Abstract
Spondylodiscitis may involve the vertebral bodies, intervertebral discs, paravertebral structures and spinal canal, with potentially high morbidity and mortality rates.
A rise in the susceptible population and improved diagnosis have increased the reported incidence of the disease in recent years.
Blood cultures, appropriate imaging and biopsy are essential for diagnosis and treatment.
Most patients are successfully treated by conservative means; however, some patients may require surgical treatment.
Surgical indications include doubtful diagnosis, progressive neurological deficits, progressive spinal deformity, failure to respond to treatment, and unresolved pain.
Cite this article: EFORT Open Rev 2017;2:447–461. DOI: 10.1302/2058-5241.2.160062
Keywords: spondylodiscitis, spondylitis, discitis, spinal infection, vertebral osteomyelitis
Infections of the spine have a large spectrum of clinical manifestations. The vertebral bodies, intervertebral discs, spinal canal and paravertebral structures may be involved.1 Spinal infections can be aetiologically classified as pyogenic (bacterial), granulomatous (tuberculous or fungal) and parasitic (Echinococcosis).2 Based on the specific anatomical elements involved, spinal infections may also be classified as spondylitis (vertebral osteomyelitis), discitis, epidural abscess or facet joint arthropathy.3 Nevertheless, alternative anatomical classifications also exist.4
Historically, spinal infection is reported as an ancient clinical condition. There is evidence of such infection in human skeletons dating back to the Iron Age.5 Spinal surgery was initially developed as an effort to treat vertebral infection. The first spinal fusion was performed in 1911 by Hibbs, on a patient with spinal tuberculosis (TB) in an attempt to prevent disease progression.6
Although the course and the consequences of the disease are well recognised, optimal treatment is still controversial and precise recommendations are few. In recent years, with the advent of systemic antibiotic agents and better understanding of the natural history of the disease, conservative treatment has become the standard of care. However, surgical indications for the treatment of spinal infections still exist.7 This review discusses the epidemiology, pathogenesis, clinical manifestations, diagnosis and treatment of spondylodiscitis, based on current evidence.
Epidemiology
Spondylodiscitis represents 0.15% to 5% of all osteomyelitis cases.8,9 At the same time, it constitutes the main manifestation of haematogenously-spread osteomyelitis in patients over 50 years of age.10 In the developed world, the estimated incidence of the disease ranges from four to 24 patients per million per year.11-14 Vertebral osteomyelitis demonstrates a male predominance, with a male-to-female ratio of 1.5 to 2:1.12,15 In recent decades, the incidence of vertebral infection has risen. This has been attributed to the increase in susceptible population (advanced age, immunocompromised states such as HIV infection, intravenous drug users), healthcare-associated infections, expansion of spinal surgery indications and improved diagnostic modalities.16
Risk factors
A low socioeconomic status has been associated with the presence of spinal infection. People who live in poverty, with inadequate sanitation and limited access to healthcare often remain undiagnosed until the peak of their symptoms, and sometimes present in a precarious health state.17 The most common predisposing factors include a previous infection such as of the skin and soft tissues or intravascular implants, or genitourinary, gastrointestinal, respiratory and oral cavity infections,16 advanced age,14 intravenous drug use,18 HIV infection,19 immunosuppression20 and underlying co-morbidities such as renal failure, rheumatological disease and hepatic cirrhosis.21 Urinary tract infections are common (17%) and range from cystitis to pyelonephritis.16 An underlying malignancy is also reported to be a risk factor for spondylodiscitis, and therefore its potential presence should be investigated.22 Furthermore, previous spinal surgery and outpatient spinal procedures are considered to be major risk factors (39.2% of cases).1
Pathogens
TB used to represent the most frequent cause of spinal infection. In the late 1950s, a study concluded that TB was the aetiologic agent in 59% of cases.23 In recent decades the responsible agents have changed. A study in 2010 showed that the majority of spinal infections were pyogenic in origin, whilst TB infection was detected in less than a quarter of all cases.24 In recent years, Staphylococcus aureus has become the most frequent bacterium responsible for vertebral infections, accounting for 20% to 84% of all cases.16,25-27 Additionally, Enterobacteriae spp. are implicated in 7% to 33% of pyogenic vertebral infections. Escherichia coli is the most common pathogen in this group, followed by Proteus, Klebsiella and Enterobacteriae spp. These isolates are often associated with urinary or gastrointestinal tract infections, advanced age, states of immunosuppression and diabetes.16,28 In the same group, Salmonella spinal infection is reported to be extremely rare, seen mostly in children with sickle-cell disease.29 Likewise, Pseudomonas aeruginosa is responsible for only a few cases and is associated with intravenous drug abuse, even though Staphylococcus aureus is still the predominant aetiologic agent in drug users.30,31 Streptococci and Enterococci are also common causes,32 being responsible for 5% to 20% of cases, whereas anaerobes are isolated in less than 4%.16 Staphylococcus epidermidis is associated with implant-related infections, whereas coagulase-negative Staphylococci and Streptococcus viridans may be a cause of indolent infections, due to their low virulence. On the other hand, the common zoonotic bacterium Brucella is still endemic in some Mediterranean and Middle Eastern countries. Spinal involvement in brucellosis is seen in 6% to 12% of cases, representing an important cause of spinal infection in these regions.33 Fungal infections are extremely rare, mostly responsible for opportunistic infections in immunocompromised individuals. Similarly, Echinococcal spine infection is quite rare, mostly described in endemic areas; these are countries of the temperate zones including southern South America, the entire Mediterranean littoral, southern and central parts of the former Soviet Union, central Asia, China, Australia and parts of Africa.34 In children, the most common isolates in spondylodiscitis are Staphylococcus aureus and Streptococcus spp., whereas strains of Kingella kingae constitute another very significant cause.35 In approximately one-third of patients the implicated organism may not be isolated.36 Despite the wide range of pathogens that have been associated with spondylodiscitis, it is considered to be a mono-microbial rather than a poly-microbial infection, most of the time. The latter concerns < 10% of cases and it is mostly reported after contiguous infection spread.37
A distant infection site has been identified in almost half of spondylodiscitis patients.16,37 Common distant infection sites include the genitourinary tract (17%), the heart (endocarditis, 12%), skin and soft tissue (11%), intravascular devices (5%), gastrointestinal tract (5%), respiratory tract (2%) and oral cavity (2%).
Pathogenesis
There are two main infection routes that may contribute to the development of spinal infection. Infectious spread may be haematogenous or non-haematogenous, the latter being either the result of direct external bacterial inoculation or extension from a contiguous infectious site. Haematogenous spread is the most common route, allowing bacteria from distant sites to contaminate the spine in the setting of bacteremia. The origin of infection in these patients may be the oral cavity, the skin, the respiratory, urinary or gastrointestinal tract, or any infected implanted device. Wiley and Trueta38 demonstrated how metaphysial and cartilaginous end-plates constitute potential areas of inoculation after bacterial spread in the arteriolar network. Infection in pyogenic spondylodiscitis begins when bacteria reach the metaphyseal vascular arcades, whereas the intervertebral disc is destroyed by the release of bacterial proteolytic enzymes, a process similar to cartilage destruction in septic arthritis. On the other hand, TB infection usually begins after venous spread through Batson's venous plexus. In contrast to pyogenic spondylodiscitis, the latter type of infection typically preserves the adjacent vertebral discs until late in the disease process.39
The pathophysiology of spinal infection is somewhat different in adults and children. Discitis is common in children, due to the persistent vascularisation of the vertebral disc that may provide a nidus for bacterial inoculation in the setting of bacteremia.40 Accordingly, the vertebral body is relatively protected in children. The metaphysis in children has a rich blood network with anastomotic arterioles that protects the body from extensive destruction in case of infarction by septic emboli. In adults, however, intra-osseous arteries are end-arteries and septic emboli may become entrapped, resulting in extensive destruction, leading to wedging or collapse of the body.41 Infection extending to the adjacent disc and vertebra creates the typical lesions of spondylodiscitis. In contrast to TB and fungal infections, the posterior vertebral structures are rarely involved in haematogenous pyogenic infection.42 The poor blood supply of these structures explains their limited involvement.43 Extensive vertebral destruction may result in deformity, compromise stability or cause spinal cord compression. Uncontrolled infection may lead to the formation of paravertebral abscesses or may spread into the spinal canal. Such contamination may result in meningitis, epidural or subdural abscess formation, neurological impairment and high morbidity and mortality rates.44
Direct bacterial inoculation is mainly iatrogenic. A diagnostic or therapeutic spinal procedure may inoculate bacteria and contaminate the spine. Iatrogenic inoculation accounts for 14% to 26% of spinal infections.45 Contiguous spread is extremely rare. Infection in these cases may spread from adjacent infected tissues such as a ruptured oesophagus or an infected aortic graft.46,47
Spinal regions
Hematogenous pyogenic spondylodiscitis affects mostly the lumbar spine followed by the thoracic, cervical and sacral regions.48 TB commonly affects the thoracolumbar spine;49-52 TB involvement of the cervical and lumbosacral spine is less common, whereas TB of the cranio-vertebral junction is rare.53 Patients with pyogenic infection mostly present with isolated lesions, involving one or two adjacent vertebrae. In contrast, most of the patients suffering from TB spondylodiscitis present with more than two infected vertebrae and about 25% of them with multifocal skip lesions.49
Clinical manifestations
Clinical presentation of spondylodiscitis is generally vague and non-specific.54 The most common symptom is back or neck pain, typically worse at night. During physical examination, in the majority of cases, tenderness, paravertebral muscle spasm and restricted spinal range of movement are observed.55 High fever is reported in about half of spondylodiscitis cases, being less common in patients with TB.56 Moreover, TB is usually associated with a more insidious clinical course and a delayed onset of symptoms, as compared with pyogenic infections.9 Spinal TB typically presents with back pain, tenderness mostly in the thoracolumbar spine and localised back deformity. In addition, cervical spondylodiscitis may cause dysphagia or torticollis.57 Other non-specific symptoms include malaise, lethargy, confusion, nausea, vomiting, anorexia and weight loss.58 Neurological deficits at presentation are not infrequent (27%); these include paralysis, sensory deficits, muscle weakness, radiculopathy and sphincter dysfunction as a result of spinal cord or cauda equina compression.58 Non-specific cardiac symptoms such as malaise, weakness, excessive sweat and fever may suggest subacute bacterial endocarditis. In this setting, an echocardiogram is an important diagnostic test in patients with haematogenous spinal infections.16,37
In children, abdominal pain may be present, especially when the lumbar spine is affected. Other symptoms may include low-grade fever, difficulty in walking, irritability and inability to bend over. Back pain that worsens with movement is commonly the leading complaint. Obtaining a detailed history and physical examination may be problematic in children, due to communication difficulties. As compared with adults, neurological deficit in these patients is uncommon.59
In the elderly, the diagnosis of spondylodiscitis is frequently delayed. The reasons for this include a higher prevalence of co-morbidities such as degenerative back pain that may cloud diagnostic thinking. Clinical assessment may be difficult because of pre-existing cognitive impairment or intercurrent delirium and atypical or subtler symptoms and biochemical marker abnormalities. Relatively few spinal infection studies specifically deal with older populations, and data on spinal infections in adults is similarly sparse.60
Laboratory findings
Laboratory tests are usually inconclusive in diagnosing spondylodiscitis. White blood cell count (WBC) is of limited diagnostic value, as it is commonly non-specific, being elevated in less than half of patients.21 Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels are more helpful.55 ESR is a sensitive laboratory test, being elevated in > 90% of patients, with a typical range of 43 mm to 87 mm per hour.61 CRP is more sensitive than ESR, with its levels also elevated in most cases.55 Nevertheless, these markers are also non-specific, since they are unable to distinguish between a pyogenic, granulomatous or other inflammatory process. Obviously, patients with acute disease may demonstrate a significant increase of these markers, while in chronic infections (as in TB infections) these values may be normal or only slightly elevated.8
WBC is not very useful for the diagnosis of spinal infection, but should be part of the work-up, as it may provide information concerning response to treatment. CRP levels and ESR are more reliable to evaluate treatment response or detect the onset of a post-operative infection after a spinal procedure.21 CRP presents higher clinical value as it normalises faster than ESR after appropriate treatment.55 An increase or decrease of CRP levels is directly related to deterioration or improvement of the clinical course, respectively.62 Other findings include anaemia (approximately 70% of patients) and elevated serum levels of alkaline phosphatase (approximately 50% of patients).16
If spondylodiscitis is suspected, it is essential to obtain blood and urine cultures before administration of antibiotics. Multiple blood samples are recommended. The pathogen may be detected not only in the febrile or the critically ill, but also in afebrile patients.63 Blood cultures are reported to be positive in 58% of cases (range, 30% to 78%), and approximately 25% to 59% of positive cultures identify the causative organism.37,61,64 However, in post-operative infections, blood cultures are often negative.21
Biopsy
In general, when spinal infection is suspected and blood cultures are negative, biopsy should be performed. Biopsy is considered to be a superior diagnostic tool and may also be performed to verify diagnosis in suspicious cases, or when a polymicrobial infection is suspected, regardless of the presence of positive blood culture.18,65
Percutaneous CT-guided biopsy is the standard of care for the diagnosis of spondylodiscitis of unidentified origin.65 The yield of CT-guided biopsy is reported to be up to 75%.3 In cases of negative results, some authors advise the conduction of a second percutaneous biopsy, whereas others advise the conduction of an open biopsy.66,67 CT-guided biopsy can also be performed in children with an accuracy up to 80%. However, open biopsy is argued due to increased local morbidity.59 Lower yield is expected if patients were treated with antibiotics before blood cultures or biopsy, and false negative results are frequent. In such cases, providing that the patient is stable, biopsy should preferably be performed 48 hours after the most recent antibiotic dose.68 Nonetheless, some authors reported significant false negative results after CT-guided biopsy in the absence of prior antibiotic administration (39%) and confirmation of the infection with further histological evaluation.69 For this reason, for optimum diagnosis, fluid and tissue specimens for aerobic, anaerobic, fungal and mycobacterial cultures, as well as histology, should be submitted.67 Histopathological analysis is able to distinguish between infection and contamination, as well as between pyogenic and granulomatous disease.42,68 Additionally, it may reveal a potential underlying malignancy.
Other laboratory tests
Even though they are not able to differentiate between active and latent disease, important information may also be obtained by the tuberculin skin test and interferon-gamma release assays, if a TB infection is suspected,70 and serological tests may be performed if Brucella infection is suspected.71 Another useful diagnostic method is amplification-based DNA analysis via polymerase chain reaction (PCR). PCR seems to have higher sensitivity as compared with cultures, but it is inferior in cases of polymicrobial infections. Therefore, it is a useful adjunct to cultures, rather than a substitute for them. It may be used to confirm culture results in cases of unusual pathogens, to recognise sample contamination and to identify promptly slow-growing bacteria and contribute to a species-specific therapeutic approach.72
Radiographs
Even though radiographs of the spine have a low specificity for the evaluation of spondylodiscitis (57%), they are regarded as a vital baseline examination.65 They may provide evidence suggestive of vertebral infection, paravertebral abscess formation or spinal deformity, and may also differentiate between other clinical conditions, such as bone metastases or osteoporotic fractures (Fig. 1). Radiographs may reveal the extent of bone destruction and indicate coronal or sagittal malalignment secondary to disease progression.61 Radiographs are reported to show abnormalities of the infected spine in almost 90% of cases.37 However, sensitivity and specificity of radiographs are expected to be lower in early disease stages, as the first radiographic signs may appear two to eight weeks after the onset of infection.45 In pyogenic spondylodiscitis, narrowing of the disc space represents the earliest finding, followed by blurring and irregularity of the end-plate.61 This happens due to disc and bone degeneration by the released proteolytic enzymes. In early TB, however, considering the absence of these enzymes, the disc space is generally preserved.9 Nonetheless, after eight to 12 weeks, significant bone destruction is obvious.61
Fig. 1.
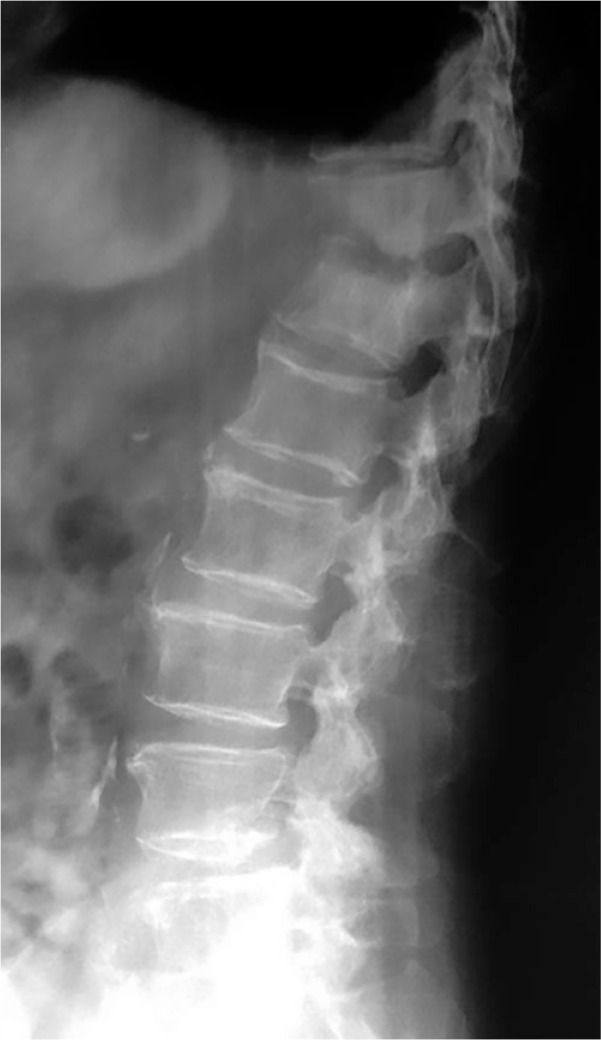
Lateral radiograph of the thoracolumbar spine of a 70-year-old patient with pyogenic spondylodiscitis that originated from an infected pacemaker shows partial collapse of the T11 and T12 vertebral bodies and T11 to T12 disc degeneration.
In children, four radiographic phases of discitis have been described; these include the latent, acute, healing and late phase. In the latent phase, plain radiographs are normal, whilst in the acute phase (two to four weeks later) erosion of the disc space is evident. In the healing phase (two to three months later), sclerosis of the contours of the vertebral bodies is evident, and in the late phase, narrowing of the involved disc space is observed.73
Computed tomography
Computed tomography (CT) can further characterise bone lesions, as it is more sensitive than radiographs, due to its higher soft-tissue contrast resolution. In early disease stages, end-plate destruction, reduction of paravertebral fat and disc hypodensity may be evident. During disease progression, erosion of the end-plates and sequestra formation may be seen. Bone necrosis and pathological calcification suggestive of TB infection, as well as paravertebral disease extension or epidural abscess formation, can also be detected.74 Even if CT provides a more conclusive evaluation of bone integrity and it is more sensitive to earlier changes than radiographs, it is inferior to magnetic resonance imaging (MRI), being generally reserved for the conduction of CT-guided biopsy or if MRI is contraindicated.
MRI
MRI is considered the imaging modality of choice for the detection and evaluation of spondylodiscitis, giving 96% sensitivity, 92% specificity and 94% accuracy.75 In acute spinal infections, an increase in fluid signal due to marrow oedema is demonstrated. The infection mostly begins in the anterolateral vertebral body near the end-plates, presenting irregular signal intensity on T1-weighted images, while the associated oedema typically affects most of the body and adjacent disc.75 In this setting, typical findings of pyogenic infection include low signal on T1- and high signal on T2-weighted images, with enhancement of the involved vertebral bodies on T1-weighted images after contrast medium administration (Fig. 2). Additionally, high signal on T2 images, loss of intranuclear cleft and peripheral enhancement of the infected disc are shown.76,77
Fig. 2.
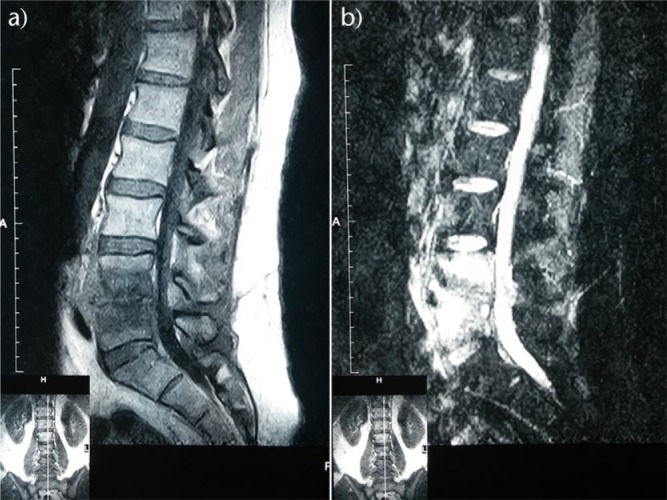
Sagittal (a) T1-weighted and (b) T2-weighted MRI of the lumbar spine of a 45-year-old patient with spontaneous pyogenic spondylodiscitis showing reduction of the L4 to L5 vertebral disc height and erosion of the adjacent vertebral end-plates.
Intra-osseous abscesses, large paravertebral abscesses, skip lesions, contiguous subligamentous spread, involvement of the posterior elements and encroachment on the spinal canal and nerve roots are suggestive of TB spondylodiscitis.77,78 Another MRI feature in patients with TB infection is the relative preservation of the disc, whilst it is also reported that the chronic course of the disease contributes to the formation of thinner and smoother abscess walls.79 In Brucella infection, intact vertebral architecture is observed, despite the evidence of diffuse vertebral infection. An increase in signal intensity of the disc on T2-weighted and contrast-enhanced images, as well as facet joint involvement are also characteristic findings.80 Paravertebral abscesses tend to be smaller than those in TB infections. However, it can be difficult to differentiate between Brucella and TB spinal infections.81 Most fungal spinal infections present with non-specific findings on MRI, whist vertebral body destruction in this type of disease may mimic TB infections. Absence of signal increase on T2-weighted images, and low or undetectable enhancement after contrast administration may be MRI features of fungal infection.76
As MRI in spinal infections can result in images that mimic malignancy, biopsy may be warranted.66 Additionally, MRI may overestimate the extent of the infected tissue. Therefore, additional information from radiographs or CT may be necessary for the definition of the real amount of tissue necrosis.61 Finally, the results of MRI in both pyogenic and TB spondylodiscitis are not always in agreement with the clinical course of the disease. Abnormal findings may persist even after successful treatment. An early sign of healing (from a few weeks to a few months after successful treatment) on MRI is the reduction of contrast enhancement. The absence of enhancement and return to normal signal pattern are reliable features of healing after spondylodiscitis. However, some degree of contrast uptake may be present for many months, being more likely to involve the disc rather than the vertebral body.82 In this context, the interpretation of MRI findings in the follow-up period should be treated with caution.
Nuclear imaging
In the absence of abnormal radiographic findings or when MRI is inconclusive, radionuclide imaging modalities may aid diagnosis. Three-phase bone scintigraphy with technetium-99m is very sensitive (90%) within a few days of infection onset.75 However, it gives limited specificity and is inferior in terms of resolution of the relevant anatomical structures (Fig. 3). Gallium-67 (Ga-67) scintigraphy with single-photon-emission CT (SPECT) is more reliable in the evaluation of spinal infections, with a reported accuracy of 67%. This method, though, has comparative results with MRI and for this reason it is mostly reserved for cases in which MRI is inconclusive or contraindicated.83 The combination of 99m-Tc methylene diphosphonate (MDP) and Ga-67 scintigraphy and conventional CT (SPECT-CT) is reported to offer even higher accuracy.84 Fluorine-18 fluorodeoxyglucose positron emission tomography is another promising method, reported to have higher sensitivity, specificity and accuracy in spinal infections.85 Results are comparable with MRI in patients with high and medium grade spinal infections, whereas it seems to be superior in detecting low grade infections.86 However, this method is not widely available and evidence regarding its usefulness and cost-effectiveness is still limited.87
Fig. 3.
(a) Lateral radiograph of the thoracic spine, (b) 99mTc-MDP bone scan, (c) sagittal and (d) axial CT, and sagittal (e) T1-weighted and (f) T2-weighted MRI of the spine of an 83-year-old woman with tuberculosis spondylodiscitis showing increased radionuclide uptake and destruction of the T9 to T10 vertebrae.
Conservative treatment
The goal of treatment for patients with spondylodiscits is eradication of infection, preservation or restoration of spinal structure, stability and neurological deficits, and relief of pain. Conservative treatment constitutes the standard of care and most patients are successfully treated with non-operative means. In this context, appropriate antibiotics in combination with non-pharmacological regimes, such as immobilisation and physical therapy, are effective in most cases.
Immobilisation with bed rest or with bracing decreases pain, stabilises the spine and prevents deformity. Initially, bed rest is recommended in the first stages of disease, until acute pain resolves, generally lasting no longer than two weeks. Ambulation using a brace is recommended thereafter. If the cervical spine is involved, a collar or a halo-vest may be applied. A reclining brace may be indicated for thoracic spine lesions. This brace immobilises the affected spine in the reclining position, reducing the load on the infected vertebrae by distributing weight to the unaffected facet joints. Accordingly, a thoracolumbar or lumbosacral brace may be applied, if the thoracolumbar or lumbar spine is affected. In the presence of major defects in the anterior column in the lower lumbar or the lumbosacral spine, bed rest is claimed to be necessary for at least six weeks. Nevertheless, immobilisation-related morbidity, especially in the elderly, should be considered. Bed rest may result in skin ulcerations, deep vein thrombosis, pulmonary embolism or pneumonia. In addition, high rates of pseudarthrosis and instability that may result in spinal deformity and chronic pain have been reported (16% to 50%). Therefore, conservative treatment discontinuation may be advisable after four to six weeks. If there are no radiological signs of bone fusion, destruction progresses or there is no clinical improvement,8,88 prompt treatment with antibiotics is essential. However, antibiotic administration should be initiated preferably after isolation of the pathogenic organism and consideration of the relevant susceptibility data. This is feasible in most cases, provided that the patient does not receive antibiotic agents before cultures or biopsy. The initiation of treatment may be withheld until a conclusive result, as long as the patient’s condition is stable.89
A variety of agents may be employed for the management of spinal infections. In cases of methicillin-susceptible Staphylococci, an anti-staphylococcal penicillin or a first-generation cephalosporin are the antibiotic agents of choice. For methicillin-resistant organisms, including most of Staphylococcus epidermidis cases, a glycopeptide such as vancomycin may be administered. Linezolid and quinupristin-dalfopristin are alternative options. Additionally, in cases of Streptococcus spp., penicillin G is the agent of choice. For gram-negative bacteria, a cephalosporin or a quinolone could be administered, whereas in cases of anaerobes, metronidazole or clindamycin may be used. In TB spondylodiscitis, multiple agents are administered due to potential resistance.90 The antimicrobial regime classically includes isoniazid, rifampicin, ethambutol and pyrazinamide. In Brucella infections, treatment with doxycycline and streptomycin or gentamicin may be needed. Fungal infections may require treatment with an azole or amphotericin B.89 It should be emphasised, however, that the antibiotic regime has to be tailored to the specific pathogen according to cultures and sensitivities.
The duration of antimicrobial therapy is controversial. There are diverse recommendations for duration of treatment, ranging from four to six weeks to three months, based mostly on observational studies and expert opinions.68,91 A recent randomised controlled trial including 359 patients showed that six weeks of antibiotic treatment was not inferior to 12 weeks. The authors assigned 176 patients to a six-week and 175 patients to a 12-week antibiotic regime. Almost 91% of patients in the first group and 91% in the second had a clinical cure, with similar rates of adverse events in both groups; death rate was 8% versus 7%, antibiotic intolerance 7% versus 5%, cardiorespiratory failure 4% versus 7% and neurological complications 4% versus 2%. The authors suggested that the standard antibiotic treatment duration for patients with pyogenic spondylodiscitis could be limited to six weeks.92
Likewise, the optimal duration of treatment in Brucella spondylodiscitis is unknown, but treatment of at least three to six months should be beneficial.93 In TB spondylodiscitis, antimicrobial agents are generally administered for nine to 12 months, which may be extended to 18 to 24 months to allow complete healing and prevent relapse.8,89 A full antimicrobial regime is recommended for the first two months, followed by part of the regime according to sensitivities.89 In these patients, compliance is essential to prevent multi-drug resistance, especially when the patients are immunocompromised.94 On the other hand, the duration of antifungal treatment needs to be individualised with respect to side-effects and clinical response.95 Finally, in patients with undrained abscesses or infected spinal instrumentation, prolonged antibiotic treatment is generally recommended.68
In general, administration of intravenous antibiotics as initial treatment for at least two to four weeks is recommended to improve bioactivity.88 Higher treatment failure rates are reported in cases with parenteral administration for less than four weeks.96 However, switching to an oral regimen after a two-week intravenous treatment is also reported to be effective, if enlarged epidural or paravertebral abscesses have been drained and CRP levels have decreased.97 Peroral agents with high bioavailability, such as fluoroquinolones, allow for an early switch to oral administration.68 Clindamycin has good bioavailability and is effective for prolonged treatment of chronic staphylococcal osteomyelitis, but there are limited data for treatment of acute staphylococcal disease in adults. Furthermore, the low bioavailability of beta-lactam antibiotics does not allow for oral administration.68
Antibiotics should not be started in children before pathogen isolation, provided that they are not critically ill. However, there are reports that support empirical antibiotic treatment in children, considering the likely probability of the involved pathogen as well as the related risk factors. Considering that the most common isolates are Staphylococcus aureus and Streptococcus, a combined regime of a third generation cephalosporin and oxacillin/clindamycin is recommended.59
As in adults, there are no standardised guidelines for the duration of antibiotic treatment in children. Intravenous administration for one to three weeks followed by oral antibiotic agents until clinical improvement is generally employed. According to the patient’s response, the total duration of treatment may range from two weeks to six months.59 Conversely, some authors doubt the bacterial origin of spondylodiscitis in childhood, questioning the need of antimicrobial regimes, as self-limiting disc inflammation that resolved without antibiotics has been observed.40 Regardless of a patient’s age, the criteria for antimicrobial treatment discontinuation are clinical improvement, resolution of symptoms and normalisation of ESR and CRP values. A weekly decrease of CRP levels by 50% has been proposed as an adequate progress index.16,59 In contrast, lack of clinical improvement over a four-week period, continued fever, pain and persistently high CRP levels (above 30 mg/lt) are predictors of treatment failure.68
Surgical treatment
Conservative treatment is the standard of care for patients with spondylodiscitis, using multidisciplinary approaches involving microbiologists, infectious disease consultants, anaesthetists, intensivists and geriatricians, with public health physicians for contact tracing. The morbidity and mortality of patients with spondylodiscitis treated conservatively has fallen from 56% to 25% over the last 15 years.7,9 However, careful selection of patients who need surgical treatment is necessary.
Surgical treatment is absolutely indicated in patients with spinal cord or cauda equina compression with progressive neurological deficits. Relative surgical indications include spinal instability due to extensive bone destruction, significant deformity or conservative treatment failure.61,98,99 Considering the fact that the majority of patients can be treated successfully by conservative means, surgical intervention mainly aims to acquire bacteriological or histological verification when CT-guided biopsy is inconclusive.37 However, a surgical operation may also be necessary to drain enlarged abscesses,100 despite the fact that sufficient drainage can be achieved with a CT-guided percutaneous catheter. Surgical drainage, bone debridement and reconstruction are indicated when an anterior abscess is larger than 2.5 cm on radiographs.98 Only 10% to 20% of adult patients with pyogenic spondylodiscitis require open surgery;61 in children, surgical treatment is rarely indicated, as conservative treatment is effective in most cases.59
Principles of surgical treatment are important; surgery should aim for decompression of neurological deficits and reconstruction for spinal stability and deformities. Timing of surgical treatment is critical; surgical decompression should be done as an emergency in patients with spinal nerve or cord compression, as prognosis may be poor after 48 hours from the onset of symptoms and signs, despite some authors’ claims of good results even after long-term paralysis.101 Septicaemia is also a surgical emergency.3 Epidural abscesses should be treated before neurological impairment ensues.102 Moreover, prolonged unsuccessful conservative treatment may compromise the remaining healthy bone stock, leading to major reconstructive operations.
Recommendations regarding optimal surgical management are controversial.65,88,98 The main goals of surgery include prompt decompression and stabilisation, aggressive debridement and tissue sample harvesting.65 There is a wide spectrum of surgical options, including anterior, posterior or combined approaches and single-stage or two-stage procedures, with or without instrumentation. Less invasive endoscopic techniques for debridement and reconstruction have also been described. However, open surgery remains the standard of care, particularly in cases of extensive bone destruction.103 Due to lack of specific guidelines for the optimal technique, surgeons often come to a decision taking into account the general health status, the most suitable spinal approach and instrumentation tailored to the characteristics of each patient and the individual surgeon’s preference.7
Because spinal infections commonly involve the vertebral body, an anterior surgical approach decompression and debridement via an anterior approach, followed by anterior fusion is usually recommended.61 After debridement, strut grafting with autografts, allografts or cages has been reported to be safe, provided that most of the infected tissue has been removed.104-106 However, anterior fusion alone does not result in primary stability and when applied should be followed by prolonged bed rest and bracing. Additionally, in multi-segmental lesions, high rates of complications such as pseudarthrosis, graft displacement and spinal deformity have been reported.107 For this reason, supplementary posterior stabilisation is usually required.98 A posterior approach is preferred in the presence of an epidural abscess in the lumbar spine.98,108 The posterior elements should not be destabilised by laminectomy alone.88 Abscess drainage without fusion has been suggested in some reports,109 but it may result in further instability and neurological deterioration. Consequently, a transpedicular instrumentation is usually advised.98 Nevertheless, in some cases, an additional posterior approach is necessary for the correction and preservation of the sagittal alignment and augmentation of the stability of the affected segments.110,111 Finally, posterior percutaneous instrumentation has been also postulated to be safe and effective in relieving pain, preventing deformity and neurological compromise in non-complicated cases involving the lower thoracic or lumbar spine.112,113
In the cervical spine, an anterior approach followed by grafting and plating may be performed. Locking plates with or without posterior stabilisation may be required in multilevel lesions.114 In the thoracic spine, considering that stability is maintained by the thoracic cage, a posterior approach with or without instrumentation is usually sufficient. An anterior approach alone may be effective for decompression and fusion in cases of single level involvement without posterior infectious spread.115 In the presence of extensive anterior destruction and collapse, an anterior approach for debridement, decompression and fusion, followed by additional posterior instrumentation, is recommended.88,114 If the thoracolumbar junction or lumbar spine is affected and the patient experiences neurological deficit or extended epidural infection, stabilisation is necessary after decompression. In cases of single segment disease with limited anterior involvement and minor kyphosis, an anterior fusion with bone grafts may be adequate.107 Posterior lumbar interbody fusion with autologous iliac crest bone graft is also reported to be successful in cases of minor bone destruction.116
Instrumentation in the setting of infection constitutes a controversy in spinal surgery. Implantation of metallic implants into the infected site carries the risk of colonisation and persistent infection. However, a recent study recorded a higher re-operation rate after decompression alone compared with decompression with internal stabilisation.117 Spinal instrumentation can be successful after thorough debridement and concomitant antibiotic administration.8,88,118 Apparently, staphylococcal infections may heavily colonise spinal rods covering them with a thick biofilm, whist only a few biofilm-covered colonies of Mycobacterium tuberculosis have been observed on stainless steel rods. These findings are in favour of anterior instrumentation at the time of surgical debridement, especially with titanium implants that have lower bacterial adherence.119 Bone grafting seems to be safe even in the setting of an active infection,120 with tri-cortical iliac autograft being usually the first choice. Structural allografts can also be used to avoid the associated donor site morbidity and to reduce operation time.121 Titanium mesh cages also seem to be safe and successful for reconstruction after anterior debridement.122 Pedicle screws are used in these cases to secure fixation. Finally, even if bacterial biofilm formation is reported to be more frequent with use of polyetheretherketone (PEEK) as compared with titanium in a laboratory setting,123 recent reports advocate the use of PEEK cages for interbody fusion as a feasible and safe alternative.124,125
One-stage or two-stage procedure is another subject of debate, as risk of implant contamination by residual bacteria demands caution. In a study of patients with pyogenic and TB spondylodiscitis that received a two-stage procedure, advantages such as less operation time, less blood loss and safety for patients with poor general health have been reported.126 In contrast, it has been reported that a single-stage procedure is a safe and efficient way to control infection and simultaneously reconstruct the spine.127,128 Both techniques seem to be efficient; however, two-stage operations increase hospital stay and the already compromised patients may not be able to be withstand a second surgical procedure. The decision for the appropriate procedure should be based on surgical experience and general health status.
The Medical Research Council Working Party on Tuberculosis of the Spine, which was formed in 1963, designed the prospective multicentre clinical trials that took place in Hong Kong, Korea, Rhodesia and South Africa.129-138 They studied various combinations of conservative treatments and compared conservative treatment to debridement alone, radical debridement and strut grafting. They described and popularised the ‘Hong Kong operation’, developed by Hodgson, that is thorough excision of the tuberculous focus, posteriorly as far as the dura mater, and cephalad and caudad, until healthy, bleeding cancellous bone is exposed, to create surfaces suitable for docking of a strut graft obtained from a cut rib.130 They provided strict criteria for a favourable outcome for the patients, including no symptoms, full physical activity at work or school, no evidence of central nervous system involvement, no residual sinus or abscess detectable clinically or radiologically and radiographic evidence of healing of the spinal lesion.138 In the short term, the Hong Kong operation provided faster bone union and resolution of abscesses compared with ambulatory chemotherapy, and similar results compared with debridement.132,135,138 Additionally, in the long term, up to 15 years follow-up, the Hong Kong operation resulted in less kyphotic deformity, which is an important determinant not only of cosmetic well-being but also of possible future neurologic impairment.139
Strengths and weaknesses of the literature
A more recent literature review was published in 2016140 and a second report using the GRADE approach141 in order to obtain an evidence-based assessment of the literature on the management of patients with spondylitis (Table 1). From a total of 1662 related articles found in the four electronic databases, the systematic literature search for the purpose of that article resulted in 25 suitable included studies.140 According to the GRADE approach, this systematic review concluded that 20 of the included articles had a very low level of evidence resulting in difficulty in producing definitive guidelines. Potential selection bias included inclusion of articles published only in the English language, articles published after 2000, exclusion of case reports and a small series focusing on a decision-making approach, and inherent limitations of the published studies such as the heterogeneity of the study populations with respect to age, gender, type of infections (pyogenic and TB; primary and post-operative) and treatments, and small sample sizes.140 However, there was a strong level of recommendation for six weeks of systemic antibiotic treatment in uncomplicated pyogenic spondylodiscitis, as shown in one randomised control trial (RCT),142 and a strong level of recommendation that, if surgical treatment is indicated, an isolated anterior approach can result in better clinical outcomes compared with more extensive combined anteroposterior procedures, as shown in a prospective comparative study105 and a RCT.143 This study concluded that a prognostic systematic review regarding the effect of patient characteristic on the outcome of spondylodiscitis could be a valuable addition to the available literature, and that emerging less invasive surgical techniques should be studied more extensively.140
Table 1.
Summary of the most recent published studies on spondylodiscitis
| Study | Study design | Patients (number) | Type of infection | Positive cultures | Treatment | Antibiotics (duration) | Outcome | Follow-up (mean) |
|---|---|---|---|---|---|---|---|---|
| Hadjipavlou et al3 | RCS | 101 | Pyogenic | 75.5% | Conservative (57.4% required surgical treatment) | 12 weeks | Relapse 2%, failure 0%, mortality 1% | Not given |
| Loibl et al25 | RCS | 105 | Pyogenic (n=102) TB (n=3) |
56.2% | Conservative (53.3% required surgical treatment) | Not given | Mortality 12.4% | 31.5 months |
| Legrand et al26 | RCS | 110 | Pyogenic | 72.8% | Conservative (brace, 89.1%) | 103 days | Mortality 1% | 3 months |
| Parra et al27 | RCS | 108 | Pyogenic (n=67) Postoperative (n=30) TB (n=11) |
69.4% | Conservative (25% required surgical treatment) | 5.2 weeks IV | Mortality 10% | 73 months |
| Mulleman et al32 | RCS | 136 | Pyogenic | 100% | Conservative (brace, 74%; 8.9% required surgical treatment) | 122 days | Mortality 4.6% | Not given |
| Kotil et al50 | PCS | 44 | TB | 100% | Conservative (4.6% required surgical treatment) | 17 months | Favorable 95.4% | 24 months |
| Hassan et al51 | RCOMPS | 42 | TB | Not given | Surgical (anterior, n=20; posterior, n=22) | At least 2 weeks before and at least 9 months after surgery | Not given | 15 months |
| Park et al52 | RCS | 116 | TB | 88.3% | Conservative (84.4% required surgical treatment) | 6-9 months (n=20) vs > 12 months (n=96) | Favorable 70% vs. 83.3% | Not given |
| Jensen et al54 | RCS | 133 | Pyogenic | 40% | Conservative | 2 to >10 weeks | Relapse 10%, failure 14%, mortality 14% | Not given |
| Shi et al60 | RCS | 967 | TB | 100% | Conservative (76.5% required surgical treatment) | Not given | Relapse 0.6%, failure 0%, mortality 0% | Not given |
| Aagaard et al64 | RCS | 100 | Pyogenic | 90% | Conservative (41% required surgical treatment) | 91 days | Relapse 4%, failure 0%, mortality 8% | 12 months |
| Roblot et al91 | RCOMPS | 120 | Pyogenic (n=98), Postoperative (n=22) |
100% | Conservative | <6 weeks (n=36) vs. >6 weeks (n=84) | Relapse 0%, mortality 8% vs. relapse 7.1%, mortality 12% | 6 months (n= 120), 41 months (n= 91) |
| Bernard et al92 | RCT | 351 | Pyogenic | 100% | Conservative | 6 weeks (n=176) vs.12 weeks (n=175) | Relapse 2.3%, failure 9.1%, mortality 8% vs. relapse 0%, failure 9.1%, mortality 7% | 12 months |
| Valancius et al99 | RCOMPS | 196 | Pyogenic | 72.9% | Conservative (n=91) and surgical (n=105) | At least 2 weeks IV and 3–6 months per os | Relapse 7.6%, failure 13.1%, mortality 8.7% vs. relapse 2.9%, failure 0%, mortality 1.9% | 12 months |
| Rossbach et al100 | RCS | 135 | Pyogenic (n=127) TB (n=8) |
59.5% | Conservative (brace) and surgical (n=75) | Not given | Not given | Not given |
| Si et al105 | PCOMPS | 23 | Pyogenic | Not given | Surgical (anterior, n=12; posterior fusion, n=11) | Not given | Relapse 0%, failure 0% vs. relapse 0%, failure 0% | 38 months |
| Pee et al106 | RCOMPS | 60 | Pyogenic | 50% | Surgical (anterior cages and pedicle screw fixation, n=37; anterior struts and pedicle screw fixation, n=23) | At least 6 weeks IV and at least 6 weeks per os | Infection resolution in all patients | 35.8 months |
| Vcelak et al108 | RCOMPS | 31 | Pyogenic (n=27) TB (n=4) |
100% | Surgical (dorsal transmuscular, n=23; two-stage posterior-anterior, n=8) | Not given | Relapse 8.7%, failure 4.3%, mortality 4.3% vs relapse 0%, failure 0%, mortality 0% | 12 months |
| Ozturk et al110 | RCOMPS | 56 | Pyogenic (n=40) TB (n=16) |
100% | Surgical (sequential, n=29; simultaneous anterior and posterior surgery, n=27) | 6 weeks IV and 3 months per os (pyogenic) 9 months (TB) |
Failure 0% | 78 months |
| Lee et al111 | RCOMPS | 26 | Pyogenic (n=24), TB (n=2) |
42% | Surgical (transpedicular curettage and drainage, n=10; combined anterior-posterior approach, n=26) | 91.1 days vs 65 days | Relapse 10%, failure 0%, mortality 0% vs relapse 0%, failure 0%, mortality 0% |
57 months |
| Nasto et al112 | RCOMPS | 27 | Pyogenic | 100% | Conservative (brace, n=15) and surgical (n=12) | 76-84 days | Relapse 0%, failure 0%, mortality 0% | 9 months |
| Lin et al113 | RCOMPS | 45 | Pyogenic | 84% | Surgical (combined anterior-posterior, n=25; combined anterior-percutaneous posterior, n=20) | 28-83 days | Relapse 8%, mortality 0% vs relapse 5%, mortality 0% | 24 months |
| Karadimas et al117 | RCS | 163 | Pyogenic (n=141) TB (n=22) |
59% | Conservative (brace, n=70) and surgical (without, n=56; with instrumentation, n=37) | 2-7 months | Failure 0 %, mortality 11.4% vs. failure 12.5%, mortality 13.5% | 12 months |
| Pourtaheri et al118 | RCOMPS | 104 | Pyogenic | Not given | Surgical (instrumented, n=57; non-instrumented, n=47) | Not given | Infection resolution 54%, mortality 9% vs. infection resolution 42.5%, mortality 17% | 43 months |
| Schomacher et al125 | RCOMPS | 37 | Pyogenic | 70.3% | Surgical (PEEK cages, n=21; titanium cages, n=16) | 2–4 weeks IV, 8–10 weeks per os | Reinfection was not observed in any patient | 20.4 months |
| Chen et al128 | RCS | 24 | Brucella | 100% | Surgical (posterior debridement, bone grafting and instrumentation) | 6.5 months | Infection resolution | 14.3 months |
| Linhardt et al143 | RCT | 22 | Pyogenic (n=17) TB (n=5) |
Not given | Surgical (ventro-dorsal spondylodesis, n=20; ventral spondylodesis, n=10) | 23.8-24.1 weeks | Relapse 8 %, failure 0%, mortality 25% vs relapse 0%, failure 0%, mortality 10% | 63 months |
Note: TB: tuberculosis; RCS: retrospective case series; RCT: randomized controlled trial; RCOMPS: retrospective comparative study; PCS: prospective cohort study; PCOMPS: prospective comparative study; PEEK: polyether ether ketone
Conclusion
Spondylodiscitis may involve the vertebral bodies, the intervertebral disc, the paravertebral structures and the spinal canal with potentially high morbidity and mortality rates. The incidence has risen in recent years due to an increase in the susceptible population and an improved diagnostic accuracy with advanced imaging. However, diagnosis remains challenging because the disease may have an insidious onset, with subtle and misleading clinical features. The management of spondylodiscitis requires a multidisciplinary approach involving radiologists, infectious diseases specialists, spine surgeons and rehabilitation personnel. Conservative treatment is the basic standard of care. Surgical treatment via an anterior and/or posterior approach, in the setting of a one- or a two-stage procedure, with or without instrumentation, may be needed occasionally. Unfortunately, the majority of the published studies have a low level of evidence, with very few studies showing strong levels of recommendation for a treatment approach. Therefore, further research is necessary to provide high levels of evidence for the management of patients with spondylodiscitis.
Footnotes
ICMJE Conflict of interest statement: None declared.
Funding
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Jeong SJ, Choi SW, Youm JY, et al. Microbiology and epidemiology of infectious spinal disease. J Korean Neurosurg Soc 2014;56:21-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaufman DM, Kaplan JG, Litman N. Infectious agents in spinal epidural abscesses. Neurology 1980;30:844-850. [DOI] [PubMed] [Google Scholar]
- 3. Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ. Hematogenous pyogenic spinal infections and their surgical management. Spine (Phila Pa 1976) 2000;25:1668-1679. [DOI] [PubMed] [Google Scholar]
- 4. Calderone RR, Larsen JM. Overview and classification of spinal infections. Orthop Clin North Am 1996;27:1-8. [PubMed] [Google Scholar]
- 5. Tayles N, Buckley HR. Leprosy and tuberculosis in Iron Age Southeast Asia? Am J Phys Anthropol 2004;125:239-256. [DOI] [PubMed] [Google Scholar]
- 6. Hibbs RA. An operation for progressive spinal deformities: a preliminary report of three cases from the service of the orthopaedic hospital. 1911. Clin Orthop Relat Res 2007;460:17-20. [DOI] [PubMed] [Google Scholar]
- 7. Mavrogenis AF, Igoumenou V, Tsiavos K, et al. When and how to operate on spondylodiscitis: a report of 13 patients. Eur J Orthop Surg Traumatol 2016;26:31-40. [DOI] [PubMed] [Google Scholar]
- 8. Sobottke R, Seifert H, Fätkenheuer G, et al. Current diagnosis and treatment of spondylodiscitis. Dtsch Arztebl Int 2008;105:181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee KY. Comparison of pyogenic spondylitis and tuberculous spondylitis. Asian Spine J 2014;8:216-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jensen AG, Espersen F, Skinhøj P, Rosdahl VT, Frimodt-Møller N. Increasing frequency of vertebral osteomyelitis following Staphylococcus aureus bacteraemia in Denmark 1980-1990. J Infect 1997;34:113-118. [DOI] [PubMed] [Google Scholar]
- 11. Chelsom J, Solberg CO. Vertebral osteomyelitis at a Norwegian university hospital 1987-97: clinical features, laboratory findings and outcome. Scand J Infect Dis 1998;30:147-151. [DOI] [PubMed] [Google Scholar]
- 12. Grammatico L, Baron S, Rusch E, et al. Epidemiology of vertebral osteomyelitis (VO) in France: analysis of hospital-discharge data 2002-2003. Epidemiol Infect 2008;136:653-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krogsgaard MR, Wagn P, Bengtsson J. Epidemiology of acute vertebral osteomyelitis in Denmark: 137 cases in Denmark 1978-1982, compared to cases reported to the National Patient Register 1991-1993. Acta Orthop Scand 1998;69:513-517. [DOI] [PubMed] [Google Scholar]
- 14. Joughin E, McDougall C, Parfitt C, Yong-Hing K, Kirkaldy-Willis WH. Causes and clinical management of vertebral osteomyelitis in Saskatchewan. Spine (Phila Pa 1976) 1991;16:261-264. [DOI] [PubMed] [Google Scholar]
- 15. Sapico FL, Montgomerie JZ. Pyogenic vertebral osteomyelitis: report of nine cases and review of the literature. Rev Infect Dis 1979;1:754-776. [DOI] [PubMed] [Google Scholar]
- 16. Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother 2010;65:iii11-iii24. [DOI] [PubMed] [Google Scholar]
- 17. Sai Kiran NA, Vaishya S, Kale SS, Sharma BS, Mahapatra AK. Surgical results in patients with tuberculosis of the spine and severe lower-extremity motor deficits: a retrospective study of 48 patients. J Neurosurg Spine 2007;6:320-326. [DOI] [PubMed] [Google Scholar]
- 18. Patzakis MJ, Rao S, Wilkins J, Moore TM, Harvey PJ. Analysis of 61 cases of vertebral osteomyelitis. Clin Orthop Relat Res 1991;264:178-183. [PubMed] [Google Scholar]
- 19. Weinstein MA, Eismont FJ. Infections of the spine in patients with human immunodeficiency virus. J Bone Joint Surg [Am] 2005;87-A:604-609. [DOI] [PubMed] [Google Scholar]
- 20. Rezai AR, Woo HH, Errico TJ, Cooper PR. Contemporary management of spinal osteomyelitis. Neurosurgery 1999;44:1018-1025. [DOI] [PubMed] [Google Scholar]
- 21. Dufour V, Feydy A, Rillardon L, et al. Comparative study of postoperative and spontaneous pyogenic spondylodiscitis. Semin Arthritis Rheum 2005;34:766-771. [DOI] [PubMed] [Google Scholar]
- 22. Enoch DA, Cargill JS, Laing R, et al. Value of CT-guided biopsy in the diagnosis of septic discitis. J Clin Pathol 2008;61:750-753. [DOI] [PubMed] [Google Scholar]
- 23. Leong JC. Tuberculosis of the spine. J Bone Joint Surg [Br] 1993;75:173-175. [DOI] [PubMed] [Google Scholar]
- 24. Yee DK, Samartzis D, Wong YW, Luk KD, Cheung KM. Infective spondylitis in Southern Chinese: a descriptive and comparative study of ninety-one cases. Spine (Phila Pa 1976) 2010;35:635-641. [DOI] [PubMed] [Google Scholar]
- 25. Loibl M, Stoyanov L, Doenitz C, et al. Outcome-related co-factors in 105 cases of vertebral osteomyelitis in a tertiary care hospital. Infection 2014;42:503-510. [DOI] [PubMed] [Google Scholar]
- 26. Legrand E, Flipo RM, Guggenbuhl P, et al. Management of nontuberculous infectious discitis. treatments used in 110 patients admitted to 12 teaching hospitals in France. Joint Bone Spine 2001;68:504-509. [DOI] [PubMed] [Google Scholar]
- 27. Cebrián Parra JL, Saez-Arenillas Martín A, Urda Martínez-Aedo AL, et al. Management of infectious discitis. Outcome in one hundred and eight patients in a university hospital. Int Orthop 2012;36:239-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cottle L, Riordan T. Infectious spondylodiscitis. J Infect 2008;56:401-412. [DOI] [PubMed] [Google Scholar]
- 29. Broner FA, Garland DE, Zigler JE. Spinal infections in the immunocompromised host. Orthop Clin North Am 1996;27:37-46. [PubMed] [Google Scholar]
- 30. D’Agostino C, Scorzolini L, Massetti AP, et al. A seven-year prospective study on spondylodiscitis: epidemiological and microbiological features. Infection 2010;38:102-107. [DOI] [PubMed] [Google Scholar]
- 31. Chuo CY, Fu YC, Lu YM, et al. Spinal infection in intravenous drug abusers. J Spinal Disord Tech 2007;20:324-328. [DOI] [PubMed] [Google Scholar]
- 32. Mulleman D, Philippe P, Senneville E, et al. Streptococcal and enterococcal spondylodiscitis (vertebral osteomyelitis). High incidence of infective endocarditis in 50 cases. J Rheumatol 2006;33:91-97. [PubMed] [Google Scholar]
- 33. Ulu-Kilic A, Karakas A, Erdem H, et al. Update on treatment options for spinal brucellosis. Clin Microbiol Infect 2014;20:O75-O82. [DOI] [PubMed] [Google Scholar]
- 34. Charles RW, Govender S, Naidoo KS. Echinococcal infection of the spine with neural involvement. Spine (Phila Pa 1976) 1988;13:47-49. [DOI] [PubMed] [Google Scholar]
- 35. Tyagi R. Spinal infections in children: A review. J Orthop 2016;13:254-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maslen DR, Jones SR, Crislip MA, et al. Spinal epidural abscess. Optimizing patient care. Arch Intern Med 1993;153:1713-1721. [PubMed] [Google Scholar]
- 37. Mylona E, Samarkos M, Kakalou E, Fanourgiakis P, Skoutelis A. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum 2009;39:10-17. [DOI] [PubMed] [Google Scholar]
- 38. Wiley AM, Trueta J. The vascular anatomy of the spine and its relationship to pyogenic vertebral osteomyelitis. J Bone Joint Surg [Br] 1959;41-B:796-809. [DOI] [PubMed] [Google Scholar]
- 39. Rivas-Garcia A, Sarria-Estrada S, Torrents-Odin C, Casas-Gomila L, Franquet E. Imaging findings of Pott’s disease. Eur Spine J 2013;22:567-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kayser R, Mahlfeld K, Greulich M, Grasshoff H. Spondylodiscitis in childhood: results of a long-term study. Spine (Phila Pa 1976) 2005;30:318-323. [DOI] [PubMed] [Google Scholar]
- 41. Ratcliffe JF. An evaluation of the intra-osseous arterial anastomoses in the human vertebral body at different ages. A microarteriographic study. J Anat 1982;134:373-382. [PMC free article] [PubMed] [Google Scholar]
- 42. Turunc T, Demiroglu YZ, Uncu H, Colakoglu S, Arslan H. A comparative analysis of tuberculous, brucellar and pyogenic spontaneous spondylodiscitis patients. J Infect 2007;55:158-163. [DOI] [PubMed] [Google Scholar]
- 43. Babinchak TJ, Riley DK, Rotheram EB., Jr Pyogenic vertebral osteomyelitis of the posterior elements. Clin Infect Dis 1997;25:221-224. [DOI] [PubMed] [Google Scholar]
- 44. Moritani T, Kim J, Capizzano AA, et al. Pyogenic and non-pyogenic spinal infections: emphasis on diffusion-weighted imaging for the detection of abscesses and pus collections. Br J Radiol 2014;87:20140011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Govender S. Spinal infections. J Bone Joint Surg [Br] 2005;87-B:1454-1458. [DOI] [PubMed] [Google Scholar]
- 46. Mavrogenis AF, Triantafyllopoulos GK, Kokkinis K, et al. Continuous L3 spondylitis caused by an infected endovascular aortic graft. Surg Infect (Larchmt) 2014;15:861-862. [DOI] [PubMed] [Google Scholar]
- 47. Megaloikonomos PDAT, Antoniadou T, Dimopoulos L, et al. Spondylitis transmitted from infected aortic grafts: a review. J Bone Jt Infect 2017;2:96-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jaramillo-de la Torre JJ, Bohinski RJ, Kuntz C. Vertebral osteomyelitis. Neurosurg Clin N Am 2006;17:339-351. [DOI] [PubMed] [Google Scholar]
- 49. Griffith JF, Kumta SM, Leung PC, et al. Imaging of musculoskeletal tuberculosis: a new look at an old disease. Clin Orthop Relat Res 2002;398:32-39. [DOI] [PubMed] [Google Scholar]
- 50. Kotil K, Alan MS, Bilge T. Medical management of Pott disease in the thoracic and lumbar spine: a prospective clinical study. J Neurosurg Spine. 2007;6:222-228. [DOI] [PubMed] [Google Scholar]
- 51. Hassan K, Elmorshidy E. Anterior versus posterior approach in surgical treatment of tuberculous spondylodiscitis of thoracic and lumbar spine. Eur Spine J 2016;25:1056-1063. [DOI] [PubMed] [Google Scholar]
- 52. Park DW, Sohn JW, Kim EH, et al. Outcome and management of spinal tuberculosis according to the severity of disease: a retrospective study of 137 adult patients at Korean teaching hospitals. Spine (Phila Pa 1976) 2007;32:E130-E135. [DOI] [PubMed] [Google Scholar]
- 53. Megaloikonomos PD, Igoumenou V, Antoniadou T, Mavrogenis AF, Soultanis K. Tuberculous Spondylitis of the Craniovertebral Junction. J Bone Jt Infect 2016;1:31-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jensen AG, Espersen F, Skinhøj P, Frimodt-Møller N. Bacteremic Staphylococcus aureus spondylitis. Arch Intern Med 1998;158:509-517. [DOI] [PubMed] [Google Scholar]
- 55. Euba G, Narváez JA, Nolla JM, et al. Long-term clinical and radiological magnetic resonance imaging outcome of abscess-associated spontaneous pyogenic vertebral osteomyelitis under conservative management. Semin Arthritis Rheum 2008;38:28-40. [DOI] [PubMed] [Google Scholar]
- 56. Fantoni M, Trecarichi EM, Rossi B, et al. Epidemiological and clinical features of pyogenic spondylodiscitis. Eur Rev Med Pharmacol Sci 2012;16:2-7. [PubMed] [Google Scholar]
- 57. Schimmer RC, Jeanneret C, Nunley PD, Jeanneret B. Osteomyelitis of the cervical spine: a potentially dramatic disease. J Spinal Disord Tech. 2002;15:110-117. [DOI] [PubMed] [Google Scholar]
- 58. Butler JS, Shelly MJ, Timlin M, Powderly WG, O’Byrne JM. Nontuberculous pyogenic spinal infection in adults: a 12-year experience from a tertiary referral center. Spine (Phila Pa 1976) 2006;31:2695-2700. [DOI] [PubMed] [Google Scholar]
- 59. Fucs PM, Meves R, Yamada HH. Spinal infections in children: a review. Int Orthop 2012;36:387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Amadoru S, Lim K, Tacey M, Aboltins C. Spinal infections in older people: an analysis of demographics, presenting features, microbiology and outcomes. Intern Med J 2017;47:182-188. [DOI] [PubMed] [Google Scholar]
- 61. Cheung WY, Luk KD. Pyogenic spondylitis. Int Orthop 2012;36:397-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kim CJ, Song KH, Jeon JH, et al. A comparative study of pyogenic and tuberculous spondylodiscitis. Spine (Phila Pa 1976) 2010;35:E1096-E1100. [DOI] [PubMed] [Google Scholar]
- 63. Nolla JM, Ariza J, Gómez-Vaquero C, et al. Spontaneous pyogenic vertebral osteomyelitis in nondrug users. Semin Arthritis Rheum 2002;31:271-278. [DOI] [PubMed] [Google Scholar]
- 64. Aagaard T, Roed C, Dragsted C, Skinhøj P. Microbiological and therapeutic challenges in infectious spondylodiscitis: a cohort study of 100 cases, 2006-2011. Scand J Infect Dis 2013;45:417-424. [DOI] [PubMed] [Google Scholar]
- 65. Duarte RM, Vaccaro AR. Spinal infection: state of the art and management algorithm. Eur Spine J 2013;22:2787-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Grados F, Lescure FX, Senneville E, et al. Suggestions for managing pyogenic (non-tuberculous) discitis in adults. Joint Bone Spine 2007;74:133-139. [DOI] [PubMed] [Google Scholar]
- 67. Lew DP, Waldvogel FA. Osteomyelitis. Lancet 2004;364:369-379. [DOI] [PubMed] [Google Scholar]
- 68. Zimmerli W. Clinical practice. Vertebral osteomyelitis. N Engl J Med 2010;362:1022-1029. [DOI] [PubMed] [Google Scholar]
- 69. Michel SCA, Pfirrmann CWA, Boos N, Hodler J. CT-guided core biopsy of subchondral bone and intervertebral space in suspected spondylodiskitis. AJR Am J Roentgenol 2006;186:977-980. [DOI] [PubMed] [Google Scholar]
- 70. Trecarichi EM, Di Meco E, Mazzotta V, Fantoni M. Tuberculous spondylodiscitis: epidemiology, clinical features, treatment, and outcome. Eur Rev Med Pharmacol Sci 2012;16:58-72. [PubMed] [Google Scholar]
- 71. Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. N Engl J Med 2005;352:2325-2336. [DOI] [PubMed] [Google Scholar]
- 72. Lecouvet F, Irenge L, Vandercam B, et al. The etiologic diagnosis of infectious discitis is improved by amplification-based DNA analysis. Arthritis Rheum 2004;50:2985-2994. [DOI] [PubMed] [Google Scholar]
- 73. Crawford AH, Kucharzyk DW, Ruda R, Smitherman HC., Jr Diskitis in children. Clin Orthop Relat Res 1991;266:70-79. [PubMed] [Google Scholar]
- 74. Jevtic V. Vertebral infection. Eur Radiol 2004;14:E43-E52. [DOI] [PubMed] [Google Scholar]
- 75. Modic MT, Feiglin DH, Piraino DW, et al. Vertebral osteomyelitis: assessment using MR. Radiology 1985;157:157-166. [DOI] [PubMed] [Google Scholar]
- 76. Maiuri F, Iaconetta G, Gallicchio B, Manto A, Briganti F. Spondylodiscitis. Clinical and magnetic resonance diagnosis. Spine (Phila Pa 1976) 1997;22:1741-1746. [DOI] [PubMed] [Google Scholar]
- 77. Thurnher MM, Bammer R. Diffusion-weighted magnetic resonance imaging of the spine and spinal cord. Semin Roentgenol 2006;41:294-311. [DOI] [PubMed] [Google Scholar]
- 78. Sharif HS. Role of MR imaging in the management of spinal infections. AJR Am J Roentgenol 1992;158:1333-1345. [DOI] [PubMed] [Google Scholar]
- 79. Galhotra RD, Jain T, Sandhu P, Galhotra V. Utility of magnetic resonance imaging in the differential diagnosis of tubercular and pyogenic spondylodiscitis. J Nat Sci Biol Med 2015;6:388-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ozaksoy D, Yücesoy K, Yücesoy M, et al. Brucellar spondylitis: MRI findings. Eur Spine J 2001;10:529-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sharif HS, Aideyan OA, Clark DC, et al. Brucellar and tuberculous spondylitis: comparative imaging features. Radiology 1989;171:419-425. [DOI] [PubMed] [Google Scholar]
- 82. An HS, Seldomridge JA. Spinal infections: diagnostic tests and imaging studies. Clin Orthop Relat Res 2006;444:27-33. [DOI] [PubMed] [Google Scholar]
- 83. Love C, Patel M, Lonner BS, Tomas MB, Palestro CJ. Diagnosing spinal osteomyelitis: a comparison of bone and Ga-67 scintigraphy and magnetic resonance imaging. Clin Nucl Med 2000;25:963-977. [DOI] [PubMed] [Google Scholar]
- 84. Tamm AS, Abele JT. Bone and Gallium Single-Photon Emission Computed Tomography-Computed Tomography is Equivalent to Magnetic Resonance Imaging in the Diagnosis of Infectious Spondylodiscitis: A Retrospective Study. Can Assoc Radiol J 2017;68:41-46. [DOI] [PubMed] [Google Scholar]
- 85. Love C, Palestro CJ. Nuclear medicine imaging of bone infections. Clin Radiol 2016;71:632-646. [DOI] [PubMed] [Google Scholar]
- 86. Gratz S, Dörner J, Fischer U, et al. 18F-FDG hybrid PET in patients with suspected spondylitis. Eur J Nucl Med Mol Imaging 2002;29:516-524. [DOI] [PubMed] [Google Scholar]
- 87. Strobel K, Stumpe KD. PET/CT in musculoskeletal infection. Semin Musculoskelet Radiol 2007;11:353-364. [DOI] [PubMed] [Google Scholar]
- 88. Zarghooni K, Röllinghoff M, Sobottke R, Eysel P. Treatment of spondylodiscitis. Int Orthop 2012;36:405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tsiodras S, Falagas ME. Clinical assessment and medical treatment of spine infections. Clin Orthop Relat Res 2006;444:38-50. [DOI] [PubMed] [Google Scholar]
- 90. Shi T, Zhang Z, Dai F, et al. Retrospective Study of 967 Patients With Spinal Tuberculosis. Orthopedics 2016;39:e838-e843. [DOI] [PubMed] [Google Scholar]
- 91. Roblot F, Besnier JM, Juhel L, et al. Optimal duration of antibiotic therapy in vertebral osteomyelitis. Semin Arthritis Rheum 2007;36:269-277. [DOI] [PubMed] [Google Scholar]
- 92. Bernard L, Dinh A, Ghout I, et al. ; Duration of Treatment for Spondylodiscitis (DTS) study group. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet 2015;385:875-882. [DOI] [PubMed] [Google Scholar]
- 93. Chelli Bouaziz M, Ladeb MF, Chakroun M, Chaabane S. Spinal brucellosis: a review. Skeletal Radiol 2008;37:785-790. [DOI] [PubMed] [Google Scholar]
- 94. Govender S, Parbhoo AH, Kumar KP, Annamalai K. Anterior spinal decompression in HIV-positive patients with tuberculosis. A prospective study. J Bone Joint Surg [Br] 2001;83-B:864-867. [DOI] [PubMed] [Google Scholar]
- 95. Govender S, Kumar KPS. Aspergillus spondylitis in immunocompetent patients. Int Orthop 2001;25:74-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sapico FL, Montgomerie JZ. Vertebral osteomyelitis. Infect Dis Clin North Am 1990;4:539-550. [PubMed] [Google Scholar]
- 97. Babouee Flury B, Elzi L, Kolbe M, et al. Is switching to an oral antibiotic regimen safe after 2 weeks of intravenous treatment for primary bacterial vertebral osteomyelitis? BMC Infect Dis 2014;14:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Guerado E, Cerván AM. Surgical treatment of spondylodiscitis. An update. Int Orthop 2012;36:413-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Valancius K, Hansen ES, Høy K, et al. Failure modes in conservative and surgical management of infectious spondylodiscitis. Eur Spine J 2013;22:1837-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Roßbach BP, Niethammer TR, Paulus AC, et al. Surgical treatment of patients with spondylodiscitis and neurological deficits caused by spinal epidural abscess (SEA) is a predictor of clinical outcome. J Spinal Disord Tech 2014;27:395-400. [DOI] [PubMed] [Google Scholar]
- 101. Liebergall M, Chaimsky G, Lowe J, Robin GC, Floman Y. Pyogenic vertebral osteomyelitis with paralysis. Prognosis and treatment. Clin Orthop Relat Res 1991;269:142-150. [PubMed] [Google Scholar]
- 102. Darouiche RO. Spinal epidural abscess. N Engl J Med 2006;355:2012-2020. [DOI] [PubMed] [Google Scholar]
- 103. Ito M, Abumi K, Kotani Y, Kadoya K, Minami A. Clinical outcome of posterolateral endoscopic surgery for pyogenic spondylodiscitis: results of 15 patients with serious comorbid conditions. Spine (Phila Pa 1976) 2007;32:200-206. [DOI] [PubMed] [Google Scholar]
- 104. Osenbach RK, Hitchon PW, Menezes AH. Diagnosis and management of pyogenic vertebral osteomyelitis in adults. Surg Neurol 1990;33:266-275. [DOI] [PubMed] [Google Scholar]
- 105. Si M, Yang ZP, Li ZF, Yang Q, Li JM. Anterior versus posterior fixation for the treatment of lumbar pyogenic vertebral osteomyelitis. Orthopedics 2013;36:831-836. [DOI] [PubMed] [Google Scholar]
- 106. Pee YH, Park JD, Choi YG, Lee SH. Anterior debridement and fusion followed by posterior pedicle screw fixation in pyogenic spondylodiscitis: autologous iliac bone strut versus cage. J Neurosurg Spine 2008;8:405-412. [DOI] [PubMed] [Google Scholar]
- 107. Klöckner C, Valencia R, Weber U. Alignment of the sagittal profile after surgical therapy of nonspecific destructive spondylodiscitis: ventral or ventrodorsal method–a comparison of outcomes. Orthopade 2001;30:965-976.(In German) [DOI] [PubMed] [Google Scholar]
- 108. Včelák J, Chomiak J, Toth L. Surgical treatment of lumbar spondylodiscitis: a comparison of two methods. Int Orthop. 2014;38:1425-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. No authors listed. Spine MRCWPoTot. A controlled trial of anterior spinal fusion and débridement in the surgical management of tuberculosis of the spine in patients on standard chemotherapy: a study in two centres in South Africa. Seventh Report of the Medical Research Council Working Party on tuberculosis of the spine. Tubercle 1978;59:79-105. [DOI] [PubMed] [Google Scholar]
- 110. Ozturk C, Aydinli U, Vural R, Sehirlioglu A, Mutlu M. Simultaneous versus sequential one-stage combined anterior and posterior spinal surgery for spinal infections (outcomes and complications). Int Orthop 2007;31:363-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lee BH, Park JO, Kim HS, et al. Transpedicular curettage and drainage versus combined anterior and posterior surgery in infectious spondylodiscitis. Indian J Orthop 2014;48:74-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Nasto LA, Colangelo D, Mazzotta V, et al. Is posterior percutaneous screw-rod instrumentation a safe and effective alternative approach to TLSO rigid bracing for single-level pyogenic spondylodiscitis? Results of a retrospective cohort analysis. Spine J 2014;14:1139-1146. [DOI] [PubMed] [Google Scholar]
- 113. Lin TY, Tsai TT, Lu ML, et al. Comparison of two-stage open versus percutaneous pedicle screw fixation in treating pyogenic spondylodiscitis. BMC Musculoskelet Disord 2014;15:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Pola E, Rossi B, Nasto LA, Colangelo D, Logroscino CA. Surgical treatment of tuberculous spondylodiscitis. Eur Rev Med Pharmacol Sci 2012;16:79-85. [PubMed] [Google Scholar]
- 115. Schinkel C, Gottwald M, Andress HJ. Surgical treatment of spondylodiscitis. Surg Infect (Larchmt) 2003;4:387-391. [DOI] [PubMed] [Google Scholar]
- 116. Lee JS, Suh KT. Posterior lumbar interbody fusion with an autogenous iliac crest bone graft in the treatment of pyogenic spondylodiscitis. J Bone Joint Surg [Br] 2006;88-B:765-770. [DOI] [PubMed] [Google Scholar]
- 117. Karadimas EJ, Bunger C, Lindblad BE, et al. Spondylodiscitis. A retrospective study of 163 patients. Acta Orthop. 2008;79:650-659. [DOI] [PubMed] [Google Scholar]
- 118. Pourtaheri S, Issa K, Stewart T, et al. Comparison of Instrumented and Noninstrumented Surgical Treatment of Severe Vertebral Osteomyelitis. Orthopedics 2016;39:e504-e508. [DOI] [PubMed] [Google Scholar]
- 119. Oga M, Arizono T, Takasita M, Sugioka Y. Evaluation of the risk of instrumentation as a foreign body in spinal tuberculosis. Clinical and biologic study. Spine (Phila Pa 1976) 1993;18:1890-1894. [DOI] [PubMed] [Google Scholar]
- 120. Stone JL, Cybulski GR, Rodriguez J, Gryfinski ME, Kant R. Anterior cervical debridement and strut-grafting for osteomyelitis of the cervical spine. J Neurosurg 1989;70:879-883. [DOI] [PubMed] [Google Scholar]
- 121. Lu DC, Wang V, Chou D. The use of allograft or autograft and expandable titanium cages for the treatment of vertebral osteomyelitis. Neurosurgery 2009;64:122-129. [DOI] [PubMed] [Google Scholar]
- 122. Korovessis P, Vardakastanis K, Fennema P, Syrimbeis V. Mesh cage for treatment of hematogenous spondylitis and spondylodiskitis. How safe and successful is its use in acute and chronic complicated cases? A systematic review of literature over a decade. Eur J Orthop Surg Traumatol 2016;26:753-761. [DOI] [PubMed] [Google Scholar]
- 123. Rochford ET, Poulsson AH, Salavarrieta Varela J, et al. Bacterial adhesion to orthopaedic implant materials and a novel oxygen plasma modified PEEK surface. Colloids Surf B Biointerfaces 2014;113:213-222. [DOI] [PubMed] [Google Scholar]
- 124. Shiban E, Janssen I, da Cunha PR, et al. Safety and efficacy of polyetheretherketone (PEEK) cages in combination with posterior pedicel screw fixation in pyogenic spinal infection. Acta Neurochir (Wien) 2016;158:1851-1857. [DOI] [PubMed] [Google Scholar]
- 125. Schomacher M, Finger T, Koeppen D, et al. Application of titanium and polyetheretherketone cages in the treatment of pyogenic spondylodiscitis. Clin Neurol Neurosurg 2014;127:65-70. [DOI] [PubMed] [Google Scholar]
- 126. Fukuta S, Miyamoto K, Masuda T, et al. Two-stage (posterior and anterior) surgical treatment using posterior spinal instrumentation for pyogenic and tuberculotic spondylitis. Spine (Phila Pa 1976) 2003;28:E302-E308. [DOI] [PubMed] [Google Scholar]
- 127. Safran O, Rand N, Kaplan L, Sagiv S, Floman Y. Sequential or simultaneous, same-day anterior decompression and posterior stabilization in the management of vertebral osteomyelitis of the lumbar spine. Spine (Phila Pa 1976) 1998;23:1885-1890. [DOI] [PubMed] [Google Scholar]
- 128. Chen Y, Yang JS, Li T, et al. One-stage surgical management for lumbar brucella spondylitis by posterior debridement, autogenous bone graft and instrumentation: a case series of 24 patients. Spine (Phila Pa 1976) 2017;42:E1112-E1118. [DOI] [PubMed] [Google Scholar]
- 129. No authors listed. A controlled trial of plaster-of-paris jackets in the management of ambulant outpatient treatment of tuberculosis of the spine in children on standard chemotherapy. A study in Pusan, Korea. Second report of the Medical Research Council Working Party on Tuberculosis of the Spine. Tubercle 1973;54:261-282. [DOI] [PubMed] [Google Scholar]
- 130. No authors listed. A controlled trial of anterior spinal fusion and débridement in the surgical management of tuberculosis of the spine in patients on standard chemotherapy: a study in Hong Kong. Br J Surg 1974;61:853-866. [DOI] [PubMed] [Google Scholar]
- 131. No authors listed. A controlled trial of ambulant out-patient treatment and in-patient rest in bed in the management of tuberculosis of the spine in young Korean patients on standard chemotherapy a study in Masan, Korea. First report of the Medical Research Council Working Party on Tuberculosis of the Spine. J Bone Joint Surg [Br] 1973;55-B:678-697. [PubMed] [Google Scholar]
- 132. No authors listed. A 10-year assessment of controlled trials of inpatient and outpatient treatment and of plaster-of-Paris jackets for tuberculosis of the spine in children on standard chemotherapy. Studies in Masan and Pusan, Korea. Ninth report of the Medical Research Council Working Party on Tuberculosis of the Spine. J Bone Joint Surg [Br] 1985;67-B:103-110. [DOI] [PubMed] [Google Scholar]
- 133. No authors listed. A five-year assessment of controlled trials of in-patient and out-patient treatment and of plaster-of-Paris jackets for tuberculosis of the spine in children on standard chemotherapy. Studies in Masan and Pusan, Korea. Fifth report of the Medical Research Council Working Party on tuberculosis of the spine. J Bone Joint Surg [Br] 1976;58-B:399-411. [DOI] [PubMed] [Google Scholar]
- 134. No authors listed. A controlled trial of anterior spinal fusion and débridement in the surgical management of tuberculosis of the spine in patients on standard chemotherapy: a study in two centres in South Africa. Seventh Report of the Medical Research Council Working Party on tuberculosis of the spine. Tubercle 1978;59:79-105. [DOI] [PubMed] [Google Scholar]
- 135. No authors listed. Five-year assessments of controlled trials of ambulatory treatment, debridement and anterior spinal fusion in the management of tuberculosis of the spine. Studies in Bulawayo (Rhodesia) and in Hong Kong. Sixth report of the Medical Research Council Working Party on Tuberculosis of the Spine. J Bone Joint Surg [Br] 1978;60-B:163-177. [DOI] [PubMed] [Google Scholar]
- 136. No authors listed. A 10-year assessment of a controlled trial comparing debridement and anterior spinal fusion in the management of tuberculosis of the spine in patients on standard chemotherapy in Hong Kong. Eighth Report of the Medical Research Council Working Party on Tuberculosis of the Spine. J Bone Joint Surg [Br] 1982;64-B:393-398. [DOI] [PubMed] [Google Scholar]
- 137. No authors listed. A controlled trial of six-month and nine-month regimens of chemotherapy in patients undergoing radical surgery for tuberculosis of the spine in Hong Kong. Tenth report of the Medical Research Council Working Party on Tuberculosis of the Spine. Tubercle 1986;67:243-259. [DOI] [PubMed] [Google Scholar]
- 138. No authors listed. A 15-year assessment of controlled trials of the management of tuberculosis of the spine in Korea and Hong Kong. Thirteenth Report of the Medical Research Council Working Party on Tuberculosis of the Spine. J Bone Joint Surg [Br] 1998;80-B:456-462. [DOI] [PubMed] [Google Scholar]
- 139. Mak KC, Cheung KMC. Surgical treatment of acute TB spondylitis: indications and outcomes. Eur Spine J 2013;22:603-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Rutges JP, Kempen DH, van Dijk M, Oner FC. Outcome of conservative and surgical treatment of pyogenic spondylodiscitis: a systematic literature review. Eur Spine J 2016;25:983-999. [DOI] [PubMed] [Google Scholar]
- 141. Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Bernard L, Dinh A, Ghout I, et al. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet 2015;385:875-882. [DOI] [PubMed] [Google Scholar]
- 143. Linhardt O, Matussek J, Refior HJ, Krödel A. Long-term results of ventro-dorsal versus ventral instrumentation fusion in the treatment of spondylitis. Int Orthop 2007;31:113-119. [DOI] [PMC free article] [PubMed] [Google Scholar]