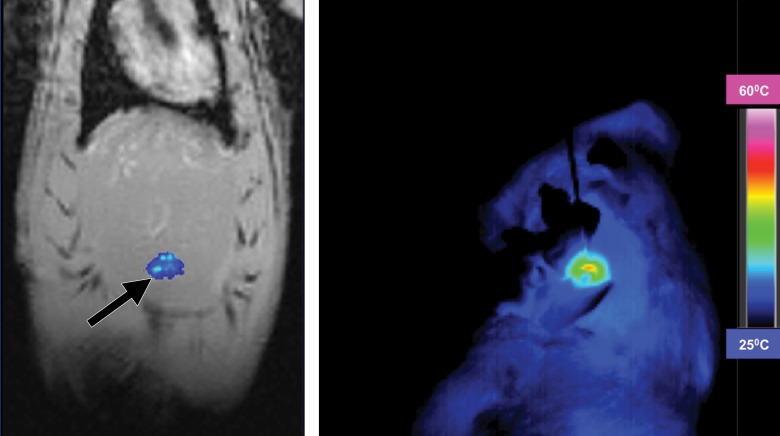
In this issue of Radiology, White et al introduced their work on developing biofunctionalized hybrid magnetic gold nanoparticles as catalysts for photothermal ablation of colorectal liver metastases.
Summary
Image-guided percutaneous thermal ablation has been one of the principal tools in management of unresectable liver malignancies, including colorectal liver metastases (CRLM) (1). Currently, however, this technique is suitable mainly for tumors less than 4–5 cm in diameter and also results in incomplete ablation at tumor margins (2). To solve these problems, efforts have been made to combine thermal ablation with other treatment options, such as systemic and intra-arterial administration of therapeutics (3–5). In this issue of Radiology, White et al (6) introduced their work on development of an alternative approach by using biofunctionalized hybrid magnetic gold nanoparticles (HNPs) as catalysts for photothermal ablation of CRLM. They found that (a) the targeted (anti-MG1) HNPs are noncytotoxic and have greater than 20% intratumoral accumulation and (b) systemic administration of anti-MG1 HNPs can enlarge a tumor’s necrotic zone with photothermal ablation. The results of this study establish the proof of the concept that targeted HNPs can enhance the therapeutic effect of photothermal ablation, which presents an exciting strategy for complete removal of CRLM by integrating two rapidly advancing scientific fields—interventional radiology and nanotechnology.
The Setting
Colorectal cancer is the third most common cancer and the third most common cause of cancer-related death in the United States (7). Approximately 70% of patients with colorectal cancer develop liver metastases (CRLM). Only 25% of patients with CRLM are candidates for surgical resection (8). The remaining patients must consider alternative treatment options, such as systemic chemotherapy and image-guided tumor ablation and chemoembolization (9). Although image-guided percutaneous thermal ablation is an important tool for ablative removal of unresectable CRLM, the thermal ablation techniques are primarily applicable to tumors smaller than 4–5 cm and have shown limited success in tumors adjacent to normal structures, such as bile ducts, vasculatures, the colon, the stomach, and the diaphragm, which are prone to thermal injury. In addition, these ablation techniques can result in incomplete ablation at tumor margins, because of several factors, such as decreased ablation heating from blood in neighboring vessels (from the “heat sink” effect), intentional avoidance of heating adjacent critical structures, or off-center positioning of the ablation probes (2,4,5). Ultimately, these drawbacks result in local tumor recurrence from residual tumor cells, which poses a critical issue in the clinical utility of image-guided thermal ablation.
To overcome these challenges, efforts have been focused on combining ablation with other treatment options, such as systemic and intra-arterial administration of various therapeutics (3–5), which are aimed primarily to shrink and devascularize tumors before ablation (4,5). However, systemic chemotherapy cannot achieve adequate therapeutic doses at the tumor site without causing substantial undesired toxicity to other vital organs. Similarly, successful intra-arterial therapy depends on the presence of sufficient vessels supplying the tumor.
Results of recent studies from different groups have confirmed that nonablative hyperthermia (at approximately 42°C–44°C) can greatly enhance image-guided direct intratumoral gene therapy and chemotherapy of various malignancies (10,11). The recognized mechanisms of nonablative hyperthermia–enhanced therapies include fracturing tissue by means of heating, higher permeability of the cytoplasmic membrane, higher cellular metabolism, and activation of the heat shock protein pathway (12–14). These mechanisms facilitate the entrance of therapeutics into target tumor cells, thereby promoting the effective destruction of tumor tissue.
Rapid progress of nanoscience and the application of nanotechnology in medicine are changing the traditional processes of prevention, diagnosis, and treatment of diseases. Innovation and expansion as well as application of a wide spectrum of nano-scale particles have opened new avenues for medical imaging and image-guided interventions (15,16). The construction of nanoparticles by using imaging-detectable biomaterials enables the production of different types of diagnostic imaging agents, while modification of the physical and chemical properties of these imaging-detectable particles (such as the conjugation of ligands onto the particle surface) permits target-specific imaging of diseases at the molecular level—a process termed molecular imaging (17). Furthermore, the loading or coating of therapeutic agents (such as genes, chemotherapeutic agents, stem cells, and radiopharmaceuticals) into the targeted imaging nanoparticles promotes the combination of target-specific imaging and target-specific therapies called theranostics (18).
The Science
In this issue of Radiology, White et al (6) introduced their work on developing biofunctionalized HNPs as catalysts for photothermal ablation of CRLM. This study represents one of the recent efforts on effective treatment of local-regional CRLM by integrating the advantages of two rapidly advancing scientific fields: interventional radiology and nanotechnology.
The authors successfully synthesized HNPs that are conjugated with anti-MG1 monoclonal antibodies. They created rat models with orthotopic CRLM by implanting CC-531 rat CRLM cells. Then, with photothermal ablation of these CRLM cells, they systemically infused the anti-MG1–targeted HNPs. The coupling efficiencies were determined by using in-vivo magnetic resonance (MR) imaging by following up and comparing the HNP-mediated tumor signal changes among different animal groups. Findings on MR images were correlated with subsequent histologic examination results. The authors concluded that anti-MG1–targeted HNP can serve as a catalyst for photothermal ablation of CRLM, the evidence of which is a significant increase of tumor ablation zones and tumor necrosis (6) (Fig 3c, 3e).
Evidence of achievement of such effective treatment of CRLM with the approach introduced by the authors is shown in several findings. First, the HNPs permit specific targeting of MG1-expressing CRLM cells, which thus enable local accumulation of HNPs in the treated CRLM masses. Second, these targeted HNPs were synthesized with magnetic gold nanostructures, which allow not only MR imaging visualization and quantification (19), but also photothermal sensitization (20). All of these findings together promote the enhanced efficacy of photothermal ablation on CRLM.
The Practice
By providing tumor cell targeting and photothermal sensitization, the multifunctional HNPs described by White et al can improve the efficacy of thermal ablation. If proven successful in humans, this technical development may have the following implications: (a) Specific targeting of tumors through the binding and internalizing of HNPs by tumor cells will allow local accumulation of HNPs in the treated tumors; (b) photothermal sensitization by gold nanoparticles will result in larger necrotic zones to minimize residual viable tumor cells at peripheries, which will in turn reduce or delay recurrence; and (c) encapsulation of iron oxide within the nanoparticles will enable longitudinal monitoring of ablation efficacy by using quantitative imaging.
Further investigations could include the coloading or coating of antitumor therapeutic agents in these targeted HNPs to facilitate therapeutic accumulation at the tumor sites via the theranostic mechanism (21). Image-guided percutaneous or intraluminal interventional approaches for direct intratumoral delivery of theranostic HNPs will further expand effective treatment of local-regional malignancies, not only in the liver but also in other organs, harnessing the benefits of integrating interventional radiology and nanotechnology (22).
Footnotes
See also White et al.
This study was supported by a US National Institutes of Health grant R01 EB012467 and the Center for Scientific Review (R01 EB012467).
Disclosures of Conflicts of Interest: X.Y. disclosed no relevant relationships.
References
- 1.Gillams A, Goldberg N, Ahmed M, et al. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, The Interventional Oncology Sans Frontières meeting 2013. Eur Radiol 2015;25(12):3438–3454. doi:10.1007/s00330-015-3779-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Künzli BM, Abitabile P, Maurer CA. Radiofrequency ablation of liver tumors: Actual limitations and potential solutions in the future. World J Hepatol 2011;3(1):8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamakado K, Inaba Y, Sato Y, et al. Radiofrequency ablation combined with hepatic arterial chemoembolization using degradable starch microsphere mixed with mitomycin C for the treatment of liver metastasis from colorectal cancer: a prospective multicenter study. Cardiovasc Intervent Radiol 2017;40(4):560–567. doi:10.1007/s00270-016-1547-3 [DOI] [PubMed] [Google Scholar]
- 4.Hwang JE, Kim SH, Jin J, et al. Combination of percutaneous radiofrequency ablation and systemic chemotherapy are effective treatment modalities for metachronous liver metastases from gastric cancer. Clin Exp Metastasis 2014;31(1):25–32. doi:10.1007/s10585-013-9606-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iezzi R, Cesario V, Siciliani L, et al. Single-step multimodal locoregional treatment for unresectable hepatocellular carcinoma: balloon-occluded percutaneous radiofrequency thermal ablation (BO-RFA) plus transcatheter arterial chemoembolization (TACE). Radiol Med (Torino) 2013;118(4):555–569. doi:10.1007/s11547-012-0914-7 [DOI] [PubMed] [Google Scholar]
- 6.White SB, Kim DH, Guo Y, et al. Biofunctionalized hybrid magnetic gold nanoparticles as catalysts for photothermal ablation of colorectal liver metastases. Radiology 2017;285(3):809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975-2014, featuring survival. J Natl Cancer Inst 2017;109(9):1–22. doi:10.1093/jnci/djx030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viganò L, Russolillo N, Ferrero A, Langella S, Sperti E, Capussotti L. Evolution of long-term outcome of liver resection for colorectal metastases: analysis of actual 5-year survival rates over two decades. Ann Surg Oncol 2012;19(6):2035–2044. doi:10.1245/s10434-011-2186-1 [DOI] [PubMed] [Google Scholar]
- 9.Kallini JR, Gabr A, Abouchaleh N, et al. New developments in interventional oncology: liver metastases from colorectal cancer. Cancer J 2016;22(6):373–380. doi:10.1097/PPO.0000000000000226 [DOI] [PubMed] [Google Scholar]
- 10.Shi Y, Wang J, Bai Z, et al. Radiofrequency hyperthermia-enhanced herpes simplex virus-thymidine kinase/ganciclovir direct intratumoral gene therapy of esophageal squamous cancers. Am J Cancer Res 2016;6(9):2054–2063. [PMC free article] [PubMed] [Google Scholar]
- 11.Jung BK, Lee YK, Hong J, Ghandehari H, Yun CO. Mild hyperthermia induced by gold nanorod-mediated plasmonic photothermal therapy enhances transduction and replication of oncolytic adenoviral gene delivery. ACS Nano 2016;10(11):10533–10543. doi:10.1021/acsnano.6b06530 [DOI] [PubMed] [Google Scholar]
- 12.Antanavičiūtė I, Mildažienė V, Stankevičius E, Herdegen T, Skeberdis VA. Hyperthermia differently affects connexin43 expression and gap junction permeability in skeletal myoblasts and HeLa cells. Mediators Inflamm 2014;2014:748290. doi:10.1155/2014/748290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer 2014;14(3):199–208. doi:10.1038/nrc3672 [DOI] [PubMed] [Google Scholar]
- 14.Haviv YS, Blackwell JL, Li H, Wang M, Lei X, Curiel DT. Heat shock and heat shock protein 70i enhance the oncolytic effect of replicative adenovirus. Cancer Res 2001;61(23):8361–8365. [PubMed] [Google Scholar]
- 15.Bogart LK, Pourroy G, Murphy CJ, et al. Nanoparticles for imaging, sensing, and therapeutic intervention. ACS Nano 2014;8(4):3107–3122. doi:10.1021/nn500962q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X. Nano- and microparticle-based imaging of cardiovascular interventions: overview. Radiology 2007;243(2):340–347. doi:10.1148/radiol.2432060307 [DOI] [PubMed] [Google Scholar]
- 17.Weissleder R, Mahmood U. Molecular imaging. Radiology 2001;219(2):316–333. doi:10.1148/radiology.219.2.r01ma19316 [DOI] [PubMed] [Google Scholar]
- 18.Warner S. Diagnostics plus therapy = theranostics. Scientist 2004;18:38–39. [Google Scholar]
- 19.Schmid-Tannwald C, Strobl FF, Theisen D, et al. Diffusion-weighted MRI before and after robotic radiosurgery (Cyberknife®) in primary and secondary liver malignancies: a pilot study. Technol Cancer Res Treat 2015;14(2):191–199. [DOI] [PubMed] [Google Scholar]
- 20.Park JH, von Maltzahn G, Xu MJ, et al. Cooperative nanomaterial system to sensitize, target, and treat tumors. Proc Natl Acad Sci U S A 2010;107(3):981–986. doi:10.1073/pnas.0909565107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muthu MS, Mehata AK, Viswanadh MK. Upconversion nanotheranostics: emerging designs for integration of diagnosis and therapy. Nanomedicine (Lond) 2017;12(6):577–580. doi:10.2217/nnm-2017-0010 [DOI] [PubMed] [Google Scholar]
- 22.Yang X. Interventional molecular imaging. Radiology 2010;254(3):651–654. doi:10.1148/radiol.09091264 [DOI] [PMC free article] [PubMed] [Google Scholar]