Abstract
Unipolar depression has been characterized as involving diminished approach motivation and reward sensitivity. A psychophysiological indicator of approach motivation involves an asymmetry in frontal electroencephalographic (EEG) activity, such that greater left relative to right frontal cortical activity indicates increased approach motivation. Consistent with the perspective of reduced approach motivation tendencies, depression has been associated with decreased relative left frontal cortical activity. To date, supporting research has primarily relied on categorical diagnoses or composite symptom counts. However, given the heterogeneity in depression, it is unclear what specific symptom dimensions relate to decreased relative left frontal cortical activity. The present study examined the association between multiple depression symptom dimensions and asymmetrical frontal cortical activity while anticipating reward in separate undergraduate (n = 75) and clinical samples (current major depressive disorder [n = 68] and never depressed controls [n = 67]). All participants completed the Inventory of Depression and Anxiety Symptoms, a self-report measure of factor-analytically derived symptom dimensions. Frontal cortical activity was assessed during a computerized slot machine task while participants anticipated potential monetary reward or no incentive. In undergraduates with low depression symptoms and never depressed controls, reward trials relative to no incentive trials elicited greater relative left frontal cortical activity. Furthermore, in both samples across all participants, increased dysphoria and lassitude symptoms were associated with decreased relative left frontal cortical activity while anticipating reward. The present study suggests that depression symptoms consistent with motivational disengagement are associated with decreased relative left frontal cortical activity.
Keywords: asymmetry, depression, dysphoria, EEG, lassitude, reward
Introduction
Abnormalities in approach motivation and reward sensitivity have long been considered fundamental features of depression (Klein, 1974; Meehl, 1975). A psychophysiological indicator of approach motivation tendencies is an asymmetry in electroencephalographic (EEG) activity between left and right frontal brain regions (Allen, Coan, & Nazarian, 2004; Coan & Allen, 2004).1 Although several psychobiological models have been proposed for asymmetrical frontal cortical activity, it has often been explained using the approach-withdrawal model (Davidson, 1992, 1998), which proposes that there are two separate systems of behavior and motivation.2 The approach system controls appetitive behavior and approach motivation and is implemented by a neural circuit incorporating left frontal regions. The withdrawal system underlies behavioral inhibition and avoidance and is implemented by a neural circuit incorporating right frontal regions. According to the model, individual differences in motivational tendencies are measured by examining the relative difference in cortical activity between the left and right hemispheres, such that greater left relative to right frontal cortical activity indicates increased approach motivation.
The approach-withdrawal model proposes that depression is associated with decreased relative left frontal cortical activity (Davidson, 1992, 1998). Indeed, a number of studies have found that, relative to controls, individuals with unipolar depressive disorders (e.g., Bruder et al., 1997; Henriques & Davidson, 1991) and increased depressive symptoms (e.g., Diego, Field, & Hernandez-Reif, 2001; Feng et al., 2012; see Thibodeau, Jorgensen, & Kim, 2006 for a meta-analysis) exhibit decreased relative left frontal cortical activity at rest. These results support the hypothesis that depression is associated with decreased approach-motivation tendencies. However, there have been inconsistencies and null findings in the literature (e.g., Quinn, Rennie, Harris, & Kemp, 2014; Reid, Duke, & Allen, 1998).
One issue that may contribute to the mixed results is the measurement of frontal cortical activity while at rest. For example, one study found that frontal cortical activity measured during an emotional challenge, compared to while at rest, produced a stronger relationship with criterion measures (Coan, Allen, & McKnight, 2006). To further address this issue, Shankman and colleages (2007) examined frontal cortical activity during a computerized slot machine task designed to elicit approach motivation in depressed and control participants. Results indicated that adults with childhood or adolescent (i.e., early) onset depression exhibited decreased relative left frontal cortical activity compared to controls and adult onset depressives, who did not differ. Importantly, there were no group differences in relative left frontal cortical activity when measured at rest. Other studies have found similar results using different emotional challenges (Stewart, Coan, Towers, & Allen, 2014), suggesting that the association between depression and relative left frontal cortical activity is strongest during the manipulation of approach motivation.
Another issue is the heterogeneity of depression, such that two individuals who receive a depression diagnosis can present with very different symptom profiles (Mineka, Watson, & Clark, 1998). Most studies have examined categorical diagnoses or composite symptom counts, making it difficult to understand what particular aspects of depression are associated with decreased relative left frontal cortical activity. Theoretical models of depression provide an ideal foundation to better understand this unanswered question. For example, the tripartite model proposes that depression is characterized by high negative affect and low positive affect (Clark & Watson, 1991; Clark, Watson, & Mineka, 1994; Watson, 2009), which have both been associated with decreased relative left frontal cortical activity (Gollan et al., 2014; Tomarken, Davidson, & Henriques, 1990; Tomarken, Davidson, Wheeler, & Doss, 1992; Wheeler, Davidson, & Tomarken, 1993). Therefore, it is possible that depression symptoms consistent with negative and positive affective disturbances will be associated with decreased relative left frontal cortical activity. However, there are multiple problems with this valence-based (i.e., positive vs. negative affect) explanation. First, anger, a negative valence emotion that occurs from the disruption of approach-motivated states, has been associated with increased relative left frontal cortical activity (Harmon-Jones & Allen, 1998; Stewart et al., 2008). Second, relative left frontal cortical activity has been associated with both positive and negative approach-oriented motivational states (Poole & Gable, 2014).
An alternative approach is to use a motivational framework to explain the association between depression and decreased relative left frontal cortical activity (Carver & Harmon-Jones, 2009; Nusslock, Walden, & Harmon-Jones, 2015). The motivational perspective transverses emotional valence and instead emphasizes the motivational deficits and behavioral disengagement embedded in particular depression symptoms. To test this framework and better understand the relationship between depression and decreased relative left frontal cortical activity, we evaluated multiple depression symptom dimensions across separate undergraduate and clinical samples.
The present article examined the association between multiple depression symptom dimensions and asymmetrical frontal cortical activity while anticipating reward. To this end, participants completed the Inventory of Depression and Anxiety Symptoms (IDAS; Watson et al., 2007, 2008), a self-report measure of factor analytically-derived symptom dimensions. In regard to depression, the IDAS includes a dysphoria scale, which contains symptoms assessing depressed mood, loss of interest, worry, worthlessness, guilt, hopelessness, cognitive disturbance, and psychomotor disturbance. In addition to this broad factor, there are several specific symptom scales, including lassitude (i.e., anergia, fatigue, and hypersomnia), insomnia, suicidality, appetite loss, appetite gain, and (low) well-being (i.e., positive affect). It is important to note that all nine Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; American Psychiatric Association, 2000) diagnostic criteria for a major depressive episode are jointly captured by the IDAS scales dysphoria, lassitude, suicidality, appetite loss, and appetite gain. While we examined all the IDAS depression scales, we were primarily interested in the two symptom dimensions most consistent with motivational deficits—dysphoria and lassitude—as well as the positive affect dimension (well-being) as a test of the specificity of the hypotheses to motivational rather than strictly affective constructs.
The present article consisted of two separate samples. The first sample (Study 1) included 75 undergraduates with mild to moderate levels of depression symptoms. The second sample (Study 2) included a subset of community-dwelling participants from Shankman et al. (2013), which consisted of 135 adults with current major depressive disorder (MDD) (n = 68) or no lifetime history of a depressive disorder (n = 67). For Study 1, consistent with the motivational model outlined above (Carver & Harmon-Jones, 2009; Nusslock et al., 2015), we hypothesized that increased dysphoria and lassitude symptoms would be associated with decreased relative left frontal cortical activity while anticipating reward.
Study 1
Method
Participants
The sample included 75 Introduction to Psychology students who participated for course credit. Exclusion criteria were an inability to read or write in English, a history of head trauma with loss of consciousness, or being left-handed as confirmed by the Edinburgh Handedness Inventory (range of laterality quotient: +55 to +100; Oldfield, 1971). The sample was college-aged (M = 19.01, SD = 1.41), predominately female (75.6%), and ethnically diverse, including 34.9% Caucasian, 31.4% Asian, 25.6% Latino, 3.5% Black, and 4.8% “Other”. No participants were currently taking psychiatric medication. Informed consent was obtained prior to participation and all procedures were approved by the University of Illinois at Chicago's Institutional Review Board.
Measures
Inventory of Depression and Anxiety Symptoms
The IDAS (Watson et al., 2007) is a 64-item factor-analytically derived self-report inventory of empirically distinct dimensions of depression and anxiety symptoms. Each item assesses symptoms over the past two weeks on a five-point Likert scale ranging from 1 (not at all) to 5 (extremely). The IDAS provides a general depression scale (20 items), which is similar to most self-report depression measures (e.g., Beck Depression Inventory-II; Beck, Steer, & Brown, 1996; Center for Epidemiological Studies Depression Scale; Radloff, 1977), and specific content subscales, including dysphoria (10 items; e.g., “I felt depressed”, “I felt discouraged about things”), lassitude (6 items; “I felt exhausted”, “It took a lot of effort for me to get going”), insomnia (6 items; “I slept less than usual”, “I slept very poorly”), suicidality (6 items; “I had thoughts of suicide”, “I thoughts about hurting myself”), appetite loss (3 items; “I did not have much of an appetite”, “I felt like eating less than usual”), appetite gain (3 items; “I thought a lot about food”, “I ate more than usual”), and well-being (8 items; “I was proud of myself”, “I felt optimistic”). The general depression scale is a composite of the ten dysphoria items as well as two items each from the specific content subscales lassitude, insomnia, suicidality, appetite loss, and well-being (items are reverse-keyed). The IDAS has demonstrated good internal consistency, test-retest reliability, and convergent and discriminant validity with diagnoses and other self-report measures (Watson et al., 2007, 2008).
Procedure
After providing informed consent, participants completed the IDAS questionnaire. Next, the EEG electrodes were applied to the participant's head. Finally, participants were seated in an electronically shielded sound-attenuated booth approximately 3.5 ft. from a 19-in. computer monitor where they completed a slot machine task (described below).
Slot task and physiological recordings
A computerized slot machine task was used to assess frontal cortical activity while anticipating reward (Shankman et al., 2013, 2007). The task included three reels of fruit or numbers, which spun simultaneously for 11 s and then landed on a result. To start the reels spinning, participants pressed a button with both thumbs that pulled a lever on the computer screen. The task consisted of 60 spins that were divided into two possible outcomes (30 trials each) of a reward condition in which participants won money if the reels landed on three pieces of fruits, and a no incentive condition in which participants were ineligible to win money regardless of the outcome. Thus, the reward condition was designed to elicit anticipation of reward, and the no incentive condition served as a control for several aspects of the reward condition (e.g., anticipating an outcome, visual input). The amount of money that could be won during each reward trial ranged from $0.50 to $3.00. In both conditions participants did not lose money if the reels did not land on three pieces of fruit.
Trials were presented in a pseudorandom order, and there were never more than two consecutive trials of a similar type or outcome. Participants began the game with $2.00 and were told the specific condition (reward or no incentive) prior to each trial, but not the potential dollar amount in each reward condition. Unbeknownst to the participant, half of the trials in each condition landed on three pieces of fruit. Trials were divided into three blocks, and at the end of the task participants were given their winnings ($12.00) in cash.
EEG data were recorded from Ag/AgCl electrodes in a 64-channel stretch-lycra electrode cap (Compumedics Neuroscan 4.4, Charlotte, NC). The ground electrode was at the frontal pole (AFz) and the online reference was near the vertex (between Cz and CPz). Electromyography electrodes placed at the right supra- and infraorbital sites were used to monitor vertical eye movements (VEOG) and electrodes placed at the right and left outer canthi were used to monitor horizontal eye movements (HEOG). Electrode impedances were under 5,000 Ω, and homologous sites were within 1,500 Ω of each other. Data were recorded through a Neuroscan Synamp2 data acquisition system at a gain of 10K (5K for eye channels) with a bandpass of DC-200 Hz. Data were acquired and digitized continuously at a rate of 1,000 Hz. EEG data were re-referenced offline by computing a digitally derived linked mastoids reference using data from the left and right mastoid.
Physiological data processing
EEG data from the 11-s period while the slot machine reels were spinning were segmented into consecutive 1.024-s epochs every 0.512 s (50% overlap). After referencing to a linked mastoid reference offline and then applying a baseline correction, every epoch was visually inspected and was excluded from analyses if it was contaminated by blinks, eye movements, or movement-related artifacts. The EEG was tapered over the entire 1.024-s epoch by a Hanning window to suppress spectral side lobes. Artifact-free data were recovered in adjacent (overlapping) epochs and power spectra were computed offline from EEG data by using a fast Fourier transform. Subsequently, the average absolute alpha power was computed for each electrode and then natural log transformed to normalize the data. Consistent with previous studies (Bruder et al., 1997), the alpha band was defined as 7.81–12.70 Hz and used as an inverse measure of regional brain activity. Shankman et al. (2013) found a statistical trend suggesting that the MDD diagnostic group difference was most pronounced in lateral electrodes (i.e., F7 and F8); therefore, the present study focused on the electrodes F7 and F8.
Data Analysis
The slot machine task was designed to elicit greater relative left frontal cortical activity during the reward trials compared to the no incentive trials. For depression, we expected that decreased relative left frontal cortical activity would be demonstrated via a relative right frontal cortical asymmetry (i.e., greater cortical activity in the right relative to left hemisphere). However, decreased relative left frontal cortical activity could also be demonstrated via symmetrical cortical activity between left and right hemispheres. Thus, across all participants the opposing asymmetries of nondepressed (relative left frontal cortical activity) and depressed (relative right frontal cortical activity or symmetrical frontal cortical activity) participants may cancel each other out, making it difficult to determine whether the slot task successfully elicited approach motivation. Therefore, as a manipulation check to determine whether the slot task successfully impacted frontal cortical activity, a Condition (no incentive vs. reward) × Hemisphere (left [F7] vs. right [F8]) repeated-measures analysis of variance (ANOVA) was conducted in participants with low IDAS general depression scores, determined via a median split (median = 40). To follow-up a Condition × Hemisphere interaction, difference scores were calculated by subtracting alpha power during the reward condition from the no incentive condition (i.e., no incentive alpha power – reward alpha power), with greater (i.e., more positive) values indicating increased brain activity during the reward condition, and comparing the left and right hemispheres on these difference scores.
For the depression symptom analyses, across all participants asymmetry scores were computed by subtracting alpha power at the left frontal electrode from alpha power at the homologous right frontal electrode (i.e., F8 – F7), so that higher values reflected greater activity in left relative to right frontal regions (i.e., relative left frontal cortical activity). Difference scores were then computed by subtracting relative left frontal cortical activity during the no incentive condition from the reward condition (i.e., reward trials relative left frontal cortical activity – no incentive trials relative left frontal cortical activity). The difference score helped control for the presentation of a stimulus (during the no incentive condition), with the resulting difference representing relative left frontal cortical activity while anticipating potential reward. Pearson's correlations were conducted to examine the bivariate association between depression symptom dimensions and relative left frontal cortical activity while anticipating reward. To control for multiple comparisons, the Benjamini-Hochberg procedure was applied to reduce false discovery rate (Benjamini & Hochberg, 1995). Multiple regression was also used to examine the unique association between depression symptom dimensions and relative left frontal cortical activity. All analyses were conducted in IBM SPSS Statistics, Version 22.0 (Armonk, NY, USA).
Results
Slot Machine Task
Across all participants, there was an average of 364.03 (SD = 121.37) artifact-free trials during the reward condition and 345.70 (SD = 119.65) artifact-free trials during the no incentive condition. The first analysis was a manipulation check of the task effect and tested whether frontal cortical activity differed between the reward and no incentive trials in participants with low IDAS general depression. Results indicated a main effect of hemisphere, F(1, 39) = 5.33, p = .026, ηp2 = .12, which was qualified by a Condition × Hemisphere interaction, F(1, 39) = 4.27, p = .045, ηp2 = .10. As shown in Figure 1 (top), the reward condition elicited greater brain activity in the left relative to right hemisphere, suggesting that, in participants with low levels of depression, the reward condition elicited increased approach motivation.
Figure 1.
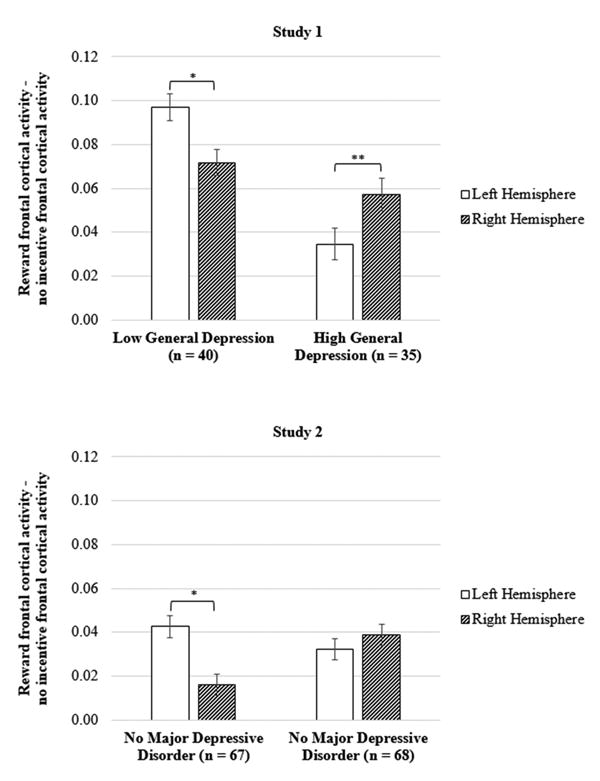
Frontal cortical activity while anticipating reward during the slot machine task for participants in Study 1 (top) and Study 2 (bottom). Participants with low versus high IDAS general depression scores in Study 1 were determined via a median split (median = 40). The y-axis represents increased frontal cortical activity during the reward relative to no incentive condition (i.e., reward – no incentive), and separate bars are presented for the left and right hemispheres. IDAS = Inventory of Depression and Anxiety Symptoms. * p < .05, ** p < .01
Depression Symptom Dimensions
Table 1 (top) presents descriptive statistics and bivariate correlations between the IDAS subscales in the undergraduate sample. The IDAS subscales demonstrated no to moderate associations with each other. Table 2 (left) presents bivariate correlations between the IDAS subscales and relative left frontal cortical activity while anticipating reward. As shown in Table 2 (left column), the composite general depression scale was negatively associated with relative left frontal cortical activity, such that greater depression symptom severity was associated with decreased approach motivation. For the IDAS depression subscales, as shown in Figure 2, both dysphoria and lassitude were negatively associated with relative left frontal cortical activity.3 Next, a multiple regression was conducted with dysphoria and lassitude entered as simultaneous independent variables to determine whether they were independently associated with relative left frontal cortical activity. Results indicated dysphoria and lassitude explained a significant proportion of variance in relative left frontal cortical activity, R2 = .11, F(2, 74) = 4.26, p = .018; however, neither dysphoria, t(72) = -1.36, β = -.22, ns, nor lassitude, t(72) = -0.86, β = -.14, ns, were independently associated with relative left frontal cortical activity.4
Table 1.
Descriptive statistics and Pearson's r correlation coefficients for the Inventory of Depression and Anxiety Symptoms subscales in the undergraduate (top) and clinical (bottom) samples
| Undergraduate Sample (N=75) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| 1. General Depression | - | .93 | .71 | .75 | .47 | .61 | .22 | -.37 | |
| 2. Dysphoria | - | .68 | .62 | .38 | .41 | .30 | -.29 | ||
| 3. Lassitude | - | .53 | .12 | .42 | .17 | -.10 | |||
| 4. Insomnia | - | .34 | .50 | .10 | -.08 | ||||
| 5. Suicidality | - | .41 | .25 | -.08 | |||||
| 6. Appetite Loss | - | .04 | -.10 | ||||||
| 7. Appetite Gain | - | .08 | |||||||
| 8. Well-Being | - | ||||||||
|
| |||||||||
| M | 42.49 | 21.64 | 14.39 | 12.65 | 7.09 | 4.87 | 6.95 | 27.09 | |
| SD | 12.65 | 7.66 | 4.55 | 5.01 | 2.74 | 3.15 | 3.00 | 7.25 | |
| Cronbach's α | .90 | .87 | .69 | .78 | .80 | .95 | .75 | .90 | |
|
| |||||||||
| Clinical Sample (N=135) | |||||||||
|
| |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
|
| |||||||||
| 1. General Depression | - | .97 | .85 | .77 | .61 | .65 | .37 | -.77 | |
| 2. Dysphoria | - | .82 | .69 | .59 | .54 | .44 | -.71 | ||
| 3. Lassitude | - | .57 | .52 | .48 | .29 | -.59 | |||
| 4. Insomnia | - | .35 | .55 | .36 | -.53 | ||||
| 5. Suicidality | - | .31 | .21 | -.42 | |||||
| 6. Appetite Loss | - | -.04 | -.50 | ||||||
| 7. Appetite Gain | - | -.21 | |||||||
| 8. Well-Being | - | ||||||||
|
| |||||||||
| M | 45.67 | 22.79 | 13.65 | 13.45 | 7.39 | 5.22 | 5.73 | 22.08 | |
| SD | 18.82 | 11.12 | 5.71 | 6.96 | 2.76 | 3.16 | 3.04 | 8.62 | |
| Cronbach's α | .96 | .95 | .82 | .91 | .77 | .96 | .86 | .95 | |
Note. IDAS general depression is a composite of items from the specific content subscales dysphoria, lassitude, insomnia, suicidality, appetite loss, appetite gain, and well-being. Bolded correlation coefficients were significant at p < .05. M = mean; SD = standard deviation.
Table 2.
Pearson's r correlation coefficients for the Inventory of Depression and Anxiety Symptoms subscales and relative left frontal cortical activity while anticipating reward
| IDAS Scale | Relative Left Frontal Cortical Activity | |||
|---|---|---|---|---|
|
| ||||
| Undergraduate Sample (N = 75) | Clinical Sample (N = 135) | |||
| r | p | r | p | |
| General Depression | -.298 | .009* | -.197 | .022* |
| Dysphoria | -.311 | .007* | -.213 | .013* |
| Lassitude | -.288 | .012* | -.194 | .024* |
| Insomnia | -.150 | .198 | -.135 | .119 |
| Suicidality | -.018 | .881 | -.035 | .686 |
| Appetite Loss | -.168 | .149 | -.050 | .564 |
| Appetite Gain | -.110 | .347 | -.153 | .077 |
| Well-Being | .010 | .932 | .116 | .182 |
Note. IDAS general depression is a composite of items from the specific content subscales dysphoria, lassitude, insomnia, suicidality, appetite loss, appetite gain, and well-being. Relative left frontal cortical activity during the no incentive condition was subtracted from the reward condition (i.e., reward – no incentive), with the resulting difference representing approach motivation while anticipating reward. IDAS = Inventory of Depression and Anxiety Symptoms.
= p-value statistically significant after using the Benjamini-Hochberg procedure to control for false discovery rate.
Figure 2.
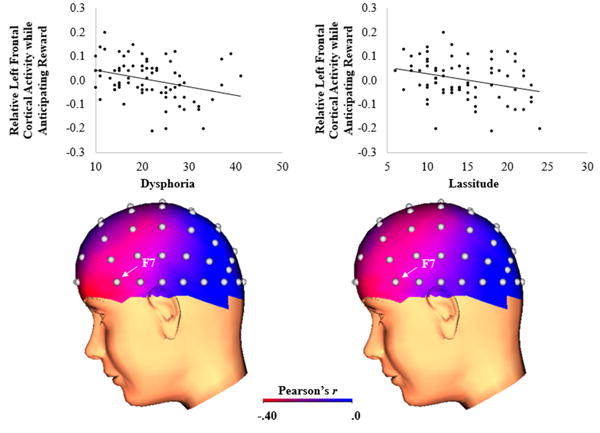
Scatterplots (top) and three-dimensional correlation coefficient head maps (bottom) depicting the association between IDAS dysphoria (left) and lassitude (right) symptom dimensions and relative left frontal cortical activity while anticipating reward relative to no incentive (i.e., reward – no incentive) in the undergraduate sample (N = 75). In the scatterplots, the y-axis represents increased relative left frontal cortical activity while anticipating reward. In the correlation coefficient head maps, brighter colors represent a stronger negative correlation between the symptom dimension and relative left frontal cortical activity while anticipating reward. IDAS = Inventory of Depression and Anxiety Symptoms.
The IDAS dysphoria subscale contains one item that assesses anhedonia (“I had little interest in my usual hobbies and activities”), which is conceptually similar to low well-being (i.e., positive affect). Therefore, we re-calculated the dysphoria subscale while excluding this anhedonia item and examined whether it was associated with relative left frontal cortical activity while anticipating reward. Results indicated that the modified dysphoria subscale was again negatively associated with relative left frontal cortical activity, r(75) = -.33, p = .004.
Discussion
Study 1 indicated that greater IDAS general depression was associated with decreased relative left frontal cortical activity while anticipating reward. Furthermore, examination of the specific content subscales revealed that this relationship was primarily due to dysphoria and lassitude. Interestingly, there was no association between well-being (i.e., positive affect) and relative left frontal cortical activity. Together, these results are consistent with previous studies identifying an association between depression and relative left frontal cortical activity (Thibodeau et al., 2006), and provide novel evidence that this relationship is driven by depression symptoms consistent with motivational disengagement.
A limitation of Study 1 is the use of an undergraduate sample that had, on average, a low level of general depression (M = 42.49, SD = 12.65) compared to a psychiatric sample (M = 56.04, SD = 15.42; Watson et al., 2007). Study 2 attempted to replicate Study 1 and extend the findings to a sample of 135 adults with current MDD (n = 68) or no lifetime history of a depressive disorder (n = 67). Similar to the results for Study 1, we hypothesized that greater general depression would be associated with decreased relative left frontal cortical activity while anticipating reward, and this relationship would be primarily due to the dysphoria and lassitude symptom dimensions.
Study 2
Participants
The sample included 135 of the 191 participants from Shankman et al. (2013) who completed the IDAS and slot machine task. Participants who did versus did not complete the IDAS were comparable on all clinical and demographic variables. The sample for the present study consisted of 68 individuals with current MDD and 67 individuals with no lifetime history of a depressive disorder. As part of the primary aims of Shankman et al., both of these groups contained participants with current panic disorder (64.7% and 29.9%, respectively). Panic disorder participants were allowed to meet criteria for other anxiety disorders, which included current or lifetime diagnoses of generalized anxiety disorder (n = 4), obsessive-compulsive disorder (n = 9), posttraumatic stress disorder (n = 16), social anxiety disorder (n = 14), and specific phobia (n = 12). Axis I diagnoses were determined via the Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 1997) which were conducted by either a Ph.D. level clinical psychologist or advanced clinical psychology doctoral students.
Depressed participants were required to have an age of onset of first affective disorder (dysthymia or MDD) before age 18 as Shankman et al. (2007) found that it was only those with an early onset depression who exhibited decreased relative left frontal cortical activity during the slot task. Exclusion criteria were a lifetime diagnosis of a psychotic disorder, bipolar disorder, or dementia; inability to read or write in English; history of head trauma with loss of consciousness; or left-handedness (range of Edinburgh Handedness Inventory laterality quotient: +20 to +100; Oldfield, 1971). Participants were recruited from the community (via flyers, Internet postings, etc.) and local mental health clinics. Informed consent was obtained prior to participation and all procedures were approved by the University of Illinois at Chicago's Institutional Review Board.
The sample of 135 participants had a mean age of 32.69 years (SD = 12.62) and was predominately female (61.5%). Participant racial/ethnic background was 45.3% Caucasian, 26.4% Black, 9.5% Latino, and 18.9% Asian. Educational history included 2.0% with 7 to 12 years of education (without graduating high school), 4.7% with a high school or GED diploma, 41.2% with some college education, 33.8% with a bachelor's degree, 11.5% with part graduate/professional education, and 6.8% with completed graduate/professional education.
Procedure
The IDAS, slot task, physiological recording and processing, and data analysis for Study 2 were identical to Study 1. The present study conducted analyses that were independent of the categorical diagnostic group differences reported in Shankman et al. (2013).
Results
Slot Machine Task
Similar to Study 1, the first analysis for Study 2 was a manipulation check and tested whether frontal brain activity differed between the reward trials and no incentive trials. These analyses were limited to participants with no lifetime history of a depressive disorder so that we could test whether the slot task successfully impacted frontal cortical activity. Results indicated a main effect of condition, F(1, 66) = 4.34, p = .041, ηp2 = .06, which was qualified by a Condition × Hemisphere interaction, F(1, 66) = 7.20, p = .009, ηp2 = .10. As shown in Figure 1 (right), the reward condition elicited greater brain activity in the left relative to right hemisphere, suggesting that the reward condition increased approach motivation.
Depression Symptom Dimensions
Table 1 (bottom) presents descriptive statistics and bivariate correlations between the IDAS subscales in the clinical sample. The IDAS subscales demonstrated moderate to strong relationships with each other. Table 2 (right) displays the bivariate correlations between the IDAS subscales and relative left frontal cortical activity while anticipating reward. The Study 2 clinical sample produced an identical pattern of results as the Study 1 undergraduate sample. As shown in Figure 1 (right column), the composite IDAS depression scale was negatively associated with relative left frontal cortical activity, such that greater depression symptom severity was associated with decreased approach motivation. For the IDAS depression subscales, as shown in Figure 3, both dysphoria and lassitude were negatively associated with relative left frontal cortical activity. A multiple regression with dysphoria and lassitude entered as simultaneous independent variables explained a significant proportion of variance in relative left frontal cortical activity, R2 = .05, F(2, 132) = 3.21, p = .044. However, neither dysphoria, t(132) = -1.10, β = -.16, ns, nor lassitude, t(132) = -0.39, β = -.06, ns, were independently associated with relative left frontal cortical activity.
Figure 3.
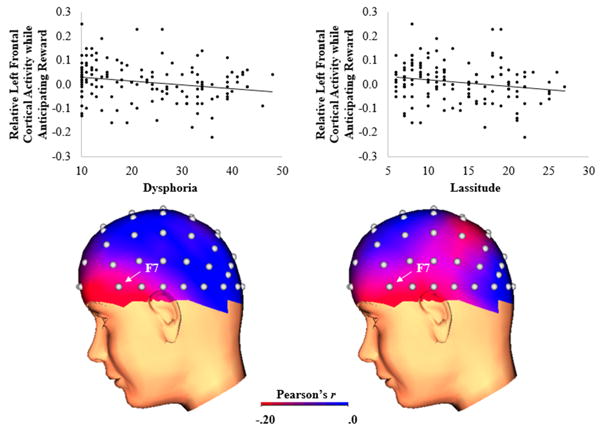
Scatterplots (top) and three-dimensional correlation coefficient head maps (bottom) depicting the association between IDAS dysphoria (left) and lassitude (right) symptom dimensions and relative left frontal cortical activity while anticipating reward relative to no incentive (i.e., reward – no incentive) in the clinical sample (N = 135). In the scatterplots, the y-axis represents increased relative left frontal cortical activity while anticipating reward. In the correlation coefficient head maps, brighter colors represent a stronger negative correlation between the symptom dimension and relative left frontal cortical activity while anticipating reward. IDAS = Inventory of Depression and Anxiety Symptoms.
Similar to Study 1, we re-calculated the dysphoria subscale while excluding the single anhedonia item and examined whether it was associated with relative left frontal cortical activity. Results indicated that the modified dysphoria subscale was again negatively associated with relative left frontal cortical activity, r(135) = -.21, p = .013.
Discussion
Study 2 replicated the results of Study 1 in a clinical sample. Specifically, greater IDAS general depression was associated with decreased relative left frontal cortical activity while anticipating reward, and this relationship was primarily due to the dysphoria and lassitude symptom dimensions.
General Discussion
The present study examined the association between multiple depression symptom dimensions and relative left frontal cortical activity while anticipating reward. Across separate undergraduate and clinical samples, greater overall depression symptom severity was associated with decreased relative left frontal cortical activity. Examination of the specific content scales indicated that this relationship was primarily due to the dysphoria and lassitude symptoms. In contrast, there was no association between well-being (i.e., positive affect) and relative left frontal cortical activity. Together, these results suggest that depression symptoms consistent with motivational disengagement, rather than just affective disturbance, are associated with decreased relative left frontal cortical activity (Carver & Harmon-Jones, 2009; Nusslock et al., 2015).
Previous studies on depression and relative left frontal cortical activity have identified inconsistent and, at times, null results (e.g., Quinn et al., 2014; Reid et al., 1998). The present study suggests there are at least two factors that may have contributed to these mixed findings. Specifically, one factor is whether relative left frontal cortical activity is measured at rest or during a task (Coan et al., 2006). Frontal cortical activity has most frequently been examined while at rest, an approach consistent with a dispositional model that emphasizes measuring emotional and motivation tendencies while minimizing situational demands. In contrast, measuring relative left frontal cortical activity during an approach-oriented task is consistent with a capability model, which aims to assess the degree to which individuals are capable of approach motivation. Across two separate samples, we found an association between depression and relative left frontal cortical activity during the anticipation of potential reward compared to the anticipation of no incentive. These results are consistent with previous investigations suggesting that the relationship between relative left frontal cortical activity and criterion measures is strongest during the manipulation of approach motivation (Shankman et al., 2007; Stewart et al., 2014). A second important factor is the heterogeneity of depression. Indeed, we found that relative left frontal cortical activity was only associated with specific symptom clusters of depression (dysphoria and lassitude). In previous investigations, the focus on broad categorical diagnoses or composite symptom counts may have obscured underlying relationships between relative left frontal cortical activity and depression symptoms consistent with motivational disengagement.
The present study supports theoretical models of biobehavioral motivational systems that suggest both the approach/appetitive and avoidance/withdrawal systems can give rise to positive or negative emotions, depending on how well the action is serving the motive (Carver, 2000; Higgins, Shah, & Friedman, 1997). For example, the emotion sadness can arise from failing to approach a reward, whereas elation can stem from successfully avoiding a threat. Thus, it is possible that individuals who demonstrate decreased relative left frontal cortical activity are less inclined to put forth the appropriate amount of effort necessary to obtain an incentive, increasing the likelihood of failure experiences, and subsequently eliciting feelings of despondency and sadness.
In regard to the positive valence system, we found no association between relative left frontal cortical activity and low well-being (i.e., positive affect). Reward anticipation and hedonic reactions are complex processes that involve multiple psychological components (Berridge & Robinson, 2003; Schultz, 2006). It is possible that relative left frontal cortical activity and the IDAS well-being scale indexed nonoverlapping aspects of the broader positive valence system, further supporting the notion that relative left frontal cortical activity is related to the motivational, rather than just affective, disturbance. It is important to note that the IDAS contains a single anhedonia item that loads onto the dysphoria scale (Watson et al., 2007), and the association between dysphoria and relative left frontal cortical activity remained significant even after excluding the single anhedonia item. Future studies should consider alternative approaches toward assessing anhedonia to better understand the relationship between relative left frontal cortical activity and psychopathology (Rizvi, Pizzagalli, Sproule, & Kennedy, 2016).
Depression and decreased relative left frontal cortical activity is often interpreted in the context of a diathesis-stress model (Coan & Allen, 2004; Davidson, 1998). The diathesis-stress perspective suggests that individuals with decreased relative left frontal cortical activity are less likely to engage in approach-oriented behaviors or pursue pleasurable activities, which buffer against the effects of stress on affect and motivation. While this investigation only focused on cross-sectional analyses, there is growing evidence that decreased relative left frontal cortical activity is a risk factor for the development of depression. Specifically, decreased relative left frontal cortical activity has been observed in the offspring of depressed individuals who have never experienced a depressed episode (Dawson, Frey, Panagiotides, Osterling, & Hessl, 1997; Goldstein et al., 2016; Tomarken, Dichter, Garber, & Simien, 2004), associated with genetic risk for depression (Bismark et al., 2010), and shown to prospectively predict increased depressive symptoms (Pössel, Lo, Fritz, & Seemann, 2008) and first-onset depression (Nusslock et al., 2011). In addition, a recent investigation found that decreased relative left frontal cortical activity interacted with stressful life events to predict increased internalizing symptoms in children at familial risk for depression (Lopez-Duran, Nusslock, George, & Kovacs, 2012).
Decreased relative left frontal cortical activity has also been identified as a potential target for prevention and treatment efforts. Indeed, mindfulness meditation has been shown to increase relative left frontal cortical activity (Barnhofer et al., 2007; Keune et al., 2013; although see Keune et al., 2011), and biofeedback training using frontal cortical activity has been shown to influence subsequent emotional responses (Allen, Harmon-Jones, & Cavender, 2001). In addition, frontal cortical activity has been shown to predict treatment response, including mood improvement following cognitive restructuring (Deldin & Chiu, 2005), negative affect reduction after behavioral activation treatment (Gollan et al., 2014), and SSRI treatment response (Bruder et al., 2001).
The present study had several important limitations that warrant consideration. First, in line with previous research (Coan & Allen, 2004), we examined the relative difference between the left and right hemispheres (i.e., an asymmetry difference score). While relative left frontal cortical activity was examined during an approach motivation task (i.e., reward trials of a slot machine task), the use of an asymmetry difference score limits our ability to determine whether depression symptoms were associated with decreased approach and/or increased withdrawal motivation. Second, analyses focused on lateral frontal electrodes (F7/8), while some previous studies have examined medial frontal electrodes (e.g., F3/F4). It is important to note that there is still no clear consensus regarding which electrode pair optimally measures approach motivation tendencies. Third, in Study 2 a large number of participants had panic disorder, which has also been associated with decreased relative left frontal cortical activity (Wiedemann et al., 1999). Future studies should attempt to replicate the depression symptom dimension and frontal cortical activity findings in a more heterogeneous clinical sample that can better determine the potential role of comorbid anxiety. Finally, we only examined unipolar depression symptoms (largely due to the undergraduate sample in Study 1 and the fact that bipolar disorder was an exclusion criterion in Study 2). However, bipolar depression, a condition characterized by elevated approach motivation (Alloy & Abramson, 2010), has been associated with increased relative left frontal cortical activity (Harmon-Jones et al., 2008; Nusslock et al., 2012). In addition, attention-deficit/hyperactivity disorder, which is similarly regarded as a disorder of elevated approach motivation (Mitchell, 2010), has been associated with an increased relative left frontal cortical activity despite the presence of comorbid depression (Hale et al., 2009; Keune, Schönenberg, et al., 2011; Keune, Wiedemann, Schneidt, & Schönenberg, 2015). Future studies are needed to determine whether dysphoria symptoms in other forms of psychopathology are also related to relative left frontal cortical activity or whether there is a different pathophysiological mechanism.
A major tenet of the National Institute of Mental Health's Research Domain Criteria (RDoC; Insel et al., 2010; Sanislow et al., 2010) initiative is to understand biobehavioral dimensions (e.g., approach motivation) that span the entire normal to abnormal spectrum, rather than restricting definitions of psychopathology to categories such as those used in the DSM. Another recent initiative across psychological science is the increased emphasis on demonstrating the reproducibility of research findings (Open Science Collaboration, 2015). Consistent with both of these goals, the present study replicated the association between dysphoria and lassitude symptom dimensions and relative left frontal cortical activity across both undergraduate and clinical samples. Future research should continue to pursue these efforts in conjunction with other forms of psychopathology that have been associated with aberrations in approach motivation tendencies and reward system functioning (e.g., attention-deficit/hyperactivity disorder, bipolar disorder, schizophrenia, and substance use disorder).
Acknowledgments
Funding information: National Institute of Health grant R21MH080689 awarded to S.A.S. and the University of Illinois at Chicago Chancellor's Discovery Fund
Footnotes
Frontal cortical activity has most often been examined using alpha power as an inverse measure of cortical activity, such that greater alpha power over right relative to left frontal regions is traditionally thought to reflect increased left relative to right frontal brain activity. This perspective is supported by research indicating that alpha power is inversely associated with other measures of brain activity, including functional magnetic resonance imaging (Goldman, Stern, Engel, & Cohen, 2002), positron emission tomography (Oakes et al., 2004), and neuropsychological test performance (Davidson, Chapman, Chapman, & Henriques, 1990). However, the role of EEG alpha power has been challenged in recent years and more likely represents a change in neuronal communication rather than the absence of brain activity (Miller, Crocker, Spielberg, Infantolino, & Heller, 2013). In this manuscript, the terminology relative left frontal cortical activity is used to indicate increased brain activity in left relative to right frontal regions (i.e., increased alpha power in right relative to left frontal regions).
Increased relative left frontal cortical activity is not necessarily a unique indicator of approach motivation and has been affiliated with other psychological processes (e.g., cognitive control, inhibitory executive control; Grimshaw & Carmel, 2014; Parvaz, MacNamara, Goldstein, & Hajcak, 2012).
There is some evidence that women, relative to men, demonstrate stronger associations between depression and relative left frontal cortical activity (Miller et al., 2002; Smit, Posthuma, Boomsma, & De Geus, 2007; Stewart, Bismark, Towers, Coan, & Allen, 2010). Therefore, we conduction linear regressions and examined whether participant sex moderated the relationship between dysphoria and lassitude symptom dimensions and relative left frontal cortical activity while anticipating reward. Across both the undergraduate and clinical samples, results indicated no significant Dysphoria × Sex or Lassitude × Sex interactions (ps > .51).
Alternative theoretical models (e.g., valence-arousal model; Heller, Nitschke, Etienne, & Miller, 1997; Heller, 1993) propose that depression is also associated with abnormal posterior brain asymmetries, and previous studies have demonstrated that depression is associated with decreased relative right posterior cortical activity (Blackhart, Minnix, & Kline, 2006; Bruder et al., 1997; Kentgen et al., 2000; Mathersul, Williams, Hopkinson, & Kemp, 2008; Metzger et al., 2004; Rabe, Debener, Brocke, & Beauducel, 2005). To examine the specificity of our findings, we also examined the association between depression symptom dimensions and relative right posterior (P7/P8) cortical activity while anticipating reward (i.e., relative right posterior cortical activity during reward trials – relative right parietal cortical activity during no incentive trials). Across both the undergraduate and clinical samples, no depression symptom dimension was associated with relative right posterior cortical activity while anticipating reward (ps > .10).
Contributor Information
Brady D. Nelson, Department of Psychology, Stony Brook University, Stony Brook, New York
Ellen M. Kessel, Department of Psychology, Stony Brook University, Stony Brook, New York
Daniel N. Klein, Department of Psychology, Stony Brook University, Stony Brook, New York
Stewart A. Shankman, Department of Psychology, University of Illinois at Chicago, Chicago, IL
References
- Allen JJB, Coan JA, Nazarian M. Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biological Psychology. 2004;67:183–218. doi: 10.1016/j.biopsycho.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Allen JJB, Harmon-Jones E, Cavender JH. Manipulation of frontal EEG asymmetry through biofeedback alters self-reported emotional responses and facial EMG. Psychophysiology. 2001;38:685–693. doi: 10.1111/1469-8986.3840685. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY. The role of the behavioral approach system (BAS) in bipolar spectrum disorders. Current Directions in Psychological Science. 2010;19:189–194. doi: 10.1177/0963721410370292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. American Psychiatric Association 2000 [Google Scholar]
- Barnhofer T, Duggan D, Crane C, Hepburn S, Fennell MJV, Williams JMG. Effects of meditation on frontal alpha-asymmetry in previously suicidal individuals. Neuroreport. 2007;18:709–712. doi: 10.1097/WNR.0b013e3280d943cd. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck depression inventory-II. San Antonio, TX: Psychological Corporation 1996 [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57:289–300. doi: 10.2307/2346101. [DOI] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends in Neurosciences. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Bismark AW, Moreno FA, Stewart JL, Towers DN, Coan JA, Oas J, Allen JJB. Polymorphisms of the HTR1a allele are linked to frontal brain electrical asymmetry. Biological Psychology. 2010;83:153–158. doi: 10.1016/j.biopsycho.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackhart GC, Minnix JA, Kline JP. Can EEG asymmetry patterns predict future development of anxiety and depression? A preliminary study. Biological Psychology. 2006;72:46–50. doi: 10.1016/j.biopsycho.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Fong R, Tenke CE, Leite P, Towey JP, Stewart JE, Quitkin FM. Regional brain asymmetries in major depression with or without an anxiety disorder: A quantitative electroencephalographic study. Biological Psychiatry. 1997;41:939–948. doi: 10.1016/S0006-3223(96)00260-0. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Stewart JW, Tenke CE, McGrath PJ, Leite P, Bhattacharya N, Quitkin FM. Electroencephalographic and perceptual asymmetry differences between responders and nonresponders to an SSRI antidepressant. Biological Psychiatry. 2001;49:416–425. doi: 10.1016/s0006-3223(00)01016-7. doi:0.1016/S0006-3223(00)01016-7. [DOI] [PubMed] [Google Scholar]
- Carver CS. Negative affects deriving from the behavioral approach system. Emotion. 2000;4:3–22. doi: 10.1037/1528-3542.4.1.3. [DOI] [PubMed] [Google Scholar]
- Carver CS, Harmon-Jones E. Anger is an approach-related affect: Evidence and implications. Psychological Bulletin. 2009;135:183–204. doi: 10.1037/a0013965. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100:316–336. doi: 10.1037/0021-843X.100.3.316. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, Mineka S. Temperament, personality, and the mood and anxiety disorders. Journal of Abnormal Psychology. 1994;103:103–116. doi: 10.1037/0021-843X.103.1.103. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB. Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology. 2004;67:7–49. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB, McKnight PE. A capability model of individual differences in frontal EEG asymmetry. Biological Psychology. 2006;72:198–207. doi: 10.1016/j.biopsycho.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Emotion and affective style: Hemispheric substrates. Psychological Science. 1992;3:39–43. doi: 10.1111/j.1467-9280.1992.tb00254.x. [DOI] [Google Scholar]
- Davidson RJ. Affective style and affective disorders: Perspectives from affective neuroscience. Cognition & Emotion. 1998;12:307–330. doi: 10.1080/026999398379628. [DOI] [Google Scholar]
- Davidson RJ, Chapman JP, Chapman LJ, Henriques JB. Asymmetrical brain electrical activity discriminates between psychometrically-matched verbal and spatial cognitive tasks. Psychophysiology. 1990;27:528–543. doi: 10.1111/j.1469-8986.1990.tb01970.x. [DOI] [PubMed] [Google Scholar]
- Dawson G, Frey K, Panagiotides H, Osterling J, Hessl D. Infants of depressed mothers exhibit atypical frontal brain activity: A replication and extension of previous findings. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1997;38:179–186. doi: 10.1111/j.1469-7610.1997.tb01852.x. [DOI] [PubMed] [Google Scholar]
- Deldin PJ, Chiu P. Cognitive restructuring and EEG in major depression. Biological Psychology. 2005;70:141–151. doi: 10.1016/j.biopsycho.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Diego MA, Field T, Hernandez-Reif M. CES-D depression scores are correlated with frontal EEG alpha asymmetry. Depression and Anxiety. 2001;13:32–37. doi: 10.1002/1520-6394(2001)13:1<32∷AID-DA5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Feng X, Forbes EE, Kovacs M, George CJ, Lopez-Duran NL, Fox NA, Cohn JF. Children's depressive symptoms in relation to EEG frontal asymmetry and maternal depression. Journal of Abnormal Child Psychology. 2012;40:265–276. doi: 10.1007/s10802-011-9564-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) 1997 [Google Scholar]
- Goldman RI, Stern JM, Engel J, Cohen MS. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport. 2002;13:2487–2492. doi: 10.1097/01.wnr.0000047685.08940.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BL, Shankman SA, Kujawa A, Torpey-Newman DC, Olino TM, Klein DN. Developmental changes in electroencephalographic frontal asymmetry in young children at risk for depression. Journal of Child Psychology and Psychiatry. 2016;57:1075–1082. doi: 10.1111/jcpp.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan JK, Hoxha D, Chihade D, Pflieger ME, Rosebrock L, Cacioppo J. Frontal alpha EEG asymmetry before and after behavioral activation treatment for depression. Biological Psychology. 2014;99:198–208. doi: 10.1016/j.biopsycho.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimshaw GM, Carmel D. An asymmetric inhibition model of hemispheric differences in emotional processing. Frontiers in Psychology. 2014;5 doi: 10.3389/fpsyg.2014.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale TS, Smalley SL, Hanada G, Macion J, McCracken JT, McGough JJ, Loo SK. Atypical alpha asymmetry in adults with ADHD. Neuropsychologia. 2009;47:2082–2088. doi: 10.1016/j.neuropsychologia.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E, Abramson LY, Nusslock R, Sigelman JD, Urosevic S, Turonie LD, Fearn M. Effect of bipolar disorder on left frontal cortical responses to goals differing in valence and task difficulty. Biological Psychiatry. 2008;63:693–698. doi: 10.1016/j.biopsych.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJ. Anger and frontal brain activity: EEG asymmetry consistent with approach motivation despite negative affective valence. Journal of Personality and Social Psychology. 1998;74:1310–1316. doi: 10.1037/0022-3514.74.5.1310. [DOI] [PubMed] [Google Scholar]
- Heller W. Neuropsychological mechanisms of individual differences in emotion, personality, and arousal. Neuropsychology. 1993;7:476–489. doi: 10.1037/0894-4105.7.4.476. [DOI] [Google Scholar]
- Heller W, Nitschke JB, Etienne Ma, Miller GA. Patterns of regional brain activity differentiate types of anxiety. Journal of Abnormal Psychology. 1997;106:376–385. doi: 10.1037/0021-843X.106.3.376. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. Journal of Abnormal Psychology. 1991;100:535–545. doi: 10.1037/0021-843X.100.4.535. [DOI] [PubMed] [Google Scholar]
- Higgins ET, Shah J, Friedman R. Emotional responses to goal attainment: Strength of regulatory focus as moderator. Journal of Personality and Social Psychology. 1997;72:515–525. doi: 10.1037/0022-3514.72.3.515. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Wang P. Research Domain Criteria (RDoC): Toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Kentgen LM, Tenke CE, Pine DS, Fong R, Klein RG, Bruder GE. Electroencephalographic asymmetries in adolescents with major depression: Influence of comorbidity with anxiety disorders. Journal of Abnormal Psychology. 2000;109:797–802. doi: 10.1037//0021-843X.109.4.797. [DOI] [PubMed] [Google Scholar]
- Keune PM, Bostanov V, Hautzinger M, Kotchoubey B. Mindfulness-based cognitive therapy (MBCT), cognitive style, and the temporal dynamics of frontal EEG alpha asymmetry in recurrently depressed patients. Biological Psychology. 2011;88:243–252. doi: 10.1016/j.biopsycho.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Keune PM, Bostanov V, Hautzinger M, Kotchoubey B. Approaching dysphoric mood: State-effects of mindfulness meditation on frontal brain asymmetry. Biological Psychology. 2013;93:105–113. doi: 10.1016/j.biopsycho.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Keune PM, Schönenberg M, Wyckoff S, Mayer K, Riemann S, Hautzinger M, Strehl U. Frontal alpha-asymmetry in adults with attention deficit hyperactivity disorder: Replication and specification. Biological Psychology. 2011;87:306–310. doi: 10.1016/j.biopsycho.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Keune PM, Wiedemann E, Schneidt A, Schönenberg M. Frontal brain asymmetry in adult attention-deficit/hyperactivity disorder (ADHD): Extending the motivational dysfunction hypothesis. Clinical Neurophysiology. 2015;126:711–720. doi: 10.1016/j.clinph.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Klein DF. Endogenomorphic depression: A conceptual and terminological revision. Archives of General Psychiatry. 1974;31:447–454. doi: 10.1001/archpsyc.1974.01760160005001. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Nusslock R, George C, Kovacs M. Frontal EEG asymmetry moderates the effects of stressful life events on internalizing symptoms in children at familial risk for depression. Psychophysiology. 2012;49:510–521. doi: 10.1111/j.1469-8986.2011.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathersul D, Williams LM, Hopkinson PJ, Kemp AH. Investigating models of affect: Relationships among EEG alpha asymmetry, depression, and anxiety. Emotion. 2008;8:560–572. doi: 10.1037/a0012811. [DOI] [PubMed] [Google Scholar]
- Meehl PE. Hedonic capacity: Some conjectures. Bulletin of the Menniger Clinic. 1975;39:295–307. [PubMed] [Google Scholar]
- Metzger LJ, Paige SR, Carson MA, Lasko NB, Paulus LA, Pitman RK, Orr SP. PTSD arousal and depression symptoms associated with increased right-sided parietal EEG asymmetry. Journal of Abnormal Psychology. 2004;113:324–329. doi: 10.1037/0021-843X.113.2.324. [DOI] [PubMed] [Google Scholar]
- Miller A, Fox NA, Cohn JF, Forbes EE, Sherrill JT, Kovacs M. Regional patterns of brain activity in adults with a history of childhood-onset depression: Gender differences and clinical variability. The American Journal of Psychiatry. 2002;159:934–940. doi: 10.1176/appi.ajp.159.6.934. [DOI] [PubMed] [Google Scholar]
- Miller GA, Crocker LD, Spielberg JM, Infantolino ZP, Heller W. Issues in localization of brain function: The case of lateralized frontal cortex in cognition, emotion, and psychopathology. Frontiers in Integrative Neuroscience. 2013;7:1–9. doi: 10.3389/fnint.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineka S, Watson D, Clark LA. Comorbidity of anxiety and unipolar mood disorders. Annual Review of Psychology. 1998;49:377–412. doi: 10.1146/annurev.psych.49.1.377. [DOI] [PubMed] [Google Scholar]
- Mitchell JT. Behavioral approach in ADHD: Testing a motivational dysfunction hypothesis. Journal of Attention Disorders. 2010;13:609–617. doi: 10.1177/1087054709332409. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Harmon-Jones E, Alloy LB, Urosevic S, Goldstein K, Abramson LY. Elevated left mid-frontal cortical activity prospectively predicts conversion to bipolar I disorder. Journal of Abnormal Psychology. 2012;121:592–601. doi: 10.1037/a0028973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Shackman AJ, Harmon-Jones E, Alloy LB, Coan JA, Abramson LY. Cognitive vulnerability and frontal brain asymmetry: Common predictors of first prospective depressive episode. Journal of Abnormal Psychology. 2011;120:497–503. doi: 10.1037/a0022940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Walden K, Harmon-Jones E. Asymmetrical frontal cortical activity associated with differential risk for mood and anxiety disorder symptoms: An RDoC perspective. International Journal of Psychophysiology. 2015;98:249–261. doi: 10.1016/j.ijpsycho.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Oakes TR, Pizzagalli DA, Hendrick AM, Horras KA, Larson CL, Abercrombie HC, Davidson RJ. Functional coupling of simultaneous electrical and metabolic activity in the human brain. Human Brain Mapping. 2004;21:257–270. doi: 10.1002/Hbm.20004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assesment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Open Science Collaboration. Estimating the reproducibility of psychological science. Science. 2015;349:aac4716. doi: 10.1126/science.aac4716. [DOI] [PubMed] [Google Scholar]
- Parvaz MA, MacNamara A, Goldstein RZ, Hajcak G. Event-related induced frontal alpha as a marker of lateral prefrontal cortex activation during cognitive reappraisal. Cognitive, Affective, & Behavioral Neuroscience. 2012;12:730–740. doi: 10.3758/s13415-012-0107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole BD, Gable PA. Affective motivational direction drives asymmetric frontal hemisphere activation. Experimental Brain Research. 2014;232:2121–2130. doi: 10.1007/s00221-014-3902-4. [DOI] [PubMed] [Google Scholar]
- Pössel P, Lo H, Fritz A, Seemann S. A longitudinal study of cortical cortical activity in adolescents. Biological Psychology. 2008;78:173–178. doi: 10.1016/j.biopsycho.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Quinn CR, Rennie CJ, Harris AWF, Kemp AH. The impact of melancholia versus non-melancholia on resting-state, EEG alpha asymmetry: Electrophysiological evidence for depression heterogeneity. Psychiatry Research. 2014;215:614–617. doi: 10.1016/j.psychres.2013.12.049. [DOI] [PubMed] [Google Scholar]
- Rabe S, Debener S, Brocke B, Beauducel A. Depression and its relation to posterior cortical activity during performance of neuropsychological verbal and spatial tasks. Personality and Individual Differences. 2005;39:601–611. doi: 10.1016/j.paid.2005.02.005. [DOI] [Google Scholar]
- Radloff LS. The CES-D scale: A self report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Reid SA, Duke LM, Allen JJ. Resting frontal electroencephalographic asymmetry in depression: Inconsistencies suggest the need to identify mediating factors. Psychophysiology. 1998;35:389–404. doi: 10.1111/1469-8986.3540389. [DOI] [PubMed] [Google Scholar]
- Rizvi SJ, Pizzagalli DA, Sproule BA, Kennedy SH. Assessing anhedonia in depression: Potentials and pitfalls. Neuroscience and Biobehavioral Reviews. 2016;65:21–35. doi: 10.1016/j.neubiorev.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, Cuthbert BN. Developing constructs for psychopathology research: research domain criteria. Journal of Abnormal Psychology. 2010;119:631–639. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annual Review of Psychology. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Klein DN, Tenke CE, Bruder GE. Reward sensitivity in depression: A biobehavioral study. Journal of Abnormal Psychology. 2007;116:95–104. doi: 10.1037/0021-843X.116.1.95. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Nelson BD, Sarapas C, Robison-Andrew EJ, Campbell ML, Altman SE, Gorka SM. A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. Journal of Abnormal Psychology. 2013;122:322–338. doi: 10.1037/a0030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit DJA, Posthuma D, Boomsma DI, De Geus EJC. The relation between frontal EEG asymmetry and the risk for anxiety and depression. Biological Psychology. 2007;74:26–33. doi: 10.1016/j.biopsycho.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Stewart JL, Bismark AW, Towers DN, Coan JA, Allen JBJB. Resting frontal EEG asymmetry as an endophenotype for depression risk: Sex-specific patterns of frontal brain asymmetry. Journal of Abnormal Psychology. 2010;119:502–512. doi: 10.1037/a0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Coan JA, Towers DN, Allen JJB. Resting and task-elicited prefrontal EEG alpha asymmetry in depression: Support for the capability model. Psychophysiology. 2014;51:446–455. doi: 10.1111/psyp.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Levin-Silton R, Sass SM, Heller W, Miller GA. Anger style, psychopathology, and regional brain activity. Emotion. 2008;8:701–713. doi: 10.1037/a0013447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau R, Jorgensen RS, Kim S. Depression, anxiety, and resting frontal EEG asymmetry: A meta-analytic review. Journal of Abnormal Psychology. 2006;115:715–729. doi: 10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Henriques JB. Resting frontal brain asymmetry predicts affective responses to films. Journal of Personality and Social Psychology. 1990;59:791–801. doi: 10.1037/0022-3514.59.4.791. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE, Doss RC. Individual-differences in anterior brain asymmetry and fundamental dimensions of emotion. Journal of Personality and Social Psychology. 1992;62:676–687. doi: 10.1037/0022-3514.62.4.676. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Dichter GS, Garber J, Simien C. Resting frontal brain activity: linkages to maternal depression and socio-economic status among adolescents. Biological Psychology. 2004;67:77–102. doi: 10.1016/j.biopsycho.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Watson D. Differentiating the mood and anxiety disorders: a quadripartite model. Annual Review of Clinical Psychology. 2009;5:221–247. doi: 10.1146/annurev.clinpsy.032408.153510. [DOI] [PubMed] [Google Scholar]
- Watson D, O'Hara MW, Chmielewski M, McDade-Montez EA, Koffel E, Naragon K, Stuart S. Further validation of the IDAS: Evidence of convergent, discriminant, criterion, and incremental validity. Psychological Assessment. 2008;20:248–259. doi: 10.1037/a0012570. [DOI] [PubMed] [Google Scholar]
- Watson D, O'Hara MW, Simms LJ, Kotov R, Chmielewski M, McDade-Montez EA, Stuart S. Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS) Psychological Assessment. 2007;19:253–268. doi: 10.1037/1040-3590.19.3.253. [DOI] [PubMed] [Google Scholar]
- Wheeler RE, Davidson RJ, Tomarken AJ. Frontal brain asymmetry and emotional reactivity: A biological substrate of affective style. Psychophysiology. 1993;30:82–89. doi: 10.1111/j.1469-8986.1993.tb03207.x. [DOI] [PubMed] [Google Scholar]
- Wiedemann G, Pauli P, Dengler W, Lutzenberger W, Birbaumer N, Buchkremer G. Frontal brain asymmetry as a biological substrate of emotions in patients with panic disorders. Archives of General Psychiatry. 1999;56:78–84. doi: 10.1001/archpsyc.56.1.78. [DOI] [PubMed] [Google Scholar]


