Abstract
The physical demands for swimming and feeding change dramatically over the course of development for many aquatic animals. Indeed, in teleosts, the transition from larva to adult involves major shifts in both trophic morphology and feeding behavior. A spike in thyroid hormone (TH) coordinates many developmental processes that occur during this adult transition in numerous vertebrate species. Using mutant and transgenic zebrafish, we tested the hypothesis that TH is essential for the transition from larval to adult feeding kinematic profiles. We found that every measured kinematic variable that distinguished larvae from adults also differentiated hypothyroid from wild-type (WT) euthyroid adults, suggesting that TH is indeed necessary for the onset of mature feeding behaviors. In contrast, feeding kinematics in hyperthyroid adults were extremely similar to those measured in euthyroid adults. Altered TH signaling underlies pedomorphosis in some amphibian species, and Danionella is a pedomorphic danionin genus. We therefore tested whether feeding kinematics of adult Danionella would more closely match larval zebrafish (and hypothyroid adults) than WT adult zebrafish. We found Danionella feeding kinematics resemble those of larval (and hypothyroid) zebrafish in multiple respects. Overall, we conclude that TH is essential in stimulating the onset of adult feeding kinematics in zebrafish, and that some of the underlying developmental pathways may have been lost in Danionella.
Keywords: : zebrafish, developmental biology, other aquatic model species, behavior
Introduction
Most fishes encounter extremely different aquatic environments over the course of their development. Even small species such as the zebrafish grow more than an order of magnitude from first feeding to adulthood.1,2 Larval zebrafish live in a low Reynold's number aquatic environment, in which viscous forces are relatively higher than inertial forces.2,3 This relationship is reversed as fish grow to larger sizes and inertia becomes stronger than aquatic viscosity. Similar to most aquatic vertebrates, zebrafish use suction feeding throughout their lives.2,3 The production of suction is particularly sensitive to relative fluid viscosity,4 and morphologies and behaviors that confer efficient suction performance at one size (and life stage) are unlikely to function well if scaled to a different size and Reynold's number.
Many of the physiological characters that typify adults originate during their developmental transition from larva to adult. While some teleosts undergo major body rearrangements, behavioral, and kinematic shifts during this metamorphosis (e.g., flatfishes and many reef fishes),5 zebrafish maintain the same overall bauplan, and changes in behavior shift gradually as individuals mature.3,6 Nonetheless, the adult transition in zebrafish development brings about important changes in numerous organ systems,1,5,7 including shifts in the functional morphology of feeding that promote better performance in a higher Reynold's number environment.2,3
Thyroid hormone (TH) signaling is critical for stimulating and coordinating numerous developmental and physiological processes in vertebrates. In many species that experience a spectacular metamorphic transition to a markedly different adult physiology, increased TH production and sensitivity orchestrate whole-body metamorphosis by stimulating coordinated transitions in skeletal shape, body coloration, and digestive processes.5,8,9 Most famously, TH stimulates the spectacular metamorphosis of amphibians from aquatic herbivores to terrestrial predators.8,10 In organisms that undergo less dramatic ontogenetic transitions (e.g., humans and zebrafish), development is also profoundly influenced by TH.5,7,8,11,12 In zebrafish, plasma concentrations of TH peak during the transition from larva to adult,13 and blocking TH chemically or genetically inhibits the development of multiple physical and physiological features.7,8 The craniofacial skeleton appears to be particularly sensitive to TH concentration, and hypothyroidism leads to changes in skull proportions, while hyperthyroidism induces the growth of a hypertrophic lower jaw.7
We hypothesized that TH signaling stimulates the onset of adult feeding in zebrafish, and that this hormonal signal served as a necessary cue for individuals to transition from larval to adult feeding kinematics. Craniofacial morphology and feeding kinematics change dramatically as zebrafish grow and mature,1–3 and, particularly, since TH appears to influence craniofacial shape,7 we predicted that a lack of TH would inhibit adult feeding kinematics. To test this hypothesis, we used a congenitally hyperthyroid zebrafish mutant line and a transgenic line allowing conditional ablation of the thyroid follicles.7 We analyzed feeding strikes in these TH-disrupted lines and compared them with both larval and adult euthyroid wild-type (WT) zebrafish.
Disruptions in TH signaling underlie many cases of pedomorphosis and metamorphic failure, particularly in amphibians,9,14,15 and we were curious about the kinematics of a pedomorphic relative of the zebrafish. Danionella is a recently described genus comprising miniaturized species16 that exhibit natural developmental arrest and fail to develop many adult traits, including a failure to develop many bones typical of adult zebrafish and other danionins.17,18 Indeed, Danionella dracula are known to lack a kinethmoid,17,18 which is necessary for the premaxilla protrusion typical of adult feeding. Whether Danionella exhibit reduced TH production and/or sensitivity is currently unknown. Based on their small size, immature appearance, and failure to progress to typical adult stages of skull development, we hypothesized that feeding kinematics of Danionella would resemble those of both larval WT zebrafish and adult hypothyroid zebrafish.
Methods
Specimens
All experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of Boston College and Washington State University, Tri-Cities. Fish were reared and kept under standard zebrafish conditions. Multiple groups were used, and their descriptions follow, including postembryonic developmental stage and standard length (SL), Parichy et al.1 Larval 8 days postfertilization (dpf): WT fish of the AB strain were filmed at 8 dpf; these ranged in postembryonic stage (see Parichy et al.1) from pSB+ to Fle and ranged in SL from 3.4 to 3.8 mm. Larval 30 dpf: WT AB at 30 dpf, ranging in stage from DR+ to PB, and from 6.4 to 8.3 mm SL. WT AB: adults of the AB strain (27.6–31.2 mm SL). WT dimethyl sulfoxide (DMSO): adult tg(TG:VenusV2AnfnB) treated with the vehicle DMSO at 4 dpf according to McMenamin et al.7 (22.5–26 mm SL). Hypothyroid: adult tg(TG:VenusV2AnfnB) treated with metronidazole (Sigma) at 4 dpf to ablate the thyroid follicles according to McMenamin et al.7 (21.3–24.5 mm SL). Hyperthyroid: adult hyperthyroid opallus mutants7 (21–27 mm SL). Danionella spp: specimens ranged from 9.6 to 13.3 mm SL. Danionella were obtained commercially and were sold as Danionella translucida. We cannot rule out that these specimens may have been another species within the genus, or even a mix of multiple species.
The WT DMSO fish represent the control group the hypothyroid transgenics. These fish are in the same transgenic background as the thyroid-ablated hypothyroid fish, but were treated with DMSO instead of metronidazole at 4 dpf, and therefore have intact thyroid follicles. Likewise, the WT AB fish are the control for the hyperthyroid mutants. The hyperthyroid opallus mutation was induced in an AB background,7 and this comparison should minimize any strain-specific differences.
For larval groups (AB 8 dpf and AB 30 dpf), we analyzed seven feeding strikes per group. For all adult zebrafish groups (hyperthyroid, hypothyroid, and WT), we recorded two strikes for each of four to five individuals per group. We recorded two to three strikes for each of three Danionella specimens. Fish were euthanized after the collection of feeding videos, and their eye diameter and SL were recorded. Measurements from videos were converted from pixels to mm using the measurement of the eye diameter as a known length.
Kinematic analyses and measurements
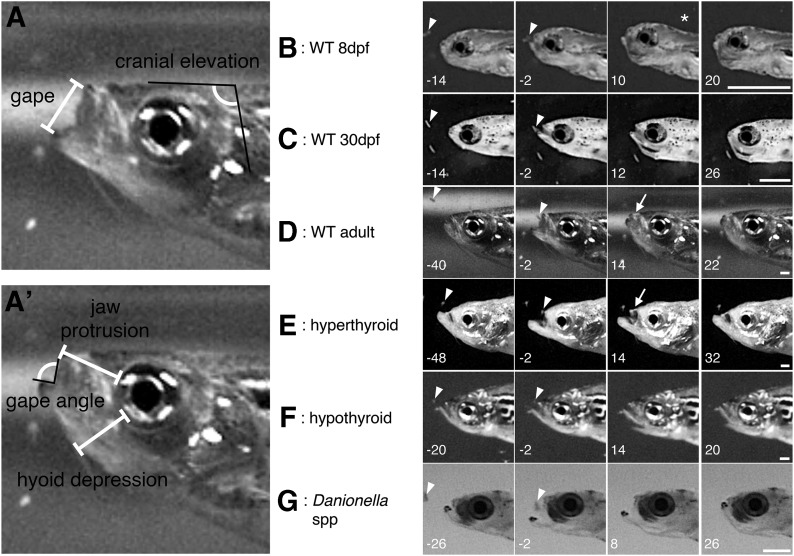
Feeding strikes were recorded at 500 frames/s with an Edgertronic Monochrome high-speed video camera (Sanstreak Corp., San Jose, CA). Larval fish were filmed while feeding on Paramecia spp., and adult fish were filmed while feeding on newly hatched brine shrimp (Artemia spp.). Portions of movies that showed successful strikes in a lateral view were extracted and analyzed. Five measurements of maximum kinematic excursions—maximum gape distance, maximum gape angle, maximum cranial elevation, maximum jaw protrusion, and maximum hyoid depression—were each measured in ImageJ.19 To maintain internal consistency, all measurements were taken by the same person. Maximum gape distance and maximum gape angle were measured as in Figure 1. Cranial elevation was measured as the angle between a fixed point on the flank and the head (Fig. 1), and maximum cranial elevation was calculated as the difference between the smallest amount of cranial elevation (before the strike began) and the largest amount of cranial elevation. Maximum hyoid depression was measured as the difference between the smallest amount of hyoid depression (before the strike) and the largest amount of hyoid depression (both measured from the edge of the eye to the most protruded portion of the hyoid, see Fig. 1). Likewise, maximum premaxilla protrusion was measured as the difference between the amount of premaxillary protrusion before the strike and the largest amount of premaxillary protrusion during the strike, both measured from the edge of the eye to the anteriormost part of the premaxilla (as in Fig. 1). Note that larval zebrafish20 and Danionella adults17,18 lack the upper jaw elements that allow premaxillary protrusion, so measurements that deviate from zero essentially represent measurement error.
FIG. 1.
Kinematic movements in different backgrounds. (A, A') Two angles and three distances were ultimately recorded from each strike; see text for measurement details. Note that these two images represent different frames and do not necessarily reflect the maximum measurement for any variable. (B–G) Example strikes from six groups of analyzed fish. Leftmost panels show the frames as the fish starts to open its mouth; next is the panel immediately before food is enveloped; next is the frame at which the fish shows maximum hyoid depression; rightmost panel shows the fish after the mouth completely closes. Arrowheads indicate prey item before envelopment. Asterisk in (B) indicates the high degree of cranial elevation in young larvae. Arrows in (D, E) indicate large amount of jaw protrusion in WT and hyperthyroid adults. Numbers in bottom left corners indicate the milliseconds from the frame at which prey item is enveloped. Scale bar = 1 mm. WT, wild type.
To analyze the relative timing of kinematic events during each strike, the frame at which the mouth began to open and the frame at which the prey item passed into the mouth were recorded. The frame at which each maximum excursion occurred was also recorded for each of the five measured variables. Each of these time measurements were standardized to the time at which the prey item entered the mouth, which was assigned as time = 0.
Analyses
Figure 2 shows raw values for maximum excursions and angles. In all other figures and analyses, each maximum kinematic excursion (maximum gape distance, maximum jaw protrusion, and maximum hyoid depression) was standardized to the SL of the fish. In Figure 3, each standardized excursion as well as each angle measurement (maximum gape angle and maximum cranial elevation) was analyzed independently with Tukey Honest Significant Differences (HSD) tests. When the two larval groups or the two WT groups did not differ (based on independent t-tests), they were pooled. All five kinematic measurements standardized to SL were used as variables in principle components analyses (PCAs).
FIG. 2.
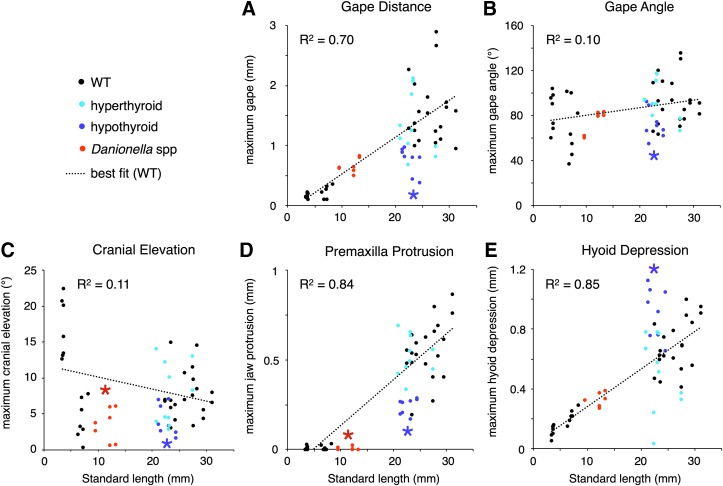
Kinematics change as fish grow, and these relationships are affected by thyroid hormone. Scatter plots show the relationships between fish size and maximum (A) gape distance, (B) gape angle, (C) cranial elevation angle, (D) jaw protrusion, and (E) hyoid depression. Measurement details in text. All WT groups shown as black dots; dashed lines show best fit lines for WT samples only. Asterisks of corresponding colors indicate that a group differs significantly from values predicted by regression (paired t test, p < 0.01).
FIG. 3.
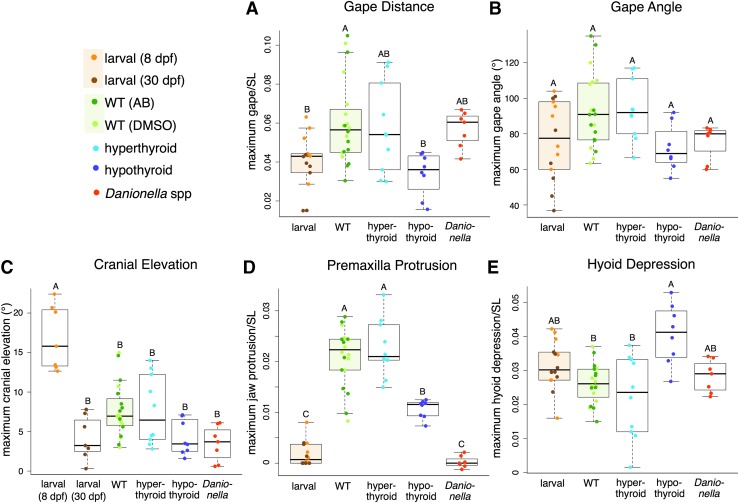
Maximum kinematic excursions show that strikes of hypothyroid and Danionella adults resemble strikes of larvae. Box plots show the relative amount of maximum (A) gape distance, (B) gape angle, (C) cranial elevation angle, (D) jaw protrusion, and (E) hyoid depression. Measurement details in text. For most factors, the groups Larval and WT contain pooled samples of 8 dpf (light orange) with 30 dpf (dark orange), and AB (dark green) and DMSO (light green), respectively. Points are jittered for clarity. Letters indicate significance groups. DMSO, dimethyl sulfoxide; dpf, days postfertilization.
Each timing variable (time at mouth opening, time at maximum gape distance, time at maximum gape angle, time at maximum cranial elevation, time at maximum hyoid depression, and time at maximum jaw protrusion; all standardized relative to time 0) was analyzed independently using Tukey HSD (Fig. 5) and all six measurements were used as variables in a timing PCA (Fig. 6), as well as a PCA including both kinematic measurements and timing variables (Supplementary Fig. S2; Supplementary Data are available online at www.liebertpub.com/zeb). All analyses were performed in Microsoft Excel 15.27, JMP® 13.1.021 or R.22
FIG. 5.
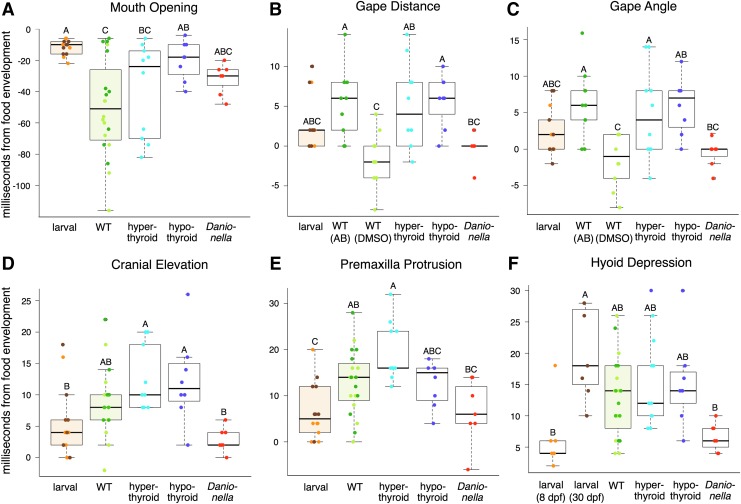
Timing measurements show that strikes of hypothyroid and Danionella adults resemble strikes of larvae. Box plots show the time in milliseconds from food envelopment to different kinematic events: (A) opening of the mouth, (B) maximum gape distance, (C) maximum gape angle, (D) maximum cranial elevation, (E) maximum premaxillary protrusion, and (F) maximum hyoid depression. Colors as in Figure 3. Letters indicate significance groups.
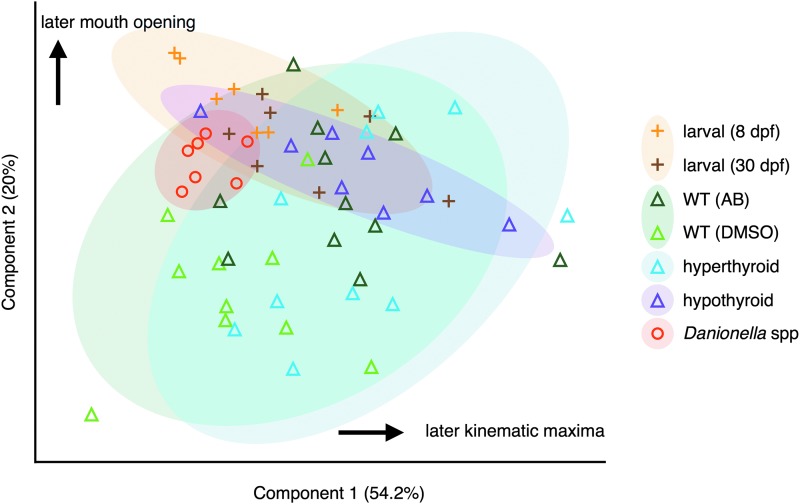
FIG. 6.
PCA of strike timing. PCA includes all measured times of events, zeroed to the time at which the food is enveloped. The groups larval and WT contain pooled samples of 8 dpf (light-orange plus signs) with 30 dpf (dark-orange plus signs), and AB (dark-green triangles) and DMSO (light-green triangles), respectively. Transparent ovals show regions of 90% coverage for each group.
Results
Variation in physical kinematic measurements
Gape, premaxilla protrusion, and hyoid depression all increased as WT fish grew and developed, while cranial elevation decreased with increasing size (Fig. 2). Hypothyroid fish showed maximal gape distance, gape angle, and premaxilla protrusion that were significantly smaller than expected by size, while showing hyoid depression significantly more extensive than expected by size (Fig. 2). Danionella showed lower cranial elevation and premaxilla protrusion than expected for their size, while hyperthyroid fish were comparable with WT fish of the same sizes for all variables (Fig. 2).
Gape distance and premaxilla protrusion increased significantly between larval stages and adulthood in WT euthyroid fish (Figs. 2 and 3), and although absolute hyoid depression increased as fish matured (Fig. 2E), size-corrected hyoid depression did not differ between larvae and adults (Fig. 3E). Gape angle did not significantly change as fish grew (Figs. 2B and 3B). Cranial elevation decreased significantly as larvae matured from 8 to 30 dpf (Figs. 2C and 3C). Chronic hyperthyroidism did not impact any of the kinematic variables that we measured (Figs. 2 and 3), but as predicted, the feeding kinematics of hypothyroid adults were found to resemble those of larval WT in several respects. Hypothyroid adults were much more similar to larval WT specimens with respect to gape distance and premaxilla protrusion than they were to adult WT fish (Figs. 2 and 3). Danionella adults exhibited very little premaxilla protrusion, and in this respect more closely resembled larval zebrafish than adults (Figs. 2D and 3D).
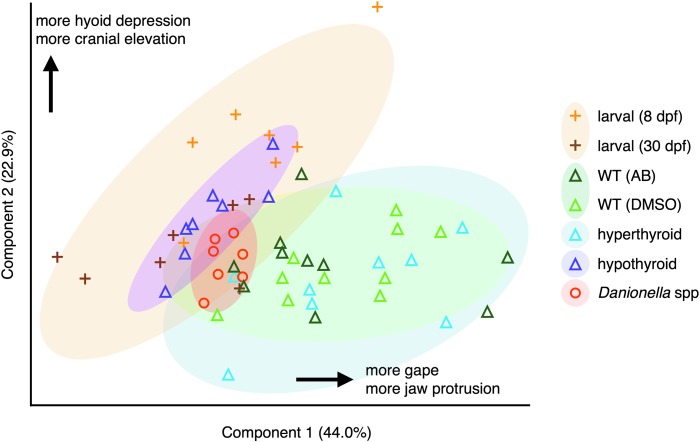
A PCA of the maximum excursion variables in each strike showed considerable separation between larval and adult WT zebrafish (Fig. 4). These life stages showed significantly different distributions along both PC1 (p < 0.001) and PC2 (p < 0.001). Older (30 dpf) larvae were more similar to adults in PC2 than were younger (8 dpf) larvae. While hyperthyroid zebrafish clustered with euthyroid WT adults, hypothyroid zebrafish clustered with euthyroid WT larvae (Fig. 4). Danionella spp. occupied an area of intermediate functional morphospace located between the regions occupied by larval and adult WT zebrafish (Fig. 4).
FIG. 4.
PCA separates larval from adult strike kinematics. PCA performed on the maximum excursion for each variable for every strike. The groups larval and WT contain pooled samples of 8 dpf (light-orange plus signs) with 30 dpf (dark-orange plus signs), and AB (dark-green triangles) and DMSO (light-green triangles), respectively. Shaded ovals show regions of 90% coverage for each group. PCA, principle components analysis.
Variation in timing
We examined differences in time at which the mouth began to open and time at which each variable reached its maximum excursion, zeroed to time at food envelopment. Overall, strike timing did not change as much over the course of development as did the physical kinematics of the strike (compare slopes and R2 values in Fig. 2 to those in Supplementary Fig. S1). Nonetheless, larval and adult WT fish differed in the relative timing of several strike components (Fig. 5). While WT adults exhibited a wide range of times at which mouth opening began (minimum = 8 ms, maximum = 116 ms, average = 50.3 ms before time 0), larvae showed little variation in this variable (minimum = 6 ms, maximum = 32 ms, average = 11.4 ms; Fig. 5A and Supplementary Fig. S1A). Hyoid depression occurred significantly later in older (30 dpf) larvae compared with younger (8 dpf) larvae, although neither group differed significantly from adults (Fig. 5F).
The two groups of euthyroid WT adult zebrafish [the AB background and DMSO-treated tg(TG:VenusV2AnfnB)] differed in the timing of maximum gape distance and maximum gape angle, with each maximum occurring earlier in the DMSO group. Hyperthyroid adults were similar to euthyroid adults in all measured aspects of timing, but they more closely resembled AB than DMSO adults in the timing of maximum gape (Fig. 5B, C). The timing of Danionella spp. strikes was similar to that of WT zebrafish in all respects except for the timing of maximum hyoid depression, which occurred much earlier than in adult WT zebrafish. The early timing of hyoid depression in Danionella spp. was similar to that observed in younger zebrafish larvae. It should be noted that larvae and Danionella cannot protrude their jaws (Figs. 2D and 3D), so measuring the time at which premaxilla protrusion is maximized (Fig. 5E) is not necessarily meaningful.
The results of a PCA of all timing variables showed considerable overlap between larval and adult WT zebrafish in PC1 (Fig. 6), but the groups showed differences in mean PC2 scores (p < 0.0001). The distributions of hypothyroid zebrafish and Danionella spp. both overlapped with those of larval and adult WT zebrafish, and the distribution of hyperthyroid zebrafish was very similar to that of WT zebrafish.
The results of a PCA of all maximum excursion and timing variables combined showed almost no overlap between larval and adult WT zebrafish (Supplementary Fig. S2). The mean scores of the two groups were significantly different for both of the first two principal components (p < 0.0002). Hypothyroid zebrafish and Danionella occupied a region intermediate between larval and adult WT zebrafish (Supplementary Fig. S2).
Discussion
Larval strikes differ from adult strikes
To establish a baseline of how feeding kinematics change over the course of zebrafish development, we tested for kinematic differences between larval and adult feeding strikes. Of the five maximum distance and angle excursions, we found that gape distance, premaxilla protrusion, and hyoid depression increased significantly as fish grew from larvae to adults (Figs. 2 and 3), although the increase in hyoid depression was not significant when values were standardized to fish size (Fig. 3). As expected from the fact that larvae do not have mobile upper jaw elements, larvae showed essentially no premaxilla protrusion, while adults showed considerable protrusion (Figs. 2D and 3D). With these aggregated differences, a PCA of kinematic measurements showed strong separation between larvae and WT adults (Fig. 4).
Previous work showed that while the absolute amount of hyoid depression increases as fish grow (consistent with Fig. 2E), when standardized for size, hyoid depression decreases as fish grow and develop.2,3 Larvae in our study did show slightly greater levels of relative hyoid depression, but these differences were not significant in a Tukey HSD test when corrected using SL (p = 0.33; Fig. 3E), or when standardized using head length, as data were standardized in Hernandez et al.3 (p = 0.08; data not shown). This incongruity is likely due to the fact that previous investigations used smaller, more immature larvae at 5 dpf.3 The youngest larvae we used were somewhat older (8 and 30 dpf) and may have already lost some of hyoid depression that characterizes very young fish.
We tested for developmental differences in the relative timing of multiple aspects of the feeding strike and found that strikes began significantly later in larvae (i.e., the mouth opened much closer to time 0, when the food was enveloped) in comparison with WT adults and that WT adults also exhibited much greater variation in the onset of mouth opening, while larvae showed uniformly late-opening mouths (Fig. 5A and Supplementary Fig. S1). Suction production is most efficient close to a food item23 and the consistency with which larval zebrafish open their mouths very soon before prey contact suggests that maximizing suction production is critical for overcoming high levels of water viscosity in their high Reynold's number environment.
The feeding strikes of the two larval ages that we examined (8 and 30 dpf) were similar in most kinematic respects, except that the youngest larvae exhibited significantly more cranial elevation, which may reflect the need to overcome particularly high water viscosity at a very small size (Figs. 1B, 2C, and 3C). Moreover, young larvae reached maximum hyoid depression more rapidly after food envelopment than did older larvae (Fig. 5F), reflecting a more rapid strike.
Interestingly, the two groups of adult WT fish (AB and DMSO-treated transgenic controls) showed differences in the time at which they achieved maximum gape distance and maximum gape angle. This may reflect slight differences between the sizes of the two groups (mean SL of AB = 28.8 mm; mean SL of DMSO = 24 mm) or strain-specific differences in feeding kinematics.
Hypothyroid adults feed as larvae
In several respects the strikes of hypothyroid adult zebrafish more closely resemble the strikes of larval than those of adult WT fish. We measured three kinematic factors in which larvae differed from adults: maximum gape, maximum premaxilla protrusion, and the timing of mouth opening (Figs. 3 and 5). In all three cases hypothyroid adults more closely resembled WT larvae than adults (Fig. 3). As euthyroid fish mature, the mouth opens earlier and wider and premaxilla protrusion eventually arises. As larvae, hypothyroid adults retain late mouth-opening behavior, utilize a small gape, and show little premaxilla protrusion (Figs. 3 and 5). We did not detect a significant developmental decrease in relative hyoid depression previously reported for WT zebrafish,3 possibly because we examined older specimens than the earlier study. Other factors likely also contributed to the difference, including the fact that we were using different strains of fish reared in a different environment, a different individual made the measurements, and we size standardized our measurements differently (by SL rather than by head length). Nonetheless, we did detect a significant increase in relative hyoid depression in hypothyroid fish relative to WT adults (Fig. 3E). The results of PCAs, including maximum kinematic excursions (Fig. 4), timing variables (Fig. 6), and both (Supplementary Fig. S2), uniformly showed that feeding kinematics of hypothyroid adults are similar to those of WT larvae. The PCA of maximum excursions showed the functional morphospace of hypothyroid specimens to be completely nested within those of larval specimens (Fig. 4). Our data therefore support the conclusion that TH is required to stimulate the onset of multiple aspects of adult feeding kinematics.
Many of the differences between adult feeding and larval feeding in WT zebrafish reflect the differences in water viscosity experienced at different life stages. As discussed, larval mouth opening may be constrained to occur immediately before food is enveloped to maximize suction production and overcome high water viscosity. Likewise, the larger degree of hyoid depression seen in larval WT zebrafish maximizes the expansion of their buccal cavity and enhances suction production.3 Although hypothyroid fish were essentially the same size as the euthyroid WTs used in this study (Fig. 2), hypothyroid adults were more similar to WT larvae in that they delayed mouth opening and used large amounts of hyoid depression. Although they experience a Reynold's number comparable to that of WT adults, hypothyroid adults behave as though they are much smaller than they actually are. However, we cannot discount the possibility that their delayed mouth opening is the result of a slow reaction time due to a depressed metabolism or other physiological factors. Moreover, the changes in maximum excursions likely stem from changes in the physical morphology of hypothyroid fish. Nonetheless, multiple kinematic variables suggest that without the endocrine trigger of TH, hypothyroid fish continue to exhibit feeding kinematics as though living in a low Reynolds number environment.
It is notable that hypothyroidism does not equally block components of feeding kinematic development. Indeed, hypothyroid adults resemble older larvae in terms of cranial elevation, but resemble younger larvae in terms of hyoid depression. These differences may reflect other triggers that induce the onset of adult feeding kinematics, or may be physiological adjustments and compensations for the altered morphology of hypothyroid skulls (see McMenamin et al.7) that are unrelated to normal zebrafish ontogeny.
Hyperthyroidism alters craniofacial proportions without altering adult feeding kinematics
Hyperthyroidism results in prominent mandibular prognathism (visible in Fig. 1E; see also7). Surprisingly, these changes in craniofacial and jaw proportions did not result in altered craniofacial kinematics during feeding. Hyperthyroid adults were comparable to euthyroid adults for all measured variables (Figs. 2, 3, and 5) and overlapped almost perfectly with WT fish in the different PCA score plots (Figs. 4 and 6 and Supplementary Fig. S2).
The fact that the upper and lower jaws are differentially affected by hyperthyroidism is suggestive of some degree of developmental independence for these structures. Previous work with cichlid fishes revealed that dermal skull bones are prone to region-specific changes that do not affect the remainder of the skull.24 We see this reflected in the different developmental responses of the upper and lower jaws to altered TH levels. The maxilla and premaxilla of the upper jaw are dermal bones, as are the articular and dentary of the lower jaw (although a portion of the small retroarticular at the posterior end of the mandible forms through endochondral ossification20). The separation of the upper and lower jaws into two developmental modules suggest that evolutionary changes to either structure may not require a corresponding change in their counterpart. Independent changes to these structures, possibly through some of the same pathways on which TH acts, might underlie evolutionary changes in jaw proportions and mouth angle that have occurred in adaptive radiations in other teleosts, including cichlids and damselfishes.25,26
Our analyses of hypothyroid zebrafish suggest that TH is necessary for the onset of adult feeding kinematics. It remains unknown whether larval hyperthyroidism is sufficient to initiate precocious adult feeding, but this possibility may be tested in future studies by examining the development of feeding kinematics in hyperthyroid backgrounds.
Danionella feed as larval and hypothyroid adult zebrafish
Danionella are relatively closely related to zebrafish27,28 and are described as pedomorphic.17,18 We therefore predicted that their feeding kinematics would more closely resemble those of larval as opposed to adult zebrafish. Furthermore, since adult Danionella are so small (9–13 mm SL), we reasoned that the aquatic environment they experience might be more similar to that of larval zebrafish (<10 mm SL). As zebrafish larvae, adult Danionella spp. showed essentially no premaxilla protrusion during a feeding strike (Figs. 2D and 3D). Also as the larvae, Danionella showed little variation in the time at which mouth opening began, although this occurred later in Danionella than in either WT larvae or adult hypothyroid zebrafish (Fig. 5A and Supplementary Fig. S1A). Similar to the older larvae and adult zebrafish of all TH profiles, Danionella showed only a small amount of cranial elevation (Figs. 2C and 3C), suggesting that they do not retain the extensive head lift of extremely young larvae.
The finding that Danionella feeding kinematics resemble those of larval zebrafish in several respects is consistent with this species being a pedomorph, and might suggest that their retention of juvenile characters includes elements of kinematics and behavior, as well as morphology. Our findings are consistent with the hypothesis that a lack of TH signaling or modifications to TH-sensitive pathways may underlie developmental arrest in these species.
Conclusions
We demonstrated that TH plays a critical role in the development of zebrafish feeding kinematics. Hypothyroidism eliminated or reduced many of the changes in feeding kinematics that occur as zebrafish transition from larvae to adults, and feeding in hypothyroid adults resembled that of both larval zebrafish and the closely related pedomorphic genus Danionella. These effects were particularly pronounced in the timing of mouth opening and the maximum amount of hyoid depression and premaxilla protrusion. Further investigation into the roles of TH in regulating feeding kinematics and sculpting craniofacial morphology is likely to be informative in both developmental and evolutionary contexts.
Supplementary Material
Acknowledgments
The authors thank Caroline Nazaire, Aisha Khalid, Nikhita Deshpande, Alexander Aguilar, and Demi Galiando for their assistance in making preliminary kinematic measurements. For valuable discussion and comments, we thank Nicolai Konow, Patricia Hérnandez, Catherine May, and two anonymous reviewers. We thank David Parichy and all members of the Parichy Laboratory, as well as the members of the McMenamin and Cooper Laboratories. We gratefully acknowledge funding sources R00 GM105874 (S.M.), R03 HD91634 (S.M.), and R01 GM111233 (David Parichy).
Authors' Contributions
W.J.C. and C.C. filmed and processed videos of fish strikes. S.M. digitized measurements from strikes, performed statistical analyses, and prepared the article with assistance from W.J.C.
Disclosure Statement
No competing financial interests exist.
References
- 1.Parichy DM, Elizondo MR, Mills MG, Gordon TN, Engeszer RE. Normal table of postembryonic zebrafish development: Staging by externally visible anatomy of the living fish. Dev Dyn 2009;238:2975–3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hernandez L. Intraspecific scaling of feeding mechanics in an ontogenetic series of zebrafish, Danio rerio. J Exp Biol 2000;203:3033–3043 [DOI] [PubMed] [Google Scholar]
- 3.Hernandez LP, Barresi MJF, Devoto SH. Functional morphology and developmental biology of zebrafish: Reciprocal illumination from an unlikely couple. Integr Comp Biol 2002;42:222–231 [DOI] [PubMed] [Google Scholar]
- 4.Yaniv S, Elad D, Holzman R. Suction feeding across fish life stages: Flow dynamics from larvae to adults and implications for prey capture. J Exp Biol 2014;217:3748–3757 [DOI] [PubMed] [Google Scholar]
- 5.McMenamin SK, Parichy DM. Metamorphosis in teleosts. Curr Top Dev Biol 2013;103:127–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staab KL, Hernandez LP. Development of the cypriniform protrusible jaw complex in Danio rerio: Constructional insights for evolution. J Morphol 2010;271:814–825 [DOI] [PubMed] [Google Scholar]
- 7.McMenamin SK, Bain EJ, McCann AE, Patterson LB, Eom DS, Waller ZP, et al. . Thyroid hormone-dependent adult pigment cell lineage and pattern in zebrafish. Science 2014;345:1358–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown DD. The role of thyroid hormone in zebrafish and axolotl development. Proc Natl Acad Sci U S A 1997;94:13011–13016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laudet V. The origins and evolution of vertebrate review metamorphosis. Curr Biol 2011;21:R726–R737 [DOI] [PubMed] [Google Scholar]
- 10.Galton VA. The role of thyroid hormone in amphibian metamorphosis. Trends Endocrinol Metab 1992;3:96–100 [DOI] [PubMed] [Google Scholar]
- 11.Buchholz DR. More similar than you think: Frog metamorphosis as a model of human perinatal endocrinology. Dev Biol 2015;408:1–8 [DOI] [PubMed] [Google Scholar]
- 12.Schreibman M, Scanes CG, Pang PKT. The Endocrinology of Growth, Development and Metabolism in Vertebrates. Academic Press, New York, 1993 [Google Scholar]
- 13.Chang J, Wang M, Gui W, Zhao Y, Yu L, Zhu G. Changes in thyroid hormone levels during zebrafish development. Zoolog Sci 2012;29:181–184 [DOI] [PubMed] [Google Scholar]
- 14.Johnson CK, Voss SR. Salamander paedomorphosis: Linking thyroid hormone to life history and life cycle evolution. Curr Top Dev Biol 2013;103:229–258 [DOI] [PubMed] [Google Scholar]
- 15.Denver RJ, Glennemeier KA, Boorse GC. Endocrinology of complex life cycles: Amphibians. Horm Brain Behav 2002;2:469–513 [Google Scholar]
- 16.Roberts TR. Danionella translucida, a new genus and species of cyprinid fish from Burma, one of the smallest living vertebrates. Environ Biol Fishes 1986;16:231–241 [Google Scholar]
- 17.Britz R, Conway KW. Danionella dracula, an escape from the cypriniform Bauplan via developmental truncation? J Morphol 2015;277:147–166 [DOI] [PubMed] [Google Scholar]
- 18.Britz R, Conway KW, Ruber L. Spectacular morphological novelty in a miniature cyprinid fish, Danionella dracula n. sp. Proc Biol Sci 2009;276:2179–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 Years of image analysis. Nat Methods 2012;9:671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cubbage CC, Mabee PM. Development of the cranium and paired fins in the zebrafish Danio rerio (Ostariophysi, Cyprinidae). J Morphol 1996;229:121–160 [DOI] [PubMed] [Google Scholar]
- 21.JMP (R), Version 12.2. SAS Institute, Inc., Cary, NC, 1989–2007 [Google Scholar]
- 22.Team RDC. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, 2008 [Google Scholar]
- 23.Wainwright PC, McGee MD, Longo SJ, Hernandez LP. Origins, innovations, and diversification of suction feeding in vertebrates. Integr Comp Biol 2015;55:134–145 [DOI] [PubMed] [Google Scholar]
- 24.Le Pabic P, Cooper WJ, Schilling TF. Developmental basis of phenotypic integration in two Lake Malawi cichlids. Evodevo 2016;7:1–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper WJ, Parsons K, McIntyre A, Kern B, McGee-Moore A, Albertson RC. Bentho-pelagic divergence of cichlid feeding architecture was prodigious and consistent during multiple adaptive radiations within African rift-lakes. PLoS One 2010;5:e9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper WJ, Westneat MW. Form and function of damselfish skulls: Rapid and repeated evolution into a limited number of trophic niches. BMC Evol Biol 2009;9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCluskey BM, Postlethwait JH. Phylogeny of zebrafish, a model species, within Danio, a model genus. Mol Biol Evol 2014;32:635–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang KL, Agnew MK, Hirt MV, Sado T, Schneider LM, Freyhof J, et al. . Systematics of the subfamily Danioninae (Teleostei: Cypriniformes: Cyprinidae). Mol Phylogen Evol 2010;57:189–214 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.