Abstract
Background
CPNE1 plays a vital role in regulating cell differentiation. The clinical and biological values of CPNE1 in prostate cancer are still unclear. The aim of this study was to investigate the clinicopathological value of CPNE1 and the association of CPNE1 with TRAF2 expression in patients with prostate cancer.
Material/Methods
CPNE1 expression in prostate cancer was analyzed using Gene Expression Omnibus (GEO) databases. The Cancer Genome Atlas (TCGA) dataset was used to investigate the association of CPNE1 expression with TRAF2 expression in prostate cancer. The association of CPNE1 expression with recurrence-free survival in patients was also analyzed using the TCGA dataset. Immunohistochemistry assay was performed to examine CPNE1 expression in 65 normal prostate samples and 114 prostate cancer samples. The recurrence-free survival in patients was evaluated using Kaplan-Meier curves and log-rank test. In addition, multivariate and univariate analyses of prognostic factors were investigated by Cox regression. The effect of CPNE1 on TRAF2 expression was explored in human prostate cancer DU-145 cells.
Results
Our results showed that expression level of CPNE1 is higher in prostate cancer than in normal prostate tissues (P=0.006). In the GSE35988 dataset, CPNE1 expression was found to be upregulated in castration-resistant prostate cancer compared with non-castration-resistant prostate cancer (P<0.001). Furthermore, we found that CPNE1 high expression was significantly related to tumor stage, Gleason score, and poorer biochemical recurrence-free survival in prostate cancer patients. Co-expression analysis of TCGA data showed that CPNE1 is significantly associated with TRAF2 expression. CPNE1 overexpression can upregulate TRAF2 expression in prostate cancer DU-145 cells as determined by Western blotting and immunofluorescence assays.
Conclusions
Overall, our findings suggest that CPNE1 is a valuable prognostic marker for evaluating recurrence-free survival and is positively related to TRAF2 expression in prostate cancer.
MeSH Keywords: Biological Markers, Prostatic Neoplasms, Urologic Neoplasms
Background
Prostate cancer is one of the most common cancer types in aging men. There were approximate 220 800 new cases and 27 540 deaths in the United States in 2015 [1,2]. The incidence rate of prostate cancer has also been increasing in Asian populations [3]. Different treatments, including surgical resection, radiological therapy, and androgen deprivation therapy (ADT), can be used to treat patients with localized prostate cancer. However, prostate cancer eventually progresses to an androgen-refractory state in most patients, which is also known as castration-resistant prostate cancer (CRPC). CRPC is relatively resistant to ADT treatment and can influence approximate 20% of prostate cancer patients in 5 years. However, the underlying mechanisms governing the development and progression of prostate cancer remain unclear.
CPNE1 is a member of the calcium-dependent lipid-binding protein family. Currently, 9 family members have been reported in studies [4,5]. It is reported that Copine1, encoded by CPNE1, is a soluble membrane-binding protein, which includes 2 tandem C2 domains at the N-terminus and an A domain at the C-terminus [6,7]. Copine1 can bind with different intracellular proteins via its A domain at the C-terminus [8,9]. In addition, Copine1 can also exhibit calcium-dependent phospholipid binding activity via its C2 domain [10]. Park et al. [7] reported that Copine1 plays a vital role in regulating neuronal differentiation of HiB5 cells, which may be related to activating Akt signaling. Akt has been found to be involved in various biological processes, including cell migration, invasion, and survival [11]. It was reported that CPNE1 can promote neuronal differentiation via phosphorylating Akt on the residue 473 (S473) [12]. Currently, the effects of CPNE1 on development and progression of prostate cancer are not well understood.
Tumor necrosis factor receptor (TNF-R)-associated factor 2 (TRAF2), as an adaptor protein, belongs to the TRAF family. Seven members (TRAF1-7) have been identified in the TRAF family. TRAF2 expression is upregulated in multiple cancer types and is a valuable prognostic biomarker in patients. It was reported that TRAF2 expression is increased in gastric cancer and can be a novel diagnostic and prognostic biomarker in gastric cancer patients [13]. In addition, the expression level of TRAF2 is higher in malignant pleural effusion cells than in normal breast samples in breast cancer patients [14]. Further study showed that TRAF2 is an important prognostic biomarker in patients [14]. Our study also indicated that TRAF2 expression in prostate cancer is upregulated and can be a valuable prognostic marker in prostate cancer patients (data not shown). However, the relationship between CPNE1 and TRAF2 expression is not clear.
In this report, our findings showed that CPNE1 expression is significantly increased in prostate cancer. CPNE1 expression is related to tumor stage and Gleason score. A novel finding is that CPNE1 expression is significantly related to TRAF2 expression. CPNE1 overexpression can upregulate TRAF2 expression in prostate cancer DU-145 cells. Overall, CPNE1 is a novel prognostic factor to predict biochemical recurrence-free survival and is positively related to TRAF2 expression in prostate cancer.
Material and Methods
Microarray analysis
Two publicly available datasets from the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) were downloaded to investigate CPNE1 mRNA expression in prostate cancer, and castration-resistant prostate cancer (CRPC). For the GSE6956 dataset, microarray gene expression data were used to examine CPNE1 mRNA expression in normal prostate samples (n=20) and prostate cancer samples (n=69) [15]. For the GSE35988 dataset, microarray gene expression data were extracted to detect CPNE1 mRNA expression in non-CRPC (n=49) and CRPC (n=27) [16]. The GEO data were processed in the R statistical environment and analyzed by SPSS 16.0 software. For the GEO microarray dataset, CPNE1 high/low mRNA expression was retrieved and identified according to median value of gene expression. The statistical differences were evaluated using the t test. Co-expression analysis of CPNE1 and TRAF2 was performed using the Cancer Genome Atlas (TCGA) dataset. In addition, we also analyzed the association of CPNE1 expression with recurrence-free survival in patients, using the TCGA dataset. For the TCGA dataset, the normalized level 3 IllumiaHiSeq RNASeqV2 microarray gene expression data were downloaded to evaluate the association of CPNE1 with TRAF2 expression in 497 prostate cancer samples.
Prostate samples
The study was conducted after the approval of the Ethics Committee of Wuxi People’s Hospital. A total of 65 normal prostate samples and 114 prostate cancer samples were collected to investigate CPNE1 expression. The patients were diagnosed and treated at Wuxi People’s Hospital (WXPH) between December 2007 and December 2015.
All patients were treated with radical prostatectomy. The surveillance included a serum prostate-specific antigen (PSA) measure at least every 3 months for the first year and every 6 months thereafter. The endpoint was time to biochemical recurrence, which was defined as the time from operation to the first of at least 2 consecutive values of 0.2 ng/mL or greater in total serum PSA level.
Prostate cancer of the patients were classified according to the 2010 AJCC/UICC TNM classification for stage and postoperative Gleason score (2005 ISUP modified) for grade [17,18]. Clinicopathological features are shown in Table 1. Follow-up was completed until October 2016.
Table 1.
Correlation between CPNE1 expression and clinicopathological factors.
| Parameters | Cases (%) | CPNE1 expression | P* | |
|---|---|---|---|---|
| Low | High | |||
| Age (years) | ||||
| ≤70 | 55 (48.2) | 38 | 17 | 0.473 |
| >70 | 59 (51.8) | 37 | 22 | |
| Tumor stage | ||||
| T2 | 65 (57.0) | 49 | 16 | 0.013 |
| T3 | 49 (43.0) | 26 | 23 | |
| Gleason score | ||||
| ≤7 | 59 (51.8) | 46 | 13 | 0.005 |
| >7 | 55 (48.2) | 29 | 26 | |
| Biochemical recurrence | ||||
| Negative | 95 (83.3) | 68 | 27 | 0.004 |
| Positive | 19 (16.7) | 7 | 12 | |
Pearson Chi-Square.
Immunohistochemistry
Immunohistochemical assay was conducted to examine CPNE1 expression in prostate samples according to the manufacturer’s protocol, as previously described [19]. Briefly, all prostate tissues on slides were deparaffinized and rehydrated. The slides were incubated with polyclonal rabbit anti-CPNE1 (1: 400 dilution, Abcam, Cambridge, UK) antibody. The CPNE1 expression was evaluated as described below. Briefly, a score represented the percentage of tumor cells with positive staining. Score 0 represented negative staining; score 1 represented positive tumor cell staining (<25%); score 2 represented positive tumor cell staining (25–50%); score 3 represented positive tumor cell staining (50–75%); and score 4 represented positive tumor cell staining (>75%). Then, a score was further assigned according to the staining intensity (score 0 indicated negative staining intensity; score 1 indicated weak staining intensity; score 2 indicated moderate staining intensity; and score 3 indicated strong staining intensity). According to scores of positive staining and staining intensity, a total score was assigned (Range: 0–7). The expression levels of CPNE1 were categorized into low expression (Scores: <4) or high expression (Scores: 4–7).
Cell culture
Human prostate cancer DU-145 cells were obtained from the American Type Culture Collection (ATCC), which were cultured in a humidified 5% CO2 atmosphere at 37°C using Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, CA, USA) with 10% fetal bovine serum. Cells were split every 3–4 days.
Lentivirus-mediated expression of CPNE1 in prostate cancer cells
Human CPNE1 cDNA was amplified and subcloned into lentiviral transfer plasmid pLVX-puro. Then, lentiviral particles were generated in 293T/17 cells by co-transfection of lentiviral transfer plasmid pLVX-puro-CPNE1 or control plasmid pLVX-puro, along with the packaging plasmids psPAX and pMD2.G. Lentiviral particles were collected 48 h after transfection and used to treat prostate cancer cells. Cells were selected using 2 μg/ml puromycin.
Immunofluorescence assay
Immunofluorescence assay was conducted according to a previously described method [20]. Briefly, cells were incubated with polyclonal rabbit anti-CPNE1 (1: 400 dilution, Abcam, Cambridge, UK) or TRAF2 (1: 400 dilution, Bioworld Technology, Minneapolis, USA) antibodies, and subsequently were incubated with goat anti-rabbit IgG Rhodamine (TRITC) for 2 h at room temperature.
Quantitative real-time RT-PCR
According to the manufacturer’s instructions, total RNA in cancer cells was obtained using the RNAiso Plus reagent (TaKaRa). Expression levels of CPNE1 and GAPDH mRNA were investigated using the SYBR® Premix Ex Taq™ II (Tli RNaseH Plus) (TaKaRa) by quantitative real-time RT-PCR (qRT-PCR). The primers for qRT-PCR were as follows: for CPNE1, 5′-CAAGAACAACCTGAACCCTACA -3′ (forward) and 5′-GTGACCCGTCACTGTCATAATC-3′ (reverse); for GAPDH, 5′-AGGTGAAGGTCGGAGTCAAC-3′ (forward) and 5′-GACAAGCTTCCCGTTCTCA-3′ (reverse). Sample amplification was performed using the following cycle scheme: 95°C for 30 s, 40 cycles of 95°C for 5 s, and 60°C for 50 s.
Western blotting
Western blotting was performed as previously described [21]. Briefly, proteins were transferred to polyvinylidene difluoride membranes when separated by SDS-polyacrylamide gel electrophoresis (PAGE). The membranes were first incubated with the following primary antibodies: Polyclonal rabbit anti-GAPDH or TRAF2 (1: 1000 dilution, Bioworld Technology, Minneapolis, USA) antibodies, and polyclonal rabbit anti-CPNE1 antibody (1: 1000 dilution, Abcam, Cambridge, UK). Then, the membranes were incubated with secondary antibody goat anti-rabbit IgG (H+L) HRP (1: 5000 dilution, Bioworld Technology, Minneapolis, USA) for 2 h at room temperature.
Statistical analysis
The recurrence-free survival in prostate cancer patients from the Wuxi cohort was analyzed by Kaplan-Meier curves and log-rank test. For the multivariate and univariate analyses, Cox regression analysis was performed to investigate the association of CPNE1 with recurrence-free survival in patients with prostate cancer. The intergroup comparison was performed using the t test. A two-sided P value <0.05 indicated statistical significance. Statistical analyses were performed using SPSS 16.0 software (SPSS Inc., USA).
Results
CPNE1 mRNA expression is upregulated in prostate cancer
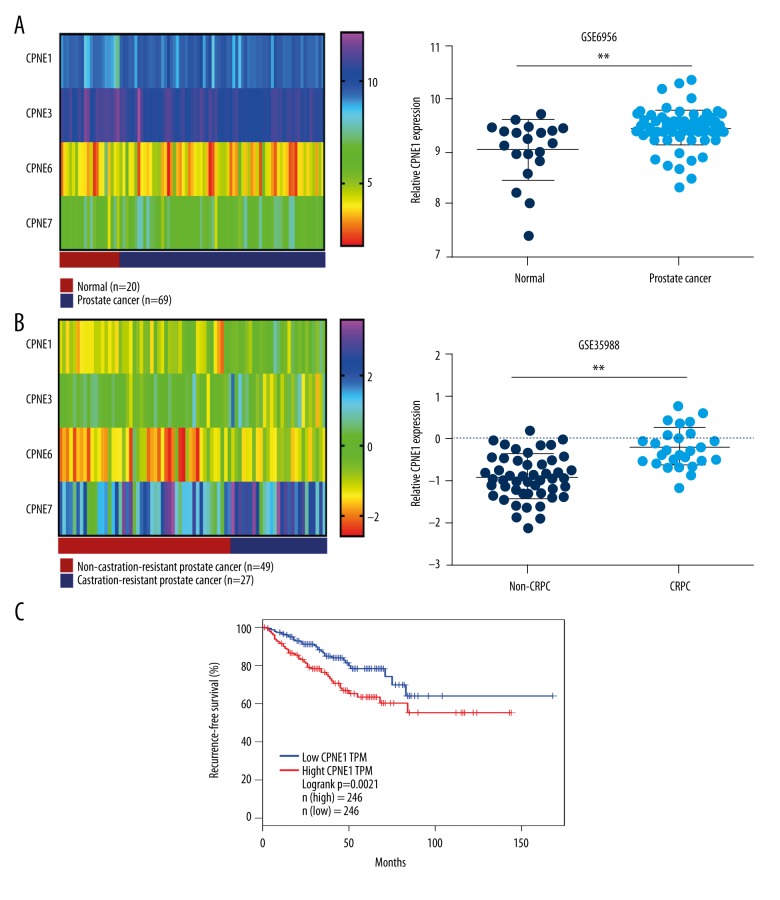
To examine CPNE1 mRNA expression in prostate cancer, the publicly available GSE6956 and GSE35988 datasets from the Gene Expression Omnibus (GEO) were downloaded in our study. For the GSE6956 dataset, 20 normal prostate samples and 69 prostate cancer samples were included to investigate the CPNE1 mRNA expression. Our findings showed that CPNE1 mRNA expression is significantly increased in prostate cancer tissues compared with normal prostate tissues (P=0.006, Figure 1A). In addition, the GSE35988 dataset was analyzed to investigate CPNE1 mRNA expression in castration-resistant prostate cancer (CRPC). Our analysis of GEO dataset showed that CPNE1 mRNA expression is significantly upregulated in CRPC compared with non-CRPC (P<0.001, Figure 1B). Furthermore, using the TCGA dataset, our analysis indicated that CPNE1 expression is significantly related to recurrence-free survival in prostate cancer patients (Figure 1C).
Figure 1.
CPNE1 expression in prostate cancer. (A) Levels of CPNE1, CPNE3, CPNE6, and CPNE7 expression are shown as a heatmap; levels of CPNE1 expression were statistically analyzed in normal prostate samples (n=20) and prostate cancer samples (n=69) from GEO dataset GSE6956. (B) Levels of CPNE1, CPNE3, CPNE6, and CPNE7 expression are shown as a heatmap; expression level of CPNE1 was statistically analyzed in non-castration-resistant (n=49) and castration-resistant (n=27) prostate cancer samples from GEO dataset GSE35988. Asterisks indicate a significant difference (** P<0.01). (C) The association of CPNE1 expression with recurrence-free survival in prostate cancer patients was analyzed using TCGA dataset.
CPNE1 expression and clinicopathological features in patients with prostate cancer
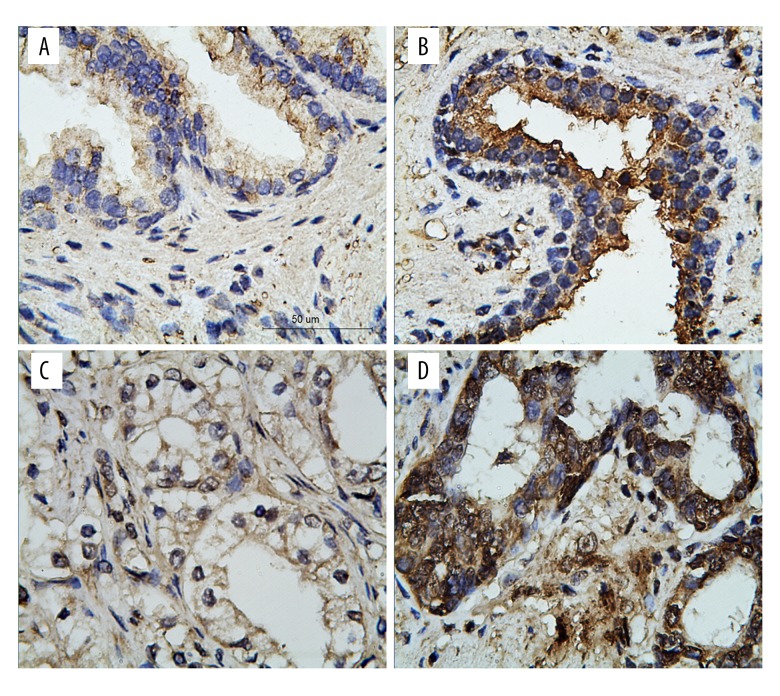
To evaluate CPNE1 protein expression in prostate cancer, 65 normal prostate samples and 114 prostate cancer samples were analyzed by immunohistochemistry. CPNE1 high expression was found in 18.5% (12/65) of normal prostate cancer samples. In addition, CPNE1 high expression was detected in 34.2% (39/114) of prostate cancer samples (Figure 2). It was also found that CPNE1 high expression is significantly associated with prostate cancer by chi-square test (P=0.025). Furthermore, CPNE1 high expression is significantly related to tumor stage (P=0.013, Table 1) and Gleason score (P=0.005, Table 1). CPNE1 high expression is significantly related to biochemical recurrence-free survival (P=0.004, Table 1).
Figure 2.
Immunohistochemical staining of CPNE1 in human prostate tissues. (A) Representative images of normal prostate CPNE1 low expression and (B) high expression. (C) Representative images of prostate cancer CPNE1 low expression and (D) high expression. Scale bar indicates 50 μm.
Effect of CPNE1 expression on biochemical recurrence-free survival in prostate cancer patients
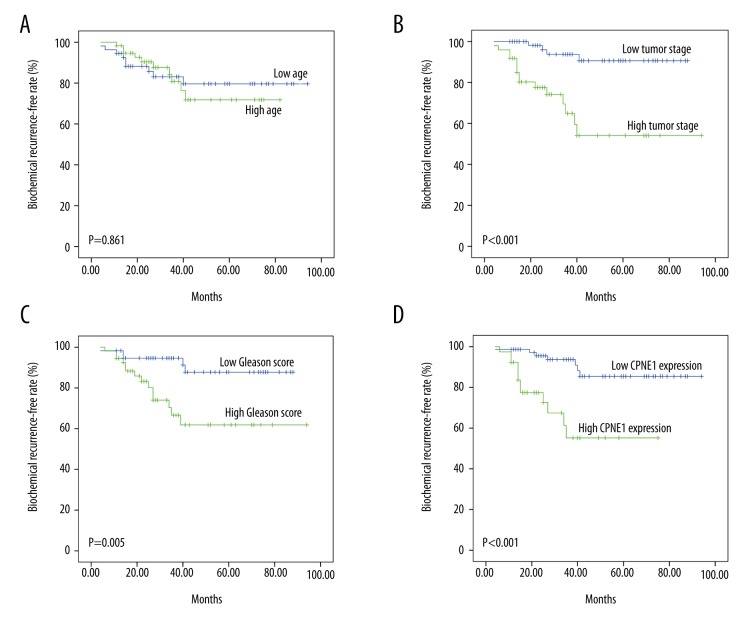
In this study, we found that the 5-year overall biochemical recurrence rate in prostate cancer patients is 16.7%. The biochemical recurrence-free rate in patients from the WXPH cohort was further investigated according to age, tumor stage, Gleason score, and CPNE1 expression. Our data showed that the biochemical recurrence-free survival is related to tumor stage (P<0.001), Gleason score (P=0.005), and CPNE1 expression (P<0.001, Figure 3).
Figure 3.
An analysis of biochemical recurrence-free survival in prostate cancer patients by the Kaplan-Meier method. (A) Kaplan-Meier curves for biochemical recurrence-free rate according to age, (B) tumor stage, (C) Gleason score, and (D) CPNE1 expression.
Evaluation of the prognostic factors in prostate cancer patients
To investigate the effects of CPNE1 expression on other pathological features of patients, the Cox proportional hazard model was used in the analyses. Univariate analysis showed that tumor stage, Gleason score, and CPNE1 expression are significant prognostic factors (Table 2). Furthermore, CPNE1 expression is a valuable prognostic factor (Table 2). Taken together, our findings show that CPNE1 is a valuable prognostic biomarker for predicting biochemical recurrence-free survival in prostate cancer patients (P=0.019).
Table 2.
Prognostic factors for recurrence-free survival in univariate and multivariate analyses.
| Parameters | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age | 1.083 (0.439–2.674) | 0.862* | 0.896 (0.353–2.271) | 0.817# |
| Tumor stage | 6.856 (2.260–20.795) | 0.001* | 4.152 (1.303–13.235) | 0.016# |
| Gleason score | 3.892 (1.394–10.868) | 0.009* | 2.323 (0.795–6.782) | 0.123# |
| CPNE1 expression | 5.013 (1.953–12.867) | 0.001* | 3.273 (1.220–8.778) | 0.019# |
Univariate analysis;
multivariate analysis.
Exogenous CPNE1 expression in prostate cancer cells
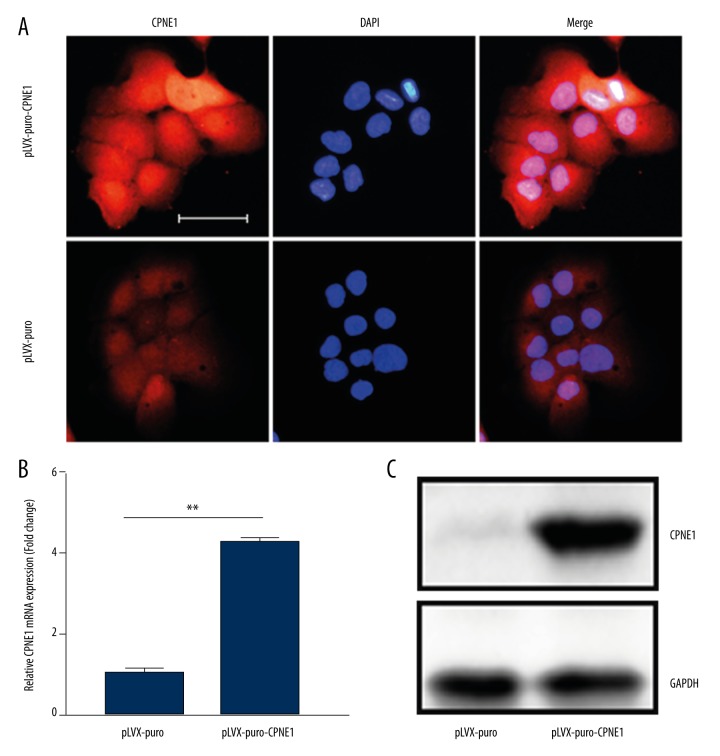
To investigate the effect of CPNE1 on prostate cancer development, prostate cancer DU-145 cells with exogenous CPNE1 expression were developed. CPNE1 overexpression in DU-145 cells was observed as determined by immunofluorescence assay (Figure 4A). Expression levels of CPNE1 mRNA and protein were found to be upregulated in DU-145 cells as determined by qRT-PCR and Western blotting assays (Figure 4B, 4C).
Figure 4.
Overexpression of CPNE1 in prostate cancer cells. (A) Representative images of CPNE1 overexpression in DU-145 cells by immunofluorescence assay. Scale bar indicates 50 μm. (B) CPNE1 overexpression in DU-145 cells was confirmed by qRT-PCR and (C) Western blotting. Quantitation of CPNE1 expression was normalized by GAPDH. Data are expressed as mean ±SD. Asterisks indicate a significant difference (** P<0.01).
Association of CPNE1 with TRAF2 expression in prostate cancer
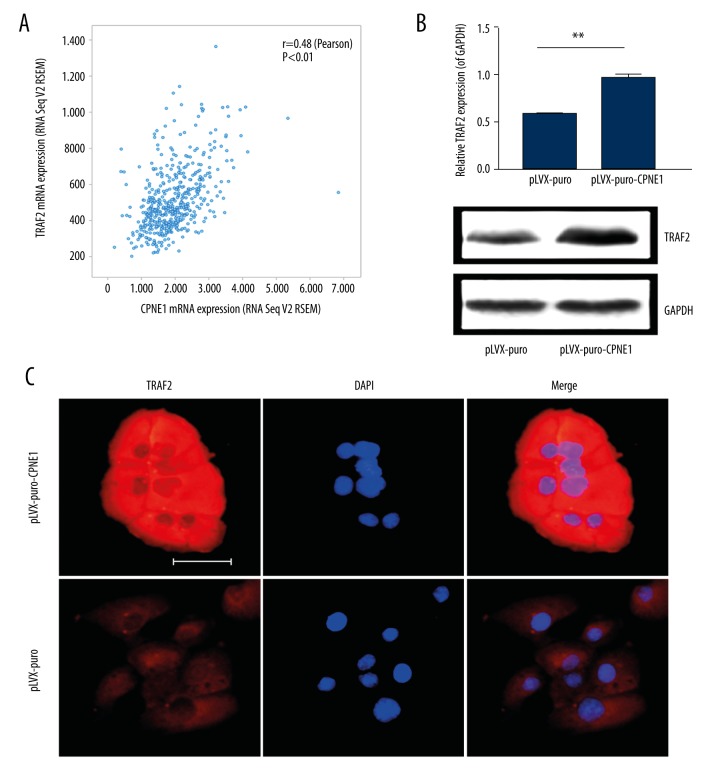
Our study has shown that TRAF2 is a valuable prognostic biomarker and can promote the growth of prostate cancer (data not shown). Here, we investigated the relationship between CPNE1 and TRAF2 expression. To determine the association of CPNE1 with TRAF2 expression, the TCGA dataset for prostate cancer was analyzed. From the TCGA dataset, there are 497 prostate cancer samples included in the analysis. Our analysis of TCGA data showed CPNE1 is significantly associated with TRAF2 expression in prostate cancer (Figure 5A).
Figure 5.
CPNE1 upregulates TRAF2 expression in prostate cancer cells. (A) Co-expression analysis of TCGA data showed CPNE1 is positively associated with TRAF2 expression in prostate cancer. TRAF2 expression was found to be upregulated in CPNE1-overexpressed DU-145 cells as determined by (B) Western blotting and (C) immunofluorescence assays. Scale bar indicates 50 μm.
CPNE1 upregulates TRAF2 expression in prostate cancer
Co-expression analysis of TCGA dataset showed that CPNE1 is related to TRAF2 expression in prostate cancer. This led us to investigate the in vitro effect of CPNE1 on TRAF2 expression in prostate cancer cells. Lentivirus containing human CPNE1 gene were used to infect DU-145 cells. Our findings showed that exogenous CPNE1 overexpression can upregulate TRAF2 expression in DU-145 cells as determined by Western blotting and immunofluorescence assays (Figure 5B, 5C). These results suggest that CPNE1 may promote the growth of prostate cancer at least partially via upregulating TRAF2 expression.
Discussion
CPNE1 is thought to be an important member of the calcium-dependent lipid-binding protein family, which is conserved in species ranging from Arabidopsis to Homo sapiens. It was reported that CPNE1 plays a vital role in regulating neuronal differentiation via activation of Akt [7,12], which suggests that CPNE1 itself may have protease activity. Multiple biomarkers have been identified to evaluate the prognosis in prostate cancer patients [22–24], but the prognostic value of CPNE1 in prostate cancer patients is still unclear. Indeed, CPNE gene expression has been found to be increased in late-stage breast and gastrointestinal tract cancers [25–27]. In addition, Choi et al. [28] reported that CPNE3 can activate downstream ErbB2 signaling and promote migration in SKBr3 breast cancer cells. Heinrich et al. [29] also found that CPNE3 RNA levels are significantly correlated with ERBB2 amplification. It was further reported that CPNE3 can interact with ErbB2 and promote tumor cell migration. Taken together, CPNE genes may promote tumor development and progression.
The present study is the first to report that CPNE1 expression is significantly upregulated in prostate cancer. Increased CPNE1 expression is related to tumor stage, Gleason score, and biochemical recurrence-free survival in prostate cancer patients. In addition, using GEO datasets, we also found that CPNE1 expression is significantly increased in castration-resistant prostate cancer (CRPC). Overall, this study suggests that CPNE1 can be a novel prognostic biomarker for predicting biochemical recurrence-free survival in prostate cancer patients, and may promote development and progression of prostate cancer.
CPNE1 had 2 tandem C2 domains (C2A and C2B) at the N-terminus and an A domain at the C-terminus [30], suggesting that this A domain is known from von Willebrand Factor, which is a plasma and extracellular matrix protein. Further study showed that this A domain has been investigated in several integrins and extracellular matrix proteins and may serve as a protein-binding domain [31]. The C2 domain is a calcium-dependent phospholipid-binding motif, which was originally found in protein kinase C [32]. The C2 domain was found to be very important for calcium and phospholipid binding [32,33]. Multiple proteins have been identified as Copine targets, including MEK1/ERK, protein phosphatase 5, and CDC42-regulated protein kinase [8,9]. Furthermore, it was reported that CPNE1 can activate Akt signaling and induce neuronal differentiation. Taken together, CPNE1 may promote the development and progression of prostate cancer via its C2 domain. However, the underlying mechanisms should be further investigated.
Using the TCGA dataset, we analyzed the association of CPNE1 expression with TRAF2 expression. We found that CPNE1 expression is positively associated with TRAF2 expression in prostate cancer. For the TCGA dataset, almost 497 samples were included in the analysis, which significantly improved the confidence of the results. Because TRAF2 plays a vital role in cancer development and progression [34–28], and this finding further shows that CPNE1 may influence the development and progression of prostate cancer, at least partially via upregulating TRAF2 expression. However, more in vitro experiments should be performed to verify the roles of CPNE1 in regulating TRAF2 expression in prostate cancer.
Conclusions
We found that CPNE1 expression is significantly increased in prostate cancer. Based on the GEO dataset, increased CPNE1 expression was further identified in CRPC, and CPNE1 expression was found to be significantly related to tumor stage and Gleason score. In addition, our results showed that CPNE1 expression is significantly associated with TRAF2 expression. Overall, CPNE1 is a valuable prognostic marker for predicting recurrence-free survival and is positively associated with TRAF2 expression in prostate cancer.
Footnotes
Conflicts of interest
None.
Source of support: This work was partially funded by grants from the Research Program of Wuxi Health and Family Planning Commission (Q201612), the Research Project of Wuxi Young Medical Talent (2016), and the Foundation of Nanjing Medical University (2013NJMU157)
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Nussbaum N, George DJ, Abernethy AP, et al. Patient experience in the treatment of metastatic castration-resistant prostate cancer: Atate of the science. Prostate Cancer Prostatic Dis. 2016;19:111–21. doi: 10.1038/pcan.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W. Cancer statistics: updated cancer burden in China. Chin J Cancer Res. 2015;27:1. doi: 10.3978/j.issn.1000-9604.2015.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomsig JL, Creutz CE. Copines: a ubiquitous family of Ca(2+)-dependent phospholipid-binding proteins. Cell Mol Life Sci. 2002;59:1467–77. doi: 10.1007/s00018-002-8522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maitra R, Grigoryev DN, Bera TK, et al. Cloning, molecular characterization, and expression analysis of Copine 8. Biochem Biophys Res Commun. 2003;303:842–47. doi: 10.1016/s0006-291x(03)00445-5. [DOI] [PubMed] [Google Scholar]
- 6.Creutz CE, Tomsig JL, Snyder SL, et al. The copines, a novel class of C2 domain-containing, calcium-dependent, phospholipid-binding proteins conserved from Paramecium to humans. J Biol Chem. 1998;273:1393–402. doi: 10.1074/jbc.273.3.1393. [DOI] [PubMed] [Google Scholar]
- 7.Park N, Yoo JC, Lee YS, et al. Copine1 C2 domains have a critical calcium-independent role in the neuronal differentiation of hippocampal progenitor HiB5 cells. Biochem Biophys Res Commun. 2014;454:228–33. doi: 10.1016/j.bbrc.2014.10.075. [DOI] [PubMed] [Google Scholar]
- 8.Tomsig JL, Snyder SL, Creutz CE. Identification of targets for calcium signaling through the copine family of proteins. Characterization of a coiled-coil copine-binding motif. J Biol Chem. 2003;278:10048–54. doi: 10.1074/jbc.M212632200. [DOI] [PubMed] [Google Scholar]
- 9.Tomsig JL, Sohma H, Creutz CE. Calcium-dependent regulation of tumour necrosis factor-alpha receptor signalling by copine. Biochem J. 2004;378:1089–94. doi: 10.1042/BJ20031654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomsig JL, Creutz CE. Biochemical characterization of copine: A ubiquitous Ca2+-dependent, phospholipid-binding protein. Biochemistry. 2000;39:16163–75. doi: 10.1021/bi0019949. [DOI] [PubMed] [Google Scholar]
- 11.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park N, Yoo JC, Ryu J, et al. Copine1 enhances neuronal differentiation of the hippocampal progenitor HiB5 cells. Mol Cells. 2012;34:549–54. doi: 10.1007/s10059-012-0235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie P. TRAF molecules in cell signaling and in human diseases. J Mol Signal. 2013;8:7. doi: 10.1186/1750-2187-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao ZJ, Ren HY, Yang F, et al. Expression, correlation, and prognostic value of TRAF2 and TRAF4 expression in malignant plural effusion cells in human breast cancer. Diagn Cytopathol. 2015;43:897–903. doi: 10.1002/dc.23330. [DOI] [PubMed] [Google Scholar]
- 15.Wallace TA, Prueitt RL, Yi M, et al. Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res. 2008;68:927–36. doi: 10.1158/0008-5472.CAN-07-2608. [DOI] [PubMed] [Google Scholar]
- 16.Grasso CS, Wu YM, Robinson DR, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–43. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng L, Montironi R, Bostwick DG, et al. Staging of prostate cancer. Histopathology. 2012;60:87–117. doi: 10.1111/j.1365-2559.2011.04025.x. [DOI] [PubMed] [Google Scholar]
- 18.Epstein JI, Allsbrook WC, Jr, Amin MB, et al. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29:1228–42. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 19.Mi Y, Yu M, Zhang L, et al. COPB2 is upregulated in prostate cancer and regulates PC-3 cell proliferation, cell cycle, and apoptosis. Arch Med Res. 2016;47:411–18. doi: 10.1016/j.arcmed.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Wang YQ, Cao Q, Wang F, et al. SIRT1 protects against oxidative stress-induced endothelial progenitor cells apoptosis by inhibiting FOXO3a via FOXO3a ubiquitination and degradation. J Cell Physiol. 2015;230:2098–107. doi: 10.1002/jcp.24938. [DOI] [PubMed] [Google Scholar]
- 21.Kim SW, Lee JH, Moon JH, et al. Niacin alleviates TRAIL-mediated colon cancer cell death via autophagy flux activation. Oncotarget. 2016;7:4356–68. doi: 10.18632/oncotarget.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen H, Zhang L, Zhou J, et al. Epidermal growth factor-containing fibulin-like extracellular matrix protein 1 (EFEMP1) acts as a potential diagnostic biomarker for prostate cancer. Med Sci Monit. 2017;23:216–22. doi: 10.12659/MSM.898809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qu Y, Zhang C, Du E, et al. Pim-3 is a critical risk factor in development and prognosis of prostate cancer. Med Sci Monit. 2016;22:4254–60. doi: 10.12659/MSM.898223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bryniarski P, Paradysz A, Fryczkowski M. PSA mass as a marker of prostate cancer progression after radical prostatectomy. Med Sci Monit. 2011;17:CR104–9. doi: 10.12659/MSM.881395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson KS, Roberts H, Leek R, et al. Differential gene expression patterns in HER2/neu-positive and -negative breast cancer cell lines and tissues. Am J Pathol. 2002;161:1171–85. doi: 10.1016/S0002-9440(10)64394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang E, Cheng SH, Dressman H, et al. Gene expression predictors of breast cancer outcomes. Lancet. 2003;361:1590–96. doi: 10.1016/S0140-6736(03)13308-9. [DOI] [PubMed] [Google Scholar]
- 27.Kim TM, Jeong HJ, Seo MY, et al. Determination of genes related to gastrointestinal tract origin cancer cells using a cDNA microarray. Clin Cancer Res. 2005;11:79–86. [PubMed] [Google Scholar]
- 28.Choi HY, Park N, Na JB, et al. Direct binding of Copine3 with Jab1 activates downstream ErbB2 signaling and motility in SKBr3 breast cancer cells. Oncol Rep. 2016;35:1147–52. doi: 10.3892/or.2015.4472. [DOI] [PubMed] [Google Scholar]
- 29.Heinrich C, Keller C, Boulay A, et al. Copine-III interacts with ErbB2 and promotes tumor cell migration. Oncogene. 2010;29:1598–610. doi: 10.1038/onc.2009.456. [DOI] [PubMed] [Google Scholar]
- 30.Perestenko PV, Pooler AM, Noorbakhshnia M, et al. Copines-1, -2, -3, -6 and -7 show different calcium-dependent intracellular membrane translocation and targeting. FEBS J. 2010;277:5174–89. doi: 10.1111/j.1742-4658.2010.07935.x. [DOI] [PubMed] [Google Scholar]
- 31.Whittaker CA, Hynes RO. Distribution and evolution of von Willebrand/integrin A domains: Widely dispersed domains with roles in cell adhesion and elsewhere. Mol Biol Cell. 2002;13:3369–87. doi: 10.1091/mbc.E02-05-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nalefski EA, Falke JJ. The C2 domain calcium-binding motif: Structural and functional diversity. Protein Sci. 1996;5:2375–90. doi: 10.1002/pro.5560051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rizo J, Sudhof TC. C2-domains, structure and function of a universal Ca2+-binding domain. J Biol Chem. 1998;273:15879–82. doi: 10.1074/jbc.273.26.15879. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Wen Z, Sun L, et al. TRAF2 regulates the cytoplasmic/nuclear distribution of TRAF4 and its biological function in breast cancer cells. Biochem Biophys Res Commun. 2013;436:344–48. doi: 10.1016/j.bbrc.2013.05.107. [DOI] [PubMed] [Google Scholar]
- 35.Shen RR, Zhou AY, Kim E, et al. TRAF2 is an NF-kappaB-activating oncogene in epithelial cancers. Oncogene. 2015;34:209–16. doi: 10.1038/onc.2013.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karl I, Jossberger-Werner M, Schmidt N, et al. TRAF2 inhibits TRAIL- and CD95L-induced apoptosis and necroptosis. Cell Death Dis. 2014;5:e1444. doi: 10.1038/cddis.2014.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Etemadi N, Chopin M, Anderton H, et al. TRAF2 regulates TNF and NF-kappaB signalling to suppress apoptosis and skin inflammation independently of Sphingosine kinase 1. Elife. 2015;4 doi: 10.7554/eLife.10592. pii: e10592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borghi A, Verstrepen L, Beyaert R. TRAF2 multitasking in TNF receptor-induced signaling to NF-kappaB, MAP kinases and cell death. Biochem Pharmacol. 2016;116:1–10. doi: 10.1016/j.bcp.2016.03.009. [DOI] [PubMed] [Google Scholar]