Abstract
The hippocampus has been consistently associated with episodic simulation (i.e., the mental construction of a possible future episode). In a recent study, we identified an anterior-posterior temporal dissociation within the hippocampus during simulation. Specifically, transient simulation-related activity occurred in relatively posterior portions of the hippocampus and sustained activity occurred in anterior portions. In line with previous theoretical proposals of hippocampal function during simulation, the posterior hippocampal activity was interpreted as reflecting a transient retrieval process for the episodic details necessary to construct an episode. In contrast, the sustained anterior hippocampal activity was interpreted as reflecting the continual recruitment of encoding and/or relational processing associated with a simulation. In the present study, we provide a direct test of these interpretations by conducting a subsequent memory analysis of our previously published data to assess whether successful encoding during episodic simulation is associated with the anterior hippocampus. Analyses revealed a subsequent memory effect (i.e., later remembered > later forgotten simulations) in the anterior hippocampus. The subsequent memory effect was transient and not sustained. Taken together, the current findings provide further support for a component process model of hippocampal function during simulation. That is, unique regions of the hippocampus support dissociable processes during simulation, which include the transient retrieval of episodic information, the sustained binding of such information into a coherent episode, and the transient encoding of that episode for later retrieval.
Keywords: episodic memory, subsequent memory, timecourse, medial temporal lobe, fMRI
Episodic memory refers to encoding, storage, and retrieval processes that give rise to our ability to recall and mentally re-experience personal past events. Episodic simulation refers to our ability to imagine and mentally pre-experience personal future events (e.g., Schacter, Addis, & Buckner, 2008). The constructive episodic simulation hypothesis (Schacter & Addis, 2007) states that episodic memory retrieval supports episodic simulation by allowing for the flexible retrieval and recombination of episodic information (e.g., people, places and objects that comprise an episode) into novel events that might occur in the future. Specifically, whereas episodic memory entails the re-construction of a previously experienced episode, episodic simulation entails the novel construction of a future event using retrieved and recombined episodic details. In support of this hypothesis, there is now a wealth of neuroimaging evidence to indicate that both episodic memory and episodic simulation are associated with a core network of brain regions, which includes the hippocampus, parahippocampal cortex, medial prefrontal cortex, and lateral and medial parietal cortex (for a meta-analysis, see Benoit & Schacter, 2015). This evidence of overlapping neural activity has been used to argue that both episodic memory and episodic simulation are supported by similar constructive processes (e.g., Buckner & Carroll, 2007; Hassabis & Maguire, 2007; Schacter, Addis, & Buckner, 2007; Schacter, Addis, Hassabis, Martin, Spreng, & Szpunar, 2012; Schacter, Benoit, & Szpunar, 2017).
Recent studies have begun to identify the specific processes supported by individual core network regions, particularly the hippocampus (e.g., Addis, Cheng, Roberts & Schacter, 2011; Addis & Schacter, 2008; Gaesser, Spreng, McLelland, Addis, & Schacter, 2013; Martin, Schacter, Corballis, & Addis, 2011; Palombo, Hayes, Peterson, Keane, & Verfaellie, 2016; Thakral, Benoit, & Schacter, 2017a; Zeidman, Mullally, & Maguire, 2016; for reviews and discussion, see Addis & Schacter, 2012; Moscovitch, Cabeza, Winocur, & Nadel, 2016; Roberts, Schacter, & Addis, 2017; Schacter, Addis, Szpunar, & 2017; Sheldon & Levine, 2016; Zeidman & Maguire, 2016). Based on some of these observations, Addis and Schacter (2012) proposed a component process model of hippocampal function during episodic simulation. They argued that simulation can be broken down into three component processes. First, episodic details must be retrieved in order to build a given simulation. Second, the retrieved details must be recombined into a coherent scenario with spatiotemporal context. Third, if a simulation is sufficiently important to guide future behavior, a simulation needs to be encoded into memory. According to Addis and Schacter (2012) distinct anterior and posterior hippocampal regions support these three processes (see also, Schacter et al., 2017). The posterior hippocampus supports access to previously experienced details. In contrast, the anterior hippocampus supports both the relational/recombination processing of episodic information into a coherent scenario and the encoding of the episode for later use.
Findings from several studies have provided support for the component process model of hippocampal function during simulation (for a review of early findings, see Addis & Schacter, 2012; see also, Lepage, Habib, and Tulving (1998) and Schacter and Wagner (1999) for discussion of evidence implicating the posterior hippocampus in retrieval as opposed to encoding). In the study of Addis and Schacter (2008), for example, hippocampal activity was examined as a function of the subjective vividness/detail for both past and future events. Activity in the posterior hippocampus was insensitive to the amount of vividness/detail for both past and future events. In contrast, anterior hippocampal activity increased as a function of vividness/detail for only future events. Under the assumption that relational/recombination processes are recruited to a greater extent for future relative to past events together with the fact that such processes likely scale with the amount of detail, the anterior hippocampal activity was taken to reflect relational/recombinatorial processes (see also, Addis et al., 2011; Weiler, Suchan & Daum 2010a). In contrast, the posterior hippocampal activity, which did not differ as a function of temporal distance, was interpreted as reflecting a common retrieval/access process recruited for both past and future events (see also, Weiler et al., 2010b).
Since the initial review of Addis and Schacter (2012), more recent data have also lent support to the component process model (for a recent review of hippocampal findings, see Schacter et al., 2017). For example, in the study of Gaesser et al., (2013) posterior hippocampal activity was greater when participants had to construct an episode containing details across disparate memories (i.e., the imagine condition) relative to an episode containing details previously simulated (i.e., the re-imagine condition). Events imagined for the first time require greater retrieval of the cued details in order to integrate them into a coherent scenario, whereas this retrieval process would have already been recruited for re-imagined events. It should be noted that this posterior hippocampal activity was observed even after controlling for differences in novelty across the two conditions. Of particular interest, and replicating earlier findings (Martin et al., 2011), a dissociable encoding effect was observed in the anterior hippocampus (i.e., later remembered > later forgotten simulations). These findings lend additional support to the component process model of Addis and Schacter (2012). Specifically, the posterior hippocampus is associated with the access to details during simulation. In contrast, encoding and relational/recombination processes are associated with the anterior hippocampus.
Directly relevant to the current study, we recently identified an anterior-posterior dissociation within the hippocampus during simulation based on the respective timecourses of activation within each of these regions (Thakral et al., 2017a). This investigation was motivated by two prior episodic memory studies (Vilberg & Rugg, 2012, 2014). Vilberg and Rugg (2012, 2014) dissociated the brain regions associated with transient memory effects (i.e., those involved in the initial reinstatement of retrieved episodic details) and sustained memory effects (i.e., those involved in the maintenance of episodic information). Both studies revealed transient episodic memory effects in the posterior hippocampus. Based on these findings and together with the proposal of Addis and Schacter (2012), we assessed whether the posterior hippocampus would demonstrate a transient profile during simulation, as predicted by the idea that it supports the initial access/retrieval of information during simulation.
In the study of Thakral et al. (2017a), participants imagined future events in response to place, person, and object cues. Cues were presented for 5 seconds followed by a variable delay fixation period for 8–12 seconds. Participants were instructed to continually simulate the future event throughout the delay period. Transient neural activity was defined as activity specific to the cue period and sustained neural activity was defined as activity associated with the delay period. In line with our prediction and replicating the episodic memory findings of Vilberg and Rugg (2012, 2014), transient activity was identified in the posterior hippocampus. Of particular interest, this posterior effect was dissociated from an anterior hippocampal effect that demonstrated a sustained timecourse. Consistent with other studies that have reported anterior hippocampal activity during both the construction and elaboration phases of simulation (Addis, Wong, & Schacter, 2007, see also, Madore, Szpunar, Addis, & Schacter, 2016), the anterior hippocampal findings were interpreted as reflecting the continual recruitment of encoding and/or relational processing during simulation (Addis & Schacter, 2012).
In the present study, we provide a direct test of these interpretations. Here, we conducted a novel subsequent memory analysis of our previously published data (Thakral et al., 2017a) to address three specific aims. First, we aimed to add to the currently limited evidence for successful encoding effects during simulation within the hippocampus (Martin et al., 2011; Gaesser et al., 2013). Second, we aimed to provide an additional test of the component process model of Addis and Schacter (2012), and determine whether successful encoding effects would be restricted to the anterior hippocampus. Lastly, our experimental design allowed us to characterize the temporal nature of successful encoding activity within the hippocampus (i.e., whether it is transient or sustained). It is unknown whether successful encoding, akin to retrieval, is a transient process during episodic simulation. In contrast, for a simulation to be successfully encoded, it is possible that anterior hippocampal activity must be sustained. Such evidence would be in line with prior research which has indicated that anterior hippocampal activity is continually recruited during simulation (Addis et al., 2007; Thakral et al., 2017a; see also, Madore et al., 2016).
Materials and Methods
The methods are described here in abbreviated form. See Thakral et al., 2017a for full details.
Participants
Twenty participants (15 females, mean age of 22.9, range 18–30, mean years of education 15.2, range 12–17) contributed to the analysis (one person was excluded due to excessive movement). Participants self-reported to be right handed, in good health, have normal or corrected-to-normal vision, and have no history of psychiatric or neurological disorder. These participants were a subset of those reported in Thakral et al., (2017a) on whom subsequent memory was assessed. The experimental protocol was approved by the Institutional Review Board of Harvard University and informed consent was obtained prior to participation.
Stimuli and Task
The study comprised two sessions. In Session 1, participants provided a list of 162 people and 108 places they were personally familiar with. For each person and place, participants provided a familiarity rating (on a scale of 1 to 9, ranging from very unfamiliar to very familiar) and a pleasantness rating (on a scale of 1 to 9, ranging from very unpleasant to very pleasant; see Benoit, Schacter, & Szpunar, 2014). In addition to providing the person and place names, participants were familiarized with a list of 378 object words. Object words were drawn from Hemera Photo Objects 50,000 Volume III (http://www.hemera.com/index.html). Participants made the same pleasantness and familiarity ratings as they did for the places and people. In addition, participants imagined performing an action with the object denoted by the word and provided a rating as to how vivid the image was (on a scale of 1 to 9, ranging from not vivid to very vivid).
Participants returned for Session 2 (median delay of 6 days, range 1–17 days) for the scanning session. During scanning, participants performed two tasks: a simulation task and a sentence task (Figure 1). The order of simulation and sentence trials was pseudorandomized such that no more than 4 trials of a given task occurred in succession. For the simulation task, participants were presented with trial-unique combinations of 3, 4, or 5 episodic details (i.e., person, place, and object words from Session 1) and were instructed to imagine a specific future episode where they were interacting with the cued details in a location-specific manner. They were further instructed to start imagining the episode as soon as possible and continue to simulate their episode throughout the fixation/delay period till the onset of a probe question. At the end of each simulate trial, participants were asked to judge the plausibility of the episode on a 5 point scale. For the sentence task, participants were presented with trial-unique combinations of 3, 4, or 5 object words from Session 1 and were instructed to covertly create a sentence that ranked the object words according to their size. As in the simulate task, participants were instructed to generate the sentence as quick as possible. Once generated, participants were instructed to either elaborate about the meaning of each of the words, including imagining the objects, or continually repeat the generated sentence for the rest of the trial. At the end of each sentence trial, participants were asked to rate how difficult it was to create the sentence on a 5 point scale. The sentence task is similar to the simulate task in that it requires the generation and integration of semantic and visual information, but without the requirement to generate a coherent episodic event. Therefore, the sentence task serves an appropriate non-episodic control task (for other uses of the sentence task as a control, see Addis et al., 2011; Gaesser et al., 2013; Martin et al., 2011; van Mulukom, Schacter, Corballis, Addis, 2013; for a discussion of the validity of the sentence task as a control, see Thakral et al., 2017a).
Figure 1.
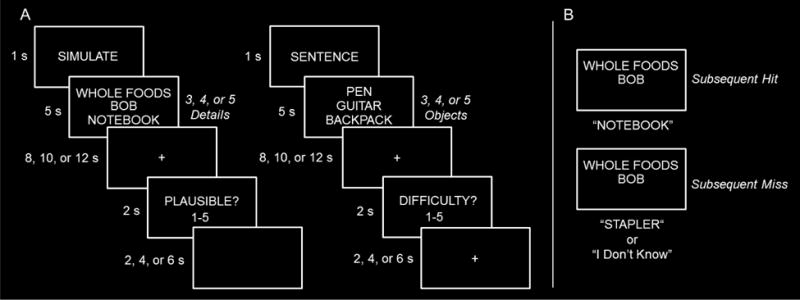
A. While undergoing fMRI, participants alternated between performing 2 tasks: a simulation task and a sentence task. On each trial of the simulation task, participants were cued with 3, 4, or 5 person, place and object and asked to simulate a hypothetical future episode which involved all of the cued episodic details. On each trial of the sentence task participants were cued with 3, 4, or 5 objects and asked to generate a sentence that sorted those objects by their respective size. B. In a post-scan session, participants were presented with every previous trial with a single randomly missing item (i.e., 2, 3, or 4 of the previous details and/or object words) and asked to recall the missing item. Subsequent hits were those trials where participants successfully recalled the associated item and subsequent misses were those where participants either recalled the incorrect associated item or failed to recall any information.
Across 6 fMRI sessions, participants completed 108 simulation trials and 54 sentence trials (one participant only completed 5 sessions due to technical error). Simulation and sentence trials were subdivided equally across the respective 3, 4, and 5 amount conditions and an equal proportion of each fixation delay period (8, 10, and 12 s) and inter-trial interval (2, 4, and 6 s) occurred for each task type. Approximately 10 minutes after exiting the scanner, participants completed a surprise cued-recall task for simulation and sentence trials. Participants were presented every previous trial with a single missing detail (i.e., 2, 3, or 4 of the previous details and/or object words) and were asked to recall the missing item (Figure 1). Across all simulate trials, memory was tested equally often for each type of detail (i.e., place, person, or object). Participants responded by typing the missing detail for each trial into a computer. This phase was self-paced. Subsequent hits were those trials where participants successfully recalled the associated detail and subsequent misses were those where participants either recalled the incorrect detail or failed to recall any information.
Image acquisition and analysis
Images were acquired on a 3 Tesla Siemens Prisma scanner equipped with a 32-channel head coil. Anatomic images were acquired with a magnetization-prepared rapid gradient echo sequence (1 mm3 resolution). Functional images were acquired with a multiband echo-planar imaging sequence (University of Minnesota C2P sequence: TR = 2 s, TE = 30 ms, matrix size of 136 × 136, 84 slices (3 slices acquired simultaneously), 1.5 mm3 resolution, multiband factor of 3, in-plane GRAPPA acceleration factor of 2; Moeller et al., 2009; Feinberg et al., 2010; Xu et al., 2013). Slices were auto-aligned to an angle 20° towards coronal from anterior-posterior commissure alignment (van der Kouwe et al., 2005).
Analyses were conducted using Statistical Parametric Mapping (SPM12, Wellcome Department of Cognitive Neurology, London, UK). Functional data preprocessing included, slice-time correction, two-pass spatial realignment, and normalization into Montreal Neurological Institute (MNI) space (no resampling). Functional data was smoothed with a 3 mm full-width-half-maximum Gaussian kernel. Anatomic images were normalized into MNI space.
Univariate analysis was conducted in a two stage general linear model (GLM). Consistent with earlier studies examining transient and sustained activity during episodic memory (Vilberg & Rugg, 2012, 2014) and episodic simulation (Thakral et al., 2017a), transient and sustained activity was modeled separately for each trial. Specifically, a 5 s boxcar function that onset concurrently with the details/object words was used to model transient neural activity and sustained activity was modeled with a variable duration boxcar (8, 10, or 12 s) that onset with the fixation period following stimulus offset. The associated BOLD response was modeled by convolving the two boxcar functions with a canonical hemodynamic response function to yield regressors in a GLM. The purpose of the variable jitter for the delay period and inter-trial interval was to reduce the collinearity between the transient and sustained regressors in the GLM (see also, Thakral et al., 2017a; Vilberg & Rugg, 2012, 2014).
Consistent with our previously reported findings from the same dataset (Thakral et al., 2017a), we found no evidence for any differences in hippocampal subsequent memory effects as a function of the amount of cued detail (i.e., 3, 4, or 5). Thus, we created two events of subsequent hits and misses, each collapsed across the three amount conditions (mean ± 1 standard error subsequent hit simulate trials (out of a possible 36) for each of the three amount conditions (3, 4, and 5) was 25.9 ± 1.6, 19.0 ± 2.0, and 13.5 ± 1.6, respectively). We also conducted an analysis examining subsequent memory as a function of the content (i.e., recall of people, place or object detail). This analysis failed to identify content-sensitive hippocampal subsequent memory effects, and thus the subsequent hits and misses were collapsed across the three content types (mean ± 1 standard error subsequent hit simulate trials (out of a possible 36) for each of the three details (people, place, and object) was 20.8 ± 1.8, 17.9 ± 1.7, and 19.7 ± 2.2, respectively).
As we collapsed across both the amount and content conditions, it was important to assess whether subsequent memory performance varied across these two factors. An ANOVA including factors detail (person, place, or object) and number (3, 4, or 5 details), with the number of subsequent memory hits serving as the dependent variable, revealed solely a main effect of number of detail (F(2, 38) = 77.02, p < 0.001). As expected, follow-up pairwise t-tests revealed that the number of hits decreased as a function of the number of details simulated (i.e., 3 > 4 > 5; ts(19) < 6.43, ps < 0.001)1. There was no reliable detail by number interaction or main effect of detail (Fs < 2.41, ps > 0.05). These null findings suggest that memory did not reliably differ as a function of the type of content. We also conducted a follow-up analysis to determine whether the delay between Session 1 and Session 2 affected subsequent memory of the simulations. When repeating the same ANOVA with delay as a covariate (i.e., number of days), we again found only a significant main effect of number (F(2, 36) = 29.64, p < 0.001). Thus, there was no evidence that memory performance was reliably affected by the delay between Session 1 and Session 2.
There were insufficient numbers of trials to separate sentence trials as a function of subsequent memory, and therefore sentence trials were modeled as a single event Mean number of subsequent hit sentence trials (± 1 standard error) was 5.6 ± 2.0. The model thus contained a total of 6 events of interest (i.e., transient and sustained regressors for subsequent hits, subsequent misses, and sentence trials). Six regressors representing movement related variance (three for rotation and three for rigid-body translation) and regressors modeling each scan session were also entered into the design matrix. An AR(1) model was used to estimate and correct for nonsphericity of the error covariance (Friston et al., 2002).
The participant-specific parameter estimates for each of the 6 events of interest were carried forward to a repeated measures ANOVA where participants served as a random effect. The ANOVA model employed factors of condition (subsequent hit, subsequent miss, and sentence trials) and regressor (transient and sustained). An individual voxel threshold of p < 0.001 was employed with a cluster extent threshold of 19 voxels (corrected threshold of p < 0.05; see Thakral et al., 2017a). This was computed using a Monte Carlo simulation with 10,000 iterations with an estimated spatial autocorrelation of 11.13 mm (Slotnick et al., 2003; Slotnick, 2017). Given our a priori interest in the hippocampus, results are reported within an anatomically defined hippocampal mask created by manually tracing the hippocampus in both hemispheres using the across-participant mean anatomic image based on standard anatomical landmarks (Frisoni et al., 2015; for similar approaches, see Thakral, Yu, & Rugg, 2015; Thakral, Wang, & Rugg, 2017b; see supplemental material for a list of whole-brain subsequent memory results). In addition to the GLM analysis, we also conducted an analysis employing a finite-impulse response (FIR) model to estimate the timecourses associated with each of the events described above (see, Thakral, et al., 2017a; see also, Vilberg and Rugg, 2012, 2014). We provide the timecourses for illustrative purposes only.
Results
We first identified subsequent memory-related simulation effects that were transient and sustained. Second, because the present analyses were conducted on a subset of the participants comprising our previously published data (i.e., those on whom subsequent memory was tested; Thakral et al., 2017a), we then replicated our original results and identified simulation effects that were transient and sustained. As the original analysis was conducted without considering subsequent memory, it is unknown whether our prior effects reflect, in part, encoding-related processes during simulation. In a novel extension of our prior study, we then conducted a set of analyses to identify simulation effects dissociable from those sensitive to encoding-related processes.
Subsequent memory-related simulation effects
Transient Effects
Transient hippocampal subsequent memory effects were identified with the contrast of subsequent hits > subsequent misses for the transient regressor at a threshold of p < 0.001. The resulting statistical map was exclusively masked with the identical contrast for the sustained regressor at a threshold of p < 0.05 (note that the more liberal an exclusive mask threshold, the more conservative the approach). This procedure identifies those voxels sensitive to encoding success specific to the transient regressor (for similar approaches see, Vilberg & Rugg, 2012, 2014). Transient subsequent memory effects were identified in two clusters that fell in the right hippocampus (Figure 2), one with a peak voxel of 26, −9, −13 (20 voxels, peak Z of 4.71) and the other with a peak voxel of 26, −16, −18 (20 voxels, peak Z of 3.71)2. We consider these subsequent memory effects to fall within the anterior hippocampus as the y coordinate for each cluster was greater than -21 (Poppenk, Evensmoen, Moscovitch, & Nadel, 2013). In accordance with how these effects were identified, the extracted parameter estimates demonstrate that the subsequent memory effect (i.e., hit > miss) was present for the transient but not the sustained regressor (Figure 2).
Figure 2.
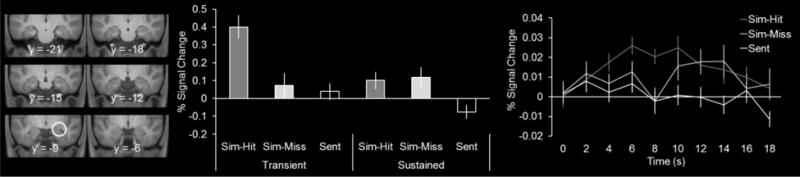
Transient successful encoding effects during episodic simulation in green. Parameter estimates as well as the timecourses are shown extracted from the respective peak voxel in the right hemisphere (denoted by white circles, see main text for coordinates). Results are overlaid onto the coronal sections of the mean T1-weighted anatomical image.
Because the encoding effects were identified using solely the simulation trials (i.e., subsequent hits > misses), it is unknown whether subsequent hits and subsequent misses elicited significantly greater activity than the sentence trials (i.e., evidence for a simulation effect). Upon inspection of the parameter estimates and timecourse extracted from the left anterior hippocampal effect, a sustained simulation effect appeared to be present (i.e., hits and misses elicited greater activity relative to sentence trials for both regressors, Figure 2). Follow-up paired t-tests conducted on the parameter estimates confirmed this observation. Simulate trials (collapsed across hits and misses) elicited significantly greater activity than sentence trials for each regressor in each of the two clusters identified to be sensitive to subsequent memory (ts(19) > 2.77, ps < 0.05; note that these contrasts are unbiased with respect to the statistical procedure used to identify the activity). These results are consistent with the expected pattern of sustained activity given the anatomic location of the effect (i.e., the anterior hippocampus should exhibit a sustained simulation effect, see Introduction). In a follow-up analysis to more directly test whether the transient subsequent memory effect exhibited a sustained simulation effect, we inclusively masked the transient-specific subsequent memory effect (Figure 2) with the contrast of simulate > sentence trials (collapsed across subsequent memory) for the sustained regressor at a threshold of p < 0.001. This analysis revealed a cluster in the right anterior hippocampus with a peak voxel of 24, −12, −15 (8 voxels, peak Z of 3.71).
Sustained Effects
Sustained hippocampal subsequent memory effects were identified with the contrast of subsequent hits > subsequent misses for the sustained regressor at a threshold of p < 0.001. This contrast failed to identify any significant effects, even when the threshold was relaxed to p < 0.01.
Simulation effects
Transient effects
Transient hippocampal simulation effects were identified with the contrast of simulate > sentence trials (collapsed across subsequent memory) for the transient regressor at a threshold of p < 0.001. Replicating our previous findings (Thakral et al., 2017a), transient simulation effects were identified in the hippocampus with a peak voxel in the left hemisphere of −27, −21, −15 (113 voxels, peak Z of 5.44) and in the right hemisphere of 29, −19, −16 (21 voxels, peak Z of 4.56). Although the peak voxels fell near the boundary of anterior/posterior hippocampus (y = −21), the transient effects extended primarily into the posterior hippocampus in each hemisphere.
If, as predicted by the component process model of hippocampal function during simulation (Addis and Schacter, 2012; see also; Schacter et al., 2017), the posterior hippocampus is associated with retrieval-related processes during simulation, then the transient-specific simulation effects should be distinct from those that exhibit encoding effects (i.e., subsequent hits > subsequent misses). To examine this possibility, we removed voxels sensitive to subsequent memory by exclusively masking the outcome of the analysis used to identify transient-specific simulation effects with the contrast of subsequent hits > subsequent misses for the transient regressor (threshold of p < 0.05). This analysis identified simulation effects in the left posterior hippocampus with a peak voxel of −27, −21, −16 (92 voxels, peak Z of 5.34)3. Consistent with the unmasked results (see preceding paragraph), the transient effects extended primarily into the posterior hippocampus (Figure 3). In accordance with how these effects were identified, the extracted parameter estimates and timecourses in Figure 3 illustrate that the simulation effects were specific to the transient regressor and transient in temporal profile, respectively. As these posterior effects were insensitive to encoding (i.e., subsequent hits > subsequent misses), together with the fact that they replicate the pattern of hippocampal activity observed during episodic memory (Vilberg & Rugg, 2012, 2014), these results support the idea that the transient and posterior hippocampal effects do reflect retrieval-related processes.
Figure 3.
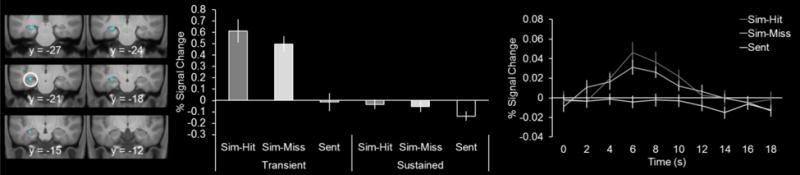
Transient episodic simulation effects in cyan. Parameter estimates as well as the timecourses are shown extracted from the respective peak voxel in the left hemisphere (denoted by white circles, see main text for coordinates). Results are overlaid onto the coronal sections of the across-participants mean T1-weighted anatomical image.
Sustained effects
Sustained hippocampal simulation effects were identified with the contrast of simulate > sentence trials (collapsed across subsequent memory) for the sustained regressor at a threshold of p < 0.001. Replicating our previous findings (Thakral et al., 2017a), sustained simulation effects were identified in the hippocampus with a peak voxel in the left hemisphere of −21, −13, −13 (526 voxels, peak Z of 6.11) and in the right hemisphere of 23, −21, −16 (441 voxels, peak Z of 6.47). Although the peak fell near the anterior/posterior boundary (y = -21), particularly in the right hemisphere, the sustained effects extended predominately into the anterior hippocampus.
According to the component process model of Addis and Schacter (2012) the anterior hippocampus supports two dissociable processes (i.e., encoding and relational/recombination processes, see Introduction). To test this idea, we examined the extent to which the sustained simulation effects were distinct from those sensitive to encoding. We exclusively masked the sustained simulation effect (i.e., simulate > sentence contrast for the sustained regressor; see preceding paragraph) with the subsequent memory contrast (i.e., subsequent hits > subsequent misses for the both the transient and sustained regressors; exclusive mask threshold of p < 0.05). This analysis revealed the identical peak voxels as the original unmasked analysis (263 voxels in the left hemisphere (peak Z of 5.89) and 205 voxels in the right hemisphere (peak Z of 6.07). The extracted parameter estimates and timecourses in Figure 4 illustrate that the simulation effects were present for both the transient and sustained regressor and sustained in their temporal profile, respectively. These findings indicate that the anterior hippocampus supports at least two processes during simulation, an encoding process (Figure 2) and one distinct from encoding (Figure 4).
Figure 4.
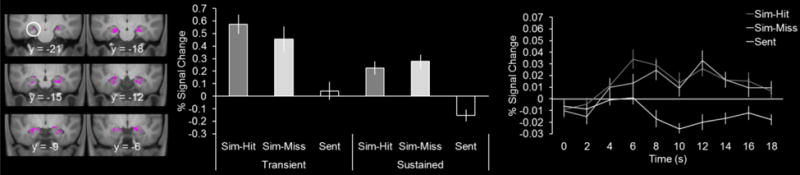
Sustained episodic simulation effects in magenta. Parameter estimates as well as the timecourses are shown extracted from the respective peak voxel in the left hemisphere (denoted by white circles, see main text for coordinates). Results are overlaid onto the coronal sections of the across-participants mean T1-weighted anatomical image.
Discussion
According to the component process model of hippocampal function during simulation (Addis and Schacter, 2012; see also; Schacter et al., 2017), the posterior hippocampus supports the retrieval of past details during simulation and the anterior hippocampus supports the encoding of a simulation into memory and the relational/recombination processes necessary to create a coherent simulation. Within a single experiment, we provide novel evidence that the hippocampus supports each of these three processes by dissociating hippocampal function as a function of timecourse and spatial location. We identified a transient retrieval process in the posterior hippocampus, a transient encoding process in the anterior hippocampus, and a sustained simulation process in the anterior hippocampus. Figure 5 illustrates the spatial distribution of each of these three effects. Taken together, these results support the model of Addis and Schacter (2012).
Figure 5.
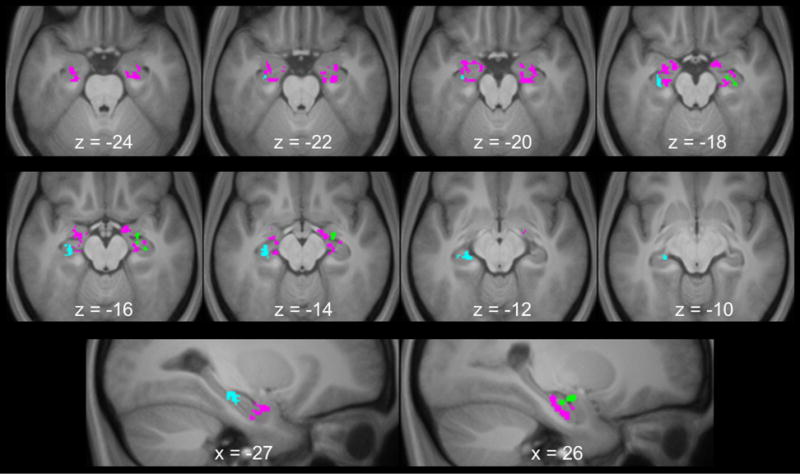
Long-axis dissociation of the hippocampal simulation effects. Those in cyan illustrate the transient simulation effects, those in magenta illustrate the sustained simulation effects, and those in green illustrate the transient subsequent memory effects during simulation. Each of the contrasts used to identify the three effects was exclusively masked with the respective alternative contrast to demonstrate the anatomical dissociation (see text for further details). Results are overlaid onto the axial and sagittal sections of the across-participants mean T1-weighted anatomical image.
There is an important caveat to our interpretation concerning specifically the anterior hippocampal effects that were insensitive to subsequent memory. Given that these effects were reliably dissociated from retrieval-related activity (i.e., the posterior/transient hippocampal effects) and encoding-related activity (i.e., the anterior/transient hippocampal effects), we suggest that these effects likely reflect processes such as relational/recombination processing (see, Addis & Schacter, 2012; Schacter et al., 2017). However, if these effects do reflect relational/recombinatorial processes, such effects should have varied with the amount of detail comprising the simulation (i.e., 3, 4, or 5 cued details), as relational/recombinatorial processing should increase with the number of details within a simulation. However, we failed to identify amount-sensitive effects within the hippocampus (see Materials and Methods). As with any null finding, there are a number of possible explanations for our failure to identify such effects. We note that the anterior hippocampal effects that were insensitive to subsequent memory corroborate the findings of Addis et al. (2007). In that study, anterior hippocampal activity was observed during both the construction and elaboration phases of simulation (see also, Madore et al., 2016) and was common to both past and future events. Addis et al. (2007) interpreted the common sustained hippocampal activity as reflecting a binding process necessary to form a coherent past or future event. That is, past events require the reintegration of a memory trace and future events require the novel integration of details. Together with the known the role of the anterior hippocampus in relational processing (see, Davachi, 2006; Eichenbaum & Cohen, 2014; Schacter et al., 2017), we interpret our effects similarly. However, given the null finding, it is important to consider alternative explanations. For example, according to Zeidman and Maguire (2016), the anterior hippocampus supports the construction of scenes during memory and imagination. Future research is needed to differentiate between these and other possibilities (for a discussion see, Roberts et al., 2017).
The lack of amount-dependent effects could also be taken as inconsistent with our interpretation that the posterior and transient hippocampal effects during simulation reflect the retrieval of episodic details. If these effects do reflect retrieval, one would predict that such effects would vary with the amount of information necessary to be retrieved during simulation (e.g., 5 details versus 3 details). As noted earlier, any null finding should be treated with caution. Nevertheless, the current retrieval interpretation is supported by the fact that the transient and posterior nature of the hippocampal effects replicates prior episodic memory findings (Vilberg & Rugg, 2012, 2014).
The current findings add to currently limited evidence for the role of the hippocampus in encoding simulated future events into memory (Martin et al., 2011; Gaesser et al., 2013). These findings also bear on our understanding of the adaptive nature of episodic simulation (for a review, see Schacter, 2012). Specifically, it has been argued that in order for a simulation to be beneficial, it must be encoded for later retrieval when the simulated event is carried out (Ingvar, 1985; for a discussion, see Szpunar, Addis, McLelland, & Schacter, 2013; Schacter et al., 2017). Consistent with prior findings (Martin et al., 2011; Gaesser et al., 2013), the current results indicate that the hippocampus contributes to this adaptive function by its role in encoding simulations into memory.
Notably, the anterior hippocampal region characterized by an encoding effect (i.e., subsequent hits > subsequent misses) early in time also demonstrated a non-specific simulation effect (i.e., subsequent hits + subsequent misses > sentence trials; see timecourse in Figure 2) that was present during the later phase of a simulation. These findings are the first to suggest that portions of the anterior hippocampus support dissociable processes across time during simulation. The transient nature of the encoding effect corroborates other studies that identified subsequent memory effects primarily during the construction phase of simulation (Martin et al., 2011; Gaesser et al., 2013). Also in line with these prior studies is the spatial location of the hippocampal subsequent memory effect (i.e., the anterior hippocampus). We further emphasize that, with respect to prior encoding studies, the present study is unique in its analytic approach. Here, we exclusively masked out sustained encoding-related activity to isolate neural activity that was specifically transient (see also, Thakral et al., 2017a; Vilberg & Rugg, 2012, 2014). This exclusive masking approach was not taken in prior studies (Martin et al., 2011; Gaesser et al., 2013), where activity was identified for each phase of the trial (i.e., construction and elaboration). It thus remains possible that encoding activity in these studies may have extended into the elaboration period and, accordingly, might have been sustained. However, the consistency of the spatial location and temporal nature of the encoding effects across the current and past studies argues against this possibility. Nevertheless, we believe that it will be important for future studies to utilize an analysis similar to that in the current study to ensure the accurate identification of underlying processes, particularly those hypothesized to be recruited at a specific time during simulation, such as encoding and retrieval.
The transient nature of the subsequent memory effect raises an additional point worth considering. That is, if the episode is being continually bound together across time during simulation (as reflected in the sustained anterior hippocampal effect, Figure 4), an open question is how a transient subsequent memory effect would support the encoding of an episode (and if so, what specific aspects of the episode are being encoded). One possibility is that the transient subsequent memory effect reflects a relatively shallow encoding process, specifically related to the cue words. Of relevance to this point, in the present experiment subsequent memory was tested for only a single detail comprising the simulated episode. Thus, subsequent hits may have been supported by retrieval of the associative information between the cue words without a complete episodic memory for the simulation. We highlight that, consistent with our prior encoding studies (Martin et al., 2011; Gaesser et al., 2013), because participants were instructed during scanning to integrate all the details into a coherent event, we assume that subsequent memory for the tested details reflects how well the details were bound together into a coherent episodic simulation. Nevertheless, these points highlight the need for future studies that test subsequent memory not only for individual details comprising the simulation but also measure recollection of the simulation as a whole. It is possible that subsequent memory for the cue words is supported by a transient hippocampal process (e.g., Figure 2), whereas subsequent memory for the episode as a whole is supported by a sustained hippocampal process. This is a topic for future research.
The current findings are consistent with the component process model of Addis and Schacter (2012) in that unique hippocampal regions were shown to contribute to distinct processes during simulation (i.e., retrieval, relational, and encoding-related processes). We note that the model of Addis and Schacter (2012) is not exhaustive in its ability to accommodate all hypothetical processes engaged during simulation. There are a number of theoretical models of hippocampal function during remembering and imagining that also specify anterior-posterior dissociations (e.g., Sheldon & Levine, 2016; Zeidman & Maguire, 2016). Regardless of which model is correct, it is important to recognize the variety of hippocampal processes that support simulation and the possibility that in any given experiment these processes may be confounded (cf., Gaesser et al., 2013). For example, in one study, anterior hippocampal activity decreased as a function of the number of times a given event was simulated (van Mulukom et al., 2013). Such a decrease in hippocampal activity could be interpreted as reflecting either a decrease in the recruitment of relational or encoding processes as a function of repetition. The current experiment highlights that the success of future studies concerning episodic simulation and hippocampal activity likely depends on the use of experimental designs that allow the effective differentiation of the component processes associated with simulation.
Supplementary Material
Acknowledgments
NIMH Grant RO1MH60941 (to DLS) and NIH Shared Instrumentation Grant S10OD020039
Footnotes
Given that the number of subsequent hit simulate trials significantly differed between the three amount conditions, it is possible that the condition containing the greater number of trials was driving the fMRI results. To assess this possibility, we extracted activity for subsequent hits and misses as a function of the three amount conditions (e.g., subsequent hit-3, subsequent hit-4, and subsequent hit-5). In no region did activity between the three amount conditions significantly differ. Thus, there was no evidence that the difference in trial numbers influenced the fMRI findings.
When employing a cluster uncorrected threshold of p < 0.001, a transient subsequent memory effect was also identified in the left anterior hippocampus with a peak voxel of −26, −15, −16 (10 voxels, peak Z of 3.65).
When employing a cluster uncorrected threshold of p < 0.001, a cluster in the right posterior hippocampus was also identified (9 voxels, peak Z of 4.50).
References
- Addis DR, Cheng T, Roberts R, Schacter DL. Hippocampal contributions to the episodic simulation of specific and general future events. Hippocampus. 2011;21:1045–1052. doi: 10.1002/hipo.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Schacter DL. Constructive episodic simulation: Transient distance and detail of past and future events modulate hippocampal engagement. Hippocampus. 2008;18:227–237. doi: 10.1002/hipo.20405. [DOI] [PubMed] [Google Scholar]
- Addis DR, Schacter DL. The hippocampus and imagining the future: where do we stand? Front Hum Neurosci. 2012;5:1–15. doi: 10.3389/fnhum.2011.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, Schacter DL. Specifying the core network supporting episodic simulation and episodic memory by activation likelihood estimation. Neuropsychologia. 2015;75:450–457. doi: 10.1016/j.neuropsychologia.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, Szpunar KK, Schacter DL. Ventromedial prefrontal cortex supports affective future simulation by integrating distributed knowledge. Proc Natl Acad Sci. 2014;111:16550–16555. doi: 10.1073/pnas.1419274111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context, and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Eichenbaum HE, Cohen NJ. Can we reconcile the declarative memory and spatial navigation views on hippocampal function? Neuron. 2014;83:764–770. doi: 10.1016/j.neuron.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisoni GB, Jack CR, Bocchetta M, Bauer C, Frederiksen KS, Liu Y, Winblad B. The EADC-ADNI harmonized protocol for manual hippocampal segmentation on magnetic resonance: Evidence of validity. Alzheimers Dement. 2015;11:111–125. doi: 10.1016/j.jalz.2014.05.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Penny W, Phillips C, Kiebel S, Hinton G, Ashburner J. Classical and Bayesian inference in neuroimaging: theory. Neuroimage. 2002;16:465–483. doi: 10.1006/nimg.2002.1090. [DOI] [PubMed] [Google Scholar]
- Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Glasser MF, Miller KL, Uğurbil K, Yacoub E. Multiplexed echo planar imaging for sub-second whole brain fMRI and fast diffusion imaging. PLoS One. 2010;5:1–11. doi: 10.1371/journal.pone.0015710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaesser B, Spreng RN, Mclelland VC, Addis DR, Schacter DL. Imagining the future: Evidence for a hippocampal contribution to constructive processing. Hippocampus. 2013;23:1150–1161. doi: 10.1002/hipo.22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends Cogn Sci. 2007;11:299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Ingvar DH. “Memory of the future”: An essay on the temporal organization of conscious awareness. Hum Neurobiol. 1985;4:127–136. [PubMed] [Google Scholar]
- Lepage M, Habib R, Tulving E. Hippocampal PET activations of memory encoding and retrieval: The HIPER model. Hippocampus. 1998;8:313–322. doi: 10.1002/(SICI)1098-1063(1998)8:4<313::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Madore KP, Szpunar KK, Addis DR, Schacter DL. Episodic specificity induction impacts activity in a core brain network during construction of imagined experiences. Proc Natl Acad Sci U S A. 2016;113:10696–10701. doi: 10.1073/pnas.1612278113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin VC, Schacter DL, Corballis MC, Addis DR. A role for the hippocampus in encoding simulations of future events. Proc Natl Acad Sci U S A. 2011;108:13858–13863. doi: 10.1073/pnas.1105816108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, Uğurbil K. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med. 2009;63:1144–1153. doi: 10.1002/mrm.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M, Cabeza R, Winocur G, Nadel L. Episodic memory and beyond: The hippocampus and neocortex in transformation. Ann Rev Psychol. 2016;67:105–134. doi: 10.1146/annurev-psych-113011-143733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 2013;17:230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Palombo DJ, Hayes SM, Peterson KM, Keane MM, Verfaellie M. Medial temporal lobe contributions to episodic future thinking: Scene construction or future projection? Cereb Cortex. 2016 doi: 10.1093/cercor/bhw381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RR, Schacter DL, Addis DR. Scene construction and relational processing: Separable constructs? Cereb Cortex. 2017 doi: 10.1093/cercor/bhx081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Philos Trans R Soc B Biol Sci. 2007;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: The prospective brain. Nat Rev Neurosci. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Episodic simulation of future events: Concepts, data, and applications. Ann N Y Acad Sci. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK. The future of memory: Remembering, imagining, and the brain. Neuron. 2012;76:677–694. doi: 10.1016/j.neuron.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Szpunar KK. Escaping the past: Contributions of the hippocampus to future thinking and imagination. In: Hannula DE, Duff MC, editors. The Hippocampus from cells to systems: Structure, connectivity, and functional contributions to memory and flexible cognition. New York: Springer; 2017. pp. 439–465. [Google Scholar]
- Schacter DL, Benoit RG, Szpunar KK. Episodic future thinking: Mechanisms and functions. Curr Op Behav Sci. 2017;17:41–50. doi: 10.1016/j.cobeha.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Sheldon S, Levine B. The role of the hippocampus in memory and mental construction. Ann N Y Acad Sci. 2016;1369:76–92. doi: 10.1111/nyas.13006. [DOI] [PubMed] [Google Scholar]
- Slotnick SD. Cluster_threshold. 2017 Retrieved March, 1, 2017 from Web site: http://www2.bc.edu/~slotnics/scripts.htm.
- Slotnick SD, Moo LR, Segal JB, Hart J. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. 2003;17:75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Szpunar KK, Addis DR, McLelland VC, Schacter DL. Memories of the future: New insights into the adaptive value of episodic memory. Front Behav Neurosci. 2013;7:47. doi: 10.3389/fnbeh.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, Jacques PLS, Robbins CA, Wig GS, Schacter DL. Repetition-related reductions in neural activity reveal component processes of mental simulation. Soc Cogn Affect Neurosci. 2014;9:712–722. doi: 10.1093/scan/nst035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral PP, Benoit RL, Schacter DL. Imagining the future: The core episodic simulation network dissociates as a function of timecourse and the amount of simulated information. Cortex. 2017a doi: 10.1016/j.cortex.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral PP, Wang TH, Rugg MD. Decoding the content of recollection within the core recollection network and beyond. Cortex. 2017b;91:101–113. doi: 10.1016/j.cortex.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral PP, Yu SS, Rugg MD. The hippocampus is sensitive to the mismatch in novelty between items and their contexts. Brain Res. 2015;1602:144–152. doi: 10.1016/j.brainres.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kouwe AJW, Benner T, Fischl B, Schmitt F, Salat DH, Harder M, Sorensen AG, Dale AM. On-line automatic slice positioning for brain MR imaging. Neuroimage. 2005;27:222–230. doi: 10.1016/j.neuroimage.2005.03.035. [DOI] [PubMed] [Google Scholar]
- van Mulukom V, Schacter DL, Corballis MC, Addis DR. Re-imagining the future: Repetition decreases hippocampal involvement in future simulation. PLoS ONE. 2013;8:1–10. doi: 10.1371/journal.pone.0069596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. The neural correlates of recollection: Transient versus sustained fMRI effects. J Neurosci. 2012;32:15679–15687. doi: 10.1523/JNEUROSCI.3065-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Temporal dissociations within the core recollection network. Cogn Neurosci. 2014;5:77–84. doi: 10.1080/17588928.2013.860088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler JA, Suchan B, Daum I. Foreseeing the Future: Occurrence Probability of Imagined Future Events Modulates Hippocampal Activation. 2010a;690:685–690. doi: 10.1002/hipo.20695. [DOI] [PubMed] [Google Scholar]
- Weiler JA, Suchan B, Daum I. When the future becomes the past: Differences in brain activation patterns for episodic memory and episodic future thinking. Behav Brain Res. 2010b;212:196–203. doi: 10.1016/j.bbr.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Xu J, Moeller S, Auerbach EJ, Strupp J, Smith SM, Feinberg DA, Yacoub E, Uğurbil K. Evaluation of slice accelerations using multiband echo planar imaging at 3T. Neuroimage. 2013;83:991–1001. doi: 10.1016/j.neuroimage.2013.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidman P, Maguire EA. Anterior hippocampus: the anatomy of perception, imagination and episodic memory. Nat Rev Neurosci. 2016;17:173–182. doi: 10.1038/nrn.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidman P, Mullally SL, Maguire EA. Constructing, perceiving, and maintaining scenes: Hippocampal activity and connectivity. Cereb Cortex. 2016;25:3836–3855. doi: 10.1093/cercor/bhu266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


