Abstract
Activation of the phosphatidylinositol 3-kinase/mechanistic target of rapamycin pathway plays a role in the pathogenesis of non-Hodgkin lymphoma. This multicenter, open-label phase 2 study evaluated buparlisib (BKM120), a pan-class I phosphatidylinositol 3-kinase inhibitor, in patients with relapsed or refractory non-Hodgkin lymphoma. Three separate cohorts of patients (with diffuse large B-cell lymphoma, mantle cell lymphoma, or follicular lymphoma) received buparlisib 100 mg once daily until progression, intolerance, or withdrawal of consent. The primary endpoint was overall response rate based on a 6-month best overall response by cohort; secondary endpoints included progression-free survival, duration of response, overall survival, safety, and tolerability. Overall, 72 patients (26 with diffuse large B-cell lymphoma, 22 with mantle cell lymphoma, and 24 with follicular lymphoma) were treated. The overall response rates were 11.5%, 22.7%, and 25.0% in patients with diffuse large B-cell lymphoma, mantle cell lymphoma, and follicular lymphoma, respectively; two patients (one each with diffuse large B-cell lymphoma and mantle cell lymphoma) achieved a complete response. The most frequently reported (>20%) adverse events of any grade in the population in which safety was studied were hyperglycemia, fatigue, and nausea (36.1% each), depression (29.2%), diarrhea (27.8%), and anxiety (25.0%). The most common grade 3/4 adverse events included hyperglycemia (11.1%) and neutropenia (5.6%). Buparlisib showed activity in relapsed or refractory non-Hodgkin lymphoma, with disease stabilization and sustained tumor burden reduction in some patients, and acceptable toxicity. Development of mechanism-based combination regimens with buparlisib is warranted. (This study was funded by Novartis Pharmaceuticals Corporation and registered with ClinicalTrials.gov number, NCT01693614).
Introduction
Non-Hodgkin lymphoma (NHL) accounts for 4% of all cancers1 and resulted in an estimated 199,700 deaths worldwide in 2012.2 NHL subtypes include diffuse large B-cell lymphoma (DLBCL; 22–36% of cases of NHL), mantle cell lymphoma (MCL; 1%–14% of cases of NHL), and follicular lymphoma (FL; 8%–32% of cases of NHL).3–5 Response to treatment is heterogeneous due to the varying biology of each NHL subtype; disease course can be indolent or aggressive.6,7 The 5-year survival rate from 2006 to 2012 was 62% for patients with DLBCL, 56% for those with MCL, and 88% for those with FL.5 Combining rituximab, a monoclonal antibody targeting the CD20 antigen on B cells, with standard chemotherapy has improved NHL treatment outcomes.8 Despite high response rates to rituximab-based regimens, many patients become resistant to treatment or relapse after initially achieving a response.8
Phosphatidylinositol 3-kinase (PI3K)/mechanistic target of rapamycin (mTOR) signaling regulates many cellular activities, including proliferation, survival, angiogenesis, and glucose metabolism.9 Due to its effects on B-cell signaling, dysregulation of PI3K signaling is associated with cancer development and may play a key role in the pathogenesis of NHL.10–14 Class I PI3K, the most common forms implicated in cancer, are heterodimers with regulatory (p85) and catalytic (p110) subunits.15 There are four class I isoform catalytic subunits, p110α, p110b, p110γ, and p110d (encoded by PIK3CA, PIK3CB, PIK3CG, and PIK3CD, respectively),15 with varying levels of expression; p110α and p110b are ubiquitous, whereas p110γ and p110d are mainly expressed in hematopoietic cells.15,16 Preclinical evidence indicates that B-cell malignancies frequently overexpress PIK3CD.17,18 The PI3Kd inhibitor idelalisib showed antitumor activity in patients with relapsed or refractory indolent NHL,19,20 and was subsequently approved in 2014 for the treatment of patients with relapsed FL and chronic lymphocytic leukemia (in the latter case in combination with rituximab).21,22
Differential PI3K isoform expression levels have also been associated with different disease stages in NHL.23 For example, in MCL, PIK3CA expression increased upon progression and was implicated as an escape mechanism for PI3Kd inhibition.14,23 Other members of the PI3K pathway, including the phosphatase and tensin homolog (PTEN; a negative regulator of PI3K activity) and mTOR (a downstream effector), also play a role in NHL.14,24 Thus, treating patients with a targeted drug that can inhibit all four isoforms of PI3K may help to block escape pathways, prolong responses, and improve outcomes compared to those achieved with treatment with an inhibitor specific for a single isoform, particularly in patients with relapsed or refractory disease.25,26 Buparlisib, a potent, oral pan-PI3K inhibitor, showed antitumor activity in lymphoma cell lines, induced apoptosis in DLBCL27 and reduced myc-dependent proliferation in MCL,28 showed preclinical activity in hematologic malignancies,28,29 and had clinical efficacy in solid tumors, including breast cancer.30–33 In this open-label phase 2 study, we evaluated the efficacy and safety of buparlisib in patients with relapsed or refractory DLBCL, MCL, or FL.
Methods
Patients
Adult patients (aged ≥18 years) were eligible if they had histologically confirmed DLBCL, MCL, or FL that had relapsed or was refractory to one or more prior therapies. Patients were required to have one or more measurable nodal lesions (≥2 cm according to International Working Group criteria);34,35 if no such nodal lesion was present at baseline, one or more measurable extra-nodal lesions were required. Additional inclusion criteria included an Eastern Cooperative Oncology Group performance status of ≤2, adequate bone marrow and organ function, and fasting plasma glucose ≤120 mg/dL. Patients with DLBCL were either ineligible for autologous or allogeneic stem cell transplantation or had previously received a transplant.
Patients previously treated with PI3K inhibitors, or those with evidence of graft-versus-host disease, active or past central nervous system disease, poorly controlled diabetes mellitus (glycated hemoglobin >8%), active cardiac disease, a history of myocardial infarction within 6 months, or documented congestive heart failure or cardiomyopathy were excluded (additional information is available in the Online Supplementary Methods). Patients were excluded if they had received anticancer therapy within 4 weeks (6 weeks for nitrosourea, monoclonal antibodies, or mitomycin-C) prior to starting the study.
Objectives
The primary objective was to determine the overall response rate, defined as the proportion of patients with a best overall response of complete or partial response, according to International Working Group criteria,34,35 in three different histological subgroups of NHL (i.e., DLBCL, MCL, and FL). The primary objective was assessed locally, per investigator, independently for each cohort, and was based on a 6-month best overall response. Secondary objectives were to assess progression-free survival, duration of response, overall survival, and safety and tolerability in the three histological subgroups (additional information is available in the Online Supplementary Methods).
Study design
This was a multicenter, open-label, single-arm, phase II study in patients with relapsed or refractory NHL (NCT01693614).
Patients with previously treated DLBCL, MCL, or FL received buparlisib 100 mg daily continuously in 28-day cycles until disease progression, death, or intolerable toxicity. Treatment interruptions due to toxicity lasting ≥28 days required permanent study drug discontinuation. Up to two dose reductions (to 80 mg or 60 mg daily) were allowed; patients needing additional reductions discontinued the study.
Patients provided written informed consent prior to entering the study. The study protocol and informed consent form were reviewed and approved by the independent ethics committee or institutional review board for each center. The study was conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice, with applicable local regulations, and with the ethical principles of the Declaration of Helsinki.
Schedule of assessments
The assessment schedule is described in the Online Supplementary Methods.
Statistical analyses
The intent-to-treat population included all patients who received one or more dose of study treatment; the safety set included all patients who received one or more dose of study treatment and had one or more post-baseline safety assessments. The analysis for each cohort (i.e., DLBCL, MCL, and FL) was based on an exact binomial test comparing the overall response rate to a reference level of 10% in the intent-to-treat population, with a significance level of 5% (two-sided). An exact 95% Clopper-Pearson confidence interval (CI) for the overall response rate was calculated. Time-to-event endpoints (i.e., progression-free survival, duration of response, and overall survival) were described using Kaplan–Meier curves with associated summary statistics. For progression-free survival and duration of response, patients not known to have progressed or who died by the data cut-off date were censored at the time of last tumor assessment or before any further anticancer therapy had been given. For overall survival, patients not known to have died at the data cut-off were censored at the date of their last contact (additional information is available in the Online Supplementary Methods).
Results
Patients
Between February 28, 2013, and June 15, 2014, 72 patients were enrolled in the trial, including 26 with DLBCL, 22 with MCL, and 24 with FL (Table 1). Their median age was 65 years (range, 28–85) and 68.1% were male. Most patients (73.6%) had stage III/IV disease at initial diagnosis, and 36.1% of patients had elevated lactate dehydrogenase levels. All patients had received prior anti-neoplastic therapy, and 17 patients (23.6%) had undergone a prior autologous stem cell transplantation (6 with DLBCL, 7 with MCL, and 4 with FL). The median number of prior antineoplastic regimens was three (range, 1–12) for patients with DLBCL, two (range, 1–6) for patients with MCL, and two (range, 1–9) for patients with FL. The most frequently received prior antineoplastic therapy was an alkylating agent (n=70; 97.2%), followed by rituximab (n=68; 94.4%), anthracycline (n=60; 83.3%), and bendamustine (n=21; 29.2%). At last treatment, a best response of complete or partial response was achieved by 11 patients with DLBCL (42.3%; 15.4% complete responses and 26.9% partial responses), 13 with MCL (59.1%; 31.8% complete responses and 27.3% partial responses), and 13 with FL (54.2%; 41.7% complete responses and 12.5% partial responses): ten (38.5%), four (18.2%), and four (16.7%) patients, respectively, had refractory disease.
Table 1.
Baseline characteristics and patient disposition of the patients.
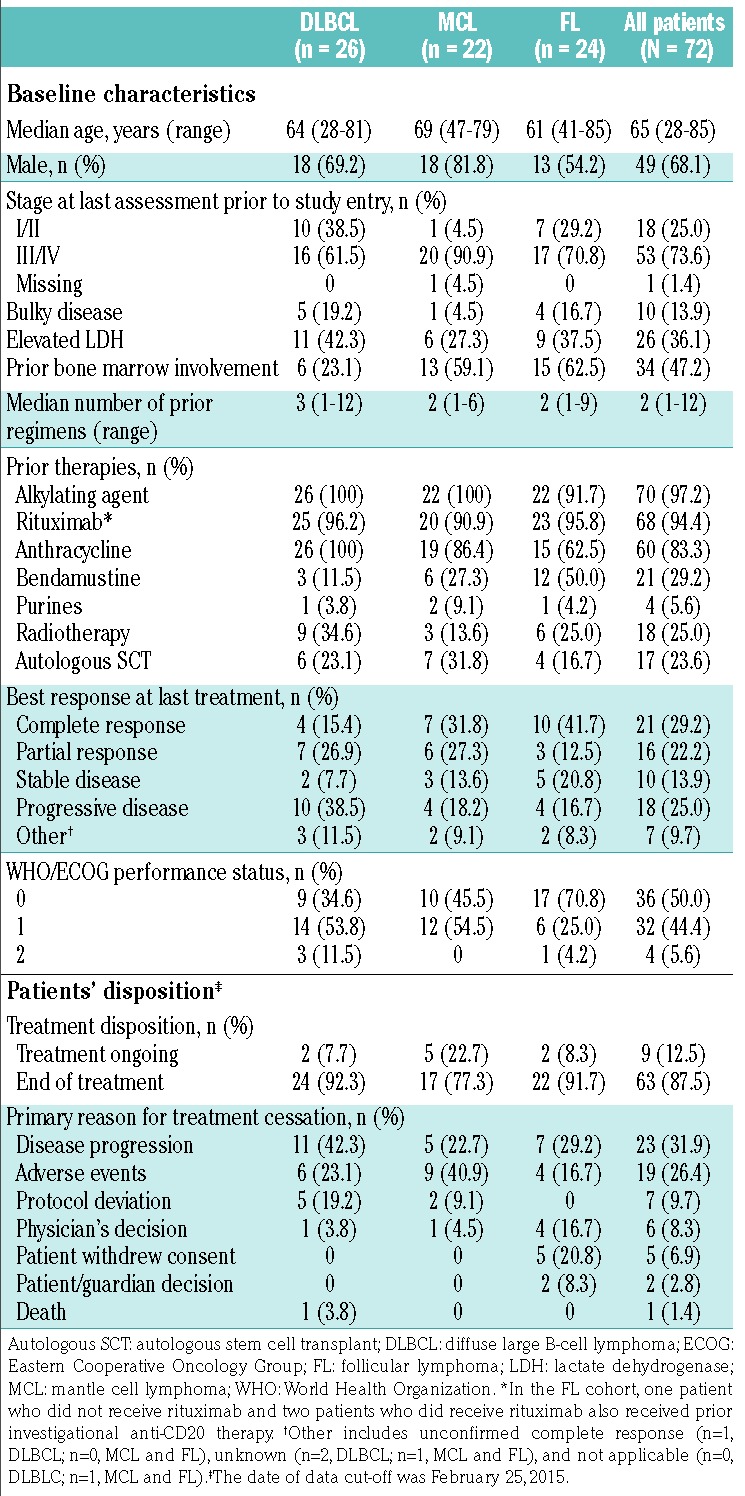
The most common reasons for discontinuing prior therapy were completion of the prescribed regimen (n=36; 50.0%), disease progression (n=24; 33.3%), and adverse events (n=5; 6.9%); other reasons were listed as “unknown” or “other”. The date of data cut-off was February 25, 2015 for all analyses, except for the analysis of best overall response, which occurred separately in each cohort when all patients within the cohort had received 6 months of treatment.
Efficacy
Overall response rate
The overall response rates in the DLBCL, MCL, and FL cohorts were 11.5% (95% CI: 2.5–30.2), 22.7% (95% CI: 7.8–45.4), and 25.0% (95% CI: 9.8–46.7), respectively (Table 2). The primary endpoint was not met, since none of the cohorts reached the minimum number of responses to establish a 35% response rate required for statistical significance at the 5% level, based on the lower limits of the 95% CI (i.e., the 95% CI lower bound was <10% for all three cohorts). As shown in waterfall plots of the best overall response during buparlisib treatment, evaluable patients in all three cohorts had a reduction in investigator-assessed tumor size (Figure 1A-C). The disease control rates in the DLBCL, MCL, and FL cohorts were 30.8% (95% CI: 14.3–51.8), 81.8% (95% CI: 59.7–94.8), and 87.5% (95% CI: 67.6–97.3), respectively.
Table 2.
Best overall response.
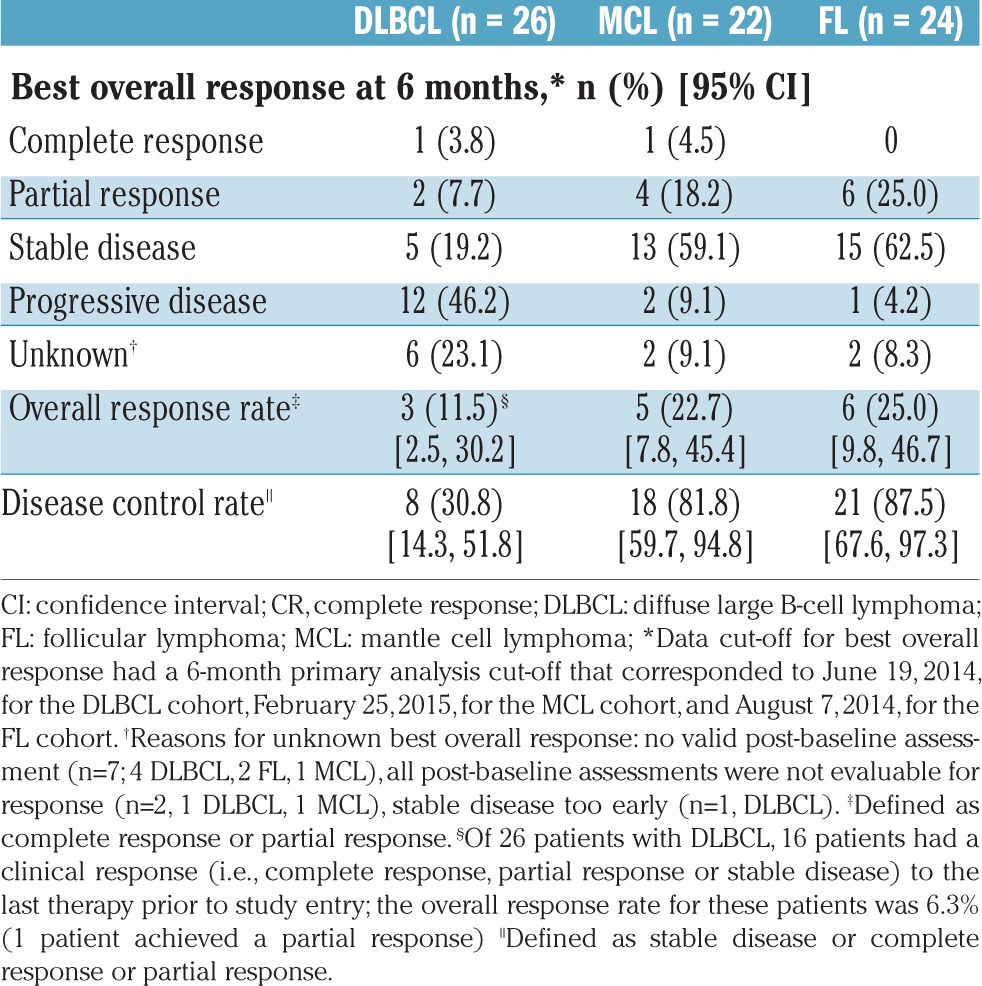
Figure 1.
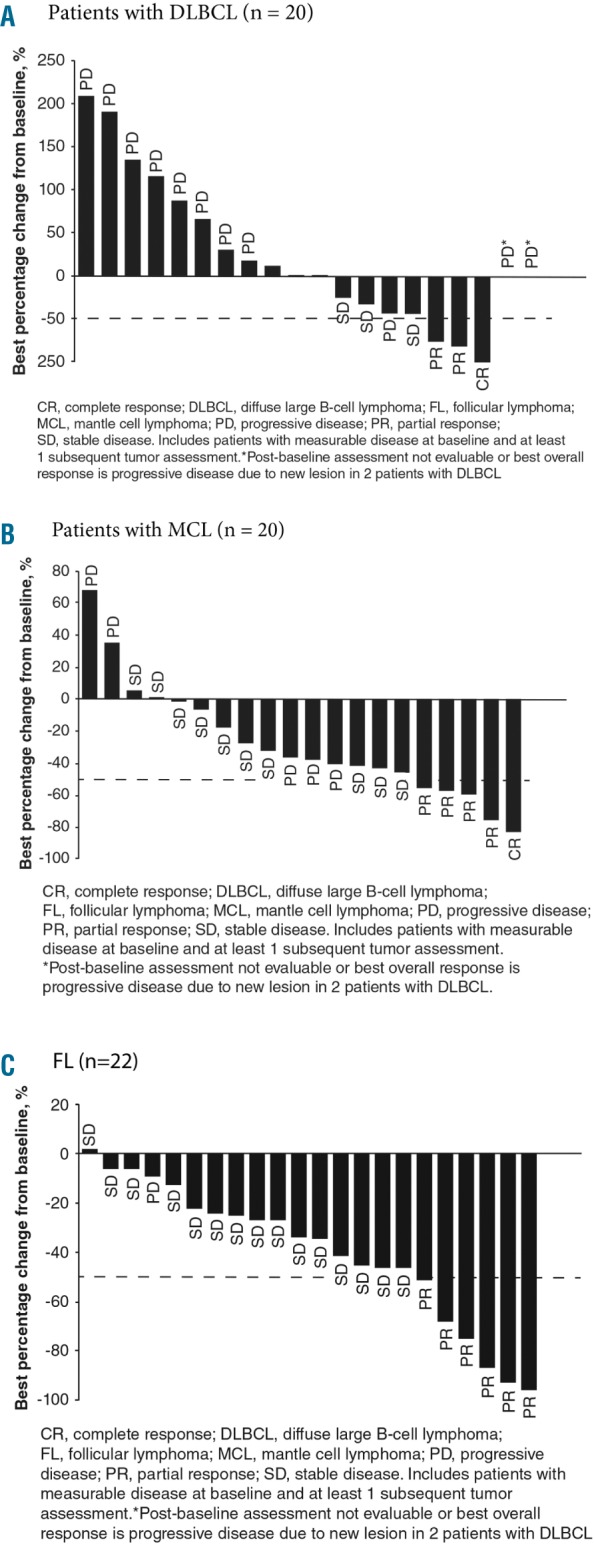
Best overall response with respect to best percentage change from baseline in investigator-assessed tumor size during buparlisib therapy for patients with measurable lesions divided by cohort The graphs include patients with measurable disease at baseline and ≥1 subsequent tumor assessments. The data cut-off was February 25, 2015, for all cohorts. (A) Patients with DLBCL (n=20), (B) patients with MCL (n=20), (C) patients with FL (n=22). Best overall response is indicated for each patient. Note that for two patients with DLBCL, the post-baseline assessment was not evaluable or best overall response was progressive disease due to a new lesion (asterisk). The dashed line shows the percentage change that represents the criterion for response, according to International Working Group criteria.34,35 CR: complete response; DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; IWG: International Working Group; MCL: mantle cell lymphoma; PD: progressive disease; PR: partial response; SD, stable disease.
Time to response and duration of response
For the patients in the DLBCL cohort who responded, the median time to response was 2.1 months (range, 1.8–3.5) and the median duration of response was 2.2 months (95% CI: 1.2-not estimable). Of the three patients who responded, two relapsed. In the MCL cohort, the median time to response was 1.8 months (range, 0.9–9.4), while the median duration of response was not reached; of the five patients who responded, one patient subsequently relapsed. In the FL cohort, the median time to response and duration of response were 3.5 months (range, 1.6–5.9) and 11.0 months (95% CI: 3.9-not estimable), respectively; of the six patients who responded, two relapsed.
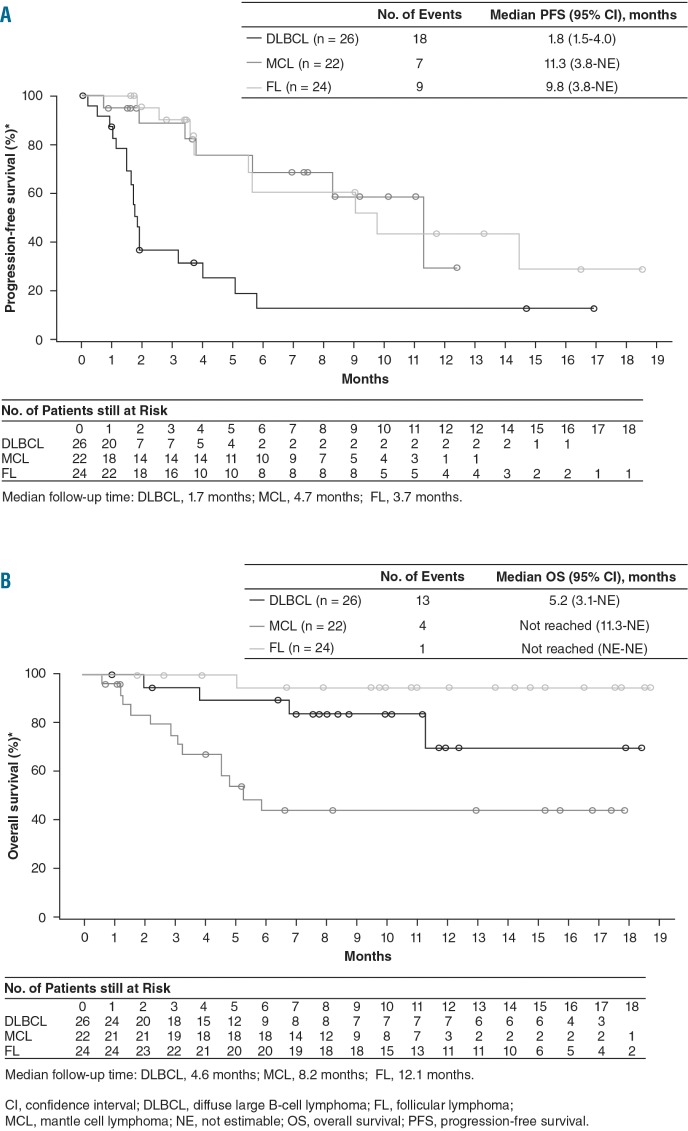
Progression-free survival
The median progression-free survival of patients with DLBCL, MCL, and FL was 1.8 months (95% CI: 1.5–4.0), 11.3 months (95% CI: 3.8-not estimable), and 9.8 months (95% CI: 3.8-not estimable), respectively (Figure 2A). The estimated 6-month progression-free survival rate was 12.6% (95% CI: 2.3–32.0) in the DLBCL cohort, 68.6% (95% CI: 39.8–85.7) in the MCL cohort, and 60.7% (95% CI: 31.7–80.6) in the FL cohort. The median follow-up time for progression-free survival was 1.7, 4.7, and 3.7 months for the DLBCL, MCL, and FL cohorts, respectively.
Figure 2.
Kaplan–Meier curves for progression-free survival and overall survival. Kaplan–Meier curves for the secondary endpoints by cohort (DLBCL, MCL, and FL) are shown here together, but the curves are not meant to be compared between cohorts as the study was not designed to compare the three cohorts. (A) Progression-free survival curves; median follow-up time: DLBCL, 1.7 months; MCL, 4.7 months; FL, 3.7 months. (B) Overall survival curves; median follow-up time: DLBCL, 4.6 months; MCL, 8.2 months; FL, 12.1 months. CI, confidence interval; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma; NE, not estimable; OS, overall survival; PFS, progression-free survival.
Overall survival
With a median follow-up time of 4.6 months, the median overall survival for the DLBCL cohort was 5.2 months (95% CI: 3.1-not estimable) (Figure 2B). For the MCL and FL cohorts the median follow-up time for overall survival was 8.2 months and 12.1 months, respectively, and the median overall survival was not reached for either cohort.
Safety
The median duration of exposure to buparlisib in the DLBCL, MCL, and FL cohorts was 7.5 weeks (range, 1.7–76.0), 20.6 weeks (range, 1.7–54.1), and 16.3 weeks (range, 3.3–81.3), respectively. As of the data cut-off, 47 patients (65.0%; 50.0%, 68.2%, and 79.2% in the DLBCL, MCL, and FL cohorts, respectively) had received buparlisib for ≥8 weeks and 21 patients (29.2%; 7.7%, 45.5%, and 37.5%, in the DLBCL, MCL, and FL cohorts, respectively) had received the drug for ≥24 weeks. Treatment was ongoing as of February 25, 2015, the data cut-off date, in nine patients (12.5%), including two with DLBCL, five with MCL, and two with FL. The remaining 63 patients (87.5%) had discontinued treatment (Table 1). The most common primary reasons for treatment discontinuation were disease progression (n=23; 31.9%) and adverse events (n=19; 26.4%).
Of the 22 patients (30.6%; DLBCL, n=7; MCL, n=7; FL, n=8) who required a dose reduction, the majority required only one dose reduction (54.5%: DLBCL, 4/7; MCL, 2/7; FL, 6/8), while only one patient in the FL cohort required ≥3 dose reductions (Online Supplementary Table S1). Of the patients requiring a dose reduction, all patients required a dose reduction due to adverse events (n=22: DLBCL, n=7; MCL, n=7; FL, n=8), and one patient in the FL cohort had an additional dose reduction due to a dosing error. The median percent of days with the full dose was 100% (range, 14.3–100%), 100% (range, 6.6–100%), and 97.4% (range, 4.2–100%) for patients in the DLBCL, MCL, and FL cohorts, respectively. Dose interruptions occurred in 36 patients (50.0%): 11 with DLBCL, 10 with MCL, and 15 with FL. Among patients whose dosing was interrupted, the reasons for the dose interruption included adverse events [n=32 (88.9%): DLBCL, n=11; MCL, n=9; FL, n=12], scheduling conflict [n=5 (13.9%): MCL, n=2; FL, n=3], and dosing error [n=4 (11.1%): MCL, n=1; FL, n=3]. The most common adverse events (occurring in >3 patients) that caused dose reduction or interruption were hyperglycemia (n=8), fatigue (n=6), and nausea (n=4).
Hyperglycemia, fatigue, and nausea (n=26; 36.1% each), depression (n=21; 29.2%), diarrhea (n=20; 27.8%), and anxiety (n=18; 25.0%) were the most frequently reported adverse events of any grade, regardless of their relationship with the study drug, occurring in >20% of patients (Table 3). The most frequently reported grade 3/4 adverse events, regardless of study drug relationship (occurring in ≥3 patients), were hyperglycemia (n=8; 11.1%), neutropenia (n=4; 5.6%), fatigue, nausea, anxiety, asthenia, confusional state, and thrombocytopenia (each n=3; 4.2%). Mood disturbances (e.g., anxiety, agitation, confusional state, depression, stress, psychiatric decompensation) of any grade were reported in 29 patients (40.3%); 24 patients (33.3%) had grade 1/2 mood disorders, and five patients (6.9%) had grade 3/4 mood disorders. Laboratory hematologic and biochemical abnormalities of any grade were reported in 63 patients (87.5%) and 71 patients (98.6%), respectively (Table 3). Grade 3/4 hematologic and biochemical abnormalities occurring in more than 10% of patients were lymphocytopenia (n=16; 22.2%), hyperglycemia (n=15; 20.8%), and neutropenia (n=9; 12.5%) (Table 3). Grade 3/4 infections were reported in four patients with DLBCL, four patients with MCL, and one patient with FL. Of these, serious infections were reported in two patients with DLBCL (pneumonia and urinary tract infection), two patients with MCL (pneumonia and septic shock), and one patient with FL (lung infection). Treatment for patients who had infectious complications was at the discretion of the investigator.
Table 3.
Most frequently reported adverse events and laboratory abnormalities, regardless of relationship with the study drug.
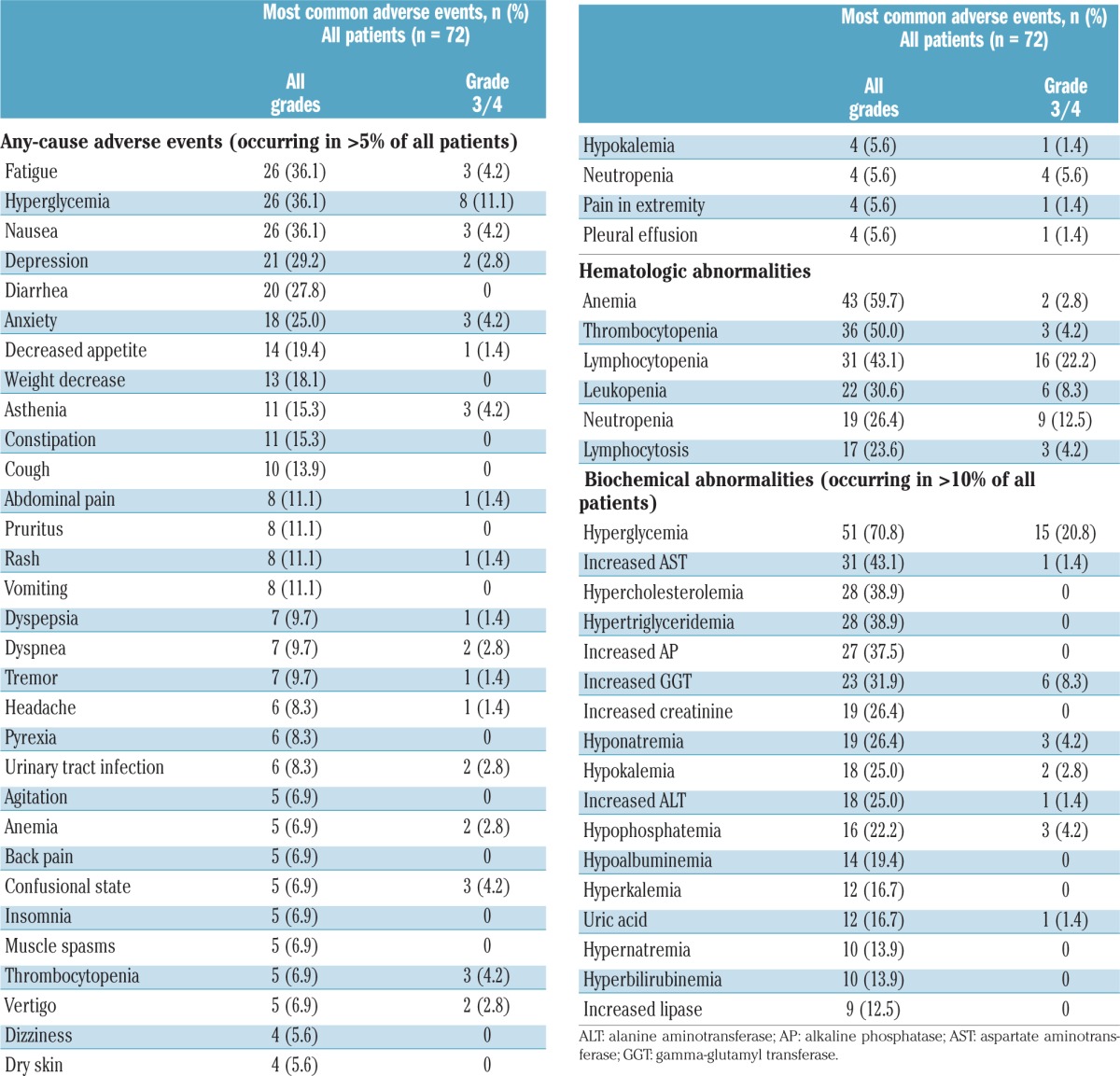
Serious adverse events, regardless of a relationship with the study medication, of any grade and grade 3/4 were reported in 30 patients (41.7%: DLBCL, n=12; MCL, n=12; FL, n=6) and 26 patients (36.1%: DLBCL, n=10; MCL, n=12; FL, n=4), respectively. The most common serious adverse events were confusional state (n=3), pleural effusion (n=3), nausea (n=2), vomiting (n=2), dysphagia (n=2), and pneumonia (n=2). Serious adverse events suspected to be related to study medication were reported in 14 patients (19.4%: DLBCL, n=3; MCL, n=9; FL, n=2), and were all grade 3/4. Overall, 18 patients died, including eight on treatment (occurring ≤30 days after study drug discontinuation): seven due to underlying DLBCL (n=6) and MCL (n=1), and one in the DLBCL cohort due to gastrointestinal hemorrhage suspected to be related to the study drug.
Exploratory analysis
Biomarkers were assessed using baseline archival tumor samples from 52 patients (72.2%) and fresh tumor samples from one patient (1.4%); all but one of the archival tumor samples were derived from the primary tumor. Of the patients with evaluable PI3KCA mutation status (19 with DLBCL, 11 with MCL, and 18 with FL) or PTEN mutation status (19 with DLBCL, 11 with MCL, and 17 with FL), none had mutations in PI3KCA or PTEN (Online Supplementary Table S2). Loss of PTEN expression was evaluable in 20, 12, and 20 patients with DLBCL, MCL, and FL, respectively; none of these patients met the criteria for loss of PTEN expression.
Discussion
Buparlisib elicited tumor reduction in patients with relapsed or refractory NHL; patients in all cohorts had reductions in tumor size, and the median progression-free survival was 1.8, 11.3, and 9.8 months in the DLBCL, MCL, and FL cohorts, respectively. The median overall survival, although not yet matured, was 5.2 months (95% CI: 3.1-not estimable) for the DLBCL cohort and was not reached for the MCL and FL cohorts.
The PI3K/mTOR pathway is frequently activated in NHL and plays an important role in the pathogenesis of this disease.14 However, mutations in key components of the PI3K pathway, such as PIK3CA, seem infrequent in this condition.10,36 Consistently, no PIK3CA or PTEN mutations were detected in the 42 tumor samples tested for mutations in this study. Despite previous reports of a reduction or loss of PTEN expression in approximately 21% of FL,36 37% of DLBCL,11 and 15% of MCL samples,23,37 no PTEN loss was observed among the 50 tumors tested in this study, which may be explained by the stringent cut-off used for defining a loss of expression (< 10% expression by immunohistochemistry). Further investigation, including a broader evaluation of PI3K activation markers or multivariate analyses of baseline characteristics, would be needed to identify subpopulations of patients who might respond to buparlisib.
Preclinical models suggest that all PI3K isoforms (PI3Kα, PI3Kb, and PI3Kd) are expressed in leukocytes,38 but that their relative levels of expression vary in NHL according to disease stage.23 Specific PI3K isoform ratios are associated with relapse and have been implicated in resistance to PI3Kd inhibitors, but not PI3Kα/PI3Kd inhibitors.23
Previous studies on PI3K inhibitors that specifically target one or two PI3K isoforms have met with some success in indolent NHL (overall response rate of 65% with duvelisib),39 in FL and in MCL (overall response rates of 45% and 40%, respectively, with idelalisib),40,41 but not in DLBCL (overall response rate of 0% with rigosertib).42 However, improved long-term outcomes (e.g., progression-free survival) with these agents have been limited in patients with relapsed or refractory disease; for example, the median progression-free survival of patients treated with idelalisib was 3.7 months among those with MCL and 7.6 to 11 months for patients with indolent NHL (the majority, 58% – 59%, with FL).20,40,41 Pan-PI3K inhibition results in similar or higher overall response rates (≤25% in DLBCL, 83% in MCL, and 20% - 50% in FL),43–45 with potentially longer progression-free survival. For example, in a study of the pan-PI3K inhibitor SAR245409, the overall response rate was 50% and the median progression-free survival was not reached after 8 months of follow-up in patients with relapsed or refractory FL.45 In our study, patients with FL on treatment with buparlisib had an overall response rate of 25.0% and a median progression-free survival of 9.8 months. Thus, it appears that sustained inhibition of all PI3K isoforms has some effect on achieving response, but may have a more pronounced benefit on long-term outcomes for patients with FL and MCL. In general, PI3K inhibitors have a manageable safety profile in NHL;46–48 however, the European Medicines Agency has recently opened a review of idelalisib due to new safety concerns over serious adverse events (mostly infections) in ongoing clinical trials.
Buparlisib was generally well tolerated. Hyperglycemia, although frequently reported, is a known on-target effect of PI3Kα inhibitors9 and, as in other buparlisib studies,30 was manageable with standard antidiabetic therapy. Mood disorders have been previously reported in studies of buparlisib49 owing, in part, to the ability of this drug to cross the blood-brain barrier.50 For this reason, in this study, patients with active psychiatric disorders at screening were excluded, and the patients enrolled were prospectively followed with self-assessment questionnaires (i.e., GAD-7 and PHQ-9). As in previous studies, some patients with NHL experienced mood disorders that were not serious and were manageable with dose reduction/interruption or treatment with appropriate concomitant medication. In addition, patients developing any of these adverse events were referred for psychiatric consultation. Loss of appetite and weight (Online Supplementary Table S3) in patients may compromise quality of life, as reported in some previous studies.51
Most treatment-related adverse events with buparlisib were grade 1/2 and could be managed with dose reduction or interruption until resolution of toxicity. The rates of grade 3/4 elevations in alanine aminotransferase and aspartate aminotransferase, pneumonia, and diarrhea were lower with buparlisib than with a selective PI3Kd inhibitor in patients with relapsed or refractory NHL.20
Although the overall response rate with buparlisib was not substantial, the observed progression-free survival in this study warrants further evaluation of this agent in hematologic cancers. Combination of buparlisib with other treatments (i.e., targeted therapies, monoclonal anti-CD20 antibodies, checkpoint inhibitors, or standard chemotherapies) should be considered, particularly for patients with FL and MCL.
Supplementary Material
Acknowledgments
We thank the patients and their caregivers for their participation in this trial and the other investigators who enrolled patients into the study: from Belgium, Marc André (n=3) and Achiel van Hoof (n=1); from France, Reda Bouabdalla (n=3) and Thierry Lamy (n=1); from Germany, Maria-Elisabeth Goebeler (n=2) and Oliver Ottmann (n=1); from Italy, Daniele Laszlo (n=5) and Paolo Corradini (n=3); from Korea, Hyeon Seok Eom (n=1); from Turkey, Fahri Sahin (n=3); and from the USA, Alice Mims (n=2) and Jason Westin (n=1).
We would also like to thank Nabanita Mukherjee for support with the statistical analysis, Joe Sulovski for trial design, study conduct, and review of early versions of the manuscript, Fabian Herbst for review of early versions of the manuscript, and Pamela Tuttle, PhD, CMPP, and Katherine Mills-Luján, PhD, of ArticulateScience, LLC for medical editorial assistance, which was funded by Novartis Pharmaceuticals Corporation.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/12/2104
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;62(2):87–108. [DOI] [PubMed] [Google Scholar]
- 3.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89(11):3909–3918. [PubMed] [Google Scholar]
- 4.Anderson JR, Armitage JO, Weisenburger DD. Epidemiology of the non-Hodgkin’s lymphomas: distributions of the major subtypes differ by geographic locations. Non-Hodgkin’s Lymphoma Classification Project. Ann Oncol. 1998;9(7):717–720. [DOI] [PubMed] [Google Scholar]
- 5.Howlader N, Noone AM, Krapcho M, et al. (eds.) SEER Cancer Statistics Review, 1975–2013, National Cancer Institute; http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016. [Google Scholar]
- 6.Armitage JO. Treatment of non-Hodgkin’s lymphoma. N Engl J Med. 1993;328(14): 1023–1030. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: non-Hodgkin’s lymphoma. Version 3. 2016: http://www.nccn.org. Accessed July 2016.
- 8.Chao MP. Treatment challenges in the management of relapsed or refractory non-Hodgkin’s lymphoma - novel and emerging therapies. Cancer Manag Res. 2013;5:251–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–675. [DOI] [PubMed] [Google Scholar]
- 10.Rudelius M, Pittaluga S, Nishizuka S, et al. Constitutive activation of Akt contributes to the pathogenesis and survival of mantle cell lymphoma. Blood. 2006;108(5):1668–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abubaker J, Bavi PP, Al-Harbi S, et al. PIK3CA mutations are mutually exclusive with PTEN loss in diffuse large B-cell lymphoma. Leukemia. 2007;21(11):2368–2370. [DOI] [PubMed] [Google Scholar]
- 12.Dal Col J, Zancai P, Terrin L, et al. Distinct functional significance of Akt and mTOR constitutive activation in mantle cell lymphoma. Blood. 2008;111(10):5142–5151. [DOI] [PubMed] [Google Scholar]
- 13.Baohua Y, Xiaoyan Z, Tiecheng Z, Tao Q, Daren S. Mutations of the PIK3CA gene in diffuse large B cell lymphoma. Diagn Mol Pathol. 2008;17(3):159–165. [DOI] [PubMed] [Google Scholar]
- 14.Jabbour E, Ottmann OG, Deininger M, Hochhaus A. Targeting the phosphoinositide 3-kinase pathway in hematologic malignancies. Haematologica. 2014;99(1): 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13(2):140–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beer-Hammer S, Zebedin E, von Holleben M, et al. The catalytic PI3K isoforms p110gamma and p110delta contribute to B cell development and maintenance, transformation, and proliferation. J Leukoc Biol. 2010;87(6):1083–1095. [DOI] [PubMed] [Google Scholar]
- 17.Chantry D, Vojtek A, Kashishian A, et al. p110delta, a novel phosphatidylinositol 3-kinase catalytic subunit that associates with p85 and is expressed predominantly in leukocytes. J Biol Chem. 1997;272(31):19236–19241. [DOI] [PubMed] [Google Scholar]
- 18.Puri KD, Gold MR. Selective inhibitors of phosphoinositide 3-kinase delta: modulators of B-cell function with potential for treating autoimmune inflammatory diseases and B-cell malignancies. Front Immunol. 2012;3:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benson DM, Kahl BS, Furman RR, et al. Final results of a phase I study of idelalisib, a selective inhibitor of PI3K, in patients with relapsed or refractory indolent non-Hodgkin lymphoma (iNHL). J Clin Oncol. 2013;31(suppl):8526. [Google Scholar]
- 20.Gopal AK, Kahl BS, de Vos S, et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370(11):1008–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zydelig idelalisib. Package insert. 2014. [Google Scholar]
- 22.Zydelig idelalisib. Summary of product characteristics. 2014. [Google Scholar]
- 23.Iyengar S, Clear A, Bödör C, et al. P110alpha-mediated constitutive PI3K signaling limits the efficacy of p110delta-selective inhibition in mantle cell lymphoma, particularly with multiple relapse. Blood. 2013;121(12):2274–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okosun J, Wolfson RL, Wang J, et al. Recurrent mTORC1-activating RRAGC mutations in follicular lymphoma. Nat Genet. 2016;48(2):183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foukas LC, Berenjeno IM, Gray A, Khwaja A, Vanhaesebroeck B. Activity of any class IA PI3K isoform can sustain cell proliferation and survival. Proc Natl Acad Sci USA. 2010;107(25):11381–11386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabe Y, Jin L, Konopleva M, et al. Class IA PI3K inhibition inhibits cell growth and proliferation in mantle cell lymphoma. Acta Haematol. 2014;131(1):59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zang C, Eucker J, Liu H, et al. Inhibition of pan-class I phosphatidyl-inositol-3-kinase by NVP-BKM120 effectively blocks proliferation and induces cell death in diffuse large B-cell lymphoma. Leuk Lymphoma. 2014;55(2):425–434. [DOI] [PubMed] [Google Scholar]
- 28.Walsh KJ, Fan S, Patel A, et al. PI3K inhibitors inhibit lymphoma growth by downregulation of MYC-dependent proliferation. Blood. 2009;114(22):1697. [Google Scholar]
- 29.Matas-Cespedes A, Rodriguez V, Kalko SG, et al. Disruption of follicular dendritic cells-follicular lymphoma cross-talk by the pan-PI3K inhibitor BKM120 (buparlisib). Clin Cancer Res. 2014;20(13):3458–3471. [DOI] [PubMed] [Google Scholar]
- 30.Rodon J, Bra AI, Siu LL, et al. Phase I dose-escalation and -expansion study of buparlisib (BKM120), an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. Invest New Drugs. 2014;32(4):670–681. [DOI] [PubMed] [Google Scholar]
- 31.Ma CX, Luo J, Naughton M, et al. A phase I study of BKM120 and fulvestrant in postmenopausal women with estrogen receptor positive metastatic breast cancer. Cancer Res. 2015;75(9):abstract PD5–6. [Google Scholar]
- 32.Mayer IA, Abramson VG, Isakoff SJ, et al. Stand up to cancer phase Ib study of pan-phosphoinositide-3-kinase inhibitor buparlisib with letrozole in estrogen receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2014;32(12):1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saura C, Bendell J, Jerusalem G, et al. Phase Ib study of buparlisib plus trastuzumab in patients with HER2-positive advanced or metastatic breast cancer that has progressed on trastuzumab-based therapy. Clin Cancer Res. 2014;20(7):1935–1945. [DOI] [PubMed] [Google Scholar]
- 34.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. [DOI] [PubMed] [Google Scholar]
- 35.Cheson BD. The International Harmonization Project for response criteria in lymphoma clinical trials. Hematol Oncol Clin North Am. 2007;21(5):841–854. [DOI] [PubMed] [Google Scholar]
- 36.Yahiaoui OI, Nunes JA, Castanier C, et al. Constitutive AKT activation in follicular lymphoma. BMC Cancer. 2014;14:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Psyrri A, Papageorgiou S, Liakata E, et al. Phosphatidylinositol 3′-kinase catalytic subunit alpha gene amplification contributes to the pathogenesis of mantle cell lymphoma. Clin Cancer Res. 2009;15(18):5724–5732. [DOI] [PubMed] [Google Scholar]
- 38.Vanhaesebroeck B, Welham MJ, Kotani K, et al. P110delta, a novel phosphoinositide 3-kinase in leukocytes. Proc Natl Acad Sci USA. 1997;94(9):4330–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flinn I, Oki Y, Patel M, et al. A phase 1 evaluation of duvelisib (IPI-145), a PI3K-δ, inhibitor, in patients with relapsed/refractory iNHL. Blood. 2014;124:(21):802. [Google Scholar]
- 40.Flinn IW, Kahl BS, Leonard JP, et al. Idelalisib, a selective inhibitor of phosphatidylinositol 3-kinase-delta, as therapy for previously treated indolent non-Hodgkin lymphoma. Blood. 2014;123(22): 3406–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kahl BS, Spurgeon SE, Furman RR, et al. A phase 1 study of the PI3Kdelta inhibitor idelalisib in patients with relapsed/refractory mantle cell lymphoma (MCL). Blood. 2014;123(22):3398–3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roschewski M, Farooqui M, Aue G, Wilhelm F, Wiestner A. Phase I study of ON 01910.Na (rigosertib), a multikinase PI3K inhibitor in relapsed/refractory B-cell malignancies. Leukemia. 2013;27(9):1920–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dreyling M, Morschhauser F, Bron D, et al. Preliminary results of a phase II study of single agent Bay 80-6946, a novel PI3K inhibitor, in patients with relapsed/refractory, indolent or aggressive lymphoma. Blood. 2013;122(21):87. [Google Scholar]
- 44.Brown JR, Davids MS, Rodon J, et al. Update on the safety and efficacy of the pan class I PI3K inhibitor SAR245408 (XL147) in chronic lymphocytic leukemia and non-Hodgkin’s lymphoma patients. Blood. 2013;122:4170. [Google Scholar]
- 45.Brown JR, Hamadani M, Arnason J, et al. SAR245409 monotherapy in relapsed/refractory follicular lymphoma: preliminary results from the phase II ARD12130 study. Blood. 2013;122(21):86. [Google Scholar]
- 46.Coutre S, Barrientos J, Brown J, et al. Safety of idelalisib in B-cell malignancies: integrated analysis of eight clinical trials. Haematologica. 2015;100(Suppl 1):P588. [Google Scholar]
- 47.O’Brien S, Faia K, White K, et al. Early clinical activity and pharmacodynamic effect of duvelisib, a PI3K-delta, gamma inhibitor, in patients with treatment-naïve CLL. Haematologica. 2015;100(Suppl 1):S434. [Google Scholar]
- 48.Burris HA, Patel MR, Fenske TS, et al. TGR-1202, a novel once daily PI3K-delta inhibitor, demonstrates clinical activity with a favorable safety profile, lacking hepatotoxicity, in patients with CLL and B-cell lymphoma. Haematologica. 2015;100(Suppl 1):S432. [Google Scholar]
- 49.Bendell JC, Rodon J, Burris HA, et al. Phase I, dose-escalation study of BKM120, an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30(3):282–290. [DOI] [PubMed] [Google Scholar]
- 50.Nanni P, Nicoletti G, Palladini A, et al. Multiorgan metastasis of human HER-2(+) breast cancer in Rag2(−/−);Il2rg(−/−) mice and treatment with PI3K inhibitor. PLoS One. 2012;7(6):e39626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simons JP, Aaronson NK, Vansteenkiste JF, et al. Effects of medroxyprogesterone acetate on appetite, weight, and quality of life in advanced-stage non-hormone-sensitive cancer: a placebo-controlled multicenter study. J Clin Oncol. 1996;14(4):1077–1084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.