Abstract
As the burden of non-communicable diseases such as cancer continues to rise in low- and middle-income countries (LMICs), it is essential to identify and invest in promising solutions for cancer control and treatment. Point-of-care technologies (POCTs) have played critical roles in curbing infectious disease epidemics in both high- and low-income settings, and their successes can serve as a model for transforming cancer care in LMICs, where access to traditional clinical resources is often limited. The versatility, cost-effectiveness, and simplicity of POCTs warrant attention for their potential to revolutionize cancer detection, diagnosis, and treatment. This paper reviews the landscape of affordable POCTs for cancer care in LMICs with a focus on imaging tools, in vitro diagnostics, and treatment technologies and aspires to encourage innovation and further investment in this space.
Keywords: Cancer, point-of-care technology, imaging, in-vitro diagnostics
This review explores the current landscape of point-of care (POC) tools available for cancer detection, diagnosis, and treatment in resource-limited settings. With a concerted effort to support international collaborations in technology development, fund promising POC technology concepts for translation in low-resource settings, and train the next generation of scientists in resource-appropriate design, the engineering research community can be a vital part of delivering quality cancer care to all patients.
I. Introduction
It is estimated that by 2030, greater than two-thirds of new cancer cases will occur in low- and middle-income countries (LMICs) (GLOBOCAN 2012). To mitigate this drastic shift in cancer burden, a range of cancer control and treatment strategies must be actively pursued to address this challenge. However, the response of the global research community to the growing cancer burden has been relatively limited compared to other disease areas in global health. Foundational to strengthening cancer care delivery is the successful development and implementation of appropriately designed, affordable technologies for cancer diagnosis and treatment that can improve both access to care and patient outcomes.
Access to point-of-care technologies (POCTs), devices used at or near the time and place of patient presentation, can revolutionize delivery of cancer care in settings with limited health infrastructure. Attractive features of POCTs include a lower-cost, increased durability, and more streamlined approaches that are useful to providers practicing outside of traditional clinical settings. Technologies adapted to low-resource settings therefore have the potential to reduce cancer disparities by increasing widespread access to higher efficacy care.
Although advances in POC cancer technology are relatively underexploited, early successes show that the field has significant potential to contribute to improving cancer detection, diagnosis, and treatment in LMICs. This paper presents the state of POCT development for cancer care in LMICs, with an emphasis on imaging tools, in vitro diagnostics, and treatment technologies.
II. Imaging Technologies for the Detection and Diagnosis of Cancer
Imaging plays a central role across the comprehensive cancer care spectrum from screening, to early detection and diagnosis, through treatment and follow-up. The ability to non- or minimally-invasively visualize anatomy and physiology empowers healthcare teams in providing optimal care to patients. It reduces unnecessary treatment and further testing, especially in already overburdened medical systems. Therefore, the development of affordable, robust imaging technologies for use in resource-limited settings is an essential component of global cancer control efforts.
A. Ultrasound
Versatile, portable, and low cost, ultrasound’s value in cancer care is currently being evaluated as a common imaging modality for diagnosis and disease staging in low-resource settings [1]. Additionally, POC ultrasound capability can enhance or introduce new methods of sample collection previously unavailable in low-resource settings.
The use of ultrasound has been shown to augment breast cancer diagnosis by mammography, particularly for women with dense breast tissue [2]–[5], and evidence continues to grow for the use of ultrasound as a primary modality of breast cancer diagnosis in resource-limited settings, including the use of ultrasound-guided fine needle aspiration [6]–[8]. Ultrasound is also used to guide targeted biopsies for prostate cancer diagnosis as well, lowering cost and overtreatment [9]. Additionally, as the development of promising, low-cost contrast agents for image-guided therapeutics continues, ultrasound has the potential to play a role in targeted treatment and disease monitoring [10].
Hardware modifications to improve portability, enhance simplicity of use, and reduce cost have helped promote the use of ultrasound devices in LMICs. There are now several products on the market that have taken such considerations into account with promising results, demonstrating that compact ultrasound can improve primary care and can be used by healthcare workers with a range of training levels [11], [12]. The average cost of a traditional ultrasound system, approximately $101,865 in 2016 (ECRI Institute 2016 Technology Price Index), is often prohibitive for health systems in LMICs. Companies are recognizing this barrier and developing low-cost models. GE Healthcare’s VSCAN Ultrasound line includes the VSCAN Mobile Pocket Ultrasound and the VSCAN Access, which are both priced more than an order of magnitude lower than traditional systems. SonoScape’s portable ultrasound models offer comparable price points. Additionally, MobiSante’s MobiUS SP1 and TC2 Systems offer smartphone and tablet-based ultrasound capabilities, respectively, for further portability and proven utility in a range of settings for a variety of clinical applications [13]–[15]. Captured in Table 1, these lower-cost options for portable ultrasound offer accessible alternatives for health systems in LMICs.
TABLE 1. A Comparison of Technologies Mentioned in this Paper Solely as Exemplars of Pre-Commercial or Commercial Devices/Assays Being Tested in LMIC Settings. Table Compares Training Levels Required, Sample or Clinical Preparation Requirements, Times to Result or Lengths of Procedure, Materials/Disposables, Power Requirements, and Primary Locations of Use. Note that this Table Only Highlights Some of the Technologies Mentioned in this Paper and is not Intended to be an Exhaustive List of POCTs for Cancer Care.
| Tech | Training Level Required | Sample or Clinical Preparation | Time to Result or Length of Procedure | Materials/ Disposables | Power Requirements | Primary Location of Use |
|---|---|---|---|---|---|---|
| Imaging Tools | ||||||
| Ultrasound | ||||||
| Portable ultrasound | Midlevel provider, GP | Minimal | Real-time, with image storage | Ultrasound Gel | Rechargeable battery | Field, Clinic |
| Optical Imaging | ||||||
| High Resolution Microendoscope [29]–[32] | Midlevel provider, GP |
 tissue staining tissue staining |
Real-time, with image analysis and storage | Fluorescent stain | Rechargeable battery | Field, Clinic, Hospital |
| Mobile colposcopy [39], [40] | Midlevel provider | Minimal | Real-time, with image storage | Acetic acid | Rechargeable battery | Field, Clinic |
| Field-ready microscopes [43], [44] | Trained lab technician | Variable – sample dependent | Real-time | Slides, objectives, illumination, illuminating mirror | Rechargeable battery | Field, Laboratory |
| Mobile phone mounted microscope attachments [50], [51] | Midlevel provider | Variable – sample dependent | Real-time, with image analysis | Fluorescent stain | Relies on user phone battery | Field |
| Lens-free, cellphone holographic microscope attachment [52] | Trained lab technician | Variable – sample dependent | Real-time, with image reconstruction | Cellphone camera with at least 5 Megapixel RGB sensor | Rechargeable battery | Field, Laboratory |
| Smartphone based chip-scale microscope [53] | Trained lab technician | Variable – sample dependent | Real-time, with image reconstruction and storage | Smartphone with built-in camera | Relies on user phone battery | Field |
| Handheld, spectral domain OCT instrument [59] | GP | Minimal | Real-time, with image storage | Computer, computer monitor | Electricity | Clinic |
| Nuclear Imaging | ||||||
| C-ARM/3D rotational x-ray [62], [63] | Trained technician | Calibration | Real-time | Radiation protection equipment | Electricity | Clinic, Hospital |
| Treatment Tools | ||||||
| Screen-and-Treat | ||||||
| Modified gas cryotherapy [104] | Midlevel provider | Minimal | <1 hour | CO2 gas tank, autoclaved probe tip | CO2 gas | Field, Clinic |
| Gasless cryotherapy [105] | Midlevel provider | Minimal | <1 hour | Ethanol, autoclaved probe tips and sheathes | Rechargeable battery | Field, Clinic |
| Portable thermocoagulator [107], [109] | Midlevel provider | Minimal | <1 hour | Electrical unit, instrument cable, autoclaved probe | Rechargeable battery | Field, Clinic |
The advent of low-cost ultrasound hardware has prompted alongside it the development of pathology-driven, clinically applicable image analysis software such as computer-aided detection and diagnosis (CADD) and image guided evaluation tools designed specifically for cancer care. Supplementing innovations in hardware by layering on disease-specific, image analysis tools provides an opportunity for improved cancer care in resource-limited settings by decreasing the cost and expertise required for accurate cancer diagnoses and staging [16], [17]. Continued enhancement of image analysis algorithms has decreased false positive rates and unnecessary biopsies, particularly in breast cancer diagnostics [7], [18]. These tools complement a trend towards task-shifting in cancer care, enabling alternate sectors of the healthcare workforce such as primary care providers, nurses, or community health workers to share the workload of over-burdened specialists [19], for example:
-
•
Computer-based algorithms combine a human image reading with a machine learning methodology as a second reader to augment early detection strategies for breast cancer [20].
-
•
Elastography techniques draw on radiofrequency data of ultrasound readings to calculate tissue elasticity and determine stage status for cervical and prostate cancers with minimal additional equipment or training [21].
-
•
Educational programs, in conjunction with these ultrasound innovations, enhance the early detection of cancer by primary care practitioners and mid-level community healthcare workers [22], [23].
While the use of ultrasound in cancer diagnostics is currently limited in low-resource settings, previous successes of ultrasound integration into primary and maternal care in these environments offer a pathway for increasing its use in cancer care. If appropriate modifications in hardware and software continue to enter the wider market, low-cost and user-friendly ultrasound technology could play an increasingly important role in cancer diagnosis and treatment modalities.
B. Optical Imaging Technologies
Optical imaging technology allows physicians to non-invasively examine organs and tissues at the cellular and molecular level. The technique generates images using non-ionizing radiation, including visible, ultraviolet, and infrared light, and these images can then be used in real-time to diagnose and treat disease.
Particularly relevant to cancer care, optical imaging tools are safe, fast, and versatile: useful for repeated procedures to evaluate disease progression and monitor treatment [24]. Rapid, POC imaging, coupled with image-analysis software and proper training programs, can change the landscape of cancer staging and diagnosis in settings where a lack of pathology services has created a bottleneck in clinical workflows. Access to pathology expertise can be as limited as 1 pathologist per 5,000,000 people in extremely low-resourced settings [25]. Novel optical tools, combined with image analysis algorithms, can enhance capacity for traditional pathology services, offer new avenues for digital telepathology, or task-shift roles to primary care providers to improve the early detection of cancer [26].
Among recent advances in optical imaging tools, several technologies have shown great promise for the advancement of cancer care capacity in healthcare systems in LMICs. These tools, summarized in Table 1, include portable, low-cost methods for micro-endoscopy, microscopy, and optical coherence tomography.
1). High Resolution Micro-Endoscopy (HRME)
High-resolution microendoscopy (HRME) allows the user to conduct high-quality, real-time imaging at the cellular level in vivo [27], [28]. HRME is therefore becoming an adjunct to established screening modalities to enhance the detection of precancerous and cancerous lesions at multiple sites, including the esophagus, colon, and cervix, as well as the oral cavity and gastrointestinal tract [29]–[32]. Particularly relevant for low-resource settings where access to traditional pathology services is limited, HRME can become a valuable tool to enhance screen-and-treat programs, especially as the development of smartphone-based image analysis programs bolsters the diagnostic capacity of the imaging modality [25], [33]. For example, an automated frame selection algorithm for HRME images enables fully-automated image analysis at the POC, a particularly important consideration for technologies used in locations where infrastructure and specialized expertise are already limited [32].
In esophageal cancer, while standard imaging techniques such as Lugol’s chromoendoscopy (LCE) lack specificity, HRME as an adjunct screening tool enhances the accuracy of LCE in correctly identifying esophageal squamous cell neoplasia, ultimately reducing unnecessary biopsies [34] (Figure 1). In colorectal cancer, the combination of HRME with standard white-light colonoscopy can be used to both detect colorectal polyp growth and to differentiate neoplastic and non-neoplastic colorectal polyps [30], [35], again offering providers real-time feedback on lesions that should be biopsied for further analysis [30], [35]. HRME also complements the widespread use of visual inspection with acetic acid (VIA) for cervical cancer screening. Without the need for extensive infrastructure, the tool can be used to deliver mobile care to patients in remote areas, bypassing the need for patients to come to a primary cancer center. Real-time evaluation, diagnosis, and staging of cervical neoplasia using HRME can assist providers in determining the necessity of immediate cryotherapy, improving the efficiency of both mobile and in-clinic see-and-treat approaches and ultimately reducing overtreatment [36], [37].
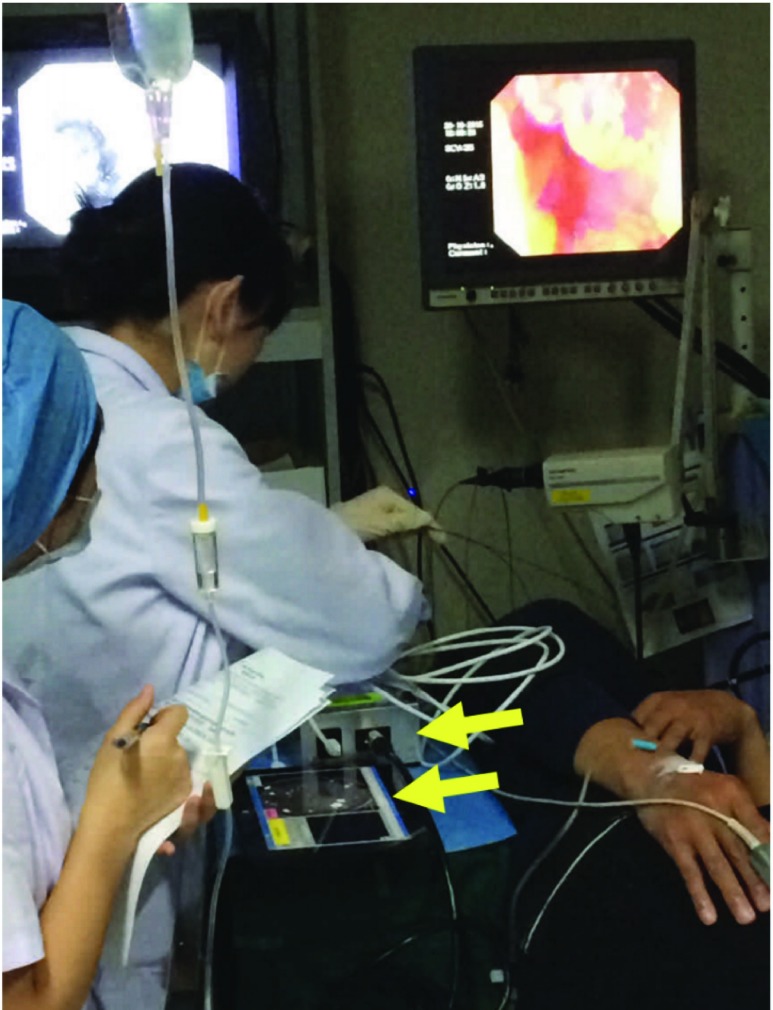
FIGURE 1.
HRME in use during a study of endoscopic screening for esophageal squamous cell carcinoma at the Cancer Institute of the Chinese Academy for Medical Science (CICAMS). Device is seen at the bottom of the photo (arrows).
Due to its application across different conditions, settings, and cancer sites, HRME is an imaging tool poised to facilitate simplified clinical diagnosis, margin determination, and endoscopic therapy for cancers in low-resource settings [29].
2). Mobile Colposcopy
Colposcopy, an examination of the cervix performed using a magnifying device called a colposcope, allows providers to identify abnormal cervical cells for biopsy and further evaluation [38]. Traditional colposcopy requires a trained provider; adequate equipment, space, and time for a pelvic exam; and a functioning colposcope. Innovative approaches to colposcopy in resource-limited settings, however, have the potential to redefine these requirements. For example, MobileODT is a device that uses image capture to aid providers in improved visualization of cervical cells. It is encased in a hard shell and is battery-powered for enhanced portability [39]. Another example, the POCkeT Colposcope, removes the need for a full pelvic exam with its tampon-like form that enables insertion into the vagina for cervical image capture. By reducing the training level required for use, this option fits well into task-shifting models that empower community-level providers to enhance early detection of cervical abnormalities at the POC [40].
3). Low-Cost Microscopy
Clinical pathology capacity remains central to the infrastructure necessary to deliver quality diagnostic services to patients in LMICs [41]. As hospital and clinic systems move to bolster pathology capabilities, the availability of low-cost microscopy could reduce the cost prohibitive nature of outfitting a laboratory in low-resource settings. Successful affordable microscopy tools are demonstrating the rugged design, power-source independence, and portability necessary for use in a range of contexts [41], [42].
Low-cost options originally engineered for Mycobacterium tuberculosis detection have leveraged advances in light-emitting diodes (LEDs) for fluorescence microscopy. PrimoStar™ iLED, a standalone LED-based fluorescent microscope (FM) designed by the ZEISS and the Foundation for Innovative New Diagnostics, enables switching between fluorescence excitation and brightfield (BF) illumination when examining samples. The simple design includes special eyecups that eliminate the need for a dark room during sample observation (ZEISS, Oberkochen, Germany). PrimoStar™ iLED compared favorably with direct smear microscopy for TB diagnosis, and the speed of slide screening was also substantially faster with LED FM [43]. Another option, the Global Focus microscope is a portable, battery-operated BF and FM with an LED-based flashlight as the light source. The microscope obtained results consistent with a laboratory grade FM in 98.4% of TB cases screened and can be manufactured at a cost of $240 USD, compared to PrimoStar™ at $1875 USD [44].
Fluorescence microscopy (FM) techniques are an important modality for the detection, diagnosis, and monitoring of cancer in ex vivo tissue samples [45]–[48]. Adopting and building on the aforementioned low-cost microscopy tools for infectious disease diagnosis can offer laboratories the ability to analyze biopsies at the point-of-care, accelerating decision making and treatment in settings where follow-up can be limited [41].
Additionally, leveraging rapid developments in mobile phone technology can further increase microscopy capabilities while driving costs down. As mobile connectivity and data-sharing become faster, more cost-efficient, and more reliable, the use of mobile platforms for microscopy in low-resource settings can drive innovation in imaging at the point-of-care [49]. Camera-enabled mobile phones offer the opportunity for point-of-care analysis:
-
•
Mobile phone-mounted microscope attachments capable of high-resolution clinical light microscopy can be adapted for high-resolution LED-based fluorescent microscopy [50], [51].
-
•
Light-weight, holographic microscope attachments to camera units of mobile phones allow lens-free digital microscopy. Samples are vertically illuminated by an LED for imaging by the phone camera. Images are then reconstructed through rapid digital processing for further analysis [52].
-
•
Smartphone-based chip-scale microscopes use ambient light sources and rely on the user’s hand motion for angular scanning. This design eliminates the need for both lenses and a light source, and can therefore be built by a simple modification of a phone’s camera module. In this modality, images are reconstructed into a high-resolution image for analysis [53].
As mobile-phone-based microscopy tools utilize smartphones as a base for the technology, their use will likely become more widespread amongst healthcare providers in low-resource settings. Adapting automatic image analysis platforms or telemedicine programs for mobile use can therefore assist in overcoming subsequent issues of training and expertise shortage [49]. While resource-appropriate microscopy tools will address aspects of these shortages, researchers are also exploring similar mobile-phone adjuncts and lens-free imaging techniques to determine molecular specificity and improve detection and diagnosis of abnormal cellular pathology [54]. Increasing pathology capacity using both microscopy and molecular diagnostics in low-resource settings has the potential to allow for more widespread use of optical biopsy technologies at the point-of-care, enhancing cancer detection and diagnosis by leveraging the extensive pre-existing mobile phone infrastructure.
4). Optical Coherence Tomography
Optical coherence tomography (OCT) uses light to capture high-resolution, 3D images of unprocessed and unstained biological tissue at the cellular level [55]. Its rising relevance in cancer care spans from detection and diagnosis to treatment monitoring and tumor margin definition [56], [57]. Because of the relatively simple and compact optics used in OCT, these new imaging tools are portable and cost-effective for use at the point-of-care [58]. Particularly in cancer care, the following OCT technologies have emerged as promising diagnostic tools for low-resource settings:
-
•
A handheld OCT instrument for use in primary care settings enables early detection of disease using image-based diagnostics. The device not only offers real-time imaging of tissues, but it draws on digital 2D and 3D datasets that can be used to assist with diagnostic decisions [59].
-
•
Full-field OCT has been shown to be effective for analysis of ex vivo human breast tissue samples, removing the need for tissue staining or preparation in the laboratory setting. This use of OCT could streamline the workflow of laboratory technicians by decreasing the amount of time and expertise necessary to prepare samples for pathology analysis [60].
In addition to detection and diagnosis capabilities, OCT offers improved visualization intraoperatively for providers performing surgical interventions for cancer patients. These tools are discussed in more depth in sections below.
C. Nuclear Imaging
While nuclear imaging such as computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography-computed tomography (PET/CT) are widely used in cancer care within high-income settings, there are few options for these types of sectional imaging tools in less-resourced settings. However, digital tomography is a low-cost method for producing sectional images by leveraging digital x-ray technology. It is important to emphasize the relevance of such imaging modalities for their potential to enhance diagnostics and treatment monitoring in hospital settings [61]. While not portable, tools such as cone-beam x-ray CT (CBCT) and C-ARM/3D rotational x-rays are potentially viable options for LMIC health systems, and evidence suggests their utility for head and neck as well as breast imaging [62], [63].
As the infrastructure for cancer care in low- and middle-income countries continues to develop, affordable imaging technologies have the potential to provide healthcare workers with the detection and diagnostic capabilities necessary to improve outcomes. Particularly in medically underserved areas where consistent interactions with the health system are rare, the need for practical, point-of-care imaging is essential as providers face higher attrition rates during patient follow-up. The tools presented here offer an important step forward in this regard, and their potential impact is wide-ranging. However, challenges to implementation and scalability remain, and the careful consideration of these complexities must be addressed for these technologies to deliver optimal quality care.
III.  Tools for the Detection and Diagnosis of Cancer
Tools for the Detection and Diagnosis of Cancer
In vitro diagnostics at the POC have formed the foundation of infectious disease (ID) control in low-resource settings [64], [65]. Their use has improved the diagnosis and subsequent treatment of malaria, parasitic infections, and sexually transmitted diseases by simplifying and reducing the cost of the diagnostic process [64], [66]. As the burden of NCDs continues to rise in LMICs, health systems can leverage the success of diagnostic technologies in ID frameworks by translating these advances into improved diagnostics for cancer care.
A. Molecular Diagnostics
In 2012, infection-associated cancers accounted for 15.4% of new cancer cases worldwide. For some countries in sub-Saharan Africa, this number jumps to more than 50%, representing the significant burden of cancers attributable to infections in LMICs [67]. Molecular diagnostics hold great potential for the early detection and diagnosis of infections such as human immunodeficiency virus (HIV), human papillomavirus (HPV), and hepatitis B and C viruses (HBV, HCV) that when identified quickly and accurately can lead to more successful interventions and prevent the development of associated cancers [67].
1). Human Immunodeficiency Virus
As the HIV/AIDS epidemic evolves, reduced mortality among HIV-positive individuals has led to an increase in the number of both AIDS- and non-AIDS-associated cancers diagnosed each year in HIV-positive patients [68], [69]. These cancers include Kaposi’s sarcoma (KS), certain high-grade non-Hodgkin’s Lymphomas (NHL), and cervical cancer [69]. Associated with human herpesvirus 8 (HHV8), Epstein-Barr virus (EBV), and HPV infection, respectively, these cancers indicate the onset of clinically significant immunosuppression [70].
POC tools aimed at the early detection of cancer-associated infections in HIV patients could help improve survival by identifying those at highest risk of cancer development, distinguishing between rapid and slow disease progression, and evaluating treatment efficacy [70]. In particular, molecular diagnostics for AIDS-associated infections can improve patient stratification and allow clinicians to select and monitor appropriate treatment regimens [70].
The oncogenic HHV8 plays a causative role in the development of KS [71]. Previously, distinguishing the clinical presentation of KS from bacillary angiomatosis and pyogenic granuloma created diagnostic challenges in settings with limited pathology capacity [72]. A novel colorimetric multiplex system was developed to address this challenge. The test can detect the DNA of each pathogen using nanoparticle aggregation that creates two independent color change reactions depending on the DNA target present in the sample [73]. Because the reactions occur in a single solution, the scheme could potentially be integrated into a microfluidic device for use with solid biopsy samples in the future [73]. While the colorimetric system is designed to detect unamplified DNA, advances have also been made in low-cost, field-deployable qPCR devices. For example, solar energy and mobile phone power can be used as a heat source for the thermal cycling process required for nucleic acid amplification. Using this method, the KS-Detect device demonstrates a complete sample-to-answer system for detecting HHV8 in human skin biopsies [74].
EBV is also classified as a group 1 carcinogen due to its causative role in the development of Burkitt’s Lymphoma (BL), a form of rapidly progressing NHL that, while not unique to HIV infection, has high incidence in HIV-positive patients. Notably, EBV also has associations with other NHLs and gastric adenocarcinoma. In EBV-associated cancers, the viral genome is localized within malignant cells. Therefore, low-cost, POC molecular methods of EBV quantification could transform care in settings where the virus is endemic and rapid diagnosis is essential to improving BL outcomes. Particularly in areas where limited access to histopathology services can delay a definitive diagnosis of rapidly advancing BL, recent advances in POC methods to detect clonal IGH gene rearrangement and EBV viral load offer critical shorter turnaround times [75], [76]. The tests can be applied to blood, aspirate, or biopsy samples to support a clinical diagnosis of cancer while awaiting confirmation with cytology or histology results [75]–[77]. With further development and validation, these tests have the potential to greatly improve the diagnosis and monitoring of EBV-associated cancers in resource-limited settings.
HIV-positive patients also have higher and faster rates of HPV progression to cervical cancer when compared to immunocompetent groups [78]. For this reason, low-cost molecular diagnostics have the potential to improve early detection rates in high-risk patients, enabling earlier interventions and improved outcomes.
2). Human Papillomavirus
Because validated protocols exist for the treatment of pre-cancerous cervical lesions in low-resource settings, early detection of infection with high-risk HPV types can greatly limit the number of cases that progress to a cancerous state [79]. Widespread use of visual inspection with acetic acid (VIA) has contributed to improved detection of cervical lesions in low-resource settings, but due to the subjective nature of a visual examination, its sensitivity and specificity remain low [80]. Molecular diagnostics, however, offer great promise for identifying patients at high risk of developing precancerous lesions by enhancing diagnostic accuracy.
Particularly relevant to HPV screening technologies is the trend of cervicovaginal self-sampling tools provided to women at the community level. The need for a direct endocervical specimen obtained by a physician has been a rate-limiting factor for widespread uptake of HPV screening. Self-sampling methods allows clinicians to bypass the need for a pelvic exam, evaluating significantly more women for HPV infection in less time [81]. Additionally, women find self-collection acceptable and even preferable to physician-collection [82]–[84]. Several studies have demonstrated similar sensitivities on both self-collected and physician-collected samples when PCR-based HPV tests are used [81], [85]–[88]. Results indicate that with further validation, PCR-based diagnostic tools could allow self-sampling to become a feasible widespread modality for women unable to participate in clinic-based HPV screening regimens.
3). Hepatitis B and Hepatitis C
In 2013, viral hepatitis was the seventh leading cause of death worldwide and responsible for 80% of all primary hepatocellular carcinoma cases [89]. Routine HBV and HCV screening has long relied on the detection of surface antigens [90]. However, due to the presence of serologically negative infections and the emergence of escape mutants, nucleic acid amplification assays have been shown to be a more reliable method of HBV/HCV infection detection [91], [92]. The advancement of affordable molecular diagnostics, therefore, can improve HBV/HCV detection and monitoring in high-burden, low-resource settings. In particular, quantitative methods such as real-time PCR and bDNA assays enable healthcare providers to more accurately monitor viral loads and treatment efficacy [93].
Current technologies for HBV/HCV detection focus predominantly on PCR as opposed to other isothermal amplification methods used for other viruses [94]. As the cost of primers and enzymes continues to fall, however, the simpler thermal requirements and faster reaction times of isothermal techniques may lead to wider use in POC diagnostics for HBV/HCV in low-resource settings. These methods include loop mediated isothermal amplification, transcription mediated amplification, ligase chain reaction, and rolling circle amplification and have been reviewed elsewhere [94], [95].
B. Laboratory Equipment Requirements
The examples of in vitro molecular assays for HPV, HBV, and HCV described above demonstrate great potential for delivering molecular diagnostic capabilities to the POC in resource-limited settings. However, the relevance of high-quality molecular targets depends heavily on the availability of equipment necessary to prepare and process samples effectively. Nucleic acid testing platforms designed with high-throughput, automated sample preparation, and rapid turnaround time address shortages in workforce capacity and optimize the number of samples processed. In addition, ruggedizing platforms to be resistant to extreme temperatures, environmental pollutants, and frequent transport is essential for successful implementation in resource-limited settings.
The Cepheid GeneXpert I-XVI Platforms, for example, are used for detection of a range of biological agents and offer a simple, rapid option for molecular diagnostics [96]. Samples are loaded into cartridges and depending on the device model, a number of samples can be tested concurrently [96]. The GeneXpert Omni, not yet available for diagnostic use, offers a ruggedized version of the analysis platform that will use the same sample cartridges and has the ability to analyze one cartridge per run [97]. While the Omni model will offer greater portability and durability to make the assays truly field-deployable, the cost of the cartridges remains high for widespread adoption in resource-limited settings. Additionally, only 12 hours of battery life limits its utility without regular access to power [97].
The KS-Detect solar thermal PCR device (Figure 2), however, presents a relevant example of ruggedization and miniaturization at a lower cost per test. The portable system amplifies target DNA by cycling samples through three temperature zones and performs subsequent analysis of amplified DNA at the POC [74]. Reduced power consumption allows an iPhone battery or solar energy to provide necessary energy for 70+ hours of use, making the device a novel solution for PCR in settings with limited access to reliable power [74], [98]. The device case includes all material to conduct sample preparation and analysis, and it can be carried in one hand [98]. While the device is currently targeted to detect Kaposi’s sarcoma herpesvirus, use of primers for other oncogenic viruses could make the system useful for POC diagnosis across a range of molecular targets [98].
FIGURE 2.
KS-Detect System in Solar Heating Mode deployed outside of a rural clinic near Kampala, Uganda.
Particularly due to its portability and minimal power requirements, the KS-Detect design offers great promise for molecular diagnostics in resource-constrained settings. When healthcare workers can conduct PCR at the POC, it enables more sophisticated sample analysis. For example, it could allow the use of novel assays such as the GeneXpert BC Strat for stratification of estrogen receptor, progesterone receptor, HER2 and Ki67 status [99]. This capability for more advanced analysis empowers providers by offering enhanced information for clinical decision making at the POC.
Management of NCDs such as cancer will increasingly rely on the decentralization of care and the extension of diagnostic and treatment capabilities to providers at the primary care level [100]. In order to achieve successful decentralization, however, it is essential to equip providers and community healthcare workers with the appropriate tools and technologies to direct clinical treatment decisions [101]. The innovative molecular assays and platforms discussed here will contribute to the effort to both diagnosis and monitor treatment progression in cancer patients across resource-settings, and evidence on their task-shifting potential should be explored as they continue into future stages of development. Note that because these assays and benchtop systems have been reviewed extensively elsewhere, they are not included in Table 1.
IV. Tools for the Treatment of Cancer
As POC imaging and in vitro tools for the detection and diagnosis of cancer continue to evolve, the parallel development of resource-appropriate treatment options for patients must also occur. Upon a cancer diagnosis, patients must enter a health system properly equipped to prevent the advancement of disease when possible, manage follow-on care, and maintain quality of life during treatment. Relevant treatment technologies include screen-and-treat, surgical, chemotherapy and radiation, and palliative care tools.
A. Screen and Treat Technologies
As discussed previously, the burden of cervical cancer falls disproportionately on women in LMICs. While screening frameworks continue to expand in these settings, POC technologies to treat precancerous cervical lesions once identified are critical to improving outcomes [102]. There are many methods by which to treat cervical pre-cancer. Methods for excision of cervical pre-cancer, such as a Loop Electrosurgical Excision Procedure (LEEP), Large Loop Excision of the Transformation Zone (LLETZ), laser conization, cold-knife conization, or hysterectomy are often used in high-income settings, but the expertise and equipment required for these procedures are not appropriate for POC treatment by non-physician providers [103]. Methods for ablation of cervical pre-cancer include laser ablation, cryotherapy, thermocoagulation (also called cold coagulation), electrocoagulation, and electrocautery. Like the excision methods, several of these options are not suitable for use at the POC in LMICs [103]. Cryotherapy and thermocoagulation, however, are methods that lend themselves to adaptation for resource-limited settings by offering portability and simple protocols that complement pre-existing screen-and-treat approaches [103]. Tools for these techniques are captured in Table 1.
Currently, gas-based cryotherapy is the standard treatment of precancerous lesions in LMICs. Although relatively low-cost, gas-based systems present significant challenges due to difficulties with safely procuring and transporting gas tanks to remote areas to support mobile screen-and-treat efforts. CryoPop, a modified gas-based system, uses CO2 gas at one tenth the amount of a traditional system per treatment, solving portability but not procurement challenges [104]. CryoPen (Figure 3), originally created for dermatologic lesions, has been adapted as a non-gas-based cryotherapy tool. CryoPen achieves cold temperatures using electricity by compression cooling technology, eliminating the need for compressed gas. When the probe is applied to cervical tissue, it freezes and crystallizes intracellular water, causing necrosis of pre-cancerous epithelial cells on the cervix. The device weighs 20 pounds, and battery power allows treatment of 24 women per day [105]. Although approximately twice the fixed cost of gas-based devices, CryoPen reduces operational costs by eliminating the need for constant gas procurement [105].
FIGURE 3.
CryoPen (left) offers a smaller, gasless alternative to CO2-based systems for cryotherapy (right). CryoPen is also enclosed in a ruggedized container for easy transport.
Thermocoagulation, although used less frequently, has cure rates approaching those of other ablative methods such as cryotherapy [106]. The Semm thermocoagulator was first developed in 1966, but with the emergence of excisional techniques, the method became less common in the treatment of precancerous lesions [106], [107]. However, in the absence of trained physicians, excision methods present significant risk to the patient during and after the procedure. Therefore, due to its safety, portability, and speed, thermocoagulation has resurfaced as a treatment option relevant for see-and-treat models in low-resource settings [106]. The Semm device is currently being updated, now called the WiSAP Cold-Coagulator. It consists of probes attached to a simple control box by cables and an enlarged probe tip to accommodate variable lesion sizes [108], [109]. The Liger Thermo Coagulator is a battery-powered, portable, cordless, and handheld thermocoagulation device currently in clinical trials to validate its effectiveness in treating cervical precancerous lesions [110].
Oral cancers also place a large burden on health systems in LMICs due to risk factors such as smoking, chewing tobacco, and exposure to infectious agents prevalent in lower-resource settings. Oral pre- and early stage cancer is well-suited to screen-and-treat approaches, but appropriately designed therapeutics must exist to improve outcomes. Photodynamic therapy, for example, uses a laser or broadband lamp to activate a photosensitizing molecule that selectively accumulates in malignant tissue, allowing site-directed tissue destruction [111]. Currently in development, a battery-operated PDT device could allow therapy at the POC by leveraging high-output LEDs connected to a multimode fiber and a specially designed oral insert [112], [113]. The insert is crafted to direct intraoral light delivery to target lesions using a smartphone-based image guidance system and aminolevulinic acid (ALA) as a contrast agent [112], [113]. Particularly in areas with limited healthcare infrastructure, acceptable methods for treating early malignancies have the potential to greatly improve oral cancer outcomes in areas with high disease incidence.
B. Surgery
While early stage squamous cell carcinomas are well-suited to the screen-and-treat approach, a majority of advanced and non-squamous cell carcinomas will require more invasive treatment. Often used in conjunction with chemotherapy and radiation, surgery often has the most important role in cancer treatment plans. Particularly in low-resource settings where patients present with later stage disease, sophisticated, easy-to-use tools to perform surgical excision or tumor resection are essential.
Real-time optical imaging of the surgical site can provide immediate feedback to clinicians on post-resection margins. HRME is an example of a small, portable device that has the potential to be used for margin determination during a procedure. It offers the surgeon critical histologic information and could lead to fewer repeat procedures by ensuring more complete tumor resection [29]. As novel applications of optical imaging at the POC continue to evolve, cancer surgeons in lower-resource settings will benefit from additional clinical decision-making tools.
C. Chemotherapy and Radiation
Capacity to provide chemotherapy and radiation therapy in conjunction with surgery is critical to cancer treatment programs in low-resource settings. In part, this capacity stems from reliable supply chains to procure essential chemo- and radiotherapy drugs. However, there is a large role for innovative, low-cost technologies to revolutionize the way that chemotherapy and radiation treatment is delivered in low-resource settings.
While there are a range of types of chemotherapy treatments, intravenous chemotherapy can require the use of an infusion pump to carefully control dosage over time. Currently in development, a low-cost infusion pump called AutoSyP offers laboratory accuracy of fluid delivery within 4% of the programmed flow rate [114]. Battery-powered and operable for 66-hours per charge, AutoSyP offers consistent drug delivery in environments with less-reliable power sources. The device can be assembled for approximately $500, which is a viable price point for hospitals in LMICs [114].
The global disparity in access to radiotherapy is particularly great relative to other cancer treatment options. In high-resource settings, the provision of radiation therapy requires the expertise of a large team that includes several levels of healthcare professionals. In lower-resource settings where human resource capacity in health systems is already limited, severe shortages in equipment and trained staff present significant challenges. Therefore, a major component of appropriately designed tools for radiotherapy is a focus on task-shifting to less specialized levels of healthcare professionals.
The application of automated radiotherapy treatment planning has great potential to promote this task-shifting and address staffing shortages. Using real-time software and cloud-based capabilities, researchers are developing automated treatment planning algorithms for cervical, breast, and head and neck cancers [115]. Preparatory steps such as isocenter detection and body segmentation are informed by the patient’s imaging and verified by a Mobius 3D quality assurance (QA) system that ensures proper dose calculations and treatment parameters. Completion of a cervical cancer radiotherapy treatment plan, for example, takes less than 30 minutes. The goal of the system is to include several layers of internal QA to enable its use by junior staff [115].
While addressing the significant shortages in human resources for radiotherapy is essential, it is also important to continue the development of novel treatment delivery technologies that increase treatment precision at an acceptable cost. Additionally, applying POC imaging or molecular diagnostic tools is also critical for ongoing treatment monitoring to evaluate patient progress during radiotherapy or chemotherapy regimens or for monitoring patients with chronic cancer conditions.
D. Palliation
Lastly, effective cancer treatment maintains the ability to preserve patient quality of life by controlling pain and physical symptoms during treatment, recovery, and at the end of life. One aspect of palliative care is pain control using low-cost, reliable drug delivery systems such as the aforementioned infusion pump [114]. Its ability to reliably function without consistent access to power while delivering accurate drug dosages makes it a promising option for cancer care units seeking cost-appropriate solutions for infusion of pain medication.
Other tools to improve quality of life, such as stents for esophageal cancer patients, can play a role in providing late-stage presenters with drastic benefits in the final months of life [116]. Self-expanding metal stents are an example of a low-cost intervention tool that can be tailored to a particular patient, markedly improving hydration and nutrition [117]. Especially in settings where late-stage presentation is common, solutions to provide relief to patients with advanced disease will add substantial benefit to cancer care regimens [118].
V. Discussion
In 2004, the WHO outlined a set of criteria, called the ASSURED guidelines, to promote affordable, sensitive, specific, user-friendly, rapid and robust, equipment-free, and deliverable to end-user POC technologies [119]. These characteristics offer a strong framework by which to evaluate tools designed for providers in less-resourced settings. A burgeoning field, POC technologies for the detection, diagnosis, and treatment of cancer have the potential to revolutionize care delivery in low-resource settings while ultimately lowering costs. The tools discussed in this paper offer solutions that can fundamentally alter patient pathways, change the standard of care, and improve cancer patient outcomes by capitalizing on a range of innovations within the ASSURED framework.
Where it does not distract from or destabilize overall health systems strengthening, task-shifting, or delegating healthcare tasks where appropriate to less-specialized healthcare workers, will continue to play a growing role in healthcare systems, particularly in LMICs. While strengthening specializations central to cancer care such as oncology, pathology, and radiology is a long-term goal of cancer control initiatives, the most successful translational research focused on POC technologies currently focuses on real-work, on-the-ground scenarios and more applicability at the POC as well as affordability and ease of use by healthcare professionals with a range of training levels. For example, software innovations built to complement resource-appropriate hardware offer capabilities such as image-guided analysis or computer automated detection and diagnosis to enhance clinical decision making for non-specialists treating cancer patients. Usability is a central component of the technologies referenced in this review, and it will be necessary to continue to show evidence that they enhance task-shifting as they enter scalable phases of development [19].
Another aspect of successful POC technologies is the ability to leverage the widespread infrastructure of mobile phone use in LMICs. Particularly for communities in which radiology and pathology subject matter expertise is limited, tools that offer image transmission to trained specialists in urban centers, for example, can crowdsource diagnosis and treatment planning decisions to qualified experts. Especially as wireless connectivity and cloud-computing capabilities improve, mobile health solutions have the potential to remove bottlenecks and offer access to high quality cancer care even in remote locations.
Particularly for NCDs, early intervention is key to improving disease outcomes. Tools that contribute to downstaging, or detecting disease at a less-advanced stage, hold great potential for patients at the primary care level in LMICs. The development of lab-based technologies useful for downstaging in high-income settings has provided an opportunity to adapt certain high-performance tools for use in LMICs. The miniaturization and ruggedization of in vitro diagnostics, for example, can improve the accuracy of diagnoses at the point-of-care for cancer patients, optimizing the use of already limited treatment resources. While these concepts are important for imaging tools at the POC as well, they are particularly essential for in vitro diagnostics. Continued emphasis on multiplexing, high-performance process automation, and compatibility with telemedicine infrastructure are also common components of the emerging cancer-specific molecular diagnostics discussed in this review.
For both imaging and in vitro diagnostics, technologies that provide rapid or real-time clinical information to providers are particularly poised to improve cancer care in LMICs. In lower-resource settings where patient loss to follow-up is a significant concern, tools that allow same-visit results can reduce this barrier. Particularly for those technologies designed for mobile clinic-style use to treat patients in remote areas, the speed of test results is an important consideration. However, to increase the effectiveness and long-term impact of the same-visit screen-and-treat options, the in vitro diagnostic POCTs must be sensitive and specific to reduce over diagnosis and over treatment.
Early detection and diagnosis tools can yield great progress towards revolutionizing cancer care systems in LMICs, but technologies for cancer treatment require equal attention. Especially as LMICs continue to integrate chronic care models into health systems, equipping providers with appropriately-designed treatment tools must be a priority. POC technologies for cancers that lend themselves well to screen-and-treat frameworks have been well-received in LMICs. However, for more complex cancers, more advanced capabilities in surgery and radiotherapy will be critical as the field progresses.
Lastly, the role of implementation science in the effective utilization and scaling-up of technology-based interventions for cancer care cannot be understated. The success of a POCT requires not only innovative and novel applications of the science but also careful attention to the health system, end-user, and patient that will ultimately use it.
VI. Conclusion
In recent years, technology development in high-income settings has rapidly advanced, particularly with respect to cancer detection, diagnosis, and treatment. However, this advancement has been slow to materialize for cancer care in less-resourced settings in both LMICs and high-income countries (HICs). It is imperative that the global research community mobilize around this issue, leveraging the ongoing POCT development mentioned in this paper to both build on successes and address challenges in the field. This mobilization should include a strong emphasis on supporting international technology development collaborations, funding promising POCT concepts for translation in low-resource settings, and training the next generation of scientists and engineers in resource-appropriate technology design. Each of these steps will ultimately contribute to equipping healthcare providers in low-resource settings with the appropriate tools for cancer control.
The burden of cancer continues to increase in LMICs, and disparities in cancer outcomes are growing in less-resourced settings of HICs. It is critical, therefore, that scientists, engineers, and clinicians continue to translate novel cancer care technologies into innovative, resource-appropriate tools for these environments. The ongoing revolutions in fields such as microfluidics and imaging science, in parallel with burgeoning telecommunications and mobile connectivity infrastructure, offer more opportunity and more obligation than ever before to revolutionize cancer care delivery for patients in less-resourced settings through appropriately designed POCTs.
Acknowledgments
The authors would like to especially thank Dr. Rebecca Richards Kortum of Rice University, Dr. Kathleen Schmeler of MD Anderson Cancer Center, Dr. David Erickson of Cornell University, and Dr. Miriam Cremer of Basic Health International for their generous contributions of clinical images of their devices being implemented. K. Haney was with the National Cancer Institute, Rockville, MD, USA.
Biographies

Karen Haney received the B.A. degree in biochemistry and cell biology from Rice University in 2015. She is currently pursuing the Medical degree with the Dell Medical School, The University of Texas at Austin. After conducting research on a Wagoner Foreign Study Fellowship in Port-au-Prince, Haiti, for a year, she joined the U.S. National Cancer Institute’s Center for Global Health as a Cancer Research Training Fellow in 2016.

Pushpa Tandon received the M.Sc. and Ph.D. degrees in biochemistry from the University of Lucknow, India. She came to the U.S. as a Fogarty Fellow, and continued her research at the U.S. Environmental Protection Agency, Boston College, and then at the Harvard Medical School before moving to Wellstat Therapeutics Corporation, Rockville, MD, USA. She joined NIH as a Scientific Review Officer with the Center for Scientific Review. She is currently a Program Director of the Cancer Imaging Program and the Division of Cancer Therapeutics and Diagnosis at NCI.

Rao Divi received the M.S. degree in biochemistry from Andhra University, and the Ph.D. degree in biochemistry from Osmania University, India, in 1993. He was involved in post-doctoral research with the National Center for Toxicological Research, U.S. Food and Drug Administration, and the Center for Cancer Research, National Cancer Institute (NCI) from 1993 to 2008. Since 2008, he has been with the Division of Cancer Control and Population Sciences, NCI, as a Program Director.

Miguel R. Ossandon received the M.S. degree in computer science from George Washington University. He was previously with Georgetown University for 11 years in cancer research, and he has been with the National Cancer Institute since 2007 as a Program Director of the Technology Development Branch. His research interests focus on technology development for low resources settings.

Houston Baker received the B.A. degree in physical sciences from Harvard University, and the Ph.D. degree in physiology and biophysics from The Ohio State University. He served as a Post-Doctoral Fellow with the Universität des Saarlandes in electrophysiology and biophysics of excitable tissues and with the Red Cross Blood Research Laboratory in cryobiology. He is currently with the National Institutes of Health’s National Cancer Institute as a Program Director with the National Cancer Institute Cancer Imaging Program. He previously served as a Business Executive of the American Society of Plant Physiologists, an Executive Officer of the American Society for Pharmacology and Experimental Therapeutics, and a Health Scientist Administrator with the Walter Reed Army Institute of Research, and with the National Institutes of Health’s National Center of Research Resources, Center for Scientific Review, and the Division of Research Grants.

Paul C. Pearlman (M’02) received the M.S., M.Phil., and Ph.D. degrees in electrical engineering from Yale University. He conducted post-doctoral research with the University Medical Center Utrecht. He has been with the U.S. National Cancer Institute’s Center for Global Health since 2013, transitioning to this role on a prestigious AAAS Science and Technology Policy Fellowship. He is currently a Program Director and the Lead for Global Health Technology. He is a member of the IEEE’s Technical Committee on Translational Engineering for Health Innovations.
References
- [1].Denny L.et al. , “Interventions to close the divide for women with breast and cervical cancer between low-income and middle-income countries and high-income countries,” Lancet, vol. 389, pp. 861–870, Feb. 2016. [DOI] [PubMed] [Google Scholar]
- [2].Corsetti V.et al. , “Breast screening with ultrasound in women with mammography-negative dense breasts: Evidence on incremental cancer detection and false positives, and associated cost,” Eur. J. Cancer, vol. 44, no. 4, pp. 539–544, 2008. [DOI] [PubMed] [Google Scholar]
- [3].Berg W. A.et al. , “Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer,” JAMA, vol. 299, no. 18, pp. 2151–2163, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ohuchi N.et al. , “Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan strategic anti-cancer randomized trial (J-START): A randomised controlled trial,” Lancet, vol. 387, pp. 341–348, Jan. 2016. [DOI] [PubMed] [Google Scholar]
- [5].Burkett B. J. and Hanemann C. W., “A review of supplemental screening ultrasound for breast cancer: Certain populations of women with dense breast tissue may benefit,” Acad. Radiol., vol. 23, no. 12, pp. 1604–1609, 2016. [DOI] [PubMed] [Google Scholar]
- [6].Tohno E., Ueno E., and Watanabe H., “Ultrasound screening of breast cancer,” Breast Cancer, vol. 16, p. 18, Jan. 2009. [DOI] [PubMed] [Google Scholar]
- [7].Berg W. A., Bandos A. I., Mendelson E. B., Lehrer D., Jong R. A., and Pisano E. D., “Ultrasound as the primary screening test for breast cancer: Analysis from ACRIN 6666,” J. Nat. Cancer Inst., vol. 108, no. 4, p. djv367, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Krishnamurthy S.et al. , “Role of ultrasound-guided fine-needle aspiration of indeterminate and suspicious axillary lymph nodes in the initial staging of breast carcinoma,” Cancer, vol. 95, no. 5, pp. 982–988, 2002. [DOI] [PubMed] [Google Scholar]
- [9].Palmeri M. L.et al. , “Identifying clinically significant prostate cancers using 3-D in vivo acoustic radiation force impulse imaging with whole-mount histology validation,” Ultrasound Med. Biol., vol. 42, no. 6, pp. 1251–1262, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Perera R. H., Hernandez C., Zhou H., Kota P., Burke A., and Exner A. A., “Ultrasound imaging beyond the vasculature with new generation contrast agents,” Wiley Interdiscip Rev. Nanomed. Nanobiotechnol., vol. 7, pp. 593–608, Jul-Aug 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Harris R. D. and Marks W. M., “Compact ultrasound for improving maternal and perinatal care in low-resource settings,” J. Ultrasound Med., vol. 28, no. 8, pp. 1067–1076, 2009. [DOI] [PubMed] [Google Scholar]
- [12].Nelson B. P., Melnick E. R., and Li J., “Portable ultrasound for remote environments, part II: Current indications,” J. Emergency Med., vol. 40, no. 3, pp. 313–321, 2011. [DOI] [PubMed] [Google Scholar]
- [13].Hwang J. Q.et al. , “An evidence-based approach to emergency ultrasound,” Emerg. Med. Pract., vol. 13, no. 3, pp. 1–27, 2011. [PubMed] [Google Scholar]
- [14].Schleder S.et al. , “Diagnosis of pericardial effusion with a new generation hand-carried ultrasound device in cardiothoracic intensive care unit patients,” Acta Radiol., vol. 53, pp. 1133–1136, Dec. 2012. [DOI] [PubMed] [Google Scholar]
- [15].Galvao L. F. and Pierri-Galvao M., “Analysis of a vascular screening program in a rural community,” J. Cardiovascular Disease Res., vol. 1, pp. 92–95, Apr. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Huang Y.-L., Chen D.-R., and Liu Y.-K., “Breast cancer diagnosis using image retrieval for different ultrasonic systems,” in Proc. Int. Conf. Image Process. (ICIP), 2004, pp. 2957–2960. [Google Scholar]
- [17].Samundeeswari E., Saranya P., and Manavalan R., “Segmentation of breast ultrasound image using regularized K-means (ReKM) clustering,” in Proc. Int. Conf. Wireless Commun., Signal Process. Netw. (WiSPNET), 2016, pp. 1379–1383. [Google Scholar]
- [18].Mammone R., Love S., Barinov L., Hulbert W., Jairaj A., and Podilchuk C., “Preprocessing for improved computer aided detection in medical ultrasound,” in Proc. IEEE Signal Process. Med. Biol. Symp. (SPMB), Dec. 2013, pp. 1–3. [Google Scholar]
- [19].Farmer P.et al. , “Expansion of cancer care and control in countries of low and middle income: A call to action,” Lancet, vol. 376, no. 9747, pp. 1186–1193, Oct. 2010. [DOI] [PubMed] [Google Scholar]
- [20].Venkatesh S. S., Levenback B. J., Sultan L. R., Bouzghar G., and Sehgal C. M., “Going beyond a first reader: A machine learning methodology for optimizing cost and performance in breast ultrasound diagnosis,” Ultrasound Med. Biol., vol. 41, pp. 3148–3162, Dec. 2015. [DOI] [PubMed] [Google Scholar]
- [21].Mousavi S. R., Sadeghi-Naini A., Czarnota G. J., and Samani A., “Towards clinical prostate ultrasound elastography using full inversion approach,” Med. Phys., vol. 41, p. 033501, Mar. 2014. [DOI] [PubMed] [Google Scholar]
- [22].Scheel J. R.et al. , “Improving breast ultrasound interpretation in uganda using a condensed breast imaging reporting and data system,” Acad. Radiol., vol. 23, pp. 1271–1277, Oct. 2016. [DOI] [PubMed] [Google Scholar]
- [23].Andersen G. N., Viset A., Mjolstad O. C., Salvesen O., Dalen H., and Haugen B. O., “Feasibility and accuracy of point-of-care pocket-size ultrasonography performed by medical students,” BMC Med. Edu., vol. 14, p. 156, Jul. 28 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Taruttis A. and Ntziachristos V., “Translational optical imaging,” Amer. J. Roentgenol., vol. 199, no. 2, pp. 263–271, 2012. [DOI] [PubMed] [Google Scholar]
- [25].The National Academies Collection: Reports funded by National Institutes of Health, Nat. Acad. Sci, Washington, DC, USA, 2016. [Google Scholar]
- [26].Boppart S. A. and Richards-Kortum R., “Point-of-care and point-of-procedure optical imaging technologies for primary care and global health,” Sci. Transl. Med., vol. 6, p. 253rv2, Sep. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dromard T., Ravaine V., Ravaine S., Leveque J. L., and Sojic N., “Remote in vivo imaging of human skin corneocytes by means of an optical fiber bundle,” Rev. Sci. Instrum., vol. 78, p. 053709, May 2007. [DOI] [PubMed] [Google Scholar]
- [28].Muldoon T. J., Pierce M. C., Nida D. L., Williams M. D., Gillenwater A., and Richards-Kortum R., “Subcellular-resolution molecular imaging within living tissue by fiber microendoscopy,” Opt. Exp., vol. 15, pp. 16413–16423, Dec. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Louie J. S., Richards-Kortum R., and Anandasabapathy S., “Applications and advancements in the use of high-resolution microendoscopy for detection of gastrointestinal neoplasia,” Clin. Gastroenterol. Hepatol., vol. 12, pp. 1789–1792, Nov. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Louie J. S., Shukla R., Richards-Kortum R., and Anandasabapathy S., “High-resolution microendoscopy in differentiating neoplastic from non-neoplastic colorectal polyps,” Best Pract. Res. Clin. Gastroenterol., vol. 29, pp. 663–673, Aug. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Grant B. D.et al. , “High-resolution microendoscopy: A point-of-care diagnostic for cervical dysplasia in low-resource settings,” Eur. J. Cancer Prev., vol. 26, no. 1, pp. 63–70, 2015. [DOI] [PubMed] [Google Scholar]
- [32].Ishijima A.et al. , “Automated frame selection process for high-resolution microendoscopy,” J. Biomed. Opt., vol. 20, p. 46014, Apr. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Adesina A.et al. , “Improvement of pathology in sub-Saharan Africa,” Lancet Oncol., vol. 14, p. e152, Apr. 2013. [DOI] [PubMed] [Google Scholar]
- [34].Protano M. A.et al. , “Low-cost high-resolution microendoscopy for the detection of esophageal squamous cell neoplasia: An international trial,” Gastroenterology, vol. 149, pp. 321–329, Aug. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Parikh N. D.et al. , “In vivo diagnostic accuracy of high-resolution microendoscopy in differentiating neoplastic from non-neoplastic colorectal polyps: A prospective study,” Amer. J. Gastroenterol., vol. 109, pp. 68–75, Jan. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pierce M. C.et al. , “A pilot study of low-cost, high-resolution microendoscopy as a tool for identifying women with cervical precancer,” Cancer Preview Res. (Phila), vol. 5, pp. 1273–1279, Nov. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Schmeler K. M., “Preventing cervical cancer globally,” Cancer Preview Res. (Phila), vol. 5, pp. 1257–1259, Nov. 2012. [DOI] [PubMed] [Google Scholar]
- [38].Mitchell M. F., Schottenfeld D., Tortolero-Luna G., Cantor S. B., and Richards-Kortum R., “Colposcopy for the diagnosis of squamous intraepithelial lesions: A meta-analysis 1,” Obstet. Gynecol., vol. 91, pp. 626–631, Apr. 1998. [DOI] [PubMed] [Google Scholar]
- [39].Lombardi T. M., Kahn B. S., Contreras S., Waalen J., and Levitz D., “Image comparison of a mobile colposcope (EVA) versus a standard colposcope for directing cervical biopsies in women with abnormal pap smears: A non-inferiority trial,” J. Minimally Invasive Gynecol., vol. 23, no. 7, p. S92, 2016. [Google Scholar]
- [40].Lam C. T.et al. , “Design of a novel low cost point of care tampon (POCkeT) colposcope for use in resource limited settings,” PLoS ONE, vol. 10, p. e0135869, Sep. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fleming K. A.et al. , “An essential pathology package for low- and middle-income countries,” Amer. J. Clin. Pathol., vol. 147, no. 1, pp. 15–32, Jan. 2017. [DOI] [PubMed] [Google Scholar]
- [42].Balsam J., Ossandon M., Bruck H. A., Lubensky I., and Rasooly A., “Low-cost technologies for medical diagnostics in low-resource settings,” Expert Opin. Med. Diagnostics, vol. 7, pp. 243–255, May 2013. [DOI] [PubMed] [Google Scholar]
- [43].Albert H.et al. , “Performance of three LED-based fluorescence microscopy systems for detection of tuberculosis in Uganda,” PLoS ONE, vol. 5, p. e15206, Dec. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Miller A. R.et al. , “Portable, battery-operated, low-cost, bright field and fluorescence microscope,” PLoS ONE, vol. 5, p. e11890, Aug. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Krishnamurthy S.et al. , “Detection of minimal residual disease in blood and bone marrow in early stage breast cancer,” Cancer, vol. 116, no. 14, pp. 3330–3337, 2010. [DOI] [PubMed] [Google Scholar]
- [46].Cohen S. J.et al. , “Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer,” J. Clin. Oncol., vol. 26, no. 19, pp. 3213–3221, 2008. [DOI] [PubMed] [Google Scholar]
- [47].Okegawa T., Nutahara K., and Higashihara E., “Prognostic significance of circulating tumor cells in patients with hormone refractory prostate cancer,” J. Urol., vol. 181, no. 3, pp. 1091–1097, 2009. [DOI] [PubMed] [Google Scholar]
- [48].Pavlova I., Williams M., El-Naggar A., Richards-Kortum R., and Gillenwater A., “Understanding the biological basis of autofluorescence imaging for oral cancer detection: High-resolution fluorescence microscopy in viable tissue,” Clin. Cancer Res., vol. 14, pp. 2396–2404, Apr. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ozcan A., “Mobile phones democratize and cultivate next-generation imaging, diagnostics and measurement tools,” Lab Chip, vol. 14, no. 17, pp. 3187–3194, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Breslauer D. N., Maamari R. N., Switz N. A., Lam W. A., and Fletcher D. A., “Mobile phone based clinical microscopy for global health applications,” PLoS ONE, vol. 4, p. e6320, Jul. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Fletcher D.et al. , “High numerical aperture telemicroscopy apparatus,” Google Patent, 2016.
- [52].Tseng D.et al. , “Lensfree microscopy on a cellphone,” Lab Chip, vol. 10, pp. 1787–1792, Jul. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lee S. A. and Yang C., “A smartphone-based chip-scale microscope using ambient illumination,” Lab Chip, vol. 14, pp. 3056–3063, May 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Im H.et al. , “Digital diffraction analysis enables low-cost molecular diagnostics on a smartphone,” Proc. Nat. Acad. Sci. USA, vol. 112, pp. 5613–5618, May 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Latrive A. and Boccara A. C., “In vivo and in situ cellular imaging full-field optical coherence tomography with a rigid endoscopic probe,” Biomed. Opt. Exp., vol. 2, pp. 2897–2904, Oct. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Nguyen F. T.et al. , “Intraoperative evaluation of breast tumor margins with optical coherence tomography,” Cancer Res., vol. 69, no. 22, pp. 8790–8796, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Adie S. G. and Boppart S. A., “Optical coherence tomography for cancer detection,” in Optical Imaging of Cancer. Springer, 2010, pp. 209–250. [Google Scholar]
- [58].Zhu H., Isikman S. O., Mudanyali O., Greenbaum A., and Ozcan A., “Optical imaging techniques for point-of-care diagnostics,” Lab Chip, vol. 13, pp. 51–67, Jan. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Jung W., Kim J., Jeon M., Chaney E. J., Stewart C. N., and Boppart S. A., “Handheld optical coherence tomography scanner for primary care diagnostics,” IEEE Trans. Biomed. Eng., vol. 58, no. 3, pp. 741–744, Mar. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Assayag O.et al. , “Large field, high resolution full-field optical coherence tomography: A pre-clinical study of human breast tissue and cancer assessment,” Technol. Cancer Res. Treatment, vol. 13, no. 5, pp. 455–468, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Dobbins J. T., III, “Tomosynthesis imaging: At a translational crossroads,” Med. Phys., vol. 36, no. 6, pp. 1956–1967, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kalender W. A., Beister M., Boone J. M., Kolditz D., Vollmar S. V., and Weigel M. C., “High-resolution spiral CT of the breast at very low dose: Concept and feasibility considerations,” Eur. Radiol., vol. 22, no. 1, pp. 1–8, 2012. [DOI] [PubMed] [Google Scholar]
- [63].Miracle A. C. and Mukherji S. K., “Conebeam CT of the head and neck, part 2: Clinical applications,” Amer. J. Neuroradiol., vol. 30, no. 7, pp. 1285–1292, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Peeling R. W. and Mabey D., “Point-of-care tests for diagnosing infections in the developing world,” Clin. Microbiol. Infection, vol. 16, no. 8, pp. 1062–1069, 2010. [DOI] [PubMed] [Google Scholar]
- [65].Niemz A., Ferguson T. M., and Boyle D. S., “Point-of-care nucleic acid testing for infectious diseases,” Trends Biotechnol., vol. 29, no. 5, pp. 240–250, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].St John A. and Price C. P., “Existing and emerging technologies for point-of-care testing,” Clin. Biochem. Rev., vol. 35, pp. 155–167, Aug. 2014. [PMC free article] [PubMed] [Google Scholar]
- [67].Plummer M., de Martel C., Vignat J., Ferlay J., Bray F., and Franceschi S., “Global burden of cancers attributable to infections in 2012: A synthetic analysis,” Lancet Global Health, vol. 4, pp. e609–e616, Sep. 2016. [DOI] [PubMed] [Google Scholar]
- [68].Shiels M. S. and Engels E. A., “Evolving epidemiology of HIV-associated malignancies,” Current Opinion HIV AIDS, vol. 12, no. 1, pp. 6–11, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Engels E. A.et al. , “Cancer risk in people infected with human immunodeficiency virus in the United States,” Int. J. Cancer, vol. 123, pp. 187–194, Jul. 2008. [DOI] [PubMed] [Google Scholar]
- [70].Flepisi B. T., Bouic P., Sissolak G., and Rosenkranz B., “Biomarkers of HIV-associated cancer,” Biomarkers Cancer, vol. 6, pp. 11–20, Jul. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Mesri E. A., Cesarman E., and Boshoff C., “Kaposi’s sarcoma and its associated herpesvirus,” Nature Rev. Cancer, vol. 10, pp. 707–719, Sep. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Tappero J. W. and Koehler J. E., “Bacillary angiomatosis or Kaposi’s sarcoma?” New England J. Med., vol. 337, p. 1888, Dec. 1997. [DOI] [PubMed] [Google Scholar]
- [73].Mancuso M., Jiang L., Cesarman E., and Erickson D., “Multiplexed colorimetric detection of Kaposi’s sarcoma associated herpesvirus and Bartonella DNA using gold and silver nanoparticles,” Nanoscale, vol. 5, pp. 1678–1686, Jan. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Jiang L., Mancuso M., Lu Z., Akar G., Cesarman E., and Erickson D., “Solar thermal polymerase chain reaction for smartphone-assisted molecular diagnostics,” Sci. Rep., vol. 4, p. 4137, Feb. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Petrara M. R.et al. , “Dried blood spot sampling for detection of monoclonal immunoglobulin gene rearrangement,” Leukemia Res., vol. 37, no. 10, pp. 1265–1270, 2013. [DOI] [PubMed] [Google Scholar]
- [76].Fellner M. D., Durand K., Rodriguez M., Irazu L., Alonio V., and Picconi M. A., “Duplex realtime PCR method for Epstein-Barr virus and human DNA quantification: Its application for post-transplant lymphoproliferative disorders detection,” Brazilian J. Infectious Diseases, vol. 18, no. 3, pp. 271–280, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Snijdewind I. J. M., van Kampen J. J. A., Fraaij P. L. A., van der Ende M. E., Osterhaus A. D. M. E., and Gruters R. A., “Current and future applications of dried blood spots in viral disease management,” Antiviral Res., vol. 93, no. 3, pp. 309–321, 2012. [DOI] [PubMed] [Google Scholar]
- [78].Palefsky J. M. and Holly E. A., “Immunosuppression and co-infection with HIV,” J. Nat. Cancer Inst. Monographs, vol. 31, pp. 41–46, Mar. 2002. [DOI] [PubMed] [Google Scholar]
- [79].WHO Guidelines for Screening and Treatment of Precancerous Lesions for Cervical Cancer Prevention, World Health Org, Geneva, Switzerland, 2013. [PubMed] [Google Scholar]
- [80].De Vuyst H.et al. , “Comparison of pap smear, visual inspection with acetic acid, human papillomavirus DNA-PCR testing and cervicography,” Int. J. Gynecol. Obstetrics, vol. 89, no. 2, pp. 120–126, 2005. [DOI] [PubMed] [Google Scholar]
- [81].Kamal E. M., El Sayed G. A., El Behery M. M., and El Shennawy G. A., “HPV detection in a self-collected vaginal swab combined with VIA for cervical cancer screening with correlation to histologically confirmed CIN,” Arch. Gynecol. Obstetrics, vol. 290, pp. 1207–1213, Dec. 2014. [DOI] [PubMed] [Google Scholar]
- [82].Barbee L.et al. , “Assessing the acceptability of self-sampling for HPV among Haitian immigrant women: CBPR in action,” Cancer Causes Control, vol. 21, no. 3, pp. 421–431, 2010. [DOI] [PubMed] [Google Scholar]
- [83].Arriba L. N., Enerson C. L., Belinson S., Novick L., and Belinson J., “Mexican cervical cancer screening study II: Acceptability of human papillomavirus self-sampler,” Int. J. Gynecol. Cancer, vol. 20, no. 8, pp. 1415–1423, 2010. [DOI] [PubMed] [Google Scholar]
- [84].Huynh J., Howard M., and Lytwyn A., “Self-collection for vaginal human papillomavirus testing: Systematic review of studies asking women their perceptions,” J. Lower Genital Tract Disease, vol. 14, no. 4, pp. 356–362, 2010. [DOI] [PubMed] [Google Scholar]
- [85].Arbyn M.et al. , “Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: A meta-analysis,” Lancet Oncol., vol. 15, pp. 172–183, Feb. 2014. [DOI] [PubMed] [Google Scholar]
- [86].Belinson J. L.et al. , “Improved sensitivity of vaginal self-collection and high-risk human papillomavirus testing,” Int. J. Cancer, vol. 130, pp. 1855–1860, Apr. 2012. [DOI] [PubMed] [Google Scholar]
- [87].Du H.et al. , “A new PCR-based mass spectrometry system for high-risk HPV, part II: Clinical trial,” Amer. J. Clin. Pathol., vol. 136, no. 6, pp. 920–923, Dec. 2011. [DOI] [PubMed] [Google Scholar]
- [88].Toliman P.et al. , “Field evaluation of xpert HPV point-of-care test for detection of human papillomavirus infection by use of self-collected vaginal and clinician-collected cervical specimens,” J. Clin. Microbiol., vol. 54, pp. 1734–1737, Jul. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Stanaway J. D.et al. , “Self-collection for vaginal human papillomavirus testing: Systematic review of studies asking women their perceptions,” Lancet, vol. 388, pp. 1081–1088, Sep. 2016. [DOI] [PubMed] [Google Scholar]
- [90].Blumberg B. S., “Hepatitis B virus, the vaccine, and the control of primary cancer of the liver,” Proc. Nat. Acad. Sci. USA, vol. 94, no. 14, pp. 7121–7125, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Weber B., “Recent developments in the diagnosis and monitoring of HBV infection and role of the genetic variability of the S gene,” Expert Rev. Molecular Diagnosticsl, vol. 5, pp. 75–91, Jan. 2005. [DOI] [PubMed] [Google Scholar]
- [92].Weber B., “Genetic variability of the S gene of hepatitis B virus: Clinical and diagnostic impact,” J. Clin. Virol., vol. 32, no. 2, pp. 102–112, 2005. [DOI] [PubMed] [Google Scholar]
- [93].Lok A. S. and McMahon B. J., “Chronic hepatitis B,” Hepatology, vol. 45, pp. 507–539, Feb. 2007. [DOI] [PubMed] [Google Scholar]
- [94].Datta S., Chatterjee S., and Veer V., “Recent advances in molecular diagnostics of hepatitis B virus,” World J. Gastroenterol., vol. 20, no. 40, pp. 14615–14625, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Craw P. and Balachandran W., “Isothermal nucleic acid amplification technologies for point-of-care diagnostics: A critical review,” Lab Chip, vol. 12, pp. 2469–2486, Jan. 2012. [DOI] [PubMed] [Google Scholar]
- [96].GeneXpert Systems, 2016. [Google Scholar]
- [97].GeneXpert Omni: The True Point of Care Molecular Diagnostic System, 2016. [Google Scholar]
- [98].Snodgrass R., Gardner A., Jiang L., Fu C., Cesarman E., and Erickson D., “KS-detect—Validation of solar thermal PCR for the diagnosis of Kaposi’s sarcoma using pseudo-biopsy samples,” PLoS ONE, vol. 11, p. e0147636, Jan. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Wu N.et al. , Abstract P1-03-03: High Concordance of ER, PR, HER2 and Ki67 by Central IHC and FISH With mRNA Measurements by GeneXpert Breast Cancer Stratifier Assay. Philadelphia, PA, USA: AACR, 2017. [Google Scholar]
- [100].Maher D., Smeeth L., and Sekajugo J., “Health transition in Africa: Practical policy proposals for primary care,” Bull. World Health Org., vol. 88, no. 12, pp. 943–948, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Weidemaier K., Carrino J., Curry A., Connor J. H., and Liebmann-Vinson A., “Advancing rapid point-of-care viral diagnostics to a clinical setting,” Future Virol., vol. 10, no. 3, pp. 313–328, 2015. [Google Scholar]
- [102].Denny L., Kuhn L., De Souza M., Pollack A. E., Dupree W., and Wright T. C. Jr., “Screen-and-treat approaches for cervical cancer prevention in low-resource settings: A randomized controlled trial,” JAMA, vol. 294, no. 17, pp. 2173–2181, 2005. [DOI] [PubMed] [Google Scholar]
- [103].Wittet S., “Cervical cancer screening and treatment in low-resource settings: Practical experience from PATH,” Tech. Rep., 2013.
- [104].Pearlman P. C.et al. , “The national institutes of health affordable cancer technologies program: Improving access to resource-appropriate technologies for cancer detection, diagnosis, monitoring, and treatment in low- and middle-income countries,” IEEE J. Transl. Eng. Health Med., vol. 4, no. 9, pp. 1–8, Sep. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Cremer M.et al. , “CryoPen, a gasless cryotherapy system adapted for low-resource settings,” in Proc. IEEE Healthcare Innov. Point Care Technol. Conf. (HI-POCT), Nov. 2016, pp. 130–133. [Google Scholar]
- [106].Dolman L., Sauvaget C., Muwonge R., and Sankaranarayanan R., “Meta-analysis of the efficacy of cold coagulation as a treatment method for cervical intraepithelial neoplasia: A systematic review,” Int. J. Obstetrics Gynaecol., vol. 121, pp. 929–942, Jul. 2014. [DOI] [PubMed] [Google Scholar]
- [107].Semm K., “New apparatus for the ‘cold-coagulation’ of benign cervical lesions,” Amer. J. Obstetrics Gynaecol., vol. 95, no. 7, pp. 963–966, 1966. [DOI] [PubMed] [Google Scholar]
- [108].Papoutsis D., Underwood M., Parry-Smith W., and Panikkar J., “Comparison of cure rates in women treated with cold-coagulation versus LLETZ cervical treatment for CIN2-3 on pretreatment cervical punch biopsies: A retrospective cohort study,” Arch. Gynecol. Obstet., vol. 295, no. 4, pp. 979–986, 2017. [DOI] [PubMed] [Google Scholar]
- [109].Naud P. S. V., Muwonge R., Passos E. P., Magno V., Matos J., and Sankaranarayanan R., “Efficacy, safety, and acceptability of thermocoagulation for treatment of cervical intraepithelial neoplasia in a hospital setting in Brazil,” Int. J. Gynecol. Obstet., vol. 133, no. 3, pp. 351–354, 2016. [DOI] [PubMed] [Google Scholar]
- [110].Chen J.et al. , “Industry-academic partnerships: An approach to accelerate innovation,” J. Surgical Res., vol. 205, pp. 228–233, Sep. 2016. [DOI] [PubMed] [Google Scholar]
- [111].Dougherty T. J.et al. , “Photodynamic therapy,” J. Nat. Cancer Inst., vol. 90, pp. 889–905, Jun. 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Liu H.et al. , “Development of low-cost devices for image-guided photodynamic therapy treatment of oral cancer in global health settings,” Proc. SPIE, vol. 9694, p. 96940C, Mar. 2016. [Google Scholar]
- [113].Hempstead J.et al. , “Low-cost photodynamic therapy devices for global health settings: Characterization of battery-powered LED performance and smartphone imaging in 3D tumor models,” Sci. Rep., vol. 5, p. 10093, May 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Juarez A.et al. , “AutoSyP: A low-cost, low-power syringe pump for use in low-resource settings,” Amer. J. Tropical Med. Hygiene, vol. 95, no. 4, pp. 964–969, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].McCarroll R.et al. , “O20. Full automation of radiation therapy treatment planning,” Phys. Med., vol. 32, p. 147, Sep. 2016. [Google Scholar]
- [116].Yim H. B., “Self-expanding metallic stents and self-expanding plastic stents in the palliation of malignant oesophageal dysphagia,” Ann. Palliative Med., vol. 3, no. 2, pp. 41–46, 2014. [DOI] [PubMed] [Google Scholar]
- [117].Felix V. N., Caetano A., Cipullo J. P., Almodova E. C., Colaiacovo W., and Zamboti A. F., “Mid-esophagus unresectable cancer treated with a low cost stent. First experience,” BMC Res. Notes, vol. 4, p. 486, Nov. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Singh P., Singh A., Singh A., Sharma G., Bhatia P. K., and Grover A. S., “Long term outcome in patients with esophageal stenting for cancer esophagus—Our experience at a rural hospital of Punjab, India,” J. Clin. Diagnostic Res., vol. 10, pp. PC06–PC09, Dec. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Kettler H., White K., and Hawkes S., Mapping the Landscape of Diagnostics for Sexually Transmitted Infections. Geneva, Switzerland: World Health Org, 2004. [Google Scholar]