Abstract
Phosphorylation has an incredible impact on the biological behavior of proteins, altering everything from intrinsic activity to cellular localization and complex formation. It is no surprise then that this post-translational modification has been the subject of intense study and that, with the advent of faster, more accurate instrumentation, the number of large-scale mass spectrometry-based phosphoproteomic studies has swelled over the past decade. Recent developments in sample preparation, phosphorylation enrichment, quantification, and data analysis strategies permit both targeted and ultra-deep phosphoproteome profiling, but challenges remain in pinpointing biologically relevant phosphorylation events. We describe here technological advances that have facilitated phosphoproteomic analysis of cells, tissues, and biofluids and note applications to neuropathologies in which the phosphorylation machinery may be dysregulated, much as it is in cancer.
Keywords: Phosphoproteomics, post-translational modifications, mass spectrometry, neurodegeneration
INTRODUCTION
Protein phosphorylation is a ubiquitous post-translational modification and crucial component of most cellular processes. In eukaryotes, it is estimated that one-third of all proteins are phosphorylated and that there are over 500 kinases responsible for phosphorylation while approximately 150 phosphatases are responsible for dephosphorylation.1 The sizeable portion of the human proteome that is dedicated to the regulation of phosphorylation underscores the intricacy of phosphorylation networks and the temporal and spatial control of phosphoproteins necessary for appropriate responses to internal and environmental stimuli. In turn, aberrant protein phosphorylation can lead to a multitude of disease states, including cancer, diabetes, and neuropathologies.
Given the druggability of kinase catalytic domains, it is no coincidence that kinases are popular therapeutic targets. Kinase drug discovery efforts showed promise after FDA approval of the monoclonal antibody Herceptin for treatment of Her2 positive breast cancer and rapidly accelerated after the 2001 approval of the small molecule imatinib (Gleevec) for treatment of Bcr-Abl positive chronic myelogenous leukemia. As of December 2016, there are 31 FDA approved small molecule kinase inhibitors in the United States,2 but with the exception of tofacitinib for rheumatoid arthritis and nintedanib for idiopathic pulmonary fibrosis, kinase drug approval has been primarily associated with oncology.3, 4 Encouragingly, there are now kinase inhibitors, often repurposed from cancer, in clinical trials for central nervous system disorders5, 6 and in preclinical studies for cardiovascular diseases.7 This highlights the importance of locating and quantifying phosphorylation changes in a wide variety of tissues and biofluids to provide insight into how signaling networks are disturbed during pathogenesis of diseases including and beyond cancer.
Despite its importance as a post-translational modification, protein phosphorylation is typically both substoichiometric and dynamic in nature,8 making it challenging subject for study. In this review, we will focus on recent advances in sample preparation techniques, phosphopeptide enrichment strategies, LC-MS data acquisition and analysis, and determination of phosphorylation abundance and stoichiometry. Finally, we will describe extensions of phospoproteomics in the areas of PTM crosstalk, kinase-substrate mapping, and biomarker discovery. When possible, we will also note applications to the study of human diseases, especially neurological disorders, which have received considerably less attention than cancer for their association with dysregulated phosphorylation networks.
Sample Preparation for Mass Spectrometry-based Phosphoproteomics
The past two decades have seen the rise of the “omics” era, so named for the fields of genomics, proteomics, and metabolomics that focus on large scale study of genes, proteins, and metabolites, respectively. Proteomics in particular takes a holistic approach to studying protein structure and function and integrates this information so pathways in complex systems can be better understood. Because protein structure and function are intimately connected, much attention has been focused on the modifications that proteins undergo after translation, particularly phosphorylation.
Serine, threonine, and tyrosine are generally the most common sites of phosphorylation in eukaryotes, composing approximately 90%, 10%, and <1% of the total phosphoproteome, respectively;8, 9 the extent of histidine and arginine phosphorylation is still unclear.10 On the other hand, histidine and aspartic acid are common sites of modification in prokaryotes, especially as part of a two component sensor, but studies suggest that the prevalence of phosphorylation of serine, threonine, tyrosine in these organisms may have be underappreciated11–13 and that arginine phosphorylation may also play an important role in cellular processes such as protein degradation.14, 15 Analysis of acid-labile phosphorylation often requires different conditions and protocols, so for the purposes of clarity, this review will discuss isolation and analysis of serine, threonine, and tyrosine phosphorylation.
The first challenges in a successful phosphoproteomic study are those derived from sample preparation, with unique considerations for cell lines, tissue samples, and biofluids such as plasma, cerebrospinal fluid (CSF), urine, and saliva. Lysis protocols for cells and tissues are largely similar; in both cases, abolishment of endogenous phosphatase activity with inhibitors or thermal denaturation is critical.16 Phosphate groups are labile, meaning that pH and temperature must be controlled accordingly during preparation; care must also be taken to minimize overall sample loss. Other problems may arise upon LC-MS analysis because of lower phosphopeptide fragmentation efficiency and variations in the retention of phosphopeptides on reversed phase chromatographic columns,8 as discussed in further detail below. Problems associated with phosphorylation analysis may also be the result of protein phosphorylation dynamics within cells. Tracking real phosphorylation changes upon disturbance of a signaling network can be impeded by changes in protein expression, necessitating measurement of phosphorylation stoichiometry. Small alterations in cellular environment due to sample collection, storage, or the period between collection and storage can also cause rapid shifts in signaling networks; the resulting distortion of the phosphorylation profile makes it difficult to quantify the true phosphorylation events of interest.17–19
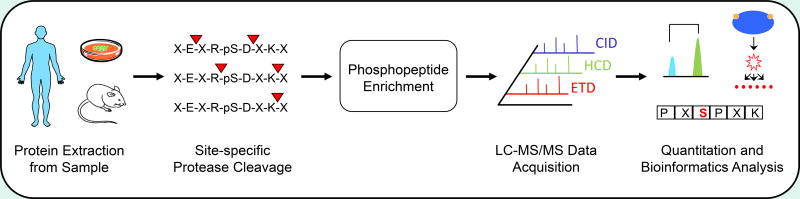
The vast majority of phosphoproteomic studies are still done in a “bottom-up” fashion, which is to say that peptides are analyzed, and protein information is inferred from the peptide identifications (Figure 1). Top-down approaches are gaining traction for analysis of simple samples because they provide opportunities to analyze isoform-specific PTMs.20 Unfortunately, fragmentation of intact proteins in mass spectrometers is still inefficient; thus, sequence coverage is problematic and the mass range is limited.21 Middle-down strategies, where long peptides of 50 or more amino acids (~3000 to 20000 Da total) are analyzed, may present a compromise.22 In the meantime, utilization of proteases of differing specificities has improved sequence coverage in bottom-up experiments. Trypsin is the most common endoprotease for phosphoprotemic studies because it is easily available, relatively inexpensive, and capable of producing positively charged peptides of suitable length for MS analyses.23, 24 However, cleavage efficiency can be particularly low near phosphorylated residues.25 Several groups have demonstrated enhanced proteomic identification and/or PTM localization rates when using alternative proteases such as Lys-C, Asp-N, Glu-C, chymotrypsin, elastase, and subtilisin, often in combination with trypsin.24, 26–29 Aside from choice of protease, digestion protocols can be optimized according to temperature, denaturing reagent, time, and so forth. Filtered assisted sample preparation (FASP) and phase transfer surfactant-aided extraction (PTS) represent significant advances for phosphoproteome analysis of membrane proteins and tissue, whether fresh30 or formalin-fixed/paraffin-embedded.31, 32 EasyPhos, a platform that uses TFE (2,2,2-trifluoroethanol) digestion buffer with enrichment and desalting in a 96 well format, provides a substantial boost in throughput.33
Figure 1.
Typical bottom-up phosphoproteomics workflow. Proteins are first extracted from cell lines, tissues, or biofluids and digested into peptides by site-specific proteases such as GluC, trypsin, or LysC. Phosphopeptides are then enriched and subjected to tandem mass spectrometry before bioinformatics analyses, including quantitation, signal transduction pathway mapping, or kinase motif extraction.
Enrichment Techniques
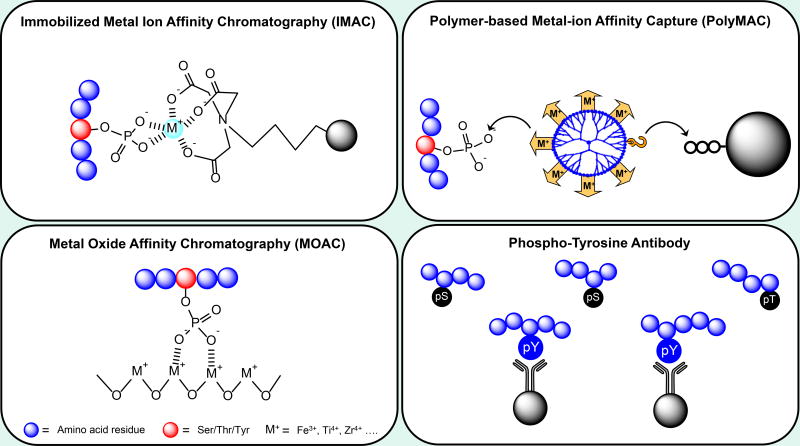
Due to the low ionization efficiency of negatively charged phosphate groups and the low stoichiometric amount of phosphoproteins in the proteome, it is very difficult to directly detect phosphopeptides by MS in complicated cellular digests or biofluids.34 To address this problem, many approaches have been developed to enrich phosphopeptides prior to MS-based analysis, including immobilized metal ion affinity chromatography (IMAC), metal oxide affinity chromatography (MOAC), polymer-based metal ion affinity capture (PolyMAC), and tyrosine phosphopeptide enrichment (Figure 2). IMAC uses the electrostatic interactions between transition metal ions (Fe3+, Ga3+, Ti4+, Zr4+, etc.)35–38 and negatively charged phosphate groups to capture phosphorylated peptides. The cations are immobilized on metal ion chelation groups, such as nitrilotriacetic acid (NTA), iminodiacetic acid (IDA), or phosphate polymer, which are on the surface of agarose-, silica-, or magnetic-based solid supports.39–41 MOAC, similar to IMAC, uses the affinity of metals in metal oxide particles (TiO2, ZrO2, Fe3O4, etc.) to retain the anionic phosphoryl groups on the matrixes.42–44
Figure 2.
Common strategies for phosphopeptide enrichment.
Enrichment specificity has historically been a serious problem that hampered the depth of phosphoproteomic profiling. The acidic non-phosphopeptides that contain glutamic or aspartic residues have been found to bind to both IMAC and MOAC materials, which interferes with the efficiency of phosphopeptide identification. To eliminate this nonspecific binding, many groups have refined enrichment protocols by adjusting the pH value of the loading buffer45 or using different organic acids46, solvents47 and additives48 to remove unwanted peptides. As of now, more than 10,000 phosphopeptides with 90% enrichment specificity can be identified in a one-shot MS analysis.33 Due to the complexity of phosphoproteome, different strategies have shown methodological preference for enriching distinct physiochemical types of phosphopeptides.49 This may be attributed to the different affinity of metal ions toward phosphate groups50 or different sample handling steps in each protocol.51 For example, Fe3+ and Ga3+-based IMAC strategies are often biased toward enrichment of multiply phosphorylated peptides, while TiO2-based chromatography is typically biased toward mono-phosphorylated peptides.49, 52, 53 Physiological loading buffers such as PBS and certain additives to prevent non-specific binding of acidic peptides (e.g. citric acid) have also been shown to bias enrichments toward multiply phosphorylated peptides, presumably because peptides with several phosphorylation groups have stronger metal ion affinity.54 Thus, the combination of different metal ions or approaches has been used to achieve wider coverage of the whole phosphoproteome.55, 56
Recently, Fe3+-IMAC has become more popular because of extensive optimization of the enrichment format. To mitigate the variation and sample loss from each manual enrichment step, the automated HPLC column57 and cartridge platform for Fe3+-IMAC58 have been developed. The benefit of column-based enrichment is that the performance is not perturbed by the bead-to-sample ratio and variation from manual steps. The automated Fe3+-IMAC format was shown to outperform batch-based Ti4+-IMAC as well as tip-based TiO2 by identifying higher number of phosphopeptide identification with lower variation between replicates. However, the heterogeneous conditions of solid-phase enrichment material and solution-phase samples also contributes to enrichment variability and poor binding efficiency to phosphopeptides. To address the drawbacks of solid-phase phosphopeptide extraction, our group developed a PolyMAC strategy by immobilizing the metal ions on the surface of the water-soluble dendrimer to capture phosphopeptides in the homogenous aqueous environment.59, 60 This strategy has demonstrated outstanding selectivity, sensitivity, and recovery when compared to Fe-IMAC and TiO2 for complex samples, and this strategy has been applied to study the phosphoproteome of animals and plants.61–64
Despite large improvement in identification of phosphoserine/phosphothreonine (pS/pT) sites, the identification of phosphotyrosine (pY) sites is still challenging because the abundance of pY is significantly lower than that of pS/pT.65 To selectively enrich pY peptides from a complex proteome, immunoprecipitation by pY antibodies has been exploited for large-scale identification.66, 67 However, the depth of pY proteome is still limited and highly reliant on the efficiency of phosphatase inhibitors. Abe et al. reported an improved protocol by optimizing the elution buffer and the LC-MS/MS parameters.68 This protocol provided more than 1,000 pY sites from 8 mg of lysate without the use of phosphatase inhibitors. Bian et al. recently developed a SH2 (Src Homology 2) superbinder-based approach to systemically identify tyrosine phosphoproteins.69 Instead of using a low affinity wild-type SH2 domain, they designed a mutated SH2 domain superbinder which has much higher affinity toward pY sites. Encouragingly, the superbinder permitted identification of more than 10,000 pY sites from nine cell lines.
Peptide Chromatographic Separations
Due to the scan speed of mass spectrometers, the depth of phosphoproteome is still limited in a single shot analysis. Thus, multidimensional chromatographies such as strong cation exchange (SCX),70 strong anion exchange (SAX),71 hydrophilic interaction chromatography (HILIC),72 electrostatic repulsion-hydrophilic interaction liquid chromatography (ERLIC),73, 74 and high pH reverse phase (RP) fractionation75–77 have been coupled with RP LC-MS/MS to analyze low abundance phosphopeptides and to enlarge the coverage of the phosphoproteome. Two types of separation approaches are used for simplifying complex samples: one is the HPLC column-based format, in which the beads are packed into a column and connected with an HPLC system. Because the column format uses linear mobile gradients, it provides better reproducibility as well as separation between fractions. The other is the StageTip-based format, in which a 3M extraction membrane is packed into a loading tip.78 The advantages of StageTip-based fractionation are high throughput, low cost, and no need for HPLC equipment. Overall, basic pH RP fractionation outperforms SCX in terms of phosphopeptide identification,75 which may be attributed to lack of an additional desalting step before MS analysis. Adachi et al. improved the performance of SCX StageTip fractionation by using acid-based elution instead of salt-based elution, and the results showed that more than 20,000 phosphopeptides from seven fractions were identified with high reproducibility.79 Due to the high selectivity of phosphopeptide enrichment, fractionation of phosphopeptides followed by phosphopeptide enrichment, often called the “enrichment-fractionation” approach, is prevalent in a variety of recent reports.57, 79, 80
Tandem MS Technologies
One of the most important roles of MS-based phosphoproteomic analysis is to localize and quantify thousands of phosphate groups on the peptides themselves, which provides useful information for biological studies. Many MS/MS fragmentation technologies including collision-induced dissociation (CID),81 high-energy collisional dissociation (HCD),82 and electron transfer dissociation (ETD)83 have been implemented in phosphoproteomic analyses. Conventionally, CID is utilized for peptide identification. However, the low-energy dissociation of CID usually fragments the labile bond between the phosphate groups and peptides, causing a dominant neutral loss peak in the spectra, and the information of phosphorylation site and peptide sequence is lost.84 To address the problem of neutral loss, ETD and HCD have been incorporated to improve phosphorylation site identification and localization. Despite the fact that ETD fragments the phosphopeptides without generating a neutral loss peak in the spectra, the poor dissociation of low charge density peptides hampers phosphopeptide identification. Recently, hybrid electron-transfer and high-energy collision dissociation (EThcD) fragmentation85 and ultraviolet photodissociation (UVPD)86, 87 have been utilized for large-scale phosphopeptide identification. Although these technologies did not show significant identification improvement, they provided complementary coverage over ETD in terms of sequence information and site localization. In general, HCD is currently selected as the routine fragmentation approach for phosphopeptide identification because of the robustness of implementation and reduced problems with neutral loss in the spectra.
Phosphorylation Site Identification
Phosphopeptide MS/MS spectra can be difficult to interpret because pS and pT peptides can produce neutral losses of H3PO4 (98 m/z) that dominate the spectra.88 In addition, pY peptides can lose the smaller PO3− ion (80 m/z), increasing not only the difficulty of site localization but also the possibility of confusing phosphorylation with another PTM of the same molecular weight such as sulfation.89 As a result, many algorithms have been developed for deciphering the MS/MS fragmentation spectra and phosphorylation sites localization. For example, the Mascot Delta score,90 PhosphoRS localization probability,91 and PTM score of MaxQuant92 are three common phosphorylation localization tools. These algorithms match the spectra generated from the mass spectrometer with a protein sequence database and use probabilistic models to assign the probabilities of potential phosphorylation sites. Although these algorithms speed up the identification of phosphorylation sites, it is difficult to ascertain whether the matching results are reliable or not. To estimate searching accuracy and reproducibility, a large number of library phosphopeptides with known phosphorylation sites were synthesized and analyzed by LC-MS/MS.93 This phosphopeptide library and its corresponding fragmentation spectra provide a resource for informatics researchers to further examine and improve the accuracy and reliability of phosphorylation localization algorithms.
Quantitation Strategies for Shotgun LC-MS
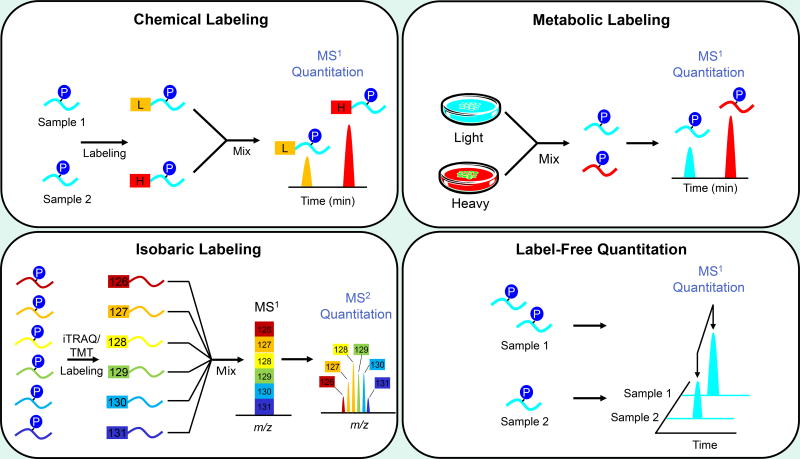
A variety of enrichment strategies and MS/MS activation methods have been established to ameliorate the difficulties associated with analyzing low abundance phosphorylation. Similarly, multiple methods—each with its own advantages and disadvantages—have been developed to improve the ease and accuracy with which changes in protein phosphorylation can be quantified (Figure 3). Phosphoproteomic experiments are frequently concerned with relative quantitation, that is, comparison of samples for determination of the biological differences at the molecular or phenotypic level. Quantitation techniques for such comparisons fall into roughly two categories, depending on whether isotopic labels are incorporated or whether the strategy is “label-free.”
Figure 3.
Popular approaches for quantitative LC-MS.
Quantitation by metabolic labeling utilizes an organism’s own cellular processes to gradually incorporate isotope labeled amino acids into its proteins, often through15N enriched media/diet94–96 or stable isotope labeling by amino acids in cell culture (SILAC).97 A significant benefit of this strategy is that labeled samples can be combined early, diminishing bias due to sample preparation. Great success has been demonstrated with the SILAC technique, which has been used to quantify thousands of phosphorylation sites in cell culture systems, including HeLa,65 MCF-7,98 human stem cells,99 and yeast.100 Interestingly, SILAC has also been applied in a study of Fmr1 knockout murine cell lines to examine phosphorylation signaling pathways that may be dysregulated in Fragile X syndrome, permitting quantification of over 5000 proteins and 6000 unique phosphorylation events.101 Direct SILAC labeling of primary cells may still be out of reach, but researchers have demonstrated that SILAC can be applied to larger systems. Spike-in SILAC, in which several SILAC cell lines that represent a particular tissue are added to the sample, has been used to quantify over 20,000 phosphorylation sites in murine liver. Similarly, tissue lysate from SILAC mice has been spiked into skin cancer samples extracted from non-labeled mice to quantify over 5200 phosphorylation sites.102
As in metabolic labeling, chemical labeling produces isotopic variants that can be distinguished from one another at the MS1 level. Labeling may be accomplished through, for example, reductive dimethylation103 or proteolytic digestion in 18O enriched water.104 Chemical isotopic labeling is generally applicable to more sample types than metabolic labeling, but depending on preexisting sample complexity, further convoluting the MS1 spectra may be undesirable.105, 106 An answer to this predicament is pre-enrichment of a subset of the proteome. Kettenbach and co-workers showed that dimethyl labeling could be performed after phosphopeptide enrichment, and their results were on par with SILAC.107 Melo-Braga et al. applied dimethyl labeling between membrane purification and phosphopeptide enrichment of human embryonic and neural stem cells.108
In contrast to other chemical labels, isobaric labeled peptides have the same chromatographic behavior and cannot be distinguished from one another at the MS1 level. Instead, activation for tandem MS causes fragmentation of the label, releasing the reporter ions that provide quantitative information.105 This labeling strategy has an obvious advantage in multiplexing capability. Commercial isobaric tags for relative and absolute quantitation (iTRAQ)109, 110 and tandem mass tag (TMT)111, 112 reagents can have up to 8 or 10 channels, respectively. Frost et al. recently demonstrated that their own isobaric dimethyl leucine (DiLeu) tags can reach 12-plex quantitation.113 A potential pitfall is precursor ion interference, which can produce falsely high reporter ion intensities. When using a high speed mass spectrometer, a solution to ratio distortion is the introduction of a MS3 event, which has been demonstrated to eliminate interference.80, 114, 115 With respect to neuropathologies, isobaric tags have been applied to phosphoproteomic studies on, for example, signaling pathways in primary neurons116 and neurofilaments from AD brain tissue.117
Isotope labeled samples are combined for one data acquisition, but label-free strategies are employed after samples have been successively analyzed by LC-MS. Quantitation is achieved through comparison of precursor/product ion chromatogram peak areas (often called extracted ion chromatograms, XICs) or through spectral counting. Although the number of times a protein is selected for tandem MS correlates roughly to its abundance, phosphoproteins may be identified by only one or two phosphopeptides that generate few MS/MS spectra; thus, ion intensity-based approaches can be more sensitive than spectral counts for phosphoproteomic studies. In comparison to isotopic labeling, label-free approaches in general permit deeper proteome coverage. This may be due to the absence of dilution effects or to the added ability to quantify spectral features across several runs after chromatogram alignment, even if those peptides were not consistently selected for MS/MS (e.g. the “match between runs” function in MaxQuant).118 However, the fact that the samples are never combined means quantitative accuracy is largely affected by the robustness of the LC-MS system. Run to run variations, particularly in chromatographic performance and ionization efficiency, can be assessed using spiked-in peptide standards.119, 120 Our group recently introduced the Library-Assisted eXtracted Ion Chromatogram (LAXIC) approach, which uses a spiked-in peptide library designed to elute across the entire chromatogram for local normalization of signal throughout the analysis.121, 122 Significant effort has also been devoted to the development of data analysis tools, including Mascot Distiller, MaxQuant,118 Skyline,123 and OpenMS,124 among others, that can align and normalize MS data for accurate label-free analyses.
Targeted LC-MS and Determination of Phosphorylation Stoichiometry
In certain cases, for example in clinical biomarker verification, it may be critical to quantify a particular set of phosphopeptides with high precision and sensitivity. Targeted approaches can be optimized for limited sample amounts and have traditionally been more amenable to absolute quantitation than shotgun experiments, due at least in part to the stochastic selection of precursor ions in data dependent acquisition (DDA).125, 126 The combination of absolute quantitation (AQUA) stable isotope labeled standard peptides with selected reaction monitoring (SRM) mass spectrometry (sometimes called multiple reaction monitoring, MRM) has been particularly effective for quantifying sets of phosphoproteins.127–129 In this approach, the ion intensities of endogenous phosphopeptides are compared to their corresponding spiked-in standards. However, as all forms of a peptide need to be monitored for accurate protein quantitation, chemical synthesis of isotope labeled standards can be costly. Some have addressed this problem through generation of “signature” proteolytic peptides through expression in E. coli130 or cell-free systems131 with heavy isotope labeled arginine and lysine. SRM-MS limits of detection are superior to those of DDA for a given set of target peptides, but SRM methods must be defined in advance for the analyte of interest, including selection of proteotypic peptides, peptide fragments, and fragmentation conditions.132 Thus, lengthy method development hinders application to more extensive phosphoproteomic studies, particularly when PTMs significantly increase the number of transitions needed to monitor a specific protein.
Parallel reaction monitoring (PRM), which takes advantage of high resolution/accurate mass instruments equipped with a quadrupole (e.g. the Q Exactive quadrupole-Orbitrap) and does not require preselection of fragment ions, has shown promise in decreasing both assay development time and cost while maintaining high sensitivity.133–135 For example, Lawrence and colleagues introduced a method to mine a large database of previous LC-MS analyses to assist with phosphopeptide selection and retention time scheduling; interestingly, for a set of 101 peptides, their “plug and play” PRM approach outperformed both DDA and DIA strategies in reproducibility and number of peptides identified.136 Urisman et al. recently demonstrated that label-free PRM multiplexing could be expanded to quantify over 800 peptides from 150 kinases in a single analysis using the separation capabilities of a long monolithic silica-C18 capillary column.133 Spiked in stable isotope labeled standards can also be used to trigger analysis of the corresponding endogenous peptide, thus optimizing data acquisition time and augmenting multiplexing capabilities.137 An interesting twist on IS-PRM incorporates TMTzero labeling of the standard trigger peptides and TMT 10plex labeling of samples to generate a targeted analysis that is multiplexed in both target peptide number and sample number.138 It is not yet clear whether these IS-PRM methods will translate from proteomics to phosphoproteomics, but increased multiplexing/throughput is a necessity for targeted MS methods to find their way toward routine use in a clinical setting.
An important but challenging aspect of quantitative phosphoproteomics is determination of site-specific phosphorylation stoichiometry, that is, the fraction of a protein that is phosphorylated at a particular site relative to the total amount of that protein.139 Site stoichiometry indicates whether changes in phosphorylation networks are due to kinase/phosphatase regulation or alterations in protein expression and provides hints as to whether a site has biological function.140 In a groundbreaking study, Olsen et al. calculated the stoichiometry of over 5000 phosphorylation sites using three SILAC-derived ratios (protein, phosphopeptide, and unmodified peptide) acquired over different mitotic states.141 Others have utilized phosphatase treatment and stable isotope labeling142, 143 or spiked-in internal standards130 to calculate site stoichiometries for a single cellular state. More recently, Tsai et al. demonstrated a kinase motif-targeting approach in which cell lysate was treated with or without a phosphatase and differentially labeled.139 After phosphopeptide enrichment, the enrichment flow-through was subjected to a kinase reaction and a second enrichment. The ratio between light and heavy phosphopeptides was then used to estimate the phosphorylation stoichiometry for over 1000 sites. The primary benefit of this strategy is reduction of sample complexity before quantitative LC-MS, but the overall success depends upon having a kinase with sufficient in vitro activity.
Kinase-substrate Mapping
Phosphorylation quantitation alone, whether relative or absolute, does not indicate distinct kinase-substrate relationships. Because kinases are both critical mediators of the very signaling pathways at the center of a variety of diseases, it is important to define specific kinase-substrate relationships. Traditional approaches to kinase substrate mapping include gene interaction screens,144 yeast two-hybrid assays,145 and in vitro kinase assays with purified kinase, γ-[32P]-ATP and putative substrates (e.g. cell lysate, protein arrays, or peptide libraries). However, evolution of better tools for phosphorylation enrichment and quantitation has facilitated development of mass spectrometry-based strategies that can complement or even replace traditional methods. For example, affinity purification can be combined with mass spectrometry (AP-MS) for enhanced sensitivity and throughput in identification of kinase interacting partners that may also be potential substrates.146 Li et al. recently demonstrated a logical extension of AP-MS for kinase-substrate mapping in which rapid isolation of native cyclin-dependent kinase complexes was followed by on-bead kinase reactions with heavy labeled ATP and LC-MS.147
The analog sensitive (AS) kinase allele approach pioneered by Shokat and colleagues is another popular technique that was initially coupled to traditional detection methods (i.e. autoradiography) and has since been integrated with MS. Genetically engineered AS kinases accommodate bulky ATP analogs that cannot be used by endogenous kinases but still retain their substrate specificity;148 thus, the phosphorylation resulting from in vitro assays with AS kinases and cell lysate is far more specific for the kinase of interest.149 In more current studies, one of the γ-phosphoryl oxygen atoms of the ATP analog has been substituted for a sulfur atom. The unique chemistry of this thiophosphate moiety allows for selective capture and identification of substrates by mass spectrometry after the kinase reaction.150–152 The quantitative identification of kinase substrates (QIKS) strategy is another extension of AS kinase technology that employs differential labeling to obtain relative quantitation; when QIKS was applied to MEK1, only ERK1/2 were identified as substrates, which may be due to either the high specificity of MEK1 or the use of only cytosolic proteins as candidate substrates.153
Instead of in vitro kinase activity, others have focused on the response of the endogenous phosphoproteome to the modification of either kinase expression or activity. This type of large-scale in vivo study highlights potential kinase substrates but does not differentiate between direct substrates and substrates that are further downstream in the affected signaling pathway. One work-around is to screen the phosphoproteome several times in response to different perturbations of kinase activity; phosphorylation sites that are altered in multiple screens are more likely to be true kinase substrates. Of particular interest is a recent study from Steger et al. that examined the substrates of leucine-rich repeat kinase 2 (LRRK2), mutations in which are a common cause of hereditary Parkinson’s disease (PD).154 In the first screen, they treated mouse embryonic fibroblasts containing a hyperactive form of LRRK2 with two structurally distinct LRRK2 inhibitors; phosphorylation events strongly regulated by both treatments were likely substrates. In the second screen, they treated wild type or inhibitor-insensitive LRRK2 knock-in cells with a selective LRRK2 inhibitor; phosphorylation events altered in the wild type but not the inhibitor-insensitive cells were putative LRRK2 cells. Although they identified over 9000 phosphorylation sites in each replicate, once they set stringent cut-offs for fold change and overlapped the results from each screen, they were left with only two phosphorylation sites: a site on LRRK2 itself and a site on the small GTPase Rab10. Interestingly, a subset of Rab GTPases were identified in a separate large-scale SILAC-based phosphoproteomic screen as the substrates of PTEN-induced kinase 1 (PINK1), a PD-related kinase that is activated by mitochondrial depolarization.155
Another way to account for problems stemming from the missing physiological context of an in vitro reaction and the downstream effects of in vivo phosphoproteomics is to look at the overlap between these two types of experiments. In the simplest form of kinase assay linked phosphoproteomics (KALIP), an in vitro reaction is performed with purified kinase and whole cell lysate and analyzed by LC-MS. Kinase activity is then altered in vivo (e.g. by small molecule inhibition), and quantitative phosphoproteomics is used to determine kinase-dependent phosphorylation. Finally, the results from both screens are overlapped with one another, and putative substrates appearing in both are further validated. The KALIP approach has been used to extensively examine the direct substrates of the spleen tyrosine kinase SYK63, 156 as well as the S/T kinase ERK1.157 In the case of ERK1, a stable isotope labeled form of ATP was introduced to further enhance the sensitivity of the in vitro reaction; since cell lysate is used as the candidate substrate pool, addition of a heavy phosphate group makes it significantly easier to differentiate between ERK1 activity and background endogenous phosphorylation.
Finally, it should be noted that kinases are only part of the cellular phosphorylation machinery; perhaps equally important are the phosphatases responsible for the removal of protein phosphorylation. There are fewer encoded phosphatases than kinases, leading to the assumption that phosphatases lack specificity. In reality, structural variety (e.g. docking motifs or different subunit combinations) provides substantial specificity.158 There has been some success in examining phosphatase interaction partners with a mammalian two-hybrid system159 or cross-linking mass spectrometry,160 but because these studies indicate what proteins simply associate with phosphatase complexes, additional work is needed to verify their identities as substrates. Another strategy involves mutation of the phosphatase catalytic domain so that it binds tightly to potential substrates. This substrate trapping approach has been combined with phosphoproteomics to identify substrates of a Drosophila melanogaster protein tyrosine phosphatase.161 However, significant work remains to fully characterize physiological phosphatase substrates, an issue made more pressing by the fact that phosphatases have been proposed as therapeutic targets in certain types of cancer,162 epilepsy,163 PD,164 and Alzheimer’s disease (AD).165
PTM Crosstalk
There is burgeoning evidence of phosphorylation crosstalk, where one phosphorylation event promotes or impedes the phosphorylation of another site, outside of well-characterized histone tails.166–168 For instance, priming phosphorylation of a tyrosine residue in the activation loop of ABL stimulates its kinase activity,169 while phosphorylation of an N-terminal serine residue in GSK3 inhibits its kinase activity.170 PTMs may act in concert, as in the activation of MAPK kinases through diphosphorylation of a TXY motif.171 However, phosphorylation does not occur in isolation. Other modifications such as methylation, acetylation, SUMOylation, neddylation, ubiquitination, and O-GlcNAcylation often occur at or in close proximity to phosphorylation sites,168, 172–174 adding combinatorial specificity and nuance in signal transduction. There are a variety of mechanisms by which PTMs participate in crosstalk. For instance, one modification may directly compete with another for the same site (e.g. methylation, acetylation, and ubiquitination of lysine residues or nitration, sulfation, and phosphorylation of tyrosine residues).168, 175 A PTM may also serve as a signal in cis for addition/removal of a second modification or modulate other modifications in trans by providing or blocking a recognition site, thereby determining whether necessary binding partners can access the second modification site.168, 176, 177
There are many examples of both cis and trans phosphorylation-ubiquitination interplay in the literature. In one example, Tong and co-workers used affinity purification LC-MS/MS to examine the changes in phosphorylation and ubiquitination of EGFR in response to its endocytotic internalization and found that EGF stimulation resulted in initial increases in Y phosphorylation and K ubiquitination; upon receptor internalization, these PTMs decreased in favor of increasing S/T phosphorylation.178 On a larger scale, Swaney et al. utilized SILAC and proteasome inhibition along with serial enrichment to identify co-occurring phosphorylation and ubiquitination in S. cerevisiae. They used SCX to enrich doubly charged peptides which were then subjected to diGly immunoaffinity purification to identify phosphopeptides that also included the diGly artifact typically left on ubiquitinated lysine residues after trypsin digestion. In total, 466 proteins with over 2,000 co-occurring phosphorylation and ubiquitination sites were identified along with several novel phosphodegron motifs, short sequences that promote ubiquitination upon phosphorylation.179 Another group examined the phosphoproteome and ubiquitylome separately in S. cerevisiae after rapamycin treatment to identify pathways co-regulated by the two PTMs in response to TOR inhibition.100 Finally, Mertins et al. employed extensive basic pH reversed-phase fractionation with serial enrichments of different post-translational modifications (SEPTM) to examine PTMs in SILAC-labeled human cell lines upon proteasome inhibition; using this approach, they were able to identify more than 20,000 phosphorylation, 15,000 ubiquitination and 3,000 acetylation sites from nearly 8,000 proteins.180
It is becoming apparent that cross-talk between phosphorylation and other PTMs can play an important role in disease, including neuropathologies. It was discovered through large-scale proteomic studies that ubiquitin itself can be phosphorylated on several residues.179, 181 Interestingly, the Parkinson’s disease related protein kinase PINK1 can phosphorylate both ubiquitin and the E3 ligase Parkin; phosphorylated ubiquitin then recruits and activates Parkin, which ubiquitinates other proteins,182–184 which may be additional PINK1 substrates.185 The interactions of PINK1, ubiquitin, and Parkin help mark damaged mitochondria for mitophagy, a process that can be disrupted in hereditary and sporadic PD.186 Aside from ubiquitination, methylation and O-GlcNAcylation are examples of PTMs that have known interactions with phosphorylation. For instance, it was recently demonstrated that a critical voltage gated sodium channel in rat brains is reciprocally regulated by methylation and phosphorylation after seizure induction.187 Another group utilized SILAC followed by O-GlcNAcylation chemoenzymatic tagging/enrichment and MOAC to show that overexpression of O-GlcNAc transferase (OGT) in HeLa cells altered the phosphorylation of around 25% of sites identified in mitotic spindles and midbodies by more than two-fold.188 Others have demonstrated a reciprocal relationship between phosphorylation and O-GlcNAcylation of tau, a protein critical in Alzheimer’s disease.189
We have only scratched the surface of PTM interplay and how different combinations of PTMs generate “codes” for fine tuning biological responses to diverse stimuli with a relatively limited gene set. PTMs have traditionally been studied separately, but with the advent of large publicly available mass spectrometry data sets, it is possible to mine these repositories to predict PTM motifs and identify potential PTM crosstalk. Peng and co-workers developed a computational approach that extracts peptides from the PhosphoSitePlus database190 that contain adjacent PTMs (e.g. phosphorylation and acetylation), determines their motifs with Motif-X,191 analyzes their evolutionary conservation, and then generates an enrichment analysis based on gene ontologies and protein-protein interactions.192 Another group utilized PTM sequence and structural proximity along with co-evolution among vertebrates to build a naïve Bayesian model for prediction of PTM crosstalk.193 Finally, it is possible to use text mining to find PTM-dependent protein-protein interactions with enzymes that modify the same protein at another site;194 this last approach does not definitively indicate PTM crosstalk but provides sites for further study.
Biomarker Discovery
Mass spectrometry-based proteomics initially showed great promise in the search for biomarkers that could be used to diagnose, predict, or monitor disease progress. Despite significant efforts, few if any of the proposed protein biomarkers are routinely analyzed by mass spectrometry in a clinical setting.195–197 However, new advances in targeted MS analyses, especially in high resolution/accurate mass instrumentation and SRM/PRM method development, could be key for clinical translation.195 We also posit that rather than quantifying whole proteins, it may be more helpful to define and monitor certain protein PTMs. Phosphoproteins are particularly promising potential biomarkers for CNS disorders because the extent of phosphorylation can be descriptive of disease state.196, 198, 199 In fact, phosphoprotein biomarkers have been proposed for several neurological disorders using other analytical techniques such as ELISA (Table 1).
Table 1.
Selection of putative phosphoprotein biomarkers for neurological diseases
| Disease | Phosphoprotein | Biological Source | References |
|---|---|---|---|
|
| |||
| Multiple Sclerosis | Tau | CSF | 218, 219 |
|
| |||
| Autism Spectrum Disorders | Histatin | Saliva | 220, 221 |
| Slatherin | |||
| Acidic proline-rich phosphoprotein | |||
|
| |||
| Traumatic Brain Injury | Neurofilament-H | Blood | 222 |
|
| |||
| Amyotrophic Lateral Sclerosis | Neurofilament-H | CSF | 223, 224 |
|
| |||
| Blood | 225 | ||
|
| |||
| Creutzfeldt-Jakob Disease | Neurofilament-H | CSF | 226, 227 |
| Tau | |||
The clinical diagnosis of many neurodegenerative diseases often occurs relatively late in their progression, after significant neurodegeneration has already occurred. The inherent heterogeneity of these diseases also makes sensitive and specific diagnosis extremely difficult, spurring the drive to identify biomarkers in patient proximal biofluids that can correlate clinical cognitive assessments. As an example, the most extensively studied protein biomarkers for Alzheimer’s disease are amyloid beta peptide (Aβ1–42), total Tau (T-Tau), and phosphorylated Tau (P-Tau).200–202 Interestingly, the activity of the glycogen synthase kinase-3 (GSK3), which phosphorylates Tau on several of its 39 known phosphosites, has also been implicated in stimulating production of Aβ peptide via phosphorylation of its precursor protein APP.203 An abnormal, hyperphosphorylated form of Tau is responsible for the neurofibrillary tangles found in the brains of AD patients, making Tau protein an attractive biomarker for the disease.204, 205 However, It is still not clear whether the aggregation of hyperphosphorylated Tau is responsible for AD pathology or if it is simply a symptom.
An important consideration for biomarker discovery and validation via MS-based phosphoproteomics is the sample source. For example, although patient cerebrospinal fluid (CSF) is a source of putative AD biomarkers, studies using plasma have been less conclusive.205, 206 It has also been demonstrated that the ratio of circulating T-Tau and P-Tau in the CSF can distinguish patients with AD from those with other Tauopathies, including Creutzfeldt-Jakob disease.204, 205, 207 These biomarker studies have historically relied upon enzyme-linked immunosorbent assays (ELISA), but recent phosphoproteomic experiments have confirmed these findings and identified additional novel phosphoprotein biomarkers for AD.205, 208
Small, extracellular vesicles called exosomes that are present in biofluids have emerged as a prospective source of biomarkers for several diseases.209, 210 The cargo carried by exosomes includes miRNA, DNA, proteins, and lipids, and the composition of this cargo changes to modulate various pathological processes and communicate messages to other cells.209, 211 Biomarker discovery efforts in exosomes have largely focused on their miRNA content, but considering the early success of such studies for AD,210, 212 exosomal proteins and their post translationally modified forms are a promising direction for biomarker discovery. Importantly, exosomal phosphoproteins are encapsulated inside a lipid membrane, protecting them from external proteases and phosphatases. This could make them more useful as clinical biomarkers than circulating proteins in the blood or CSF.213, 214 In a recent study, Chen et al. isolated exosomal phosphoproteins from plasma and used label-free quantitative LC-MS to identify over 100 phosphoproteins that were significantly more abundant in breast cancer patients compared to healthy controls; they then validated a subset of this list in individual patients using PRM-MS to demonstrate the utility of exosomal phosphoproteins as biomarkers for breast cancer.215 For CNS diseases in which protein phosphorylation is critical to pathology, exosomal phosphoproteins may also become increasingly important markers of disease.
Future Perspectives
The field of phosphoproteomics has long been concerned with deep coverage of protein phosphorylation,69, 216, 217 but while these broad surveys are incredibly important, they are still essentially snapshots taken at different points during the cellular cycle. It is possible to identify tens of thousands of phosphorylation sites, especially with extensive pre-fractionation, but issues with stochastic sampling remain in shotgun experiments, and the choice of protease, enrichment strategy, quantitation approach, and sample storage method can still introduce slight bias. With these considerations in mind, now may be the time to move away from efforts to cover 100% of the phosphoproteome in a single experiment and return to more narrowly focused analyses. Targeted analyses in particular may substantially benefit personalized medicine efforts, especially if multiplexing capabilities are further enhanced to facilitate throughput. Phosphoproteomics already has a large role in oncology, but based on our evolving understanding of protein phosphorylation signaling and stoichiometry, we foresee application to additional studies of neuropathologies with an emphasis on analysis of clinical samples.
ABBREVIATIONS
- AD
Alzheimer’s disease
- AQUA
Absolute quantification
- CID
Collision induced dissociation
- ETD
Electron transfer dissociation
- HCD
Higher energy collision induced dissociation
- IMAC
Immobilized metal affinity chromatography
- LC-MS
Liquid chromatography-mass spectrometry
- KALIP
Kinase assay linked phosphoproteomics
- MOAC
Metal oxide affinity chromatography
- MS/MS
Tandem mass spectrometry
- PD
Parkinson’s disease
- PolyMAC
Polymer-based metal ion affinity capture
- PRM-MS
Parallel reaction monitoring-mass spectrometry
- PTM
Post-translational modification
- SRM-MS
Selected reaction monitoring-mass spectrometry
- SCX
Strong cation exchange chromatography
- SILAC
Stable isotope labeling by amino acids in cell culture
- XIC
Extracted ion chromatogram
References
- 1.Moorhead GB, Trinkle-Mulcahy L, Ulke-Lemee A. Nat Rev Mol Cell Biol. 2007;8:234–244. doi: 10.1038/nrm2126. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Salminen A, Yang X, Luo Y, Wu Q, White M, Greenhaw J, Ren L, Bryant M, Salminen W, Papoian T, Mattes W, Shi Q. Arch Toxicol. 2016 doi: 10.1007/s00204-016-1918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu P, Nielsen TE, Clausen MH. Drug Discov Today. 2016;21:5–10. doi: 10.1016/j.drudis.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Knapp S, Sundstrom M. Curr Opin Pharmacol. 2014;17:58–63. doi: 10.1016/j.coph.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Nygaard HB, Wagner AF, Bowen GS, Good SP, MacAvoy MG, Strittmatter KA, Kaufman AC, Rosenberg BJ, Sekine-Konno T, Varma P, Chen K, Koleske AJ, Reiman EM, Strittmatter SM, van Dyck CH. Alzheimers Res Ther. 2015;7:35. doi: 10.1186/s13195-015-0119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bucchia M, Ramirez A, Parente V, Simone C, Nizzardo M, Magri F, Dametti S, Corti S. Clin Ther. 2015;37:668–680. doi: 10.1016/j.clinthera.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 7.Rask-Andersen M, Zhang J, Fabbro D, Schioth HB. Trends Pharmacol Sci. 2014;35:604–620. doi: 10.1016/j.tips.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Nita-Lazar A, Saito-Benz H, White FM. Proteomics. 2008;8:4433–4443. doi: 10.1002/pmic.200800231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mann M, Ong SE, Gronborg M, Steen H, Jensen ON, Pandey A. Trends Biotechnol. 2002;20:261–268. doi: 10.1016/s0167-7799(02)01944-3. [DOI] [PubMed] [Google Scholar]
- 10.Trentini DB, Fuhrmann J, Mechtler K, Clausen T. Mol Cell Proteomics. 2014;13:1953–1964. doi: 10.1074/mcp.O113.035790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cousin C, Derouiche A, Shi L, Pagot Y, Poncet S, Mijakovic I. FEMS Microbiol Lett. 2013;346:11–19. doi: 10.1111/1574-6968.12189. [DOI] [PubMed] [Google Scholar]
- 12.Macek B, Mijakovic I, Olsen JV, Gnad F, Kumar C, Jensen PR, Mann M. Mol Cell Proteomics. 2007;6:697–707. doi: 10.1074/mcp.M600464-MCP200. [DOI] [PubMed] [Google Scholar]
- 13.Macek B, Gnad F, Soufi B, Kumar C, Olsen JV, Mijakovic I, Mann M. Mol Cell Proteomics. 2008;7:299–307. doi: 10.1074/mcp.M700311-MCP200. [DOI] [PubMed] [Google Scholar]
- 14.Elsholz AK, Turgay K, Michalik S, Hessling B, Gronau K, Oertel D, Mader U, Bernhardt J, Becher D, Hecker M, Gerth U. Proc Natl Acad Sci U S A. 2012;109:7451–7456. doi: 10.1073/pnas.1117483109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trentini DB, Suskiewicz MJ, Heuck A, Kurzbauer R, Deszcz L, Mechtler K, Clausen T. Nature. 2016;539:48–53. doi: 10.1038/nature20122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lundby A, Secher A, Lage K, Nordsborg NB, Dmytriyev A, Lundby C, Olsen JV. Nature communications. 2012;3:876. doi: 10.1038/ncomms1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Gould TD, Yuan P, Manji HK, Chen G. Neuropsychopharmacology. 2003;28:1017–1025. doi: 10.1038/sj.npp.1300112. [DOI] [PubMed] [Google Scholar]
- 18.Shabihkhani M, Lucey GM, Wei B, Mareninov S, Lou JJ, Vinters HV, Singer EJ, Cloughesy TF, Yong WH. Clin Biochem. 2014;47:258–266. doi: 10.1016/j.clinbiochem.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mertins P, Yang F, Liu T, Mani DR, Petyuk VA, Gillette MA, Clauser KR, Qiao JW, Gritsenko MA, Moore RJ, Levine DA, Townsend R, Erdmann-Gilmore P, Snider JE, Davies SR, Ruggles KV, Fenyo D, Kitchens RT, Li S, Olvera N, Dao F, Rodriguez H, Chan DW, Liebler D, White F, Rodland KD, Mills GB, Smith RD, Paulovich AG, Ellis M, Carr SA. Mol Cell Proteomics. 2014;13:1690–1704. doi: 10.1074/mcp.M113.036392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunner AM, Lossl P, Liu F, Huguet R, Mullen C, Yamashita M, Zabrouskov V, Makarov A, Altelaar AF, Heck AJ. Anal Chem. 2015;87:4152–4158. doi: 10.1021/acs.analchem.5b00162. [DOI] [PubMed] [Google Scholar]
- 21.McLafferty FW, Breuker K, Jin M, Han X, Infusini G, Jiang H, Kong X, Begley TP. FEBS J. 2007;274:6256–6268. doi: 10.1111/j.1742-4658.2007.06147.x. [DOI] [PubMed] [Google Scholar]
- 22.Switzar L, Giera M, Niessen WM. J Proteome Res. 2013;12:1067–1077. doi: 10.1021/pr301201x. [DOI] [PubMed] [Google Scholar]
- 23.Burkhart JM, Schumbrutzki C, Wortelkamp S, Sickmann A, Zahedi RP. J Proteomics. 2012;75:1454–1462. doi: 10.1016/j.jprot.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Guo X, Trudgian DC, Lemoff A, Yadavalli S, Mirzaei H. Mol Cell Proteomics. 2014;13:1573–1584. doi: 10.1074/mcp.M113.035170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dickhut C, Feldmann I, Lambert J, Zahedi RP. J Proteome Res. 2014;13:2761–2770. doi: 10.1021/pr401181y. [DOI] [PubMed] [Google Scholar]
- 26.Giansanti P, Aye TT, van den Toorn H, Peng M, van Breukelen B, Heck AJ. Cell reports. 2015;11:1834–1843. doi: 10.1016/j.celrep.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 27.Bian Y, Ye M, Song C, Cheng K, Wang C, Wei X, Zhu J, Chen R, Wang F, Zou H. J Proteome Res. 2012;11:2828–2837. doi: 10.1021/pr300242w. [DOI] [PubMed] [Google Scholar]
- 28.Gonczarowska-Jorge H, Dell'Aica M, Dickhut C, Zahedi RP. Methods Mol Biol. 2016;1355:225–239. doi: 10.1007/978-1-4939-3049-4_15. [DOI] [PubMed] [Google Scholar]
- 29.Linke D, Koudelka T, Becker A, Tholey A. Rapid Commun Mass Spectrom. 2015;29:919–926. doi: 10.1002/rcm.7185. [DOI] [PubMed] [Google Scholar]
- 30.Wisniewski JR, Nagaraj N, Zougman A, Gnad F, Mann M. J Proteome Res. 2010;9:3280–3289. doi: 10.1021/pr1002214. [DOI] [PubMed] [Google Scholar]
- 31.Wakabayashi M, Yoshihara H, Masuda T, Tsukahara M, Sugiyama N, Ishihama Y. J Proteome Res. 2014;13:915–924. doi: 10.1021/pr400960r. [DOI] [PubMed] [Google Scholar]
- 32.Ostasiewicz P, Zielinska DF, Mann M, Wisniewski JR. J Proteome Res. 2010;9:3688–3700. doi: 10.1021/pr100234w. [DOI] [PubMed] [Google Scholar]
- 33.Humphrey SJ, Azimifar SB, Mann M. Nat Biotechnol. 2015;33:990–995. doi: 10.1038/nbt.3327. [DOI] [PubMed] [Google Scholar]
- 34.Lemeer S, Heck AJ. Curr Opin Chem Biol. 2009;13:414–420. doi: 10.1016/j.cbpa.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 35.Andersson L, Porath J. Anal Biochem. 1986;154:250–254. doi: 10.1016/0003-2697(86)90523-3. [DOI] [PubMed] [Google Scholar]
- 36.Posewitz MC, Tempst P. Anal Chem. 1999;71:2883–2892. doi: 10.1021/ac981409y. [DOI] [PubMed] [Google Scholar]
- 37.Zhou H, Ye M, Dong J, Corradini E, Cristobal A, Heck AJ, Zou H, Mohammed S. Nat Protoc. 2013;8:461–480. doi: 10.1038/nprot.2013.010. [DOI] [PubMed] [Google Scholar]
- 38.Zhou H, Xu S, Ye M, Feng S, Pan C, Jiang X, Li X, Han G, Fu Y, Zou H. J Proteome Res. 2006;5:2431–2437. doi: 10.1021/pr060162f. [DOI] [PubMed] [Google Scholar]
- 39.Abelin JG, Trantham PD, Penny SA, Patterson AM, Ward ST, Hildebrand WH, Cobbold M, Bai DL, Shabanowitz J, Hunt DF. Nat Protoc. 2015;10:1308–1318. doi: 10.1038/nprot.2015.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou H, Ye M, Dong J, Han G, Jiang X, Wu R, Zou H. J Proteome Res. 2008;7:3957–3967. doi: 10.1021/pr800223m. [DOI] [PubMed] [Google Scholar]
- 41.Wu HT, Hsu CC, Tsai CF, Lin PC, Lin CC, Chen YJ. Proteomics. 2011;11:2639–2653. doi: 10.1002/pmic.201000768. [DOI] [PubMed] [Google Scholar]
- 42.Larsen MR, Thingholm TE, Jensen ON, Roepstorff P, Jorgensen TJD. Molecular & Cellular Proteomics. 2005;4:873–886. doi: 10.1074/mcp.T500007-MCP200. [DOI] [PubMed] [Google Scholar]
- 43.Wolschin F, Wienkoop S, Weckwerth W. Proteomics. 2005;5:4389–4397. doi: 10.1002/pmic.200402049. [DOI] [PubMed] [Google Scholar]
- 44.Kweon HK, Hakansson K. Anal Chem. 2006;78:1743–1749. doi: 10.1021/ac0522355. [DOI] [PubMed] [Google Scholar]
- 45.Tsai CF, Wang YT, Chen YR, Lai CY, Lin PY, Pan KT, Chen JY, Khoo KH, Chen YJ. J Proteome Res. 2008;7:4058–4069. doi: 10.1021/pr800364d. [DOI] [PubMed] [Google Scholar]
- 46.Sugiyama N, Masuda T, Shinoda K, Nakamura A, Tomita M, Ishihama Y. Molecular & Cellular Proteomics. 2007;6:1103–1109. doi: 10.1074/mcp.T600060-MCP200. [DOI] [PubMed] [Google Scholar]
- 47.Ye J, Zhang X, Young C, Zhao X, Hao Q, Cheng L, Jensen ON. J Proteome Res. 2010;9:3561–3573. doi: 10.1021/pr100075x. [DOI] [PubMed] [Google Scholar]
- 48.Fukuda I, Hirabayashi-Ishioka Y, Sakikawa I, Ota T, Yokoyama M, Uchiumi T, Morita A. J Proteome Res. 2013;12:5587–5597. doi: 10.1021/pr400546u. [DOI] [PubMed] [Google Scholar]
- 49.Thingholm TE, Jensen ON, Robinson PJ, Larsen MR. Mol Cell Proteomics. 2008;7:661–671. doi: 10.1074/mcp.M700362-MCP200. [DOI] [PubMed] [Google Scholar]
- 50.Lai AC, Tsai CF, Hsu CC, Sun YN, Chen YJ. Rapid Commun Mass Spectrom. 2012;26:2186–2194. doi: 10.1002/rcm.6327. [DOI] [PubMed] [Google Scholar]
- 51.Kyono Y, Sugiyama N, Imami K, Tomita M, Ishihama Y. J Proteome Res. 2008;7:4585–4593. doi: 10.1021/pr800305y. [DOI] [PubMed] [Google Scholar]
- 52.Bodenmiller B, Mueller LN, Mueller M, Domon B, Aebersold R. Nat Methods. 2007;4:231–237. doi: 10.1038/nmeth1005. [DOI] [PubMed] [Google Scholar]
- 53.Yue X, Schunter A, Hummon AB. Anal Chem. 2015;87:8837–8844. doi: 10.1021/acs.analchem.5b01833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jensen SS, Larsen MR. Rapid Commun Mass Spectrom. 2007;21:3635–3645. doi: 10.1002/rcm.3254. [DOI] [PubMed] [Google Scholar]
- 55.Tsai CF, Hsu CC, Hung JN, Wang YT, Choong WK, Zeng MY, Lin PY, Hong RW, Sung TY, Chen YJ. Anal Chem. 2014;86:685–693. doi: 10.1021/ac4031175. [DOI] [PubMed] [Google Scholar]
- 56.Sun Z, Hamilton KL, Reardon KF. Anal Biochem. 2014;445:30–37. doi: 10.1016/j.ab.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruprecht B, Koch H, Medard G, Mundt M, Kuster B, Lemeer S. Mol Cell Proteomics. 2015;14:205–215. doi: 10.1074/mcp.M114.043109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Post H, Penning R, Fitzpatrick MA, Garrigues LB, Wu W, MacGillavry HD, Hoogenraad CC, Heck AJ, Altelaar AF. J Proteome Res. 2017;16:728–737. doi: 10.1021/acs.jproteome.6b00753. [DOI] [PubMed] [Google Scholar]
- 59.Iliuk AB, Martin VA, Alicie BM, Geahlen RL, Tao WA. Mol Cell Proteomics. 2010;9:2162–2172. doi: 10.1074/mcp.M110.000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iliuk A, Jayasundera K, Wang WH, Schluttenhofer R, Geahlen RL, Tao WA. International journal of mass spectrometry. 2015;377:744–753. doi: 10.1016/j.ijms.2014.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang PC, Xue L, Batelli G, Lee S, Hou YJ, Van Oosten MJ, Zhang HM, Tao WA, Zhu JK. P Natl Acad Sci USA. 2013;110:11205–11210. doi: 10.1073/pnas.1308974110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Radu M, Rawat SJ, Beeser A, Iliuk A, Tao WA, Chernoff J. J Biol Chem. 2013;288:21117–21125. doi: 10.1074/jbc.M113.459719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xue L, Wang WH, Iliuk A, Hu L, Galan JA, Yu S, Hans M, Geahlen RL, Tao WA. Proc Natl Acad Sci U S A. 2012;109:5615–5620. doi: 10.1073/pnas.1119418109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Searleman AC, Iliuk AB, Collier TS, Chodosh LA, Tao WA, Bose R. Electrophoresis. 2014;35:3463–3469. doi: 10.1002/elps.201400022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M. Cell. 2006;127:635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 66.Di Palma S, Zoumaro-Djayoon A, Peng M, Post H, Preisinger C, Munoz J, Heck AJ. J Proteomics. 2013;91:331–337. doi: 10.1016/j.jprot.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 67.van der Mijn JC, Labots M, Piersma SR, Pham TV, Knol JC, Broxterman HJ, Verheul HM, Jimenez CR. J Proteomics. 2015;127:259–263. doi: 10.1016/j.jprot.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 68.Abe Y, Nagano M, Tada A, Adachi J, Tomonaga T. J Proteome Res. 2017;16:1077–1086. doi: 10.1021/acs.jproteome.6b00576. [DOI] [PubMed] [Google Scholar]
- 69.Bian Y, Li L, Dong M, Liu X, Kaneko T, Cheng K, Liu H, Voss C, Cao X, Wang Y, Litchfield D, Ye M, Li SS, Zou H. Nat Chem Biol. 2016;12:959–966. doi: 10.1038/nchembio.2178. [DOI] [PubMed] [Google Scholar]
- 70.Hennrich ML, van den Toorn HW, Groenewold V, Heck AJ, Mohammed S. Anal Chem. 2012;84:1804–1808. doi: 10.1021/ac203303t. [DOI] [PubMed] [Google Scholar]
- 71.Dong M, Ye M, Cheng K, Song C, Pan Y, Wang C, Bian Y, Zou H. J Proteome Res. 2012;11:4673–4681. doi: 10.1021/pr300503z. [DOI] [PubMed] [Google Scholar]
- 72.Zhou H, Di Palma S, Preisinger C, Peng M, Polat AN, Heck AJ, Mohammed S. J Proteome Res. 2013;12:260–271. doi: 10.1021/pr300630k. [DOI] [PubMed] [Google Scholar]
- 73.Zarei M, Sprenger A, Gretzmeier C, Dengjel J. J Proteome Res. 2012;11:4269–4276. doi: 10.1021/pr300375d. [DOI] [PubMed] [Google Scholar]
- 74.Loroch S, Zahedi RP, Sickmann A. Anal Chem. 2015;87:1596–1604. doi: 10.1021/ac502708m. [DOI] [PubMed] [Google Scholar]
- 75.Batth TS, Francavilla C, Olsen JV. J Proteome Res. 2014;13:6176–6186. doi: 10.1021/pr500893m. [DOI] [PubMed] [Google Scholar]
- 76.Yue XS, Hummon AB. J Proteome Res. 2013;12:4176–4186. doi: 10.1021/pr4005234. [DOI] [PubMed] [Google Scholar]
- 77.Fleitz A, Nieves E, Madrid-Aliste C, Fentress SJ, Sibley LD, Weiss LM, Angeletti RH, Che FY. Anal Chem. 2013;85:8566–8576. doi: 10.1021/ac401691g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rappsilber J, Mann M, Ishihama Y. Nat Protoc. 2007;2:1896–1906. doi: 10.1038/nprot.2007.261. [DOI] [PubMed] [Google Scholar]
- 79.Adachi J, Hashiguchi K, Nagano M, Sato M, Sato A, Fukamizu K, Ishihama Y, Tomonaga T. Anal Chem. 2016;88:7899–7903. doi: 10.1021/acs.analchem.6b01232. [DOI] [PubMed] [Google Scholar]
- 80.Erickson BK, Jedrychowski MP, McAlister GC, Everley RA, Kunz R, Gygi SP. Anal Chem. 2015;87:1241–1249. doi: 10.1021/ac503934f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Crowe MC, Brodbelt JS. J Am Soc Mass Spectrom. 2004;15:1581–1592. doi: 10.1016/j.jasms.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 82.Nagaraj N, D'Souza RC, Cox J, Olsen JV, Mann M. J Proteome Res. 2010;9:6786–6794. doi: 10.1021/pr100637q. [DOI] [PubMed] [Google Scholar]
- 83.Syka JE, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. Proc Natl Acad Sci U S A. 2004;101:9528–9533. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown R, Stuart SA, Houel S, Ahn NG, Old WM. J Am Soc Mass Spectrom. 2015;26:1128–1142. doi: 10.1007/s13361-015-1109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Frese CK, Altelaar AF, van den Toorn H, Nolting D, Griep-Raming J, Heck AJ, Mohammed S. Anal Chem. 2012;84:9668–9673. doi: 10.1021/ac3025366. [DOI] [PubMed] [Google Scholar]
- 86.Fort KL, Dyachenko A, Potel CM, Corradini E, Marino F, Barendregt A, Makarov AA, Scheltema RA, Heck AJ. Anal Chem. 2016;88:2303–2310. doi: 10.1021/acs.analchem.5b04162. [DOI] [PubMed] [Google Scholar]
- 87.Robinson MR, Taliaferro JM, Dalby KN, Brodbelt JS. J Proteome Res. 2016;15:2739–2748. doi: 10.1021/acs.jproteome.6b00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Steen H, Jebanathirajah JA, Rush J, Morrice N, Kirschner MW. Mol Cell Proteomics. 2006;5:172–181. doi: 10.1074/mcp.M500135-MCP200. [DOI] [PubMed] [Google Scholar]
- 89.Gharib M, Marcantonio M, Lehmann SG, Courcelles M, Meloche S, Verreault A, Thibault P. Mol Cell Proteomics. 2009;8:506–518. doi: 10.1074/mcp.M800327-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Savitski MM, Lemeer S, Boesche M, Lang M, Mathieson T, Bantscheff M, Kuster B. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M110.003830. M110 003830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Taus T, Kocher T, Pichler P, Paschke C, Schmidt A, Henrich C, Mechtler K. J Proteome Res. 2011;10:5354–5362. doi: 10.1021/pr200611n. [DOI] [PubMed] [Google Scholar]
- 92.Cox J, Mann M. Nature Biotechnology. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 93.Marx H, Lemeer S, Schliep JE, Matheron L, Mohammed S, Cox J, Mann M, Heck AJ, Kuster B. Nat Biotechnol. 2013;31:557–564. doi: 10.1038/nbt.2585. [DOI] [PubMed] [Google Scholar]
- 94.Conrads TP, Alving K, Veenstra TD, Belov ME, Anderson GA, Anderson DJ, Lipton MS, Pasa-Tolic L, Udseth HR, Chrisler WB, Thrall BD, Smith RD. Anal Chem. 2001;73:2132–2139. doi: 10.1021/ac001487x. [DOI] [PubMed] [Google Scholar]
- 95.McClatchy DB, Dong MQ, Wu CC, Venable JD, Yates JR., 3rd J Proteome Res. 2007;6:2005–2010. doi: 10.1021/pr060599n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oda Y, Huang K, Cross FR, Cowburn D, Chait BT. Proc Natl Acad Sci U S A. 1999;96:6591–6596. doi: 10.1073/pnas.96.12.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, Mann M. Mol Cell Proteomics. 2002;1:376–386. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- 98.Imami K, Sugiyama N, Tomita M, Ishihama Y. Molecular bioSystems. 2010;6:594–602. doi: 10.1039/b921379a. [DOI] [PubMed] [Google Scholar]
- 99.Rigbolt KT, Prokhorova TA, Akimov V, Henningsen J, Johansen PT, Kratchmarova I, Kassem M, Mann M, Olsen JV, Blagoev B. Sci Signal. 2011;4:rs3. doi: 10.1126/scisignal.2001570. [DOI] [PubMed] [Google Scholar]
- 100.Iesmantavicius V, Weinert BT, Choudhary C. Mol Cell Proteomics. 2014;13:1979–1992. doi: 10.1074/mcp.O113.035683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Matic K, Eninger T, Bardoni B, Davidovic L, Macek B. J Proteome Res. 2014;13:4388–4397. doi: 10.1021/pr5006372. [DOI] [PubMed] [Google Scholar]
- 102.Zanivan S, Meves A, Behrendt K, Schoof EM, Neilson LJ, Cox J, Tang HR, Kalna G, van Ree JH, van Deursen JM, Trempus CS, Machesky LM, Linding R, Wickstrom SA, Fassler R, Mann M. Cell reports. 2013;3:552–566. doi: 10.1016/j.celrep.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 103.Boersema PJ, Raijmakers R, Lemeer S, Mohammed S, Heck AJ. Nat Protoc. 2009;4:484–494. doi: 10.1038/nprot.2009.21. [DOI] [PubMed] [Google Scholar]
- 104.Yao X, Freas A, Ramirez J, Demirev PA, Fenselau C. Anal Chem. 2001;73:2836–2842. doi: 10.1021/ac001404c. [DOI] [PubMed] [Google Scholar]
- 105.Rauniyar N, Yates JR., 3rd J Proteome Res. 2014;13:5293–5309. doi: 10.1021/pr500880b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mertins P, Udeshi ND, Clauser KR, Mani DR, Patel J, Ong SE, Jaffe JD, Carr SA. Mol Cell Proteomics. 2012;11 doi: 10.1074/mcp.M111.014423. M111 014423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kettenbach AN, Sano H, Keller SR, Lienhard GE, Gerber SA. J Proteomics. 2015;114:48–60. doi: 10.1016/j.jprot.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Melo-Braga MN, Schulz M, Liu Q, Swistowski A, Palmisano G, Engholm-Keller K, Jakobsen L, Zeng X, Larsen MR. Mol Cell Proteomics. 2014;13:311–328. doi: 10.1074/mcp.M112.026898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin DJ. Mol Cell Proteomics. 2004;3:1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 110.Choe L, D'Ascenzo M, Relkin NR, Pappin D, Ross P, Williamson B, Guertin S, Pribil P, Lee KH. Proteomics. 2007;7:3651–3660. doi: 10.1002/pmic.200700316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dayon L, Hainard A, Licker V, Turck N, Kuhn K, Hochstrasser DF, Burkhard PR, Sanchez JC. Anal Chem. 2008;80:2921–2931. doi: 10.1021/ac702422x. [DOI] [PubMed] [Google Scholar]
- 112.McAlister GC, Huttlin EL, Haas W, Ting L, Jedrychowski MP, Rogers JC, Kuhn K, Pike I, Grothe RA, Blethrow JD, Gygi SP. Anal Chem. 2012;84:7469–7478. doi: 10.1021/ac301572t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Frost DC, Greer T, Li L. Anal Chem. 2015;87:1646–1654. doi: 10.1021/ac503276z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ting L, Rad R, Gygi SP, Haas W. Nat Methods. 2011;8:937–940. doi: 10.1038/nmeth.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McAlister GC, Nusinow DP, Jedrychowski MP, Wuhr M, Huttlin EL, Erickson BK, Rad R, Haas W, Gygi SP. Anal Chem. 2014;86:7150–7158. doi: 10.1021/ac502040v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yu P, Pisitkun T, Wang G, Wang R, Katagiri Y, Gucek M, Knepper MA, Geller HM. PLoS One. 2013;8:e59285. doi: 10.1371/journal.pone.0059285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rudrabhatla P, Grant P, Jaffe H, Strong MJ, Pant HC. Faseb J. 2010;24:4396–4407. doi: 10.1096/fj.10-157859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cox J, Hein MY, Luber CA, Paron I, Nagaraj N, Mann M. Molecular & Cellular Proteomics. 2014;13:2513–2526. doi: 10.1074/mcp.M113.031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Beasley-Green A, Bunk D, Rudnick P, Kilpatrick L, Phinney K. Proteomics. 2012;12:923–931. doi: 10.1002/pmic.201100522. [DOI] [PubMed] [Google Scholar]
- 120.Kim YJ, Zhan P, Feild B, Ruben SM, He T. Anal Chem. 2007;79:5651–5658. doi: 10.1021/ac070200u. [DOI] [PubMed] [Google Scholar]
- 121.Xue L, Wang P, Wang L, Renzi E, Radivojac P, Tang H, Arnold R, Zhu JK, Tao WA. Molecular & cellular proteomics : MCP. 2013;12:2354–2369. doi: 10.1074/mcp.O113.027284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Arrington JV, Xue L, Tao WA. Methods Mol Biol. 2014;1156:407–416. doi: 10.1007/978-1-4939-0685-7_27. [DOI] [PubMed] [Google Scholar]
- 123.Schilling B, Rardin MJ, MacLean BX, Zawadzka AM, Frewen BE, Cusack MP, Sorensen DJ, Bereman MS, Jing E, Wu CC, Verdin E, Kahn CR, Maccoss MJ, Gibson BW. Mol Cell Proteomics. 2012;11:202–214. doi: 10.1074/mcp.M112.017707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rost HL, Sachsenberg T, Aiche S, Bielow C, Weisser H, Aicheler F, Andreotti S, Ehrlich HC, Gutenbrunner P, Kenar E, Liang X, Nahnsen S, Nilse L, Pfeuffer J, Rosenberger G, Rurik M, Schmitt U, Veit J, Walzer M, Wojnar D, Wolski WE, Schilling O, Choudhary JS, Malmstrom L, Aebersold R, Reinert K, Kohlbacher O. Nat Methods. 2016;13:741–748. doi: 10.1038/nmeth.3959. [DOI] [PubMed] [Google Scholar]
- 125.Liu H, Sadygov RG, Yates JR., 3rd Anal Chem. 2004;76:4193–4201. doi: 10.1021/ac0498563. [DOI] [PubMed] [Google Scholar]
- 126.Gillet LC, Navarro P, Tate S, Rost H, Selevsek N, Reiter L, Bonner R, Aebersold R. Mol Cell Proteomics. 2012;11 doi: 10.1074/mcp.O111.016717. O111 016717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gerber SA, Rush J, Stemman O, Kirschner MW, Gygi SP. Proc Natl Acad Sci U S A. 2003;100:6940–6945. doi: 10.1073/pnas.0832254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kettenbach AN, Rush J, Gerber SA. Nat Protoc. 2011;6:175–186. doi: 10.1038/nprot.2010.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mayya V, Rezual K, Wu L, Fong MB, Han DK. Mol Cell Proteomics. 2006;5:1146–1157. doi: 10.1074/mcp.T500029-MCP200. [DOI] [PubMed] [Google Scholar]
- 130.Johnson H, Eyers CE, Eyers PA, Beynon RJ, Gaskell SJ. J Am Soc Mass Spectrom. 2009;20:2211–2220. doi: 10.1016/j.jasms.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 131.Narumi R, Shimizu Y, Ukai-Tadenuma M, Ode KL, Kanda GN, Shinohara Y, Sato A, Matsumoto K, Ueda HR. Proc Natl Acad Sci U S A. 2016;113:E3461–3467. doi: 10.1073/pnas.1603799113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bauer M, Ahrne E, Baron AP, Glatter T, Fava LL, Santamaria A, Nigg EA, Schmidt A. J Proteome Res. 2014;13:5973–5988. doi: 10.1021/pr500860c. [DOI] [PubMed] [Google Scholar]
- 133.Urisman A, Levin RS, Gordan JD, Webber JT, Hernandez H, Ishihama Y, Shokat KM, Burlingame AL. Mol Cell Proteomics. 2017;16:265–277. doi: 10.1074/mcp.M116.058172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gallien S, Duriez E, Crone C, Kellmann M, Moehring T, Domon B. Mol Cell Proteomics. 2012;11:1709–1723. doi: 10.1074/mcp.O112.019802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Peterson AC, Russell JD, Bailey DJ, Westphall MS, Coon JJ. Mol Cell Proteomics. 2012;11:1475–1488. doi: 10.1074/mcp.O112.020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lawrence RT, Searle BC, Llovet A, Villen J. Nat Methods. 2016;13:431–434. doi: 10.1038/nmeth.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gallien S, Kim SY, Domon B. Mol Cell Proteomics. 2015;14:1630–1644. doi: 10.1074/mcp.O114.043968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Erickson BK, Rose CM, Braun CR, Erickson AR, Knott J, McAlister GC, Wuhr M, Paulo JA, Everley RA, Gygi SP. Molecular cell. 2017;65:361–370. doi: 10.1016/j.molcel.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tsai CF, Wang YT, Yen HY, Tsou CC, Ku WC, Lin PY, Chen HY, Nesvizhskii AI, Ishihama Y, Chen YJ. Nature communications. 2015;6:6622. doi: 10.1038/ncomms7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Olsen JV, Mann M. Mol Cell Proteomics. 2013;12:3444–3452. doi: 10.1074/mcp.O113.034181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Olsen JV, Vermeulen M, Santamaria A, Kumar C, Miller ML, Jensen LJ, Gnad F, Cox J, Jensen TS, Nigg EA, Brunak S, Mann M. Sci Signal. 2010;3:ra3. doi: 10.1126/scisignal.2000475. [DOI] [PubMed] [Google Scholar]
- 142.Wu R, Haas W, Dephoure N, Huttlin EL, Zhai B, Sowa ME, Gygi SP. Nat Methods. 2011;8:677–683. doi: 10.1038/nmeth.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang X, Jin QK, Carr SA, Annan RS. Rapid Commun Mass Spectrom. 2002;16:2325–2332. doi: 10.1002/rcm.864. [DOI] [PubMed] [Google Scholar]
- 144.Fiedler D, Braberg H, Mehta M, Chechik G, Cagney G, Mukherjee P, Silva AC, Shales M, Collins SR, van Wageningen S, Kemmeren P, Holstege FCP, Weissman JS, Keogh MC, Koller D, Shokat KM, Krogan NJ. Cell. 2009;136:952–963. doi: 10.1016/j.cell.2008.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang X, Hubbard EJ, Carlson M. Science. 1992;257:680–682. doi: 10.1126/science.1496382. [DOI] [PubMed] [Google Scholar]
- 146.Varjosalo M, Sacco R, Stukalov A, van Drogen A, Planyavsky M, Hauri S, Aebersold R, Bennett KL, Colinge J, Gstaiger M, Superti-Furga G. Nat Methods. 2013;10:307–314. doi: 10.1038/nmeth.2400. [DOI] [PubMed] [Google Scholar]
- 147.Li Y, Cross FR, Chait BT. Proc Natl Acad Sci U S A. 2014;111:11323–11328. doi: 10.1073/pnas.1409666111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Witucki LA, Huang X, Shah K, Liu Y, Kyin S, Eck MJ, Shokat KM. Chem Biol. 2002;9:25–33. doi: 10.1016/s1074-5521(02)00091-1. [DOI] [PubMed] [Google Scholar]
- 149.Shah K, Shokat KM. Chem Biol. 2002;9:35–47. doi: 10.1016/s1074-5521(02)00086-8. [DOI] [PubMed] [Google Scholar]
- 150.Hertz NT, Berthet A, Sos ML, Thorn KS, Burlingame AL, Nakamura K, Shokat KM. Cell. 2013;154:737–747. doi: 10.1016/j.cell.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Blethrow JD, Glavy JS, Morgan DO, Shokat KM. Proc Natl Acad Sci U S A. 2008;105:1442–1447. doi: 10.1073/pnas.0708966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schaffer BE, Levin RS, Hertz NT, Maures TJ, Schoof ML, Hollstein PE, Benayoun BA, Banko MR, Shaw RJ, Shokat KM, Brunet A. Cell Metab. 2015;22:907–921. doi: 10.1016/j.cmet.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Morandell S, Grosstessner-Hain K, Roitinger E, Hudecz O, Lindhorst T, Teis D, Wrulich OA, Mazanek M, Taus T, Ueberall F, Mechtler K, Huber LA. Proteomics. 2010;10:2015–2025. doi: 10.1002/pmic.200900749. [DOI] [PubMed] [Google Scholar]
- 154.Steger M, Tonelli F, Ito G, Davies P, Trost M, Vetter M, Wachter S, Lorentzen E, Duddy G, Wilson S, Baptista MA, Fiske BK, Fell MJ, Morrow JA, Reith AD, Alessi DR, Mann M. Elife. 2016;5 doi: 10.7554/eLife.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lai YC, Kondapalli C, Lehneck R, Procter JB, Dill BD, Woodroof HI, Gourlay R, Peggie M, Macartney TJ, Corti O, Corvol JC, Campbell DG, Itzen A, Trost M, Muqit MM. The EMBO journal. 2015;34:2840–2861. doi: 10.15252/embj.201591593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xue L, Geahlen RL, Tao WA. Molecular & cellular proteomics : MCP. 2013;12:2969–2980. doi: 10.1074/mcp.O113.027722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xue L, Wang P, Cao P, Zhu JK, Tao WA. Molecular & cellular proteomics : MCP. 2014;13:3199–3210. doi: 10.1074/mcp.O114.038588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Roy J, Cyert MS. Sci Signal. 2009;2:re9. doi: 10.1126/scisignal.2100re9. [DOI] [PubMed] [Google Scholar]
- 159.Yao Z, Darowski K, St-Denis N, Wong V, Offensperger F, Villedieu A, Amin S, Malty R, Aoki H, Guo H, Xu Y, Iorio C, Kotlyar M, Emili A, Jurisica I, Neel BG, Babu M, Gingras AC, Stagljar I. Molecular cell. 2017;65:347–360. doi: 10.1016/j.molcel.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Herzog F, Kahraman A, Boehringer D, Mak R, Bracher A, Walzthoeni T, Leitner A, Beck M, Hartl FU, Ban N, Malmstrom L, Aebersold R. Science. 2012;337:1348–1352. doi: 10.1126/science.1221483. [DOI] [PubMed] [Google Scholar]
- 161.Chang YC, Lin SY, Liang SY, Pan KT, Chou CC, Chen CH, Liao CL, Khoo KH, Meng TC. J Proteome Res. 2008;7:1055–1066. doi: 10.1021/pr700801p. [DOI] [PubMed] [Google Scholar]
- 162.Oaks JJ, Santhanam R, Walker CJ, Roof S, Harb JG, Ferenchak G, Eisfeld AK, Van Brocklyn JR, Briesewitz R, Saddoughi SA, Nagata K, Bittman R, Caligiuri MA, Abdel-Wahab O, Levine R, Arlinghaus RB, Quintas-Cardama A, Goldman JM, Apperley J, Reid A, Milojkovic D, Ziolo MT, Marcucci G, Ogretmen B, Neviani P, Perrotti D. Blood. 2013;122:1923–1934. doi: 10.1182/blood-2013-03-492181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zheng P, Shultz SR, Hovens CM, Velakoulis D, Jones NC, O'Brien TJ. Mol Neurobiol. 2014;49:1532–1539. doi: 10.1007/s12035-013-8601-9. [DOI] [PubMed] [Google Scholar]
- 164.Park HJ, Lee KW, Park ES, Oh S, Yan R, Zhang J, Beach TG, Adler CH, Voronkov M, Braithwaite SP, Stock JB, Mouradian MM. Ann Clin Transl Neurol. 2016;3:769–780. doi: 10.1002/acn3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sontag JM, Sontag E. Front Mol Neurosci. 2014;7:16. doi: 10.3389/fnmol.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schweiger R, Linial M. Biol Direct. 2010;5:6. doi: 10.1186/1745-6150-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cohen P. Trends Biochem Sci. 2000;25:596–601. doi: 10.1016/s0968-0004(00)01712-6. [DOI] [PubMed] [Google Scholar]
- 168.Hunter T. Molecular cell. 2007;28:730–738. doi: 10.1016/j.molcel.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 169.Dorey K, Engen JR, Kretzschmar J, Wilm M, Neubauer G, Schindler T, Superti-Furga G. Oncogene. 2001;20:8075–8084. doi: 10.1038/sj.onc.1205017. [DOI] [PubMed] [Google Scholar]
- 170.Frame S, Cohen P, Biondi RM. Molecular cell. 2001;7:1321–1327. doi: 10.1016/s1097-2765(01)00253-2. [DOI] [PubMed] [Google Scholar]
- 171.Cargnello M, Roux PP. Microbiol Mol Biol Rev. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yang XJ, Seto E. Molecular cell. 2008;31:449–461. doi: 10.1016/j.molcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lamoliatte F, McManus FP, Maarifi G, Chelbi-Alix MK, Thibault P. Nature communications. 2017;8:14109. doi: 10.1038/ncomms14109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Annu Rev Biochem. 2011;80:825–858. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pan Z, Liu Z, Cheng H, Wang Y, Gao T, Ullah S, Ren J, Xue Y. Sci Rep. 2014;4:7331. doi: 10.1038/srep07331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lin DI, Barbash O, Kumar KG, Weber JD, Harper JW, Klein-Szanto AJ, Rustgi A, Fuchs SY, Diehl JA. Molecular cell. 2006;24:355–366. doi: 10.1016/j.molcel.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rajbhandari P, Schalper KA, Solodin NM, Ellison-Zelski SJ, Ping Lu K, Rimm DL, Alarid ET. Oncogene. 2014;33:1438–1447. doi: 10.1038/onc.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tong J, Taylor P, Moran MF. Mol Cell Proteomics. 2014;13:1644–1658. doi: 10.1074/mcp.M114.038596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Swaney DL, Beltrao P, Starita L, Guo A, Rush J, Fields S, Krogan NJ, Villen J. Nat Methods. 2013;10:676–682. doi: 10.1038/nmeth.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mertins P, Qiao JW, Patel J, Udeshi ND, Clauser KR, Mani DR, Burgess MW, Gillette MA, Jaffe JD, Carr SA. Nat Methods. 2013;10:634–637. doi: 10.1038/nmeth.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Peng J, Schwartz D, Elias JE, Thoreen CC, Cheng D, Marsischky G, Roelofs J, Finley D, Gygi SP. Nat Biotechnol. 2003;21:921–926. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- 182.Kane LA, Lazarou M, Fogel AI, Li Y, Yamano K, Sarraf SA, Banerjee S, Youle RJ. J Cell Biol. 2014;205:143–153. doi: 10.1083/jcb.201402104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kazlauskaite A, Kondapalli C, Gourlay R, Campbell DG, Ritorto MS, Hofmann K, Alessi DR, Knebel A, Trost M, Muqit MM. Biochem J. 2014;460:127–139. doi: 10.1042/BJ20140334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Koyano F, Okatsu K, Kosako H, Tamura Y, Go E, Kimura M, Kimura Y, Tsuchiya H, Yoshihara H, Hirokawa T, Endo T, Fon EA, Trempe JF, Saeki Y, Tanaka K, Matsuda N. Nature. 2014;510:162–166. doi: 10.1038/nature13392. [DOI] [PubMed] [Google Scholar]
- 185.Yau R, Rape M. Nature cell biology. 2016;18:579–586. doi: 10.1038/ncb3358. [DOI] [PubMed] [Google Scholar]
- 186.Pickrell AM, Youle RJ. Neuron. 2015;85:257–273. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Baek JH, Rubinstein M, Scheuer T, Trimmer JS. J Biol Chem. 2014;289:15363–15373. doi: 10.1074/jbc.M114.562785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang Z, Udeshi ND, Slawson C, Compton PD, Sakabe K, Cheung WD, Shabanowitz J, Hunt DF, Hart GW. Sci Signal. 2010;3:ra2. doi: 10.1126/scisignal.2000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhu Y, Shan X, Yuzwa SA, Vocadlo DJ. J Biol Chem. 2014;289:34472–34481. doi: 10.1074/jbc.R114.601351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hornbeck PV, Kornhauser JM, Tkachev S, Zhang B, Skrzypek E, Murray B, Latham V, Sullivan M. Nucleic Acids Res. 2012;40:D261–270. doi: 10.1093/nar/gkr1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Schwartz D, Gygi SP. Nat Biotechnol. 2005;23:1391–1398. doi: 10.1038/nbt1146. [DOI] [PubMed] [Google Scholar]
- 192.Peng M, Scholten A, Heck AJ, van Breukelen B. J Proteome Res. 2014;13:249–259. doi: 10.1021/pr4005579. [DOI] [PubMed] [Google Scholar]
- 193.Huang Y, Xu B, Zhou X, Li Y, Lu M, Jiang R, Li T. Mol Cell Proteomics. 2015;14:761–770. doi: 10.1074/mcp.M114.037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ross KE, Huang H, Ren J, Arighi CN, Li G, Tudor CO, Lv M, Lee JY, Chen SC, Vijay-Shanker K, Wu CH. Methods Mol Biol. 2017;1558:333–353. doi: 10.1007/978-1-4939-6783-4_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gillette MA, Carr SA. Nat Methods. 2013;10:28–34. doi: 10.1038/nmeth.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Iliuk AB, Arrington JV, Tao WA. Electrophoresis. 2014;35:3430–3440. doi: 10.1002/elps.201400153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Crutchfield CA, Thomas SN, Sokoll LJ, Chan DW. Clin Proteomics. 2016;13:1. doi: 10.1186/s12014-015-9102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hunter T. Cell. 2000;100:113–127. doi: 10.1016/s0092-8674(00)81688-8. [DOI] [PubMed] [Google Scholar]
- 199.Kabuyama Y, Resing KA, Ahn NG. Curr Opin Genet Dev. 2004;14:492–498. doi: 10.1016/j.gde.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 200.Craig-Schapiro R, Fagan AM, Holtzman DM. Neurobiol Dis. 2009;35:128–140. doi: 10.1016/j.nbd.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Simonsen AH, McGuire J, Podust VN, Davies H, Minthon L, Skoog I, Andreasen N, Wallin A, Waldemar G, Blennow K. Neurobiol Aging. 2008;29:961–968. doi: 10.1016/j.neurobiolaging.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 202.Mulder C, Verwey NA, van der Flier WM, Bouwman FH, Kok A, van Elk EJ, Scheltens P, Blankenstein MA. Clinical chemistry. 2010;56:248–253. doi: 10.1373/clinchem.2009.130518. [DOI] [PubMed] [Google Scholar]
- 203.Hooper C, Killick R, Lovestone S. J Neurochem. 2008;104:1433–1439. doi: 10.1111/j.1471-4159.2007.05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Iqbal K, Alonso Adel C, Chen S, Chohan MO, El-Akkad E, Gong CX, Khatoon S, Li B, Liu F, Rahman A, Tanimukai H, Grundke-Iqbal I. Biochim Biophys Acta. 2005;1739:198–210. doi: 10.1016/j.bbadis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 205.Portelius E, Hansson SF, Tran AJ, Zetterberg H, Grognet P, Vanmechelen E, Hoglund K, Brinkmahn G, Westman-Brinkmalm A, Nordhoff E, Blennow K, Gobom J. J Proteome Res. 2008;7:2114–2120. doi: 10.1021/pr7008669. [DOI] [PubMed] [Google Scholar]
- 206.Doecke JD, Laws SM, Faux NG, Wilson W, Burnham SC, Lam CP, Mondal A, Bedo J, Bush AI, Brown B, De Ruyck K, Ellis KA, Fowler C, Gupta VB, Head R, Macaulay SL, Pertile K, Rowe CC, Rembach A, Rodrigues M, Rumble R, Szoeke C, Taddei K, Taddei T, Trounson B, Ames D, Masters CL, Martins RN. I. Alzheimer's Disease Neuroimaging, B. Australian Imaging and G. Lifestyle Research. Arch Neurol. 2012;69:1318–1325. doi: 10.1001/archneurol.2012.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Riemenschneider M, Wagenpfeil S, Vanderstichele H, Otto M, Wiltfang J, Kretzschmar H, Vanmechelen E, Forstl H, Kurz A. Mol Psychiatry. 2003;8:343–347. doi: 10.1038/sj.mp.4001220. [DOI] [PubMed] [Google Scholar]
- 208.Di Domenico F, Sultana R, Barone E, Perluigi M, Cini C, Mancuso C, Cai J, Pierce WM, Butterfield DA. J Proteomics. 2011;74:1091–1103. doi: 10.1016/j.jprot.2011.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kanninen KM, Bister N, Koistinaho J, Malm T. Bba-Mol Basis Dis. 2016;1862:403–410. doi: 10.1016/j.bbadis.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 210.Giau VV, An SSA. Journal of the Neurological Sciences. 2016 [Google Scholar]
- 211.Ailawadi S, Wang X, Gu H, Fan GC. Biochim Biophys Acta. 2015;1852:1–11. doi: 10.1016/j.bbadis.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cheng L, Doecke JD, Sharples RA, Villemagne VL, Fowler CJ, Rembach A, Martins RN, Rowe CC, Macaulay SL, Masters CL, Hill AF B. Australian Imaging and G. Lifestyle Research. Mol Psychiatry. 2015;20:1188–1196. doi: 10.1038/mp.2014.127. [DOI] [PubMed] [Google Scholar]
- 213.Palmisano G, Jensen SS, Le Bihan MC, Laine J, McGuire JN, Pociot F, Larsen MR. Mol Cell Proteomics. 2012;11:230–243. doi: 10.1074/mcp.M111.012732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cocucci E, Meldolesi J. Trends in cell biology. 2015;25:364–372. doi: 10.1016/j.tcb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 215.Chen IH, Xue L, Hsu CC, Paez JS, Pan L, Andaluz H, Wendt MK, Iliuk AB, Zhu JK, Tao WA. Proc Natl Acad Sci U S A. 2017 doi: 10.1073/pnas.1618088114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Huang FK, Zhang G, Lawlor K, Nazarian A, Philip J, Tempst P, Dephoure N, Neubert TA. J Proteome Res. 2017;16:1121–1132. doi: 10.1021/acs.jproteome.6b00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Riley NM, Coon JJ. Anal Chem. 2016;88:74–94. doi: 10.1021/acs.analchem.5b04123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Szalardy L, Zadori D, Simu M, Bencsik K, Vecsei L, Klivenyi P. J Neurol Sci. 2013;331:38–42. doi: 10.1016/j.jns.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 219.Bartosik-Psujek H, Archelos JJ. J Neurol. 2004;251:414–420. doi: 10.1007/s00415-004-0336-0. [DOI] [PubMed] [Google Scholar]
- 220.Wetie AGN, Wormwood KL, Russell S, Ryan JP, Darie CC, Woods AG. Autism Research. 2015 doi: 10.1002/aur.1450-338-350. [DOI] [PubMed] [Google Scholar]
- 221.Castagnola M, Messana I, Inzitari R, Fanali C, Cabras T, Morelli A, Pecoraro AM, Neri G, Torrioli MG, Gurrieri F. J Proteome Res. 2008;7:5327–5332. doi: 10.1021/pr8004088. [DOI] [PubMed] [Google Scholar]
- 222.Zurek J, Bartlova L, Fedora M. Brain Inj. 2011;25:221–226. doi: 10.3109/02699052.2010.541895. [DOI] [PubMed] [Google Scholar]
- 223.Zetterberg H, Jacobsson J, Rosengren L, Blennow K, Andersen PM. Eur J Neurol. 2007;14:1329–1333. doi: 10.1111/j.1468-1331.2007.01972.x. [DOI] [PubMed] [Google Scholar]
- 224.Brettschneider J, Petzold A, Sussmuth SD, Ludolph AC, Tumani H. Neurology. 2006;66:852–856. doi: 10.1212/01.wnl.0000203120.85850.54. [DOI] [PubMed] [Google Scholar]
- 225.Boylan K, Yang C, Crook J, Overstreet K, Heckman M, Wang Y, Borchelt D, Shaw G. J Neurochem. 2009;111:1182–1191. doi: 10.1111/j.1471-4159.2009.06386.x. [DOI] [PubMed] [Google Scholar]
- 226.Hyeon JW, Kim SY, Lee J, Park JS, Hwang KJ, Lee SM, An SA, Lee MK, Ju YR. Sci Rep. 2015;5:15283. doi: 10.1038/srep15283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.van Eijk JJ, van Everbroeck B, Abdo WF, Kremer BP, Verbeek MM. J Alzheimers Dis. 2010;21:569–576. doi: 10.3233/JAD-2010-090649. [DOI] [PubMed] [Google Scholar]