Abstract
This study shows the effects of dietary supplementation with Lactobacillus acidophilus on the gut microbiota of broiler chickens challenged with Clostridium perfringens infection during a 21-day period according to pyrosequencing of the 16S ribosomal RNA gene. In a 2 × 2 factorial arrangement of treatments, 308 1-day-old male Arbor Acres broiler chicks were analyzed for the effects of the probiotic (groups without or with L. acidophilus supplementation), pathogen challenge (groups without or with C. perfringens), and the effects of interaction. The infection decreased the number of Observed species, Chao1, and ACE of ileal microbiota and increased Chao1 of cecal microbiota of broilers, whereas L. acidophilus supplementation decreased the Shannon index of the ileal microbiota. Shannon index and Simpson indices were lower in the ileal microbiota than in the cecal microbiota. In the ileal microbiota, the control group had higher relative abundance of Lachnospiraceae and Ruminococcaceae in comparison with the other groups; however, the relative abundance of Gammaproteobacteria was significantly higher in the challenge group than in the other groups. C. perfringens infection tended to increase lactate concentration and decreasedconcentrations of formate, acetate and propionate in the ileum; decreased isobutyrate concentration; and tended to decrease isovalerate concentration in the cecum. Besides, L. acidophilus supplementation increased the concentration of lactate and butyrate and decreased concentrations of formate and propionate in the ileum, and increased concentrations of lactate and valerate in the cecum. In conclusion, C. perfringens infection and/or dietary supplementation with L. acidophilus modulated the relative abundance of some bacteria taxa, and the L. acidophilus supplementation helped to restore the microbial community disrupted by C. perfringens infection.
Introduction
Necrotic enteritis (NE) is a universal poultry disease, which is mainly caused by Clostridium perfringens type A [1]. Globally, it costs $ 2 billion per year to the poultry industry owing to the poor growth performance, mortality of the birds and prevention expenses [2]. With the withdrawal of antibiotic growth promoters from poultry feed, this infection tends to break out [3] and has been a threat to poultry welfare and human food security.
Lactobacillus has been widely used as probiotics in feed to improve poultry growth performance and overall health [4, 5]. Some studies have shown that Lactobacillus acidophilus can suppress intestinal pathogens [6, 7]. Our previous study revealed that the probiotic L. acidophilus (used in the present study) can ameliorate the inflammatory response, and inhibit the colonization of some pathogens. Nonetheless, far less is known about the L. acidophilus-mediated changes in the C. perfringens challenged chicken microbiota. In this study, we used high-throughput sequencing of the V3-V4 region of the 16S ribosomal RNA (rRNA) gene to assess the ileal and cecal microbiota of unchallenged or challenged chickens fed diets without or with L. acidophilus supplementatioin. We also measured the concentrations of lactate and short-chain fatty acids (SCFAs) in the ileum and cecum.
Methods
Birds, diet, and experimental design
The experiment was approved by the China Agricultural University Animal Care and Use Committee.
A total of 308 1-day-old male Arbor acres broilers were obtained from a local commercial hatchery and used in a 2 × 2 factorial arrangement of treatments to study the effects of a probiotic (groups without or with L. acidophilus supplementation), a pathogen challenge (groups without or with C. perfringensinfection) and their interactive effects. All the birds were weighed and randomly assigned to four treatments [Control group (CTL), L. acidophilus supplementationgroup (LA), Challenge group (CLG), and Challenge group supplemented with L. acidophilus (LACLG)] with seven replicates per treatment. All the birds were offered free access to feed and water throughout the 21-d study period.
A corn—soybean meal basal diet in mash form was formulated to meet the nutrient requirements recommended by the feeding standard of China (NY/T 2004) for broilers (Additional file: S1 Table). The probiotic L. acidophilus (Synlac Material Technology Co., Ltd, Nanjing, China) was added to the basal diet at 40 mg/kg to provide 4 × 105 colony-forming units (cfu)/(kg of feed). The probiotic was progressively diluted and mixed with basal diet homogeneously every 4 d.
The C. perfringens challenge
The C. perfringens challenge method used in our experiment was developed by Dahiya et al. (2005) [8] and modified by Liu et al. (2010) [9]. Briefly, a chicken C. perfringens type A field strain (CVCC 2030) was isolated from a clinical case of NE, which was obtained from the China Veterinary Culture Collection Center (Beijing, China). The organism was cultured anaerobically on tryptose-sulfite-cycloserine for 18 h at 37°C and then aseptically inoculated into a cooked meat medium and incubated anaerobically for 8h at 37°C. The birds of challenged groups were orally gavaged once a day with actively growing culture of wild-type C. perfringens (2.0 × 108 cfu/mL, 1.0 mL/bird) to cause gut damage on days 14–20, and the unchallenged birds were gavaged with the same volume of the sterilized cooked meat medium.
Sample collection and DNA extraction
Five birds per treatment were randomly chosen from different replicates and chickens were rendered unconscious by intravenously injection of pentobarbital sodium (100 mg/[kg body weight]) just before slaughter by exsanguination; next, the digesta samples were collected in the ileum and cecum. The content of the terminal half of the ileum (defined as the region between Meckel’s diverticulum until the point 2-cm cranial to the ileo-cecal junction) and cecum were collected within 5 min of euthanasia, immediately placed in cryogenic vials, snap-frozen in liquid nitrogen, delivered to the laboratory and stored at −80°C until DNA extraction. Genomic DNA was isolated from ~200 mg of digesta from the ileum and caecum using a commercial kit (QIAamp DNA Stool Mini Kit, Qiagen Inc., Valencia, CA). The DNA concentration and purity were determined on a NanoDrop 2000 spectrophotometer (Thermo Scientific, MA, USA).
Pyrosequencing
The purified genomic DNA at the normalized concentration (20 ng/uL) served as a template for analysis of the microbial communities. The V3-V4 region of the 16S rRNA gene was amplified with the universal eubacterial primers (341 F: 5′-CCTAYGGGRBGCASCAG-3′ and 806R: 5′-GGACTACNNGGGTATCTAAT-3′). Primer sequences were modified by adding Illumina adaptor A to the 5’ ends of the forward primers, and adaptor B followed by 12 nucleotide barcode sequences to the 3’ ends of the reverse primers. The 50 μL reaction system consists of 20 μL Premix Ex Taq (Takara Biotechnology, Dalian, China), 0.4 μL of each primer (10 μM), 4 μL of five-fold diluted template DNA (1–10 ng) and 25.2 μL of sterilized water. Thermal cycling conditions were as follows: initial denaturation of 3 min at 94°C; six touch down cycles of 45 s at 94°C, 60 s from 65 to 58°C, and 70 s at 72°C; followed by 22 cycles of 45 s at 94°C, 60 s at 58°C, and 60 s at 72°C, with final extension at 72°C for 10 min. The PCR products were purified with the Qiagen Gel Extraction Kit (Qiagen, Germany), and then sequenced on the HiSeq2500 platform (Illumina, San Diego, CA, USA) at Novogene, Beijing, China.
Data processing
Raw sequences generated by HiSeq paired-end sequencing were merged by means of fast length adjustment of short reads (FLASH) [10] with Q30 of clean full-length reads ranging from 95.0% to 95.8%. Barcodes and primers were subjected to trimming, where maximal two-base differences in barcodes and no primer mismatches were permitted. Sequences were excluded if they did not meet the default QIIME quality criteria. Sequences with an average quality score less than 25 in a sliding window of 50 nucleotides were also discarded. The sequence data were denoised using the denoise_wrapper.py command in QIIME. Chimeras were identified by means of the Uparse software and removed. Then the Effective Tags were obtained and applied to further analyses.
Uparse was used to select operational taxonomic units (OTUs) at 97% similarity [11] on the Bio-Linux platform. Representative sequences were processed via the QIIME pipeline [12]. LCA alignment assignment was carried out based on the Silva database [13]. Resampling according to the minimal sequence numbers across all the samples was performed before the downstream analyses. Community composition provides the classification information at different taxonomic levels. Shifts in bacterial community composition were visualized byprincipal component analysis (PCA) based on the 97% OTU similarity across the four treatment groups.
Ileal and cecal SCFA and lactate concentrations
A 0.5-g sample of ileal or cecal digesta was weighed into a 10-mL polypropylene tube, then 8 mL of deionized water was added, an ultrasonic bath was applied for 30min, and the mixture was centrifuged for 10 min at 8000 revolutions per minute. The resulting suspension was diluted 10-fold and passed through a 0.22 μm-filter. Next, 25 μL of the extracted sample solution was studiedon a high performance ion chromatography system (ICS-3000; Dionex, USA) and analyzed by conductivity detection. The organic acids were separated on an AS11 analytical column (250 × 4mm) and an AG11 guard column under the following gradient conditions (the gradient was based on potassium hydroxide): 0-5min, 0.8–1.5mM; 5–10 min, 1.5–2.5 mM; and 10-15min, 2.5mM; the flow rate was 1.0 mL/min. The units of measurement of lactate and SCFAs were μg/(g digesta).
Statistical analyses
To compare the microbial community structures, a plot of PCA was constructed in QIIME. ANOSIM with 999 permutations was employed to detect statistical significance of difference between microbial communities in different groups. This test yields a value of R, normally on the scale from 0 to 1, which is based on the average rank similarity among groups and replicates within each group [14]. R = 0 indicates that two groups are similar whereas R = 1 shows perfect separation between groups. Differentially abundant taxa were identified by the linear discriminant anylysis (LDA) effect size (LEfSe) method [15]. The LEfSe algorithm involves the nonparametric factorial Kruskal-Wallis test (α = 0.05) to analyze differences between classes (treatments). To normalize sequencing depth, the lowest counts among samples were randomly subsampled in each library 1,000 times and average values were selected to measure diversity indices. To evaluate the α-diversity in samples, the number of observed OTUs, Shannon index, Simpson index, Chao1, and ACE were computed in QIIME. Differences between the mean values were identified by two-factorial analysis of variance and Duncan’s multiple-comparison test in the SPSS 20.0 software. The relation between bacterial genera and SCFA concentrations was evaluated by the Pearson correlation test. The differences in within-community (α) diversity indices of gut microbiota and in SCFA concentrations among the treatment groups were subjected to two-factorial analysis of variance in the SPSS 20.0 software, and each cage was considered an experimental unit.
Results
The quality of sequencing data
In total, 3,678,796 and 3,896,271 pyrosequencing reads were obtained from 20 ileal samples and 20 cecal samples, respectively. After quality was checked and chimeric sequences were removed, the average number of reads generated from the ileal samples per bird was 53,708 (±7,093 [standard deviation; SD]) and 56,017 (±11,769) for the cecal samples, with the median read length of 419 (± 6.08) base pairs and 411 (±3.42) base pairs, respectively. The estimate of Good’s coverage reached > 99.6% for all the ileal and cecal samples. Rare OTUs (< 0.005% of all OTUs) were removed, and then 1,229 OTUs were retained for subsequent analysis.
Effects of the C. perfringens challenge and L. acidophilus supplementation on the ileal microbiota
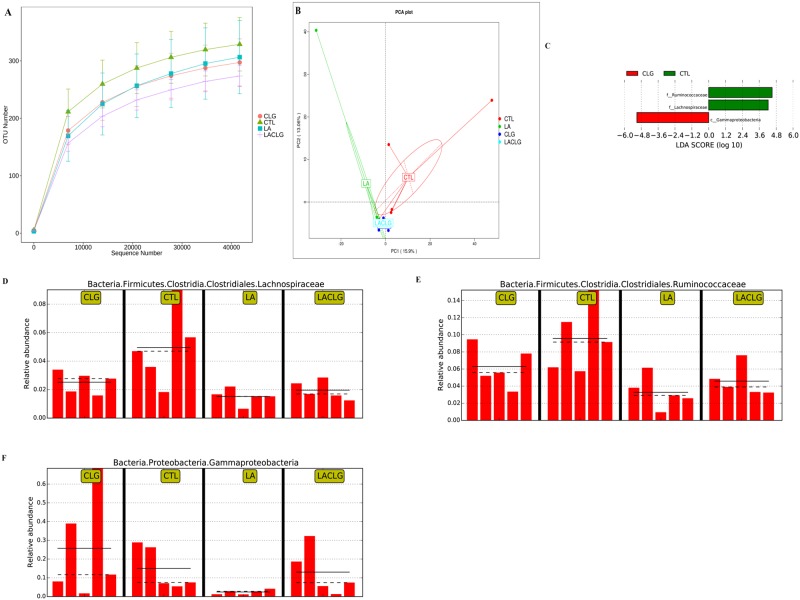
The number of observed species and Shannon, Simpson, Chao1 and ACE indices were calculated to assess α diversity (Table 1). According to the rarefaction curves for the observed OTUs (Fig 1A), the sequencing depth in the present study was sufficient to achieve high sampling coverage (99.9%) of all OTUs present in the ileal samples. The C. perfringens challenge decreased the number of observed species (P < 0.05), Chao 1 (P < 0.05), and ACE (P < 0.05) in the ileum. L. acidophilus addition to the diet decreased the ileal Shannon index (P < 0.05), irrespective of the C. perfringens challenge. By contrast, the Simpson index was not affected either by the challenge (P > 0.05) or by L. acidophilus supplementation (P > 0.05). To determine the similarities between pairs of microbial communities (β-diversity, Fig 1B), principal component analysis (PCA) was performed. Due to the high inter-individual variation, no distinguishable clustering of the ileal samples by dietary and/or infectious interventions was evident.
Table 1. The α diversity of ileal samples.
| Items | Observed species | Shannon | Simpson | Chao1 | ACE |
|---|---|---|---|---|---|
| CTL | 343.83 | 3.49 | 0.73 | 414.33 | 417.82 |
| LA | 308.83 | 2.48 | 0.60 | 386.37 | 400.33 |
| CLG | 284.83 | 3.36 | 0.74 | 334.64 | 337.75 |
| LACLG | 281.67 | 2.92 | 0.70 | 343.70 | 344.67 |
| SEM | 10.297 | 0.167 | 0.032 | 13.288 | 13.044 |
| Main effects | |||||
| L. acidophilus | |||||
| No addition | 314.33 | 3.42 | 0.73 | 374.49 | 377.79 |
| Addition | 295.25 | 2.70 | 0.65 | 365.04 | 372.50 |
| C. perfringens challenge | |||||
| Negative | 326.33 | 2.98 | 0.67 | 400.35 | 409.08 |
| Positive | 283.25 | 3.14 | 0.72 | 339.17 | 341.21 |
| P-value | |||||
| C. perfringens challenge | 0.035 | 0.606 | 0.431 | 0.022 | 0.009 |
| L. acidophilus | 0.329 | 0.030 | 0.231 | 0.705 | 0.823 |
| C. perfringens challenge × L. acidophilus | 0.414 | 0.369 | 0.525 | 0.460 | 0.606 |
Fig 1. Diversity and composition of ileal microbiota.
Rarefaction curves of the observed OTUs (A) for ileal samples. The community structure among the treatment groups did not differ according to the Principle component analysis (PCA) of 20 ileal samples (B). Linear discriminant analysis (LDA) effect size (LEfSe) showed the phylotypes that differ among treatment groups with statistical and biological significance (C). Histograms indicate the highest relative abundance of the families Lachnospiraceae (D) and Ruminococcaceae (E) in the ileal microbiota of the CTL group, and of the class Gammaproteobacteria (F) in the ileal microbiota of the CLG group. CTL: control group; LA: L. acidophilus supplementation group; CLG: Challenge group; LACLG: challenge group supplemented with L. acidophilus.
OTUs were taxonomically categorized via the LCA classifier trained on the Silva database with a minimal confidence score of 0.8. The relative abundance of OTUs was analyzed at different ranking levels: from phyla to genera. At the phylum level, the ileal microbiota was mainly composed of Firmicutes (>68%), followed by Proteobacteria, Bacteroidetes, and Cyanobacteria, whereas the probiotic L. acidophilus supplementation increased the relative abundance of Firmicutes in the ileal microbiota (S1A Fig). The C. perfringens infection numerically decreased relative abundance of the Peptostreptococcaceae family, and numerically increased the relative abundance of the Enterobacteriaceae family which was mainly composed of Escherichia-Shigella genera. In contrast, the probiotic L. acidophilus numerically increased the relative abundance of Lactobacillaceae, which was mainly composed of the Lactobacillus genus, and numerically decreased the relative abundance of Escherichia-Shigella genera (S1B and S1C Fig). Three taxonomic biomarkers (LDA score > 2) in the ileal microbial communities of groups CTL and CLG were identified by LEfSe method (Fig 1C). The relative abundance of Lachnospiraceae (Fig 1D) and Ruminococcaceae (Fig 1E) was significantly (LDA score > 2) higher in the CTL group than in other treatment groups; however, the relative abundance of Gammaproteobacteria was significantly (LDA score > 2) higher in the CLG group than in the treatment groups (Fig 1F).
Effects of the C. perfringens challenge and L. acidophilus supplementation on the cecal microbiota
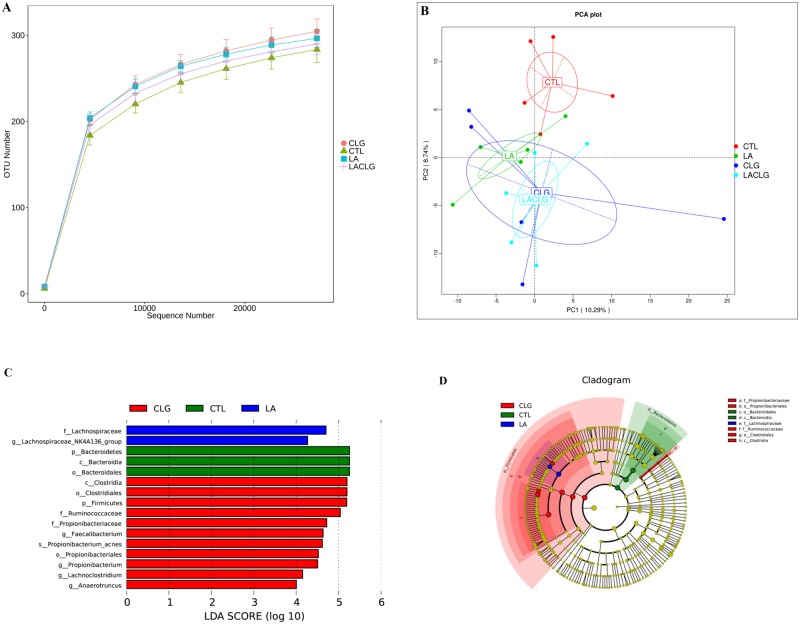
Rarefaction curves of the observed OTUs in the cecum indicated that there were comparable numbers of OTUs among treatment groups (Fig 2A and Table 2). The number of observed species, and Shannon and Simpson indices of the CTL group were the lowest among the four groups (P < 0.05), and the C. perfringens challenge decreased the Chao1 index of the cecal bacterial community (P < 0.05). This community’s structure (β-diversity) in broiler chickens was compared among the four treatment groups by PCA. Principal component axis 1 explained 10.29% of the variation in the bacterial diversity, whereas the second axis explained 8.74% (Fig 2B).
Fig 2. Diversity and composition of cecal microbiota.
There were significant interaction effects of L. acidophilus and the infectious challenge on the number of observed OTUs (A). The PCA plot shows separation of bacterial communities between the CTL group and LA group (R = 0.42, P = 0.024), between the CTL group and CLG group (R = 0.404, P = 0.027), and between the CTL group and LACLG group (R = 0.50, P = 0.012) (B). Key phylotypes in the cecum responding to treatments were identified by the LEfSe algorithm (C). The circular cladogram (D) shows the taxa that are significantly associated with treatments.
Table 2. The α diversity of cecal samples.
| Items | Observed species | Shannon | Simpson | Chao1 | ACE |
|---|---|---|---|---|---|
| CTL | 316.50b | 4.67b | 0.86b | 370.00 | 374.66 |
| LA | 337.33a | 5.54a | 0.94a | 365.88 | 359.96 |
| CLG | 332.67a | 5.31a | 0.91a | 384.72 | 389.04 |
| LACLG | 331.17a | 5.22a | 0.91a | 385.41 | 382.39 |
| SEM | 2.150 | 0.094 | 0.009 | 4.060 | 5.985 |
| Main effects | |||||
| C. perfringens challenge | |||||
| Negative | 326.92 | 5.11 | 0.90 | 367.94 | 367.31 |
| Positive | 331.92 | 5.27 | 0.91 | 385.07 | 385.71 |
| L. acidophilus | |||||
| No addition | 324.58 | 4.99 | 0.89 | 377.36 | 381.85 |
| Addition | 334.25 | 5.38 | 0.93 | 375.64 | 371.17 |
| P-value | |||||
| C. perfringens challenge | 0.139 | 0.295 | 0.392 | 0.039 | 0.135 |
| L. acidophilus | 0.007 | 0.015 | 0.019 | 0.829 | 0.379 |
| C. perfringens challenge × L. acidophilus | 0.002 | 0.003 | 0.013 | 0.762 | 0.738 |
With the Silva classifier, > 99.60% of the total sequence reads were assigned to the bacterial phyla. At the phylum level, the cecal microbiota was dominated by Firmicutes (> 45%), followed by Bacteroidetes, Proteobacteria and Tenericutes (S2A Fig). The C. perfringens infection increased the relative abundance of the Escherichia-Shigella genus (S2B Fig) in the cecal microbiota. LEfSe detected a marked increase (LDA score > 4) in the relative abundance of the Clostridiales family in the chicks of the CLG group compared with other groups (Fig 2C and 2D).
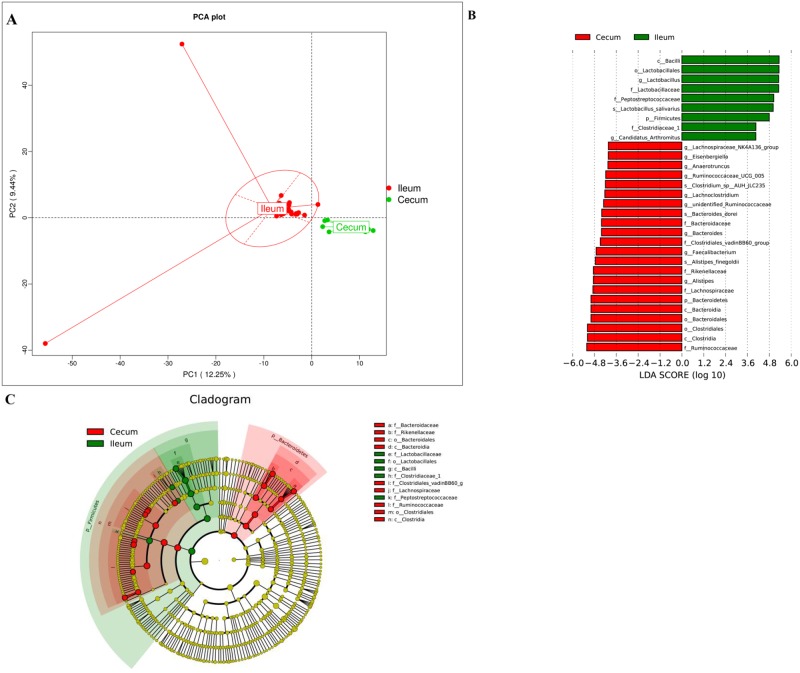
Comparison of the microbiota between the ileum and cecum
The α diversity indices of Shannon and Simpson were higher (P < 0.05) in the cecal samples than in the corresponding ileal samples (Table 3), indicating that the cecal microbiota was more diverse than the ileal microbiota. PCA of OTUs from the microbiotas in the ileum and cecum (Fig 3A) also revealed that the bacterial community structure differed significantly by sampling site (ANOSIM: R = 0.9138, P = 0.001). According to the LEfSe results, there were 31 differentially abundant bacterial clades at all taxonomic levels (LDA score > 2.0) between the ileal and cecal microbiotas (Fig 3B). Peptostreptococcaceae, Lactobacillaceae and Enterobacteriaceae were the dominant families in the ileum while the cecum was inhabited mostly by families Ruminococcaceae, Rikenellaceae and Lachnospiraceae (S3A Fig). The most dominant genus in the ileum was Lactobacillus accounting for more than 43% of all the observed sequence reads (S3B Fig).
Table 3. A comparison of α-diversity between ileal and cecal samples.
| Items | Observed species | Shannon | Simpson | Chao1 | ACE |
|---|---|---|---|---|---|
| Ileum | 300.75 | 2.90 | 0.67 | 370.16 | 370.56 |
| Cecum | 306.75 | 5.14 | 0.91 | 360.17 | 355.30 |
| SEM | 6.097 | 0.209 | 0.027 | 8.398 | 7.944 |
| P value | 0.629 | < 0.001 | < 0.001 | 0.559 | 0.343 |
Fig 3. A comparison of diversity and composition between ileal and cecal microbiotas.
The PCA plot (A) shows that the bacterial profile differed strongly by sampling site (R = 0.9138, P = 0.001). Taxa significantly associated with communities of ileum versus cecum were identified by the LEfSe algorithm (B) and are shown in the circular cladogram (C).
The concentration of lactate and SCFAs in the ileum and cecum
The lactate and SCFA concentrations in the ileum (Table 4) and cecum (Table 5) were measured to test whether the observed microbial changes due to the infectious challenge and L. acidophilus supplementation affected intestinal function. In the ileum, the C. perfringens challenge tended to increase the lactate concentration (P = 0.061), and decreased the concentrations of formate (P < 0.05), acetate (P < 0.05) and propionate (P < 0.05), whereas L. acidophilus addition to the diet increased the concentration of lactate (P < 0.05) and butyrate, and decreased the concentrations of formate (P < 0.05) and propionate (P < 0.05). In contrast, isobutyrate, isovalerate and valerate were not detected in the ileum.
Table 4. Lactate and SCFA concentrations in the ileum.
| Items1 | Lactic acid | Formate | Acetate | Propionate | Butyrate |
|---|---|---|---|---|---|
| CTL | 1347.41 | 111.14 | 173.45 | 5.79 | 0.73 |
| LA | 4205.59 | 79.46 | 92.76 | 3.26 | 3.18 |
| CLG | 2450.45 | 72.52 | 63.76 | 3.72 | 2.12 |
| LACLG | 7453.46 | 62.57 | 28.54 | 2.62 | 3.72 |
| SEM | 702.105 | 4.610 | 18.719 | 0.326 | 0.390 |
| Main effects | |||||
| C. perfringens challenge | |||||
| Negative | 2776.50 | 95.30 | 133.10 | 4.52 | 1.96 |
| Positive | 4951.95 | 67.54 | 46.15 | 3.17 | 2.92 |
| L. acidophilus | |||||
| No addition | 1898.93 | 91.83 | 118.60 | 4.57 | 1.42 |
| Addition | 5829.53 | 71.01 | 60.65 | 2.94 | 3.45 |
| P value | |||||
| C. perfringens challenge | 0.061 | < 0.001 | 0.014 | 0.008 | 0.161 |
| L. acidophilus | 0.002 | 0.001 | 0.087 | 0.001 | 0.006 |
| C. perfringens challenge × L. acidophilus | 0.339 | 0.068 | 0.489 | 0.133 | 0.529 |
Table 5. Lactate and SCFA concentrations in the cecum.
| Items1 | Lactic acid | Formate | Acetate | Propionate | Isobutyrate | Butyrate | Isovalerate | Valerate |
|---|---|---|---|---|---|---|---|---|
| CTL | 55.25 | 140.10 | 3476.65 | 340.32 | 28.31 | 930.79 | 17.53 | 59.73 |
| LA | 62.22 | 164.61 | 3310.78 | 399.83 | 24.87 | 1337.39 | 16.78 | 75.29 |
| CLG | 29.04 | 169.42 | 3384.29 | 230.14 | 15.86 | 1128.09 | 5.95 | 47.73 |
| LACLG | 140.63 | 126.69 | 3344.76 | 291.89 | 11.08 | 1052.34 | 14.78 | 70.51 |
| SEM | 15.135 | 9.022 | 120.136 | 36.174 | 2.891 | 72.924 | 1.985 | 4.648 |
| Main effects | ||||||||
| C. perfringens challenge | ||||||||
| Negative | 58.74 | 152.36 | 3398.72 | 370.07 | 26.59 | 1134.09 | 17.16 | 67.51 |
| Positive | 84.84 | 148.06 | 3364.52 | 261.01 | 13.47 | 1090.21 | 10.36 | 59.12 |
| L. acidophilus | ||||||||
| No addition | 42.14 | 154.76 | 3435.47 | 285.23 | 22.09 | 1029.44 | 11.74 | 53.73 |
| Addition | 101.43 | 145.65 | 3327.77 | 345.86 | 17.97 | 1194.86 | 15.78 | 72.90 |
| P value | ||||||||
| C. perfringens challenge | 0.338 | 0.810 | 0.895 | 0.148 | 0.024 | 0.760 | 0.083 | 0.350 |
| L. acidophilus | 0.037 | 0.612 | 0.678 | 0.412 | 0.454 | 0.257 | 0.290 | 0.041 |
| C. perfringens challenge × L. acidophilus | 0.063 | 0.072 | 0.793 | 0.988 | 0.903 | 0.104 | 0.212 | 0.685 |
In the cecum, the C. perfringens challenge significantly decreased the isobutyrate concentration (P < 0.05), and tended to decrease the isovelerate concentration (P = 0.081). L. acidophilus supplementation significantly increased the concentration of lactate (P < 0.05) and valerate (P < 0.05).
The correlation analyses indicated that the relative abundance of the ileal Lactobacillus genus positively correlated with ileal lactate production (R = 0.728, P < 0.001), whereas relative abundance of the Propionibacterium genus in the ileum of broiler chickens correlated with cecal valerate concentration negatively (R = -0.443, P < 0.05).
Discussion
The gut microbiota is of great importance to host health and production. Many studies have revealed that the gut microbiota can provide the host with vitamin E, SCFAs, and other benefits [16]. Furthermore, gut microbiota is useful for the development of the host immune system [17]. Any alteration in the intestinal microbiota may have functional consequences for the health of the host [18]. Nowadays, analysis of 16S rRNA gene amplicons is a popular method for identifying the functional diversity [19] and variability [20] of the microbiome in the intestine of broiler chickens. In the present study, we used high-throughput sequencing of the V3-V4 region of the 16S rRNA gene to monitor the ileal and cecal microbiota of individual unchallenged or challenged broiler chickens fed a diet without or with L. acidophilus supplementation in a battery cage trial.
In our previous study, impaired growth performance of broilers, increased mortality and intestinal lesion scores after a C. perfringens challenge indicated that an experimental NE model was successfully established, and the dietary supplementation with L. acidophilus improved the growth performance and intestinal health of broilers (manuscript under review). Our current results indicate that the C. perfringens challenge and L. acidophilus supplementation modified the proportion of specific OTUs and these changes were associated with ileal lactate production.
In general, a more diverse microbial community shows stronger homeostasis of the intestinal microbial community and resistance to pathogens [21]. Increased gut microbial diversity is healthier in the elderly [22]. Another published study showed that the gut microbial diversity of athletes is higher than that of controls [23]. Low diversity of microorganisms is associated with a plethora of diseases, such as inflammatory bowel diseases [24, 25]. One study revealed that a C. perfringens challenge numerically decreases the α-diversity index of the small intestinal microbial community [18], as proved in our study. Nevertheless, other studies indicate that a C. perfringens challenge in the absence of predisposing factors does not cause significant changes in either α or β diversity of the cecal microbiota [26, 27]. The discrepancy in findings may be due to difference in C. perfringens strains and dissimilar diet types. Here, L. acidophilus supplementation of the diet decreased the ileal Shannon index, namely decreased the α-diversity of the ileal microbiota; this result can be explained as follows: L. acidophilus dietary supplementation made L. acidophilus the dominant bacterium in the ileum.
Our results on β-diversity revealed that the ileal microbial communities of four treatment groups were not separated clearly; this finding impliesthat chickens shared a core set of bacterial taxa in the ileum regardless of treatments. Plenty of studies indicate that Firmicutes rank first at the phylum level in terms of relative abundance in the intestinal bacterial community of broiler chickens [28–30]. In the present study, the C. perfringens challenge decreased the relative abundance of Firmicutes and increased the relative abundance of Proteobacteria. In the ileum, enrichment of the phylum Firmicutes and a reduction in the phylum Proteobacteria were observed after the probiotic L. acidophilus supplementation. We identified a class of bacteria that is associated with the CLG treatment, namely, Gammaproteobacteria, which belong to the phylum Proteobacteria. Firmicutes have a positive correlation with high energy efficiency, and the Firmicutes-to-Bacteroides ratio affects the amount of energy extracted from the diet [31–34]. Consequently, these parameters are directly related to the growth performance of broilers [35, 36]. Proteobacteria include many pathogens, such as subgroup Salmonella, E. coli, and Shigella, which can colonize both humans and chickens [37] and may trigger some specific disease. Therefore, the increase in Firmicutes and a reduction in Proteobacteria in the ileum may be associated with the improvement of growth performance and intestinal health of broilers fed with L. acidophilus. The ileal microbiota in this study was mainly composed of the genera Lactobacillus and Escherichia-Shigella, a finding that is consistent with other studies [29, 38]. In cecal samples here, most of the OTUs belonged to genera Alistipes, Bacteroides, Faecalibacterium, and Escherichia-Shigella.
Lactobacillus, as a typical probiotic bacterium, promotes the homeostasis of immune cells and intestinal health of the host [39, 40]. After a C. perfringens-challenge, Lactobacillus is suppressed at the early stage [18]. Nonetheless, other studies have shown that the number of is increased by an infectious challenge [9, 41], as confirmed in our present study. Some Lactobacillus species, such as L. aviarius, are suppressed by C. perfringens infection in the ileum of broilers [42]. The Lactobacillus genus was not dominant in the cecal microbiota in this study and was not affected by the infection. The Enterobacteriaceae family mainly consists of the Escherichia-Shigella genus, whose relative abundance was increased numerically by the infection in the present study, was and it represents major pathogenic microbes coexisting with NE in the intestine [43]. The probiotic L. acidophilus supplementation numerically decreased the relative abundance of Escherichia-Shigella genus both in the ileum and cecum and thereby further improved intestinal health.
SCFAs are defined as the class of fatty acids with fewer than six carbons, including formic acid (C1), acetic acid (C2), propionic acid (C3), butyric acid (C4) and valeric acid (C5) [44]. SCFAs are necessary for intestinal functionality and integrity [45], energy intake of enterocytes and cellular proliferation and differentiation within the intestinal mucosa [46]. These by-products are beneficial to broiler energy metabolism and contribute to lower pH of the broiler gut environment; this acidic pH may inhibit the overgrowth of intestinal pathogens [47]. Nutrients in the diets can be fermented by the gut microbiota, accompanied by the production of SCFAs and lactate [48]. The probiotic L. acidophilus supplementation increased the relative abundance of Lactobacillus in the intestine but might have suppressed some SCFA-producing bacteria, such as Faecalibacterium spp. (S1C Fig). Our results indicate that the ileal microbiota of chickens in the control group contained a higher proportion of butyrate-producing families Ruminococcaceae and Lachnospiraceae, which might have been suppressed in other experimental groups by the C. perfringens challenge or L. acidophilus supplementation. The lactate produced by Lactobacillus species, can be converted to butyrate, propionate, and acetate [49–51]. This notion was substantiated in the current study by the increased butyrate concentration in the ileum after L. acidophilus supplementation in comparison with control groups. Some SCFAs, mainly butyrate, are metabolized by the intestinal epithelial cells [52], and the others enter the diverse carbohydrate and lipid metabolic routes: propionate will mainly participate in gluconeogenesis while acetate and butyrate will be mostly processed in lipid biosynthesis [53]. Acetate has been proved to be the key Bifidobacteria-generated inhibitor of enteropathogens’ growth [54]. Butyrate nourishes the enterocytes, increases intestinal mucin production, which may change the bacterial adhesion [55] and improve tight-junction integrity [56], takes part in cellular differentiation and proliferation within the intestinal mucosa [46], and can reduce an inflammatory response as an anti-inflammatory effector [57]. C. perfringens infection damages the intestinal mucosa of broilers [9, 58] and disrupts homeostasis of the intestinal microbiota [18]. Another study revealed that a C. perfringens challenge in the absence of predisposing factors does not cause significant changes in concentrations of SCFAs [26]. In the present study, however, the infection decreased the ileal concentration of formate, acetate, and propionate and cecal isobutyrate concentration; these alterations might have resulted frominhibition of the growth of a few bacterial taxa that can produce SCFAs. The probiotic L. acidophilus supplementation increased the relative abundance of Lactobacillus and concentrations of lactate and SCFAs in the intestine of broilers. Our results showed that the ileal concentrations of lactate and butyric acid and cecal concentrations of lactate and valerate increased with the L. acidophilus supplementation, and consequently alleviated the damage caused by C. perfringens infection and restored the disrupted gut microbiota.
Conclusion
We found that dietary supplementation with probiotic L. acidophilus increases the relative abundance of beneficial bacteria and decreases the relative abundance of pathogens in the intestine of broilers challenged by C. perfringens infection. Increased population of Lactobacillus, elevated concentrations of lactate and butyrate, and decreased relative abundance of Escherichia-Shigella may promote intestinal health and contribute to the recovery of an intestinal microbial community disrupted by C. perfringens infection.
Supporting information
(TIF)
(TIF)
(TIF)
(DOCX)
Data Availability
All sequence files are available from the NCBI Sequence Read Archive database (accession number(s) PRJNA397003).
Funding Statement
This work was supported by the Natural Science foundation of China (http://www.nsfc.gov.cn/). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Van Immerseel F, Rood JI, Moore RJ, Titball RW. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends in Microbiology. 2009;17(1):32–6. doi: 10.1016/j.tim.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 2.Wvd Sluis, W van der Sluis. Clostridial enteritis is an often underestimated problem. World Poultry. 2000;16(7):42–3. [Google Scholar]
- 3.Hofacre CL, Beacorn T, Collett S, Mathis G. Using competitive exclusion, mannan-oligosaccharide and other intestinal products to control necrotic enteritis. Journal of Applied Poultry Research. 2003;12(1):60–4. [Google Scholar]
- 4.Peng Q, Zeng XF, Zhu JL, Wang S, Liu XT, Hou CL, et al. Effects of dietary Lactobacillus plantarum B1 on growth performance, intestinal microbiota, and short chain fatty acid profiles in broiler chickens. Poultry Science. 2016;95(4):893–900. doi: 10.3382/ps/pev435 [DOI] [PubMed] [Google Scholar]
- 5.Bull M, Plummer S, Marchesi J, Mahenthiralingam E. The life history of Lactobacillus acidophilus as a probiotic: a tale of revisionary taxonomy, misidentification and commercial success. Fems Microbiology Letters. 2013;349(2):77–87. doi: 10.1111/1574-6968.12293 [DOI] [PubMed] [Google Scholar]
- 6.Lin C-K, Tsai H-C, Lin P-P, Tsen H-Y, Tsai C-C. Lactobacillus acidophilus LAP5 able to inhibit the Salmonella choleraesuis invasion to the human Caco-2 epithelial cell. Anaerobe. 2008;14(5):251–5. doi: 10.1016/j.anaerobe.2008.07.003 [DOI] [PubMed] [Google Scholar]
- 7.Tsai CC, Hsih HY, Chiu HH, Lai YY, Liu JH, Yu B, et al. Antagonistic activity against Salmonella infection in vitro and in vivo for two Lactobacillus strains from swine and poultry. International Journal of Food Microbiology. 2005;102(2):185–94. doi: 10.1016/j.ijfoodmicro.2004.12.014 [DOI] [PubMed] [Google Scholar]
- 8.Dahiya JP, Hoehler D, Wilkie DC, Van Kessel AG, Drew MD. Dietary glycine concentration affects intestinal Clostridium perfringens and lactobacilli populations in broiler chickens. Poultry Science. 2005;84(12):1875–85. [DOI] [PubMed] [Google Scholar]
- 9.Liu D, Guo Y, Wang Z, Yuan J. Exogenous lysozyme influences Clostridium perfringens colonization and intestinal barrier function in broiler chickens. Avian Pathology. 2010;39(1):17–24. doi: 10.1080/03079450903447404 [DOI] [PubMed] [Google Scholar]
- 10.Magoc T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27(21):2957–63. doi: 10.1093/bioinformatics/btr507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nature Methods. 2013;10(10):996–+. doi: 10.1038/nmeth.2604 [DOI] [PubMed] [Google Scholar]
- 12.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nature Methods. 2010;7(5):335–6. doi: 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Research. 2013;41(D1):D590–D6. doi: 10.1093/nar/gks1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke KR. NONPARAMETRIC MULTIVARIATE ANALYSES OF CHANGES IN COMMUNITY STRUCTURE. Australian Journal of Ecology. 1993;18(1):117–43. doi: 10.1111/j.1442-9993.1993.tb00438.x [Google Scholar]
- 15.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biology. 2011;12(6). doi: 10.1186/gb-2011-12-6-r60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanley D, Hughes RJ, Moore RJ. Microbiota of the chicken gastrointestinal tract: influence on health, productivity and disease. Applied Microbiology and Biotechnology. 2014;98(10):4301–10. doi: 10.1007/s00253-014-5646-2 [DOI] [PubMed] [Google Scholar]
- 17.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352(6285):539–44. doi: 10.1126/science.aad9378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fasina YO, Newman MM, Stough JM, Liles MR. Effect of Clostridium perfringens infection and antibiotic administration on microbiota in the small intestine of broiler chickens. Poultry Science. 2016;95(2):247–60. doi: 10.3382/ps/pev329 [DOI] [PubMed] [Google Scholar]
- 19.Sergeant MJ, Constantinidou C, Cogan TA, Bedford MR, Penn CW, Pallen MJ. Extensive Microbial and Functional Diversity within the Chicken Cecal Microbiome. Plos One. 2014;9(3). doi: 10.1371/journal.pone.0091941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanley D, Geier MS, Hughes RJ, Denman SE, Moore RJ. Highly Variable Microbiota Development in the Chicken Gastrointestinal Tract. Plos One. 2013;8(12). doi: 10.1371/journal.pone.0084290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konstantinov SR, Favier CF, Zhu WY, Williams BA, Kluss J, Souffrant WB, et al. Microbial diversity studies of the porcine gastrointestinal ecosystem during weaning transition. Animal Research. 2004;53(4):317–24. doi: 10.1051/animres:2004019 [Google Scholar]
- 22.Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–+. doi: 10.1038/nature11319 [DOI] [PubMed] [Google Scholar]
- 23.Clarke SF, Murphy EF, O’Sullivan O, Lucey AJ, Humphreys M, Hogan A, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63(12):1913–20. doi: 10.1136/gutjnl-2013-306541 [DOI] [PubMed] [Google Scholar]
- 24.Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, Folsch UR, et al. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53(5):685–93. doi: 10.1136/gut.2003.025403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas S, Izard J, Walsh E, Batich K, Chongsathidkiet P, Clarke G, et al. The Host Microbiome Regulates and Maintains Human Health: A Primer and Perspective for Non-Microbiologists. Cancer Research. 2017;77(8):1783–812. doi: 10.1158/0008-5472.CAN-16-2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanley D, Wu S-B, Rodgers N, Swick RA, Moore RJ. Differential Responses of Cecal Microbiota to Fishmeal, Eimeria and Clostridium perfringens in a Necrotic Enteritis Challenge Model in Chickens. Plos One. 2014;9(8). doi: 10.1371/journal.pone.0104739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanley D, Keyburn AL, Denman SE, Moore RJ. Changes in the caecal microflora of chickens following Clostridium perfringens challenge to induce necrotic enteritis. Veterinary Microbiology. 2012;159(1–2):155–62. doi: 10.1016/j.vetmic.2012.03.032 [DOI] [PubMed] [Google Scholar]
- 28.Antonissen G, Eeckhaut V, Van Driessche K, Onrust L, Haesebrouck F, Ducatelle R, et al. Microbial shifts associated with necrotic enteritis. Avian Pathology. 2016;45(3):308–12. doi: 10.1080/03079457.2016.1152625 [DOI] [PubMed] [Google Scholar]
- 29.Pourabedin M, Guan LL, Zhao X. Xylo-oligosaccharides and virginiamycin differentially modulate gut microbial composition in chickens. Microbiome. 2015;3 doi: 10.1186/s40168-015-0079-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaufi MAM, Sieo CC, Chong CW, Gan HM, Ho YW. Deciphering chicken gut microbial dynamics based on high-throughput 16S rRNA metagenomics analyses. Gut Pathogens. 2015;7 doi: 10.1186/s13099-015-0051-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bervoets L, Van Hoorenbeeck K, Kortleven I, Van Noten C, Hens N, Vael C, et al. Differences in gut microbiota composition between obese and lean children: a cross-sectional study. Gut Pathogens. 2013;5 doi: 10.1186/1757-4749-5-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mariat D, Firmesse O, Levenez F, Guimaraes VD, Sokol H, Dore J, et al. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. Bmc Microbiology. 2009;9 doi: 10.1186/1471-2180-9-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology—Human gut microbes associated with obesity. Nature. 2006;444(7122):1022–3. doi: 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- 34.Jumpertz R, Duc Son L, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, et al. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. American Journal of Clinical Nutrition. 2011;94(1):58–65. doi: 10.3945/ajcn.110.010132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torok VA, Ophel-Keller K, Loo M, Hughes RJ. Application of methods for identifying broiler chicken gut bacterial species linked with increased energy metabolism. Applied and Environmental Microbiology. 2008;74(3):783–91. doi: 10.1128/AEM.01384-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh KM, Shah T, Deshpande S, Jakhesara SJ, Koringa PG, Rank DN, et al. High through put 16S rRNA gene-based pyrosequencing analysis of the fecal microbiota of high FCR and low FCR broiler growers. Molecular Biology Reports. 2012;39(12):10595–602. doi: 10.1007/s11033-012-1947-7 [DOI] [PubMed] [Google Scholar]
- 37.Mora A, Herrera A, Mamani R, Lopez C, Pilar Alonso M, Blanco JE, et al. Recent Emergence of Clonal Group O25b:K1:H4-B2-ST131 ibeA Strains among Escherichia coli Poultry Isolates, Including CTX-M-9-Producing Strains, and Comparison with Clinical Human Isolates. Applied and Environmental Microbiology. 2010;76(21):6991–7. doi: 10.1128/AEM.01112-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gong J, Si W, Forster RJ, Huang R, Yu H, Yin Y, et al. 16S rRNA gene-based analysis of mucosa-associated bacterial community and phylogeny in the chicken gastrointestinal tracts: from crops to ceca. Fems Microbiology Ecology. 2007;59(1):147–57. doi: 10.1111/j.1574-6941.2006.00193.x [DOI] [PubMed] [Google Scholar]
- 39.Van Tassell ML, Miller MJ. Lactobacillus Adhesion to Mucus. Nutrients. 2011;3(5):613–36. doi: 10.3390/nu3050613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ren CC, Zhang QX, de Haan BJ, Zhang H, Faas MM, de Vos P. Identification of TLR2/TLR6 signalling lactic acid bacteria for supporting immune regulation. Scientific Reports. 2016;6 doi: 10.1038/srep34561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun Q, Liu D, Guo S, Chen Y, Guo Y. Effects of dietary essential oil and enzyme supplementation on growth performance and gut health of broilers challenged by Clostridium perfringens. Animal Feed Science and Technology. 2015;207:234–44. doi: 10.1016/j.anifeedsci.2015.06.021 [Google Scholar]
- 42.Feng Y, Gong J, Yu H, Jin Y, Zhu J, Han Y. Identification of changes in the composition of ileal bacterial microbiota of broiler chickens infected with Clostridium perfringens. Veterinary Microbiology. 2010;140(1–2):116–21. doi: 10.1016/j.vetmic.2009.07.001 [DOI] [PubMed] [Google Scholar]
- 43.Du E, Gan L, Li Z, Wang W, Liu D, Guo Y. In vitro antibacterial activity of thymol and carvacrol and their effects on broiler chickens challenged with Clostridium perfringens. Journal of Animal Science and Biotechnology. 2015;6 doi: 10.1186/s40104-015-0055-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun M, Wu W, Liu Z, Cong Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. Journal of Gastroenterology. 2017;52(1):1–8. doi: 10.1007/s00535-016-1242-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meimandipour A, Shuhaimi M, Soleimani AF, Azhar K, Hair-Bejo M, Kabeir BM, et al. Selected microbial groups and short-chain fatty acids profile in a simulated chicken cecum supplemented with two strains of Lactobacillus. Poultry Science. 2010;89(3):470–6. doi: 10.3382/ps.2009-00495 [DOI] [PubMed] [Google Scholar]
- 46.Rinttila T, Apajalahti J. Intestinal microbiota and metabolites-Implications for broiler chicken health and performance. Journal of Applied Poultry Research. 2013;22(3):647–58. doi: 10.3382/japr.2013-00742 [Google Scholar]
- 47.van der Wielen P, Biesterveld S, Notermans S, Hofstra H, Urlings BAP, van Knapen F. Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth. Applied and Environmental Microbiology. 2000;66(6):2536–40. doi: 10.1128/aem.66.6.2536-2540.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walugembe M, Hsieh JCF, Koszewski NJ, Lamont SJ, Persia ME, Rothschild MF. Effects of dietary fiber on cecal short-chain fatty acid and cecal microbiota of broiler and laying-hen chicks. Poultry Science. 2015;94(10):2351–9. doi: 10.3382/ps/pev242 [DOI] [PubMed] [Google Scholar]
- 49.Duncan SH, Louis P, Flint HJ. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Applied and Environmental Microbiology. 2004;70(10):5810–7. doi: 10.1128/AEM.70.10.5810-5817.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elferink S, Krooneman J, Gottschal JC, Spoelstra SF, Faber F, Driehuis F. Anaerobic conversion of lactic acid to acetic acid and 1,2-propanediol by Lactobacillus buchneri. Applied and Environmental Microbiology. 2001;67(1):125–32. doi: 10.1128/AEM.67.1.125-132.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bjerrum L, Engberg RM, Leser TD, Jensen BB, Finster K, Pedersen K. Microbial community composition of the ileum and cecum of broiler chickens as revealed by molecular and culture-based techniques. Poultry Science. 2006;85(7):1151–64. [DOI] [PubMed] [Google Scholar]
- 52.Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. Fems Microbiology Letters. 2002;217(2):133–9. doi: 10.1111/j.1574-6968.2002.tb11467.x [DOI] [PubMed] [Google Scholar]
- 53.Rios-Covian D, Ruas-Madiedo P, Margolles A, Gueimonde M, de los Reyes-Gavilan CG, Salazar N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Frontiers in Microbiology. 2016;7 doi: 10.3389/fmicb.2016.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469(7331):543–U791. doi: 10.1038/nature09646 [DOI] [PubMed] [Google Scholar]
- 55.Jung T-H, Park JH, Jeon W-M, Han K-S. Butyrate modulates bacterial adherence on LS174T human colorectal cells by stimulating mucin secretion and MAPK signaling pathway. Nutrition Research and Practice. 2015;9(4):343–9. doi: 10.4162/nrp.2015.9.4.343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peng L, Li Z-R, Green RS, Holzman IR, Lin J. Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly via Activation of AMP-Activated Protein Kinase in Caco-2 Cell Monolayers. Journal of Nutrition. 2009;139(9):1619–25. doi: 10.3945/jn.109.104638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–+. doi: 10.1038/nature12721 [DOI] [PubMed] [Google Scholar]
- 58.Liu D, Guo S, Guo Y. Xylanase supplementation to a wheat-based diet alleviated the intestinal mucosal barrier impairment of broiler chickens challenged by Clostridium perfringens. Avian Pathology. 2012;41(3):291–8. doi: 10.1080/03079457.2012.684089 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
(DOCX)
Data Availability Statement
All sequence files are available from the NCBI Sequence Read Archive database (accession number(s) PRJNA397003).