Abstract
Glucocorticoid receptors (GR) in the paraventricular nucleus of the hypothalamus (PVN) are important regulators of negative feedback regulation of the hypothalamic-pituitary-adrenal (HPA) axis. Previous evaluation of endogenous PVN GR function in adult mice demonstrated that mice with loss of GR exon 3 in the PVN (Sim1Cre-GRe3Δ) have a hyperactive HPA axis, growth impairment, and metabolic disruptions. Here, we hypothesized that lack of negative feedback inhibition of the HPA axis through PVN GR, as demonstrated through loss of PVN GR early in life, will have developmental-stage-specific consequences. Immunofluorescence revealed that Sim1Cre-GRe3Δ mice display PVN GR loss as early as postnatal day 2 compared to control mice. Sim1Cre-GRe3Δ mice compared to controls also displayed increased corticotropin-releasing hormone (CRH) mRNA in the PVN at postnatal day 10, as shown by in situ hybridization. Corticosterone radioimmunoassay revealed that the disruptions in PVN GR and CRH expression led to elevated basal corticosterone secretion in male Sim1Cre-GRe3Δ mice by early adolescence and increased stress-induced (restraint) corticosterone secretion in late adolescence into adulthood. In comparison, female Sim1Cre-GRe3Δ mice did not display corticosterone disruption until adulthood. Circadian rhythmicity of corticosterone secretion was normal for male and female mice at all age groups regardless of genotype with one exception. In late adolescence, female Sim1Cre-GRe3Δ mice had disrupted circadian corticosterone secretion due to significantly elevated circulating levels at nadir. We conclude that PVN GR function matures at an earlier developmental time point in male than in female mice and thus leads to later differential stress responsiveness between sexes.
Keywords: Adolescent, Corticosterone, Corticotropin-releasing hormone (CRH), Glucocorticoids, Paraventricular nucleus of the hypothalamus (PVN), Sex-specific
INTRODUCTION
Regulation of the endocrine stress response is governed in major part by the hypothalamic-pituitary-adrenal (HPA) axis, which is ultimately characterized by the release of glucocorticoids from the adrenal cortex. This glucocorticoid secretion is stimulated by the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary, which is targeted by upstream action of corticotropin-releasing hormone (CRH) from the paraventricular nucleus of the hypothalamus (PVN). Proper HPA axis function is important for normal stress reactivity, and disruptions lead to maladaptive neuroendocrine and behavioral phenotypes (Carpenter et al. 2007, Tyrka et al. 2008, Schroeder et al. 2013). Glucocorticoids acting on glucocorticoid receptors (GR) regulate many organismal functions including tissue development, metabolism and stress responses (Cole et al. 1995, Sapolsky et al. 2000, Wang 2005). During the stress response in rodents, HPA axis activity returns to baseline through negative feedback of corticosterone on GR at the level of the PVN, anterior pituitary, and forebrain to inhibit future activation.(Yi et al. 1993, Russell et al. 2010, Herman et al. 2012) This negative feedback is necessary to suppress overactivation of the HPA axis and prevent pathological effects of excess corticosterone.
HPA axis function varies at different stages of development. The early stages of neonatal life are characterized by a stress-hyporesponsive period (SHRP) during which there is limited capability of stress to evoke secretion of glucocorticoids (Sapolsky and Meaney 1986, Levine 1994, Schmidt et al. 2003). This pattern of regulation is influenced by corticosteroid-binding globulin (CBG) which binds to glucocorticoids and reduces their bioavailability. During this early postnatal period levels of CBG are low and thus in part account for relatively high glucocorticoid concentrations which are less influenced by stress (Viau et al. 1996). This SHRP serves as a protective mechanism against the deleterious effects of excess glucocorticoids in the brain and periphery, and is mediated by GR-mediated feedback at the anterior pituitary (Sapolsky and Meaney 1986, Schmidt et al. 2005). Deletion of pituitary GR results in increased HPA axis activity, leading to increased plasma corticosterone concentrations, particularly during the first week of postnatal life (Schmidt et al. 2009). In comparison, GR antagonists in the PVN have little effect on HPA axis output in the first week of postnatal life but begin to cause increases in PVN CRH mRNA and plasma corticosterone in the second week of postnatal life (Yi et al. 1993). Given that GR-mediated feedback appears to develop in distinct brain regions at different developmental time points, we hypothesized here that PVN GR plays a role in shaping the ontogeny of the HPA axis. To examine this HPA axis development, we focussed on the adolescent period, the transition between early postnatal life and adulthood (McCormick and Mathews 2007).
The brain continues to undergo development during adolescence in humans and other mammals. Morphological and connectivity changes in a number of brain regions (Durston et al. 2001, Sowell et al. 2002, Giedd 2008) make the adolescent brain more vulnerable to the effects of environmental stimuli (Steinberg 2005, Wheeler et al. 2013, Schindler et al. 2014). Moreover, in adolescent rodents the HPA axis undergoes reorganization (Meaney et al. 1985, Sowell et al. 1999, Arnsten and Shansky 2004), making this developmental period sensitive to the effects of stress. Due to the propensity of the developing adolescent brain to be influenced by external stimuli, we further hypothesize that adolescent mice will be more susceptible to the effects of PVN GR disruption. Here we aimed to define the developmental trajectory of PVN GR function by evaluating the effects of PVN GR loss during early life on neuroendocrine activity in male and female mice in early (postnatal days 30–50) and late (postnatal days 51–70) adolescence..
Our data provide neuroendocrine evidence demonstrating a sex-specific developmental role for PVN GR in HPA axis regulation.
MATERIALS AND METHODS
Animals
Animal protocols were performed in accordance with National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committees of Cincinnati Children’s Research Foundation (Cincinnati, OH). The animal room temperature was 22.2°C and humidity ranged between 30% – 70%. Mice were housed on a 14 h/10 h light/dark cycle with ad libitum access to rodent chow and water. Lights were on from 06:00 to 20:00 Eastern time during daylight savings time (DST) and is set back one hour when DST ends. All mice were housed with litter mates of the same sex with 2–4 mice per cage. Mice were generated as previously described (Laryea et al. 2013). Briefly, Sim1Cre-GRe3Δ mice were generated by initially mating male Sim1Cre mice (Balthasar et al. 2005) to female mice with a floxed GR exon 3 transgene (Tronche et al. 1999). Subsequent breeding to mice with the floxed GR allele bred to homozygosity was used to establish matings of Sim1Cre-GRe3Δ mice to homozygous floxed GREe3 mice. Controls were littermates that were Cre negative but floxed GR exon 3 homozygous. Floxed GR exon 3 mice were obtained from Dr. Günther Schütz, German Cancer Research Center, Heidelberg, Germany. Sim1Cre mice were the gift of Dr. Brad Lowell, Beth Israel Deaconess Medical Center, Boston, MA. Sample sizes ranged from 3–17 control mice and 3–15 Sim1Cre-GRe3Δ mice.
Immunohistochemistry
Mice were anesthetized by intraperitoneal injection of a solution containing 17.7 mg/mL ketamine (Ketaset, Fort Dodge IAO) and 2.6 mg/mL xylazine (TranquiVed, Vedco, St. Joseph MO) in phosphate buffered saline (PBS) (0.10mL/100g body weight). Mice were anesthetized at postnatal day 2 (P2), where P0 was the day of birth. Mice were then perfused with 1× Diethylpyrocarbonate (DEPC) PBS followed by 4% DEPC paraformaldehyde (PFA). We post-fixed the brains in 4% DEPC PFA at 4°C overnight and immersed the brains in 70% ethanol to process for paraffin embedding. A microtome was used to cut coronal sections of paraffin-embedded brains at 8 µm onto Superfrost+ slides (VWR). We deparaffinized the slides, rehydrated them in decreasing concentrations of ethanol, and reconstituted antigens in 10mM Citrate Buffer (pH 6.0). Slides were washed in PBS and blocked for 1 hour in 3% normal goat serum/ 0.25% Triton-X-100 in PBS. GR: Primary antibodies were 1:200 rabbit m20-anti-GR, directed against the N-terminus of GR, (Santa Cruz Biotech. Cat# sc-1004) and 1:200 mouse anti-NeuN (neuron-specific nuclear protein; Millipore, Cat # MAB377); Secondary antibodies were 1:250 biotinylated goat anti-rabbit IgG (Vector Laboratories, Cat# Pk-6101) and 1:200 Alexa Fluor 488 goat anti-mouse IgG (Invitrogen, Cat# A11001). We washed slides, incubated them in avidin/biotin complex (Vector Laboratories, Burlingame CA), followed by Cyanine-3 tyramide amplification (Perkin Elmer) and coverslipped with Vectashield containing 4’, 6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame CA).
Corticosterone radioimmunoassay
Mice between P30–50 (early adolescence) and P51–70 (late adolescent) from the same cage were double-housed for one week. Submandibular bleeds were performed in mice using a 5mm point Goldenrod animal lancet (Braintree Scientific, MA) to puncture a small vascular bundle located at the back of the mouse’s jaw. Blood samples (50–60µl/bleed) were collected at nadir (07:00h), peak (18:00h), and immediately after a 20-minute restraint stress performed at nadir (restraint completed in a 50mL conical tube with ventilation holes) with two days between bleeds. Blood was collected into 1.5mL EDTA-treated centrifuge tubes. We centrifuged the blood at 16,900 × g for 6 minutes and stored the plasma at −80°C until a radioimmunoassay (RIA) was performed. The Corticosterone Double Antibody - 125I RIA Kit (MP Biomedicals, Solon, OH) was used to perform the RIA. Assay sensitivity of the kit was 7.7 ng/ml and inter-assay variability was 6.33% for low controls and 3.24% for high controls.
CRH in situ hybridization
Mice were anesthetized at P10 with ketamine/xylazine as above, perfused with DEPC 1× PBS followed by 4% DEPC PFA, and the brains were post-fixed overnight in 4% DEPC PFA. After 48-hour immersion in 20% DEPC sucrose solution at 4°C, brains were embedded in Optimal Cutting Temperature (OCT) compound. 16µm coronal sections were obtained from brains at −17°C in a cryostat and mounted onto Superfrost+ slides (VWR), vacuum-dried overnight, and stored at −80°C until in situ hybridization was performed. We used P33-UTP to label an RNA probe complementary to a 0.32 kb fragment of exon 2 of the CRH mRNA (Kolber et al. 2010). We hybridized the labeled probe to sections at 60°C for 20 hours and washed in 0.1× SSC at 60°C for 30 min. Slides were exposed to Maximum resonance film (Kodax BioMAx, Rochester N.Y.) for 3 days. The autoradiographic images were scanned into Adobe Photoshop on an Epson Perfection V600 scanner at 12600 dots per cm and quantified using NIH Image J software. As the values for male mice did not significantly differ from female mice, the sexes were combined for analysis.
Statistical analysis
The results are presented as mean ± SEM after subjection to Student t-test or two-way ANOVA (Prism 5.0 software; GraphPad Software, Inc., San Diego, CA). Plasma corticosterone concentrations were analyzed with two-way ANOVA with genotype (Control of Sim1Cre GRe3) and time (Nadir, Peak, or 20 minutes post-stress) as factors. Plasma corticosterone concentrations were also analyzed with two-way ANOVA using sex (male or female) and time (Nadir, Peak, or 20 minutes post-stress) as factors. Statistical significance was defined as a P value ≤ 0.05 and was analyzed using the Tukey or Bonferroni post hoc tests.
RESULTS
Deletion of GRe3 in PVN neurons expressing Sim1
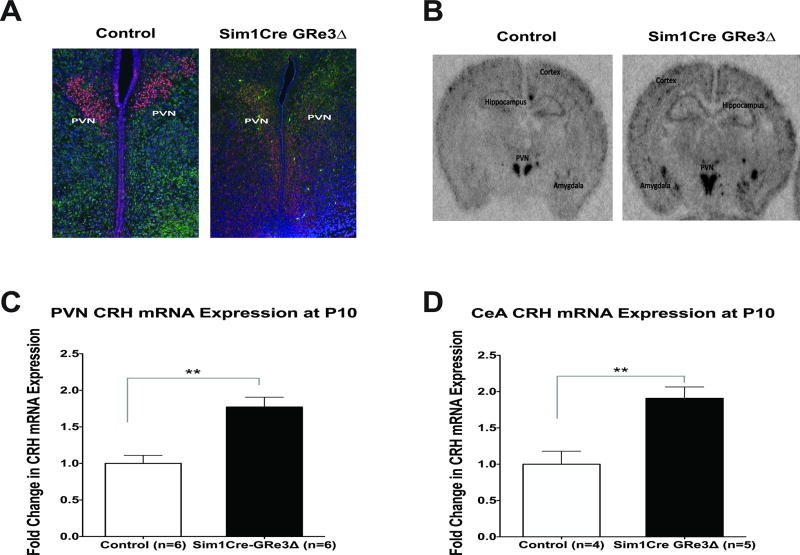
Sim1Cre-GRe3Δ transgenic mice were generated as previously described (Laryea et al. 2013), and to determine when the deletion of GR first occured, we performed fluorescent immunohistochemistry in P2 brains. We observed an absence of PVN GR protein expression in Sim1Cre-GRe3Δ mice compared to controls (mice with homozygous floxed GRe3 alleles and no Cre) at age P2 (Figure 1A). We evaluated CRH expression at a later developmental stage to allow adequate time for the consequences of GR loss to manifest. We measured CRH mRNA expression in the PVN and amygdala of controls and Sim1Cre-GRe3Δ mice at P10 using in situ hybridization to determine whether CRH mRNA was dysregulated early in postnatal development due to GR loss. Compared to control mice at P10, Sim1Cre-GRe3Δ mice had a 1.7 fold increase in CRH mRNA expression in the PVN (P < 0.002) (Figure 1B and C) and a 1.9 fold increase in amygdala CRH mRNA expression (Figure 1D).
Figure 1. Imuunofluorescence of PVN GR protein and in situ hybridization of PVN and amygdala CRH mRNA expression in control and Sim1Cre-GRe3Δ mice.
(A) Immunofluorescent images of the PVN showing GR (red), NeuN (green), and Dapi (blue) in mice at postnatal day 2 (P2), representative of 3 mice/group. Representative images depict immunofluorescent staining in male mice. (B, C, D) CRH mRNA expression is increased in the PVN and amygdala of Sim1Cre-GRe3Δ mice at postnatal day 10 (P10). (B) Representative images (females) of CRH in situ hybridization in control and Sim1Cre-GRe3Δ mice at P10. (C) Quantification of pixel density showed an increase in PVN CRH mRNA in Sim1Cre-GRe3Δ mice (n=6) compared to control mice (n=6). (D) Quantification of pixel density showed an increase in amygdala CRH mRNA in Sim1Cre-GRe3Δ mice (n=5) compared to control mice (n=4), **P < 0.01, student t-test. Data are shown as mean +/− s.e.m. Abbreviations: PVN- paraventricular nucleus of the hypothalamus; CRH- Corticotropin-releasing hormone; GR- glucocorticoid receptor; NeuN: neuron-specific nuclear protein; DAPI: 4',6-diamidino-2-phenylindole, DNA stain.
Adolescent Sim1Cre-GRE3Δ mice display sex-specific developmental differences in HPA Axis regulation
Males
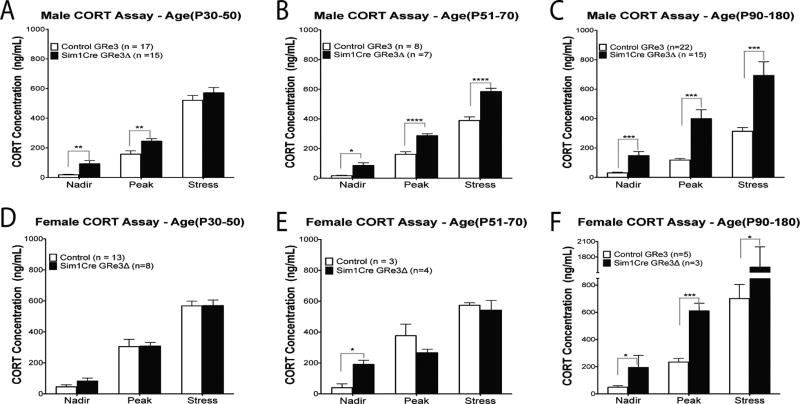
We measured plasma corticosterone concentrations in control and Sim1Cre-GRe3Δ adolescent mice to determine the developmental consequences of PVN GR loss. During the early adolescent period (P30–50) in male mice, we found a significant main effect of genotype [F(1,90)= 12.65), P=0.0006] influence by significantly elevated nadir (P = 0.001) and peak (P = 0.005) plasma corticosterone concentrations in male Sim1Cre-GRe3Δ mice compared to controls (Figure 2A). corticosterone concentrations after stress did not differ (P = 0.299) between genotypes in this age group (Figure 2A). There was also a main effect of time [F(2,90) 215.9), P<0.0001] demonstrating a circadian corticosterone rise at peak compared to nadir (P< 0.0001), and further elevations in corticosterone after stress (P< 0.0001) regardless of genotype. There was no interaction between genotype and time [F(2,90) = 0.2957), P=0.7447].
Figure 2. Plasma corticosterone concentrations measured at circadian nadir and peak, and after 20-minutes of restraint stress in males (A, B, C) and female mice (D, E, F).
(A) Male Sim1Cre-GRe3Δ mice at ages P30–P50 (P: postnatal day; n=15) display increased plasma corticosterone at nadir and peak and but not after stress compared to control mice (n=17). (B) Sim1Cre-GRe3Δ male mice at ages P51–P70 (n=7) display increased plasma corticosterone at nadir and peak, as well as after stress compared to control mice (n=8). (C) Adult Sim1Cre-GRe3Δ male mice (n=15) at ages P90–180 compared to control mice (n=22) have elevated corticosterone concentration at nadir, peak and after stress. Circadian rhythm of corticosterone secretion was maintained in males, regardless of age and genotype. (D) Between age P30–P50, corticosterone concentrations of female Sim1Cre-GRe3Δ mice (n=8) did not differ from control mice (n=13) and circadian rhythm was maintain in both genotypes. (E) Female Sim1Cre-GRe3Δ mice ages P51–P70 (n=4) display increased plasma corticosterone at nadir, but no differences in peak and stress –induced corticosterone compared to control mice (n=3). Circadian rhythm of corticosterone secretion was less in female Sim1Cre-GRe3Δ mice at ages P51–P70. (F) Adult Sim1Cre-GRe3Δ female mice (n=3) at ages P90–180 have elevated corticosterone concentration at nadir, peak and after stress compared to controls (n=5). Adult data in C and F are adapted with permission from previously published results (Fig 3, Laryea et al. 2013). *P < 0.05, **P < 0.01, ****P < 0.0001, two-way ANOVA with Bonferonni post-hoc test. Data are shown as mean +/− s.e.m. Abbrev.: CORT-corticosterone.
In late adolescence, P51–P70, we observed a significant main effect of genotype [F(1,39)= 82.75, P <0.0001] with male Sim1Cre-GRe3Δ mice showing elevated plasma corticosterone concentration at nadir (P = 0.001), peak (P < 0.0001) and after 20 minutes of restraint stress (P < 0.0001) compared to controls (Figure 2B); these findings were similar to those previously observed in adult male mice (3–6 months, P90–P180) (Figure 2C) (Laryea et al. 2013). There was also a significant main effect of time [F(2,39)= 313.1, P< 0.0001] and a genotype X time interaction [F(2,39)= 6.434, P= 0.0038].
Females
By contrast, in female Sim1Cre-GRe3Δ mice, early adolescence was characterized by a lack of differences in corticosterone secretion with regards to genotype or genotype X time. Hence, female Sim1Cre-GRe3Δ mice had no changes in basal and stress-induced plasma corticosterone concentrations compared to control mice (Figure 2D). There was however a significant main effect of time [F(2,57)= 116.3, P< 0.0001] (Figure 2D). In late adolescence, there was a significant interaction between genotype and time [F(2,57)= 4.482, P= 0.029]. Nadir plasma corticosterone concentrations in female Sim1Cre-GRe3Δ mice (P51–70) were significantly higher than controls (P = 0.011) but peak and stress-induced corticosterone concentrations did not differ (Figure 2E). Moreover, female Sim1Cre-GRe3Δ mice in this group lacked a circadian rise to peak corticosterone levels compared to controls (Figure 2E). Therefore, with the exception of these female Sim1Cre-GRe3Δ mice of ages P51–70 (Figure 2E), there was a significant main effect of time [F(2,15)= 49.12, P< 0.0001], with the circadian rhythm of corticosterone secretion maintained in female controls (P < 0.001), and stress concentrations in both genotypes elevated compared to basal corticosterone concentrations (P< 0.0001) (Figure 2E).
Sex and age differences
We compared data generated from adolescent Sim1Cre-GRe3Δ mice in this study to data previously collected from adult Sim1Cre-GRe3Δ mice (Laryea et al. 2013) to determine sex and age differences in corticosterone concentrations. In control mice, a two-way ANOVA demonstrated that at P30–50, there was a significant main effect of sex [F(1,83)= 10.91, P= 0.0014)] and time [F(1,83)= 175.8, P< 0.0001], but no interaction between the factors. In control mice at P51–70, we observed a significant main effect of sex [F(1,27)= 40.08, P< 0.0001] and time [F(1,27)= 137.5, P< 0.0001], as well as an interaction between sex and time [F(1,27)= 7.111, P= 0.0033]. In adult control mice, a two-way ANOVA displayed significant main effects of sex [F(1,74)= 58.92, P< 0.0001], time [F(1,74)= 142.5, P< 0.0001], and a sex X time interaction [F(1, 74)= 23.63, P< 0.0001]. In control mice, nadir plasma corticosterone concentrations did not vary between sexes from P30 into adulthood, (Figure 2). At circadian peak, plasma corticosterone concentrations were significantly increased in female control mice compared to males at P30–50 (Figure 2A,D; P < 0.001), P51–70 (Figure 2B,E; P < 0.0001), and in adulthood (P90–P180; Figure 2C,F; P < 0.05). Under conditions of acute stress, female control mice had significantly elevated corticosterone concentrations compared to males at P51–70 (Figure 2B,E; P < 0.001) and in adulthood (3–6 months, P90–P180;Figure 2C,F; P < 0.0001).
Two-way ANOVA in Sim1Cre-GRe3Δ mice demonstrated that at P30–50, there was a significant main effect of time [F(1,64)= 143.4, P< 0.0001] on plasma corticosterone concentrations, however no significant effects of sex or sex X time was observed.
At P51–70, we observed significant main effects of time [F(1,27)= 124.1, P< 0.0001] and interaction between sex and time [F(1,27)= 4.158, P= 0.027], but no significant effect of sex. Two-way ANOVA in adult Sim1Cre-GRe3Δ mice displayed significant main effects of sex [F(1,31)= 11.74, P= 0.0017], time [F(1,31)= 26.9, P< 0.0001], and a sex X time interaction [F(1, 31)= 5.818, P= 0.0072]. In Sim1Cre-GRe3Δ mice, nadir plasma corticosterone concentrations only differed at P51–70 (Figure 2B,E) with higher levels in females compared to males (P < 0.05). At circadian peak, plasma corticosterone concentrations did not vary between sexes from P30 into adulthood.. After an acute stressor, female Sim1Cre-GRe3Δ mice had significantly elevated corticosterone concentrations compared to males only in adulthood (P90–P180; P < 0.001).
DISCUSSION
GR function is a major contributor to control of HPA axis activity and therefore provides an important target to understand stress biology. This study was performed under the hypothesis that PVN GR loss will have developmental consequences on neuroendocrine HPA axis regulation in mice during the adolescent period. We further explored the hypothesis that the effects of PVN GR loss on HPA axis function would be sex-specific. Our results indicate that disruption of PVN GR expression leads to hyperactivation of the HPA axis with increased PVN CRH mRNA expression. Moreover, we observed a developmental trajectory in the development of PVN GR function that differs in male compared to female mice. Specifically, loss of PVN GR demonstrated that GR-mediated negative feedback of basal corticosterone secretion occurs by adolescence in male mice but not until early adulthood in female mice. Under conditions of stress, GR-mediated feedback occurs in late adolescence in male mice but not until adulthood in female mice.
HPA axis regulation during development
Constitutive GR deletion in mice has been shown to lead to neonatal mortality when exon 3 of the GR gene is targeted (Tronche et al., 1999) and approximately 80% lethality when exon 2 of the gene is targeted (Cole et al. 1995). GR disruption along all sites of HPA axis negative feedback regulation (brain and pituitary) leads to early postnatal death (Erdmann et al. 2008). These studies point to a necessity for GR function not only for neonatal organ development but also for HPA axis feedback regulation. Genetic studies have also allowed investigations to parse out the contributions of specific GR-mediated negative feedback sites in physiology. Deletion of pituitary GR (GRPOMCCre), for instance, results in HPA axis hyperactivity and growth impairments in the first few days of postnatal life, but these disruptions do not last into adulthood (Schmidt et al. 2009). In genetic mouse models of GR deletion in the forebrain (FRGRKO), substantial GR loss does not occur until adulthood, and results in HPA axis hyperactivity, indicating a role for forebrain GR in negative feedback regulation (Boyle et al. 2005, 2006, Furay et al. 2008, Solomon et al. 2012). Our data indicate that in Sim1Cre GRe3Δ mice, PVN GR loss occurs by postnatal day 2. The early disruption of PVN GR expression led to an increase in PVN CRH mRNA expression at P10 and HPA axis hyperactivity in adolescence that lasts into adulthood. Of interest is that amygdala CRH mRNA expression was also increased in Sim1Cre GRe3Δ mice at P10, compared to controls. This indicates that loss of PVN GR affects gene expression in other stress-related regions. This increase of amygada CRH mRNA level appears to occur only during early life, as in adulthood, we did not find significant differences in amygdala CRH mRNA in Sim1Cre GRe3Δ mice compared to control mice (Laryea et al. 2013).
Puberty, the period of sexual maturation, in mice begins around 40 days of age which corresponds to early adolescence in our studies (McCormick and Mathews 2007). In males, PVN GR loss in Sim1Cre GRe3Δ mice led to elevated nadir and peak plasma corticosterone concentrations during early adolescence (P30–P50) compared to controls. However, stress-induced increases in corticosterone were not different between control and Sim1Cre GRe3Δ male mice at that age, and only appeared after P51, post-puberty. This indicates that during the early adolescent period of life in males, PVN GR is important for maintaining basal regulation of glucocorticoid secretion, but it is not necessary for regulating stress-induced glucocorticoid secretion until post-puberty, late adolescence/ early adulthood. It is of note that while we did not perform a time course study of corticosterone responses, we evaluated the time point specifically that was found to be different in our adult studies in response to stress as this proved most sensitive to differences. Analyzing multiple time points, given the number of gestational ages we tested and studying both sexes would likely be of minimal biological relevance for the small transient difference that could potentially be found. However, we cannot discount the possibility that lack of GR in the PVN may not affect the maximal corticosterone response to stress but it may affect the declining phase of the response.
In females, plasma concentrations of corticosterone did not vary between control and Sim1Cre GRe3Δ mice in early adolescence. In late adolescence/ early adulthood, nadir concentrations of plasma corticosterone were increased in Sim1Cre GRe3Δ females, but peak and stress concentrations did not differ from controls. Additionally, female Sim1Cre GRe3Δ mice in late adolescence were the only group that lacked a circadian rise in corticosterone levels. In contrast to males, PVN GR in female mice was not necessary for maintaining basal regulation of glucocorticoid secretion during adolescence, and did not become necessary until adulthood.
The sex difference in corticosterone secretion between controls as well as Sim1Cre GRe3Δ mice is suggestive of modulation of GR function by sex hormones. Indeed, there is abundant evidence supporting a role of estrogen both in increasing and decreasing HPA axis activity through alteration of GR signaling pathways (Burgess and Handa 1992, Weiser and Handa 2009, Weiser et al. 2010). Moreover, androgens are also known to decrease HPA axis activity by reducing glucocorticoid secretion (Toufexis and Wilson 2012, Kalil et al. 2013). Additionally, interactions between the estrogen/androgen receptors and GR exist (Miranda et al. 2013, Lightman and George 2014) that may contribute to the observed sex differences. Thus, it is likely that the varying levels of androgens and estrogens in males compared to females would influence characteristics of physiology and produce distinct glucocorticoid signaling and HPA axis regulation between sexes. Consequently, in the absence of PVN GR, glucocorticoid feedback would be differentially regulated with respect to sex. Our data showed that in control mice, nadir levels of corticosterone did not vary from early adolescence into adulthood and did not differ between males and females. In Sim1Cre GRe3Δ mice, nadir levels of corticosterone did not vary between males and females in early adolescence or adulthood, but did differ in the transition from adolescence into adulthood (P51–70). At this age females had higher corticosterone levels than males in the absence of PVN GR. We observed that at circadian peak, levels of corticosterone in control female mice were significantly higher than those in control males from early adolescence into adulthood. This may be a result of normally higher HPA axis activity in females compared to males(Handa et al. 1994, Carpenter et al. 2007, Babb et al. 2013), possibly due to neonatal organizational effects of androgens and estrogen on decreasing and increasing HPA axis activity, respectively, in adulthood (Seale et al. 2005a, 2005b). In addition, estradiol has been shown to act on the estrogen receptor alpha to inhibit glucocorticoid negative feedback thus increasing plasma glucocorticoid concentrations (Weiser and Handa 2009, Handa and Weiser 2014). Interestingly, when glucocorticoid negative feedback was already lost in Sim1Cre GRe3Δ mice, the difference between sexes at circadian peak is also lost; suggesting perhaps that the function of estradiol may also depend on PVN GR availability. There may also be an influence of CBG which binds to circulating glucocorticoids and regulates its bioavailability (Mattos et al. 2013), and is expressed more in females than males (Mataradze et al. 1992, Tinnikov 1999, McCormick and Mathews 2007). Interactions between sex hormones, CBG, and GR may therefore contribute to the differences observed in our studies. It should be noted that females were examined randomly throughout the estrous cycle and thus the influence of hormonal variability on glucocorticoid secretion is not accounted for as a function of specific stage of the cycle. However, given that the variability (as indicated by the observed standard deviations) in our measurements did not differ drastically, we infer that hormonal variability had a limited effect on our results.
Our studies suggest that PVN GR mediation of negative feedback occurs earlier in development in males than in females and that development of the HPA axis and its regulation in males and females may occur by differing mechanisms. Based on the aforementioned findings, we propose that developmental maturation of GR feedback function is region specific. We suggest that during early postnatal development, glucocorticoid feedback is primarily regulated through pituitary GR, and that there is a switch during adolescence to PVN GR taking the role of the dominant negative feedback regulator. Forebrain GR may also contribute to this HPA axis regulation during early development, but there is little data to support this. Pituitary GR is not as involved in basal HPA axis regulation in adulthood (Schmidt et al. 2009), but PVN and forebrain GR play significant roles in modulating negative feedback during adulthood (Boyle et al. 2005, 2006, Laryea et al. 2013).
CONCLUSIONS
Taken together, our data indicate that PVN GR is important in maintaining basal plasma glucocorticoid concentrations from early adolescence onward, and it starts mediating stress-induced glucocorticoid secretion later during adulthood. Moreover, the maturation of PVN GR function appears to occur earlier in males than in females. Our findings suggest that GRs in specific brain and pituitary regions differ in developmental onset of their negative feedback function, with pituitary GR function beginning in early postnatal development and PVN GR picking up in adolescence through adulthood with input from forebrain GR. Our results lay a foundation for dissecting sex-mediated glucocorticoid-GR signaling along development to expose the mechanisms underlying sex and developmental differences in PVN GR function. Future studies would aim to identify the sex-specific effects of adolescent PVN GR loss on stress-related behavior such as anxiety and despair. These studies will help define combined role of gender and GR in the PVN in stress-related behavior.
Acknowledgments
We are grateful to Dr. Lisa Muglia and Dr. Melinda Arnett at Cincinnati Children’s Hospital Medical Center for reviewing the manuscript. We also thank the reviewers for providing insights that enhanced the quality of this manuscript.
Investigations described in this study were supported by NIH grant MH079010.
Footnotes
DECLARATION OF INTEREST: The authors have no conflicts of interest.
References
- Arnsten AFT, Shansky RM. Adolescence: vulnerable period for stress-induced prefrontal cortical function? Introduction to part IV. Ann N Y Acad Sci. 2004;1021:143–147. doi: 10.1196/annals.1308.017. [DOI] [PubMed] [Google Scholar]
- Babb JA, Masini CV, Day HEW, Campeau S. Stressor-specific effects of sex on HPA axis hormones and activation of stress-related neurocircuitry. Stress. 2013;16:664–677. doi: 10.3109/10253890.2013.840282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang C, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Boyle MP, Brewer JA, Funatsu M, Wozniak DF, Tsien JZ, Izumi Y, Muglia LJ. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci U S A. 2005;102:473–478. doi: 10.1073/pnas.0406458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle MP, Kolber BJ, Vogt SK, Wozniak DF, Muglia LJ. Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. J Neurosci. 2006;26:1971–1978. doi: 10.1523/JNEUROSCI.2173-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess LH, Handa RJ. Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology. 1992;131:1261–1269. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TJ, Blendy JA, Monaghan AP, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schutz G. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995;9:1608–1621. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- Durston S, Hulshoff Pol HE, Casey BJ, Giedd JN, Buitelaar JK, van Engeland H. Anatomical MRI of the developing human brain: what have we learned? J Am Acad Child Adolesc Psychiatry. 2001;40:1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- Erdmann G, Schütz G, Berger S. Loss of glucocorticoid receptor function in the pituitary results in early postnatal lethality. Endocrinology. 2008;149:3446–3451. doi: 10.1210/en.2007-1786. [DOI] [PubMed] [Google Scholar]
- Furay AR, Bruestle AE, Herman JP. The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology. 2008;149:5482–5490. doi: 10.1210/en.2008-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. The teen brain: insights from neuroimaging. J Adolesc Health. 2008;42:335–343. doi: 10.1016/j.jadohealth.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Weiser MJ. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front Neuroendocrinol. 2014;35:197–220. doi: 10.1016/j.yfrne.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, McKlveen JM, Solomon MB, Carvalho-Netto E, Myers B. Neural regulation of the stress response: glucocorticoid feedback mechanisms. Braz J Med Biol Res. 2012;45:292–298. doi: 10.1590/S0100-879X2012007500041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil B, Leite CM, Carvalho-Lima M, Anselmo-Franci JA. Role of sex steroids in progesterone and corticosterone response to acute restraint stress in rats: sex differences. Stress. 2013;16:452–460. doi: 10.3109/10253890.2013.777832. [DOI] [PubMed] [Google Scholar]
- Kolber BJ, Boyle MP, Wieczorek L, Kelley CL, Onwuzurike CC, Nettles Sa, Vogt SK, Muglia LJ. Transient early-life forebrain corticotropin-releasing hormone elevation causes long-lasting anxiogenic and despair-like changes in mice. J Neurosci. 2010;30:2571–2581. doi: 10.1523/JNEUROSCI.4470-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laryea G, Schütz G, Muglia LJ. Disrupting hypothalamic glucocorticoid receptors causes HPA axis hyperactivity and excess adiposity. Mol Endocrinol. 2013;27:1655–1665. doi: 10.1210/me.2013-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. The ontogeny of the hypothalamic-pituitary-adrenal axis. The influence of maternal factors. Ann N Y Acad Sci. 1994;746:275–293. doi: 10.1111/j.1749-6632.1994.tb39245.x. [DOI] [PubMed] [Google Scholar]
- Lightman SL, George CL. Nat Rev Endocrinol. Vol. 10. Nature Publishing Group; 2014. Steroid hormones in 2013: Glucocorticoids--timing, binding and environment; pp. 71–72. [DOI] [PubMed] [Google Scholar]
- Mataradze GD, Kurabekova RM, Rozen VB. The role of sex steroids in the formation of sex-differentiated concentrations of corticosteroid-binding globulin in rats. J Endocrinol. 1992;132:235–240. doi: 10.1677/joe.0.1320235. [DOI] [PubMed] [Google Scholar]
- Mattos GE, Heinzmann J-M, Norkowski S, Helbling J-C, Minni AM, Moisan M-P, Touma C. Corticosteroid-binding globulin contributes to the neuroendocrine phenotype of mice selected for extremes in stress reactivity. J Endocrinol. 2013;219:217–229. doi: 10.1530/JOE-13-0255. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav. 2007;86:220–233. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Sapolsky RM, McEwen BS. The development of the glucocorticoid receptor system in the rat limbic brain. I. Ontogeny and autoregulation. Brain Res. 1985;350:159–164. doi: 10.1016/0165-3806(85)90259-7. [DOI] [PubMed] [Google Scholar]
- Miranda TB, Voss TC, Sung M-H, Baek S, John S, Hawkins M, Grøntved L, Schiltz RL, Hager GL. Reprogramming the chromatin landscape: interplay of the estrogen and glucocorticoid receptors at the genomic level. Cancer Res. 2013;73:5130–5139. doi: 10.1158/0008-5472.CAN-13-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell GM, Henley DE, Leendertz J, Douthwaite JA, Wood SA, Stevens A, Woltersdorf WW, Peeters BWMM, Ruigt GSF, White A, Veldhuis JD, Lightman SL. Rapid glucocorticoid receptor-mediated inhibition of hypothalamic-pituitary-adrenal ultradian activity in healthy males. J Neurosci. 2010;30:6106–6115. doi: 10.1523/JNEUROSCI.5332-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schindler AG, Tsutsui KT, Clark JJ. Chronic Alcohol Intake During Adolescence, but not Adulthood, Promotes Persistent Deficits in Risk-Based Decision Making. Alcohol Clin Exp Res. 2014;38:1622–1629. doi: 10.1111/acer.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MV, Enthoven L, van der Mark M, Levine S, de Kloet ER, Oitzl MS. The postnatal development of the hypothalamic–pituitary–adrenal axis in the mouse. Int J Dev Neurosci. 2003;21:125–132. doi: 10.1016/s0736-5748(03)00030-3. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Schmidt M, Levine S, Oitzl MS, van der Mark M, Müller MB, Holsboer F, de Kloet ER. Glucocorticoid receptor blockade disinhibits pituitary-adrenal activity during the stress hyporesponsive period of the mouse. Endocrinology. 2005;146:1458–1464. doi: 10.1210/en.2004-1042. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Sterlemann V, Wagner K, Niederleitner B, Ganea K, Liebl C, Deussing JM, Berger S, Schütz G, Holsboer F, Müller MB. Postnatal glucocorticoid excess due to pituitary glucocorticoid receptor deficiency: differential short- and long-term consequences. Endocrinology. 2009;150:2709–2716. doi: 10.1210/en.2008-1211. [DOI] [PubMed] [Google Scholar]
- Schroeder M, Sultany T, Weller A. Prenatal stress effects on emotion regulation differ by genotype and sex in prepubertal rats. Dev Psychobiol. 2013;55:176–192. doi: 10.1002/dev.21010. [DOI] [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL. Postnatal masculinization alters the HPA axis phenotype in the adult female rat. J Physiol. 2005a;563:265–274. doi: 10.1113/jphysiol.2004.078212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Lightman SL, Harbuz MS. Endocrinology. Vol. 146. Endocrine Society; 2005b. Organizational role for testosterone and estrogen on adult hypothalamic-pituitary-adrenal axis activity in the male rat; pp. 1973–1982. [DOI] [PubMed] [Google Scholar]
- Solomon MB, Furay AR, Jones K, Packard AEB, Packard BA, Wulsin AC, Herman JP. Neuroscience. Vol. 203. Elsevier Inc; 2012. Deletion of forebrain glucocorticoid receptors impairs neuroendocrine stress responses and induces depression-like behavior in males but not females; pp. 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends Cogn Sci. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Tinnikov AA. Responses of serum corticosterone and corticosteroid-binding globulin to acute and prolonged stress in the rat. Endocrine. 1999;11:145–150. doi: 10.1385/ENDO:11:2:145. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Wilson ME. Dihydrotestosterone differentially modulates the cortisol response of the hypothalamic-pituitary-adrenal axis in male and female rhesus macaques, and restores circadian secretion of cortisol in females. Brain Res. 2012;1429:43–51. doi: 10.1016/j.brainres.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schütz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Reichardt HM, Schütz G. Genetic dissection of glucocorticoid receptor function in mice. Curr Opin Genet Dev. 1998;8(5):532–538. doi: 10.1016/s0959-437x(98)80007-5. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, Carpenter LL. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biol Psychiatry. 2008;63:1147–1154. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Sharma S, Meaney MJ. Changes in plasma adrenocorticotropin, corticosterone, corticosteroid-binding globulin, and hippocampal glucocorticoid receptor occupancy/translocation in rat pups in response to stress. J Neuroendocrinol. 1996;8:1–8. doi: 10.1111/j.1365-2826.1996.tb00680.x. [DOI] [PubMed] [Google Scholar]
- Wang M. The role of glucocorticoid action in the pathophysiology of the Metabolic Syndrome. Nutr Metab (Lond) 2005;2:3. doi: 10.1186/1743-7075-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MJ, Foradori CD, Handa RJ. Brain Res. Vol. 1336. Elsevier B.V; 2010. Estrogen receptor beta activation prevents glucocorticoid receptor-dependent effects of the central nucleus of the amygdala on behavior and neuroendocrine function; pp. 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009;159:883–895. doi: 10.1016/j.neuroscience.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler AL, Lerch JP, Chakravarty MM, Friedel M, Sled JG, Fletcher PJ, Josselyn SA, Frankland PW. Adolescent cocaine exposure causes enduring macroscale changes in mouse brain structure. J Neurosci. 2013;33:1797–1803a. doi: 10.1523/JNEUROSCI.3830-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi SJ, Masters JN, Baram TZ. Effects of a specific glucocorticoid receptor antagonist on corticotropin releasing hormone gene expression in the paraventricular nucleus of the neonatal rat. Brain Res Dev Brain Res. 1993;73:253–259. doi: 10.1016/0165-3806(93)90145-z. [DOI] [PubMed] [Google Scholar]