Abstract
Objective
To assess the effects of socioeconomic factors on the association between parity and long-term maternal mortality.
Methods
This was a population-based cohort study of mothers with births registered in the Medical Birth Registry of Norway (MBRN) during the period 1967 to 2009. We estimated age-specific (40 to 69 years) cardiovascular and non-cardiovascular mortality ratios by number of births using Cox proportional-hazard models. To assess effect modification by mothers’ attained education we stratified on low (<11 years) and high (≥11 years) educational level. We further evaluated fathers’ mortality by number of births using the same analytical approach.
Results
Mothers with low education had higher mortality (cardiovascular: hazard ratio (HR) 2.62, 95% confidence interval (CI) 2.34–2.93, non-cardiovascular: HR 1.67, 95% CI 1.62–1.73). Among mothers with low education, cardiovascular mortality increased linearly with each additional birth above one, (p-trend=0.02). In contrast, among mothers with high education, cardiovascular mortality declined with added births, (p-trend=0.045). For non-cardiovascular mortality there was no association among mothers with low education, while mortality declined with increasing number of births among mothers with high education, (p-trend<0.01). Father’s mortality showed similar associations with number of births when stratified on maternal education.
Conclusions
Women’s long-term mortality rose with number of births only for cardiovascular causes of death, and only among mothers with low education. Partners of women with low education had similar increasing risk with increasing number of births. Maternal educational level is a strong modifier of the association between parity and long-term mortality.
Introduction
Marriage and having children are associated with longevity.(1) A population-based study of Norwegian women found that mortality for late middle-aged women was highest for the childless and next highest for mothers with one child, compared with mothers having two children. Higher party was found protective.(2) In contrast, a study of mothers from England and Wales found significantly higher mortality among mothers with five or more children.(3) Several studies have reported a J-shaped association between women’s number of children and risk of cardiovascular disease.(4–8) Underlying mechanisms are not established, and competing explanations for the association of cardiovascular mortality and parity, presented in a recent review, range from biological to social.(9) Biological theory suggests that a pregnancy offsets negative physiological changes in the mother that accumulate with increasing parity. Selection bias and inadequate control for negative social factors are the basis of the social explanatory model. Higher parity is associated with early motherhood, low socioeconomic position and less support in later life.(10–12) Controlling for these factors is challenging and makes interpretation of results difficult. These diverging explanations suggest different implications for clinical screening and public health.
We used national registries in Norway to assess effect modification by attained education (as a measure of socioeconomic differences) on the association between parity and long-term cardiovascular and non-cardiovascular mortality. Maternal mortality was the main focus, but we also assessed paternal mortality by number of births to further explore the effects of social compared with biological factors underlying the association of maternal mortality by parity.
Materials and Methods
We conducted a population-based cohort study of mothers with births registered in the Medical Birth Registry of Norway. The Medical Birth Registry of Norway has recorded delivery data since 1967, with registration legally mandated for all births starting at 16 weeks of gestation. The registry includes data on demographics, maternal diseases, pregnancy and delivery, as well as infant outcomes. Our study covered the period from 1967 to 2009, the most recent year for which mortality data were available. Causes of death came from the Norwegian population-based cause-of-death-registry. Cardiovascular causes of death were defined as ischemic heart diseases, I20-I25 (ICD-10), 410–414 (ICD-8 and ICD-9) and cerebrovascular diseases (stroke), I60-I69 (ICD-10), 430–438 (ICD-8 and ICD-9). Non-cardiovascular causes of death were defined as all other than cardiovascular. The Medical Birth Registry of Norway is routinely matched with the Central Person Register, which provides every live-born infant in Norway with a unique national identification number. Mothers (and fathers) are also registered with their unique identification numbers, which enables all births to a given mother to be linked in sibling files with the mother as the observation unit. The data were also linked to the national education database for information on maternal educational level. The data were almost complete, with 0.3 per cent missing information on education.
The main analyses included mothers with a complete birth record within the registry period. In sub-analyses we excluded women with plural pregnancies, preeclampsia and pre-existing chronic diseases (cardiac, renal, hypertensive and diabetic disorders). In further sensitivity analyses, we expanded the sample to include mothers who delivered after 1967 but also had at least one birth before 1967. This provided additional numbers for mortality analysis, at the cost of slightly reduced quality of parity information (based in part on self-report).
All births registered in the Medical Birth Registry of Norway were included, of which 0.2% were late miscarriages (less than 22 weeks). To make the cohort more homogeneous, we excluded women born outside Norway. Further, in order to allow enough time for women to complete their reproduction, we included only women who had their first birth before 1991 (providing 19 additional years of follow up). About 90% of mothers with five children completed their reproduction within 19 years.
To assess the effects of socioeconomic factors, we evaluated the association between mortality and parity in strata of maternal education: low education (<11 years) and high education (≥11 years). Education in Norway is divided in primary school (7 years), lower secondary school (3 years), upper secondary school (3 years) and higher education. The first 10 years are mandatory. Analyses with three levels of education (<11, 11–14 and ≥15 years) gave the same results for the two highest levels and justified the dichotomization. We did analyses overall and in educational strata. The stratified analyses were done twice: First, mothers with two births were the reference group within each education stratum, and then mothers with two births and high education were used as the reference for both education groups. Similarly, we studied fathers’ cardiovascular and non-cardiovascular mortality risk in relation to number of births, stratified on the two levels of mothers’ education. We included only mothers with the same father for all her children.
We used Cox proportional hazard regression models (SPSS for Windows, version 20–22, www.spss.com) to estimate hazard ratios of age-specific (40–69 years) cardiovascular and non-cardiovascular mortality by number of births. The underlying time variable in the Cox model was the mothers’ (or fathers’) birth year. In order to handle effect differences by calendar time, we adjusted for birth year of the mother (or father) using a linear term. Due to small numbers at the highest parities, we pooled women with five or more births. We further used the regression models to evaluate linear trends in the association between number of births above one and mortality. To determine whether maternal education modified the associations between parity and long-term mortality, we carried out interaction analyses using a multiplicative model.
The study was approved by the internal review board of the Medical Birth Registry of Norway and by the regional ethics committee, REK Vest, Norway (2009/1868).
Results
A total of 527,964 mothers with 1,258,075 births were eligible for analysis (Table 1). Fifteen per cent of mothers had only one birth, 46% had two, 29% had three, 8% had four, and 3% had five or more. The median year of birth for mothers was 1954 (range 1916 to 1976). In total, 16,664 (3%) mothers died between the ages of 40 and 69 years during the follow-up period, with 53 years as median age at death (Inter Quartile Range (IQR): 47–58 years). The median follow-up time from birth of the mother to death (or censoring) was 54 years. The distributions of deaths and parities in the two strata of education are listed in Table 1.
Table 1.
Baseline characteristics of the mothers by education in years.
| TOTAL (%) | EDUCATION
|
|||
|---|---|---|---|---|
| <11 YEARS (%) | ≥11 YEARS (%) | <11/≥11 * RATIO | ||
|
|
|
|
|
|
| NUMBER OF MOTHERS | 527964 (100) | 283912 (53.9) | 242402 (46.1) | 1.2 |
| TOTAL NUMBER OF DEATHS † | 16664 (100) | 11887 (71.6) | 4710 (28.4) | 2.5 |
| CARDIOVASCULAR DEATHS † ‡ | 1924 (100) | 1523 (79.6) | 391 (20.4) | 3.9 |
| NON-CARDIOVASCULAR DEATHS † § | 14740 (100) | 10364 (70.6) | 4319 (29.4) | 2.4 |
| BIRTHS | 1 258075 (100) | 669942 (53.3) | 584653 (46.5) | 1.1 |
|
MOTHERS BY NUMBER OF BIRTHS |
||||
| 1 | 77241 (14.6) | 45190 (15.9) | 31419 (13.0) | 1.2 |
| 2 | 244323 (46.3) | 132112 (46.5) | 111698 (46.1) | 1.0 |
| 3 | 151464 (28.7) | 76766 (27.0) | 74397 (30.7) | 0.9 |
| 4 | 41994 (8.0) | 22347 (7.9) | 19513 (8.0) | 1.0 |
| ≥5 | 12942 (2.5) | 7497 (2.6) | 5375 (2.2) | 1.2 |
|
|
|
|
|
|
| Total | 527964 (100) | 283912 (100) | 242402 (100) | 1.0 |
|
| ||||
| MISSING INFORMATION ON EDUCATION: N=1 650 (0.3%) | ||||
Data are defined; <11/≥11 RATIO: The ratio between low and high education mothers, numbers used in calculations are percentages in each row.
Data are presented for age specific deaths, 40–69 years.
Data are defined; cardiovascular causes of death: Ischemic heart diseases and cerebrovascular diseases combined (see material and method).
Data are defined; non-cardiovascular causes of death: All other causes than cardiovascular causes (see material and method).
Overall, 54% of mothers had less than 11 years of education (low) and 46% had 11 or more years (high). Mothers with low education had a substantially higher risk of mortality, independent of their number of births. The hazard ratio for cardiovascular mortality risk among low- compared with high-education mothers was 2.62 (95% confidence interval (CI) 2.34–2.93). This excess risk was lower for non-cardiovascular mortality, but still elevated, HR 1.67 (1.62–1.73). The excess mortality with low education has increased over time. The hazard ratio for cardiovascular mortality was 2.42 (2.10–2.78) for those born before 1950, versus 3.02 (2.51–3.63) for those born in 1950 or later.
Consistent with the literature (2, 13, 14), we found a high mortality risk in women with only one birth in their lifetimes, relative women with two births. This high risk was present for cardiovascular and non-cardiovascular mortality, and was significantly higher among low-education mothers (Figures 1 and 2).
Figure 1.
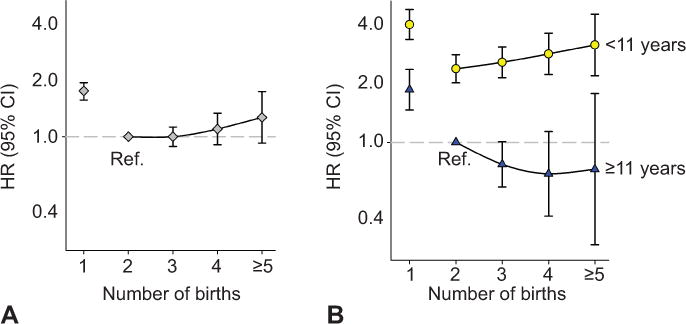
Maternal cardiovascular mortality by number of births, overall (A) and stratified by the mother’s education in years (B). Norwegian-born women, first birth after 1966 and before 1991. Adjusted for the mothers’ birth year. HR, hazard ratio; CI, confidence interval; ref, reference.
Norwegian born women. 1st birth >1966 and <1991. Adjusted mothers’ birth year.
Figure 2.
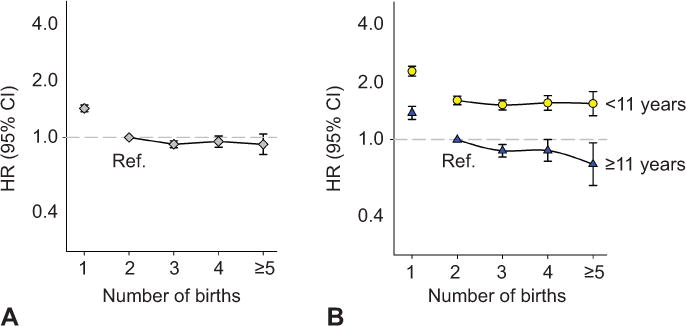
Maternal noncardiovascular mortality by number of births, overall (A) and stratified by the mother’s education in years (B). Norwegian-born women, first birth after 1966 and before 1991. Adjusted for the mothers’ birth year. HR, hazard ratio; CI, confidence interval; ref, reference.
Norwegian born women. 1st birth >1966 and <1991. Adjusted mothers’ birth year.
Overall, the hazard ratios for cardiovascular mortality by number of births followed a slightly J-shaped curve (Figure 1A). The trend with increasing parity was non-significant (p-trend=0.16). Non-cardiovascular mortality declined with increasing number of births (p-trend<0.01) (Figure 2A).
We found significant interactions between number of births and educational level for both cardiovascular (p<0.01) and non-cardiovascular mortality (p=0.02) (multiplicative model, mothers with one birth excluded). Because of these significant interactions, we explored the pattern of parity and mortality in mothers with different levels of education. Using a single reference group for both education strata (mothers with two births and eleven or more years of education), the cardiovascular hazard ratio among low-education mothers increased linearly with number of births from two to five or more, (hazard ratio 2.37 (2.01–2.80) to 3.14 (2.19–4.51), p-trend=0.02). In contrast, the cardiovascular mortality trend declined with number of births among high-education mothers (p-trend=0.045) (Table 2, Figure 1B). For causes of death other than cardiovascular, there was no association of number of births above one with mortality in low-educated mothers (p-trend=0.11), while among high-educated mothers the risk declined with parity (p-trend<0.01) (Table 2, Figure 2B).
Table 2.
Maternal cardiovascular and non-cardiovascular mortality, age 40 to 69 years, by number of births stratified by education.
| CARDIOVASCULAR CAUSES *
| |||||
|---|---|---|---|---|---|
| EDUCATION | NUMBER OF BIRTHS | NUMBER OF MOTHERS | MATERNAL DEATHS (PER 1000) | HR (95% CI) †
|
HR (95% CI) †
|
| ref.: mothers with 2 births | ref.: mothers with 2 births and 11+ years of education | ||||
| LOW (<11 YEARS) | 1 | 45190 | 429 (9.5) | 1.7 (1.5–1.9) | 4.0 (3.3–4.8) |
| 2 | 132112 | 617 (4.7) | 1.0 (ref.) | 2.4 (2.0–2.8) | |
| 3 | 76766 | 340 (4.4) | 1.1 (0.94–1.2) | 2.6 (2.1–3.1) | |
| 4 | 22347 | 102 (4.6) | 1.2 (0.96–1.5) | 2.8 (2.2–3.6) | |
| ≥5 | 7497 | 35 (4.7) | 1.3 (0.94–1.9) | 3.1 (2.2–4.5) | |
| p-trend=0.02 ‡ | |||||
|
| |||||
| TOTAL | 283 912 | 1523 (5.4) | 2.6 (2.3–2.9) | ||
|
| |||||
| HIGH (≥11 YEARS) | 1 | 31419 | 111 (3.5) | 1.8 (1.4–2.3) | 1.9 (1.5–2.4) |
| 2 | 111698 | 181 (1.6) | 1.0 (ref.) | 1.0 (ref.) | |
| 3 | 74397 | 77 (1.0) | 0.78 (0.60–1.02) | 0.77 (0.59–1.01) | |
| 4 | 19513 | 17 (0.9) | 0.70 (0.42–1.2) | 0.69 (0.42–1.1) | |
| ≥5 | 5375 | 5 (0.9) | 0.74 (0.30–1.8) | 0.73 (0.30–1.8) | |
| p-trend=0.045 ‡ | |||||
|
| |||||
| TOTAL | 242 402 | 391 (1.6) | 1.0 (ref.) | ||
|
| |||||
| NON-CARDIOVASCULAR CAUSES * | |||||
|
| |||||
| LOW (<11 YEARS) | 1 | 44 761 | 2 533 (56.6) | 1.4 (1.4–1.5) | 2.3 (2.2–2.4) |
| 2 | 131 495 | 4 678 (35.6) | 1.0 (ref.) | 1.6 (1.5–1.7) | |
| 3 | 76 426 | 2 308 (30.2) | 0.94 (0.90–0.99) | 1.5 (1.4–1.6) | |
| 4 | 22 245 | 646 (29.0) | 0.96 (0.89–1.1) | 1.6 (1.4–1.7) | |
| ≥5 | 7 462 | 199 (26.7) | 0.95 (0.83–1.1) | 1.6 (1.3–1.8) | |
| p-trend=0.11 ‡ | |||||
|
| |||||
| TOTAL | 282 389 | 10 364 (36.7) | 1.7 (1.6–1.7) | ||
|
| |||||
| HIGH (≥11 YEARS) | 1 | 31 308 | 903 (28.4) | 1.4 (1.3–1.5) | 1.4 (1.3–1.5) |
| 2 | 111 517 | 2 075 (18.6) | 1.0 (ref.) | 1.0 (ref.) | |
| 3 | 74 320 | 1 026 (13.8) | 0.89 (0.83–0.96) | 0.88 (0.81–0.94) | |
| 4 | 19 496 | 255 (13.1) | 0.90 (0.79–1.02) | 0.88 (0.77–1.00) | |
| ≥5 | 5 370 | 60 (11.2) | 0.76 (0.59–0.98) | 0.75 (0.58–0.96) | |
| p-trend<0.01 ‡ | |||||
|
| |||||
| TOTAL | 242 011 | 4 319 (17.8) | 1.0 (ref.) | ||
Data are defined; Cardiovascular causes of death: Ischemic heart diseases and cerebrovascular disease combined. Non-cardiovascular causes of death: All other causes than cardiovascular causes (see material and method).
Data are presented with ratios; HR=Hazard Ratio with 95% confidence interval, adjusted for mother’s birth year.
Data are assessed for linearity; P-trend: linear association between maternal mortality and increasing number of births exceeding one.
Sensitivity analyses in which we excluded plural pregnancies and pre-existing chronic diseases (cardiac, renal, hypertensive and diabetic disorders) did not alter the results. Excluding women with pre-eclampsia increased differences between the two education strata and strengthened the associations. When we added 143,739 mothers (14,414 deaths) with self-reported births before 1967, the trends were confirmed.
In order to explore the role of unmeasured social confounding, we assessed fathers’ cardiovascular and non-cardiovascular mortality rate by number of children (Table 3). As for the mothers, there were strong interactions between number of children and mothers’ education on fathers’ cardiovascular and non-cardiovascular mortality (both interactions p<0.01, multiplicative model, fathers with one child excluded). Fathers living with a mother with low education had higher risk of cardiovascular mortality with higher number of births (p-trend=0.03), while fathers with a mother with high education had decreasing risk (p-trend<0.01) (Figure 3A). Mortality from all other causes decreased with parity in both groups of fathers (p-trends<0.01) (Figure 3B).
Table 3.
Paternal cardiovascular and non-cardiovascular mortality, age 40 to 69 years, by number of births stratified by mothers education.
|
|
CARDIOVASCULAR CAUSES *
|
NON-CARDIOVASCULAR CAUSES *
|
|||||
|---|---|---|---|---|---|---|---|
| EDUCATION (MOTHERS) | NUMBER OF BIRTHS | NUMBER OF FATHERS | PATERNAL DEATHS (PER 1000) | HR (95% CI) † | NUMBER OF FATHERS | PATERNAL DEATHS (PER 1000) | HR (95% CI) † |
| LOW (<11 YEARS) | 1 | 39758 | 1250 (31.4) | 1.8 (1.6–1.9) | 38508 | 3230 (83.9) | 1.8 (1.7–1.9) |
| 2 | 113412 | 2263 (20.0) | 1.4 (1.3–1.5) | 111148 | 6242 (56.2) | 1.3 (1.3–1.4) | |
| 3 | 58113 | 1104 (19.0) | 1.4 (1.3–1.5) | 57009 | 2818 (49.4) | 1.2 (1.1–1.3) | |
| 4 | 14482 | 311 (21.5) | 1.5 (1.4–1.8) | 14171 | 748 (52.8) | 1.3 (1.2–1.4) | |
| ≥5 | 4280 | 99 (23.1) | 1.7 (1.4–2.0) | 4181 | 191 (45.7) | 1.10 (0.95–1.3) | |
| p-trend=0.03 ‡ | p-trend<0.01 ‡ | ||||||
|
|
|
|
|||||
| TOTAL | 230045 | 5027 (21.9) | 225017 | 13229 (58.8) | |||
|
| |||||||
| HIGH (≥11YEARS) | 1 | 27035 | 497 (18.4) | 1.3 (1.2–1.5) | 26538 | 1500 (56.5) | 1.4 (1.3–1.5) |
| 2 | 95373 | 1092 (11.4) | 1.0 (ref.) § | 94281 | 3347 (35.5) | 1.0 (ref.) § | |
| 3 | 58220 | 457 (7.8) | 0.79 (0.71–0.88) | 57763 | 1569 (27.2) | 0.86 (0.81–0.91) | |
| 4 | 13589 | 115 (8.5) | 0.86 (0.71–1.04) | 13474 | 348 (25.8) | 0.82 (0.73–0.91) | |
| ≥5 | 3426 | 28 (8.2) | 0.75 (0.52–1.09) | 3398 | 81 (23.8) | 0.70 (0.56–0.87) | |
| p-trend<0.01 ‡ | p-trend<0.01 ‡ | ||||||
|
|
|
|
|||||
| TOTAL | 197643 | 2189 (11.1) | 195454 | 6845 (35.0) | |||
Data are defined; Cardiovascular causes of death: Ischemic heart diseases and cerebrovascular disease combined. Non-cardiovascular causes of death: All other causes than cardiovascular causes (see material and method).
Data are presented with ratios; HR=Hazard Ratio with 95% confidence interval, adjusted for father’s birth year.
Data are assessed for linearity; P-trend: linear association between paternal mortality and increasing number of births exceeding one.
Data are defined; Reference (ref.): fathers living with a mother with two births and 11+ years of education.
Figure 3.
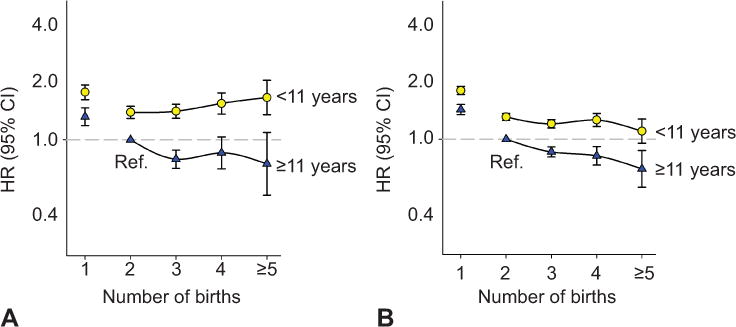
Paternal mortality by number of births stratified by the mother’s education in years. Cardiovascular (A), noncardiovascular (B). Norwegian-born fathers and mothers, first birth after 1966 and before 1991 with same paternity. Adjusted for fathers’ birth year. HR, hazard ratio; CI, confidence interval; ref, reference.
Norwegian born fathers and mothers. 1st birth >1966 and <1991. Same paternity. Adjusted fathers’ birth year.
Discussion
Using linked data from the Medical Birth Registry of Norway, the cause-of-death register, and the national education database, we found that the association between number of births and long-term mortality was significantly modified by the mothers’ level of education. Mortality increased with higher parity only among low-educated mothers, and only for cardiovascular causes of death.
Educational level and occupation are key indicators of socioeconomic status, and these influence mortality risk in later life.(15) The Norwegian welfare state allows women to raise a family without compromising their own education and career.(16) However, high-parity mothers are more likely to have low education. Most previous studies on mortality by parity tend to be biased by an even more skewed distribution of low socioeconomic status women at higher parities, contributing to higher mortality at higher parity. In Norway, the overall ratio for cardiovascular death was 3.9 between low- and high-educated mothers (Table 1). Given the interaction between education and parity on cardiovascular mortality, adjusting for education would not be warranted.
The sum of a woman’s physiological alterations in pregnancy and postpartum resolution may depend on pre-existing health status, complications in pregnancy and lifestyle factors. Cardiovascular changes in pregnancy resemble changes associated with physical training.(17) Weight retention(18) and long-term declines in high-density lipoprotein (HDL) (19) after pregnancy adversely affect long-term risk of cardiovascular disease. Pre-pregnant high Body-Mass-Index (BMI) is associated with low education, and is inversely correlated to physical activity.(20) Low-education mothers are more likely to experience a complicated pregnancy.(21, 22) Women with gestational diabetes have a 7-fold increased risk of later type-2 diabetes.(23) Sattar and Greer suggest that pregnancy act as a stress test to reveal subclinical vascular or metabolic disease.(24)
The so-called “weathering” hypothesis suggests that an exposure can have stronger effects among socially vulnerable subgroups.(25) While we cannot rule out the possibility of a biological interaction in this group of disadvantaged mothers, selection of higher-risk women into higher parities seems the most likely explanation for the higher cardiovascular mortality among women with many births. The fact that paternal cardiovascular mortality followed the same pattern with parity supports the role of selection by social factors. Any association of father’s cardiovascular mortality risk with number of births would be unrelated to the physiologic events of pregnancy.
We found protective effects of multi-parity on mortality in high-education women. This could be consistent with a beneficial physiologic effect of pregnancy that is outweighed in low-education women by other factors. However, the similar findings among fathers (Figure 3) suggests once again that selection factors are at play. Women with higher education might choose a family size depending on their own health and resources, and more children may stimulate an even more beneficial lifestyle in advantaged mothers. In later life, children of educated parents are more likely to provide support.(12)
Consistent with the literature (2, 26), we found that mothers with only one birth, compared to mothers with two births, had higher mortality, especially from cardiovascular causes. The explanation for this association is not fully established, but may reflect reduced fertility underlying conditions predisposing to cardiovascular disease.(14, 27) However, the doubling of this risk among mothers with low education again indicates effect modification by social factors.
Post hoc evaluation of mothers with four or more births gave, among low relative high education mothers, a 3.1 fold excess risk of cardiovascular death, HR 4.1 (2.6–6.5). This result highlights the importance of effect modification by social factors on the association of multi-parity and later life mortality.
The strengths of this study are the large population-based material and extended follow-up. Registration of parity, education, and mortality was prospective and virtually complete, with educational level missing for only 0.3% of mothers. The Norwegian setting allows women to pursue education and career without sacrificing childbearing and rearing. Despite the beneficial setting in Norway the results have external generalizability. Given the strength of the associations with maternal education even in Norway, we expect that the patterns would be even more extreme elsewhere. Weaknesses of the study are the lack of data on cardiovascular risk factors such as smoking, alcohol intake, and BMI. There are also relatively few deaths in the group of mothers with higher education, reflected in the wide confidence intervals (especially for cardiovascular mortality).
Maternal educational level is a strong modifier of the association between parity and long-term mortality. Our results suggest that the higher cardiovascular mortality associated with higher parity is caused by accumulation of negative lifestyle factors in mothers with low socioeconomic status.
Acknowledgments
The authors thank the Medical Birth Registry of Norway for providing the data for this analysis.
Funding
Supported by the Norwegian Research Council through the University of Bergen, and in part by the Intramural Program of the US National Institute of Environmental Health Sciences (NIEHS), NIH. The Norwegian Research Council, University of Bergen, and NIEHS had no role in the design and conduct of the study; in the collection, analysis, the interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
Contributor Information
Frode Halland, Department of Global Public Health and Primary Care, University of Bergen, Bergen, Norway; Department of Obstetrics & Gynaecology, Haukeland University Hospital, Bergen, Norway.
Nils-Halvdan Morken, Department of Global Public Health and Primary Care, University of Bergen, Bergen, Norway; Department of Clinical Sciences, University of Bergen; Department of Obstetrics & Gynaecology, Haukeland University Hospital, Bergen, Norway.
Lisa A DeRoo, Department of Global Public Health and Primary Care, University of Bergen, Bergen, Norway; Epidemiology Branch, NIEHS/NIH, Durham, North Carolina, USA.
Kari Klungsøyr, Department of Global Public Health and Primary Care, University of Bergen, Bergen, Norway; Medical Birth Registry of Norway; Norwegian Institute of Public Health, Bergen, Norway.
Allen J Wilcox, Epidemiology Branch, NIEHS/NIH, Durham, North Carolina, USA.
Rolv Skjærven, Department of Global Public Health and Primary Care, University of Bergen, Bergen, Norway; Medical Birth Registry of Norway; Norwegian Institute of Public Health, Bergen, Norway.
References
- 1.Roos E, Burstrom B, Saastamoinen P, Lahelma E. A comparative study of the patterning of women’s health by family status and employment status in Finland and Sweden. Social science & medicine. 2005 Jun;60(11):2443–51. doi: 10.1016/j.socscimed.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 2.Grundy E, Kravdal O. Reproductive history and mortality in late middle age among Norwegian men and women. American journal of epidemiology. 2008 Feb 1;167(3):271–9. doi: 10.1093/aje/kwm295. [DOI] [PubMed] [Google Scholar]
- 3.Grundy E, Tomassini C. Fertility history and health in later life: a record linkage study in England and Wales. Social science & medicine. 2005 Jul;61(1):217–28. doi: 10.1016/j.socscimed.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 4.Green A, Beral V, Moser K. Mortality in women in relation to their childbearing history. BMJ. 1988 Aug 6;297(6645):391–5. doi: 10.1136/bmj.297.6645.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ness RB, Harris T, Cobb J, Flegal KM, Kelsey JL, Balanger A, et al. Number of pregnancies and the subsequent risk of cardiovascular disease. N Engl J Med. 1993 May 27;328(21):1528–33. doi: 10.1056/NEJM199305273282104. [DOI] [PubMed] [Google Scholar]
- 6.Hardy R, Lawlor DA, Black S, Wadsworth ME, Kuh D. Number of children and coronary heart disease risk factors in men and women from a British birth cohort. BJOG. 2007 Jun;114(6):721–30. doi: 10.1111/j.1471-0528.2007.01324.x. [DOI] [PubMed] [Google Scholar]
- 7.Lawlor DA, Emberson JR, Ebrahim S, Whincup PH, Wannamethee SG, Walker M, et al. Is the association between parity and coronary heart disease due to biological effects of pregnancy or adverse lifestyle risk factors associated with child-rearing? Findings from the British Women’s Heart and Health Study and the British Regional Heart Study. Circulation. 2003 Mar 11;107(9):1260–4. doi: 10.1161/01.cir.0000053441.43495.1a. [DOI] [PubMed] [Google Scholar]
- 8.Parikh NI, Cnattingius S, Dickman PW, Mittleman MA, Ludvigsson JF, Ingelsson E. Parity and risk of later-life maternal cardiovascular disease. American heart journal. 2010 Feb;159(2):215–21 e6. doi: 10.1016/j.ahj.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Rich-Edwards JW, Fraser A, Lawlor DA, Catov JM. Pregnancy Characteristics and Women’s Future Cardiovascular Health: An Underused Opportunity to Improve Women’s Health? Epidemiol Rev. 2013 Sep 11; doi: 10.1093/epirev/mxt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maughan B, Lindelow M. Secular change in psychosocial risks: the case of teenage motherhood. Psychological medicine. 1997 Sep;27(5):1129–44. doi: 10.1017/s0033291797005576. [DOI] [PubMed] [Google Scholar]
- 11.Lund E, Arnesen E, Borgan JK. Pattern of childbearing and mortality in married women–a national prospective study from Norway. Journal of epidemiology and community health. 1990 Sep;44(3):237–40. doi: 10.1136/jech.44.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serbin LA, Karp J. The intergenerational transfer of psychosocial risk: mediators of vulnerability and resilience. Annual review of psychology. 2004;55:333–63. doi: 10.1146/annurev.psych.54.101601.145228. [DOI] [PubMed] [Google Scholar]
- 13.Grundy E, Kravdal O. Fertility history and cause-specific mortality: a register-based analysis of complete cohorts of Norwegian women and men. Soc Sci Med. 2010 Jun;70(11):1847–57. doi: 10.1016/j.socscimed.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Skjaerven R, Wilcox AJ, Klungsoyr K, Irgens LM, Vikse BE, Vatten LJ, et al. Cardiovascular mortality after pre-eclampsia in one child mothers: prospective, population based cohort study. BMJ. 2012;345:e7677. doi: 10.1136/bmj.e7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartley M. Health inequality: an introduction to theories, concepts, and methods. Cambridge: Polity Press; 2004. [Google Scholar]
- 16.Rindfuss RR, Guilkey DK, Morgan SP, Kravdal O. Child-care availability and fertility in Norway. Population and development review. 2010;36(4):725–48. doi: 10.1111/j.1728-4457.2010.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter S, Robson SC. Adaptation of the maternal heart in pregnancy. British heart journal. 1992 Dec;68(6):540–3. doi: 10.1136/hrt.68.12.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gore SA, Brown DM, West DS. The role of postpartum weight retention in obesity among women: a review of the evidence. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2003 Oct;26(2):149–59. doi: 10.1207/S15324796ABM2602_07. [DOI] [PubMed] [Google Scholar]
- 19.Gunderson EP, Lewis CE, Murtaugh MA, Quesenberry CP, Smith West D, Sidney S. Long-term plasma lipid changes associated with a first birth: the Coronary Artery Risk Development in Young Adults study. American journal of epidemiology. 2004 Jun 1;159(11):1028–39. doi: 10.1093/aje/kwh146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorbye LM, Klungsoyr K, Samdal O, Owe KM, Morken NH. Pre-pregnant body mass index and recreational physical activity: effects on perinatal mortality in a prospective pregnancy cohort. BJOG : an international journal of obstetrics and gynaecology. 2015 Sep;122(10):1322–30. doi: 10.1111/1471-0528.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matijasevich A, Victora CG, Lawlor DA, Golding J, Menezes AM, Araujo CL, et al. Association of socioeconomic position with maternal pregnancy and infant health outcomes in birth cohort studies from Brazil and the UK. Journal of epidemiology and community health. 2012 Feb;66(2):127–35. doi: 10.1136/jech.2010.108605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savitz DA, Kaufman JS, Dole N, Siega-Riz AM, Thorp JM, Jr, Kaczor DT. Poverty, education, race, and pregnancy outcome. Ethnicity & disease. 2004 Summer;14(3):322–9. [PubMed] [Google Scholar]
- 23.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009 May 23;373(9677):1773–9. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 24.Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ. 2002 Jul 20;325(7356):157–60. doi: 10.1136/bmj.325.7356.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geronimus AT. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethnicity & disease. 1992 Summer;2(3):207–21. [PubMed] [Google Scholar]
- 26.Dior UP, Hochner H, Friedlander Y, Calderon-Margalit R, Jaffe D, Burger A, et al. Association between number of children and mortality of mothers: results of a 37-year follow-up study. Annals of epidemiology. 2013 Jan;23(1):13–8. doi: 10.1016/j.annepidem.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parikh NI, Cnattingius S, Mittleman MA, Ludvigsson JF, Ingelsson E. Subfertility and risk of later life maternal cardiovascular disease. Human reproduction. 2012 Feb;27(2):568–75. doi: 10.1093/humrep/der400. [DOI] [PMC free article] [PubMed] [Google Scholar]


