Abstract
Prion transmission between species is governed in part by primary sequence similarity between the infectious prion aggregate, PrPSc, and the cellular prion protein of the host, PrPC. A puzzling feature of prion formation is that certain PrPC sequences, such as that of bank vole, can be converted by a remarkably broad array of different mammalian prions, whereas others, such as rabbit, show robust resistance to cross-species prion conversion. To examine the structural determinants that confer susceptibility or resistance to prion conversion, we systematically tested over 40 PrPC variants of susceptible and resistant PrPC sequences in a prion conversion assay. Five key residue positions markedly impacted prion conversion, four of which were in steric zipper segments where side chains from amino acids tightly interdigitate in a dry interface. Strikingly, all five residue substitutions modulating prion conversion involved the gain or loss of an asparagine or glutamine residue. For two of the four positions, Asn and Gln residues were not interchangeable, revealing a strict requirement for either an Asn or Gln residue. Bank voles have a high number of Asn and Gln residues and a high Asn:Gln ratio. These findings suggest that a high number of Asn and Gln residues at specific positions may stabilize β-sheets and lower the energy barrier for cross-species prion transmission, potentially because of hydrogen bond networks from side chain amides forming extended Asn/Gln ladders. These data also suggest that multiple PrPC segments containing Asn/Gln residues may act in concert along a replicative interface to promote prion conversion.
Keywords: amyloid, fibril, neurodegeneration, prion, prion disease, steric zipper, transmission
Introduction
Prion diseases are fatal neurodegenerative disorders of humans and animals caused by prion protein aggregates accumulating in the brain and spinal cord (1). β-Sheet–rich prion aggregates, known as PrPSc, template the misfolding of the cellular prion protein monomer, PrPC, similar to seeding mechanisms that occur with other amyloidogenic proteins such as amyloid-β, α-synuclein, and islet amyloid polypeptide (2, 3). PrPSc-templated conversion of PrPC monomers typically requires a high degree of sequence similarity (4–6); however, conversion of dissimilar PrP sequences can occur and induce prion disease in other species; for example, bovine spongiform encephalopathy prions have infected humans, cats, and zoo bovids (7, 8).
Mammalian PrPC is highly conserved in both sequence and structure, consisting of ∼210 amino acids with a disordered N terminus (residues 23–120) and a globular, C-terminal domain arranged as three α-helices and a short anti-parallel β-sheet (9, 10). We found that conversion of mouse or human PrPC by elk chronic wasting disease (CWD)3 prions occurs efficiently when the PrPC sequence within a loop segment rich in polar and aromatic residues, the β2–α2 loop, is mutated to match the elk sequence (VDQYNNQNTF) (11, 12). Further studies of the β2–α2 loop sequence revealed that substituting Tyr169 with glycine, leucine, or glutamine inhibited prion conversion, whereas substitutions of bulky aromatic residues (Y169W and Y169F) enabled conversion, indicating a requirement for highly specific amino acid side chain properties, in this case, an aromatic side chain (13). Collectively, these findings suggest that prion conversion between different PrP sequences does not require an exact match in the side chain between PrPC and PrPSc; however, side chain complementarity in amyloid-prone segments is essential. The β2–α2 loop has been identified as a steric zipper segment of PrP, in which side chains emerging from two β-sheets tightly interdigitate, forming a dry interface (14). Although residue mismatches between species may diminish steric zipper formation, polar, hydrophobic, and aromatic residues in steric zipper segments may also stabilize early aggregates via aromatic residue stacks, serine stacks, and asparagine ladders and thus may promote conversion to a β-sheet–rich isoform (15, 16)
The bank vole is a rodent that has proven remarkably susceptible to a diverse array of prions from humans and animals, and bank vole PrPC has recently been termed the “universal acceptor” sequence (17–21). In contrast, other PrPC sequences, such as that of rabbit, are more resistant to seeded conversion by prions from another species or cross-seeded conversion (22). Notably, certain residues in the bank vole and rabbit were identified as influencing seeded prion conversion (22–25). Yet in general the residues and underlying mechanisms controlling cross-species prion conversion are unclear. Here we used PrPC sequences from susceptible and resistant species in an in vitro prion conversion assay, cell-lysate protein misfolding cyclic amplification (clPMCA) (12, 13, 26), to identify the key residue positions that promote or inhibit prion conversion. We systematically substituted single residues in bank vole, rabbit, and human PrPC and seeded with dissimilar elk or mouse prions to examine the mechanism of prion conversion. Conversion of three mammalian PrPC sequences with single or multiple substitutions has revealed Asn/Gln residues in PrP, often within known steric zippers, as powerful promoters of prion conversion among dissimilar sequences, suggesting that within a β-sheet, the linear chains of hydrogen bonds among the amides in Asn/Gln side chains stabilize and enhance prion assembly.
Results
Replacing asparagine and glutamine residues in bank vole PrPC inhibits its conversion
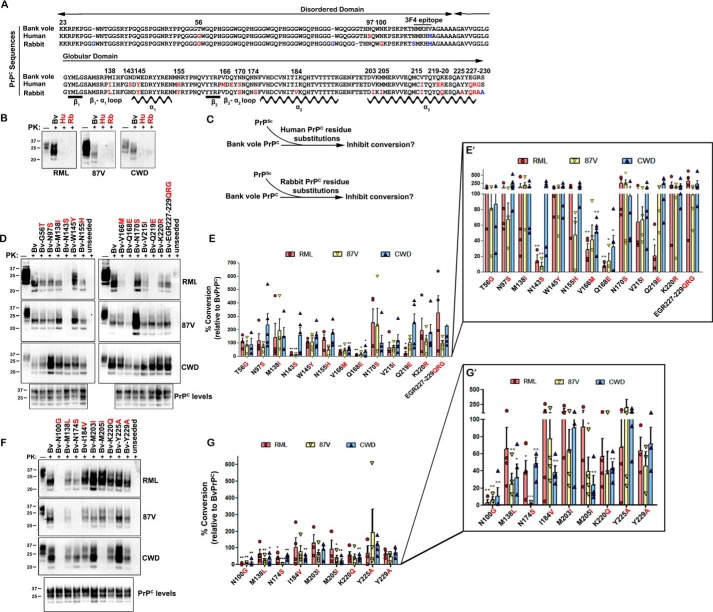
The amino acid sequences of bank vole, rabbit, and human PrPC differ at 27 positions in fully processed PrP (Fig. 1A), yet show highly similar secondary and tertiary structure by NMR spectroscopy (27–29). To identify the residues that impact prion cross-seeding, we first compared the conversion of bank vole, human, and rabbit PrPC seeded by elk and mouse prions. The two mouse prions used, RML and 87V, differ in amino acid sequence in two positions (supplemental Fig. S1) and in the PrPSc biochemical properties and disease phenotype in mice (30). Each PrPC sequence was expressed in PrP-deficient RK13 cells, and cell lysates were seeded with prions or were unseeded (supplemental Fig. S2) and subjected to clPMCA. Samples were then analyzed for proteinase K (PK)–resistant PrPSc by Western blotting, using the anti-PrP 3F4 antibody epitope for detection of newly converted PrPSc (31). Bank vole PrPC (BvPrPC) was readily seeded by elk CWD as well as mouse 87V and RML prions, whereas human (HuPrPC) and rabbit PrPC (RbPrPC) were not converted by any of the prions (Fig. 1B), confirming that the conversion efficiency reported for BvPrPC, HuPrPC, and RbPrPC (32–35) could be reproduced in the clPMCA assay.
Figure 1.
Human and rabbit amino acid substitutions inhibit conversion of bank vole PrPC. A, alignment of the PrPC sequences from bank vole, human, and rabbit reveals amino acid sequence differences between species at 27 positions (labeled in red and blue), 21 of which were investigated here (in red). Locations of the β-strands and α-helices are shown. B, mouse RML, mouse 87V, and elk CWD prions convert bank vole BvPrPC, but not HuPrPC or RbPrPC. C, schematic for experimental approach. BvPrPC with HuPrPC or RbPrPC substitutions was seeded with mouse or elk prions, and the newly converted PrPSc was measured after 24 h. D, representative immunoblots show conversion of BvPrPC with amino acid substitutions from HuPrPC. E, quantitative analysis shows that substitutions N143S, Q168E, and Q219E strongly inhibit BvPrPC conversion, depending on the PrPSc seed. F, representative immunoblots show conversion of BvPrPC with amino acid substitutions from RbPrPC. G, quantitative analysis shows that the N100G and N174S substitutions strongly inhibit conversion, depending on the PrPSc seed. E′ and G′ show the quantified data with a segmented y axis. For A, the GenBankTM accession numbers for bank vole, human, and rabbit PrPC are AF367624, DQ408531, and U28334, respectively. Quantified data are from three to five independent experiments (E and G). The error bars indicate the observed variance. *, p < 0.05; **, p < 0.01; ***, p < 0.001, one-sample t test. One-way ANOVA with Tukey post hoc test revealed statistically significant differences for CWD between residue substitutions at positions 100 and 203, positions 138 and 225, and positions 205 and 225 (*, p < 0.05), as well as between residues 100 and 225 (**, p < 0.01).
To identify the HuPrPC residues that obstruct prion conversion, bank vole PrPC with human residue substitutions was used as a substrate in clPMCA (Fig. 1C). Fifteen residue substitutions were tested singly or grouped (positions 227–229) (Fig. 1A), five of which had a major effect on conversion. BvPrPC with the V166M or Q168E substitutions from HuPrPC (β2–α2 loop) markedly reduced conversion when seeded with mouse RML, mouse 87V, or elk CWD (∼10–50% conversion relative to BvPrPC) (Fig. 1, D and E). The N143S substitution (β1–α1 loop) was strongly inhibitory for RML and 87V mouse prions (14 and 7% conversion, respectively), but not CWD (>100% conversion) (Fig. 1, D and E). Additionally, N155H was inhibitory for 87V-induced conversion (47% conversion), and Q219E was inhibitory for RML-induced conversion (20% conversion) (Fig. 1, D and E). Given that all three PrPSc sequences include Asn143 and Gln219 (supplemental Fig. S1), successful conversion is not likely due simply to a primary sequence match with PrPC but instead was influenced by the PrPSc conformation. BvPrPC with 10 other human residue substitutions was converted to high PrPSc levels by all three prions (Fig. 1, D and E), indicating that most human residues do not inhibit cross-seeding by CWD or certain mouse prions. Thus, substitution of two human PrPC residues (Met166 and Glu168) inhibited conversion of BvPrPC by all prions tested, and three other substitutions (Ser143, His155, Glu219) inhibited prion conversion to 50% or less in a PrPSc sequence- and/or conformation-specific manner.
Rabbits have resisted intracerebral challenge with Creutzfeldt–Jakob disease, kuru, sheep scrapie, and mouse-adapted scrapie prions (strain ME7) (32, 33), and are considered one of the most highly prion-resistant species. We next measured the conversion of BvPrPC having nine single or grouped rabbit-specific residue substitutions (Fig. 1C). The N100G substitution showed the most dramatic inhibition, with ≤10% conversion, whereas K220Q resulted in only 37–57% conversion by any prion (Fig. 1, F and G). The M138L, I184V, and M205I rabbit substitutions reduced CWD-seeded conversion to ∼30, 40, and 25% conversion, respectively (Fig. 1, F and G). Additionally, N174S completely blocked mouse 87V-seeded conversion of BvPrPC but only reduced RML-seeded conversion to ∼40% (Fig. 1, F and G). Thus, six of nine rabbit substitutions diminished conversion of bank vole PrPC by more than 50%. Collectively, these results demonstrate that human and rabbit residues impede prion conversion to varying levels, depending on the PrPSc sequence and/or conformation. Remarkably, the five most inhibitory substitutions, N100G, N143S, Q168E, N174S, and Q219E, involved the loss of an asparagine or glutamine residue from the bank vole sequence, suggesting that Asn/Gln residues are important for the conversion of bank vole PrP.
Bank vole asparagine and glutamine residues enable prion conversion of human and rabbit PrPC
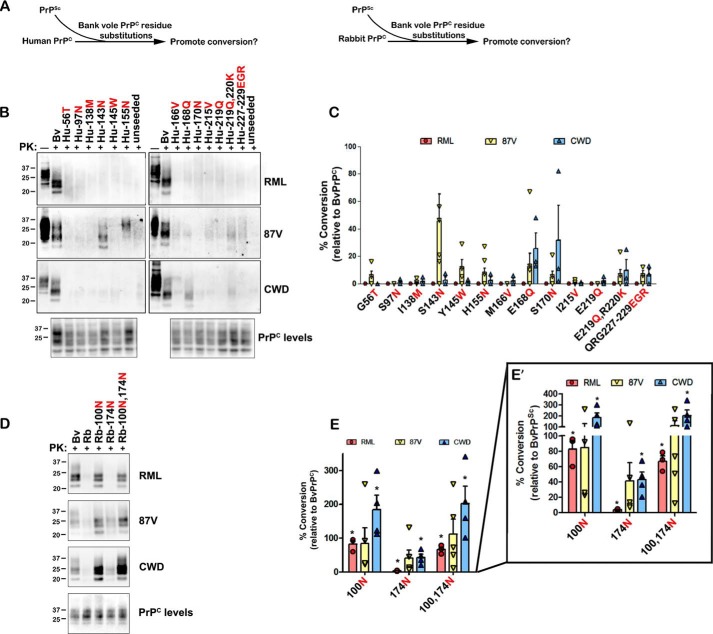
Because the N100G, N143S, Q168E, N174S, and Q219E substitutions inhibited conversion of BvPrPC, we reasoned that the converse bank vole amino acid substitutions may enable prion conversion of otherwise resistant HuPrPC and RbPrPC (Fig. 2A). We measured seeded conversion of HuPrPC having the S143N, E168Q, or E219Q substitutions and RbPrPC containing the G100N or S174N substitutions. HuPrPC with the S143N or E168Q substitutions seeded with 87V or CWD prions, respectively, led to low PrPSc levels (Fig. 2, B and C), whereas E219Q had no effect on conversion (Fig. 2, B and C). In contrast, mouse RML prions did not convert any of the HuPrPC sequences with single substitutions (Fig. 2, B and C). To identify any other residues that impact cross-seeding of HuPrPC, we assessed 12 other Hu-to-Bv amino acid substitutions including the adjacent residues 219–220 and 227–229. Only the Q168E and S170N substitutions in HuPrPC resulted in modest conversion when seeded by CWD prions (Fig. 2, B and C), as previously reported (12), whereas no other substitution enabled conversion by mouse RML or 87V prions.
Figure 2.
Key bank vole substitutions enable conversion of human and rabbit PrPC. A, schematic for experimental approach. HuPrPC or RbPrPC with BvPrPC substitutions was seeded with mouse or elk prions, and the newly converted PrPSc was measured after 24 h. B, representative immunoblots show the impact of single BvPrPC substitutions on conversion of HuPrPC by RML, 87V, or CWD prions. C, quantitative analysis shows that the S143N, E168Q, and S170N substitutions promote conversion of HuPrPC for certain PrPSc seeds. D, representative immunoblots show the conversion of RbPrPC with the G100N, S174N, or G100N,S174N substitutions. E, quantitative analysis shows that the G100N and G100N,S174N promote conversion of RbPrPC. E′ shows the quantified data with a segmented y axis. Quantified data in C and E are from three to seven or three to five independent experiments, respectively. Standard error bars indicate the observed variance. *, p < 0.05, one-sample t test. One-way ANOVA with Tukey post hoc test revealed statistically significant differences between the following residues: for C, for 87V between residue 143 and residue 56, 155, 170, 219, and 219/220 (*, p < 0.05), as well as between residue 143 and residues 97, 138, 166, and 215 (**, p < 0.01); for E, for RML between residue 100 or 174 and 100/174 (***, p < 0.001), and for CWD, between residue 100 or 100/174 and 174 (*, p < 0.01).
We next measured conversion of RbPrPC with the bank vole substitutions G100N or S174N. Remarkably, the single G100N substitution resulted in efficient cross-seeding by CWD, 87V, and RML prions (>100, 84, and 83%, respectively) (Fig. 2, D and E), indicating that the Asn100 residue is critical for conversion by all three prions. The single S174N substitution had minimal impact on conversion by RML, 87V, and CWD prions (4, 41, and 43%, of BvPrPC, respectively) (Fig. 2, D and E), whereas RbPrPC containing both G100N and S174N was converted to levels similar to RbPrPC containing the single G100N substitution (Fig. 2, D and E). These results suggest that certain asparagine and glutamine residues in BvPrPC (Asn100, Asn143, Gln168, Asn170) promote cross-species prion conversion, even in the context of a different PrP sequence.
Bank vole substitutions variably promote conversion of human PrPC in a prion-dependent manner
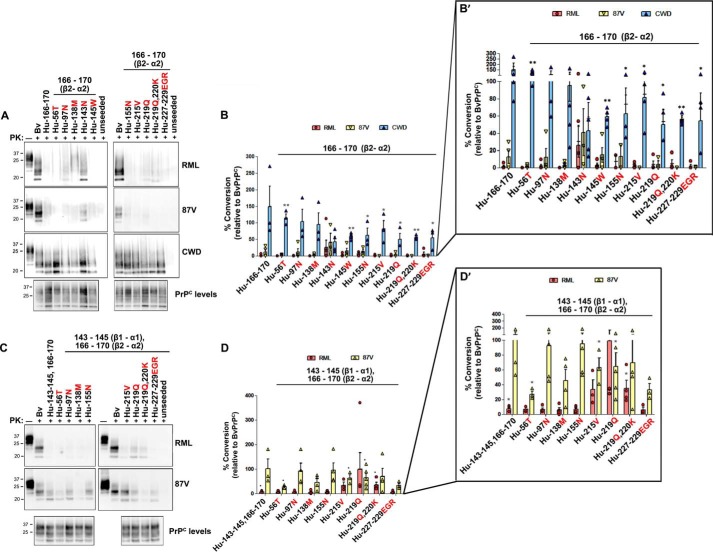
Because no single BvPrP substitution enabled conversion of HuPrPC by more than 50%, we next assessed conversion of HuPrPC having multiple substitutions. HuPrPC with the complete bank vole β2–α2 loop sequence (M166V, E168Q, and S170N substitutions) was converted by CWD prions as efficiently as bank vole PrPC; no additional bank vole substitutions further enhanced conversion (Fig. 3, A and B). These results indicate that the bank vole β2–α2 loop sequence is necessary and sufficient for elk CWD cross-seeding of HuPrPC.
Figure 3.
Bank vole substitutions in human PrPC promote 87V or RML prion conversion. A, representative immunoblots demonstrate conversion of HuPrPC with the BvPrPC β2–α2 loop sequence together with additional BvPrPC amino acid substitutions. B, quantitative analysis reveals that HuPrPC with the BvPrPC β2–α2 loop is converted by CWD, but not RML or 87V prions. C, representative immunoblots show conversion of HuPrPC sequences containing the BvPrPC β1–α1 and β2–α2 loop sequences. D, quantitative analysis indicates that HuPrPC with the BvPrPC β1–α1 and β2–α2 loop sequences was converted by 87V prions. Conversion by RML prions additionally required the I215V or E219Q substitution. Note that four of five measurements of Hu-Gln219 ranged from 28 to 35% conversion, whereas one measurement was >300. B′ and D′ show the quantified data with a segmented y axis. Quantified data in B and D are from three to five independent experiments. Standard error bars indicate the observed variance. *, p < 0.05; **, p < 0.01, one sample t test.
In contrast to elk CWD, mouse prions RML and 87V minimally converted HuPrPC containing the bank vole β2–α2 loop sequence (<15%; Fig. 3, A and B). Because the N143S substitution in the β1–α1 loop had inhibited BvPrP conversion (Fig. 1E), we next tested whether HuPrPC with the bank vole β1–α1 and β2–α2 loop segments (S143N, Y145W, M166V, E168Q, and S170N) would be converted by mouse prions. These five-residue exchanges, which add three more Asn/Gln residues to the human sequence and remove a charged residue, led to substantially higher levels of HuPrPC conversion by 87V prions (101%), but not by RML prions (8%) (Fig. 3, C and D). These results indicate that in two critical segments known to form steric zippers, the β1–α1 loop (positions 143–145) (36) and the β2–α2 loop (positions 166–170) (14), bank vole residues enable efficient conversion of HuPrPC seeded by mouse 87V prions. Because RML was not converted, these data also suggest that mouse RML and 87V prions differ in the number and location of PrPC segments required for efficient conversion.
To identify the residues controlling conversion of HuPrPC by RML prions, 10 additional BvPrPC substitutions (in groups of 1–3) were incorporated into the HuPrPC–bank vole β1–α1/β2–α2 chimera and tested for conversion. Of the 10 residues tested, RML prions only converted to >20% PrPC having the additional I215V, E219Q, or E219Q, R220K substitutions (Fig. 3, C and D; note for Glu219, four of five measurements resulted in 28–35% conversion). These experiments indicate that four Asn/Gln substitutions, which were in the β1–α1 and β2–α2 loop segments (Asn143, Gln168, and Asn170), and the C terminus of α-helix 3 (residue 219), enable limited conversion of human PrPC by RML. The Val215 substitution also enhanced conversion by RML. Interestingly, HuPrPC with the bank vole β2–α2 loop, together with the individual substitutions S143N, I215V, or E219Q, was not converted to high levels by RML prions (Fig. 3, A and B), indicating that exchanges in all three segments were essential for conversion of HuPrPC by RML prions.
Prion conversion enhanced by Asn/Gln substitutions is highly position- and side chain-dependent
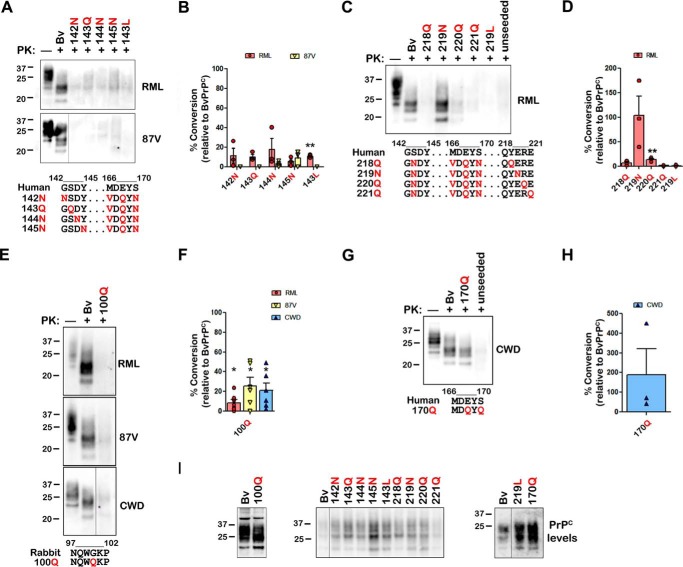
Strikingly, five of five single substitutions that inhibit conversion of BvPrPC to less than 20% or that promote conversion of HuPrPC and RbPrPC to greater than 20% involved the loss or gain of Asn/Gln residues, respectively. Mammalian PrPC from 24 species revealed 26–31 Asn/Gln residues scattered throughout the protein; bank vole PrPC has 31 Asn/Gln residues, an unusually high number, and a notably high Asn:Gln ratio (0.94) (Tables 1 and 2). To determine whether the position of the Asn or Gln substitution within a segment impacts conversion, Asn/Gln substitutions were placed at sites flanking positions 143 and 219 in HuPrPC. For RML and 87V prions, there was little to no conversion when the Asn143 substitution was transposed to 142, 144, or 145 (Fig. 4, A, B, and I) Similarly, for RML prions, there was little to no conversion when the Gln219 substitution was transposed to position 218, 220, or 221 (Fig. 4, C, D, and I); thus, the promoting effect of the Asn143 and Gln219 was highly position-dependent.
Table 1.
Total number of asparagine and glutamine residues in the PrP sequence of 24 species
| Species | Asn | Gln | Asn/Gln total | Asn:Gln ratio |
|---|---|---|---|---|
| Rodents | ||||
| Bank vole (Myodes glareolus) | 15 | 16 | 31 | 0.94 |
| Meadow vole (Microtus pennsylvanicus) | 15 | 16 | 31 | 0.94 |
| Deer mouse (Peromyscus maniculatus bairdii) | 15 | 16 | 31 | 0.94 |
| Syrian golden hamster (Mesocricetus auratus) | 15 | 16 | 31 | 0.94 |
| Chinese hamster (Cricetulus griseus) | 15 | 16 | 31 | 0.94 |
| Armenian hamster (Cricetulus migratorius) | 14 | 16 | 30 | 0.88 |
| Mouse (Mus musculus) | 13 | 16 | 29 | 0.81 |
| Primates | ||||
| Black-handed spider monkey (Ateles geoffroyi) | 14 | 15 | 29 | 0.93 |
| Common marmoset (Callithrix jacchus) | 13 | 16 | 29 | 0.81 |
| Squirrel monkey (Saimiri sciureus) | 12 | 17 | 29 | 0.71 |
| Tufted capuchin (Sapajus apella) | 12 | 16 | 28 | 0.75 |
| Macaque (Macaca mulatta, Macaca fascicularis) | 11 | 16 | 27 | 0.69 |
| Human (Homo sapiens) | 11 | 15 | 26 | 0.73 |
| Chimp (Pan troglodytes) | 10 | 16 | 26 | 0.63 |
| Ruminants | ||||
| Deer (Odocoileus sp.) | 12 | 17 | 29 | 0.71 |
| Cow (Bos taurus) | 12 | 17 | 29 | 0.71 |
| Sheep (Ovis aries) ARR/ARQ | 12 | 16/17 | 28/29 | 0.71–0.75 |
| Elk (Cervus canadensis) | 12 | 16 | 28 | 0.75 |
| Carnivores | ||||
| Cat (Felis catus) | 12 | 16 | 28 | 0.75 |
| Ferret (Mustela putorius furo) | 11 | 17 | 28 | 0.65 |
| Mink (Neovison vison) | 11 | 16 | 27 | 0.69 |
| Raccoon (Procyon lotor) | 11 | 16 | 27 | 0.69 |
| Dog (Canis familiaris) | 11 | 16 | 27 | 0.69 |
| Leporids | ||||
| Rabbit (Oryctolagus cuniculus) | 10 | 18 | 28 | 0.56 |
Table 2.
PrP residue differences between species at positions 100, 143, 168, 170, 174, and 219
Bold italic residues indicate amino acids that may confer resistance to prion conversion.
| Species | 100 | 143 | 168 | 170 | 174 | 219 |
|---|---|---|---|---|---|---|
| Bank vole | Asn | Asn | Gln | Asn | Asn | Gln |
| Hamster | Asn | Asn | Gln | Asn | Asn | Gln |
| Elk | Asn | Asn | Gln | Asn | Thr | Gln |
| Mouse | Asn | Asn | Gln | Ser | Asn | Gln |
| Cow | Asn | Asn | Gln | Ser | Asn | Gln |
| Squirrel monkey | Asn | Asn | Gln | Ser | Asn | Glu |
| Macaque | His | Asn | Gln | Ser | Asn | Glu |
| Sheep | Asn | Asn | Gln/Arg | Ser | Asn | Gln |
| Human | Asn | Ser | Glu | Ser | Asn | Glu |
| Rabbit | Gly | Asn | Gln | Ser | Ser | Gln |
| Dog | Gly | Asn | Gln | Ser | Asn | Gln |
| Ferret | Gly | Asn | Gln | Ser | Asn | Gln |
| Raccoon | Gly | Asn | Gln | Ser | Asn | Gln |
Figure 4.
The impact of Asn/Gln residues on prion conversion is highly position-dependent. A and B, HuPrPC with the BvPrPC β2–α2 loop and the 143Q or 143L substitution is not converted by RML or 87V prions. Additionally, transposing the asparagine substitution from position 143 to flanking positions in HuPrPC no longer promotes conversion by RML or 87V prions. C and D, HuPrPC with the E219N, but not the E219L, substitution together with the BvPrPC β1–α1 and β2–α2 loop sequences is efficiently converted by RML prions. Shifting the glutamine substitution from position 219 to flanking positions does not promote conversion by RML prions. E and F, RbPrPC with the 100Q substitution was minimally converted by RML, 87V, or elk CWD prions. G and H, HuPrPC with the Gln170 substitution was converted by elk CWD prions. I, PrPC in lysates used for A–H show similar PrPC levels. The blots in A, E, and I show single Western blots at the same exposure with intervening lanes removed for clarity. PrPC sequence changes are shown in red below immunoblots. Quantified data are from three to five (B), three or four (F), or three (H) independent experiments. Standard error bars indicate the observed variance. *, p < 0.05; **, p < 0.01, one-sample t test. One-way ANOVA with Tukey post hoc test revealed statistically significant differences for RML between Asn219 and residue Asn218, Asn220, Asn221, and Leu219 (*, p < 0.05).
To assess the specificity of the asparagine versus glutamine side chain on conversion, we exchanged Asn/Gln residues at four key positions of bank vole PrP (100, 143, 170, and 219). Strikingly, position 100 required an asparagine for cross-species prion conversion, because RbPrPC-100Q was not converted efficiently by RML, 87V, or CWD prions (9, 26, and 21%, respectively; Fig. 4, E, F, and I). Similarly, position 143 also required an asparagine, because glutamine did not enable conversion of HuPrPC by RML or 87V prions (Fig. 4, A and B). In contrast, at positions 170 and 219, asparagine and glutamine were interchangeable, because Hu-Bv PrPC chimeras with 170N/170Q or Gln219/219N residues were efficiently converted by CWD or RML prions, respectively (≥100%; Fig. 4, C, D, and G–I). Because Asn and Gln side chains differ by only one methylene group, our findings suggest that the promoting effect of Asn/Gln substitutions is highly dependent on side chain length in certain positions (100 and 143) but not others (170 and 219). Interestingly, although leucine is somewhat structurally similar to asparagine in volume, the Leu143 and Leu219 substitutions in Hu-Bv PrPC were not converted by 87V or RML, respectively (0–1%) (Fig. 4, A–D), suggesting that the side chain hydrogen bonding of the amide in the Asn/Gln is critical to prion conversion. These results indicate that the strong promoting effect of the Asn100 and Asn143 residues on prion conversion is highly position- and side chain-dependent, requiring specifically an asparagine residue.
Discussion
Prion transmission can be exquisitely sensitive to primary sequence differences between PrPC and PrPSc, because even one mismatched residue can obstruct prion conversion (37–39). Bank vole PrPC, however, is extraordinarily susceptible to conversion seeded by prions from other species, despite sequence differences (19, 21, 24, 25, 35, 40–42). Here we have investigated the residues that govern cross-species prion conversion by systematically testing over 40 mutated bank vole, human, and rabbit PrPC sequences seeded by three dissimilar PrPSc seed sequences. Our studies revealed five amino acid substitutions that profoundly promote or inhibit prion conversion, all of which involve the gain or loss of an asparagine or glutamine residue (positions 100, 143, 168, 174, and 219). Remarkably, substituting Asn/Gln residues into PrP conferred susceptibility to sequences that otherwise resisted conversion. The relevant positions of the Asn/Gln residues varied with the incoming prion seed, consistent with PrPSc conformation determining the critical PrPC–PrPSc interaction domains. Additionally, we have also established that certain positions tolerate either an Asn or a Gln, whereas others strictly require an Asn or a Gln for prion conversion.
The addition of Asn/Gln residues resulted in a sequence match with the PrPSc seed (supplemental Fig. S1), which could explain why conversion was enhanced. However, six other substitutions led to a sequence match with the seed but did not promote conversion. Additionally, for at least two positions, 170 and 219, a mismatched polar residue also promoted conversion, indicating that the side chain structure did not require a precise match with the PrPSc seed but did require a structurally similar residue. Notably, the position of the Asn/Gln residue was also important, because there were Asn/Gln residues in positions that did not significantly impact prion conversion (S97N or H155N), even in combination with the bank vole β1–α1 and β2–α2 residues. Among the five key Asn/Gln residues, four residues (at positions 100, 168, 174, and 219) were located within steric zipper segments identified by the Zipper DB 3D profiling method using the seven-residue zipper (Rosetta energies below an energetic threshold of −27 kcal/mol) (Table 3), and two segments have been crystallized (positions 143 and 174) (14, 36). Taken together, these findings suggest that PrPC–PrPSc interactions in short segments regulate prion conversion.
Table 3.
Rosetta energy calculations for five steric zipper segments identified in the prion protein
The position of the N or Q residue is shown as bold text.
| Asn/Gln position | Zipper segments identified by ZipperDB | Rosetta energy |
|---|---|---|
| kcal/mol | ||
| 100 | QWNKPSK | −29.2 |
| 143 | MIHGNDW | −26 |
| 168 | QYNNQNN | −34.6 |
| 174 | QYNNQNN | −34.6 |
| 219 | QYQKESQ | −31.2 |
Prion seeding specificity is widely recognized to be controlled by one or two key residues (22, 23), for example, PrP residues 138/139 in the β1–α1 loop control seeding specificity between mouse and hamsters (38, 39) and residues 168 and 170 in the β2–α2 loop impact deer CWD–human transmission barriers (12). The mechanism underlying how residues in the β1–α1 and β2–α2 loop control conversion may be explained by microcrystal structures of the 138–143 and the 170–175 segments determined to less than 3 Å, where amino acid side chain differences result in microcrystals having different symmetry (14, 36). Thus, it is not entirely surprising that residue differences in these particular zipper segments had such a remarkable impact on prion transmission barriers. The surprising element here was that the residues that most profoundly impacted cross-species prion conversion were largely dominated by Asn/Gln residues.
The critical Asn/Gln residue positions for cross-species conversion differed depending on the PrPSc seed (Fig. 5), consistent with the recognized role of PrPSc conformation in prion conversion. The results using mutant PrP have enabled a partial mapping of interacting residues critical for certain prions, suggesting segments that may be exposed in PrPSc. PrPC–PrPSc interaction at residues 215 and 219 was important for RML only, indicating that the distal C terminus is a key intermolecular contact segment for RML, but not 87V or CWD prions. Additionally, the β2–α2 loop was key for CWD-seeded conversion but insufficient for RML or 87V. Findings from our lab and others that the key interaction domains in PrPC vary with the PrPSc conformation are consistent with those from Sup35 yeast prions, showing that short segments control prion nucleation, conformation, and species barriers (43).
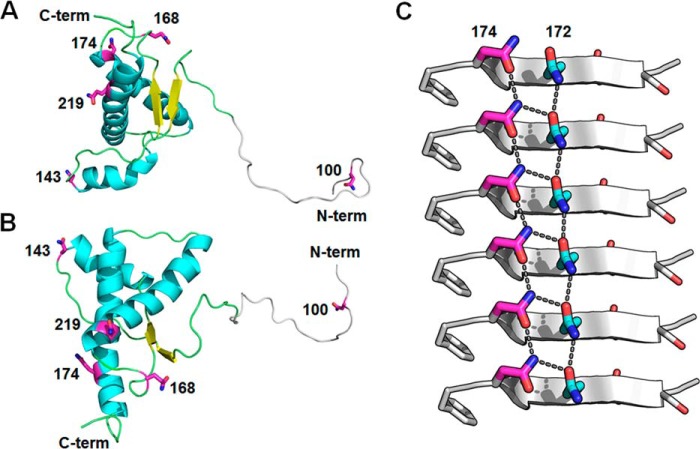
Figure 5.
Proposed mechanism for prion conversion involving asparagine/glutamine residues in discrete segments of bank vole PrPC. A, a ribbon diagram of the NMR BvPrPC structure (Protein Data Bank code 2K56; Ref. 27) with critical Asn/Gln residues shown in magenta. The N-terminal domain of PrPC (residues 97–118, gray) is modeled using Rosetta software (63). Residues 23–96 are not shown. B, another view of the NMR BvPrPC structure rotated horizontally by 90°. C, zipper structure from PrPSc peptide (residues 170–175; Protein Data Bank code 3VFA; Ref. 36) fibril demonstrating that alignment of Asn/Gln residues along the length of the fibril axis promotes side chain hydrogen bonds in a motif known as an asparagine/glutamine-ladder. β-Strands are represented by gray arrows, Asm174 residues are shown in magenta, Gln172 residues are in cyan, and side chain hydrogen bonds in the asparagine/glutamine-ladder are indicated by black dotted lines.
How do asparagine and glutamine residues lower the energy barrier for prion cross-seeding? Of relevance to Huntington's disease, fibril assembly of the huntingtin protein is caused by expansion of poly(Q) tracts (44, 45). Perutz et al. (46) have proposed that Asn/Gln residues mediate protein-protein interactions in huntingtin and other Asn/Gln-rich aggregating proteins through the formation of hydrogen bond networks that stabilize β-strands, termed “polar zippers.” Within a β-sheet, asparagine and glutamine residues were proposed to markedly enhance β-strand stability through intrasheet hydrogen bonds formed between both side chain and main chain amides (Fig. 5). These additional hydrogen bonds, more typically between amides of identical side chains in adjacent strands, greatly increase the stability of the β-sheet, because the parallel arrays of aligned bonds are hyperpolarized (47) and are even stronger than those in ice (48, 49). We suggest a model in which intermolecular interactions between complementary PrP segments that have a strong, hyperpolarized hydrogen bond network, such as the β2–α2 loop, stabilize newly incorporated PrP monomers onto β-sheet-rich fibrils, driving aggregation of dissimilar PrP sequences. These intermolecular contact sites may also serve as critical templating sites, directing PrP into a particular PrPSc fold. This model would be consistent with experimental findings from the β1–α1 and β2–α2 loop, where sequence differences can switch the prion conformation (11, 50, 51).
Several yeast prions, such as Ure2, Sup35, and Rnq1, contain Asn/Gln-rich domains that are required for assembly into functional prion fibrils (52, 53) and may mediate prion cross-seeding (54). The Asn/Gln residues at the N terminus of Ure2p and Sup35p play a particularly critical role in stabilizing the amyloid state (55). This Asn/Gln-rich domain is also modular, causing aggregation when introduced into other proteins (56). By comparison, mammalian prions contain one short Asn/Gln-rich domain (β2–α2 loop), with additional Asn/Gln residues interspersed throughout the protein. Thus, the Asn/Gln residues within PrP are not constrained to a single Asn/Gln-rich domain but seem to collectively promote conversion from multiple segments. Stacking of Asn/Gln residues into ladders along the length of a prion fibril would maximize interstrand hydrogen bonding and would be compatible with a parallel, in-register β-sheet structure for PrPSc.
Our studies afford insight into the mechanism of prion conversion. Asn/Gln substitutions at positions flanking Asn143 or Gln219 (positions 142, 144, 145, 218, 220, and 221) had little effect on conversion of HuPrPC. This finding indicates that the precise Asn/Gln position within the segment was critical for prion conversion, which is consistent with steric zipper formation at segment 138–143. For instance, Gly142 is located at a tight turn in the crystal structure of the 138–143 segment (36). Asn/Gln substitutions at this position would be predicted to clash with zipper formation and thus hinder prion conversion. Additionally, other substitutions in HuPrPC that increase total Asn/Gln content, such as S97N or H155N, did not strongly promote conversion, supporting the importance of the specific position of the Asn/Gln residue in facilitating prion conversion.
Were asparagine and glutamine residues in the key positions interchangeable? PrP positions 100 and 143 required an asparagine and not a glutamine for conversion, indicating that a specific side chain length was crucial in certain positions (Fig. 5). On the other hand, positions 170 and 219 tolerated either an asparagine or glutamine; however, leucine substitutions at position 143 or 219 blocked conversion. Together, these findings underscore the importance of the hydrogen bond stabilizing ladder provided by the asparagine or glutamine side chain. In Sup35 yeast prions, asparagine residues enhance prion formation more than glutamine residues (57). Asparagine side chains are also critical for islet amyloid polypeptide fibril assembly (58).
The bank vole PrPC sequence contains 31 Asn/Gln residues, more than most other mammals assessed here (Table 1). In comparing the Asn/Gln ratio across species, an interesting trend emerges. The species that are highly susceptible to diverse prions, such as the bank vole, have an extraordinarily high Asn/Gln ratio (0.94 in bank vole versus 0.56 in rabbit PrPC), and a particularly dense stretch of Asn residues in the β2–α2 loop. The β2–α2 loop (QYNNQNN) is a segment suspected to be involved early in PrP nucleation and is favored by asparagine residues (59, 60). Our studies suggest that the high number of key interspersed Asn/Gln residues (Table 2) may stabilize nascent β-strands through a hydrogen bond ladder and may explain the elevated promiscuity of bank voles to cross-species prion conversion, as well as the spontaneous assembly of BvPrPC in transgenic mice (61).
These findings argue that certain interspersed asparagine and glutamine residues may facilitate the anchoring of PrPC to PrPSc through strengthening a replicative interface, driving prion conversion between dissimilar sequences and lowering the barrier for aggregation. Identifying the key prion segments and specific residue interactions in early stages of conversion may facilitate predictions of cross-species prion transmission. We expect that the importance of interspersed asparagine and glutamine residues in protein aggregation may be a more general phenomena applicable to other amyloidogenic proteins and may present target segments for rational therapeutic design.
Experimental procedures
Cell-lysate protein misfolding cyclic amplification
The pcDNA3.1 vector (Invitrogen) with the mouse, human, bank vole, or rabbit Prnp encoding the 3F4 epitope (Met109 and Met112 human numbering) was used as a template for site-directed mutagenesis (QuikChange site-directed mutagenesis kit; Agilent). PrP-deficient RK13 cells (ATCC) were transfected with 5–10 μg of plasmid DNA using Lipofectamine 3000 (Invitrogen). At 24 h post-transfection, the cells were washed twice in PBS, harvested in 1 ml of PBS, and centrifuged for 1 min at 1,000 × g. The pellet was resuspended in PMCA buffer (PBS containing 1% Triton X-100, 150 mm NaCl, and 5 mm EDTA plus CompleteTM protease inhibitors), passed repeatedly through a 27-gauge needle, and clarified by centrifuging at 2,000 × g for 1 min.
RML prions from C57BL/6 mice (Prnp encoding Leu109, Thr189, human numbering), 87V prions from VM/DK mice (Prnp encoding Phe109 and Val189), and CWD prions from elk (Met129) were used to seed human, bank vole, and rabbit PrPC (Prnp sequences shown in Fig. 1 and supplemental Fig. S1). The PrPC was newly prepared for each independent experiment. The prion seeds were derived from brain homogenate that was pooled from mice inoculated with the same prion strain or from naturally infected elk. The brain homogenate samples pooled to generate the seeds were consistent between the experiments. Prion-infected brain homogenate (10% w/v) was added into PrPC-expressing RK13 cell lysate (1:10, PrPSc:PrPC by volume) and subjected to repeated 5-s sonication pulses (S4000, QSonica) with 10 min of incubation between each pulse over a total period of 24 h. Sonication power was maintained at 50–60%, and samples were continuously rotated in a water bath at 37 °C. Samples were then digested with 200 μg/ml PK for 30 min at 37 °C and analyzed by Western blot using the anti-PrP monoclonal antibody 3F4 (62). PrPC levels were measured by blotting 1–2 μl from unseeded lysates. Signals were quantified using a Fujifilm LAS-4000 imager and multi-gauge software and compared by the percentage of conversion compared with control samples (considered 100%). PK-digested unseeded lysates were included in all experiments to exclude PrPSc contamination of the PMCA substrates and spontaneous assembly of mutant PrPC protein. At least three independent experimental replicates were performed for each mutant and each prion strain used as seed.
Statistical analysis
One-way ANOVA with Tukey post hoc test and a one-sample t test were used to analyze the conversion data from each mutant PrP. For the one-sample t test, the conversion of human or rabbit substitutions in bank vole PrPC was compared against a mean of 100 (null hypothesis) to assess whether the mutation(s) inhibited PrP conversion. The conversion of bank vole substitutions in human or rabbit PrPC was compared against a mean of 0 to assess whether the mutation(s) promoted PrP conversion.
Author contributions
T. D. K. and C. J. S. designed the study and wrote the paper. T. D. K. and P. A.-C. performed the experiments, and N. A. provided technical assistance. T. D. K., P. A.-C., L. J., J. A. R., D. S. E., and C. J. S. analyzed the experiments. All authors reviewed the results and approved the final version of the manuscript.
Supplementary Material
Acknowledgment
We thank Dr. Steven Edland for discussion of the statistical analyses.
This work was supported by National Institutes of Health grants NS069566 (to C. J. S.), NS076896 (to C. J. S.), OD019919 (to T. D. K.), and OD017853 (to T. D. K.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains supplemental Figs. S1 and S2.
- CWD
- chronic wasting disease
- PMCA
- protein misfolding cyclic amplification
- clPMCA
- cell-lysate PMCA
- PK
- proteinase K
- Hu
- human
- Bv
- bank vole
- Rb
- rabbit
- ANOVA
- analysis of variance
- RML
- Rocky Mountain Laboratory.
References
- 1. Prusiner S. B. (1982) Novel proteinaceous infectious particles cause scrapie. Science 216, 136–144 [DOI] [PubMed] [Google Scholar]
- 2. Jarrett J. T., and Lansbury P. T. Jr. (1993) Seeding “one-dimensional crystallization” of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie? Cell 73, 1055–1058 [DOI] [PubMed] [Google Scholar]
- 3. Krotee P., Rodriguez J. A., Sawaya M. R., Cascio D., Reyes F. E., Shi D., Hattne J., Nannenga B. L., Oskarsson M. E., Philipp S., Griner S., Jiang L., Glabe C. G., Westermark G. T., Gonen T., et al. (2017) Atomic structures of fibrillar segments of hIAPP suggest tightly mated β-sheets are important for cytotoxicity. eLife 6, e19273b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clouscard C., Beaudry P., Elsen J. M., Milan D., Dussaucy M., Bounneau C., Schelcher F., Chatelain J., Launay J. M., and Laplanche J. L. (1995) Different allelic effects of the codons 136 and 171 of the prion protein gene in sheep with natural scrapie. J. Gen. Virol. 76, 2097–2101 [DOI] [PubMed] [Google Scholar]
- 5. Asante E. A., Smidak M., Grimshaw A., Houghton R., Tomlinson A., Jeelani A., Jakubcova T., Hamdan S., Richard-Londt A., Linehan J. M., Brandner S., Alpers M., Whitfield J., Mead S., Wadsworth J. D., et al. (2015) A naturally occurring variant of the human prion protein completely prevents prion disease. Nature 522, 478–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Priola S. A., Chabry J., and Chan K. (2001) Efficient conversion of normal prion protein (PrP) by abnormal hamster PrP is determined by homology at amino acid residue 155. J. Virol. 75, 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bruce M. E., Will R. G., Ironside J. W., McConnell I., Drummond D., Suttie A., McCardle L., Chree A., Hope J., Birkett C., Cousens S., Fraser H., and Bostock C. J. (1997) Transmissions to mice indicate that “new variant” CJD is caused by the BSE agent. Nature 389, 498–501 [DOI] [PubMed] [Google Scholar]
- 8. Kirkwood J. K., and Cunningham A. A. (1994) Epidemiological observations on spongiform encephalopathies in captive wild animals in the British Isles. Vet. Rec. 135, 296–303 [DOI] [PubMed] [Google Scholar]
- 9. Haire L. F., Whyte S. M., Vasisht N., Gill A. C., Verma C., Dodson E. J., Dodson G. G., and Bayley P. M. (2004) The crystal structure of the globular domain of sheep prion protein. J. Mol. Biol. 336, 1175–1183 [DOI] [PubMed] [Google Scholar]
- 10. Riek R., Hornemann S., Wider G., Billeter M., Glockshuber R., and Wüthrich K. (1996) NMR structure of the mouse prion protein domain PrP (121–231). Nature 382, 180–182 [DOI] [PubMed] [Google Scholar]
- 11. Sigurdson C. J., Nilsson K. P., Hornemann S., Manco G., Fernández-Borges N., Schwarz P., Castilla J., Wüthrich K., and Aguzzi A. (2010) A molecular switch controls interspecies prion disease transmission in mice. J. Clin. Invest. 120, 2590–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kurt T. D., Jiang L., Fernández-Borges N., Bett C., Liu J., Yang T., Spraker T. R., Castilla J., Eisenberg D., Kong Q., and Sigurdson C. J. (2015) Human prion protein sequence elements impede cross-species chronic wasting disease transmission. J. Clin. Invest. 125, 1485–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kurt T. D., Jiang L., Bett C., Eisenberg D., and Sigurdson C. J. (2014) A proposed mechanism for the promotion of prion conversion involving a strictly conserved tyrosine residue in the β2–α2 loop of PrPC. J. Biol. Chem. 289, 10660–10667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sawaya M. R., Sambashivan S., Nelson R., Ivanova M. I., Sievers S. A., Apostol M. I., Thompson M. J., Balbirnie M., Wiltzius J. J., McFarlane H. T., Madsen A. Ø., Riekel C., and Eisenberg D. (2007) Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature 447, 453–457 [DOI] [PubMed] [Google Scholar]
- 15. Gazit E. (2002) A possible role for pi-stacking in the self-assembly of amyloid fibrils. FASEB J. 16, 77–83 [DOI] [PubMed] [Google Scholar]
- 16. Eisenberg D. S., and Sawaya M. R. (2017) Structural studies of amyloid proteins at the molecular level. Annu. Rev. Biochem. 86, 69–95 [DOI] [PubMed] [Google Scholar]
- 17. Chandler R. L. (1971) Experimental transmission of scrapie to voles and Chinese hamsters. Lancet 1, 232–233 [DOI] [PubMed] [Google Scholar]
- 18. Nonno R., Di Bari M. A., Cardone F., Vaccari G., Fazzi P., Dell'Omo G., Cartoni C., Ingrosso L., Boyle A., Galeno R., Sbriccoli M., Lipp H. P., Bruce M., Pocchiari M., and Agrimi U. (2006) Efficient transmission and characterization of Creutzfeldt–Jakob disease strains in bank voles. PLoS Pathog. 2, e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Di Bari M. A., Chianini F., Vaccari G., Esposito E., Conte M., Eaton S. L., Hamilton S., Finlayson J., Steele P. J., Dagleish M. P., Reid H. W., Bruce M., Jeffrey M., Agrimi U., and Nonno R. (2008) The bank vole (Myodes glareolus) as a sensitive bioassay for sheep scrapie. J. Gen. Virol. 89, 2975–2985 [DOI] [PubMed] [Google Scholar]
- 20. Heisey D. M., Mickelsen N. A., Schneider J. R., Johnson C. J., Johnson C. J., Langenberg J. A., Bochsler P. N., Keane D. P., and Barr D. J. (2010) Chronic wasting disease (CWD) susceptibility of several North American rodents that are sympatric with cervid CWD epidemics. J. Virol. 84, 210–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Di Bari M. A., Nonno R., Castilla J., D'Agostino C., Pirisinu L., Riccardi G., Conte M., Richt J., Kunkle R., Langeveld J., Vaccari G., and Agrimi U. (2013) Chronic wasting disease in bank voles: characterisation of the shortest incubation time model for prion diseases. PLoS Pathog. 9, e1003219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vorberg I., Groschup M. H., Pfaff E., and Priola S. A. (2003) Multiple amino acid residues within the rabbit prion protein inhibit formation of its abnormal isoform. J. Virol. 77, 2003–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Agrimi U., Nonno R., Dell'Omo G., Di Bari M. A., Conte M., Chiappini B., Esposito E., Di Guardo G., Windl O., Vaccari G., and Lipp H. P. (2008) Prion protein amino acid determinants of differential susceptibility and molecular feature of prion strains in mice and voles. PLoS Pathog. 4, e1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Espinosa J. C., Nonno R., Di Bari M., Aguilar-Calvo P., Pirisinu L., Fernández-Borges N., Vanni I., Vaccari G., Marín-Moreno A., Frassanito P., Lorenzo P., Agrimi U., and Torres J. M. (2016) PrPC governs susceptibility to prion strains in bank vole, while other host factors modulate strain features. J. Virol. 90, 10660–10669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Piening N., Nonno R., Di Bari M., Walter S., Windl O., Agrimi U., Kretzschmar H. A., and Bertsch U. (2006) Conversion efficiency of bank vole prion protein in vitro is determined by residues 155 and 170, but does not correlate with the high susceptibility of bank voles to sheep scrapie in vivo. J. Biol. Chem. 281, 9373–9384 [DOI] [PubMed] [Google Scholar]
- 26. Mays C. E., Yeom J., Kang H. E., Bian J., Khaychuk V., Kim Y., Bartz J. C., Telling G. C., and Ryou C. (2011) In vitro amplification of misfolded prion protein using lysate of cultured cells. PLoS One 6, e18047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Christen B., Pérez D. R., Hornemann S., and Wüthrich K. (2008) NMR structure of the bank vole prion protein at 20 degrees C contains a structured loop of residues 165–171. J. Mol. Biol. 383, 306–312 [DOI] [PubMed] [Google Scholar]
- 28. Zahn R., Liu A., Lührs T., Riek R., von Schroetter C., López García F., Billeter M., Calzolai L., Wider G., and Wüthrich K. (2000) NMR solution structure of the human prion protein. Proc. Natl. Acad. Sci. U.S.A. 97, 145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wen Y., Li J., Yao W., Xiong M., Hong J., Peng Y., Xiao G., and Lin D. (2010) Unique structural characteristics of the rabbit prion protein. J. Biol. Chem. 285, 31682–31693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bett C., Joshi-Barr S., Lucero M., Trejo M., Liberski P., Kelly J. W., Masliah E., and Sigurdson C. J. (2012) Biochemical properties of highly neuroinvasive prion strains. PLoS Pathog. 8, e1002522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lund C., Olsen C. M., Tveit H., and Tranulis M. A. (2007) Characterization of the prion protein 3F4 epitope and its use as a molecular tag. J. Neurosci. Methods 165, 183–190 [DOI] [PubMed] [Google Scholar]
- 32. Barlow R. M., and Rennie J. C. (1976) The fate of ME7 scrapie infection in rats, guinea-pigs and rabbits. Res. Vet. Sci. 21, 110–111 [PubMed] [Google Scholar]
- 33. Gibbs C. J. Jr., and Gajdusek D. C. (1973) Experimental subacute spongiform virus encephalopathies in primates and other laboratory animals. Science 182, 67–68 [DOI] [PubMed] [Google Scholar]
- 34. Raymond G. J., Bossers A., Raymond L. D., O'Rourke K. I., McHolland L. E., Bryant P. K. 3rd, Miller M. W., Williams E. S., Smits M., and Caughey B. (2000) Evidence of a molecular barrier limiting susceptibility of humans, cattle and sheep to chronic wasting disease. EMBO J. 19, 4425–4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Watts J. C., Giles K., Patel S., Oehler A., DeArmond S. J., and Prusiner S. B. (2014) Evidence that bank vole PrP is a universal acceptor for prions. PLoS Pathog. 10, e1003990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Apostol M. I., Wiltzius J. J., Sawaya M. R., Cascio D., and Eisenberg D. (2011) Atomic structures suggest determinants of transmission barriers in mammalian prion disease. Biochemistry 50, 2456–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Collinge J. (2016) Mammalian prions and their wider relevance in neurodegenerative diseases. Nature 539, 217–226 [DOI] [PubMed] [Google Scholar]
- 38. Priola S. A., and Chesebro B. (1995) A single hamster PrP amino acid blocks conversion to protease-resistant PrP in scrapie-infected mouse neuroblastoma cells. J. Virol. 69, 7754–7758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vanik D. L., Surewicz K. A., and Surewicz W. K. (2004) Molecular basis of barriers for interspecies transmissibility of mammalian prions. Mol. Cell 14, 139–145 [DOI] [PubMed] [Google Scholar]
- 40. Orrú C. D., Groveman B. R., Raymond L. D., Hughson A. G., Nonno R., Zou W., Ghetti B., Gambetti P., and Caughey B. (2015) Bank vole prion protein as an apparently universal substrate for RT-QuIC-based detection and discrimination of prion strains. PLoS Pathog. 11, e1004983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pirisinu L., Di Bari M. A., D'Agostino C., Marcon S., Riccardi G., Poleggi A., Cohen M. L., Appleby B. S., Gambetti P., Ghetti B., Agrimi U., and Nonno R. (2016) Gerstmann–Straussler–Scheinker disease subtypes efficiently transmit in bank voles as genuine prion diseases. Sci. Rep. 6, 20443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nonno R., Di Bari M. A., Cardone F., Vaccari G., Fazzi P., Dell'Omo G., Cartoni C., Ingrosso L., Boyle A., Galeno R., Sbriccoli M., Lipp H. P., Bruce M., Pocchiari M., and Agrimi U. (2006) Efficient transmission and characterization of Creutzfeldt–Jakob disease strains in bank voles. PLoS Pathog. 2, e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tessier P. M., and Lindquist S. (2007) Prion recognition elements govern nucleation, strain specificity and species barriers. Nature 447, 556–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scherzinger E., Lurz R., Turmaine M., Mangiarini L., Hollenbach B., Hasenbank R., Bates G. P., Davies S. W., Lehrach H., and Wanker E. E. (1997) Huntingtin-encoded polyglutamine expansions form amyloid-like protein aggregates in vitro and in vivo. Cell 90, 549–558 [DOI] [PubMed] [Google Scholar]
- 45. Scherzinger E., Sittler A., Schweiger K., Heiser V., Lurz R., Hasenbank R., Bates G. P., Lehrach H., and Wanker E. E. (1999) Self-assembly of polyglutamine-containing huntingtin fragments into amyloid-like fibrils: implications for Huntington's disease pathology. Proc. Natl. Acad. Sci. U.S.A. 96, 4604–4609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Perutz M. F., Johnson T., Suzuki M., and Finch J. T. (1994) Glutamine repeats as polar zippers: their possible role in inherited neurodegenerative diseases. Proc. Natl. Acad. Sci. U.S.A. 91, 5355–5358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mompeán M., Nogales A., Ezquerra T. A., and Laurents D. V. (2016) Complex system assembly underlies a two-tiered model of highly delocalized electrons. J. Phys. Chem. Lett. 7, 1859–1864 [DOI] [PubMed] [Google Scholar]
- 48. Tsemekhman K., Goldschmidt L., Eisenberg D., and Baker D. (2007) Cooperative hydrogen bonding in amyloid formation. Protein Sci. 16, 761–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Eisenberg D., and Jucker M. (2012) The amyloid state of proteins in human diseases. Cell 148, 1188–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bett C., Fernández-Borges N., Kurt T. D., Lucero M., Nilsson K. P., Castilla J., and Sigurdson C. J. (2012) Structure of the β2–α2 loop and interspecies prion transmission. FASEB J. 26, 2868–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jones E. M., and Surewicz W. K. (2005) Fibril conformation as the basis of species- and strain-dependent seeding specificity of mammalian prion amyloids. Cell 121, 63–72 [DOI] [PubMed] [Google Scholar]
- 52. Serio T. R., and Lindquist S. L. (1999) [PSI+]: an epigenetic modulator of translation termination efficiency. Annu. Rev. Cell Dev. Biol. 15, 661–703 [DOI] [PubMed] [Google Scholar]
- 53. Wickner R. B., Taylor K. L., Edskes H. K., Maddelein M. L., Moriyama H., and Roberts B. T. (1999) Prions in Saccharomyces and Podospora spp.: protein-based inheritance. Microbiol. Mol. Biol. Rev. 63, 844–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Osherovich L. Z., and Weissman J. S. (2001) Multiple Gln/Asn-rich prion domains confer susceptibility to induction of the yeast [PSI+] prion. Cell 106, 183–194 [DOI] [PubMed] [Google Scholar]
- 55. DePace A. H., Santoso A., Hillner P., and Weissman J. S. (1998) A critical role for amino-terminal glutamine/asparagine repeats in the formation and propagation of a yeast prion. Cell 93, 1241–1252 [DOI] [PubMed] [Google Scholar]
- 56. Wickner R. B., Taylor K. L., Edskes H. K., and Maddelein M. L. (2000) Prions: portable prion domains. Curr. Biol. 10, R335–R337 [DOI] [PubMed] [Google Scholar]
- 57. Halfmann R., Alberti S., Krishnan R., Lyle N., O'Donnell C. W., King O. D., Berger B., Pappu R. V., and Lindquist S. (2011) Opposing effects of glutamine and asparagine govern prion formation by intrinsically disordered proteins. Mol. Cell 43, 72–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Koo B. W., Hebda J. A., and Miranker A. D. (2008) Amide inequivalence in the fibrillar assembly of islet amyloid polypeptide. Protein Eng. Des. Sel. 21, 147–154 [DOI] [PubMed] [Google Scholar]
- 59. Avbelj M., Hafner-Bratkoviĉ I., and Jerala R. (2011) Introduction of glutamines into the B2-H2 loop promotes prion protein conversion. Biochem. Biophys. Res. Commun. 413, 521–526 [DOI] [PubMed] [Google Scholar]
- 60. Reddy G., Straub J. E., and Thirumalai D. (2009) Dynamics of locking of peptides onto growing amyloid fibrils. Proc. Natl. Acad. Sci. U.S.A. 106, 11948–11953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Watts J. C., Giles K., Stöhr J., Oehler A., Bhardwaj S., Grillo S. K., Patel S., DeArmond S. J., and Prusiner S. B. (2012) Spontaneous generation of rapidly transmissible prions in transgenic mice expressing wild-type bank vole prion protein. Proc. Natl. Acad. Sci. U.S.A. 109, 3498–3503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kascsak R. J., Rubenstein R., Merz P. A., Tonna-DeMasi M., Fersko R., Carp R. I., Wisniewski H. M., and Diringer H. (1987) Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J. Virol. 61, 3688–3693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bradley P., Misura K. M., and Baker D. (2005) Toward high-resolution de novo structure prediction for small proteins. Science 309, 1868–1871 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.