastomosis to native small bowel. Transplantation. 2000;69:1927–1932. doi: 10.1097/00007890-200005150-00031. [DOI] [PubMed] [Google Scholar]
- 86.Perez A., Grikscheit T.C., Blumberg R.S., Ashley S.W., Vacanti J.P., Whang E.E. Tissue-engineered small intestine: ontogeny of the immune system. Transplantation. 2002;74:619–623. doi: 10.1097/00007890-200209150-00006. [DOI] [PubMed] [Google Scholar]
- 87.Grikscheit T.C., Siddique A., Ochoa E.R., Srinivasan A., Alsberg E., Hodin R.A., Vacanti J.P. Tissue-engineered small intestine improves recovery after massive small bowel resection. Ann Surg. 2004;240:748–754. doi: 10.1097/01.sla.0000143246.07277.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sala F.G., Matthews J.A., Speer A.L., Torashima Y., Barthel E.R., Grikscheit T.C. A multicellular approach forms a significant amount of tissue-engineered small intestine in the mouse. Tissue Eng Part A. 2011;17:1841–1850. doi: 10.1089/ten.tea.2010.0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Levin D.E., Barthel E.R., Speer A.L., Sala F.G., Hou X., Torashima Y., Grikscheit T.C. Human tissue-engineered small intestine forms from postnatal progenitor cells. J Pediatr Surg. 2013;48:129–137. doi: 10.1016/j.jpedsurg.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 90.Grant C.N., Mojica S.G., Sala F.G., Hill J.R., Levin D.E., Speer A.L., Barthel E.R., Shimada H., Zachos N.C., Grikscheit T.C. Human and mouse tissue-engineered small intestine both demonstrate digestive and absorptive function. Am J Physiol Gastrointest Liver Physiol. 2015;308:G664–G677. doi: 10.1152/ajpgi.00111.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Finkbeiner S.R., Freeman J.J., Wieck M.M., El-Nachef W., Altheim C.H., Tsai Y.H., Huang S., Dyal R., White E.S., Grikscheit T.C., Teitelbaum D.H., Spence J.R. Generation of tissue-engineered small intestine using embryonic stem cell-derived human intestinal organoids. Biol Open. 2015;4:1462–1472. doi: 10.1242/bio.013235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Workman M.J., Mahe M.M., Trisno S., Poling H.M., Watson C.L., Sundaram N., Chang C.F., Schiesser J., Aubert P., Stanley E.G., Elefanty A.G., Miyaoka Y., Mandegar M.A., Conklin B.R., Neunlist M., Brugmann S.A., Helmrath M.A., Wells J.M. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med. 2017;23:49–59. doi: 10.1038/nm.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martín M.G., Turk E., Lostao M.P., Kerner C., Wright E.M. Defects in Na+/glucose cotransporter (SGLT1) trafficking and function cause glucose-galactose malabsorption. Nat Genet. 1996;12:216–220. doi: 10.1038/ng0296-216. [DOI] [PubMed] [Google Scholar]
- 94.Qiu A., Jansen M., Sakaris A., Min S.H., Chattopadhyay S., Tsai E., Sandoval C., Zhao R., Akabas M.H., Goldman I.D. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006;127:917–928. doi: 10.1016/j.cell.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 95.Jackson R.S., Creemers J.W., Farooqi I.S., Raffin-Sanson M.L., Varro A., Dockray G.J., Holst J.J., Brubaker P.L., Corvol P., Polonsky K.S., Ostrega D., Becker K.L., Bertagna X., Hutton J.C., White A., Dattani M.T., Hussain K., Middleton S.J., Nicole T.M., Milla P.J., Lindley K.J., O'Rahilly S. Small-intestinal dysfunction accompanies the complex endocrinopathy of human proprotein convertase 1 deficiency. J Clin Invest. 2003;112:1550–1560. doi: 10.1172/JCI18784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tait I.S., Evans G.S., Flint N., Campbell F.C. Colonic mucosal replacement by syngeneic small intestinal stem cell transplantation. Am J Surg. 1994;167:67–72. doi: 10.1016/0002-9610(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 97.Avansino J.R., Chen D.C., Woolman J.D., Hoagland V.D., Stelzner M. Engraftment of mucosal stem cells into murine jejunum is dependent on optimal dose of cells. J Surg Res. 2006;132:74–79. doi: 10.1016/j.jss.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 98.Avansino J.R., Chen D.C., Hoagland V.D., Woolman J.D., Stelzner M. Orthotopic transplantation of intestinal mucosal organoids in rodents. Surgery. 2006;140:423–434. doi: 10.1016/j.surg.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 99.Avansino J.R., Chen D.C., Hoagland V.D., Woolman J.D., Haigh W.G., Stelzner M. Treatment of bile acid malabsorption using ileal stem cell transplantation. J Am Coll Surg. 2005;201:710–720. doi: 10.1016/j.jamcollsurg.2005.06.270. [DOI] [PubMed] [Google Scholar]
- 100.Morris S.A., Cahan P., Li H., Zhao A.M., San Roman A.K., Shivdasani R.A., Collins J.J., Daley G.Q. Dissecting engineered cell types and enhancing cell fate conversion via CellNet. Cell. 2014;158:889–902. doi: 10.1016/j.cell.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fukuda M., Mizutani T., Mochizuki W., Matsumoto T., Nozaki K., Sakamaki Y., Ichinose S., Okada Y., Tanaka T., Watanabe M., Nakamura T. Small intestinal stem cell identity is maintained with functional Paneth cells in heterotopically grafted epithelium onto the colon. Genes Dev. 2014;28:1752–1757. doi: 10.1101/gad.245233.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.O'Rourke K.P., Loizou E., Livshits G., Schatoff E.M., Baslan T., Manchado E., Simon J., Romesser P.B., Leach B., Han T., Pauli C., Beltran H., Rubin M.A., Dow L.E., Lowe S.W. Transplantation of engineered organoids enables rapid generation of metastatic mouse models of colorectal cancer. Nat Biotechnol. 2017;35:577–582. doi: 10.1038/nbt.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roper J., Tammela T., Cetinbas N.M., Akkad A., Roghanian A., Rickelt S., Almeqdadi M., Wu K., Oberli M.A., Sánchez-Rivera F., Park Y.K., Liang X., Eng G., Taylor M.S., Azimi R., Kedrin D., Neupane R., Beyaz S., Sicinska E.T., Suarez Y., Yoo J., Chen L., Zukerberg L., Katajisto P., Deshpande V., Bass A.J., Tsichlis P.N., Lees J., Langer R., Hynes R.O., Chen J., Bhutkar A., Jacks T., Yilmaz Ö.H. In vivo genome editing and organoid transplantation models of colorectal cancer and metastasis. Nat Biotechnol. 2017;35:569–576. doi: 10.1038/nbt.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sugimoto S., Ohta Y., Fujii M., Matano M., Shimokawa M., Nanki K., Date S., Nishikori S., Nakazato Y., Nakamura T., Kanai T., Sato T. Reconstruction of the human colon epithelium in vivo. Cell Stem Cell. 2017 doi: 10.1016/j.stem.2017.11.012. [in press] [DOI] [PubMed] [Google Scholar]