Abstract
The objective of this review was to assess the efficacy and tolerability of analgesics in reducing behavioral and psychological symptoms of dementia (BPSD) among older adults from published randomized controlled trials (RCTs). A literature search was conducted of PubMed, MEDLINE, SCOPUS, PsycINFO, and Cochrane collaboration databases for RCTs in the English language that evaluated the use of analgesics in reducing the severity of BPSD among older adults. Additionally, references of full-text articles that were included in this review were searched for extra studies. We identified a total of three unique RCTs that evaluated the use of analgesics among individuals with BPSD. One of the identified RCTs resulted in a total of three additional published papers in the literature, resulting in a total of six papers to be included in this review. All three RCTs identified some benefit for the use of analgesics in reducing BPSD. The analgesics appeared to be well tolerated in the included studies. Major study limitations include the use of data exclusively from published RCTs and limiting the search to English language publications. Additionally, we did not utilize statistical methods to evaluate the treatment outcomes including tolerability. In conclusion, available evidence although limited indicates that analgesics may reduce BPSD among some individuals with dementia living in nursing homes and are well tolerated.
Keywords: dementia, review, psychopharmacology, pain, analgesics, aggression, neuropsychiatry, elderly
Introduction
Behavioral and psychological symptoms of dementia (BPSD) describe a constellation of non-cognitive symptoms and behaviors that are unsafe, disruptive and impair the care of the individual with dementia in a given environment [1]. Available evidence indicates that BPSD occurs in approximately 90% of the individuals with dementia at some time during the course of the illness [2]. BPSD increases the risk for cognitive and functional decline among individuals with dementia, increase their rates of institutionalization, lower their quality of life, increases the caregiver burden, and adds to the social and economic burden of caring for individuals with dementia [3,4].
Current evidence indicates that both non-pharmacological and pharmacological treatments show modest efficacy for the management of BPSD [5]. Most treatment protocols recommend the use of non-pharmacological treatments in combination with pharmacotherapy particularly for the management of those BPSD that are non-responsive to non-pharmacological interventions [6]. To date, multiple different pharmacological agents have been used to manage BPSD including antipsychotics, antidepressants, anticonvulsants, and cognitive enhancers [7]. Unfortunately, these medications are burdened with a significant effect profile that carries substantial risks for the geriatric population [8]. Therefore, there is a great need for identifying efficacious and safe pharmacological treatments that are suitable for use among individuals with BPSD.
It is estimated that individuals with dementia frequently experience pain [9]. In addition, pain among individuals with dementia may manifest as BPSD. Furthermore, pain is considered to be one of the most important causal factors for BPSD [10]. However, provided the often complex etiology for BPSD, the causal link between pain and BPSD may be sometimes difficult to establish [11]. Given the possible association between pain and BPSD, analgesics appear to be a potential target for the management of BPSD [12].
Two systematic reviews have evaluated the use of analgesics among individuals with BPSD [12,13]. In the first review by Husebo et al. [12], the investigators included data from three crossover trials of which two had small sample sizes (<50). The investigators stated that the findings from these studies were inconsistent, although some correlations were noted between treatment of pain and their effect on BPSD. They concluded that there is a profound dearth of rigorous studies of the effect of pain treatment among individuals with dementia and agitation. Furthermore, the available studies do not support the hypothesis that pain management reduces agitation among nursing-home residents with dementia. In the review by Pieper et al. [13], the investigators included data from six studies: three randomized controlled trials (RCTs), one single-case design, one pseudo-RCT: placebo-controlled, double-blinded crossover trial, and one case report. All the studies focused on the use of analgesics and its effect on behavior among individuals with dementia. Five out of six studies showed a positive effect of analgesics on reducing BPSD. The investigators concluded that pain medications are effective in reducing challenging or disruptive behaviors among individuals with dementia. In addition, they opined that fixed dosage of analgesics may be less effective when compared to an individually tailored and stepwise approach to manage BPSD.
The objective of this review was to examine whether the use of analgesics can reduce BPSD from published RCTs. Additionally, we wanted to examine the tolerability of the analgesics when used in individuals with BPSD. Furthermore, we wanted to evaluate whether the possible new data that we obtain concurs with or contradicts the findings from the two previous reviews noted above. Establishing the evidence of the efficacy of this treatment modality, would possibly promote its use as a safe, effective, and relatively inexpensive alternative to other therapeutic agents for BPSD that have fairly significant side-effect profiles.
Methods
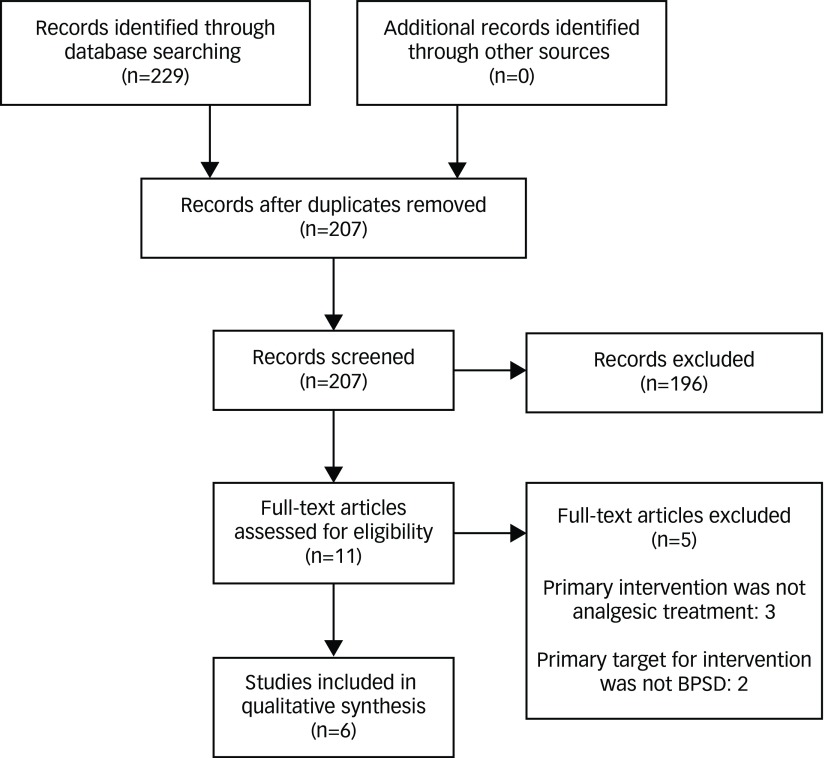
A literature search of PubMed, MEDLINE, EMBASE, PsychINFO, and Cochrane collaboration databases was conducted from June 1st to June 30th, 2017, using the following key words: pain, pain management, pain treatment, analgesic, and dementia. The search was restricted to English-language studies conducted in human subjects. In addition, references of full-text articles that were included in this review were searched for additional studies (Figure 1).
Figure 1.
Flow diagram.
All the authors reviewed the abstracts and full-text articles from the citations obtained via the search of the databases. The decision on which studies to be included or excluded from the final analysis was performed after a review of the full-text articles by all the authors. Disagreements between the authors were resolved by a consensus. Two authors (RRT and CH) abstracted the following data from each study: the year of publication of the study, country of origin, total number of participants, mean age of participants, type of setting in which the study was performed, the comparators, and the duration of the trial. Additionally, the authors collected the names of the rating scales used in each study, the primary and secondary outcomes, and the tolerability of the medications used in each of the studies.
The quality of included studies was assessed by two authors (RRT and CH) using a checklist that we created using the criteria developed by the Center for Evidence Based Medicine for the assessment of RCTs [14]. The quality of the studies was deemed as being good if all six items were met on the checklist. The quality of the study was considered fair if one or two items were absent, and the quality of the study was rated as being poor if three or more items on the checklist were absent. All studies were included in the final review irrespective of the quality of the study. Please refer Table 1 for the six items that we used to create a checklist for assessing the quality of the included RCTs.
Table 1.
Quality of the studies reviewed.
| Name of study | Randomization | Similar groups initially? | Equal treatments? | All participants accounted for? | Analyzed in groups to which they were randomized? | Objective/“blind” treatments? | Overall quality of the study |
|---|---|---|---|---|---|---|---|
| Manfredi et al. [15] | Yes | Unclear | No | Yes | Yes | Yes | Fair |
| Chibnall et al. [16] | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Husebo et al. [17] | Yes | Yes | No | Yes | Yes | Yes | Fair |
| Husebo et al. [18] | Yes | Yes | No | Yes | Yes | No | Fair |
| Husebo et al. [19] | Yes | Yes | No | Yes | Yes | No | Fair |
| Habiger et al. [20] | Yes | Yes | No | No | Yes | Yes | Fair |
Results
This literature review identified a total of six published papers that evaluated the use of analgesics among older adults (≥65 years) with BPSD [15–20]. One trial was found to be of good quality [16], whereas the other two were of fair quality [15,17]. Please refer to Table 1 for the quality rating of the included studies (Table 1).
These six publications represented three unique RCTs [15–17]. Four of the publications were based on the same trial performed in Norway among individuals with dementia that resided at nursing homes [17–20]. These separate manuscripts from the same trail were included, as they evaluated the use of analgesics for different aspects of BPSD including agitation/aggression, mood syndrome, and psychosis/agitation. Two of the three trials were performed in the United States [15,16] and were completed prior to 2010. Moreover, the other trial was performed in Norway [17] in 2011. All three trials were completed among individuals residing at nursing homes. The number of participants in the studies ranged from 25 to 352. The mean age of the participants in these studies was 86.1 years. All studies were 8 weeks in duration [15–17].
Two of the three studies involved acetaminophen as an analgesic [16,17]. The study by Manfredi et al. [15] studied the effect of long acting oxycodone or morphine compared with placebo among individuals with dementia. The four papers that reported on the treatment of pain among nursing-home residents in Norway studied the use of American Geriatrics Society (AGS) recommended standard protocol compared with usual management [17–20]. The AGS recommended standard protocol included the following: Step 1 (oral acetaminophen maximum increase to 3 g a day) or if they were already receiving treatment were adjusted to either Step 2 (oral morphine, maximum 20 mg a day) or Step 3 (buprenorphine transdermal patch, maximum 10 μg/h), or step 4 (oral pregabalin, maximum 300 mg a day) using a fixed-dose regimen. Please refer to Table 2 for details of each of the included papers (Table 2).
Table 2.
Summary of the included studies.
| Name of study/country | Total number of participants | Age of participants | Type of setting | Comparators | Duration of trial |
|---|---|---|---|---|---|
| Manfredi et al. [15]/USA | 47 | Mean age 86.7 years | Nursing home | Long acting oxycodone or morphine vs placebo | 8 weeks |
| Chibnall et al. [16]/USA | 25 | Mean age 85.9 years | Nursing home | Acetaminophen vs placebo | 8 weeks |
| Husebo et al. [17]/Norway | 352 | Mean age 85.7 years | Nursing home | AGS recommended standard protocol vs usual management | 8 weeks with additional follow-up 4 weeks after the end of treatment |
| Husebo et al. [18]/Norway | 352 | Mean age 85.7 years | Nursing home | AGS recommended standard protocol vs usual management | 8 weeks with additional follow-up 4 weeks after the end of treatment |
| Husebo et al. [19]/Norway | 352 | Mean age 85.7 years | Nursing home | AGS recommended standard protocol vs usual management | 8 weeks with additional follow-up 4 weeks after the end of treatment |
| Habiger et al. [20]/Norway | 352 | Mean age 85.7 years | Nursing home | AGS recommended standard protocol vs usual management | 8 weeks with additional follow-up 4 weeks after the end of treatment |
American Geriatrics Society (AGS).
The study by Manfredi et al. published in the International Journal of Geriatric Psychiatry in 2003 [15] showed that treatment with opioid analgesics decreased the agitation level among individuals with dementia who were ≥85 years old even after adjustment for sedation. There were no differences between the opioid and placebo groups in terms of side-effect profile although 47% of the individuals who started the study did not complete the study.
The study by Chibnall et al. published in the Journal of the American Geriatrics Society in 2005 [16] showed that although treatment with acetaminophen did not appear to improve scores on the Cohen-Mansfield Agitation Inventory (CMAI) total scores or subscale score, the Dementia Care Mapping (DCM), Type 1 behaviors (direct interaction with environment), and Type 2 behavior (passive social involvement) were significantly higher (indicating improvement) in the acetaminophen phase when compared to the placebo phase of the study. Acetaminophen was well tolerated in the study (Table 3).
Table 3.
Summary of results from included studies.
| Name of study | Outcomes and measures | Outcomes | Tolerability |
|---|---|---|---|
| Manfredi et al. [15] | MMSE, CMAI | No significant differences in agitation between the placebo and opioid phases for the 25 individuals who completed the study Among the 13 individuals who completed the study (≥85 years old), the agitation level at the end of the opioid phase was significantly lower than that at the end of the placebo phase (mean change in CMAI score −6.4) even after adjustment for sedation and inclusion of four individuals ≥85 years who had dropped out of the study after the second week of the opioid phase No correlation was noted between the number of pain diagnoses recorded at study entry and CMAI score changes |
22 individuals started but did not complete the study The main reasons for discontinuation were as follows:
|
| Chibnall et al. [16] | DCM, CMAI, BPRS | Type 1 behavior (direct interaction with environment) was significantly higher in the intervention phase when compared to the placebo phase (p=0.041) Type 2 behavior (passive social involvement) was significantly higher in the intervention phase when compared to the placebo phase (p=0.007) No differences were found between the intervention and placebo phases on the CMAI total (p=0.90) and subscale scores (p=0.48–0.88) |
Two participants experienced adverse events but it was not considered to be due to the study medication |
| Husebo et al. [17] | CMAI, NPI-NH MMSE, MOBID-2 | CMAI total score was significantly better in the intervention group when compared to the control group (p<0.001) for both last observation carried forward and treatment completers at week 8 NPI-NH total score was significantly better in the intervention group when compared to the control group (p<0.001) at week 8 |
A total 28 participants were lost in the intervention group when compared to 20 in the control group (p=0.298) Fourteen participants died during the study period; 6 in the intervention group and 8 in the control group |
| Husebo et al. [18] | CMAI, NPI-NH, FAST, MMSE, MOBID-2, ADL | When comparing the intervention group with the placebo group, from baseline to week 8, on the CMAI verbally agitated behavior items showed the largest improvement (p<0.001), followed by physically non-aggressive behavior (p=0.008) and aggressive behavior (p=0.037) Single CMAI items that showed improvements in the intervention group when compared to the placebo group, from baseline to week 8 were general restlessness (p=0.001), pacing (p=0.026), constant request for attention (p=0.034), repetitious sentences (p=0.026), complaining (p=0.001), negativism (p<0.001), and cursing and verbal aggression (p=0.043) |
Not noted |
| Husebo et al. [19] | NPI-NH, MOBID-2 | Compared to baseline, the depression and composite mood scores declined in both groups, but the changes in both these outcomes favored the intervention group when compared to the control group; composite mood syndrome (p<0.001) and depression item (p=0.025) Changes in apathy (p=0.017), nighttime behaviors (p=0.050) and appetite and eating disorders (p=0.005) favored the intervention group when compared to the control group Difference on irritability (p=0.092) and anxiety (p=0.125) were not significant between the two groups After week 8, changes in composite mood syndrome were found to be significantly correlated to change in pain (p=0.012) After week 8, change scores for depression, apathy, and irritability were also correlated with change in pain (p=0.030, p= 0.008, and p=0.002), respectively |
Not noted |
| Habiger et al. [20] | CMAI, NPI-NH, MOBID-2, MMSE, FAST, Barthel’s ADL Index | At week 8, the NPI total score was significantly better in the intervention group when compared to control group (p<0.001) At week 8, a decrease from baseline in the agitation cluster (p<0.001), agitation/aggression (p=0.01), and aberrant motor behavior (p=0.017) were noted in the intervention group when compared to the control group Among individuals with one or more symptoms of agitation at baseline, there were improvements noted in the agitation cluster (p<0.001), agitation/aggression cluster (p=0.004), and aberrant motor behavior cluster (p=0.007) in the treatment group when compared with the control group At baseline and week 8, the prevalence of delusions (p=0.8, p=0.2) and hallucinations (p=0.3 and p=0.7) were not associated with the use of opioids |
Not noted |
ADL, Activity of Daily Living; BPRS, Brief Psychiatric Rating Scale; CMAI, Cohen-Mansfield Agitation Inventory; DCM, Dementia Care Mapping; DS-DAT, Discomfort Scale of Dementia of the Alzheimer Type; FAST, Functional Assessment Staging Test; GDS, Global Deterioration Scale; MMSE, Mini-Mental Status Examination; MOBID-2, Mobilization–Observation–Behavior–Intensity–Dementia-2 pain scale; NPI-NH, Neuropsychiatric Inventory-Nursing Home version.
The study by Husebo et al. published in the British Medical Journal in 2011 [17] indicated that the AGS recommended standard protocol for treating pain when compared to usual management improved behavioral symptoms and pain without worsening cognition or function among individuals with dementia. The medications in the treatment protocol were tolerated as well as the usual treatment with no significant differences between the two groups on the dropout rates or on mortality.
In the next paper by Husebo et al. from 2014 that was published in the American Journal of Geriatric Psychiatry [18], the investigators found that the intervention group had improvements in verbally agitated behavior, physically non-aggressive behavior, and aggressive behavior when compared to placebo at 8 weeks when compared to baseline. The behaviors that showed improvements included general restlessness, pacing, constant request for attention, repetitious sentences, complaining, negativism, and cursing and verbal aggression. The adverse effects of the medications were not reported in this paper.
The following paper by Husebo et al. from 2014 that was published in the International Journal of Geriatric Psychiatry [19] found that when compared to baseline the composite mood and depression scores declined in both groups but the changes favored the intervention group when compared to the control group. Additionally, changes in apathy, nighttime behaviors, and appetite and eating disorders also favored the intervention group when compared to the control group. Furthermore, the composite mood syndrome score, scores for depression, apathy, and irritability were found to be correlated with change in pain. The adverse effects of the medications were not reported in this paper.
The paper by Habiger et al. [20] indicated that the behavioral symptoms in the intervention group were better than in the control group when compared to the baseline at week 8. Improvements were noted in the following clusters: agitation, agitation/aggression, and aberrant motor behavior. The investigators also noted that the prevalence of delusions and hallucinations were not associated with the use of opioids. The adverse effects of the medications were not reported in this paper.
Discussion
This review identified only three RCTs that evaluated the use of analgesics among individuals with BPSD [15–17]. All studies were conducted in the nursing-home setting among individuals with a mean age of ≥85 years. These studies were of short duration (8 weeks), and the number of participants was limited (25–352). There was also significant heterogeneity among the various studies. These studies were conducted in two different countries (USA and Norway) and used different validated methods to identify and manage pain and BPSD. Additionally, these studies used different drugs, dose equivalents, dosing strategies, and had varied duration of trials. Furthermore, the disease burden among the participants in these studies was varied.
The available evidence from the three studies indicates that analgesics appear reduce BPSD. Improvements were noted on a wide variety of symptoms including verbal, physical, and social behaviors. The analgesics also appeared to be generally well tolerated when compared to placebo or to usual management strategies. The findings of this review are similar to the conclusions noted from the two previous reviews on this topic by Husebo et al. [12] and Pieper et al. [13].
Improvements noted in BPSD may be due to these medications reducing the distress and discomfort associated with pain sensation by their analgesic effect. In addition, analgesics may improve social behaviors by reducing general discomfort and as a result reduce the incidence and or severity of anxiety or depressive symptoms among individuals with dementia. Furthermore, the use of narcotic analgesics in two of the studies may have contributed to the reduction in behavioral symptoms by their stress reducing effects in some of the patients. There is emerging evidence to support the stress reducing effect of narcotic analgesics [21,22].
The strengths of this study include the collection of data using different search terms from five large databases. Additionally, there were no time restrictions placed with the initial study search. The limitations of this review include the use of data exclusively from published RCTs, limiting the search to English language publications and lack of strict adherence to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [23]. Furthermore, we did not utilize statistical methods to evaluate for the heterogeneity between the included studies or to assess the efficacy or tolerability of the treatments.
The studies included in this review did not specify the nature of pain reported by the participants in terms of being acute or chronic. In addition, these studies did not report the origin of the pain as being osteopathic, musculoskeletal, or neuropathic. Furthermore, the participants in these studies did not have comorbid severe medical, psychiatric, or neurological disorders other than the diagnosis of dementia.
The limitations noted earlier prevent the findings from this review to be generalizable. It would be prudent to conclude that this review found that analgesics appear to reduce the severity of verbal, physical, and social behaviors among old–old individuals (mean age ≥ 85 years) with dementia who reside at nursing homes. These benefits were noted for a short time period. Additionally, these findings may only be limited to Caucasian individuals and women given the nationality of studies and the preponderance of women in these studies. Furthermore, the efficacy of the analgesics in treating more severe behavioral symptoms remains unclear as the populations that were studied had modest behavioral symptoms.
Although available evidence from the literature suggests an association between pain and challenging behaviors (agitation, depression) among individuals with dementia, but a common causal pathway for this association has not yet been determined. A more complete understanding of the complex nature of the relationship between pain and behaviors among individuals with dementia may help create a more comprehensive assessment for these difficult behaviors. Additionally, it may help with the development of effective management strategies for these behaviors.
Future research studies should be designed to assess whether the effectiveness of such targeted interventions is dependent on the specific types and origins of the pain. In addition, the studies should be performed to include individuals who are not living at nursing homes and those individuals who have significant medical, psychiatric, and neurological comorbidities as older adults often present with these comorbid conditions. In addition, BPSD are chronic with periods of relapses and recurrences. Hence, the studies that evaluate the use of analgesics for longer time periods are essential to determine their efficacy among individuals with BPSD. As the majority of the included interventions were only studied once, a replication of these results is warranted.
Conclusion
Although limited, available evidence from the studies included in this review indicate efficacy for analgesics in reducing BPSD particularly among the old–old (mean age ≥ 85 years) for a short duration (8 weeks). Reductions in severity were noted on a wide variety of symptoms including verbal, physical, and social behaviors. The analgesics appeared to be generally well tolerated when compared to placebo or to usual management strategies. The available data from this review on the use of analgesics in reducing BPSD should be considered as preliminary. Positive data from multiple larger studies with longer duration of treatment that are particularly designed to assess their effects on BPSD would have to be available before analgesics can be considered as definitive agents in the management algorithm for BPSD.
Footnotes
Contributions: All authors contributed equally to the development and writing of this manuscript.
Declaration of potential conflicts of interest: The authors have no conflicting interests to declare. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors are available for download at: http://www.drugsincontext.com/wp-content/uploads/2017/11/dic.212508-COI.pdf
Correct attribution: Copyright © 2017 Tampi RR, Hassell C, Joshi P, Tampi DJ. https://doi.org/10.7573/dic.212508. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252772009.
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
For all manuscript and submissions enquiries, contact the Editorial office dic.editorial@bioexcelpublishing.com
Peer review comments to author: 28 September 2017
References
- 1.Anand A, Khurana P, Chawla J, Sharma N, Khurana N. Emerging treatments for the behavioral and psychological symptoms of dementia. CNS Spectr. 2017;15:1–9. doi: 10.1017/S1092852917000530. http://dx.doi.org/10.1017/S1092852917000530. [DOI] [PubMed] [Google Scholar]
- 2.de Oliveira AM, Radanovic M, de Mello PC, Buchain PC, Vizzotto AD, Celestino DL, Stella F, Piersol CV, Forlenza OV. Nonpharmacological interventions to reduce behavioral and psychological symptoms of dementia: a systematic review. Biomed Res Int. 2015;2015:218980. doi: 10.1155/2015/218980. http://dx.doi.org/10.1155/2015/218980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maust DT, Kales HC, McCammon RJ, Blow FC, Leggett A, Langa KM. Distress associated with dementia-related psychosis and agitation in relation to healthcare utilization and costs. Am J Geriatr Psychiatry. 2017 Oct;25(10):1074–82. doi: 10.1016/j.jagp.2017.02.025. http://dx.doi.org/10.1016/j.jagp.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tible OP, Riese F, Savaskan E, von Gunten A. Best practice in the management of behavioural and psychological symptoms of dementia. Ther Adv Neurol Disord. 2017;10(8):297–309. doi: 10.1177/1756285617712979. http://dx.doi.org/10.1177/1756285617712979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forlenza OV, Loureiro JC, Pais MV, Stella F. Recent advances in the management of neuropsychiatric symptoms in dementia. Curr Opin Psychiatry. 2017;30(2):151–8. doi: 10.1097/YCO.0000000000000309. http://dx.doi.org/10.1097/YCO.0000000000000309. [DOI] [PubMed] [Google Scholar]
- 6.Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. doi: 10.1136/bmj.h369. http://dx.doi.org/10.1136/bmj.h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Yu JT, Wang HF, Meng XF, Wang C, Tan CC, Tan L. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2015;86(1):101–9. doi: 10.1136/jnnp-2014-308112. http://dx.doi.org/10.1136/jnnp-2014-308112. [DOI] [PubMed] [Google Scholar]
- 8.Mittal V, Kurup L, Williamson D, Muralee S, Tampi RR. Risk of cerebrovascular adverse events and death in elderly patients with dementia when treated with antipsychotic medications: a literature review of evidence. Am J Alzheimers Dis Other Demen. 2011;26(1):10–28. doi: 10.1177/1533317510390351. http://dx.doi.org/10.1177/1533317510390351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Achterberg P, Pieper M, van Dalen-Kok AH, de Waal MW, Husebo BS, Lautenbacher S, Kunz M, Scherder EJ, Corbett A. Pain management in patients with dementia. Clin Interv Aging. 2013;8:1471–82. doi: 10.2147/CIA.S36739. http://dx.doi.org/10.2147/CIA.S36739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corbett A, Husebo B, Malcangio M, Staniland A, Cohen-Mansfield J, Aarsland D, Ballard C. Assessment and treatment of pain in people with dementia. Nat Rev Neurol. 2012;8(5):264–74. doi: 10.1038/nrneurol.2012.53. http://dx.doi.org/10.1038/nrneurol.2012.53. [DOI] [PubMed] [Google Scholar]
- 11.Cohen-Mansfield J, Thein K, Marx MS, Dakheel-Ali M. What are the barriers to performing nonpharmacological interventions for behavioral symptoms in the nursing home? J Am Med Dir Assoc. 2012;13(4):400–5. doi: 10.1016/j.jamda.2011.07.006. http://dx.doi.org/10.1016/j.jamda.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Husebo BS, Ballard C, Aarsland D. Pain treatment of agitation in patients with dementia: a systematic review. Int J Geriatr Psychiatry. 2011;26(10):1012–18. doi: 10.1002/gps.2649. http://dx.doi.org/10.1002/gps.2649. [DOI] [PubMed] [Google Scholar]
- 13.Pieper MJ, van Dalen-Kok AH, Francke AL, van der Steen JT, Scherder EJ, Husebo BS, Achterberg WP. Interventions targeting pain or behaviour in dementia: a systematic review. Ageing Res Rev. 2013;12(4):1042–55. doi: 10.1016/j.arr.2013.05.002. http://dx.doi.org/10.1016/j.eurger.2013.07.286. [DOI] [PubMed] [Google Scholar]
- 14.Critical Appraisal Tools. 2017. [Last accessed: 15 October 2017]. Available at: http://www.cebm.net/critical-appraisal/
- 15.Manfredi P, Breuer B, Wallenstein S, Stegmann M, Bottomley G, Libow L. Opioid treatment for agitation in patients with advanced dementia. Int J Geriatr Psychiatry. 2003;18(8):700–5. doi: 10.1002/gps.906. http://dx.doi.org/10.1002/gps.906. [DOI] [PubMed] [Google Scholar]
- 16.Chibnall J, Tait R, Harman B, Luebbert RA. Effect of acetaminophen on behavior, well-being, and psychotropic medication use in nursing home residents with moderate-to-severe dementia. J Am Geriatr Soc. 2005;53(11):1921–9. doi: 10.1111/j.1532-5415.2005.53572.x. http://dx.doi.org/10.1111/j.1532-5415.2005.53572.x. [DOI] [PubMed] [Google Scholar]
- 17.Husebo B, Ballard C, Sandvik R, Nilsen OB, Aarsland D. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. BMJ. 2011;343:d4065. doi: 10.1136/bmj.d4065. http://dx.doi.org/10.1136/bmj.d4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Husebo B, Ballard C, Cohen-Mansfield J, Seifert R, Aarsland D. The response of agitated behavior to pain management in persons with dementia. Am J Geriatr Psychiatry. 2014;22(7):708–17. doi: 10.1016/j.jagp.2012.12.006. http://dx.doi.org/10.1002/ejp.523. [DOI] [PubMed] [Google Scholar]
- 19.Husebo B, Ballard C, Fritze F, Sandvik RK, Aarsland D. Efficacy of pain treatment on mood syndrome in patients with dementia: a randomized clinical trial. Int J Geriatr Psychiatry. 2014;29(8):828–36. doi: 10.1002/gps.4063. http://dx.doi.org/10.1002/gps.4063. [DOI] [PubMed] [Google Scholar]
- 20.Habiger TF, Flo E, Achterberg WP, Husebo BS. The interactive relationship between pain, psychosis, and agitation in people with dementia: results from a cluster-randomised clinical trial. Behav Neurol. 2016;2016:7036415. doi: 10.1155/2016/7036415. http://dx.doi.org/10.1155/2016/7036415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szczytkowski-Thomson JL, Lebonville CL, Lysle DT. Morphine prevents the development of stress-enhanced fear learning. Pharmacol Biochem Behav. 2013;103(3):672–7. doi: 10.1016/j.pbb.2012.10.013. http://dx.doi.org/10.1016/j.pbb.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Bershad AK, Jaffe JH, Childs E, de Wit H. Opioid partial agonist buprenorphine dampens responses to psychosocial stress in humans. Psychoneuroendocrinology. 2015;52:281–8. doi: 10.1016/j.psyneuen.2014.12.004. http://dx.doi.org/10.1016/j.psyneuen.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. http://dx.doi.org/10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]