Abstract
The immediate and long term effects of exposure to early life stress (ELS) have been documented in humans and animal models. Even relatively brief periods of stress during the first 10 days of life in rodents can impact later behavioral regulation and the vulnerability to develop adult pathologies, in particular an impairment of cognitive functions and neurogenesis, but also modified social, emotional and conditioned fear responses. The development of preclinical models of ELS exposure allows the examination of mechanisms and testing of therapeutic approaches that are not possible in humans. Here we describe limited bedding and nesting (LBN) procedures, with models that produce altered maternal behavior ranging from fragmentation of care to maltreatment of infants. The purpose of this paper is to discuss important issues related to the implementation of this chronic ELS procedure and to describe some of the most prominent endpoints and consequences, focusing on areas of convergence between laboratories. Effects on the hypothalamic-pituitary adrenal (HPA) axis, gut axis and metabolism are presented in addition to changes in cognitive and emotional functions. Interestingly, recent data have suggested a strong sex difference in some of the reported consequences of the LBN paradigm, with females being more resilient in general than males. As both the chronic and intermittent variants of the LBN procedure have profound consequences on the offspring with minimal external intervention from the investigator, this model is advantageous ecologically and has a large translational potential. In addition to the direct effect of ELS on neurodevelopmental outcomes, exposure to adverse early environments can also have intergenerational impacts on mental health and function in subsequent generation offspring. Thus, advancing our understanding of the effect of ELS on brain and behavioral development is of critical concern for the health and wellbeing of both the current population, and for generations to come.
Keywords: developmental programming, early life stress, limited bedding and nesting, maternal behavior, mental health, neonatal stress, vulnerability
1. Introduction
Cognitive and emotional disorders can arise from interactions between genes and the environment during sensitive developmental periods (Bale et al., 2010). The early postnatal brain in rodents and humans is not yet mature, and thus the perinatal period represents a sensitive stage of neural development, which organizes both beneficial and deleterious influences from the environment; among these is stress. Indeed, the influences of early-life stress (ELS) on brain development may be both robust and persistent: a relatively brief period of stress occurring during just the first week of life often has life-long consequences for both brain structure and function, and can ultimately impact behavior and vulnerability to subsequent stress (Bale, et al., 2010; Caspi et al., 2003; Chen & Baram, 2016; Everson-Rose, de Leon, Bienias, Wilson, & Evans, 2003; Gluckman & Hanson, 2008; Romeo, Patel, Pham, & So, 2016). Understanding the mechanisms for the enduring consequences of ELS on brain function has been an active area of neuroscience research, because this knowledge is critical for identifying plausible therapeutic strategies and preventive approaches.
There is strong human epidemiological and observational data regarding the critical association between early-life adversity and stress and later negative cognitive and emotional outcome, though different types of such experiences during different times of development can produce distinct neurobehavioral outcomes (Everson-Rose, et al., 2003; Kaplan et al., 2001; Lupien, McEwen, Gunnar, & Heim, 2009). Conditions of early-life adversity such as poverty, loss of a parent, maternal substance abuse or depression, are consistently associated with stress in the young offspring and an increased vulnerability to develop emotional and cognitive problems later in life. Whereas stress-related disorders, including depression, anxiety and post-traumatic stress disorders, seem particularly dependent on the effects of ELS (Caspi, et al., 2003; Dalle Molle et al., 2012; Pratchett & Yehuda, 2011), memory and executive functions are also impaired following childhood adversity (Everson-Rose, et al., 2003; Kaplan, et al., 2001).
Some of the most revealing pieces of evidence for the importance of stressful early life experience on neurobehavioral development arose from studies of institutionally reared children that were randomly assigned to foster care or remained within the institution. These institutionalized children experienced paucity of care during early life, combined with malnutrition, physical and emotional abuse, neglect and greatly reduced experience with an attachment figure. While it is difficult to pinpoint the critical causal factors in their early life experience, these children exhibited later cognitive and emotional impairments, as well as increased vulnerability to psychiatric disorders (Bos et al., 2011; Kumsta, Rutter, Stevens, & Sonuga-Barke, 2010; Nelson, Bos, Gunnar, & Sonuga-Barke, 2011; Nelson et al., 2007; Zeanah et al., 2009). These were only partially reversed by fostering, and only when fostering was introduced early (Gunnar, 2010; Nelson, et al., 2007; Tottenham et al., 2011). These findings demonstrate the gravity of the problem and its consequences, as well as the importance of understanding early life perturbations and stress during a critical developmental period.
In view of the major societal impact of ELS, and considering the number of children worldwide growing up under some form of chronic stress, there is a recognized need for research on interventions with translational and clinical potential. Many of the symptoms resulting from ELS may not emerge until later in life (Brunson et al., 2005; Kaplan, et al., 2001; Raineki, Cortés, Belnoue, & Sullivan, 2012), making it difficult to identify affected or vulnerable individuals until well past the critical developmental window. Thus, any therapeutic interventions will need to be effective when applied post-hoc, after the stress has occurred.
Notably, human studies are correlational, not enabling direct causal inferences. In addition, teasing out mechanisms is difficult in humans, with limited access to tissue and to rapid testing of mechanistic hypotheses via interventions. Thus, there has been a recognized need for animal models of ELS. Animal models facilitate mechanistic questions, distinguishing between the roles of genetic and environmental factors and controlling for parameters of interest. They also enable direct access to specific brain regions and the use of neuroanatomical, biochemical and genetic, pharmacogenetic and optogenetic approaches to identify cells, circuits, mediators and signaling cascades that might contribute to the profound consequences of ELS on outcome.
Note that in this review we employ the term “early-life stress” to denote early postnatal life, including the day of birth through the time of weaning from the mother (in most rodent species, weaning occurs around postnatal day (PND) 21). The effects of stress during other critical periods can also impact brain development and function, but will not be discussed here: e.g., prenatal(Maccari & Morley-Fletcher, 2007) and adolescence (McCormick & Green, 2013; Tzanoulinou & Sandi, 2015). Whereas it is difficult to compare developmental ages of human and rodent brain as a whole, exhaustive analyses suggest that this might be possible for individual regions and circuits (Clancy et al., 2007). For example, hippocampal development is similar in the full-term human neonate and a 5–7 day old rodent (Avishai-Eliner, Brunson, Sandman, & Baram, 2002) with the first few postnatal days in rodents perhaps corresponding to late gestation in humans. However, the amygdala of a 6–9 month old human infant might be more similar to the 10-day old rat (Avishai-Eliner, et al., 2002; Graham et al., 2016; Sullivan, Landers, Yeaman, & Wilson, 2000). Thus, research in rodents can have translational value provided behavioral and neural development are specifically defined.
1.1. Historical perspective and development of animal models of postnatal ELS
Over the past seven decades, several primate and rodent models have been established to manipulate early-life experiences in order to gain a better understanding of the mechanisms by which they impact neurobehavioral development. While the importance of the early life caregiver was acknowledged for centuries, the scientific exploration for understanding links between early life experiences and later-life outcome began in earnest in the 1950’s (Bowlby, 1952). Cross talk between different disciplines helped uncover links between disturbed maternal care and/or maternal separation and disturbed emotional and cognitive functioning (Bowlby, 1952; Harlow & Harlow, 1965).
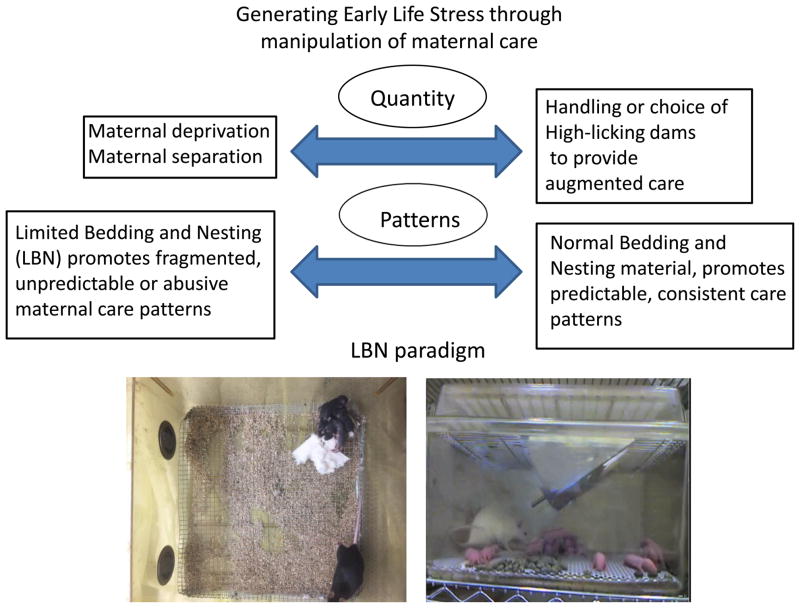
These observations were initially primarily explored by restricting maternal care, using a paradigm referred to as the maternal separation or the maternal deprivation model. Since this approach can be implemented in diverse species requiring maternal care (avian, rodent, nonhuman primate), the maternal deprivation paradigm has remained popular. Data derived with this model have been invaluable in exponentially increasing our understanding of the links between ELS and later life outcome. However, there has been little standardization of this procedure with respect to time and age of separation, whether pups are kept warm or whether offspring can hear or smell the mother. Thus, the consequence is that this procedure has produced divergent and somewhat contradictory results in terms of neurobehavioral outcomes. Maternal separation models have provided a vast amount of data on the effects of reducing (or at least altering) maternal input on pup development, however, one disadvantage of this model is that repeated or prolonged absence of the mother can induce opposite effects, and that also maternal behavior of the dam is significantly altered after return of her pups. The magnitude of this compensatory behavioral change is difficult to control experimentally (Huot, Gonzalez, Ladd, Thrivikraman, & Plotsky, 2004). In addition, data from human studies of chronic childhood stress, including war, famine and neglect/abuse suggest that the mother is typically present, but that her behavior is abnormal. Thus, alternative models which limit experimenter intervention within the cage, yet still alter maternal behavior and sensory stimulation towards the pups may provide additional insights into the processes by which early-life experience, including stress, can influence life-long resilience or vulnerability to neuropsychiatric disorders. (Figure 1)
Figure 1.
Examples of preclinical animal models used to induce early life stress through manipulation of maternal care. Some models focus more on generating changes in the quantity of maternal care and sensory stimulation provided to the pups while others might affect more the pattern of maternal care, in particular the Limited Bedding and Nesting (LBN) paradigm discussed here. Examples of LBN conditions are depicted for mouse and rat mothers with their litters kept on a wire mesh with limited nesting material. Left: The view from above for the mouse cage shows the litter being in one corner of the cage (top right) while the mother is off the nest. Right: The rat cage shows the mother being off her pups, and all pups scattered on the wire mesh above the bedding.
In parallel to the use of sensory deprivation models, research programs also developed to explore how sensory experiences guide neurobehavioral development. This was done through different rearing paradigms, for instance by varying the amount of sensory stimulation provided to artificially reared pups (Belay et al., 2011) or by exposing young animals to experimentally-controlled discrete sensory experiences. Most notable was the work of Myron Hofer, which highlighted the critical importance of the patterning and intensity of maternal stimulation of pups in providing homeostatic regulation of infant behavior and guiding neurobehavioral development (Hofer, 1984, 1996a, 1996b; Shair, Brunelli, Masmela, Boone, & Hofer, 2003). Hence the concept of “hidden regulators” emerged, which states that different stimuli and their patterns control the homeostatic balance in specific systems. Examples of this regulation include the effects of maternal tactile stimulation of pups maintaining high levels of pups’ growth hormone and neural proteins, maternal odor keeping stress hormones low, and warmth controlling behavioral motor activity levels (Chatterjee et al., 2007; Eghbal-Ahmadi, Avishai-Eliner, Hatalski, & Baram, 1999; Hofer, 1973, 1984; Hostinar, Sullivan, & Gunnar, 2014). The value of a naturalistic approach is that it can explore normal variations in maternal care within an undisturbed situation, i.e. high/low licking mothers or communal nesting, (Anacker, O’Donnell, & Meaney, 2014; Blanchard, Summers, & Blanchard, 2013). In addition, it extracts significant aspects of maternal care that are critical for the overall environmental regulation of the epigenome, proteome and behavior (Bagot et al., 2012). More recently, the crucial role of temporal patterns of maternal sensory input, and especially their fragmentation and unpredictability, has been demonstrated (Molet, Heins, et al., 2016). This fragmented care can be associated with aberrant pup sensory experiences that can go beyond programming individual differences and initiate pathways to pathology. An example of this can also be found in the human literature where unpredictable and fragmented handling and sensory stimulation provided to preterm infants during the course of intensive care has been associated with numerous adverse outcomes such as hypoxemia (Long, Philip, & Lucey, 1980) and acute heart rate increases (Zahr & Balian, 1995). Thus, minimal handling is a recommended practice for preterm infants (Álvarez et al., 2017; Symington & Pinelli, 2006; VandenBerg, 2007).
Over the past two decades, a new procedure has emerged that focuses on disrupting maternal care beyond normal experience, primarily induced by limiting the dam’s access to sufficient bedding and nesting material. The impoverished cage environment prevents her from constructing a satisfactory nest, which increases basal corticosterone levels in the dam herself on postpartum day (PPD) 9 (Ivy, Brunson, Sandman, & Baram, 2008). These stressful conditions for the dam alter the pattern and quality of maternal care she displays, resulting in fragmented and sometimes erratic nurturing behaviors (Figure 2). The altered patterns of maternal care, perhaps their unpredictability and fragmentation, induces stress in the pups evidenced by increased plasma glucocorticoid concentrations and by the presence of hypertrophied adrenal glands at the end of this one week stress period, on PND 9 in Sprague Dawley rats and in several wild-type and transgenic mice (Rice, Sandman, Lenjavi, & Baram, 2008; X. D. Wang et al., 2011). The consequences of this form of ELS are profound, including a progressive loss of cognitive function and robust derangements of emotional functions later in life in both mouse and rat studies (though variations of the procedure during different times of development can certainly produce distinct neurobehavioral outcomes).
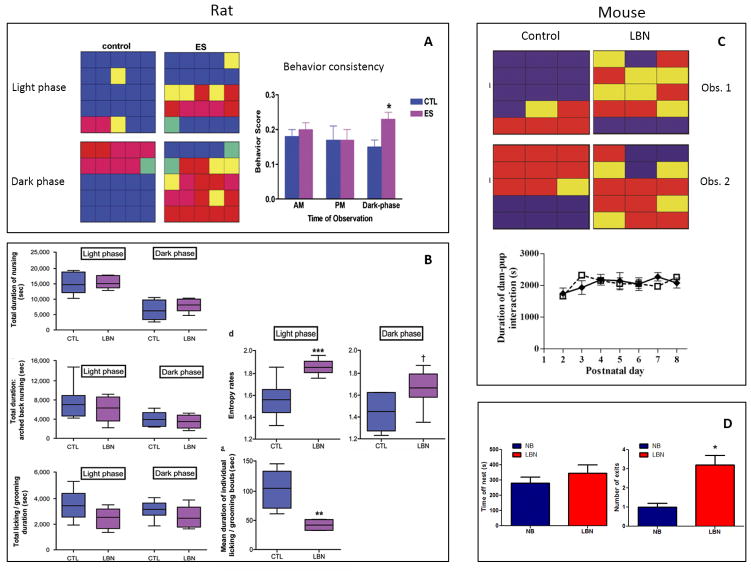
Figure 2.
Several measures of the quantity and quality of maternal care under Control or limited bedding and nesting (LBN) conditions in rats (left) or mice (right). Left Panels: A: Representative examples of maternal caring activities from one control and one early stress (ES) rat dam, during the light-phase and dark-phase observations, performed on PND5. Each individual color depicts the predominant behavior during the epoch/square. Blue: nursing; red: away from pups (off pups or out of the nest); yellow: licking and grooming pups; pink: dam eating and drinking, away from pups; green: dam licking and grooming self, away from pups. This graphic representation illustrates the fact that in control dams, each behavior typically lasted for several consecutive 3 min epochs, whereas LBN dams tended to switch behaviors frequently and unpredictably. The behavior of LBN dams (n=7 per group) was less consistent, with frequent switching from one type of activity to another, during the dark, high activity portion of the diurnal cycle. This erratic behavior is reflected by a higher behavior score, which denotes increased numbers of initiating a new behavior during consecutive observation epochs. Adapted from Ivy et al. (Ivy, et al., 2008) with permission. B: Several measures of the quantity and quality of maternal care do not distinguish dams in routine cage environment (CTL) from those in limited bedding/nesting (LBN) cages. Total duration of nursing and arched-back nursing, considered a measure of optimal quality of maternal care, were similar between groups. Duration of time spent licking/grooming pups were comparable in CTL and LBN dams (all P>0.05, Student’s t-tests). When separate analyses were performed for nurturing behaviors during the light phase or the dark phase, nursing, arched-back nursing and licking/grooming times did not differ between groups (all P>0.05, Bonferroni’s post hoc test). Values are provided in seconds, and are sum of observations over two 50-min periods per day for 8 days. (n=6 per group). Data presented as box and whisker plot show the 10th and 90th percentiles. Horizontal bars represent mean values. Entropy was employed to mathematically define the unpredictability of maternal behavior patterns. Entropy rates in LBN dams were significantly higher on average than those of CTL dams. When broken down for entropy rates during light phase and dark phase, the group differences persisted, with a stronger effect during the light phase period. Data presented as box and whisker plot show the 10th and 90th percentiles. Horizontal bars represent mean values. Black asterisks denote statistical significance using a linear model; ***P<0.001, **P<0.01, †P=0.08. CTL, control. Adapted from: Molet et al. (Molet, Heins, et al., 2016) with permission.
Right Panels: C: Representative activity grids of control and LBN mouse dams, during two matched observation periods. Each grid depicts one dam’s activity during 15 1-min epochs, and individual panes are color coded to represent the dam’s location/activity during that epoch. Blue: dam in nest in contact with pups for the total epoch; red: outside nest area; yellow: a mixed epoch. The consistency of control dam behavior is contrasted with the fragmented pattern in the LBN dam. Duration of dam-pup interaction (measured as time spent by each dam in contact with pups within the nest area) is similar for LBN (n=12) and control (n=6) dams between PND2–8. Adapted from: Rice et al. (Rice, et al., 2008) with permission. D: Parameters of maternal care in mice averaged over observations from PND2 to PND9 in control, normal bedding (NB) and LBN conditions (n=7 dams/condition). No pups were observed out of the nest in the control condition. Values are means +/− SEM. *, P<0.05 compared to NB. Adapted from: Arp et al. (Arp, et al., 2016)
The limited bedding and nesting paradigm (LBN) was initially developed in rats (Brunson, et al., 2005; Gilles, Schultz, & Baram, 1996; Ivy, et al., 2008), yet has also been successfully adopted by several other groups to provoke chronic stress in mice (Naninck et al., 2015; Rice, et al., 2008; X. D. Wang, et al., 2011). Whereas the original LBN paradigm involved continuous rearing of pups in this impoverished environment from PND 2–9 (with variations in the amount of bedding materials between mice and rats), a variation has been developed over the past decade which involves placement of dam and pups in a less severe impoverished bedding environment from PND8–12 (Moriceau, Shionoya, Jakubs, & Sullivan, 2009; Raineki, et al., 2012; Raineki et al., 2015) or intermittently from PND1–7 (Blaze, Scheuing, & Roth, 2013; T. L. Roth, Lubin, Funk, & Sweatt, 2009; T. L. Roth & Sullivan, 2005). This variation of the LBN model is referred to here as the “Scarcity model”.
It should be noted that, while this manuscript focuses on pup outcome, a few studies are now emerging on the long lasting effect of the LBN procedure on the mother. For example, the Walker lab found that multiparous mothers having been exposed to LBN during their first lactation period exhibited increased attention together with a significant increase in spine density in the infralimbic prefrontal cortex neurons in a subsequent, second lactation period (Opala, Liu, Long, & Walker, 2016). These results suggest that raising pups in a LBN environment might have long lasting effects on cognitive and attentional abilities of the mother in subsequent reproductive episodes.
2. Experimental procedures of Limited Bedding and Nesting paradigms
The main objective of the LBN experimental paradigm is to manipulate characteristics of maternal care while limiting external experimenter interventions. Both continuous and intermittent procedures have been developed to experimentally produce fragmented, unpredictable (chaotic) or adverse maternal care, in an effort to mimic the quality of care that often characterizes depressed, severely stressed, or drug abusing human mothers. One of the more powerful advantages of the LBN paradigm is its flexibility: the disruption of maternal behavior and the severity of the stress can be manipulated (by varying amounts of nesting material in the cage for instance) to assess correlations between maternal care and outcome. In addition, the age range at which pups experience LBN can be varied to assess sensitive periods. Importantly, the LBN can be used either continuously within the home cage or used for brief, daily epochs to assess duration effects. However, this flexibility can also produce procedural variability between laboratories leading to differential outcomes. Due to differences in animal facilities, the early life history of the females entering the mating procedure and strain differences in rodents, these manipulations can potentially produce very different changes in maternal behavior. For instance, total licking/grooming and nursing time might be equivalent between normal and LBN mothers in the Baram model (Ivy, et al., 2008; Molet, Heins, et al., 2016), but mothers in the Scarcity model show reduced time with the pups and more licking and grooming of pups (Moriceau, et al., 2009; Raineki, et al., 2012; Raineki, Moriceau, & Sullivan, 2010; Raineki, et al., 2015). Similarly, fragmentation of nursing episodes has been found to be significant by some investigators, but other studies report only non-significant trends towards increased fragmentation. Thus, it is critical that maternal behavior and pup responses to maternal behavior be monitored and the specific LBN procedure used validated before use as well as later. Behavioral observations during the manipulations should be done for at least 30 min a day, but preferably more frequently during the light/dark phase, to ensure that each lab’s standard care of pregnant and lactating mothers produces solid and consistent maternal care which is sufficiently altered by the limited bedding manipulation. Ideally, maternal behavior in each cohort of animals should be assessed both during the light and dark phases of the cycle and during multiple epochs through home cage video recording in order to provide a fine analysis of maternal care and historical observations. Both licking/grooming bout and nursing bout duration should be monitored; the occurrence of milk ejection reflexes could also be important variables to consider in the effect of LBN on body weight gain of the pups as observations have shown that LBN pups tend to have a lower body weight. This also depends on experimental conditions, although changes in nest temperature due to the wire mesh and changes in pup energy expenditure can be important determinants of reduction in body weight in rat pups exposed to LBN in some laboratories (McLaughlin, Verlezza, Gray, Hill, & Walker, 2016) but not others (Molet, Maras, Avishai-Eliner, & Baram, 2014). A less severe intermittent LBN procedure without rearing on a mesh (Scarcity Model) does not produce changes in weight gain (Raineki, et al., 2012; Raineki, et al., 2010; Rincón-Cortés & Sullivan, 2016; T. L. Roth & Sullivan, 2005).
As with any complex early life intervention, multiple aspects of the pup’s environment that are also altered might contribute to the resulting phenotype in addition to changes in maternal care giving behavior. For instance, changes in the amount and pattern of suckling (food intake), temperature regulation and modification in the olfactory cues from the precarious nest might also program several aspects of physiological and behavioral regulation in the long term.
2.1. Limited Bedding and Nesting using a mesh platform (Baram lab paradigm, used in many laboratories worldwide)
As devised originally in the Baram lab, the onset of the LBN procedure is on PND 2 in order to reduce mortality and cannibalism. Primiparous mothers older than 75 days are used with minimal variance. Rat or mouse pups from several litters are mixed among dams and those assigned to the LBN groups are transferred to cages with limited bedding and nesting material (Gilles, et al., 1996; Molet, et al., 2014) (Table 1). Specifically, the cages are fitted with a plastic coated aluminium mesh platform to sit approximately 2.5 cm above the cage floor. Bedding, which is placed under the mesh floor, is reduced to only cover the cage floor sparsely, and one-half of a single paper towel is provided for nesting material. The impoverished cage environment prevents the dam from constructing a satisfactory nest which might be dispersed. This leads to significant alterations in the pattern of care that manifest as fragmented and erratic nurturing behaviors, i.e. shortened bouts of each nurturing behavior and frequent shifts between different behaviors and unpredictable sequence of behaviors (Figure 2, right panels) (Ivy, et al., 2008; Molet, Heins, et al., 2016; Rice, et al., 2008). Detailed analysis of maternal behavior revealed little or no change in the overall duration of maternal care or of specific aspects of care (licking and grooming, nursing, Figure 2 left panels). (Ivy, et al., 2008; Molet, et al., 2014). However, in both mice and rats, maternal care is fragmented and unpredictable: each nurturing behavior is shorter in duration and often interrupted, and the sequence of nurturing behavior is unpredictable (Baram et al., 2012; Rice, et al., 2008). Interestingly, a hallmark of maternal behavior in any neglect/abuse situation is its unpredictable and fragmented quality (Gaudin, Polansky, Kilpatrick, & Shilton, 1996; Whipple & Webster-Stratton, 1991). It is suggested that the disrupted maternal care is a main source of chronic ELS in the pups, but changes in thermoregulation and or feeding patterns might also significantly affect the pups. When dams and pups are returned to normal bedding/nesting cages after PND9, maternal behavior returns to normal within hours, and stress hormone levels in the pups are reduced (Ivy, et al., 2008). A detailed description of this paradigm has been published recently for Sprague-Dawley, Long Evans and Wistar strains of rats (Molet, et al., 2014).
Table 1.
Setting up the limited bedding/nesting (LBN) paradigm in rats and mice (Baram lab)
| Day | Procedure |
|---|---|
| Time-pregnancy | Order time-pregnant females from your or arrange to breed females in-house. To limit the effects of previous experience on the dam’s maternal behavior and response to stress, always use virgin naïve females. Minimize disturbances and other stress sources throughout pregnancy. Check for births at least twice daily on the days surrounding expected parturition; at least two dams will give birth within the same 10–12 hour period to mix the pups across litters on the day of manipulation. |
| Postnatal day 2 |
Prepare limited bedding and nesting cages: Start with clean, empty standard housing cages. Position a fine-gauge, plastic-coated mesh platform to sit approximately 2.5 cm above the cage floor. Folding edges of mesh along the length approximately 3 cm so that platform sits above the bottom of the cage, permitting droppings to fall below the platform without trapping the pups. (Plastic-coated aluminium mesh dimensions: 0.4 × 0.9 cm. McNichols Co., Tampa, FL catalog no. 4700313244). Cover cage floor with a small amount of standard bedding (0.1cubic feet). This should not reach the top of the plastic mesh. Provide control cages with 6000 ml of corn or wood-chip bedding. Provide a limited amount of nesting material. For rats, add one-half of a single paper towel to cage. For mice, add one-half of a single NESTLET square (Ancare, Bellmore, NY). Limited bedding/nesting manipulation: To minimize genetic factors, pups from several dams are mixed and matched and assigned to CES or control dams at random. For each litter, quickly and gently remove all pups from the home cage; identify the sex of each pup (using anogenital distance) and place males and females into separate, euthermic holding cages. Repeat for each litter, keeping separate holding cages for male and female pups. Once all litters are removed and sorted, randomly assign dams to the control or limited bedding/nesting conditions. Standard cage:Place dam into fresh, clean standard cage (with normal amounts of bedding and nesting material). Randomly transfer pups from the male and female holding cages to control cage with the dam. Limited bedding/nesting cages: Place dam into cage with mesh platform and limited bedding and nesting material. Randomly transfer pups from the male and female holding cages to the experimental cage with the dam. Note: Litter size: because it influences both pup weight and hence maturation of the brain, as well as maternal behavior, we typically have 4 – 6 pups per mouse litter and 10 – 12 per rat litter. Sex ratio should be approximately 1:1. Counter-balance the order in which you replace pups with the dams between control and limited LBN conditions to limit differences in the total duration pups are separated. |
| Postnatal day 2 to postnatal day 9 | Leave control and limited bedding/nesting cages undisturbed (and unchanged) until postnatal day 9. Observe maternal behavior during that period at least twice daily, as described in Ivy et al., 2008. Alternatively, video behavior. Consider looking at continuous behaviors and patterns of care over 60– 90 min |
| Postnatal day 10 | In the morning, change all cages (control and limited bedding/nesting) to fresh, standard cages with normal bedding and nesting material. |
| Postnatal day 21 | Wean animals from dam. House same-sex litter-mates in the same cage. |
From: Molet J et al., Dev Psychobiol. 2014 (with permission)
In the Baram lab, cages are kept in rooms with strong laminar flow, minimizing the accumulation of ammonia. Lacking this, in some laboratories, the animal ethics committee requires that the bedding placed under the mesh is changed at least once during the stress period in order to lower exposure of the animals to urine-derived ammonia. The degree of bedding restriction and use of a wire mesh floor (though plastic-coated) has also been a point of concern for some laboratories, which have modified protocols to use a plastic grid providing greater comfort to the dam.
There has been reasonable consensus between laboratories regarding the implementation of this manipulation with a few instances in which the paradigm has been modified. For instance, in some laboratories, the plastic mesh has been replaced by a wire mesh for strength and ease of disinfection. This might contribute to the reduction in pup weight during ELS because of a significant temperature difference between plastic and metal mesh. For implementation of this model in mice (described in Rice et al, 2008), access to a fraction of a normal nestlet (little nest) is typically provided. In the Baram laboratory, the degree of limitation has been varied in the mouse model, and significant effects on somatic measures can be observed as a consequence of the degree of limitation of nesting materials. Complete or significant loss (less than 1/3) of a nestlet, significantly increases the risk of mortality, and leads to profound changes in weight gain of the young. Moderating the restriction to 1/2 or 2/3 of a nestlet, diminishes the effects of the stress on somatic outcomes (Rice, et al., 2008). The majority of laboratories currently use 1/2 of a standard nestlet (e.g. 2.5 × 5cm cotton nestlet) per mouse dam. In the rat, there have been some instances when the mother has been hoarding food pellets and feces in order to compensate for the deficient nesting material.
A small number of laboratories using mice, have also made modifications to the timing of maternal bedding restriction. Bath et al. chose to move the bedding and nesting restriction paradigm during the PND4–11 period. This was in part to map onto other neurodevelopmental studies of early life pharmacological exposure, but also to decrease the risk for pup cannibalism by performing stress manipulation later (Bath, Manzano-Nieves, & Goodwill, 2016). With the change in timing, Bath et al. observed similar outcomes on the weight gain and corticosterone measures, to what has been described by Baram and other laboratories.
2.2. Scarcity Model: LBN in the absence of mesh (used in the Sullivan, Weinberg and Roth laboratories)
A paradigm similar to the LBN procedure described above, called the Scarcity Model, was developed by Roth and Sullivan as a means to produce a mother that handled pups roughly to explore infant attachment learning. Most species learn to attach to their caregiver regardless of nurturing or maltreatment during caregiving (Raineki, et al., 2010; T. L. Roth & Sullivan, 2005). This procedure has been used for continuous exposure over different time intervals to explore the enduring impact of critical periods of maltreatment on neurobehavioral development, i.e. PND2–9, 1–7, 3–8, 8–12 or 10–14, with the greatest impact on neurobehavioral development if begun during the sensitive period for attachment, which ends on postnatal day 10. This paradigm also uses reduced bedding but does not incorporate the wire mesh floor. Since there is limited bedding to absorb excrement, this bedding needs to be cleaned about every second or third day, depending on the age of the pups during the manipulation. Control litters have a similar cage cleaning schedule. In this design, only multiparous mothers are used and mothers are rebred three to four times, although the assignment of LBN mother remains consistent. The use of multiparous females for LBN experiments is preferred for increased consistency of maternal care compared to primiparous mothers, although primiparous mothers produce similar results (Rincón-Cortés & Sullivan, 2016).
In the Sullivan laboratory, the Long Evans rat mother and her pups (typically 6 males and 6 females) are housed with limited (100 ml, 1–2 cm layer) nesting/bedding material compared to control mothers with abundant (5–7 cm layer) nesting/bedding material, which allows the mother to build a nest (the amount of bedding required to build a nest will differ based on the type of bedding used by the animal facility). The limited bedding environment decreases mothers’ ability to construct a nest, which results in frequent attempted nest building, spending more time away from the nest and pups, rough handling, and stepping on pups. Consequently, pups spend less time being nursed and have increased distress vocalizations. Importantly, this intermittent aversive rearing paradigm does not alter body weight but increases basal corticosterone levels (for example, at PND 9 and 13) (Moriceau, et al., 2009; Raineki, et al., 2012; Raineki, et al., 2010; Raineki, et al., 2015). Conversely, for the control group, providing abundant nest/bedding materials permits the mother to build a nest and to spend most of her time (~75%) inside the nest caring for pups.
In this paradigm, rough handling of the pups by the dam (i.e. stepping or jumping on pups, mother aggressively grooms pups, transports pups by gripping a limb) represents a strong element of maternal behavior (Moriceau, et al., 2009; Raineki, et al., 2012; Raineki, et al., 2010) that is less often reported in the LBN conditions described above.
The Scarcity Model paradigm has also been adapted as an intermittent variant using a brief daily procedure (30 min) of transferring the mother and her litter outside of the home cage for seven days (PND1–7), as in the Roth lab (Blaze, Asok, & Roth, 2015; Blaze, et al., 2013; Doherty, Forster, & Roth, 2016; T. L. Roth, et al., 2009; T. L. Roth, Matt, Chen, & Blaze, 2014). This daily manipulation allows for a powerful within-litter experimental design (depicted in Figure 3) that uniquely controls the amount of time pups are exposed to an LBN impoverished environment with aberrant maternal care, and eliminates the potential confounding effects of nutritional consequences or weight-gain differences between groups as pups are housed under standard rearing conditions (thus receiving normal maternal care) for the majority of the day (Blaze, et al., 2015; Blaze, et al., 2013; Doherty, et al., 2016; T. L. Roth, et al., 2009; T. L. Roth, et al., 2014). In this intermittent version of the Scarcity Model, Long Evans rat pups (2 males, 2 females) are removed from the home cage and exposed to a dam that has been placed in an unfamiliar environment with limited bedding resources (which is referred to as the maltreatment condition).
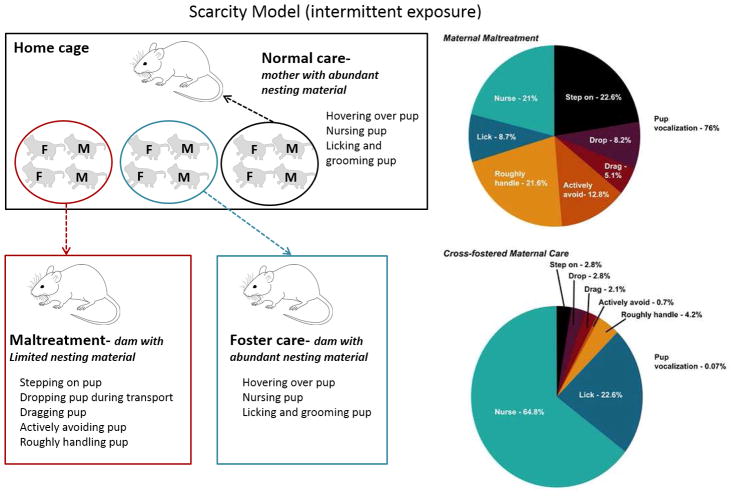
Figure 3.
Left panel: Schematic of the intermittent variation of the LBN paradigm. Utilizing a within-litter design, rat or mouse pups are repeatedly exposed for 30 minutes daily to either dams with insufficient woodchip bedding (maltreatment condition) or dams with copious amounts of woodchip bedding (foster care condition). Additional littermates are left inside the home cage (enriched with nesting material), providing normal care controls. M=male; F=female. Right panel: Qualitative assessment of the percent occurrence of pup-directed behaviors in the maltreatment condition in rats (Scarcity Model) indicates that pups experienced predominantly abusive behaviors, which resulted in considerable audible pup vocalization. (B) In sharp contrast, pups experienced significant amounts of normal maternal care behaviors in the cross-fostered maternal care condition. Pie charts represent an average of behaviors across all dams in each condition (n=15–20 dams/condition). From: Roth et al. (T. L. Roth, et al., 2009) with permission.
The combination of an unfamiliar dam not allowed to habituate to the experimental chamber prior to receiving pups, and impoverished environment is sufficient to induce caretakers to display an increased repertoire of abusive and potentially harmful behaviors such as stepping on, dropping, dragging, actively avoiding, or rough handling of the pups. From the same litter and at the same time, 2 males and 2 females are removed from the home cage and exposed to a dam that is in a familiar environment with copious amounts of bedding (referred to as the foster care condition). The combination of a familiar (dams are allowed to habituate to the experimental chamber at least 1 hour prior to receiving pups) and resourceful environment ensures that caretakers display a normal repertoire of nurturing behaviors. Since rat pups do not distinguish between their mother and another dam if they are both fed the same diet and matched for the same postpartum period (Leon, 1975), foster dams are always matched for postpartum age and diet to the biological mother. Each exposure session is for 30 minutes, and an additional control group (referred to as the normal care condition) is run where the remaining littermates (2 males, 2 females) are left with the biological mother in the home cage during the 30-minute sessions. After exposures, all pups are returned to the biological mother until the following day’s session. Stimulus dams (maltreatment and foster care) are also reunited with their biological litters immediately after each exposure session. Exposure sessions occur under low light (red) during the light phase, and are conducted at different, unpredictable times every day from PND1–7 using multiple dams (i.e. for a given litter, 3 stimulus dams are typically used across the 7 days for each condition). No first-time mothers are ever used to generate experimental litters or to serve as stimulus caregivers, and animals are derived from an in-house breeding colony.
Caregiving behaviors are scored via live observation and/or video recordings of 30 min sessions and using categories of behaviors (nurturing/abusive) within each 5-minute time-bin across each 30-minute session. Percent occurrence scores of individual types of behaviors (i.e. pup licking or rough handling) or category of behavior (nurturing or abusive) are then averaged across the 7 exposure days. Dams in the normal and foster care conditions are typically observed displaying copious amounts (at least 70% occurrence) of nurturing behaviors towards pups (Blaze, et al., 2015; Blaze, et al., 2013; T. L. Roth, et al., 2014) while those in the LBN condition more commonly (at least 50% occurrence) display abusive behaviors (Blaze, et al., 2015; Blaze, et al., 2013; Doherty, et al., 2016; T. L. Roth, et al., 2014; T. L. Roth & Sullivan, 2005). Ultrasonic (40 kHz- a distress frequency in rat pups) and audible vocalizations from pups are also scored each minute within a 30-minute session across 7 exposure sessions. Vocalization data indicate that pups respond differentially to the caregiving conditions, as they emit significantly more audible (around 50% occurrence) and ultrasonic distress (around 80% occurrence) vocalizations in the scarce environment than in the control environment (Blaze, et al., 2015; Blaze, et al., 2013; Doherty, et al., 2016; T. L. Roth, et al., 2014).
3. Proximal (neonatal) and distal (adult) phenotypes resulting from the LBN procedures
The rapid and growing expansion in the number of studies using the LBN paradigms across different laboratories has allowed documenting and understanding several of the mechanisms underlying the phenotypes resulting from these ELS paradigms. There is considerable convergence in either the intermittent or continuous LBN paradigms to impact neurobehavioral development. Published studies document changes in many central and peripheral systems in the early postnatal, preweaning, adolescence periods and in adulthood with an overall phenotype of impaired cognitive functions, increased anxiety and anhedonia, as well as altered adipose tissue metabolism, maladaptive nutrition and gut function. Adult offspring from LBN mothers are more vulnerable to the development of stress-related dysfunctions, closely mimicking the exposure to a chronically stressful early environment in children. The effect of LBN might even be observed in ageing since overall survival, amyloid processing and Alzheimer pathology have been reported to be altered after LBN and adult stress in Alzheimer mouse models (Hoeijmakers et al., 2016; Lesuis et al., 2016). These effects of LBN may, at least in part involve inflammatory changes (Brunson, et al., 2005; Green, Billings, Roozendaal, McGaugh, & LaFerla, 2006; Hoeijmakers, Lucassen, & Korosi, 2015; Hoeijmakers, et al., 2016; Lesuis, et al., 2016).
Fortunately, despite the large body of evidence pointing towards an increased vulnerability in the LBN offspring, there are some aspects of altered regulation that are more amenable to interventions and resilience, which is now the focus of many studies. In this section, we will review broad categories of documented phenotypes, with some contrasting results from different laboratories in order to illustrate some of the strengths and challenges that can arise from the use of these paradigms (see Tables 2 & 3). Thus this section is not intended to represent an exhaustive review of the literature, but to outline the usefulness of the LBN paradigms to enable us to better understand the important early life events in defining both the proximal and enduring effects on neurobehavioral outcome. Importantly, careful consideration of the type, age at manipulation and the duration of the LBN manipulation can produce divergent results, which highlights the critical importance of defining variations in the LBN manipulation to better understand how specific perturbations, during specific critical periods impacts neurobehavioral development. We also highlight some directions for the use of these LBN paradigms that are promising for future research.
Table 2.
Major Outcomes Provoked by the Limited Bedding/Nesting (LBN) or Scarcity Chronic Early-Life Stress Paradigm in Rats
| ELS Period | Sex | Strain | Acute/Long Term | Outcomes | References |
|---|---|---|---|---|---|
| PND | |||||
| Stress system perturbations | |||||
| 2–9 (LBN) | Both | Sprague-Dawley | PND 9 | Elevated basal CORT levels, higher adrenal weights |
Gilles et al. (1996) Avishai-Eliner et al. (2001), Brunson et al. (2005) |
| 1–10 (LBN) | Both | Sprague-Dawley | PND10 | No change basal ACTH or CORT concentrations, Reduced ACTH and CORT response to immobilization stress | McLaughlin et al. (2016) |
| 2–10 (LBN) | Both | Wistar | PND10 | Reduced basal CORT and adrenal weight | Moussaoui et al. (2016a) |
| Both | Wistar | PND21 | Increased basal CORT (F>M) | Moussaoui et al. (2016b) | |
| 2–9 (LBN) | Both | Sprague-Dawley | PND 9 | Reduced CRH mRNA expression in the PVN and CRF1 mRNA expression in CA1 and dentate gyrus. Reduced CRH receptor binding capacities in pituitary. Reduced GR gene expression in the PVN and frontal cortex. |
Avishai-Eliner et al. (2001) |
| 3–8 (Scarcity) | Both | Long-Evans | PND 8 | Elevated basal CORT levels. | Raineki et al. (2010) |
| Cognitive and emotional functions | |||||
| 1–7 (Scarcity) | Both | Long-Evans | PND 7 | Attachment learning deficits. | Moriceau et al. (2009) |
| 3–8 (Scarcity) | Both | Long-Evans | PND 8 | Disrupted social attachment behaviors | Raineki et al. (2010) |
| 1–7 (Scarcity) | Both | Long-Evans | PND 90 | Aberrant maternal behavior | Roth et al. (2009) |
| 8–12 (Scarcity) | Both | Long-Evans | PND 20 and 45 | Impaired social behaviors.. | Raineki et al. (2012) |
| PND 45 and 75 | Depressive-like behaviors in FST test. | Rincon-Cortes et al. (2016 | |||
| 2–9 (LBN) | Male | Wistar | PND 60 | Anxiety-like behaviors in EPM test. | Dalle Molle et al. (2012) |
| 2–9 (LBN) | Male | Sprague-Dawley | 10–12 months | Memory deficits in MWM and NOR tests. No anxiety-like behavior in EPM test |
Brunson et al. (2005) Ivy et al. (2010) |
| 2–9 (LBN) | Male | Sprague-Dawley | 10 weeks | Increased anxiety in EPM No memory deficit in NOR test. |
Maniam et al. (2016a) |
| Brain changes | |||||
| 1–7 (Scarcity) | Both | Long-Evans | PND 7 | Amygdala-locus coeruleus-olfactory bulb network perturbations. | Moriceau et al. (2009) |
| 3–8 (Scarcity) | Both | Long-Evans | PND 8 | Enhanced amygdala neural activity in mother-pup interaction test. | Raineki et al. (2010) |
| 1–7 (Scarcity) | Both | Long-Evans | PND 90 | Sex- and brain-region specific epigenetic alterations and changes in telomere length |
Asok et al. (2014) Blaze et al. (2013) (2015) Roth et al. (2009) (2014) |
| 8–12 (Scarcity) | Both | Long-Evans | PND 45 | Enhanced amygdala neural activity following FST. | Raineki et al. (2012) |
| 8–12 (Scarcity) | Both | Long-Evans | PND20–22 and 42–48 | Blunted neural activity in amygdala, mPFC and NAc following social interaction. | Rincon-Cortes et al. (2016) |
| 8–12 (Scarcity) | Male | Long-Evans | PND45 and 60 | Altered amygdala-PFC functional connectivity | Yan et al. (2017) |
| 2–9 (LBN) | Both | Wistar | PND 60 | Elevated plasma BDNF levels. | Dalle Molle et al. (2012) |
| 2–9 (LBN) | Male | Sprague-Dawley | 10–12 months | Dendritic atrophy of CA1 pyramidal cells and mossy fiber expansion in CA3. | Brunson et al. (2005) |
| Synaptic plasticity defects in CA3 and CA1 associated with physiological abnormalities in CA3. Augmented CRH expression in the hippocampus. |
Ivy et al. (2010) | ||||
| 2–9 (LBN) | Both | Sprague-Dawley | PND9 | Reduced CRH mRNA expression in the PVN and CRF1 mRNA expression in CA1 and dentate gyrus. Reduced CRH receptor binding capacities in pituitaries. Reduced GR gene expression in the PVN and frontal cortex. |
Avishai-Eliner et al. (2001) |
| 1–10 (LBN) | Male | Sprague-Dawley | PND10 and 20 | Increased spine density in BLA Increased evoked synaptic function (fEPSP) in BLA |
Guadagno et al. (2017) |
| PND70–90 | Increased conditioned fear, anxiety | ||||
| 2–9 (LBN) | Male | Sprague-Dawley | 13 weeks | Reduced hippocampal expression of genes involved in mitochondrial biogenesis, energy metabolism, neurogenesis | Maniam et al. (2016a) |
| 2–9 (LBN) | Female | Sprague-Dawley | 15 weeks | Reduced GR, NeuroD, Akt3 1 gene expression in hippocampus | Maniam et al. (2016b) |
| Brain-Gut axis and Metabolism | |||||
| 2–10 (LBN) | Both | Wistar | PND 10 | Increased gastrointestinal permeability to FD4 (M only) | Moussaoui et al. (2016a) |
| PND 21 | Increased gastrointestinal permeability to FD4 (F>M) | Moussaoui et al. (2016b) | |||
| PND 21 | Fecal microbiota alterations (increased abundance of Gram positive cocci and reduction of fiber-degrading, butyrate-producing and mucus-resident microbes) | Moussaoui et al. (2016b) | |||
| 2–9 (LBN) | Both | Wistar | PND 77–84 | Increased visceral sensitivity to CRD (M>F) | Guo et al. (2015), Holschneider et al. (2016) |
| 2–9 (LBN) | Both | Sprague-Dawley | PND 90–120 | Increased visceral sensitivity to CRD (M) | Prusator et al. (2015) |
ACTH, Adenocorticotropin Hormone; BDNF, brain-derived neurotrophic factor; BLA, basolateral amygdala; CORT, corticosterone; CRD, colorectal distension; CRH, corticotropin releasing hormone; CRF1, CRH receptor 1; EPM, elevated plus maze; FD4: FITC-dextran 4,4 kDa; FST, forced swim test, fEPSP, field excitatory postsynaptic potentials; GR, glucocorticoid receptor; LBN, Limited bedding and nesting; mPFC, medial prefrontal cortex; MWM, Morris water maze; Nac, nucleus accumbens; NOR, novel object recognition; PND, postnatal day; PVN, paraventricular nucleus of hypothalamus.
Based on: Molet et al. Dev Psychobiol 2014 with updates
Table 3.
Major Outcomes Provoked by the Limited Bedding/Nesting paradigm of Chronic Early-Life Stress (ELS) in Mice
| ELS Period (PND) | Sex | Strain | Acute/Long Term | Outcomes | References |
|---|---|---|---|---|---|
| Stress system perturbations | |||||
| 2–9 | Both | C57BL/6J | PND 9 and 4–8 months | Elevated basal corticosterone concentrations. | Rice et al. (2008) |
| 2–9 | Both | C57BL/6J | PND9,21,28. and 120 | Reduced body weight at PND9,21 Increased CORT at PND120 in males, reduced in females |
Arp et al. (2016) |
| 4–11 | Male | C57BL/6N | PND8,12,16, 21,28,38,50, and 75 | Elevated Corticosterone at PND16, Diminished brain MR and GR at PND12 | Bath et al. (2016) |
| Cognitive and emotional functions | |||||
| 2–9 | Both | C57BL/6J | PND 21, 29 and 63 | Enhanced anxiety-like behaviors in the novelty-induced hypophagia paradigm. | Malter Cohen et al. (2013) |
| 2–9 | Male | 129S2/Sv × C57BL/6J | 3 months | Enhanced anxiety-like behaviors in OF and light-dark box tests. | Wang et al. (2012) |
| 2–9 | Male | C57BL/6J | 4–8 months | Memory impairments in MWM and NOR tests No anxiety-like behavior in OF test. |
Rice et al. (2008) |
| 2–9 | Male | 129S2/Sv × C57BL/6J | 6 months | Memory impairments in MWM and Y-maze tests. | Wang et al. (2011) |
| 2–9 | Both | C57BL/6J | 3–4 months | No recognition of ‘safe’ periods after fear learning; impaired spatial memory in males | Arp et al. (2016), Kanatsou et al. (2017) |
| 4–11 | Both | C57BL/6N | PND21,28 38,50 and 75 |
Memory impairment across development in males Memory impairment at PND21 and PND38 in females |
Bath et al. (2017) |
| 4–11 | Male | C57BL/6N | PND8,12,16 21,28,38,50 and 75 |
Accelerated development of contextual fear inhibition | Bath et al. (2016) |
| Brain changes | |||||
| 2–9 | Both | C57BL/6J | PND 9 | Reduced CRH mRNA expression in the PVN. | Rice et al. (2008) |
| 2–9 | Both | C57BL/6J × 129Sv-SvJ | PND 18–26 | Upregulation of CRH expression in the PVN Astrocytic glutamate reuptake impairments and enhanced glutamatergic drive onto dorsal-medial neurons of the hypothalamus. |
Gunn et al. (2013) |
| 2–9 | Both | C57BL/6J × 129Sv-SvJ | 8 weeks | Upregulation of CRH expression in the PVN. | Gunn et al. (2013) |
| 2–9 | Male | 129S2/Sv × C57BL/6J | 3 months | No change in the gene expression of CRH and arginine vasopressin in the PVN, MR and GR in the hippocampus and CRH in the central amygdala. | Wang et al. (2012) |
| 2–9 | Male | C57BL/6J | 4–8 months | Reduced CRH mRNA expression in the PVN. | Rice et al. (2008) |
| 2–9 | Male | 129S2/Sv × C57BL/6J | 6 months | LTP deficits in CA3 Reduced number of dendritic spines in CA3 Reduced inhibitory synaptic density in CA1 and excitatory synaptic density in CA1 and CA3 Reduced Neurexin-1 mRNA levels in CA3 and Neuroligin-3 mRNA levels in CA1. |
Wang et al. (2011) |
| 4–11 | Male | C57BL/6N | PND8,12,16 21,28,38,50 and 75 |
Precocious development of PV+ and MBP mRNA, shift in ratio of NR2a:NR2b mRNA in the hippocampus. Earlier decline in Ki-67 and DCX mRNA. | Bath et al. (2016) |
| 2–9 | Both | C57BL/6J | PND9 | Increased neurogenesis in hippocampus (Ki67, Calretinin) | Naninck et al. (2015) |
| 2–9 | Male | C57BL/6J | 4 months | Reduced neurogenesis (survival of adult born neurons) | Naninck et al. (2015) |
| 2–9 | Male | C57BL/6J | PND9 and 4 months | Altered microglia (Iba1 and CD68) and cytokine expression in hippocampus | Hoeijmakers et al. (2016) |
| 2–9 | Male | C57BL/6J/APP/PS1 | 4 months 10 months |
Reduced cell associated amyloid pathology Reduced amyloid plaques |
Lesuis et al. (2016) Hoeijmakers et al. (2016) |
| Metabolic changes | |||||
| 2–9 | Both | C57BL/6J | PND 9 and 4 months | Reduced white adipose tissue (WAT) mass and reduced leptin (mRNA in WAT as well as circulating) | Yam et al. (2017) |
| 2–9 | Male | C57BL/6J | PND9 | Altered methionine in plasma and brain | Naninck et al. (2016) |
CORT, corticosterone; CRH, corticotropin releasing hormone; DCX, doublecortin; GR, glucocorticoid receptor; LTP, long-term potentiation; MBP, myelin basic protein; MR, mineralocorticoid receptor; MWM, Morris water maze; NOR, novel object recognition; NR, NMDA receptor subunit; OF, open field; PND, postnatal day; PV+, parvalbumin positive; PVN, paraventricular nucleus of hypothalamus
Based on: Molet et al. Dev Psychobiol 2014 with updates
3.1. Regulation of the hypothalamic pituitary adrenal (HPA) axis and stress responses
Since the 1950’s extensive literature on early life manipulations has indicated that most rodent and nonhuman primate developmental perturbation paradigms impact HPA development and its regulation throughout the lifespan. Activity in the HPA axis has been one of the first outcomes documented for the LBN paradigms in order to validate these models as inducing stress in the offspring (and the mother). The initial studies describing the LBN paradigm in the Baram and Sullivan laboratories both reported elevated basal plasma corticosterone (Molet, et al., 2014; Raineki, et al., 2010), although other groups have failed to observe significant differences (McLaughlin, et al., 2016) or reported a decrease (Moussaoui et al., 2017) in basal corticosterone levels in PND10 neonates (Figure 4, left panels). The Baram lab has conducted the most extensive assessment of the HPA axis and found adrenal hypertrophy in the LBN Sprague Dawley or Long Evans rat pups that is apparent already at the end of the stress (PND 9), and disappears later in adulthood (Avishai-Eliner, Gilles, Eghbal-Ahmadi, Bar-El, & Baram, 2001; Brunson, et al., 2005; Gilles, et al., 1996; Ivy, et al., 2008) (Figure 4, right panels). Elevated plasma levels of corticosterone have also been observed in LBN mice on PND9 and in adulthood (Rice, et al., 2008). In addition, increased glutamatergic innervation of corticotropin releasing hormone (CRH)-expressing, stress-sensitive hypothalamic neurons has been reported on PND 18–26 mice subjected to the LBN procedure (Gunn et al., 2013) as a result of reduced neurosteroid inhibition on these neurons. Thus, exposure to LBN promotes rapid plasticity at the level of the CRH system already during the neonatal stress period, which might contribute to long-lasting consequences.
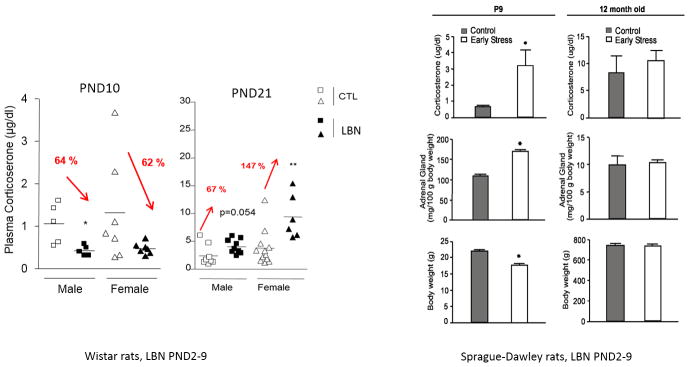
Figure 4.
Modifications in basal corticosterone plasma concentrations induced by the LBN procedure in rats. Left panels: Plasma levels in male (squares) and female (triangle) control (white) and LBN (black labelled LBS)-reared Wistar rat pups from PND2–10. At PND10, both male and female LBS pups show reduced corticosterone plasma concentrations compared to same sex controls (n=5–7 pups/condition). At PND21, LBS male and female pups exhibit increased corticosterone plasma concentrations compared to same sex controls, with higher concentrations in females (n=4–12 rats/group). *, P<0.05; **, P<0.01 compared to CTL (Student t- test) Modified from: Moussaoui et al. (Moussaoui, et al., 2017; Moussaoui, et al., 2016). Right panels: Parameters indicative of chronic stress in 9-day-old Sprague-Dawley rat pups experiencing a week of LBN; (left column) and in adult male rats (12 months of age; right column). Elevated basal corticosterone concentrations, higher adrenal weights, and modestly lower body weight are found in chronically stressed 9-day old rats (n=12/group). These changes are no longer apparent in adult rats (Control: n=5, Early stress: n=6). Values are mean ± SEM. *, P <0.05 (Student’s t-test). From:. Brunson et al.(Brunson, et al., 2005) with permission.
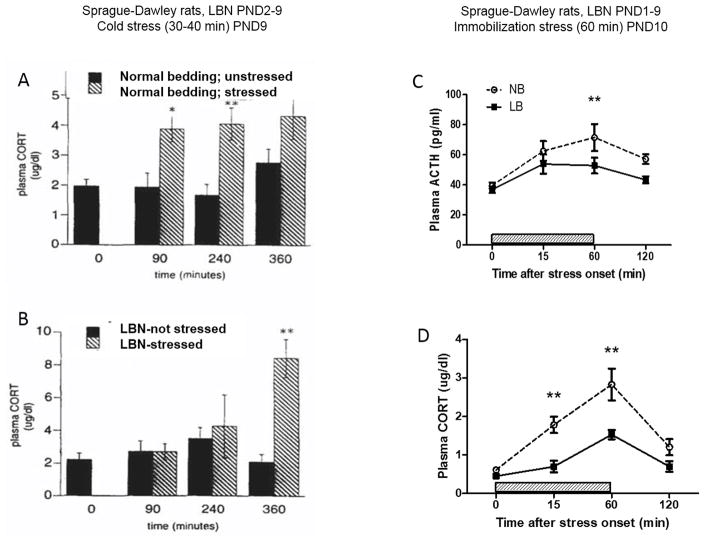
Other laboratories have found that the LBN procedure in either Sprague-Dawley or Wistar rats alters the development of the HPA axis in a time and sex-dependent manner as shown by a significant reduction of basal corticosterone plasma levels and adrenal gland weight in both male and female Wistar pups at PND10 (Moussaoui et al., 2016). In a careful assessement of the developmental trajectory of HPA development, it was found that at PND21, LBN pups exhibited increased basal plasma corticosterone levels compared to same sex controls with increased hypercorticosteronemia in females compared to males (147% vs 67% higher, Figure 4) (Moussaoui, et al., 2017), reminiscent of the exaggerated Fos response in the paraventricular nucleus of the hypothalamus observed in pre-adolescent rats after repeated restraint stress (Romeo, et al., 2016). This study found that the alteration of the HPA axis is specific to the chronic stress exposure of LBN from PND 2–10 as pups subjected to brief maternal separation (BMS; 15 min/day) during the same time period did not exhibit changes in basal plasma corticosterone levels at either PND10 (Moussaoui, et al., 2016) or PND21 (Moussaoui, et al., 2017). The lack of effect of LBN on basal ACTH and corticosterone levels was also observed in PND10 Sprague-Dawley pups (McLaughlin, et al., 2016), but this was accompanied by a significant reduction in stress-induced secretion of both ACTH and corticosterone on PND10 (Figure 5, right panels), suggesting that the stress of LBN might have either delayed the development of responsiveness in the HPA axis or significantly inhibited its activation in order to conserve available energy resources. Of interest, in both studies, LBN significantly reduced body weight gain and modified some aspect of maternal behavior towards greater fragmentation and “unsettledness”. These results indicate that LBN might limit the activation of the HPA axis during exposure to the stressful procedure and induce a hyperactive HPA axis at weaning and during the pre-adolescent period that could be more prominent in female than male pups.
Figure 5.
Stress reactivity in Sprague Dawley rat pups raised in either control (not handled, normal bedding, NB) or in limited bedding/nesting (LBN) conditions. Left panels: Comparison of the effects of rearing in the NB (top) or LBN (bottom) NB conditions on the response to cold-separation stress on PND9. Stress-induced plasma corticosterone (CORT) concentrations increased as compared with those in nonstressed “controls” in both groups. The magnitude of the stress response was significantly higher in the LBN group versus the NB group at 360 min (P < 0.05), consistent with poor recovery from the stress. (Note that the scale is increased in the bottom panel). Data are means ± SEM of 4–10 pups/group. **, P<0.01; *, P<0.05 compared to non-stressed group. From Gilles et al.(Gilles, et al., 1996) with permission. Right panels: HPA axis responsiveness to 60 min immobilization stress in PND10 rat pups in either control (normal bedding, NB) or limited bedding/nesting (LBN, termed here LB) conditions. LBN pups exhibit a significant reduction in both ACTH and corticosterone secretion compared to NB pups, most apparent at the end of the stress period (60min). Values are mean ± SEM of 5–6 rats/group. *P < 0.05. **P < 0.01 (Tukey HSD test). Redrawn from: McLaughlin et al. (McLaughlin, et al., 2016).
It is currently unclear which additional factors in the environment of the LBN paradigm might contribute to either activation or suppression of the neonatal HPA axis, proximal to the application of the chronic stressor. However, regardless of the direction of the effect on the neonatal HPA axis, it is clear that LBN has long lasting consequences beyond the application of the stressor on a number of systems that have been studied either in pre-adolescence, adolescence or adulthood. In terms of the HPA axis, the increased vulnerability to stress observed by some at weaning age (Moussaoui, et al., 2017) disappears in late adolescence (Molet, Maras, et al., 2016). However, despite restoration of HPA activity, morphological changes in the dorsal hippocampus (reduced volume and disruption of dendritic structure) was observed in LBN adolescent rats, consistent with disrupted local connectivity (Molet, Maras, et al., 2016).
3.2. Emotional responses
3.2.1. Infant attachment learning
It is well documented that infants of myriad species learn to attach to their caregiver regardless of the quality of care received and even when pain is produced by the caregiver or experienced with the caregiver. The use of the Scarcity Model has been instrumental to better understand the neural mechanism for attachment learning associated with pain induced either through maltreatment by the mother (Scarcity model) or in a more controlled learning paradigm outside the nest (odor-shock conditioning) (Moriceau, et al., 2009; Raineki, et al., 2010; Raineki, Shionoya, Sander, & Sullivan, 2009; T. L. Roth & Sullivan, 2005; Sullivan, et al., 2000). The phylogenetically old attachment system is supported by a unique infant learning system, where the reward can be either pleasant (i.e. food, warmth) or painful (shock, mother stepping on pups): pairing the maternal odor with any reward supports learned approaches to the mother, as well as social interactions and nipple attachment. The neurobiology supporting this attachment learning depends upon enhanced preference learning involving the locus coeruleus (LC) norepinephrine-dependent attachment circuitry (Moriceau & Sullivan, 2004, 2005) combined with reduced aversion/fear learning that relies on an immature amygdala that cannot support threat (fear) learning(Sullivan, et al., 2000) (Moriceau & Sullivan, 2005). This unique infant learning neurobiology provides rapid learning of attachment to the caregiver but also prevents pups from learning to avoid the caregiver inflicting pain or maltreatment on the offspring (Moriceau & Sullivan, 2005; Raineki, et al., 2010; Sullivan, et al., 2000). Importantly, with maturation, pup access to this unique attachment learning circuitry terminates, when the amygdala learning about pain and the LC no longer supports attachment learning (Moriceau, et al., 2009; Upton & Sullivan, 2010).
Using the Scarcity Model, Roth and Sullivan (T. L. Roth & Sullivan, 2005) showed that pups learn to attach to a mother exhibiting maltreatment of pups and minimal nurturing behaviors. Importantly, the learning neural circuitry used during this naturalistic maltreating attachment learning paradigm was indistinguishable from that engaged during more controlled assessment of the attachment learning circuitry outside the nest. Thus, pups in the Scarcity paradigm do not exhibit attachment deficits compared to the control pups.
Although the LBN environment supports attachment to the caregiver, there are immediate and enduring consequences of experiencing pain with the attachment figure under these conditions. First, while the amygdala is immature and not exhibiting the plasticity to support threat (fear) learning, the amygdala is still responding to the pain. The value of the maternal odor is also compromised: the deaf and blind pups depend upon their mother’s odor for interactions with the mother. While LBN pups express the survival dependent behaviors with the mother, they are significantly slower behaviorally and there is less robust connectivity between olfactory and limbic structures (Perry, Al Aïn, Raineki, Sullivan, & Wilson, 2016). Indeed, LBN pups have accelerated amygdala development, which prematurely ends the sensitive period for attachment learning (T. L. Roth et al., 2013) and disrupts social behavior with siblings (Moriceau, et al., 2009; Raineki, et al., 2012).
3.2.2. Fear learning and anxiety
Clinical and preclinical research converges to demonstrate that early-life adversity such as abuse and neglect, compromises brain development and significantly increases the vulnerability to mental health problems, including anxiety and depression. The models of ELS discussed here using both intermittent and continuous exposure to ELS have documented long term effects on fear and anxiety, allowing probing of the mechanisms by which ELS increases vulnerability to these pathologies (Krugers et al., 2016). As is documented here, the LBN paradigm produces an animal that shows increased fear but also decreased responses to safety. Application of the Scarcity Model (continuous from PND 1–6) showed that this treatment accelerated the development of the amygdala’s ability to participate in threat/fear conditioning. Specifically, rat pups are not capable of threat (fear) learning until PND10, which is causally related to functional development of the amygdala at PND10 (Sullivan, et al., 2000). However, rearing using the Scarcity Model enabled rat pups to learn amygdala-dependent threat at PND7 (Moriceau, et al., 2009).
In mice, Bath et al. also observed a significant shift in the developmental timing of contextual fear inhibition. Specifically, mice show a suppression in emotional responding to contexts that have been associated with an aversive stimulus, and that this suppressed response occurs during a defined developmental period between PND28–35. In mice exposed to LBN, the suppressed emotional responding occurred a full week earlier (PND22), which suggests that maturation of fear circuits was accelerated by the LBN procedure (Bath, et al., 2016). Using the continuous LBN paradigm, Arp et al. (Arp et al., 2016) also showed that the ability to discriminate between cue-on and ‘safe’ episodes in-between cue exposure, 24 h after fear learning, is hampered in adult male mice exposed as neonates to LBN (Arp, et al., 2016). During these cue-off periods, LBN offspring displayed a higher amount of freezing than controls, suggesting that LBN offspring, as opposed to controls, could not discriminate between cue-on and cue-off episodes.
3.2.3. Emotional processing
Depression in humans and depressive-like behavior in rodents involves a complex circuitry in which the amygdala appears to play a central role (Huang & Lin, 2006; Ressler & Mayberg, 2007; Sevelinges et al., 2011; Sibille et al., 2009). It is well known that ELS constitutes a strong risk factor for the development of adult depression and anxiety (Heim & Binder, 2012; Sanchez, Ladd, & Plotsky, 2001). Both the LBN and the Scarcity Model have recapitulated important aspects of emotional consequences of ELS in humans. Adult rat offspring raised under the Scarcity Model displayed increased time immobile in the forced swim test (FST) and this was associated with increased amygdala activation in central, lateral, and basal nuclei (Raineki, et al., 2012). Increased amygdala activation might be in part supported by morphological changes occurring as a consequence of exposure to the LBN paradigm. Morphological changes in pyramidal neurons of the basolateral amygdala have already been observed in neonatal and weaned LBN rat offspring (Guadagno, Verlezza, Long, Wong, & Walker, 2016) (Guadagno, et al., 2016) whereas they were absent after maternal deprivation (Krugers et al., 2012), suggesting that the naturalistic LBN procedure has value distinct from the maternal deprivation model, where there is prolonged removal of maternal care. The amygdala-dependent depressive-like behaviors do not emerge until pups reach independence after weaning, and persist into adulthood(Raineki, et al., 2012). Importantly, the involvement of the hyperactive amygdala in the expression of depressive-like behavior in rats exposed to the Scarcity Model was further demonstrated by the fact that temporary suppression of the amygdala activity with muscimol (a GABAA receptor agonist) was able to reverse the depressive-like behavior observed in the forced swim test, suggesting a causal link between increased amygdala activity and depressive-like behavior in these animals (Raineki, et al., 2012).
During adolescence, depressive-like behavior has been observed in rats raised under the LBN paradigm since these rats exhibit reduced sucrose preference, an indication of anhedonia (Molet, Heins, et al., 2016). Anhedonia, the reduced ability to experience pleasure, is an important emotional variable because it is often a harbinger of depression. However, in these studies, adult LBN rats did not exhibit depressive-like behaviors in the FST or anxiety-like behaviors in the open field or elevated plus maze (Molet, Heins, et al., 2016). In contrast, others have reported a sexual dimorphism in the response to the open field and the elevated plus maze with LBN males showing a higher anxiety compared to their control counterparts. No effect was seen in females(Guadagno, Wong, & Walker, 2017) (Guadagno, et al., 2017). Increased anxiety-like behaviors was also documented in the elevated plus maze in adult Wistar rats that were raised in a LBN environment (Dalle Molle, et al., 2012). Several laboratories have implemented the LBN paradigm in mice and found either no increase (Naninck, et al., 2015) or increased anxiety-like behaviors in the open field and light-dark emergence box (X. D. Wang et al., 2013).
3.3. Social behavior and aggression
The usefulness of LBN rearing as a model of rodent depressive-like behavior has been demonstrated in several studies, and in particular using the Scarcity Model (PN8–12). Pups raised in a LBN environment show dysfunctional social attachment behaviors with the mother when tested at a preweaning age (Raineki, et al., 2010), followed by deficits in social behavior with peers during adolescence and adulthood (Raineki, et al., 2012; Raineki, et al., 2015; Rincón-Cortés & Sullivan, 2016).
One important aspect of social behavior in the juvenile and adolescent periods is peer play behavior, which is a good indicator of the ability to experience pleasure. In experiments scoring peer play behavior in late adolescent-early adult rats (PND57–60), LBN rats did not differ from controls in the overall duration of social interactions although the time devoted to peer social play was significantly lower in LBN compared to control rats (Molet, Maras, et al., 2016).
Alterations in neuroligin-2 expression have previously been associated with changes in social behavior linked to psychiatric disorders, including schizophrenia and autism. A recent study reported that early-life stress, induced by LBN leads to impaired social recognition and increased aggression in adult mice, accompanied by increased expression levels of neuroligin-2 in the ventral hippocampus (Kohl et al., 2015). In these studies, a social retrieval test showed that in contrast to control mice, LBN male mice did not discriminate between the newly introduced juvenile and the one already encountered in the acquisition phase of the test. Thus it appears that in both rats and mice, the consequences of being raised in LBN conditions affects a continuum of social interactions from maternal attachment to interactions with peers in adolescence and adulthood.
3.4. Cognitive functions
3.4.1. Hippocampus
A large number of studies have investigated the consequences of ELS on cognitive functions and examined in particular the changes induced by LBN in the hippocampus and prefrontal cortex, key structures for cognition and regulation of HPA axis activity (Baram, et al., 2012). In addition to structural changes commonly related to cognitive functions such as reductions in rat hippocampal CA1 dendritic complexity (Brunson, et al., 2005; Ivy et al., 2010; Molet, Maras, et al., 2016) and spines in the hippocampal CA3 area (X. D. Wang, et al., 2011; X. D. Wang, et al., 2013), LBN has been shown to alter neurogenesis in the mouse dentate gyrus (Naninck, et al., 2015) and to lead to an earlier temporal decline in the expression of markers of hippocampal neurogenesis over the postnatal period (Bath, et al., 2016). Interestingly, in mice, the stress of LBN initially increases neurogenesis (i.e. proliferation and differentiation of newborn cells) at PND9, but at later time points (PND150), the survival of the newly born cells as well as the volume of the dentate gyrus are reduced (Naninck, et al., 2015). One way to interpret this finding is that exposure to fragmented care during this sensitive period of hippocampal development, causes or ‘forces’, subsets of hippocampal stem cells to divide that otherwise would not do so. Since this initial increase is followed by a later, lasting decrease in survival of newborn cells, this indicates that specific populations of stem cells may be depleted after their activation during ELS, and are no longer available in later life. This could be considered a measure reflecting a change in ‘overall’ hippocampal plasticity.
It remains to be determined however, whether the stem cells actually die after ELS, or whether they are rather (re-)programmed and become restricted in their subsequent proliferative capacity during adulthood. Neither is it known whether these changes are cell autonomous and whether stress triggers endogenous stem cell specific cell death programs, or whether ELS rather induces cell non-autonomous and more local changes, for instance in extracellular matrix proteins or microglia mediated phagocytosis. In line with this possibility, LBN leads to an increased expression of hippocampal CD68, a marker for microglia phagocytic activity (Hoeijmakers, et al., 2016).
Quite recently, the structural changes in hippocampus after exposure to LBN have been visualized non-invasively using high-resolution MRI (Molet, Maras, et al., 2016). These studies have shown a selective loss of dorsal hippocampal volume in LBN rats during late adolescence and a disruption of intra-hippocampal dendritic structure, consistent with disrupted local connectivity, already during this period. This suggests that hippocampal volumetric changes and alterations in fMRI connectivity may represent a non-invasive marker of incipient and overt cognitive deficits in human children who have experienced early life adversity.
Results from studies using the LBN paradigm have documented that ELS causes adaptive changes in neural mechanisms that can impair neuroplasticity in the brain, both early in life and in adulthood. In line with the structural findings mentioned above, synaptic plasticity in the hippocampus is reduced in adult LBN rats in the hippocampal CA1 and CA3 area (Brunson, et al., 2005) while synaptic plasticity is reduced only in the hippocampal CA3 area (but not hippocampal CA1 area) in the adult male mouse (X. D. Wang, et al., 2011). In the mouse, reduced hippocampus synaptic plasticity was associated with an earlier shift in NMDA receptor subunit composition (Bath, et al., 2016), which may contribute to developmental changes in circuit plasticity. The molecular and cellular mechanisms mediating the effects of LBN on morphological and synaptic plasticity are now starting to emerge. For instance, manipulation of the CRH system by blocking CRH receptor type 1 immediately after the LBN period in rats was shown to prevent the apical dendritic retraction and spine loss in the hippocampus after LBN as well as impairments in watermaze measures of spatial memory (Ivy, et al., 2010).
Genes associated with plasticity, i.e. GSKα and GSK3β, which are also a main target of antidepressant drugs are reduced in male LBN rats, but this reduction could be reversed when LBN offspring were exposed to a diet high in fat and sugar post weaning (Maniam, Antoniadis, Le, & Morris, 2016). LBN also leads to an alteration in levels of the essential amino acid methionine in the plasma and hippocampus of the offspring. Methionine is one of the key components of 1-carbon metabolism. Supplementation of the maternal diet only from PND2–9 with essential 1-carbon metabolism-associated micronutrients, not only restored methionine levels peripherally and centrally, but also rescued (some) of the effects of LBN on hippocampal cognitive measures (Naninck et al., 2016).
In behavioral paradigms, LBN reduces spatial learning performance in the water maze (Naninck, et al., 2015) as well as in stress-free tests such as object location and novel object recognition in rats and mice (Bath, et al., 2016; Brunson, et al., 2005; Molet, Maras, et al., 2016; Naninck, et al., 2015; Rice, et al., 2008). This is consistent with reduction in neurogenesis found in the LBN model in mice (Naninck, et al., 2015). Others have found that in adult rats, spatial and object recognition memory were unaltered by LBN exposure or consumption of a diet rich in fat and sugar after weaning, but the postweaning diet ameliorated the increased anxiety-like behavior induced by LBN exposure suggesting it may have “therapeutic value” (Machado et al., 2013)(Bath, et al., 2016; Brunson, et al., 2005; Naninck, et al., 2015; Rice, et al., 2008)
While hippocampal dependent learning and memory processes are generally hampered, LBN in fact enhances contextual aspects of conditioned fear as described above (Arp, et al., 2016; Krugers, et al., 2016). In particular, LBN reduces the ability to discriminate between tone-on and tone-off periods in an auditory fear-conditioning paradigm (Arp, et al., 2016). This distinction resembled the differential responses in another ELS paradigm (24hrs maternal deprivation at PND 3) where spatial memory (i.e. water maze performance) was also found to be impaired, whereas emotional learning (i.e. fear conditioning) was enhanced several weeks after maternal deprivation (Oomen et al., 2011; Oomen et al., 2010). Also here, sex specific effects were found with female rats being relatively spared compared to males (Loi, Koricka, Lucassen, & Joëls, 2014; Loi et al., 2015; Oomen et al., 2009).
3.4.2. Prefrontal cortex
Early life stress also strongly affects development of the prefrontal cortex in humans and rodents (Yang et al., 2015). In humans, exposure to ELS during the first years of life was associated with higher regional homogeneity of resting state fMRI in the lateral frontal cortex (Demir-Lira et al., 2016), broader recruitment of several brain regions and increased hippocampal-prefrontal cortex connectivity during an aversive learning paradigm (Silvers et al., 2016). In rodents, stress exposure during the first postnatal week hampers the development of dendrites in layers II/III and V pyramidal neurons in various subregions of the prefrontal cortex and reduces performance in the temporal order memory task, which assesses prefrontal cortex function (Yang, et al., 2015). In analogy to the hippocampus, manipulation of the CRH system by systemic administration of antalarmin, a CRH-R1 antagonist, prevented the apical dendritic retraction and spine loss in the prefrontal cortex after LBN as well as impairments in prefrontal cortex-dependent cognitive tasks. Dendritic regression correlated with the degree of cognitive deficits after ELS. Using another ELS paradigm, i.e. maternal separation during the entire postnatal period, it was reported that stressed females, but not males had increased infralimbic mPFC apical dendritic branch number and length at PND40 compared to controls (Farrell, Holland, Shansky, & Brenhouse, 2016). Together, these studies suggest that ELS, and in particular LBN, tends to hamper higher order cognitive functions while enhancing the emotional aspects, in particular expression of conditioned fear (Krugers, et al., 2016).
4. Metabolic aspects of the LBN paradigm
So far, the lasting effects of LBN on cognition have been mostly attributed to alterations in maternal care and neuroendocrine factors, while the role of early nutrition and metabolic hormones has been less studied. However, both aberrant maternal care and early-life malnutrition often occur simultaneously. Indeed, clinical studies show they can have similar consequences on cognition, suggesting that nutritional elements might mediate some of the ELS effects on brain structure and function. Various elements of the early-life environment (e.g. maternal care, neuroendocrine factors, nutrient availability, and metabolic hormones) are often considered for their independent actions, although they should be considered synergistic because of the intense crosstalk between stress and metabolic programming (L. Wang, Goebel-Stengel, Yuan, Stengel, & Taché, 2017). Lasting effects of LBN may then result from the synergistic action of: (i) the quality and quantity of early nutrition (ii) stress hormones and (iii) sensory stimuli from the mother (Lucassen et al., 2013). Therefore, it is important to also study if (and how) ELS alters the nutritional/metabolic environment in the mother and her offspring.
Several essential micronutrients that depend on dietary intake, including methionine, homocysteine, vitamins B6, B12, B9 (folic acid) are critical in the one-carbon (1-C) metabolism that is required for methylation, and for synthesis of proteins, phospholipids and neurotransmitters. Such methyl donors are vital for brain function and neuronal development as they are involved in processes like neurogenesis and epigenetic mechanisms, which are altered by early exposure to the LBN procedure. Moreover, impairments or deficits in these essential micronutrients have been associated with neurological disorders and developmental anomalies.
Early exposure to stress could also modulate the composition of the dam’s milk, and/or nutrient intake by the pup (Lucassen, et al., 2013; Naninck, et al., 2016; Yam et al., 2015). While this could have lasting consequences for brain structure and epigenetic regulation, it also provides interesting therapeutic opportunities for dietary interventions during sensitive early periods to rescue or prevent lasting consequences of ELS. The same applies for possible alterations in fat metabolism, which is also persistently altered by early stress exposure (Yam et al., 2017; Yam, et al., 2015). In fact, immediately following LBN, the mouse offspring exhibited reductions in white adipose tissue (WAT) mass, plasma leptin levels and in leptin mRNA expression in WAT. Furthermore, LBN exposure led to increased brown adipose tissue and browning of WAT, which was evident as a drastic increase in uncoupling protein 1 mRNA expression in the inguinal WAT at PND9. Notably, the LBN-induced reductions in WAT mass, plasma leptin and leptin expression in WAT were sustained into adulthood and were accompanied by changes in body fat distribution, such as a higher ratio between mesenteric WAT and other WAT deposits. Interestingly, while LBN exposure increased leptin receptor mRNA expression in the choroid plexus, it was unaltered in the hippocampus (Yam, et al., 2017).
In the context of the LBN paradigm, it is likely that some nutritional and metabolic aspects are altered since pup body weight is consistently reduced by 4–5% in PND10 male and female pups raised by Wistar or Sprague Dawley dams exposed to LBN from days 2–10 compared to same sex control pups (Brunson, et al., 2005; McLaughlin, et al., 2016; Molet, Heins, et al., 2016; Moussaoui, et al., 2016). A trend for lower body weight gain was still observed at PND21 in both males and female rats (Moussaoui, et al., 2017), but typically, the differences in body weight between LBN and control rats disappear in adolescence (Molet, Heins, et al., 2016) and adulthood (6 weeks) for both male and female rats. In the Scarcity Model of impoverished early environment, there is no change in body weight gain between control and stressed rat pups, possibly because of the shorter duration of the exposure, the age of exposure or alternation between a normal and abusive mother. In mice LBN exposure leads to a reduced body weight gain (~2.5 g in LBN vs 3.5 g in CTL) in both males and females. The differences in body weight are still present at around weaning and mostly disappear at around P40 (Naninck, et al., 2015; Yam, et al., 2017).
The reduction in body weight gain associates with a lower nutritional intake in LBN pups as both male and female LBN Wistar rat pups exhibit a 11–12% reduction in blood glucose on PND10, with a significant effect in LBN female pups when compared to same sex control-reared pups (Moussaoui, et al., 2016). The glucose levels measured in PND10 LBN pups were comparable to those of control pups at PND7 (Kuznetsov, Selina, & Kuznetsova, 2011), suggesting a lack of adequate age-related nutritional intake. It is unclear how much the nutritional alterations induced by the LBN paradigm and fragmentation of maternal behavior contribute to the consequences of ELS on the phenotypes described above. Recently, it was shown that LBN also alters micronutrient availability and that early nutritional intervention with micronutrients could prevent some of the LBN-induced later cognitive deficits (Naninck, et al., 2016) as well as alterations in fat tissue and metabolism (Yam, et al., 2017; Yam, et al., 2015).
Some recent studies have shown that changes in the postweaning diet can modify the effects of LBN on brain function and metabolism. Males exposed to LBN were lighter at weaning and in this experimental group, those who consumed a low fat chow diet after weaning went on to show improved glucose tolerance in adulthood compared to unstressed controls, and their male littermates who consumed a high fat, high sugar diet. They also had improved insulin sensitivity compared to controls consuming the same diet (Maniam, et al., 2016). Whether this relates to a slower growth trajectory early in life remains to be determined. It is possible that there are significant interactions between ELS and postweaning diet, which mitigate the long-term outcome of LBN exposure (Fuentes et al., 2014). A recent study showed that sucrose consumption from weaning had more marked effects on hippocampal expression of genes involved in plasticity than ELS, with no additive effects (Maniam, et al., 2016), suggesting that dietary and lifestyle exposures following ELS are likely contributing to outcomes. Given that poor diet and ELS exposure often co-exist in the general population, interventions to offset these deficits observed at the molecular level are critical. Indeed, LBN-exposed mice subjected to a Western style diet exhibit a higher increase in adiposity when compared to controls, suggesting that ES exposure might result in a higher vulnerability to develop obesity in a moderate obesogenic environment (Yam, et al., 2017). Overall, important modulating influences of diet, sex, strain and environmental conditions experienced during adulthood need to be considered in analyzing the global impact of ELS on each individual.
4.1. Brain-Gut axis
4.1.1. Epithelial permeability
In addition to a lower macro- and micronutrient intake in LBN pups, changes in the intestinal barrier permeability to macromolecules might represent an important factor impacting weight gain and metabolism. Intestinal barrier permeability is highest in neonatal rats during the first 2 weeks of suckling and declines thereafter until PDN21, although it remains permeable in weaning compared to adult rats (De Palma et al., 2015; Telemo, Weström, Ekström, & Karlsson, 1987). LBN exposure from PND2–10 significantly increases intestinal permeability in male Wistar rat pups on PND10, as measured by the increased passage into blood of a fluorescent probe (fluorescein isothiocyanate (FITC) labeled 4-kD dextran or FD4) administered orally. Female pups were not affected by LBN prior to weaning (Moussaoui, et al., 2016) although at weaning, the increase in permeability was observed in both sexes but to a greater extent in PND21 female pups. Diet and luminal factors (nutrients, bacteria, and bacterial products) can exert both beneficial and deleterious influences on the intestinal barrier (De Santis, Cavalcanti, Mastronardi, Jirillo, & Chieppa, 2015; Ulluwishewa et al., 2011; Winter, 2006). Food in the gut lumen is an important stimulus to mucosal cell growth. There is a possibility that the increase in intestinal permeability noted in LBN pups could be related to villous atrophy due to reduced nutrient intake as seen in patients and rodents suffering from malnutrition (Clough, Prykhodko, & Råberg, 2016; Norman et al., 2012). Conversely, impaired or delayed barrier function in response to the LBN stress could affect proper nutrient absorption and limit pup weight gain. The temporal sex differences observed in LBN-induced intestinal permeability was not explained by changes in corticosterone secretion or large modifications in sex hormones that modulate intestinal barrier function since these do not occur until PND30 in rats (Walker, Juenger, & Gore, 2009). However, it is still possible that LBN modifies the low level of gonadotropins present during the neonatal period (Bjelobaba, Janjic, Kucka, & Stojilkovic, 2015), thus impacting intestinal permeability in a sex-dependent manner. These findings point to a different susceptibility to LBN-related alterations in intestinal barrier function in males and females with later responsiveness occurring at weaning in females compared to males.
4.1.2. Intestinal microbiota
During the neonatal period, bacterial colonization occurs and promotes the development of food tolerance and immune function in association with the increased intestinal permeability (Turner, 2009). A few reports indicate that during this critical period of mucosal maturation, ELS may influence the microbiota which could induce long lasting effects on the gut function (De Palma, et al., 2015; R. Moloney, Stilling, Dinan, & Cryan, 2015). For instance, maternal separation stress results in a distinct fecal microbiota in adulthood (O’Mahony et al., 2009; O’Mahony, Clarke, Dinan, & Cryan, 2017) with a reduction of Lactobacillus species in the distal colon at PND20 (Gareau, Jury, MacQueen, Sherman, & Perdue, 2007; R. D. Moloney et al., 2016). In the LBN model, data are still scarce. However a recent study indicates that pups exposed to LBN from PND2–9 showed at weaning a decreased fecal microbial diversity and distinct composition characterized by the increased abundance of Gram positive cocci and reduction of fiber-degrading, butyrate-producing and mucus-resident microbes (Moussaoui, et al., 2017). Interestingly, such a pattern is similar to control animals on PND14 and suggests that LBN rats may have delayed maturation of the neonatal microbiota. Of note, no significant sex difference was observed in either the diversity or composition of fecal microbiota in weaned rats. Sustained perturbations in the intestinal microbiota related to LBN may be a potential contributing mechanism to the increased susceptibility to developing irritable bowel syndrome (IBS) later in life (Barbara et al., 2016). In addition, modifications in the neonatal intestinal microbiota induced by LBN might also be critical for regulation of postnatal HPA stress responses, behavioral alterations and neurodevelopment (Jašarević, Howerton, Howard, & Bale, 2015).
4.1.3. Visceral pain
Early life stress is now recognized as being a key factor in the development of functional pain disorders (Chaloner & Greenwood-VanMeerveld, 2013). These include functional gastrointestinal disorders like IBS, which is associated with visceral hypersensitivity (Beesley, Rhodes, & Salmon, 2010; Bradford et al., 2012; Felice, Moloney, Cryan, Dinan, & O’Mahony, 2015). Preclinical studies have demonstrated that maternal separation during early life predisposes rodents to visceral hyperalgesia as adults with a prominent role of CRH in the process and related changes in intestinal permeability and mast cell activation (Larauche, 2012; Taché & Million, 2015). Recent work also demonstrated that exposure to LBN affects the visceral pain responses of animals once they reach adulthood (Guo, Wang, Mayer, & Holschneider, 2015; Holschneider, Guo, Mayer, & Wang, 2016). Adult male and female Wistar rats that were exposed to LBN as pups exhibit a visceral hyperalgesia, as assessed by abdominal contractions to colorectal distensions monitored by electromyographic recording electrodes. Data indicate that males appear to be slightly more responsive than females at lower pressures of colorectal distension (Guo, et al., 2015; Holschneider, et al., 2016). This predisposition of LBN males to be more responsive to colorectal distension was also shown in a separate study using Sprague-Dawley rats (Prusator & Meerveld, 2015). More generally, LBN adult rats also display significant changes in functional activation of numerous regions within the pain pathway activated by colorectal distention (Holschneider, et al., 2016). Whether the increase in intestinal permeability, microbiota and/or HPA axis induced by LBN contribute to the altered viscerosensitivity in adulthood and sex difference still needs to be investigated (Million & Larauche, 2016).
5. Sex differences in outcomes
Sex is an increasingly recognized biological variable in understanding consequences of early life events (McCarthy, 2016). As mentioned in several of the earlier sections, male and female offspring do not always show comparable phenotypes after LBN. This sex-difference also appears to be age-dependent. For instance, LBN exposure from PND2–10 was found to increase significantly intestinal permeability in male but not female rat pups at PND10, whereas at PND21 permeability increases were present to a greater extent in female compared to male pups (Moussaoui, et al., 2016). Also in the cognitive domain clear male-female differences have been observed. For instance, with respect to the ability to discriminate between cue-on and cue-off episodes after fear learning, LBN conditions in early life did not lead to any deficit in performance of female mice when tested in adulthood, as opposed to the clear deficits observed in males (Arp, et al., 2016). Contextual and spatial memory formation of female mice was also not affected by LBN conditions (S Kanatsou et al., 2016), which differs from what is generally reported in males (S Kanatsou et al., 2017; Naninck, et al., 2015). However, female mice did show increased anxiety after LBN conditions in early life as observed in the open field, and somewhat reduced fear memory formation (S Kanatsou, et al., 2016).
Sexually-dimorphic consequences fit well with the overall picture emerging from a large body of studies on various models of early life adversity in rodents (Loi, et al., 2015). Impaired behavioral performance in adult rodents that had been subjected to adverse early life conditions was observed in one third of the experimental endpoints reported for males, whereas this was only 25% for females (Loi, et al., 2015). Whenever sex-dependent differences were observed, the majority (64%) of the studies reported significant effects in males but not in females. Only in about one third of cases (30%) did females but not males show significant effects. The most striking sex differences, though, appeared when taking the various cognitive domains into account. The percentage of reported experimental series showing significant changes after early life adverse conditions (total of 64 studies) in males was: depressive-like behavior (69%) > non-stressful hippocampus dependent learning (50%) > stressful learning (e.g. contextual fear conditioning) (45%) > social behavior (43%) > anxiety related behavior (40%); and for females: depressive-like behavior (50%) > stressful learning (41%) > anxiety related behavior (36%) > social behavior (33%) > non-stressful hippocampus dependent learning (29%) (Loi, et al., 2015). These observations suggest that with respect to non-stressful hippocampus dependent learning –such as object in context or object relocation tasks- considerable sex-dependent effects seem to exist. Thus, many of the later, structural and functional effects of the LBN procedure or other ELS models (i.e. maternal deprivation) are often found to be more pronounced in male offspring, while females seem to be more protected from ELS effects (Loi, et al., 2014; Loi, et al., 2015; Naninck, et al., 2015; Oomen, et al., 2011; Oomen, et al., 2010). However, recent reports indicate that females may also show modest impairments in spatial abilities following ELS, but these effects do not persist into adulthood (Bath et al., 2017)
The bases for these sex differences are not clear. It should be noted, however, that the differences may be partially a result of the tests used by investigators. For example, females (even control females) tend to perform relatively poorly in object location and recognition tests. Thus, any effect of the LBN experience might not be apparent because of a ‘floor’ effect. This possibility deserves further consideration and the development of optimal tests for females. The explanation for the sex-differences at the cellular level still remains elusive. How such sex differences in relation to an identical same stressor occur is an important open question. Whether external factors like sex-specific differences in maternal care (Oomen, et al., 2009; van Hasselt, Boudewijns, van der Knaap, Krugers, & Joëls, 2012), or in sex hormones of the pups (Naninck, Lucassen, & Bakker, 2011) are involved, or whether other early factors like nutrition and metabolism (Yam, et al., 2017; Yam, et al., 2015), or epigenetics play a role (Holland et al., 2016; Lucassen, et al., 2013; Rodgers, Morgan, Leu, & Bale, 2015) remains to be demonstrated.
Epigenetic modifications are increasingly being recognized as important for understanding sex differences in brain development and responses to early-life environments. Further, epigenetic mechanisms are known to mediate sexual differentiation of the brain (McCarthy et al., 2009; McCarthy & Nugent, 2013; Nugent et al., 2015), and sexually-dimorphic DNA methylation is observed for many genes (Ghahramani et al., 2014). Using their variation of the LBN paradigm, the Roth laboratory has shown sexually dimorphic changes in methylation of exon IV of the brain derived neurotrophic factor (BDNF) gene, an important regulatory region of the gene and sensitive to many environmental factors in both rodents and humans (Kundakovic et al., 2015; Lubin, Roth, & Sweatt, 2008; Perroud et al., 2013; E. D. Roth et al., 2015). Compared to males, females exposed to LBN environment had higher methylation in PFC (whole and medial) and lower methylation in amygdala tissue, with no change in this gene locus observed in hippocampal tissue (Blaze, et al., 2013; T. L. Roth, et al., 2009; T. L. Roth, et al., 2014). Within mPFC tissue, maltreated-females, but not males, also had less histone 3 lysine 9/14 acetylation associated with BDNF IV DNA (Blaze, et al., 2015). In addition, the LBN environment had consequences for brain telomere length that differ between males and females (Arun Asok, Bernard, Rosen, Dozier, & Roth, 2014; A Asok, Bernard, Roth, Rosen, & Dozier, 2013). Telomere length is another biomarker of stress emerging as highly sensitive to variations in the caregiving environment (Botha et al., 2012; Drury et al., 2012; Tyrka et al., 2010).
Sex differences in the outcome and consequences of ELS are critical to understand differential vulnerability in both preclinical models and clinical populations. However, it remains poorly understood how these effects can differ between human and rodent studies because in clinical reports and in human studies, it is the females that appear to be generally more sensitive to the consequences of early trauma. In addition, girls and women are more likely to develop stress-related mental disorders. The origins of discrepancy between human and rodent studies are poorly understood, and have been the focus of excellent recent reviews (Eliot & Richardson, 2016).
6. Potential interventions to reduce ELS consequences
The study of relevant animal models is useful to provide better understanding of underlying mechanisms and to enable testing of interventions. Several examples of successful interventions have been provided, focusing on exercise, food or normalization of the HPA-axis.
As an example of the first category, physical activity has been shown to improve mood across many animal studies, and to reduce stress (Kannangara et al., 2011; Schoenfeld, Rada, Pieruzzini, Hsueh, & Gould, 2013) and depressive symptoms in clinical studies (Kvam, Kleppe, Nordhus, & Hovland, 2016). Notably, previous work showed that provision of continuous voluntary exercise for 16 weeks from weaning mitigated the behavioural deficits imposed by the ELS of maternal separation (MS) including anxiety- and depression-like behaviours (Maniam & Morris, 2010). While some work has been done in relation to social isolation stress (Stranahan, Khalil, & Gould, 2006), it is not known, however, whether a shorter intervention, or late onset exercise, could be beneficial in the setting of ELS, nor has this intervention been tested with offspring exposed to the LBN paradigm. More work is required to determine the most appropriate window in which to apply preventive strategies such as modifying diet and exercise to minimize or delay long term CNS and metabolic deficits induced by ELS.
A successful intervention focusing on food intake –which may be disturbed by the LBN procedure- concerns molecules linked to neural plasticity. Thus, in male rats exposed to LBN, GSKα and GSK3β genes, were found to be profoundly down-regulated. This could be reversed by exposing LBN offspring to a diet high in fat and sugar post weaning (Maniam, et al., 2016). As indicated earlier, this diet also ameliorated the increased anxiety-like behaviour induced by LBN exposure (Machado, et al., 2013). In addition, as explained in more detail above, enriching the diet with 1C metabolites during the LBN exposure can protect partially against the LBN-induced cognitive deficits (Naninck, et al., 2016). This has high translational value, as nutritional interventions are typically non-invasive, relatively cheap and easily applicable.
As documented above, LBN may result in aberrations of distinct components of limbic-HPA-axis function. Based on this observation, offspring raised in LBN conditions were subjected to transient treatment with the glucocorticoid receptor antagonist mifepristone. This antagonist was shown to be very effective in reversing or normalizing effects of chronic stress in adulthood on hippocampal structure and function, both in rats and mice (Krugers, Goltstein, Van Der Linden, & Joels, 2006; Oomen, Mayer, De Kloet, Joëls, & Lucassen, 2007). In the case of the LBN model, male mice were treated with mifepristone between postnatal day 28 and 30, a developmental window that is known to be important for HPA axis development (Romeo, et al., 2016). As reviewed in the section on fear memory, LBN was reported to impair the ability of adult male mice to discriminate between cue-on and cue-off (presumably ‘safe’) periods 24h after fear conditioning. Interestingly, this deficit could be entirely relieved by treatment with mifepristone between PND28–30. This suggests that possible dysregulation of the HPA-axis as a consequence of the early life LBN environment may be reprogrammed during the sensitive early pubertal period. In line with this notion, restoring the balance between the glucocorticoid and mineralocorticoid receptor by genetic overexpression of the latter after LBN also alleviates the behavioral and hippocampal cellular deficits seen in adult male offspring (S Kanatsou, et al., 2017).
Notably, LBN-induced early changes in gene expression, likely via epigenetic mechanisms, have been found for CRH in hypothalamus (Gunn, et al., 2013), hippocampus (Ivy, et al., 2010) and amygdala (Singh-Taylor et al., 2017). In accord with potential hyperactivity of CRH in these brain regions induced by chronic LBN, transient blocking of the CRH receptor prevented cognitive problems in LBN animals when tested in both hippocampal and prefrontal domains. Importantly, the effects of the LBN paradigm were significantly blunted in mice lacking CRHR1 in the limbic system exclusively (X. D. Wang, et al., 2011).
These three examples emphasize that successful reversal of LBN-induced deficits is possible. However, the critical time-windows, the underlying mechanism and other potential categories of intervention targets clearly require extensive follow-up studies. Of particular interest is the importance of the genetic background which may serve as a significant modulator of risk for ELS associated pathology. Interventions causing repression or up-regulation of key genes –for example genes playing a critical role in the set point of the HPA axis- may give better insight about systems that could be targeted to reverse the deficits caused by LBN. For instance, several studies have linked genetic changes in the neurotrophin system induced by exposure to LBN and associated those changes with negative outcomes. In humans, common variants in the BDNF gene have been identified, with the most thoroughly explored being the BDNF Val66Met SNP. In human studies of international adoption, the Met variant of the BDNF gene was actually demonstrated to be protective with respect to attentional functioning in children. Thus, genetic (as well as epigenetic) screening may represent an attractive biomarker for the identification of risk/resilience in populations exposed to early adversity.
An additional potential intervention to reduce the effects of an early adverse environment would be to maintain mice exposed to the LBN paradigm in single housing conditions after puberty (between PND50 and adulthood) as this procedure, albeit detrimental to the development of normally raised female mice, was shown to attenuate the effects of LBN on adult anxiety behavior in an estrous cycle-dependent manner. These observations are in support of the match/mismatch hypothesis which suggests that matching early and late environment, whether mildly aversive or not might be a primary determinant of adult behavioral outcomes (Champagne, de Kloet, & Joëls, 2009; Nederhof & Schmidt, 2012; Santarelli et al., 2014).
Based upon the work presented here, multiple possible targets have been identified to stem the effect of ELS on neurobehavioral development, paving the road for the development and application of potential new treatments.
7. Preclinical model reproducibility
The usefulness of animal models to investigate human pathophysiological and behavioral alterations in disease states relies heavily on reproducibility of the outcomes within and across different research laboratories. Reproducibility in general is a matter of debate in animal studies (Pritt Stacy, 2017; Silverman, Macy, & Preisig, 2017), e.g. due to small differences in experimental conditions having impact on the outcome and/or issues of low power. The former do, to a certain degree, play a role in the LBN and Scarcity models, but overall there is large convergence in the data. This is remarkable because stress experiments in general are very sensitive to small variations in animal procedures, which no doubt occur between laboratories.
As highlighted throughout this review the LBN and Scarcity models represent a great example of an animal model of early-life stress that is 1) highly reproducible and 2) translationally relevant. Part of the elegance of this model is its simplicity, which leads to a robust and reproducible change in parental behavior across rodent species within as well as across laboratories and continents (Heun-Johnson & Levitt, 2016; Korosi et al., 2010; Rice, et al., 2008). In fact what appears from reviewing the data collected from the laboratories worldwide using this model, is that the main features of the paradigm that we seek to model (i.e. early-stress induced alterations in brain functioning) are highly consistent across laboratories, even when including small variations in the model. It remains very important to deeply characterize the specifics of the model that one uses (mesh, room temperature, chow, length of intervention), as subtle differences in the effects do exist. Remarkably, several of the observed phenotype closely resembles human characteristics of children exposed to various forms of adversity as for example the reduced hippocampal volume (Teicher, Anderson, & Polcari, 2012), giving a translationally powerful and an excellent tool at hand to further investigate the (neuro)biological substrates of the programming of the adult organisms exposed to early life adversity.
8. Translational potential
The focus of much of the work described here is to leverage animal models to understand the impact of early adversity, in the form of altered quality of early life care, on neurobehavioral outcomes and stress-sensitive regulatory processes. Here we have defined areas of convergence with these low bedding and nesting procedures. Moving forward, the clear description and validation of these models paves the way for using these models to identify effect of the severity of stress at different developmental periods. The sincere hope is that the information gleaned from these studies can provide novel insights into how variables such as timing of adversity, sex, and severity of the stress alter risk for the development of atypical outcomes and potentially pathological behavior. Such studies have implications for both the natural world as well as humans. For animals, human encroachment and climate change led to loss of habitat and breeding grounds and destruction of food sources that have altered the timing of reproductive events and availability of resources to care for the young. These effects lead to diminished ability of parents to care for and nurture offspring, impacting growth, development, and survival of entire populations. The effects of these forms of disturbance on the welfare of captive and domesticated species as well as health, wellbeing and survival of populations in the wild is only beginning to emerge. The current studies may provide novel insights into the drivers of these changes.
In humans, increasing civil unrest, famine, and poverty have resulted in an unprecedented 65 million individuals being displaced and nearly 650 million children worldwide who lack adequate shelter, water, or health services. With increased global population and civil unrest, millions of infants and children are exposed to stress associated with poverty, displacement, and parental stress resulting from limited access to the resources for childcare. In fact, there is data suggesting that these figures under-report the actual incidence of ELS around the world (Briere & Elliott, 2003). Furthermore, in addition to the direct effect of ELS on neurodevelopmental outcomes, there are strong data indicating that exposure to adverse early environments may have intergenerational impacts that likely affect mental health and function, not only of individuals who are directly affected, but their offspring (Gapp et al., 2014; Gröger et al., 2016; Rodgers, et al., 2015; Sanchez, et al., 2001). Thus, advancing our understanding of the effect of ELS on brain and behavioral development is of critical concern for the health and wellbeing of the population, but also for generations to come.
Acknowledgments
We would like to thank all participants of the roundtable discussion at the 4th “Neurobiology of Stress” workshop (Newport Beach, April 2016) who have enriched the discussion of the ELS models and in particular the LBN model presented here. Their contribution helped shape the present manuscript and outline some of the promising next steps in our research using this model.
Funding
The authors’ research was supported by: CDW: Canadian Institutes for Health Research (grant # MOP#114885, #) and Natural Sciences and Engineering Council of Canada (grant#138199); KGB: NPNI/BIBS New Frontiers award; MJ: ALW grant # 821-02-007 from the Netherlands Organization for Scientific Research NWO and by the Consortium on Individual Development (CID), which is funded through the Gravitation program of the Dutch Ministry of Education, Culture, and Science and the Netherlands Organization for Scientific Research (NWO grant number 024.001.003); AK: ISAO, the Netherlands Organization for Scientific Research Meervoud, NWO-FCB and JPI-NutriCog; YT and ML: National Institute of Health grants P50 DK-64539 (YT, ML), Digestive Diseases Research Center P30 DK-41301 (Animal Core, YT) and K01 DK-088937 (ML) and Veteran Administration Senior Research Career Scientist Award (YT); PJL: Alzheimer Nederland; MJM: Diabetes Australia Research Trust and National Health and Medical Research Council Project grant (#1023073); TLR: The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD; 1R01HD087509); RMS: National Institute of Health grant NIHR37HD083217; TZB: National Institute of Health grants NS28912; MH73136 and MH096889.
Footnotes
Disclosure of interest
The authors have no financial interest in, or conflict of interest with the subject and materials discussed in the present manuscript.
References
- Álvarez MJ, Fernández D, Gómez-Salgado J, Rodríguez-González D, Rosón M, Lapeña S. The effects of massage therapy in hospitalized preterm neonates: A systematic review. International Journal of Nursing Studies. 2017;69:119–136. doi: 10.1016/j.ijnurstu.2017.02.009. [DOI] [PubMed] [Google Scholar]
- Anacker C, O’Donnell KJ, Meaney MJ. Early life adversity and the epigenetic programming of hypothalamic-pituitary-adrenal function. Dialogues in Clinical Neuroscience. 2014;16:321–333. doi: 10.31887/DCNS.2014.16.3/canacker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arp JM, Ter Horst JP, Loi M, den Blaauwen J, Bangert E, Fernández G, et al. Blocking glucocorticoid receptors at adolescent age prevents enhanced freezing between repeated cue-exposures after conditioned fear in adult mice raised under chronic early life stress. Neurobiology of Learning and Memory. 2016;133:30–38. doi: 10.1016/j.nlm.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Asok A, Bernard K, Rosen JB, Dozier M, Roth TL. Infant-caregiver experiences alter telomere length in the brain. PloS One. 2014;9:e101437. doi: 10.1371/journal.pone.0101437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asok A, Bernard K, Roth TL, Rosen J, Dozier M. Parental responsiveness moderates the association between early-life stress and reduced telomere length. Development and Psychopathology. 2013;25:577–585. doi: 10.1017/S0954579413000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed-out, or in (utero)? Trends in Neurosciences. 2002;25:518–524. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S, Gilles E, Eghbal-Ahmadi M, Bar-El Y, Baram T. Altered regulation of gene and protein expression of hypothalamic-pituitary-adrenal axis components in an immature rat model of chronic stress. Journal of Neuroendocrinology. 2001;13:799–807. doi: 10.1046/j.1365-2826.2001.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot RC, Zhang T-Y, Wen X, Nguyen TTT, Nguyen H-B, Diorio J, et al. Variations in postnatal maternal care and the epigenetic regulation of metabotropic glutamate receptor 1 expression and hippocampal function in the rat. Proceedings of the National Academy of Sciences. 2012;109(Supplement 2):17200–17207. doi: 10.1073/pnas.1204599109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, et al. Early life programming and neurodevelopmental disorders. Biological Psychiatry. 2010;68:314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Davis EP, Obenaus A, Sandman CA, Small SL, Solodkin A, et al. Fragmentation and unpredictability of early-life experience in mental disorders. American Journal of Psychiatry. 2012;169:907–915. doi: 10.1176/appi.ajp.2012.11091347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbara G, Feinle-Bisset C, Ghoshal UC, Santos J, Vanner SJ, Vergnolle N, et al. The intestinal microenvironment and functional gastrointestinal disorders. Gastroenterology. 2016;150:1305–1318. e8. doi: 10.1053/j.gastro.2016.02.028. [DOI] [PubMed] [Google Scholar]
- Bath KG, Manzano-Nieves G, Goodwill H. Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Hormones and Behavior. 2016;82:64–71. doi: 10.1016/j.yhbeh.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG, Schilit-Nitenson A, Lichtman E, Lopez C, Chen W, Gallo M, et al. Early life stress leads to developmental and sex selective effects on performance in a novel object placement task. Neurobiology of Stress. 2017;7:57–67. doi: 10.1016/j.ynstr.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesley H, Rhodes J, Salmon P. Anger and childhood sexual abuse are independently associated with irritable bowel syndrome. British Journal of Health Psychology. 2010;15:389–399. doi: 10.1348/135910709X466496. [DOI] [PubMed] [Google Scholar]
- Belay H, Burton CL, Lovic V, Meaney MJ, Sokolowski M, Fleming AS. Early adversity and serotonin transporter genotype interact with hippocampal glucocorticoid receptor mRNA expression, corticosterone, and behavior in adult male rats. Behavioral Neuroscience. 2011;125:150. doi: 10.1037/a0022891. [DOI] [PubMed] [Google Scholar]
- Bjelobaba I, Janjic MM, Kucka M, Stojilkovic SS. Cell type-specific sexual dimorphism in rat pituitary gene expression during maturation. Biology of Reproduction. 2015;93:21. doi: 10.1095/biolreprod.115.129320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Summers CH, Blanchard RJ. The role of behavior in translational models for psychopathology: functionality and dysfunctional behaviors. Neuroscience & Biobehavioral Reviews. 2013;37:1567–1577. doi: 10.1016/j.neubiorev.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaze J, Asok A, Roth TL. Long-term effects of early-life caregiving experiences on brain-derived neurotrophic factor histone acetylation in the adult rat mPFC. Stress. 2015;18:607–615. doi: 10.3109/10253890.2015.1071790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaze J, Scheuing L, Roth TL. Differential methylation of genes in the medial prefrontal cortex of developing and adult rats following exposure to maltreatment or nurturing care during infancy. Developmental Neuroscience. 2013;35:306–316. doi: 10.1159/000350716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos K, Zeanah CH, Fox NA, Drury SS, McLaughlin KA, Nelson CA. Psychiatric outcomes in young children with a history of institutionalization. Harvard Review of Psychiatry. 2011;19:15–24. doi: 10.3109/10673229.2011.549773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botha M, Grace L, Bugarith K, Russell VA, Kidd M, Seedat S, et al. The impact of voluntary exercise on relative telomere length in a rat model of developmental stress. BMC Research Notes. 2012;5:697. doi: 10.1186/1756-0500-5-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby J. Maternal care and mental health. Vol. 2. WHO; Geneva: 1952. [Google Scholar]
- Bradford K, Shih W, Videlock EJ, Presson AP, Naliboff BD, Mayer EA, et al. Association between early adverse life events and irritable bowel syndrome. Clinical Gastroenterology and Hepatology. 2012;10:385–390. e383. doi: 10.1016/j.cgh.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briere J, Elliott DM. Prevalence and psychological sequelae of self-reported childhood physical and sexual abuse in a general population sample of men and women. Child Abuse & Neglect. 2003;27:1205–1222. doi: 10.1016/j.chiabu.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Brunson KL, Kramár E, Lin B, Chen Y, Colgin LL, Yanagihara TK, et al. Mechanisms of late-onset cognitive decline after early-life stress. Journal of Neuroscience. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chaloner A, Greenwood-VanMeerveld B. Early life adversity as a risk factor for visceral pain in later life: importance of sex differences. Frontiers in Neuroscience. 2013;7:13. doi: 10.3389/fnins.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne DL, de Kloet ER, Joëls M. Fundamental aspects of the impact of glucocorticoids on the (immature) brain. Paper presented at the Seminars in Fetal and Neonatal Medicine; 2009. [DOI] [PubMed] [Google Scholar]
- Chatterjee D, Chatterjee-Chakraborty M, Rees S, Cauchi J, de Medeiros CB, Fleming AS. Maternal isolation alters the expression of neural proteins during development:‘Stroking’stimulation reverses these effects. Brain Research. 2007;1158:11–27. doi: 10.1016/j.brainres.2007.04.069. [DOI] [PubMed] [Google Scholar]
- Chen Y, Baram TZ. Toward understanding how early-life stress reprograms cognitive and emotional brain networks. Neuropsychopharmacology. 2016;41:197–206. doi: 10.1038/npp.2015.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Kersh B, Hyde J, Darlington RB, Anand K, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- Clough D, Prykhodko O, Råberg L. Effects of protein malnutrition on tolerance to helminth infection. Biology Letters. 2016;12:20160189. doi: 10.1098/rsbl.2016.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle Molle R, Portella A, Goldani M, Kapczinski F, Leistner-Segala S, Salum G, et al. Associations between parenting behavior and anxiety in a rodent model and a clinical sample: relationship to peripheral BDNF levels. Translational psychiatry. 2012;2:e195. doi: 10.1038/tp.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma G, Blennerhassett P, Lu J, Deng Y, Park A, Green W, et al. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nature Communications. 2015;6:7735. doi: 10.1038/ncomms8735. [DOI] [PubMed] [Google Scholar]
- De Santis S, Cavalcanti E, Mastronardi M, Jirillo E, Chieppa M. Nutritional keys for intestinal barrier modulation. Frontiers in Immunology. 2015;6:612. doi: 10.3389/fimmu.2015.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir-Lira ÖE, Voss JL, O’Neil JT, Briggs-Gowan MJ, Wakschlag LS, Booth JR. Early-life stress exposure associated with altered prefrontal resting-state fMRI connectivity in young children. Developmental Cognitive Neuroscience. 2016;19:107–114. doi: 10.1016/j.dcn.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty TS, Forster A, Roth TL. Global and gene-specific DNA methylation alterations in the adolescent amygdala and hippocampus in an animal model of caregiver maltreatment. Behavioural brain research. 2016;298:55–61. doi: 10.1016/j.bbr.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury SS, Theall K, Gleason MM, Smyke AT, De Vivo I, Wong J, et al. Telomere length and early severe social deprivation: linking early adversity and cellular aging. Molecular Psychiatry. 2012;17:719–727. doi: 10.1038/mp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghbal-Ahmadi M, Avishai-Eliner S, Hatalski CG, Baram TZ. Differential regulation of the expression of corticotropin-releasing factor receptor type 2 (CRF2) in hypothalamus and amygdala of the immature rat by sensory input and food intake. Journal of Neuroscience. 1999;19:3982–3991. doi: 10.1523/JNEUROSCI.19-10-03982.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliot L, Richardson SS. Sex in context: Limitations of animal studies for addressing human sex/gender neurobehavioral health disparities. Journal of Neuroscience. 2016;36:11823–11830. doi: 10.1523/JNEUROSCI.1391-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson-Rose SA, de Leon CFM, Bienias JL, Wilson RS, Evans DA. Early life conditions and cognitive functioning in later life. American Journal of Epidemiology. 2003;158:1083–1089. doi: 10.1093/aje/kwg263. [DOI] [PubMed] [Google Scholar]
- Farrell M, Holland F, Shansky R, Brenhouse H. Sex-specific effects of early life stress on social interaction and prefrontal cortex dendritic morphology in young rats. Behavioural Brain Research. 2016;310:119–125. doi: 10.1016/j.bbr.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felice VD, Moloney RD, Cryan JF, Dinan TG, O’Mahony SM. Pain in Psychiatric Disorders. Vol. 30. Karger Publishers; 2015. Visceral Pain and Psychiatric Disorders; pp. 103–119. [DOI] [PubMed] [Google Scholar]
- Fuentes S, Daviu N, Garrido P, Gagliano H, Zelena D, Monasterio N, et al. Sex-dependent effects of an early life treatment in rats that increases maternal care: vulnerability or resilience? Frontiers in Behavioral Neuroscience. 2014;8:56. doi: 10.3389/fnbeh.2014.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapp K, Soldado-Magraner S, Alvarez-Sánchez M, Bohacek J, Vernaz G, Shu H, et al. Early life stress in fathers improves behavioural flexibility in their offspring. Nature Communications. 2014;5:5466. doi: 10.1038/ncomms6466. [DOI] [PubMed] [Google Scholar]
- Gareau MG, Jury J, MacQueen G, Sherman PM, Perdue MH. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut. 2007;56:1522–1528. doi: 10.1136/gut.2006.117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudin JM, Polansky NA, Kilpatrick AC, Shilton P. Family functioning in neglectful families. Child Abuse & Neglect. 1996;20:363–377. doi: 10.1016/0145-2134(96)00005-1. [DOI] [PubMed] [Google Scholar]
- Ghahramani NM, Ngun TC, Chen PY, Tian Y, Krishnan S, Muir S, et al. The effects of perinatal testosterone exposure on the DNA methylome of the mouse brain are late-emerging. Biology of Sex Differences. 2014;5:8. doi: 10.1186/2042-6410-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles EE, Schultz L, Baram TZ. Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatric Neurology. 1996;15:114–119. doi: 10.1016/0887-8994(96)00153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman P, Hanson M. Developmental and epigenetic pathways to obesity: an evolutionary-developmental perspective. International Journal of Obesity. 2008;32:S62–S71. doi: 10.1038/ijo.2008.240. [DOI] [PubMed] [Google Scholar]
- Graham AM, Buss C, Rasmussen JM, Rudolph MD, Demeter DV, Gilmore JH, et al. Implications of newborn amygdala connectivity for fear and cognitive development at 6-months-of-age. Developmental Cognitive Neuroscience. 2016;18:12–25. doi: 10.1016/j.dcn.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-β and tau pathology in a mouse model of Alzheimer’s disease. Journal of Neuroscience. 2006;26:9047–9056. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröger N, Matas E, Gos T, Lesse A, Poeggel G, Braun K, et al. The transgenerational transmission of childhood adversity: behavioral, cellular, and epigenetic correlates. Journal of Neural Transmission. 2016;123:1037–1052. doi: 10.1007/s00702-016-1570-1. [DOI] [PubMed] [Google Scholar]
- Guadagno A, Verlezza S, Long H, Wong TP, Walker CD. The Effects of Chronic Early-Life Stress on Amygdala Morphology and Function in Neonatal Rats. Paper presented at the Neurobiology of Stress Workshop; Irvine. April 12–15.2016. [Google Scholar]
- Guadagno A, Wong TP, Walker CD. Morphological and functional changes in the preweaning basolateral amygdala induced by early chronic stress associate with anxiety and fear behavior in adult male, but not female rats. Neuroscience. 2017 doi: 10.1016/j.pnpbp.2017.09.025. under review. [DOI] [PubMed] [Google Scholar]
- Gunn BG, Cunningham L, Cooper MA, Corteen NL, Seifi M, Swinny JD, et al. Dysfunctional astrocytic and synaptic regulation of hypothalamic glutamatergic transmission in a mouse model of early-life adversity: relevance to neurosteroids and programming of the stress response. Journal of Neuroscience. 2013;33:19534–19554. doi: 10.1523/JNEUROSCI.1337-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M. Reversing the effects of early deprivation after infancy: giving children families may not be enough. Frontiers in Neuroscience. 2010;4:170. doi: 10.3389/fnins.2010.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Wang Z, Mayer EA, Holschneider DP. Neonatal stress from limited bedding elicits visceral hyperalgesia in adult rats. Neuroreport. 2015;26:13. doi: 10.1097/WNR.0000000000000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow HF, Harlow MK. The affectional systems. Behavior of nonhuman primates: Modern Research Trends. 1965;2:287–334. [Google Scholar]
- Heim C, Binder EB. Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Experimental Neurology. 2012;233:102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Heun-Johnson H, Levitt P. Early-life stress paradigm transiently alters maternal behavior, dam-pup interactions, and offspring vocalizations in mice. Frontiers in Behavioral Neuroscience. 2016;10:142. doi: 10.3389/fnbeh.2016.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers L, Lucassen PJ, Korosi A. The interplay of early-life stress, nutrition, and immune activation programs adult hippocampal structure and function. Frontiers in Molecular Neuroscience. 2015;7:103. doi: 10.3389/fnmol.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers L, Ruigrok SR, Amelianchik A, Ivan D, van Dam AM, Lucassen PJ, et al. Early-life stress lastingly alters the neuroinflammatory response to amyloid pathology in an Alzheimer’s disease mouse model. Brain, Behavior, and Immunity. 2016;63:160–175. doi: 10.1016/j.bbi.2016.12.023. [DOI] [PubMed] [Google Scholar]
- Hofer MA. The effects of brief maternal separations on behavior and heart rate of two week old rat pups. Physiology & Behavior. 1973;10:423–427. doi: 10.1016/0031-9384(73)90200-x. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Relationships as regulators: A psychobiologic perspective on bereavement. Psychosomatic Medicine. 1984;46:183–197. doi: 10.1097/00006842-198405000-00001. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Multiple regulators of ultrasonic vocalization in the infant rat. Psychoneuroendocrinology. 1996a;21:203–217. doi: 10.1016/0306-4530(95)00042-9. [DOI] [PubMed] [Google Scholar]
- Hofer MA. On the nature and consequences of early loss. Psychosomatic Medicine. 1996b;58:570–581. doi: 10.1097/00006842-199611000-00005. [DOI] [PubMed] [Google Scholar]
- Holland ML, Lowe R, Caton PW, Gemma C, Carbajosa G, Danson AF, et al. Early-life nutrition modulates the epigenetic state of specific rDNA genetic variants in mice. Science. 2016;353:495–498. doi: 10.1126/science.aaf7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holschneider D, Guo Y, Mayer E, Wang Z. Early life stress elicits visceral hyperalgesia and functional reorganization of pain circuits in adult rats. Neurobiology of Stress. 2016;3:8–22. doi: 10.1016/j.ynstr.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic–pituitary–adrenocortical axis: A review of animal models and human studies across development. Psychological Bulletin. 2014;140:256. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TY, Lin CH. Role of amygdala MAPK activation on immobility behavior of forced swim rats. Behavioural Brain Research. 2006;173:104–111. doi: 10.1016/j.bbr.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Huot R, Gonzalez M, Ladd C, Thrivikraman K, Plotsky P. Foster litters prevent hypothalamic-pituitary-adrenal axis sensitization mediated by neonatal maternal separation. Psychoneuroendocrinology. 2004;29:279–289. doi: 10.1016/s0306-4530(03)00028-3. [DOI] [PubMed] [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience. 2008;154:1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS, Rex CS, Chen Y, Dubé C, Maras PM, Grigoriadis DE, et al. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. Journal of Neuroscience. 2010;30:13005–13015. doi: 10.1523/JNEUROSCI.1784-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jašarević E, Howerton CL, Howard CD, Bale TL. Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain. Endocrinology. 2015;156:3265–3276. doi: 10.1210/en.2015-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsou S, Karst H, Kortesidou D, van den Akker RA, den Blaauwen J, Harris AP, et al. Overexpression of mineralocorticoid receptors in the mouse forebrain partly alleviates the effects of chronic early life stress on spatial memory, neurogenesis and synaptic function in the dentate gyrus. Frontiers in Cellular Neuroscience. 2017;11:132. doi: 10.3389/fncel.2017.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsou S, Ter Horst JP, Harris AP, Seckl JR, Krugers HJ, Joëls M. Effects of mineralocorticoid receptor overexpression on anxiety and memory after early life stress in female mice. Frontiers in Behavioral Neuroscience. 2016;9:374. doi: 10.3389/fnbeh.2015.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannangara TS, Lucero MJ, Gil-Mohapel J, Drapala RJ, Simpson JM, Christie BR, et al. Running reduces stress and enhances cell genesis in aged mice. Neurobiology of Aging. 2011;32:2279–2286. doi: 10.1016/j.neurobiolaging.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan GA, Turrell G, Lynch JW, Everson SA, Helkala EL, Salonen JT. Childhood socioeconomic position and cognitive function in adulthood. International journal of Epidemiology. 2001;30:256–263. doi: 10.1093/ije/30.2.256. [DOI] [PubMed] [Google Scholar]
- Kohl C, Wang XD, Grosse J, Fournier C, Harbich D, Westerholz S, et al. Hippocampal neuroligin-2 links early-life stress with impaired social recognition and increased aggression in adult mice. Psychoneuroendocrinology. 2015;55:128–143. doi: 10.1016/j.psyneuen.2015.02.016. [DOI] [PubMed] [Google Scholar]
- Korosi A, Shanabrough M, McClelland S, Liu ZW, Borok E, Gao XB, et al. Early-life experience reduces excitation to stress-responsive hypothalamic neurons and reprograms the expression of corticotropin-releasing hormone. Journal of Neuroscience. 2010;30:703–713. doi: 10.1523/JNEUROSCI.4214-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugers HJ, Arp JM, Xiong H, Kanatsou S, Lesuis SL, Korosi A, et al. Early life adversity: Lasting consequences for emotional learning. Neurobiology of Stress. 2016;6:14–21. doi: 10.1016/j.ynstr.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugers HJ, Goltstein P, Van Der Linden S, Joels M. Blockade of glucocorticoid receptors rapidly restores hippocampal CA1 synaptic plasticity after exposure to chronic stress. European Journal of Neuroscience. 2006;23:3051–3055. doi: 10.1111/j.1460-9568.2006.04842.x. [DOI] [PubMed] [Google Scholar]
- Krugers HJ, Oomen CA, Gumbs M, Li M, Velzing EH, Joels M, et al. Maternal deprivation and dendritic complexity in the basolateral amygdala. Neuropharmacology. 2012;62:534–537. doi: 10.1016/j.neuropharm.2011.09.022. [DOI] [PubMed] [Google Scholar]
- Kumsta R, Rutter M, Stevens S, Sonuga-Barke EJ. IX. Risk, causation, mediation, and moderation. Monographs of the Society for Research in Child Development. 2010;75:187–211. doi: 10.1111/j.1540-5834.2010.00556.x. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Gudsnuk K, Herbstman JB, Tang D, Perera FP, Champagne FA. DNA methylation of BDNF as a biomarker of early-life adversity. Proceedings of the National Academy of Sciences. 2015;112:6807–6813. doi: 10.1073/pnas.1408355111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov S, Selina E, Kuznetsova N. The blood glucose content in newborn rats depending on level and pattern of spontaneous motor activity. Journal of Evolutionary Biochemistry and Physiology. 2011;47:374. [PubMed] [Google Scholar]
- Kvam S, Kleppe CL, Nordhus IH, Hovland A. Exercise as a treatment for depression: A meta-analysis. Journal of Affective Disorders. 2016;202:67–86. doi: 10.1016/j.jad.2016.03.063. [DOI] [PubMed] [Google Scholar]
- Larauche M. Novel insights in the role of peripheral corticotropin-releasing factor and mast cells in stress-induced visceral hypersensitivity. Neurogastroenterology & Motility. 2012;24:201–205. doi: 10.1111/j.1365-2982.2011.01867.x. [DOI] [PubMed] [Google Scholar]
- Leon M. Dietary control of maternal pheromone in the lactating rat. Physiology & Behavior. 1975;14:311–319. doi: 10.1016/0031-9384(75)90039-6. [DOI] [PubMed] [Google Scholar]
- Lesuis SL, Maurin H, Borghgraef P, Lucassen PJ, Van Leuven F, Krugers HJ. Positive and negative early life experiences differentially modulate long term survival and amyloid protein levels in a mouse model of Alzheimer’s disease. Oncotarget. 2016;7:39118–39135. doi: 10.18632/oncotarget.9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi M, Koricka S, Lucassen PJ, Joëls M. Age-and sex-dependent effects of early life stress on hippocampal neurogenesis. Frontiers in Endocrinology. 2014;5:13. doi: 10.3389/fendo.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi M, Mossink J, Meerhoff G, Den Blaauwen J, Lucassen P, Joëls M. Effects of early-life stress on cognitive function and hippocampal structure in female rodents. Neuroscience. 2017;342:101–119. doi: 10.1016/j.neuroscience.2015.08.024. [DOI] [PubMed] [Google Scholar]
- Long JC, Philip AG, Lucey JF. Excessive handling as a cause of hypoxemia. Pediatrics. 1980;65:203–207. [PubMed] [Google Scholar]
- Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. Journal of Neuroscience. 2008;28:10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucassen PJ, Naninck EFG, van Goudoever JB, Fitzsimons C, Joels M, Korosi A. Perinatal programming of adult hippocampal structure and function; emerging roles of stress, nutrition and epigenetics. Trends in Neurosciences. 2013;36:621–631. doi: 10.1016/j.tins.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Maccari S, Morley-Fletcher S. Effects of prenatal restraint stress on the hypothalamus–pituitary–adrenal axis and related behavioural and neurobiological alterations. Psychoneuroendocrinology. 2007;32:S10–S15. doi: 10.1016/j.psyneuen.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Machado TD, Dalle Molle R, Laureano DP, Portella AK, Werlang ICR, Benetti CdS, et al. Early life stress is associated with anxiety, increased stress responsivity and preference for “comfort foods” in adult female rats. Stress. 2013;16:549–556. doi: 10.3109/10253890.2013.816841. [DOI] [PubMed] [Google Scholar]
- Malter Cohen M, Jing D, Yang RR, Tottenham N, Lee FS, Casey BJ. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proceedings of the National Academy of Sciences USA. 2013;110:18274–18278. doi: 10.1073/pnas.1310163110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniam J, Antoniadis CP, Le V, Morris MJ. A diet high in fat and sugar reverses anxiety-like behaviour induced by limited nesting in male rats: impacts on hippocampal markers. Psychoneuroendocrinology. 2016;68:202–209. doi: 10.1016/j.psyneuen.2016.03.007. [DOI] [PubMed] [Google Scholar]
- Maniam J, Morris MJ. Voluntary exercise and palatable high-fat diet both improve behavioural profile and stress responses in male rats exposed to early life stress: role of hippocampus. Psychoneuroendocrinology. 2010;35:1553–1564. doi: 10.1016/j.psyneuen.2010.05.012. [DOI] [PubMed] [Google Scholar]
- McCarthy MM. Multifaceted origins of sex differences in the brain. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2016;371(1688):20150106. doi: 10.1098/rstb.2015.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, et al. The epigenetics of sex differences in the brain. Journal of Neuroscience. 2009;29:12815–12823. doi: 10.1523/JNEUROSCI.3331-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Nugent BM. Epigenetic contributions to hormonally-mediated sexual differentiation of the brain. Journal of Neuroendocrinology. 2013;25:1133–1140. doi: 10.1111/jne.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick C, Green M. From the stressed adolescent to the anxious and depressed adult: investigations in rodent models. Neuroscience. 2013;249:242–257. doi: 10.1016/j.neuroscience.2012.08.063. [DOI] [PubMed] [Google Scholar]
- McLaughlin RJ, Verlezza S, Gray JM, Hill MN, Walker CD. Inhibition of anandamide hydrolysis dampens the neuroendocrine response to stress in neonatal rats subjected to suboptimal rearing conditions. Stress. 2016;19:114–124. doi: 10.3109/10253890.2015.1117448. [DOI] [PubMed] [Google Scholar]
- Million M, Larauche M. Stress, sex, and the enteric nervous system. Neurogastroenterology & Motility. 2016;28:1283–1289. doi: 10.1111/nmo.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molet J, Heins K, Zhuo X, Mei Y, Regev L, Baram T, et al. Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Translational Psychiatry. 2016;6:e702. doi: 10.1038/tp.2015.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molet J, Maras PM, Avishai-Eliner S, Baram TZ. Naturalistic rodent models of chronic early-life stress. Developmental Psychobiology. 2014;56:1675–1688. doi: 10.1002/dev.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molet J, Maras PM, Kinney-Lang E, Harris NG, Rashid F, Ivy AS, et al. MRI uncovers disrupted hippocampal microstructure that underlies memory impairments after early-life adversity. Hippocampus. 2016;26:1618–1632. doi: 10.1002/hipo.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney R, Stilling R, Dinan T, Cryan J. Early-life stress-induced visceral hypersensitivity and anxiety behavior is reversed by histone deacetylase inhibition. Neurogastroenterology & Motility. 2015;27:1831–1836. doi: 10.1111/nmo.12675. [DOI] [PubMed] [Google Scholar]
- Moloney RD, Sajjad J, Foley T, Felice VD, Dinan TG, Cryan JF, et al. Estrous cycle influences excitatory amino acid transport and visceral pain sensitivity in the rat: effects of early-life stress. Biology of Sex Differences. 2016;7:33. doi: 10.1186/s13293-016-0086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Shionoya K, Jakubs K, Sullivan RM. Early-life stress disrupts attachment learning: the role of amygdala corticosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. Journal of Neuroscience. 2009;29:15745–15755. doi: 10.1523/JNEUROSCI.4106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Unique neural circuitry for neonatal olfactory learning. Journal of Neuroscience. 2004;24:1182–1189. doi: 10.1523/JNEUROSCI.4578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Neurobiology of infant attachment. Developmental Psychobiology. 2005;47:230–242. doi: 10.1002/dev.20093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussaoui N, Jacobs JP, Larauche M, Biraud M, Million M, Mayer E, et al. Chronic early-life stress in rat pups alters basal corticosterone, intestinal permeability, and fecal microbiota at weaning: influence of sex. Journal of Neurogastroenterology and Motility. 2017;23:135–143. doi: 10.5056/jnm16105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussaoui N, Larauche M, Biraud M, Molet J, Million M, Mayer E, et al. Limited nesting stress alters maternal behavior and in vivo intestinal permeability in male Wistar pup rats. PloS One. 2016;11:e0155037. doi: 10.1371/journal.pone.0155037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naninck EFG, Hoeijmakers L, Kakava-Georgiadou N, Meesters A, Lazic SE, Lucassen PJ, et al. Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus. 2015;25:309–328. doi: 10.1002/hipo.22374. [DOI] [PubMed] [Google Scholar]
- Naninck EFG, Lucassen P, Bakker J. Sex differences in adolescent depression: do sex hormones determine vulnerability? Journal of Neuroendocrinology. 2011;23:383–392. doi: 10.1111/j.1365-2826.2011.02125.x. [DOI] [PubMed] [Google Scholar]
- Naninck EFG, Oosterink JE, Yam KY, de Vries LP, Schierbeek H, van Goudoever JB, et al. Early micronutrient supplementation protects against early stress–induced cognitive impairments. The FASEB Journal. 2016;31:505–518. doi: 10.1096/fj.201600834R. [DOI] [PubMed] [Google Scholar]
- Nederhof E, Schmidt MV. Mismatch or cumulative stress: toward an integrated hypothesis of programming effects. Physiology & Behavior. 2012;106:691–700. doi: 10.1016/j.physbeh.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Bos K, Gunnar MR, Sonuga-Barke EJ. V. The neurobiological toll of early human deprivation. Monographs of the Society for Research in Child Development. 2011;76:127–146. doi: 10.1111/j.1540-5834.2011.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: The Bucharest Early Intervention Project. Science. 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- Norman K, Pirlich M, Schulzke J, Smoliner C, Lochs H, Valentini L, et al. Increased intestinal permeability in malnourished patients with liver cirrhosis. European Journal of Clinical Nutrition. 2012;66:1116–1119. doi: 10.1038/ejcn.2012.104. [DOI] [PubMed] [Google Scholar]
- Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, et al. Brain feminization requires active repression of masculinization via DNA methylation. Nature Neuroscience. 2015;18:690–697. doi: 10.1038/nn.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, et al. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biological Psychiatry. 2009;65:263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- O’Mahony S, Clarke G, Dinan T, Cryan J. Early-life adversity and brain development: Is the microbiome a missing piece of the puzzle? Neuroscience. 2017;342:37–54. doi: 10.1016/j.neuroscience.2015.09.068. [DOI] [PubMed] [Google Scholar]
- Oomen CA, Girardi CE, Cahyadi R, Verbeek EC, Krugers HJ, Joëls M, et al. Opposite effects of early maternal deprivation on neurogenesis in male versus female rats. PLoS One. 2009;4:e3675. doi: 10.1371/journal.pone.0003675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen CA, Mayer JL, De Kloet ER, Joëls M, Lucassen PJ. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalizes the reduction in neurogenesis after chronic stress. European Journal of Neuroscience. 2007;26:3395–3401. doi: 10.1111/j.1460-9568.2007.05972.x. [DOI] [PubMed] [Google Scholar]
- Oomen CA, Soeters H, Audureau N, Vermunt L, van Hasselt FN, Manders EM, et al. Early maternal deprivation affects dentate gyrus structure and emotional learning in adult female rats. Psychopharmacology. 2011;214:249–260. doi: 10.1007/s00213-010-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen CA, Soeters H, Audureau N, Vermunt L, van Hasselt FN, Manders EM, et al. Severe early life stress hampers spatial learning and neurogenesis, but improves hippocampal synaptic plasticity and emotional learning under high-stress conditions in adulthood. Journal of Neuroscience. 2010;30:6635–6645. doi: 10.1523/JNEUROSCI.0247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opala EA, Liu D, Long H, Walker CD. Stress response and structural plasticity in the mPFC of lactating rats. Neurobiology of Stress Workshop; April 12–15.2016. [Google Scholar]
- Perroud N, Salzmann A, Prada P, Nicastro R, Hoeppli M, Furrer S, et al. Response to psychotherapy in borderline personality disorder and methylation status of the BDNF gene. Translational Psychiatry. 2013;3:e207. doi: 10.1038/tp.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RE, Al Aïn S, Raineki C, Sullivan RM, Wilson DA. Development of odor hedonics: experience-dependent ontogeny of circuits supporting maternal and predator odor responses in rats. Journal of Neuroscience. 2016;36:6634–6650. doi: 10.1523/JNEUROSCI.0632-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratchett LC, Yehuda R. Foundations of posttraumatic stress disorder: Does early life trauma lead to adult posttraumatic stress disorder? Development and Psychopathology. 2011;23:477–491. doi: 10.1017/S0954579411000186. [DOI] [PubMed] [Google Scholar]
- Pritt Stacy LS. The Interplay of Ethics, Animal Welfare, and IACUC Oversight on the Reproducibility of Animal Studies. Comparative Medicine. 2017;67:101–105. [PMC free article] [PubMed] [Google Scholar]
- Prusator D, Meerveld GV. Gender specific effects of neonatal limited nesting on viscerosomatic sensitivity and anxiety-like behavior in adult rats. Neurogastroenterology & Motility. 2015;27:72–81. doi: 10.1111/nmo.12472. [DOI] [PubMed] [Google Scholar]
- Raineki C, Cortés MR, Belnoue L, Sullivan RM. Effects of early-life abuse differ across development: Infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. Journal of Neuroscience. 2012;32:7758–7765. doi: 10.1523/JNEUROSCI.5843-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Moriceau S, Sullivan RM. Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biological Psychiatry. 2010;67:1137–1145. doi: 10.1016/j.biopsych.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Sarro E, Rincón-Cortés M, Perry R, Boggs J, Holman CJ, et al. Paradoxical neurobehavioral rescue by memories of early-life abuse: The safety signal value of odors learned during abusive attachment. Neuropsychopharmacology. 2015;40:906–914. doi: 10.1038/npp.2014.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Shionoya K, Sander K, Sullivan RM. Ontogeny of odor-LiCl vs. odor-shock learning: Similar behaviors but divergent ages of functional amygdala emergence. Learning & Memory. 2009;16:114–121. doi: 10.1101/lm.977909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nature Neuroscience. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice CJ, Sandman CA, Lenjavi MR, Baram TZ. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149:4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón-Cortés M, Sullivan R. Emergence of social behavior deficit, blunted corticolimbic activity and adult depression-like behavior in a rodent model of maternal maltreatment. Translational Psychiatry. 2016;6:e930. doi: 10.1038/tp.2016.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers AB, Morgan CP, Leu NA, Bale TL. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proceedings of the National Academy of Sciences. 2015;112:13699–13704. doi: 10.1073/pnas.1508347112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD, Patel R, Pham L, So VM. Adolescence and the ontogeny of the hormonal stress response in male and female rats and mice. Neuroscience & Biobehavioral Reviews. 2016;70:206–216. doi: 10.1016/j.neubiorev.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth ED, Roth TL, Money KM, SenGupta S, Eason DE, Sweatt JD. DNA methylation regulates neurophysiological spatial representation in memory formation. Neuroepigenetics. 2015;2:1–8. doi: 10.1016/j.nepig.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biological Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Matt S, Chen K, Blaze J. Bdnf DNA methylation modifications in the hippocampus and amygdala of male and female rats exposed to different caregiving environments outside the homecage. Developmental Psychobiology. 2014;56:1755–1763. doi: 10.1002/dev.21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Raineki C, Salstein L, Perry R, Sullivan-Wilson T, Sloan A, et al. Neurobiology of secure infant attachment and attachment despite adversity: a mouse model. Genes, Brain and Behavior. 2013;12:673–680. doi: 10.1111/gbb.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biological Psychiatry. 2005;57:823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Development and Psychopathology. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Santarelli S, Lesuis SL, Wang XD, Wagner KV, Hartmann J, Labermaier C, et al. Evidence supporting the match/mismatch hypothesis of psychiatric disorders. European Neuropsychopharmacology. 2014;24:907–918. doi: 10.1016/j.euroneuro.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TJ, Rada P, Pieruzzini PR, Hsueh B, Gould E. Physical exercise prevents stress-induced activation of granule neurons and enhances local inhibitory mechanisms in the dentate gyrus. Journal of Neuroscience. 2013;33:7770–7777. doi: 10.1523/JNEUROSCI.5352-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevelinges Y, Mouly AM, Raineki C, Moriceau S, Forest C, Sullivan RM. Adult depression-like behavior, amygdala and olfactory cortex functions are restored by odor previously paired with shock during infant’s sensitive period attachment learning. Developmental Cognitive Neuroscience. 2011;1:77–87. doi: 10.1016/j.dcn.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shair HN, Brunelli SA, Masmela JR, Boone E, Hofer MA. Social, thermal, and temporal influences on isolation-induced and maternally potentiated ultrasonic vocalizations of rat pups. Developmental Psychobiology. 2003;42:206–222. doi: 10.1002/dev.10087. [DOI] [PubMed] [Google Scholar]
- Sibille E, Wang Y, Joeyen-Waldorf J, Gaiteri C, Surget A, Oh S, et al. A molecular signature of depression in the amygdala. American Journal of Psychiatry. 2009;166:1011–1024. doi: 10.1176/appi.ajp.2009.08121760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman J, Macy J, Preisig P. The role of the IACUC in ensuring research reproducibility. Lab Animal. 2017;46:129. doi: 10.1038/laban.1213. [DOI] [PubMed] [Google Scholar]
- Silvers JA, Lumian DS, Gabard-Durnam L, Gee DG, Goff B, Fareri DS, et al. Previous institutionalization is followed by broader amygdala–hippocampal–PFC network connectivity during aversive learning in human development. Journal of Neuroscience. 2016;36:6420–6430. doi: 10.1523/JNEUROSCI.0038-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Taylor A, Molet J, Jiang S, Korosi A, Bolton J, Noam Y, et al. NRSF-dependent epigenetic mechanisms contribute to programming of stress-sensitive neurons by neonatal experience, promoting resilience. Molecular Psychiatry. 2017 doi: 10.1038/mp.2016.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nature neuroscience. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Neurophysiology: Good memories of bad events in infancy. Nature. 2000;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symington AJ, Pinelli J. Developmental care for promoting development and preventing morbidity in preterm infants. Cochrane Database of Systematic Reviews. 2006;(2):CD001814. doi: 10.1002/14651858.CD001814.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taché Y, Million M. Role of corticotropin-releasing factor signaling in stress-related alterations of colonic motility and hyperalgesia. Journal of Neurogastroenterology and Motility. 2015;21:8–24. doi: 10.5056/jnm14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proceedings of the National Academy of Sciences. 2012;109:E563–E572. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telemo E, Weström B, Ekström G, Karlsson B. Intestinal macromolecular transmission in the young rat: influence of protease inhibitors during development. Neonatology. 1987;52:141–148. doi: 10.1159/000242703. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare T, Millner A, Gilhooly T, Zevin J, Casey B. Elevated amygdala response to faces following early deprivation. Developmental Science. 2011;14:190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JR. Intestinal mucosal barrier function in health and disease. Nature Reviews Immunology. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, Carpenter LL. Childhood maltreatment and telomere shortening: preliminary support for an effect of early stress on cellular aging. Biological Psychiatry. 2010;67:531–534. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzanoulinou S, Sandi C. The programming of the social brain by stress during childhood and adolescence: From rodents to humans. Current Topics in Behavioral Neuroscience. 2015;30:411–429. doi: 10.1007/7854_2015_430. [DOI] [PubMed] [Google Scholar]
- Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. The Journal of Nutrition. 2011;141:769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- Upton KJ, Sullivan RM. Defining age limits of the sensitive period for attachment learning in rat pups. Developmental Psychobiology. 2010;52:453–464. doi: 10.1002/dev.20448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hasselt FN, Boudewijns ZS, van der Knaap NJ, Krugers HJ, Joëls M. Maternal care received by individual pups correlates with adult CA1 dendritic morphology and synaptic plasticity in a sex-dependent manner. Journal of Neuroendocrinology. 2012;24:331–340. doi: 10.1111/j.1365-2826.2011.02233.x. [DOI] [PubMed] [Google Scholar]
- VandenBerg KA. Individualized developmental care for high risk newborns in the NICU: a practice guideline. Early Human Development. 2007;83:433–442. doi: 10.1016/j.earlhumdev.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Walker DM, Juenger TE, Gore AC. Developmental profiles of neuroendocrine gene expression in the preoptic area of male rats. Endocrinology. 2009;150:2308–2316. doi: 10.1210/en.2008-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Goebel-Stengel M, Yuan PQ, Stengel A, Taché Y. Corticotropin-releasing factor overexpression in mice abrogates sex differences in body weight, visceral fat, and food intake response to a fast and alters levels of feeding regulatory hormones. Biology of Sex Differences. 2017;8:2. doi: 10.1186/s13293-016-0122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XD, Chen Y, Wolf M, Wagner KV, Liebl C, Scharf SH, et al. Forebrain CRHR1 deficiency attenuates chronic stress-induced cognitive deficits and dendritic remodeling. Neurobiology of Disease. 2011;42:300–310. doi: 10.1016/j.nbd.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XD, Labermaier C, Holsboer F, Wurst W, Deussing JM, Muller MB, Schmidt MV. Early-life stress-induced anxiety-related behavior in adult mice partially requires forebrain corticotropin-releasing hormone receptor 1. European Journal of Neuroscience. 2012;36:2360–2367. doi: 10.1111/j.1460-9568.2012.08148.x. [DOI] [PubMed] [Google Scholar]
- Wang XD, Su YA, Wagner KV, Avrabos C, Scharf SH, Hartmann J, et al. Nectin-3 links CRHR1 signaling to stress-induced memory deficits and spine loss. Nature neuroscience. 2013;16:706–713. doi: 10.1038/nn.3395. [DOI] [PubMed] [Google Scholar]
- Whipple EE, Webster-Stratton C. The role of parental stress in physically abusive families. Child Abuse & Neglect. 1991;15:279–291. doi: 10.1016/0145-2134(91)90072-l. [DOI] [PubMed] [Google Scholar]
- Winter TA. The effects of undernutrition and refeeding on metabolism and digestive function. Current Opinion in Clinical Nutrition & Metabolic Care. 2006;9:596–602. doi: 10.1097/01.mco.0000241670.24923.5b. [DOI] [PubMed] [Google Scholar]
- Yam KY, Naninck EFG, Abbink M, la Fleur S, Schipper L, van den Beukel J, et al. Exposure to chronic early-life stress lastingly alters the adipose tissue, the leptin system and changes the vulnerability to western-style diet later in life in mice. Psychoneuroendocrinology. 2017;77:186–195. doi: 10.1016/j.psyneuen.2016.12.012. [DOI] [PubMed] [Google Scholar]
- Yam KY, Schipper L, Naninck EFG, La Fleur SE, Oosting A, van der Beek EM, et al. Involvement of fat metabolism in the early-life stress induced cognitive impairments. Psychoneuroendocrinology. 2015;61:34. [Google Scholar]
- Yan CG, Rincón-Cortés M, Raineki C, Sarro E, Colcombe S, Guilfoyle DN, … Castellano FX. Aberrant development of intrinsic brain activity in a rat model of caregiver maltreatment of offspring. Tranlsational Psychiatry. 2017;7:e1005. doi: 10.1038/tp.2016.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XD, Liao XM, Uribe-Mariño A, Liu R, Xie XM, Jia J, et al. Stress during a critical postnatal period induces region-specific structural abnormalities and dysfunction of the prefrontal cortex via CRF1. Neuropsychopharmacology. 2015;40:1203–1215. doi: 10.1038/npp.2014.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr LK, Balian S. Responses of premature infants to routine nursing interventions and noise in the NICU. Nursing Research. 1995;44:179–185. [PubMed] [Google Scholar]
- Zeanah CH, Egger HL, Smyke AT, Nelson CA, Fox NA, Marshall PJ, et al. Institutional rearing and psychiatric disorders in Romanian preschool children. American Journal of Psychiatry. 2009;166:777–785. doi: 10.1176/appi.ajp.2009.08091438. [DOI] [PubMed] [Google Scholar]